Abstract
Background:
NAC has been proven to increase BCS rates, but data are limited on conversion rates from BCS-ineligible (BCSi) to BCS-eligible (BCSe), specifically, in patients with large tumors.
Methods:
Consecutive patients with stage I-III breast cancer treated with NAC from 11/2013–03/2019 were identified. BCS-eligibility pre-/post-NAC was prospectively determined. Patients deemed BCSi pre-NAC due to large tumor size were studied. Statistical analyses were conducted using t, Wilcoxon rank sum, and chi-square tests, and logistic regression.
Results:
Of 1353 cancers, 600 were BCSi with large tumors; 69% were non-BCS candidates, 31% were borderline-BCS (bBCS) candidates. Of non-BCS candidates, 69% became BCSe post-NAC; 66% chose BCS, and 90% were successful. Among bBCS candidates, 87% were BCSe post-NAC, 73% chose BCS, and 96% were successful. On univariate analysis, bBCS candidacy, lower cT stage, cN0 status, absence of calcifications, HER2+/triple negative (TN) receptor status, poor differentiation, ductal histology, and breast pCR were associated with conversion to BCS-eligibility. On multivariable analysis, receptor status (HR+/HER2− ref, odds ratio [OR] HER2+ 1.63, p=0.047;HR-/HER2− OR 2.26, p=0.003) and breast pCR (OR 2.62, p<0.001) predicted successful downstaging, while larger clinical tumor size (OR 0.86,p=0.003), non-BCS candidacy (OR 0.46,p=0.003), cN+ status (OR 0.54, p=0.008), and calcifications (OR 0.56, p=0.007) predicted lower downstaging rates.
Conclusion:
In patients with large tumors precluding BCS, conversion to BCS-eligibility was high with NAC, particularly in bBCS candidates. HER2+/TN receptor status predicted successful downstaging, while lower downstaging rates were observed with larger tumors, cN+ status, and calcifications. These factors should be considered when selecting patients for NAC.
Keywords: neoadjuvant chemotherapy, breast-conserving surgery, unfavorable tumor-to-breast ratio, breast cancer, breast surgery, downstaging
Neoadjuvant chemotherapy (NAC), initially used in patients with inoperable breast cancer to improve resectability, is now commonly used in patients with large, operable breast cancer to downstage the primary tumor and to convert patients from mastectomy to breast-conservation candidates.1,2 In a patient-level meta-analysis of 10 randomized trials performed in the pre-trastuzumab era comparing neoadjuvant to adjuvant chemotherapy in patients with early-stage operable breast cancer, rates of breast conservation were 65% with NAC compared to 49% with upfront surgery.3 However, some patients in these studies were candidates for breast-conserving surgery (BCS) prior to NAC, so the true rate of downstaging to BCS cannot be determined from these studies. In patients receiving modern systemic chemotherapy and HER2 targeted therapy, response rates in the breast to NAC have improved, with breast pathologic complete response (pCR) rates reported to be approximately 50–60% in HER2 positive (HER2+) breast cancers, 30–50% in triple negative breast cancers, and 5–15% in hormone receptor (HR) positive (HR+)/HER2 negative (HER2−) breast cancers4,5, suggesting that a large number of patients will become eligible for breast conservation and will benefit from this approach. Breast pCR is not required for successful downstaging from mastectomy to BCS, and the presence of residual disease in the breast after NAC does not preclude breast conservation if the total volume of disease is limited.
While randomized and retrospective trials have assessed rates of BCS with NAC compared to upfront surgery, few studies have prospectively evaluated conversion rates from BCS-ineligible to BCS-eligible with NAC. The Cancer and Leukemia Group B (CALBG) 40601 and 40603 trials prospectively evaluated BCS conversion rates in HER2+ and triple negative breast cancer patients, respectively, with the most common reason for BCS-ineligibility in these studies reported as “tumor too large” or “probable poor cosmetic outcome”, but these studies also included patients with multicentric and T4 disease at presentation—characteristics which traditionally preclude surgical downstaging in the breast.6,7 Therefore, an accurate assessment of BCS conversion rates in patients ineligible for BCS because of a large tumor size relative to breast size is needed to understand the clinical benefit of NAC in this population. We sought to prospectively evaluate rates of BCS conversion with modern NAC in BCS-ineligible patients presenting with a large clinical tumor size and assess factors associated with successful downstaging.
METHODS
Beginning in 2013, our team of 15 surgeons from the Breast Service, Department of Surgery, Memorial Sloan Kettering Cancer Center prospectively collected data on all patients with invasive breast cancer treated with NAC at our institution into a prospective Health Insurance Portability and Accountability Act (HIPAA) compliant database. Patients who had a clear indication for systemic chemotherapy because of tumor biology, receptor subtype, nodal status, or tumor size were considered for NAC, to allow downstaging for BCS or avoidance of axillary dissection. Patients in whom the selection of chemotherapy approach was felt to be dependent upon surgical pathology findings underwent primary surgery. Surgeons prospectively assessed BCS eligibility prior to NAC and at the completion of NAC, based on physical exam and imaging findings, and reasons for ineligibility were documented (Fig. 1). Patients considered BCS-ineligible prior to NAC were further categorized as non-BCS candidates vs borderline BCS candidates.
Fig. 1.
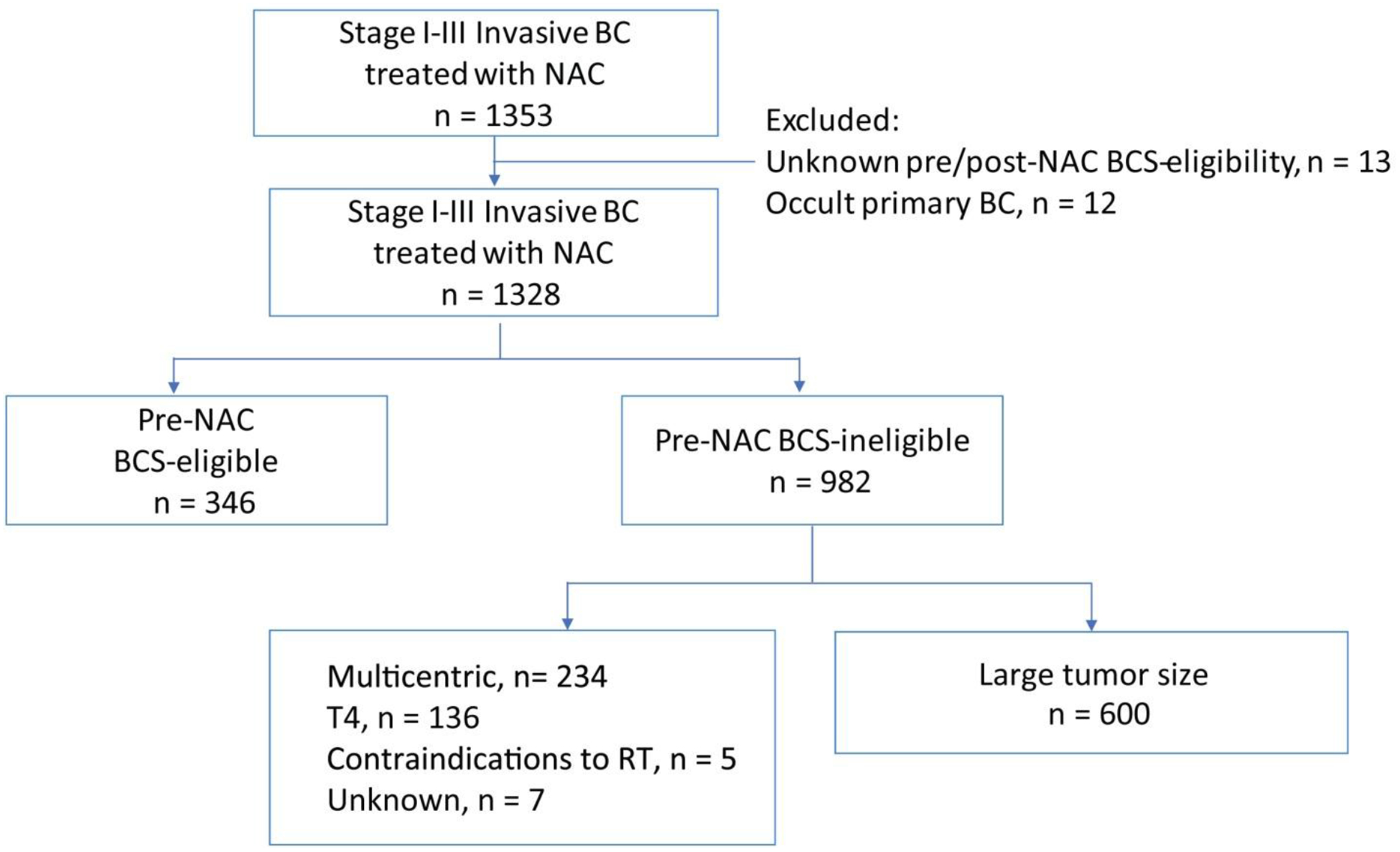
Study selection.
NAC neoadjuvant chemotherapy, BCS breast-conserving surgery, BC breast cancer, RT radiation therapy
After institutional review board approval, consecutive patients with invasive breast cancer treated with NAC and subsequent surgery between November 2013 and March 2019 were identified. Patients with occult primary breast cancer and those with unknown pre- or post-NAC BCS eligibility were excluded. Patients who were determined by the treating surgeon to be BCS-ineligible pre-NAC because of a large tumor size relative to breast size comprised the study cohort. Prior to NAC, 99% of patients in the cohort had a mammogram and ultrasound, and 89% had a pre-treatment breast MRI. Following NAC, 81% had a mammogram, 25% had an ultrasound, and 81% had a breast MRI. NAC regimens included dose-dense doxorubicin, cyclophosphamide, and a taxane in 92%. Of HER2 overexpressing patients, 99% received dual blockade with trastuzumab and pertuzumab.
Clinical characteristics between non-BCS and borderline BCS candidates pre-NAC were compared in a univariate analysis using Wilcoxon’s rank sum test for continuous variables, and Chi-square or Fischer’s exact test for categorical variables. A similar univariate analysis was performed to identify clinicopathologic factors associated with conversion to BCS eligibility. Multivariable logistic regression analysis was used to study the association between post-NAC BCS candidacy and the clinicopathological variables found to be significant in the univariate analysis. The final list of variables for the multivariable model was obtained by backward elimination using a p-value of > 0.05 as being eligible for exclusion from the model. The type I error rate (α) was set to 0.05 for all the statistical tests. All statistical analyses were performed using R 3.5.3 (R Core Development Team, Vienna, Austria).
RESULTS
From November 2013 to March 2019, 1329 patients with 1353 stage I–III invasive breast cancers (24 bilateral cancers) received NAC followed by surgery at Memorial Sloan Kettering Cancer Center, with 96% having stage II–III breast cancer. Overall, 346 (26%) were BCS-eligible prior to NAC, 982 (73%) were BCS-ineligible, and 25 (1%) had occult primary breast cancer or unknown pre-or post-NAC BCS eligibility and were excluded. Of BCS-ineligible cancers, 600 (61%) had a large tumor size relative to breast size as the reason for ineligibility and comprised our study cohort; the remainder were ineligible for downstaging because of multicentric disease, inflammatory or other T4 disease, or contraindications to radiotherapy (Fig. 1). Of the 600 cancers, the median clinical tumor size was 4.0 cm, with 94% having clinical T2/T3 tumors (Table 1). Overall, 62% of patients were clinically node positive, with a higher incidence of clinical nodal positivity observed among HR+/HER2− patients receiving NAC (72%) compared to HER2+ (61%) or triple negative (54%) patients.
TABLE 1.
Clinicopathologic characteristics of study cohort stratified by non-BCS vs. borderline-BCS candidacy
| Overall n = 600 | Non-BCS candidate n = 412 | Borderline BCS candidate n = 188 | p value | |
|---|---|---|---|---|
| Age, median, years (range) | 49 (25,87) | 48 (25,87) | 51 (26,82) | 0.2 |
| Clinical tumor size, median, cm (range)# | 4.0(0.9,12.0) | 4.5(1.1,12.0) | 3.5(0.9,10.7) | <0.001 |
| Clinical T stage | <0.001 | |||
| T1 | 37 (6%) | 20 (5%) | 17 (9%) | |
| T2 | 393 (66%) | 237 (57%) | 156 (83%) | |
| T3 | 170 (28%) | 155 (38%) | 15 (8%) | |
| Clinical N stage | 0.006 | |||
| N0 | 225 (38%) | 139 (34%) | 86 (46%) | |
| N+ | 375 (62%) | 273 (66%) | 102 (54%) | |
| Calcs on pre-NAC MMG | 0.2 | |||
| No | 413 (69%) | 277 (67%) | 136 (72%) | |
| Yes | 187 (31%) | 135 (33%) | 52 (28%) | |
| Receptors | 0.14 | |||
| HR+/HER2− | 196 (32%) | 145 (35%) | 51 (27%) | |
| HER2+ | 227 (38%) | 149 (36%) | 78 (41%) | |
| TN | 177 (30%) | 118 (29%) | 59 (31%) | |
| Differentiation | 0.2 | |||
| Well | 7 (1%) | 3 (1%) | 4 (2%) | |
| Moderate | 141 (24%) | 101 (24%) | 40 (21%) | |
| Poor | 452 (75%) | 308 (75%) | 144 (77%) | |
| Histology§ | 0.030 | |||
| Ductal | 554 (93%) | 376 (91%) | 178 (95%) | |
| Lobular | 20 (3%) | 16 (4%) | 4 (2%) | |
| Mixed | 19 (3%) | 17 (4%) | 2 (1%) | |
| Other* | 6 (1.0%) | 2 (1%) | 4 (2%) | |
| pCR | 0.97 | |||
| No | 433 (72%) | 298 (72%) | 135 (72%) | |
| Yes | 167 (28%) | 114 (28%) | 53 (28%) |
BCS breast-conserving surgery, Calcs calcifications, NAC neoadjuvant chemotherapy, MMG mammogram, HR hormone receptor, HER2 human epidermal growth factor receptor 2, TN triple negative, pCR pathologic complete response
Unknown (tumor size), n = 16
Unknown (histology), n = 1
other = 4 metaplastic, 1 anaplastic, 1 mucinous
Of patients with large tumors precluding BCS, 69% (n = 412) were non-BCS candidates and 31% (n = 188) were borderline BCS candidates, as determined by the treating surgeon. Compared to borderline BCS candidates, non-BCS candidates had larger tumors (median 4.5 cm vs. 3.5 cm, p < 0.001) and a higher proportion of clinical T3 tumors (38% vs. 8%, p < 0.001). Non-BCS candidates were also more likely to be clinically node positive (66% vs. 54%, p = 0.006) and have non-ductal histology (9% vs. 5%, p = 0.03)(Table 1).
BCS Conversion Rates
Overall Cohort
Among 600 BCS-ineligible cancers 75% (n = 450) became BCS eligible after NAC. Of these, 68% of patients (n = 308) elected BCS, which was successful in 93% (n = 285). Overall, 48% (285/600) of BCS-ineligible cancers with large clinical tumor size at presentation avoided mastectomy with preoperative chemotherapy.
Non-BCS Candidates
Of 412 non-BCS candidates, 69% (n = 286) became eligible for BCS post-NAC. One hundred and twenty-six remained BCS-ineligible due to a tumor size that was too large (n = 88, 70%) or scattered residual disease on imaging (n = 36, 29%)(1% unknown). Of the 286 BCS-eligible patients post-NAC, 66% (n = 188) chose BCS and 90% (n = 170) were successful (Fig. 2). Reasons for mastectomy in BCS-eligible patients were primarily patient preference (79%) or high-risk status (20%)(1% unknown).
Fig. 2.

Conversion to BCS-eligibility post-NAC stratified by borderline vs. non-BCS candidates.
NAC neoadjuvant chemotherapy, BCS breast-conserving surgery, pCR pathologic complete response
Borderline BCS Candidates
Of 188 borderline BCS candidates, 87% (n = 164) became eligible for BCS post-NAC and 13% (n = 24) remained BCS-ineligible due to large tumor size (n = 12, 50%), scattered residual disease (n = 8, 33%), or disease progression (n = 4, 17%). Of BCS-eligible patients post-NAC, 73% (n = 120) chose BCS and 96% (n = 115) were successful (Fig. 2). Reasons for mastectomy in BCS-eligible patients were patient preference (86%) and high-risk status (14%).
Predictors of Conversion to BCS Eligibility
On univariate analysis, smaller clinical tumor size at presentation, borderline BCS candidacy (vs. non-BCS candidacy), lower clinical T stage, HER2+/triple negative receptor status, poor differentiation, ductal histology, and breast pCR were associated with conversion to BCS, while clinical node positivity and presence of pre-NAC mammographic calcifications were associated with a lower likelihood of conversion (Table 2). Notably, although patients who achieved breast pCR were more likely to become BCS eligible (87%), approximately 70% of patients who did not achieve pCR also became BCS-eligible post-NAC.
TABLE 2.
Predictors of conversion to BCS-eligibility with NAC
| Characteristic | Overall n = 600 | Post-NAC BCS candidate | Univariable | Multivariable | |||
|---|---|---|---|---|---|---|---|
| No n = 150 | Yes n = 450 | p value | Odds ratio | 95% Cl | p value | ||
| Age at diagnosis, median, years (range) | 49 (25,87) | 49 (27,87) | 49 (25,82) | 0.3 | -- | -- | |
| Clinical tumor size, median, cm (range)# | 4.0 cm (0.9,12) | 5.0 cm (0.9,12) | 4.0 cm (1.0,12) | <0.001 | 0.86 | 0.78–0.95 | 0.003 |
| No | 412 | 126 (31%) | 286 (69%) | 0.46 | 0.27–0.76 | ||
| T3 | 170 | 61 (36%) | 109 (64%) | ||||
| cN+ | 375 | 114(30%) | 261 (70%) | 0.54 | 0.34–0.84 | ||
| Yes | 187 | 67 (36%) | 120 (64%) | 0.56 | 0.36–0.85 | ||
| HR-/HER2− | 177 | 29 (16%) | 148 (84%) | 2.26 | 1.33–3.91 | 0.003 | |
| Poor | 452 | 101 (22%) | 351 (78%) | ||||
| Other* | 6 | 2(33) | 4 (67%) | ||||
| Yes | 167 | 21 (13%) | 146 (87%) | 2.62 | 1.54–4.66 | ||
BCS breast-conserving surgery, NAC neoadjuvant chemotherapy, CI confidence interval, HR hormone receptor, HER2 human epidermal growth factor receptor 2, pCR pathologic complete response
Unknown (tumor size), n = 16
Unknown (histology) n = 1
other = 4 metaplastic, 1 anaplastic, 1 mucinous
On multivariable analysis, receptor status (HR+/HER2− ref, OR HER2+ 1.63, p = 0.047; HR negative/HER2− OR 2.26, p = 0.003) and achievement of breast pCR (odds ratio [OR] 2.62, p < 0.001) were independently associated with post-NAC BCS-eligibility. Larger clinical tumor size, non-BCS candidacy, clinical node positivity, and pre-NAC mammographic calcifications were associated with a lower likelihood of downstaging (Table 2).
DISCUSSION
In patients with clinical T1–3 breast cancer, BCS eligibility is based on tumor location and tumor size relative to the breast size, and requires surgeon judgment to determine whether a cosmetically acceptable breast-conserving surgery can be performed. In patients who are not candidates for BCS at initial presentation because of a large tumor size in relation to breast size, NAC can be utilized to downstage the breast and facilitate breast conservation.2–4 Patients with large primary tumors that preclude breast conservation are ideal candidates for consideration of NAC, as a decrease in tumor size allows for a smaller volume of tissue to be removed commensurate with the residual volume of disease.8 The majority of trials of NAC examined survival or pCR as endpoints, and did not distinguish between patients eligible for BCS at presentation and those who required downstaging to undergo BCS. Thus, data on differences in rates of BCS from these studies underestimate the benefit of NAC for downstaging. The National Surgical Adjuvant Breast and Bowel Project (NSABP) B18 and the European Organisation for Research and Treatment of Cancer (EORTC) 10902 trials specifically examined conversion to BCS in patients felt to require mastectomy after 4 cycles of an anthracycline and cyclophosphamide, and reported conversion to BCS in 27% and 23%, respectively.9,10 In more recent trials in triple negative and HER2+ patients, Golshan et al. reported 42–53% conversion rates from BCS-ineligible to BCS-eligible with NAC6,7,11, with BCS ineligibility inclusive of patients with large tumors, multicentric disease, and cT4 disease.
We chose to examine the subset of women ineligible for BCS secondary to large clinical tumor size, since this was the most common reason for BCS ineligibility in our study, observed in 61% of BCS-ineligible patients. The conversion rate of 75% from BCS-ineligible to BCS-eligible with NAC observed in our study is higher than that reported in other studies, reflecting improvements in systemic therapy as well as the exclusion of patients from our study with multicentric or T4 disease who were not eligible for downstaging.
Despite increased eligibility for BCS after NAC, others have demonstrated low rates of acceptance of BCS among patients post-NAC.6,7,11 Golshan et al. prospectively evaluated the role of NAC in facilitating BCS and found that only 56% of BCS-eligible patients post-NAC chose BCS, with a lower BCS rate in North American patients compared to European and Asian patients (55% vs. 80%, respectively).11 Furthermore, BCS-eligible patients post-NAC who opt for mastectomy often choose bilateral over unilateral mastectomy, with Christian et al. demonstrating a 3-fold higher incidence of bilateral vs. unilateral mastectomy among post-NAC BCS candidates.12 In our study, 68% of BCS-eligible patients post-NAC opted for BCS, higher than the rate reported by Golshan et al., and mastectomy was avoided in 48% of patients deemed BCS-ineligible at presentation. Surgical de-escalation and avoidance of mastectomy with the use of NAC has the potential to reduce surgical morbidity and improve long-term quality of life for patients, with accumulating evidence demonstrating improved satisfaction with breasts in patients treated with BCS vs. mastectomy.13,14 These findings reinforce that NAC should be considered for downstaging in BCS-ineligible patients with large tumors who are desirous of breast conservation, provided that chemotherapy is otherwise indicated.
In selecting patients for NAC, our study demonstrated high rates of conversion to BCS-eligibility among triple negative and HER2+ breast cancer patients with large primary tumors (84% and 79%, respectively), underscoring that these aggressive subtypes are ideally suited for downstaging with NAC, especially in the absence of mammographic calcifications. While the decision to give NAC as an initial treatment approach in stage II-III triple negative and HER2+ breast cancer patients is relatively straightforward due their high-risk biology and excellent response rates, the decision for NAC versus upfront surgery among patients with HR+/HER2− cancers is more complex. Central to this decision is the understanding that NAC should only be considered for surgical downstaging in HR+/HER2− patients in whom chemotherapy would otherwise be indicated, highlighted by the fact that over 70% of HR+/HER2− patients in our study cohort receiving NAC were also clinically node positive. Among our cohort of patients with HR+/HER2− cancer selected for NAC, 62% of BCS-ineligible patients became BCS-eligible with NAC, emphasizing that pCR is not required for successful downstaging to breast conservation. While the rate of downstaging to BCS is lower compared to triple negative and HER2+ patients, if chemotherapy is otherwise indicated because of clinical nodal positivity or other high-risk factors, our study provides evidence that a substantial proportion of high -risk HR+/HER− patients with large tumors that preclude breast conservation will convert to BCS-eligible and may derive a substantial clinical benefit from NAC.
Patients who are borderline for BCS at presentation in whom upfront surgery would result in a poor cosmetic outcome represent a subgroup of patients in whom the decision for upfront surgery versus NAC is more challenging, given their smaller tumor size compared to non-BCS candidates. However, we observed a high conversion rate to BCS eligibility among borderline BCS patients (87%), reflecting that only a small reduction in tumor volume is needed to convert these patients into BCS candidates. Furthermore, among patients who chose BCS, 96% were successful, allowing for 61% of borderline BCS patients to avoid mastectomy with NAC. In borderline BCS candidates who have a clear indication to receive chemotherapy, there is little rationale to proceeding with upfront mastectomy if the patient is desirous of breast conservation.
Our study has several limitations. Firstly, prospective assessment of BCS eligibility pre- and post-NAC for an individual patient was determined by the patient’s treating surgeon and therefore remains a subjective assessment. As there is no standard cutoff for tumor size to determine BCS-eligibility, it is possible that variability exists between surgeons in judging appropriateness for breast conservation. While we did not analyze individual surgeon biases and decision making, 94% of patients considered ineligible for BCS had clinical T2/3 tumors at presentation with a median clinical tumor size of 4.0 cm, suggesting some consistency in what individual surgeons considered to be a tumor size too large for BCS. In assessing BCS-eligibility post-NAC, Golshan et al. observed a 35% pCR rate in patients deemed to be poor BCS candidates11, while we observed a 14% pCR rate among patients determined to be BCS-ineligible post-NAC, underscoring that surgeon assessment sometimes fails to identify patients who could potentially be candidates for BCS. However, surgeon assessment relies strongly on post-NAC imaging, which may demonstrate persistent abnormalities in the breast, such as calcifications, that may require more extensive surgery due to uncertainty surrounding the presence of residual disease.15,16 Secondly, statistical analysis to identify factors associated with BCS eligibility was performed for the entire cohort. Even though a statistical analysis stratified by pre-NAC candidacy would have been ideal to uncover the favorable factors associated with conversion to BCS candidacy in borderline candidates and non-BCS candidates, it was not feasible because of the small number of pre-NAC borderline candidates who did not convert (13%).
In conclusion, among BCS-ineligible patients with a large tumor size relative to breast size, rates of conversion to BCS eligibility with NAC were high, particularly in borderline BCS candidates. HER2+ and triple negative receptor status predict for successful conversion to BCS, although, notably, > 60% of HR+/HER2− breast cancer patients selected for NAC also became BCS eligible. Overall, mastectomy was avoided in 48% of BCS-ineligible patients with the use of preoperative systemic therapy, suggesting that NAC can successfully be used for surgical de-escalation in the breast with a substantial clinical benefit, provided that systemic chemotherapy is otherwise indicated.
Synopsis:
Here we evaluate BCS conversion rates with NAC in patients with tumors too large for conservation. Borderline-BCS candidacy, HER2+/triple negative receptor status, tumor size, cN+ status, and mammographic calcifications are factors to consider when selecting patients for downstaging with NAC.
ACKNOWLEDGMENTS
The preparation of this manuscript was funded in part by NIH/NCI Cancer Center Support Grant No. P30 CA008748 to Memorial Sloan Kettering Cancer Center, and this study has been accepted for presentation in podium format at the SSO 2020 International Conference on Surgical Cancer Care, March 25–28, 2020, Boston, MA. Dr. Monica Morrow has received speaking honoraria from Genomic Health. Dr. Andrea V. Barrio has received speaking honoraria from Roche. Dr. Giacomo Montagna is supported by the Ticino Cancer League, the Hanne Liebermann Foundation, the Fondation Ancrage, and the HEMMI-Stiftung. All other authors have no conflict of interests to disclose.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Mieog JS, van der Hage JA, van de Velde CJ. Preoperative chemotherapy for women with operable breast cancer. Cochrane Database Syst Rev. April 18 2007(2):Cd005002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. February 10 2008;26(5):778–785. [DOI] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists’ Group (EBCTCG). Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. January 2018;19(1):27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boughey JC, McCall LM, Ballman KV, et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial. Ann Surg. October 2014;260(4):608–614; discussion 614–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mamtani A, Barrio AV, King TA, et al. How Often Does Neoadjuvant Chemotherapy Avoid Axillary Dissection in Patients With Histologically Confirmed Nodal Metastases? Results of a Prospective Study. Ann Surg Oncol. October 2016;23(11):3467–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golshan M, Cirrincione CT, Sikov WM, et al. Impact of neoadjuvant chemotherapy in stage II-III triple negative breast cancer on eligibility for breast-conserving surgery and breast conservation rates: surgical results from CALGB 40603 (Alliance). Ann Surg. September 2015;262(3):434–439; discussion 438–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golshan M, Cirrincione CT, Sikov WM, et al. Impact of neoadjuvant therapy on eligibility for and frequency of breast conservation in stage II–III HER2-positive breast. cancer: surgical results of CALGB 40601 (Alliance). Breast Cancer Res Treat. November 2016;160(2):297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boughey JC, Peintinger F, Meric-Bernstam F, et al. Impact of preoperative versus postoperative chemotherapy on the extent and number of surgical procedures in patients treated in randomized clinical trials for breast cancer. Ann Surg. September 2006;244(3):464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. July 1997;15(7):2483–2493. [DOI] [PubMed] [Google Scholar]
- 10.van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. November 15 2001;19(22):4224–4237. [DOI] [PubMed] [Google Scholar]
- 11.Golshan M, Loibl S, Wong SM, et al. Breast Conservation After Neoadjuvant Chemotherapy for Triple-Negative Breast Cancer: Surgical Results From the BrighTNess Randomized Clinical Trial. JAMA Surg. January 8 2020:e195410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christian N, Zabor EC, Cassidy M, Flynn J, Morrow M, Gemignani ML. Contralateral Prophylactic Mastectomy Use After Neoadjuvant Chemotherapy. Ann Surg Oncol. March 2020;27(3):743–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenberg SM, O’Neill A, Sepucha K, et al. Abstract No. GS6–05. The impact of breast cancer surgery on quality of life: Long term results from E5103. Presented at the 41st Annual San Antonio Breast Cancer Symposium, December 4–10, 2018, San Antonio, TX. [Google Scholar]
- 14.Dominici LS, Hu J, King TA, et al. Abstract No. GS6–06. Local therapy and quality of life outcomes in young women with breast cancer. Presented at the 41st Annual San Antonio Breast Cancer Symposium, December 4–10, 2018, San Antonio, TX. [Google Scholar]
- 15.Adrada BE, Huo L, Lane DL, Arribas EM, Resetkova E, Yang W. Histopathologic correlation of residual mammographic microcalcifications after neoadjuvant chemotherapy for locally advanced breast cancer. Ann Surg Oncol. April 2015;22(4):1111–1117. [DOI] [PubMed] [Google Scholar]
- 16.Feliciano Y, Mamtani A, Morrow M, Stempel MM, Patil S, Jochelson MS. Do Calcifications Seen on Mammography After Neoadjuvant Chemotherapy for Breast Cancer Always Need to Be Excised? Ann Surg Oncol. June 2017;24(6):1492–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]


