Abstract
Objectives
Infections contribute to morbidity and mortality in lupus, here we asked if they might also play a role in its pathogenesis. Uropathogenic E. coli (UPEC) cause urinary tract infections (UTIs) and form biofilms, multicellular communities of bacteria that are strengthen by amyloids such as curli. We previously reported that curli naturally form complexes with DNA (curli/eDNA) and these complexes induce hallmarks of lupus disease in mouse models.
Methods
We investigated 96 SLE patients who met at least 4 SLICC criteria. We tested the presence of anti-curli/eDNA complex antibodies for both IgG and IgA subclasses. Results were compared to 54 age, sex and race matched healthy controls. We then correlated the levels of anti-curli/eDNA antibodies with clinical parameters, lupus disease status and frequency of bacteriuria.
Results
We found anti-curli/eDNA antibodies in SLE and controls plasma, and their levels correlated with asymptomatic persistent bacteriuria and disease flares in lupus patients. Persistent bacteriuria contained curli-producing UPEC and associated with an inflammatory phenotype. Finally, curli/eDNA complexes cross-reacted with lupus autoantigens such as dsDNA in binding autoantibodies.
Conclusions
We propose that UTIs or persistent bacteriuria are environmental triggers of lupus and its flares. Antibodies against curli/eDNA could serve as a sign of systemic exposure to bacterial products in lupus.
INTRODUCTION
The leading cause of morbidity and mortality in lupus is infections, particularly bacterial infections(1) that involve the upper respiratory tract, the skin, and the urinary tract, and the most common responsible microorganisms are S. pneumoniae, S. aureus, and E. coli, respectively(1–6). Whether they influence SLE pathogenesis is an important area of research. Interestingly, these infections are characterized by the generation of biofilms, multicellular communities of bacteria that serve several important functions, such as bacterial cell-cell adhesion and protection from oxidative stress and antimicrobial agents(7). A major component of the extracellular matrix of enteric bacterial biofilm is the bacterial amyloid curli(8, 9). Curli-producing bacteria such as E. coli can trigger the innate immune system via a variety of pathways, such as TLR1/2, TLR4, TLR9, and the inflammasome(10, 11), thereby constituting a potential immune trigger for individuals genetically predisposed to SLE. We have discovered that curli amyloid is naturally complexed to bacterial extracellular DNA (curli/eDNA) and this complex is a powerful inducer of lupus autoimmunity in mouse models(12). Importantly, curli/eDNA complexes stimulate dendritic cells(12) and macrophages(13) and trigger the production of type I IFNs, a fundamental pathway in lupus pathogenesis. Moreover, the induction of lupus in mice is TLR2- and TLR9-dependent, suggesting that the amyloid part of curli stimulates TLR2 and the eDNA part triggers TLR9(13). The current work aimed at investigating if curli/eDNA complexes induce an immune response in SLE patients and healthy controls, and if they play a role in human lupus pathogenesis.
PATIENTS AND METHODS
Patients and Controls
Plasma samples from 96 SLE patients randomly selected from the Temple Lupus Cohort (TLC) were included in this study. The TLC is an IRB-approved prospective cohort of lupus patients followed in the LKSOM Temple University Lupus Program. After informed consent was obtained, blood and urine were obtained from patients who fulfilled at least four of the SLICC Criteria(14). Blood was processed as described previously (15). At each clinic visit each TLC participant has a complete history and physical exam, medication, laboratory data, including general labs, anti-dsDNA levels, C3/C4 levels as well as urine spot protein to creatinine ratio (P/C) and urine microscopic analysis. We used the latter to determine the levels of bacteriuria, which was graded with routine analysis by commercial laboratories as follows: none, +, ++, +++ based on the number of bacteria per high power field. Reflexed culture was performed only in those few with ≥ +++ and the majority was E. coli. Disease activity and damage indices (SLEDAI and SLICC/ACR DI) are also recorded as they are built within the electronic medical record used at Temple University. All the variables are extracted monthly. Plasma and urine are collected, aliquoted and stored from each patient at −80°C. We enrolled and tested plasma from up to 85 females and 11 males SLE patients, and 54 normal age-, sex-, race and ethnicity-matched controls in the study. A Ficoll Plaque plus (GE Sciences catalog 17–1440-02) gradient was used to separate plasma by centrifuging at 400XG for 30 minutes at room temperature, brake off. For bacteriuria studies we enrolled patients who had at least three urine samples analyzed in their patient history in the span of two years. Patients’ demographics are described in Table 1.
Table 1.
Patients’ Demographics
| Bacteriuria | Never | Intermittent | Persistent | p-value* |
|---|---|---|---|---|
| Age (median years) | 48 (10.3) | 37.2 (13.4) | 39.2 (12.2) | n.s. |
| Sex (% female) | 66 | 100 | 100 | <0.02 |
| Race/Ethnicity (n) | ||||
| African American | 6 | 7 | 6 | n.s. |
| Hispanic | 1 | 3 | n.s. | |
| Other | 1 | 2 | 1 | n.s. |
| Medications (% usage) | ||||
| Hydroxychloroquine | 100 | 100 | 80 | n.s. |
| Immunosuppressants | 75 | 88 | 80 | n.s. |
| Prednisone | 62.5 | 88 | 80 | n.s. |
Values ≤ 0.05 were considered significant. n.s.= not significant
Bacterial preparations and Curli/eDNA complexes
Curli-DNA complexes were prepared using the protocol previously described(12). Briefly, overnight cultures of E.coli or Salmonella Typhimurium were grown in LB with proper antibiotic selection with shaking (200 rpm) at 37°C. Overnight cultures were diluted 1:100 in YESCA broth with 4% DMSO to enhance curli formation(13), and grown in an incubator at 26°C for 72 hours with shaking (200 rpm). Bacterial pellets were collected and resuspended in 10 mM Tris-HCl, pH 8.0 and treated with 0.1 mg/mL RNase A (Sigma, R5502) from bovine pancreas, 0.1 mg/mL DNase I (Sigma, DN25), and 1 mM MgCl2 for 20 minutes at 37°C. The bacterial cells were broken by sonication (30% amplification for 30 seconds, repeated twice). Lysozyme (1 mg/mL, Sigma, L6876) was added, and the mixture was incubated at 37°C. After 40 minutes, 1% SDS was added, and samples were incubated for 20 minutes at 37°C with shaking (200 rpm). The fibers were pelleted by centrifugation (10,000 rpm for 10 minutes at room temperature) and resuspended in 10 mL Tris-HCL, pH 8 and boiled for 10 minutes. A second digestion with RNase A, DNase I, and lysozyme, followed by boiling, was performed as described above. The fibers were pelleted (10,000 rpm for 10 minutes at room temperature). Samples were boiled in 1X SDS-PAGE buffer and run on a 12% running/3–5% stacking gel for 5 hours at 20 mA. The fibers that accumulated at the top of the gel were collected, washed three times with sterile water and then extracted twice with 95% ethanol. Only the curli amyloid remains at the end of this procedure.
Before being used, the fibers were sonicated at 30% amplitude for 30 seconds to disrupt any large aggregates. Due to the partial solubility of the curli fibers, the final prep was further centrifuged at 5,000 rpm for 5 minutes to discard the largest complexes. A clear supernatant was therefore obtained and the concentration of the curli fibers was determined using BCA reagents according to the manufacturer’s protocol (Pierce BCA 23225). We controlled for consistent concentration of curli/eDNA coated on plates using the same internal controls in all the ELISA performed.
GST-CsgA purification
Plasmid pSW5–50, containing the CsgA gene cloned in the gluthathione S transferase (GST) fusion protein vector pGEX-4T-2 was described previously (16). Fusion proteins GST-CsgA were purified from E. coli strains DH5α (pGEX-4T-2) using a GST purification kit with 1 g of bacterial lysate. Gel electrophoresis and Coomassie blue staining determined purified GST-CsgA. (Millipore 70794–3).
Anti-curli/eDNA ELISA
We generated a new ELISA to measure anti-curli/eDNA Abs. Briefly, on day 1, plates were coated with curli/eDNA complexes, purified as described above (12), at a concentration at 1.5 μg/mL in Borate solution (BBS) on polyvinyl microtiter plates (Thermofisher 2801). Plates were incubated overnight at 4°C. On day 2, blocking buffer (0.5% BSA, 0.4% tween, in BBS) was added for three hours. Plates were then washed 5 times with BBS and human plasma was added to each well at 1:250 dilution and incubated overnight at 4°C. On day 3, plates were washed 5 times with BBS. Goat anti-human IgG or goat anti-human IgA biotinylated antibody (1:5,000 dilution) was used for 2 hours at room temperature (Jackson ImmunoResearch). The plates were washed, then avidin phosphatase (1:8000 dilution) was added for 2 hrs at room temperature (Sigma). Finally, plates were washed and a para-nitrophenylphosphate (PNPP) solution 1mg/ml (Sigma) was freshly made and added to the plates (100 μL). Plates were read at 450nm emission wavelength every half hour for two hours. Since it was reported that sepsis patients have anti-curli antibodies(17), a septic patient with high levels of anti-curli antibodies was used as a standard curve and positive control.
Polyclonal antibody anti-curli/eDNA
To check the specificity of our ELISA, in a separate test a rabbit polyclonal anti-curli antibody was used as the primary antibody. Briefly, serum from rabbits injected with curli/eDNA complexes was used as the primary antibody in our anti-curli/eDNA ELISA as described previously(18). As described above, curli/eDNA complexes were coated on ELISA plates. Then, the rabbit polyclonal anti-curli/eDNA antibody (1:1,000) was added to the coated plates. After 2 hours incubation, the plates were washed and secondary anti-rabbit IgG conjugated with alkaline phosphatase (1:5,000, Jackson ImmunoResearch) was added. After 2 hours incubation, plates were washed with BSS. PNPP was used as substrate and read at 450nm wavelength.
Anti-dsDNA ELISA
Monoclonal antibody anti-dsDNA
A mouse monoclonal anti-dsDNA antibody (1:2,000 dilution, Abcam [215906]) was used to test if anti-dsDNA Abs could bind to curli/eDNA complexes. This was followed by an anti-mouse secondary Ab conjugated with alkaline phosphatase. As a positive control, we found this antibody could bind to calf thymus dsDNA (2.5 μg/mL). In addition, anti-dsDNA mAb could bind to curli/eDNA complexes (1.5 μg/mL) isolated from Salmonella bacteria.
Anti-Bacterial-DNA ELISA
We performed anti-DNA ELISA in which ultrapure antigen DNA, extracted from Escherichia coli (Strain K-12) genomic DNA (Sigma) was used as Ag coated on the ELISA plate. Alternatively, E. coli K12 dsDNA, bought from InvivoGen (catalog tlrl-ecdna) and suspended in sterile water, was used as antigen.
Competitive ELISA
A competitive ELISA was performed using SLE plasma (1:250 dilution) incubated with curli/eDNA (200 μg/mL), or calf thymus dsDNA (200 μg/mL) for 2.5 hours in a 1.5 mL tube with BBS on a plate shaker set at 100 rpm. The tubes with the co-incubation solutions were spun at 14,000 rpm at 4°C for 15 minutes to allow large curli/eDNA complexes to precipitate and then we compared in ELISA the two preparations. We noted that there was no difference in the final O.D. when we used spun and unspun samples. We transferred the samples onto a curli/eDNA coated ELISA plate or dsDNA coated ELISA plate. Then the ELISA protocol was followed as described above for anti-curli/eDNA. For anti-dsDNA ELISA, we coated ELISA plates with poly-lysine for 1 hour at room temperature. Plates were washed with 1x BBS then calf thymus DNA (2.5 μg/mL) was added to the plates and incubated overnight at 4°C. Plates were blocked with 3% goat serum in BBS. We added SLE plasma, which had been co-incubated or not with dsDNA or curli/eDNA complexes as described above, and then on plates for 2 hours at room temperature. Then plates were washed, and the goat anti-human IgG secondary antibody conjugated with alkaline phosphatase (1:5,000 dilution) was added. Plates were developed with para-nitrophenylphosphate as above.
Bacterial biofilms from clinical isolates
Uropathogenic E. coli bacteria were isolated from urine samples and grown using MacConkey agar. For visual characterization of curli expression in clinical isolates, bacterial strains were grown at 37 °C overnight in LB broth and then 5 μL of culture was spotted on yeast extract supplemented with casamino acids (YESCA) agar plates supplemented with 40 μg/mL Congo Red and 20 μg/mL Coomassie Blue and grown at 28 °C for 72 hours (19). To assess the biofilm formation capacity of the bacterial isolates, the crystal violet assay, with minor modifications of a previously reported method by Crawford et al. (20), was performed. Briefly, 96-well microplate wells were inoculated with 200 μL of LB low salt and 5 μL of overnight bacterial culture grown in LB broth. Plates were incubated for 72 hours at 28°C. Next, excess cells were removed, wells were washed thrice with PBS, dried at room temperature for an hour, and then a solution of 0.5% crystal violet (in isopropanol-methanol-1x PBS 1:1:18) was added to each well for 5 min at room temperature. The plates were rinsed with double-distilled water five times. Crystal violet was extracted with 33% acetic acid and quantified with optical density readings at 570 nm.
Statistical Analysis
Experiments were analyzed using Graphpad Prism. Unpaired two-tailed t-test was used to analyze differences among groups for parametric data and Welch’s t-test was used to compare two unequal sample sizes. ANOVA was used when multiple groups were compared. Non-parametric data comparisons were analyzed by the Mann Whitney-U test. Statistical significance was determined by a p value of <0.05.
RESULTS
Lupus patients and healthy controls generate antibodies against curli/eDNA complexes
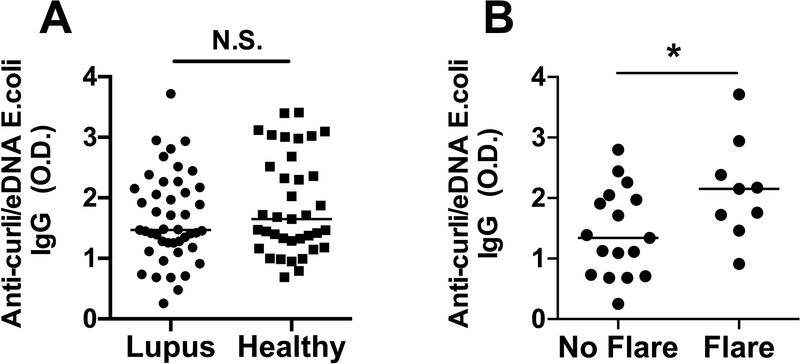
Using a newly established ELISA, we measured the levels of antibodies against curli/eDNA complexes in plasma of SLE patients from the Lupus Program at Temple University and of age- race- and sex-matched healthy controls. Both SLE patients (median O.D. = 1.47, mean ± SEM = 1.65 ± 0.10) and healthy subjects (median O.D. = 1.65, mean ± SEM = 1.87 ± 0.13) had variable levels of IgG against curli/eDNA complexes, with no statistically significant difference between the two groups (Figure 1A). We found similar results when we measured the IgG Abs against curli/eDNA purified from E. coli (Figure 1) and S. Typhimurium biofilms (Supplementary Fig. 1A), as predicted due to the similarities in three-dimensional structure and sequence between the two curli amyloids (18, 21). The biological significance and specificity of these antibodies were confirmed by the higher levels of anti-curli/eDNA IgG in both SLE patients and healthy controls compared to a patient with common variable immune deficiency (CVID), who is deficient in antibody production (Supplementary Fig. 1B). These results indicate that most human subjects, regardless of their health status, have been exposed to enterobacterial species expressing curli and developed specific antibodies. Our results indicate that the human immune system mounts a humoral immune response against these important components of bacterial biofilms.
Figure 1. Plasma anti-curli/eDNA Abs correlate with flares in SLE patients.
A) IgG Abs against curli/eDNA purified from E. coli biofilm were measured by ELISA in plasma of SLE patients, median = 1.47 O.D. and age- and sex-matched healthy controls median = 1.65 O.D.. B) Levels of IgG anti-curli/eDNA Abs in SLE patients in flare, median = 2.15, and in remission, median = 1.34 O.D.. Statistical significance was calculated with unpaired t-test with Welch correction.
Anti-curli/eDNA antibodies correlate with lupus flares
Next, we addressed whether the humoral immune response to curli/eDNA correlates with the occurrence of lupus flares as measured by increased disease activity. We defined a flare as an increase of ≥3 points in SLEDAI from the prior visit (22) and we found that SLE patients during flares had significantly higher levels of anti-curli/eDNA IgG (median 2.15, mean ± SEM = 2.13 ± 0.27) compared to those in remission (median 1.34, mean ± SEM = 1.42 ± 0.17) (Figure 1B with curli/eDNA from E.Coli)(Supplementary Fig. 1C with curli/eDNA from Salmonella), suggesting that an immune response against curli/eDNA is associated with lupus flares.
Curli/eDNA complexes cross-react with lupus autoantigen dsDNA
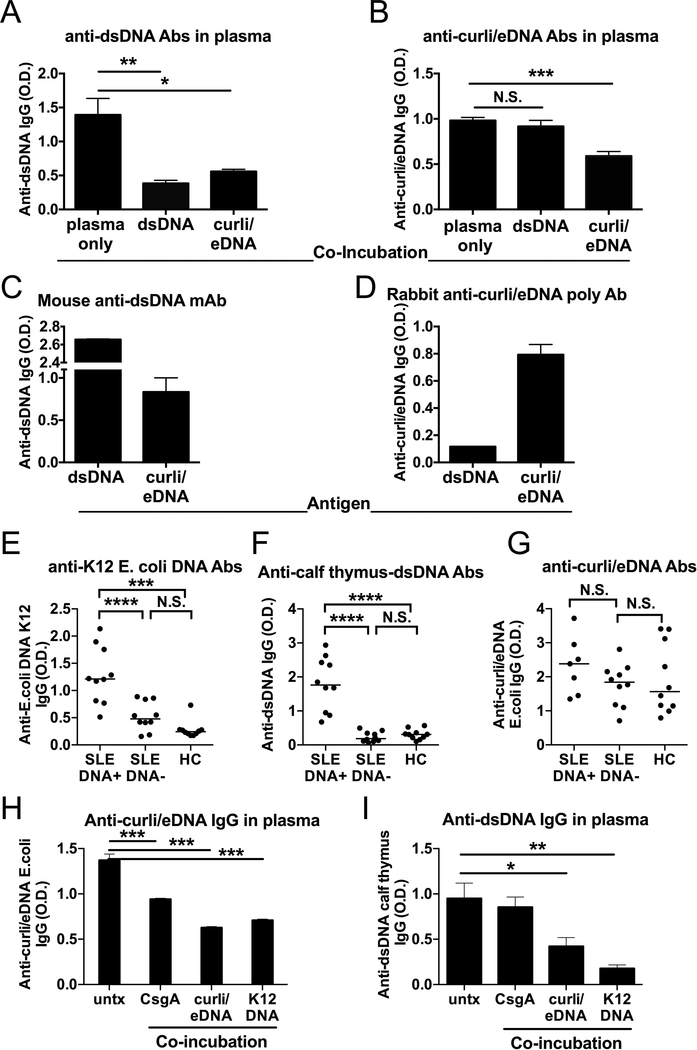
We performed a series of competitive ELISAs where dsDNA or curli/eDNA were alternatively used as soluble competitors and as antigens coated on the ELISA plates. Using dsDNA from calf thymus, which is normally used as antigen in the ELISA to detect anti-dsDNA autoAbs, we found that curli/eDNA could block both the binding of IgG Abs to curli/eDNA itself and to dsDNA, (Figure 2A–B), suggesting that both anti-curli/eDNA Abs and anti-dsDNA autoAbs can bind structures on curli/eDNA. We used plasma from a representative SLE patient with high anti-dsDNA and anti-curli/eDNA antibodies. For the competitive co-incubation, we chose the dose of 200 μg/mL of curli/eDNA or dsDNA after performing dose-titrations using 2, 20 and 200 μg/mL of each molecule to separately co-incubate with plasmas (Supplementary Fig. 1D–E). In contrast, dsDNA could only block the binding of anti-dsDNA autoAbs, and not of anti-curli/eDNA Abs (Figure 2A–B), suggesting that most of the anti-curli/eDNA Abs either are specific for the protein curli or for epitopes that are a combination of DNA and curli rather than DNA itself (Figure 2A–B). We confirmed the binding to dsDNA contained in the curli/eDNA complexes using a commercially available monoclonal antibody against dsDNA. This mAb was able to bind curli/eDNA complexes (Figure 2C) as much as one polyclonal antibody specific for curli, which could bind curli/eDNA but not dsDNA when these were used as antigens in the ELISA (Figure 2D). The fact that the levels of anti-curli/eDNA Abs were not significantly different in SLE patients positive or negative for anti-dsDNA autoAbs (Supplementary Fig. 2A) indicates that most of the anti-curli/eDNA Abs recognize curli/eDNA as complexes and are not anti-dsDNA autoAbs binding curli/eDNA, confirming their specificity for the bacterial product. It is worth mentioning that the levels of anti-dsDNA autoAbs were not different between SLE patients flaring (mean ± SEM = 2.80±0.43) or in remission (mean ± SEM = 2.06±0.38) (Supplementary Fig. 2B), as it was shown before (23) and in contrast to what we found for anti-curli/eDNA IgG (Figure 1B).
Figure 2. Curli/eDNA complexes cross-react with lupus autoantigen dsDNA.
ELISA for A) anti-dsDNA IgG Abs and (B) anti-curli/eDNA IgG Abs was performed on a representative SLE patient after co-incubation with calf thymus dsDNA or curli/eDNA complexes in suspension. C) ELISA used dsDNA as antigen and a mouse monoclonal Ab against dsDNA. D) ELISA used curli/eDNA as antigen and a rabbit polyclonal Ab against curli/eDNA complexes. In A-D, results are representative of 3 experiments. ELISA for anti-K12 E. coli DNA IgG (E), anti-calf thymus dsDNA IgG (F) and anti-curli/eDNA IgG Abs (G) in SLE patients anti-dsDNA Ab positive or negative by hospital records vs Healthy Controls (HC). Competitive ELISA with SLE plasma for anti-curli/eDNA IgG Abs (H) and anti-dsDNA Abs (I) after co-incubation with CsgA, curli/eDNA, and K12 DNA. Statistical significance was calculated with ANOVA and post-hoc t-test (E-I).
Antibodies specific for bacterial DNA have been previously described in SLE patients, in part cross-reacting and in part distinct from antibodies against mammalian DNA (24). Therefore, we compared the levels of anti-curli/eDNA IgG Abs with the pattern of antibodies binding mammalian DNA vs. DNA from E. coli. Healthy individuals and Lupus patients had similar levels of anti-curli/eDNA IgG Abs (Figure 1A and 2G), while only Lupus patients who were positive for anti-dsDNA Abs, as measured by hospital routine lab work, had high levels of antibodies recognizing DNA from the commensal E. coli K12 strain (Figure 2E) and mammalian DNA from calf thymus (Figure 2F). Lupus patients who were negative for anti-dsDNA Abs had lower levels of IgG against E. coli DNA and no antibodies recognizing calf thymus DNA, as little as the healthy controls. Our results suggest that the healthy immune system produces antibodies against curli/eDNA complexes but not against pure E. coli DNA, which instead occur in SLE patients, possibly leading to the cross-reaction with host DNA. As previously reported (24), some SLE patients had antibodies against bacterial DNA without antibodies against host DNA and vice versa, suggesting that bacteria are pathogenic in a subset of SLE patients.
To determine the role of the protein component of curli/eDNA in the binding of lupus antibodies, we also used pure CsgA protein, the main structural subunit of curli, in competitive ELISAs. Figure 2H–I show that CsgA, which does not contain DNA, blocked anti-curli/eDNA Ab ELISA but not anti-mammalian dsDNA Ab ELISA, the latter instead was inhibited by E. coli K12 DNA. The opposite was true for the anti-dsDNA Ab ELISA, while curli/eDNA inhibited both bindings of anti-curli/eDNA and anti-dsDNA Abs. These results further demonstrate that anti-curli/eDNA IgG Abs are specific for the complex. Unfortunately, our CsgA amyloid is not ideal as it is GST-tagged and therefore not truly representing the natural amyloid. It is not presently possible to extract curli amyloid from curli/eDNA complexes without fully degrade the protein (12).
IgA anti-curli/eDNA antibodies are higher in Lupus patients than healthy controls
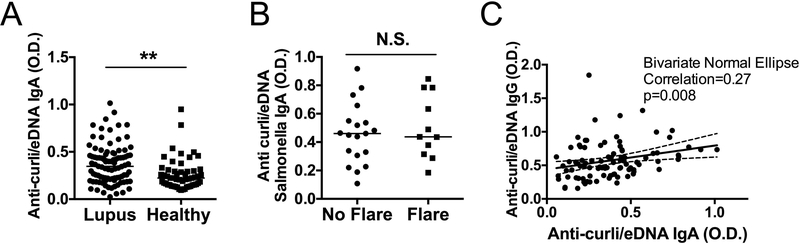
Most infections in SLE occur in mucosal organs (1, 2, 4). Being the IgA the most abundant antibody class at the mucosal surface (25), we analyzed the plasma levels of anti-curli/eDNA IgA Abs, as markers of mucosal immunity to curli. Anti-curli/eDNA IgA levels were significantly higher in SLE patients (median 0.35) compared to healthy controls (median 0.22) (Figure 3A), with no correlation with clinical flare (Figure 3B). The levels of anti-curli/eDNA IgA showed a small but significant correlation with the anti-curli/eDNA IgG, suggesting that a subgroup of SLE patients have higher levels of both Ig classes of anti-curli/eDNA Abs (Figure 3C).
Figure 3. IgA anti-curli/eDNA antibodies are higher in Lupus patients than healthy controls without correlation with flares.
A) Anti-curli/eDNA IgA Abs were measured by specific ELISA in plasma of SLE patients and age- and sex-matched healthy controls. B) Levels of anti-curli/eDNA IgA for SLE patients in flare and in remission. C) Correlation between levels of anti-curli/eDNA IgG and IgA Abs in SLE patients. *p <0.05 **p <0.01. Statistical significance was calculated with unpaired t-test with Welch correction (A-B) and Bivariate Normal Ellipse (C).
Frequency of bacteriuria correlates with anti-curli/eDNA Ab levels, lupus flares and pro-inflammatory phenotype
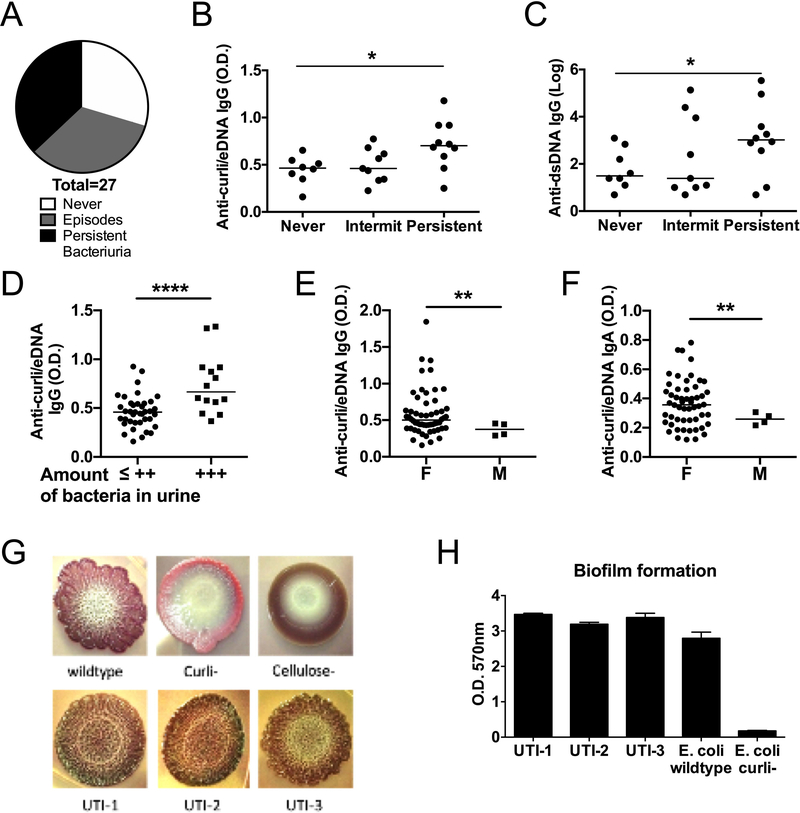
Since we found that, compared to healthy controls, all SLE patients had higher IgA and some had higher IgG specific for curli/eDNA, we hypothesized that their immune system encountered curli/eDNA complexes during a mucosal infection. Therefore, to look for the origin of this mucosal infection, we tested a group of SLE patients whose urine analyses were available for a period of two consecutive years, performed at four visits or more. We found that the majority of these SLE patients, who did not have signs and symptoms of UTI at the time of the visits, had episodes of asymptomatic bacteriuria during the two years of this analysis and 35% had persistent bacteriuria (Figure 4A). SLE patients with persistent bacteriuria had higher levels of IgG against curli/eDNA complexes (median 0.70, mean ± SEM = 0.71±0.05) than those with episodes of bacteriuria (intermittent) (median 0.46, mean ± SEM = 0.48±0.05) or who always tested negative for bacteriuria (never)(median 0.46, mean ± SEM = 0.44±0.05) (Figure 4B). The levels of anti-curli/eDNA IgG Abs correlated with the amounts of bacteria in the urine, measured using curli/eDNA from Salmonella (Figure 4D) and from E. coli (not shown but gave equivalent results). Interestingly, the levels of anti-dsDNA IgG Abs correlated with the persistence of bacteriuria too (Figure 4C). Moreover, the group of patients with persistent bacteriuria exhibited a pattern of chronic inflammatory state and disease activity, such as elevation of ESR, anemia and lymphopenia, hypocomplementemia, and elevation of globulins and anti-dsDNA Abs (Table 2). Finally, the frequency of bacteriuria was associated with an increased likelihood of flares (Table 2). These results suggest that subclinical infections or colonization by Gram-negative bacteria like Uropathogenic E. coli can expose SLE patients to curli-eDNA complexes, elicit anti-curli humoral immune responses, induce a chronic inflammatory state, and predispose to flares. The levels of plasma anti-curli/eDNA IgA were not different among the SLE patients stratified by bacteriuria frequency (Supplementary Figure 2D), suggesting that while many SLE patients mount an IgA response against this amyloid (Figure 3) and produce also anti-dsDNA IgA Abs (Supplementary Fig. 2C), persistent bacteriuria promotes an IgG response (Figure 4A–B), possibly because the chronic tissue damage leads to a leak in the epithelial barrier and to systemic exposure of bacterial products. Future studies are needed to correlate the levels of plasma vs urine IgA, a further sign of chronic urinary infections (25).
Figure 4. Association between Bacteriuria, IgG anti-curli/eDNA antibodies and disease activity.
A) Frequency of bacteriuria in SLE patients as measured by routine UA ranged from never, to intermittent (<100%), to persistent at each visit (100%). B) Levels of anti-curli/eDNA Abs (IgG) and C) anti-dsDNA IgG Abs were compared between patients with increasing frequency of bacteriuria. Wilcoxon test. D) Levels of anti-curli/eDNA against Salmonella (shown) are compared between patients with bacteriuria, as detected by routine urine analyses with microscopy and calculated using a +, ++, +++ grading system. Unpaired two tailed t-test. E-F) Anti-curli/eDNA IgG and IgA Abs levels were compared between sexes in patients who had at least three urinalyses. Welch’s t-test. G) UPEC in lupus patients produce curli containing biofilms. Colony morphologies of E. coli wild type, curli and cellulose mutants as well as three UTI isolates from SLE patients with bacteriuria were grown using YESCA plates supplemented with Congo Red and Brilliant Blue. Curli production is shown by the wrinkled rdar morphology. H). Crystal violet assay was performed to measure biomass of the biofilm generated by three E. coli isolates from urine of SLE patients in comparison to the reference E. coli WT (UTI89) and its isogenic CsgA deficient mutant (curli-).
Table 2.
Inflammation and disease activity markers correlate with Bacteriuria
| Bacteriuria | Never | Intermittent | Persistent | p-value |
|---|---|---|---|---|
| Anti-Curli/eDNA Abs | 0.44 (±0.14) | 0.48 (±0.16) | 0.71* (±0.25) | 0.001 |
| Hgb | 11.19 (±1.47) | 11.06 (±1.8) | 10.76* (±1.86) | 0.01 |
| Lymph (Absol. count) | 1527 (±1269) | 694* (±878) | 575* (±697) | 0.0001 |
| PLTs (Absol. count) | 230.7 (±103.3) | 253.04* (±109.6) | 257.5* (±86.5) | 0.004 |
| PMNs (%) | 57 (±12.70) | 58.9 (±15.3) | 67.88*+ (±11.1) | 0.0004 |
| ESR | 33.72 (±24) | 51.36* (±36.7) | 66.3* (±35.9) | 0.0001 |
| C3 | 113.57 (±15.4) | 113.75 (±30.80) | 96.38*+ (±27.12) | 0.0001 |
| Globulin | 3.38 (±0.51) | 3.65* (±0.6) | 3.92*+ (±0.75) | 0.0001 |
| anti-dsDNA | 7.65 (±8.17) | 35.26 (±32.52) | 87.9*+ (±101.81) | 0.0001 |
| Flares (%) | 50 | 66 | 90 | 0.03 |
Averages and ± SD of laboratory results from 27 SLE patients. O.D. of anti-curli/eDNA Abs in plasma; Peripheral blood Absolute counts of Lymphocytes (Lymph) and platelets (PLTs); % of polymorphonucleated cells (PMNs); Erythrocyte Sedimentation Rate (ESR); Complement 3 (C3) mg/dl; Globulin; % of SLE patients who had Flares. Results from one-way ANOVA are reported as in the column of the right. Results from the post-hoc t-tests of the comparisons between Intermittent and Persistent vs Never are signaled with * close to the numbers, while the comparisons between Intermittent vs Persistent with +. Values ≤ 0.05 were considered significant. n.s.= not significant.
p < 0.05
p < 0.01
p < 0.001
Anti-curli/eDNA levels correlate with female sex
UTIs are more frequent in the female sex, and although we found some bacteriuria in male SLE patients, it was not persistent (Table 1). The levels of both anti-curli/eDNA IgG and IgA Abs were significantly higher in female than in male SLE patients (Figure 4E–F), supporting a role for bacteriuria in eliciting these Abs primarily in females.
Uropathogenic E. coli (UPEC) from Lupus patients produce curli/eDNA complexes
To confirm the ability of bacteriuria to expose the immune system to curli/eDNA complexes, we isolated uropathogenic E. coli (UPEC) from the urine of three SLE patients with bacteriuria from the Temple Lupus Program (Figure 4G–H). The clinical isolates (UTI-1, UTI-2 and UTI-3) produced colonies, with a classic red, dry and rough (rdar) morphology, signs of production of curli amyloids and cellulose, on YESCA plates supplemented with Congo Red and Brilliant Blue to stain curli and cellulose, respectively (Figure 2G). Moreover, the SLE isolates generated vigorous biofilms, with a thickness similar to the standard lab strain (UTI89) (Figure 2H), while the curli-mutant produced a thin biofilm as reported (12). These results indicate that SLE patients harbor bacteria able to generate biofilms expressing curli/eDNA complexes, as it was previously suggested by the transcriptional signature of genes responsible for the synthesis of curli found in patients during UPEC UTI (26).
DISCUSSION
The source of immunogenic autoAgs in SLE remains unclear, including the DNA that is recognized by the pathognomonic anti-dsDNA autoAbs(27). Bacterial DNA has been previously proposed to play a role in lupus etiology as antigenic mimicker of self-DNA (24, 28). Our results show that curli/eDNA complexes expose bacterial DNA that can be recognized by specific Abs present in SLE patients and suggest that both anti-curli/eDNA Abs and anti-dsDNA autoAbs can bind structures on curli/eDNA. Moreover, curli/eDNA complexes can cross-react with self-DNA and trigger or amplify the production of autoAbs in lupus patients. Our results are in agreement with previous work that showed that SLE patients have Abs against E. coli DNA while healthy controls do not (29). These results also suggest that the repertoire of epitopes that curli/eDNA exposes is large and spans from amyloid-curli specific residues to bacterial DNA and it can mimic host dsDNA. Our findings are reminiscent of those of Azzouz and colleagues, where a similar mechanism was demonstrated with the gut commensal Ruminococcus gnavus(30). Therefore, our results expand the concept of autoimmune cross-reactivity to curli/eDNA complexes.
E. coli and S
Typhimurium, two members of the Enterobacteriaceae family, are characterized by the production of curli/eDNA complexes which form biofilms and are associated with bacteremia in SLE (31). Interestingly, activation of the innate immune system by bacteremia is supported by the report that lupus patients have increased plasma levels of LPS and peripheral blood mononuclear cells with a transcriptome suggestive of chronic exposure to bacterial products (32, 33). We have previously demonstrated that systemic injection of curli/eDNA complexes accelerated lupus disease in lupus-prone mice, as did systemic infections with E. coli or S. Typhimurium in a curli-dependent manner(12). Our newly established protocol provides a highly sensitive measurement of the humoral immune response against curli/eDNA complexes that were purified from biofilms of E. coli and Salmonella Typhimurium. Although previous literature showed the presence of similar antibodies in patients who survived sepsis, it did not examine the general population (17). Here we show that the general population is positive for anti-curli/eDNA Abs, suggesting curli/eDNA as important antigen in the recognition of enteric bacteria. However, the levels of anti-curli/eDNA Abs in lupus patients are elevated and correlate with flares and persistent bacteriuria, suggesting that previously considered benign recurrent infections such as UTIs might instead be a constant autoimmune trigger in genetically predisposed individuals.
Indeed, it is important to remark the new finding that 35% of SLE patients had asymptomatic bacteriuria, a result that open a new perspective on lupus pathogenesis. Infections of the urinary tract are frequent in women of reproductive age, the peak of SLE onset, and many of them are caused by UPEC. Although immune suppression may increase the rate of UTI and/or bacteriuria in SLE, in our cohort it did not seem the case (Table 1). UPEC produce curli/eDNA complexes, increasing their capacity to colonize the bladder and invade the blood stream (34). Interestingly, not only we found that female lupus patients had frequent bacteriuria, but importantly we found that those female lupus patients had significantly higher levels of anti-curli/eDNA IgG Abs, of anti-dsDNA Abs and stronger signs of inflammation, suggesting an active immune stimulation. Our results suggest that female common infections such as UTIs and even asymptomatic bacteriuria can trigger and/or sustain systemic autoimmunity, providing a new mechanism for the increased prevalence of lupus in females.
In summary, the higher levels of anti-curli/eDNA IgA in SLE patients provides a pathogenic role for curli/eDNA during mucosal infections in lupus, and the increased anti-curli/eDNA IgG during flares signals disease activity. Our results also show a clinical relevance for the asymptomatic presence and persistence of curli-expressing bacteria in the urine of SLE patients and link these pathogens to human autoimmunity as agents of cross-reactivity to elicit a humoral immune response to DNA. A limitation of our study is the number of patients, and therefore further studies with larger number of lupus patients and from different cohorts are warranted. Nevertheless, anti-curli/eDNA IgG correlate with both bacteriuria and flares, suggesting a pathogenic link between a systemic exposure to bacterial products and increase in disease severity. Together with bacteriuria and other parameters of disease activity and inflammation, an increase in anti-curli/eDNA Abs may pinpoint a subgroup of patients who might benefit from antibiotic therapy as adjuvant to standard of care.
Supplementary Material
Acknowledgments
We thank Philip L. Cohen for critically reading the manuscript and the numerous suggestions.
Funding: This work was supported by the NIH grant R56 AR072115-01 (to RC), Lupus Research Alliance (RC), NIH grant R21 AI119947 and Lupus Research Institute (Currently Lupus Research Alliance) (SG), NIH grants AI137541, AI132996, AI148770 and AI151893 (CT).
Footnotes
COI: Authors have no financial conflicts.
References
- 1.Doaty S, Agrawal H, Bauer E, Furst DE. Infection and Lupus: Which Causes Which? Curr Rheumatol Rep. 2016;18(3):13. [DOI] [PubMed] [Google Scholar]
- 2.Danza A, Ruiz-Irastorza G. Infection risk in systemic lupus erythematosus patients: susceptibility factors and preventive strategies. Lupus. 2013;22(12):1286–94. [DOI] [PubMed] [Google Scholar]
- 3.Pisetsky DS, Vrabie IA. Antibodies to DNA: infection or genetics? Lupus. 2009;18(13):1176–80. [DOI] [PubMed] [Google Scholar]
- 4.Gladman DD, Hussain F, Ibanez D, Urowitz MB. The nature and outcome of infection in systemic lupus erythematosus. Lupus. 2002;11(4):234–9. [DOI] [PubMed] [Google Scholar]
- 5.Pasoto SG, Ribeiro AC, Bonfa E. Update on infections and vaccinations in systemic lupus erythematosus and Sjogren’s syndrome. Curr Opin Rheumatol. 2014;26(5):528–37. [DOI] [PubMed] [Google Scholar]
- 6.Petri M Infection in systemic lupus erythematosus. Rheum Dis Clin North Am. 1998;24(2):423–56. [DOI] [PubMed] [Google Scholar]
- 7.Barnhart MM, Chapman MR. Curli biogenesis and function. Annu Rev Microbiol. 2006;60:131–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapman MR, Robinson LS, Pinkner JS, Roth R, Heuser J, Hammar M, et al. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science. 2002;295(5556):851–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Gerven N, Klein RD, Hultgren SJ, Remaut H. Bacterial amyloid formation: structural insights into curli biogensis. Trends Microbiol. 2015;23(11):693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tukel C, Nishimori JH, Wilson RP, Winter MG, Keestra AM, van Putten JP, et al. Toll-like receptors 1 and 2 cooperatively mediate immune responses to curli, a common amyloid from enterobacterial biofilms. Cell Microbiol. 2010;12(10):1495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tursi SA, Tukel C. Curli-Containing Enteric Biofilms Inside and Out: Matrix Composition, Immune Recognition, and Disease Implications. Microbiol Mol Biol Rev. 2018;82(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallo PM, Rapsinski GJ, Wilson RP, Oppong GO, Sriram U, Goulian M, et al. Amyloid-DNA Composites of Bacterial Biofilms Stimulate Autoimmunity. Immunity. 2015;42(6):1171–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tursi SA, Lee EY, Medeiros NJ, Lee MH, Nicastro LK, Buttaro B, et al. Bacterial amyloid curli acts as a carrier for DNA to elicit an autoimmune response via TLR2 and TLR9. PLoS Pathog. 2017;13(4):e1006315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruce IN, O’Keeffe AG, Farewell V, Hanly JG, Manzi S, Su L, et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort. Ann Rheum Dis. 2015;74(9):1706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jog NR, Blanco I, Lee I, Putterman C, Caricchio R. Urinary high-mobility group box-1 associates specifically with lupus nephritis class V. Lupus. 2016;25(14):1551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Humphries A, Deridder S, Baumler AJ. Salmonella enterica serotype Typhimurium fimbrial proteins serve as antigens during infection of mice. Infect Immun. 2005;73(9):5329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bian Z, Brauner A, Li Y, Normark S. Expression of and cytokine activation by Escherichia coli curli fibers in human sepsis. J Infect Dis. 2000;181(2):602–12. [DOI] [PubMed] [Google Scholar]
- 18.Tukel C, Wilson RP, Nishimori JH, Pezeshki M, Chromy BA, Baumler AJ. Responses to amyloids of microbial and host origin are mediated through Toll-like receptor 2. Cell Host and Microbe. 2009;6(1):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collinson SK, Doig PC, Doran JL, Clouthier S, Trust TJ, Kay WW. Thin, aggregative fimbriae mediate binding of Salmonella enteritidis to fibronectin. J Bacteriol. 1993;175(1):12–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crawford RW, Gibson DL, Kay WW, Gunn JS. Identification of a bile-induced exopolysaccharide required for Salmonella biofilm formation on gallstone surfaces. Infect Immun. 2008;76(11):5341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romling U, Bian Z, Hammar M, Sierralta WD, Normark S. Curli fibers are highly conserved between Salmonella typhimurium and Escherichia coli with respect to operon structure and regulation. J Bacteriol. 1998;180(3):722–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castrejon I, Tani C, Jolly M, Huang A, Mosca M. Indices to assess patients with systemic lupus erythematosus in clinical trials, long-term observational studies, and clinical care. Clin Exp Rheumatol. 2014;32(5 Suppl 85):S-85–95. [PubMed] [Google Scholar]
- 23.Anjo C, Mascaro JM Jr., Espinosa G, Cervera R. Effectiveness and safety of belimumab in patients with systemic lupus erythematosus in a real-world setting. Scand J Rheumatol. 2019:1–5. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton KJ, Schett G, Reich CF 3rd, Smolen JS, Pisetsky DS. The binding of sera of patients with SLE to bacterial and mammalian DNA. Clin Immunol. 2006;118(2–3):209–18. [DOI] [PubMed] [Google Scholar]
- 25.Breedveld A, van Egmond M. IgA and FcalphaRI: Pathological Roles and Therapeutic Opportunities. Front Immunol. 2019;10:553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subashchandrabose S, Hazen TH, Brumbaugh AR, Himpsl SD, Smith SN, Ernst RD, et al. Host-specific induction of Escherichia coli fitness genes during human urinary tract infection. Proc Natl Acad Sci U S A. 2014;111(51):18327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Putterman C, Caricchio R, Davidson A, Perlman H. Systemic lupus erythematosus. Clin Dev Immunol. 2012;2012:437282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilkeson GS, Grudier JP, Karounos DG, Pisetsky DS. Induction of anti-double stranded DNA antibodies in normal mice by immunization with bacterial DNA. J Immunol. 1989;142(5):1482–6. [PubMed] [Google Scholar]
- 29.Karounos DG, Grudier JP, Pisetsky DS. Spontaneous expression of antibodies to DNA of various species origin in sera of normal subjects and patients with systemic lupus erythematosus. J Immunol. 1988;140(2):451–5. [PubMed] [Google Scholar]
- 30.Azzouz D, Omarbekova A, Heguy A, Schwudke D, Gisch N, Rovin BH, et al. Lupus nephritis is linked to disease-activity associated expansions and immunity to a gut commensal. Ann Rheum Dis. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marcos M, Fernandez C, Soriano A, Marco F, Martinez JA, Almela M, et al. Epidemiology and clinical outcomes of bloodstream infections among lupus patients. Lupus. 2011;20(9):965–71. [DOI] [PubMed] [Google Scholar]
- 32.Shi L, Zhang Z, Yu AM, Wang W, Wei Z, Akhter E, et al. The SLE transcriptome exhibits evidence of chronic endotoxin exposure and has widespread dysregulation of non-coding and coding RNAs. PLoS One. 2014;9(5):e93846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogunrinde E, Zhou Z, Luo Z, Alekseyenko A, Li QZ, Macedo D, et al. A Link Between Plasma Microbial Translocation, Microbiome, and Autoantibody Development in First-Degree Relatives of Systemic Lupus Erythematosus Patients. Arthritis Rheumatol. 2019;71(11):1858–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hung C, Marschall J, Burnham CA, Byun AS, Henderson JP. The bacterial amyloid curli is associated with urinary source bloodstream infection. PLoS One. 2014;9(1):e86009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.