Abstract
Introduction:
Essential thrombocythemia (ET) and polycythemia vera (PV) belong to the BCR-ABL1-negative myeloproliferative neoplasms and are characterized by the clonal proliferation of hematopoietic stem and progenitor cells. The contribution of aberrant immune regulation within the bone marrow microenvironment to ET and PV pathogenesis as well as the underlying molecular landscape is becoming increasingly understood.
Areas covered:
Authors searched PubMed and conference abstracts in August 2020 for preclinical and clinical studies to provide an overview of the immune pathobiology in ET and PV and the rationale for several novel agents. A discussion of recent clinical trials on interferon and ruxolitinib in ET and PV patients is provided followed by an outline of the future challenges in the field particularly for novel therapeutics and an increasingly individualized, molecularly-driven approach to treatment selection. Several novel agents are currently being actively evaluated and are reviewed herein as well.
Expert opinion:
While hydroxyurea remains the first-line treatment for cytoreduction in most high-risk ET and PV patients, the disease-modifying potential of IFN is promising and could make it a preferred option for selected patients. Advances in molecular testing will enable a more individualized approach to prognostication and treatment selection.
Keywords: Essential thrombocythemia, polycythemia vera, immunology, interferon, ruxolitinib, imetelstat, novel therapies
1. Introduction
Essential thrombocythemia (ET) and polycythemia vera (PV) are part of the BCR-ABL1-negative myeloproliferative neoplasms (MPN) that are characterized by the clonal proliferation of hematopoietic stem and progenitor cells [1,2]. Both disease entities are rare with incidence rates in the United States between 2001 and 2016 reported as 0.3 cases per 100,000 and 1.18 per 100,000 for ET and PV, respectively [3]. Disease presentations can occur along a spectrum of asymptomatic blood count elevations with a normal life expectancy to increased thrombosis risk, reduced quality of life related to vasomotor symptoms and splenomegaly, and progression to myelofibrosis (MF) and acute myeloid leukemia (AML) [4,5].
Thanks to advances in diagnostic techniques, the mutational landscape of ET and PV continues to be characterized in greater detail with the vast majority of patients harboring driver mutations in janus kinase 2 (JAK2 [primarily V617F and exon 12]), calreticulin (CALR) and myeloproliferative leukemia virus (MPL) genes [4,6,7]. However, the influence of co-mutations in other genes and immunologic processes in the bone marrow niche between hematopoietic stem cells, bone marrow stromal cells, and immune cells is still incompletely understood but offers the opportunity for novel therapies with a disease-modifying potential [1]. For this review, we searched PubMed and recent conference abstracts in August 2020 for relevant preclinical and clinical studies on ET and PV. References of selected studies were reviewed to identify additional relevant literature. However, as this was not a systematic review we did not use an a priori defined search strategy or inclusion/exclusion criteria for study selection.
2. Current treatment landscape of ET and PV
As the prognosis for ET and PV varies substantially between patients, both the National Comprehensive Cancer Network (NCCN) and the European Leukemia Net (ELN) recommend a risk-stratified approach to the treatment of an individual patient [4,8]. This is exemplified by two large retrospective studies evaluating prognostic factors and outcomes among patients with MPNs [9,10]. Conventionally, patients age ≥ 60 years or with prior thrombosis are classified as high-risk [4]. However, the association of a higher thrombosis risk with the presence of JAK2/MPL mutations in ET patients is increasingly recognized and included in the validated International Prognostic Score of Thrombosis in ET (IPSET) [5,11]. The impact of other factors such as leukocytosis in PV patients or the influence of co-mutations continues to evolve and is not part of the current guideline recommended approach to treatment selection [5,6,12–14].
Treatment focuses primarily on mitigation of thrombosis risk and most patients with ET and PV should receive low-dose aspirin [4,8,15]. The randomized phase III CYTO-PV trial that compared risk of death from cardiovascular causes or major thrombotic events in 365 PV patients assigned to either a more stringent phlebotomy regimen (target hematocrit <45%) or a more lenient approach (target hematocrit 45–50%) showed a benefit in patients assigned to the group with the lower hematocrit target establishing phlebotomy to achieve a hematocrit <45% as standard of care [16]. In patients with high-risk PV additional cytoreduction with hydroxyurea (HU) or recombinant interferon-α (IFN) is recommended [4,8,15]. In ET patients, cytoreduction with HU in high-risk patients has been shown to be superior to anagrelide in terms of risk for a composite outcome of thrombotic and hemorrhagic events in a randomized phase III trial [17]. Anagrelide and IFN serve as alternative second-line therapies [4,8]. However, with the development of novel IFN formulations, JAK2 inhibitors such as ruxolitinib and a better understanding of the underlying pathophysiology in the bone marrow microenvironment, several promising new agents have been or are being tested and will likely broaden the therapeutic landscape allowing for a more individualized, genetically-driven treatment approach.
3. Modulation of inflammatory processes in the bone marrow as therapeutic targets in ET and PV
Both ET and PV are characterized by driver mutations that activate the JAK-STAT pathway although significant heterogeneity between individual patients exists [1,18–20]. The JAK-STAT pathway is a central regulator of inflammatory signaling and aberrant expression of genes regulated by this pathway has been shown in neutrophils of patients with MPNs compared to healthy controls [21]. Notably, the transcriptional signature differed based on the presence of co-mutations such as CALR or TET2 and increased JAK2 activity was also detected in patients without JAK2 mutations [21]. An overexpression of additional cytokine signaling pathways including the KRAS pathway was also observed suggesting a combination of dysregulated signaling as the driver of MPN pathogenesis [21]. More recently, Rodriguez-Meira et al. conducted single-cell RNA sequencing and were able to show differences in JAK2 homozygous cells and wild-type cells from MPN patients as well as healthy controls with distinct patterns of upregulation of pro-inflammatory signaling pathways [19]. These results suggest that JAK2 mutated cells could lead to a pro-inflammatory phenotype of wild-type bystander cells and contributing to genome instability and proliferation of the mutant clone [18,22].
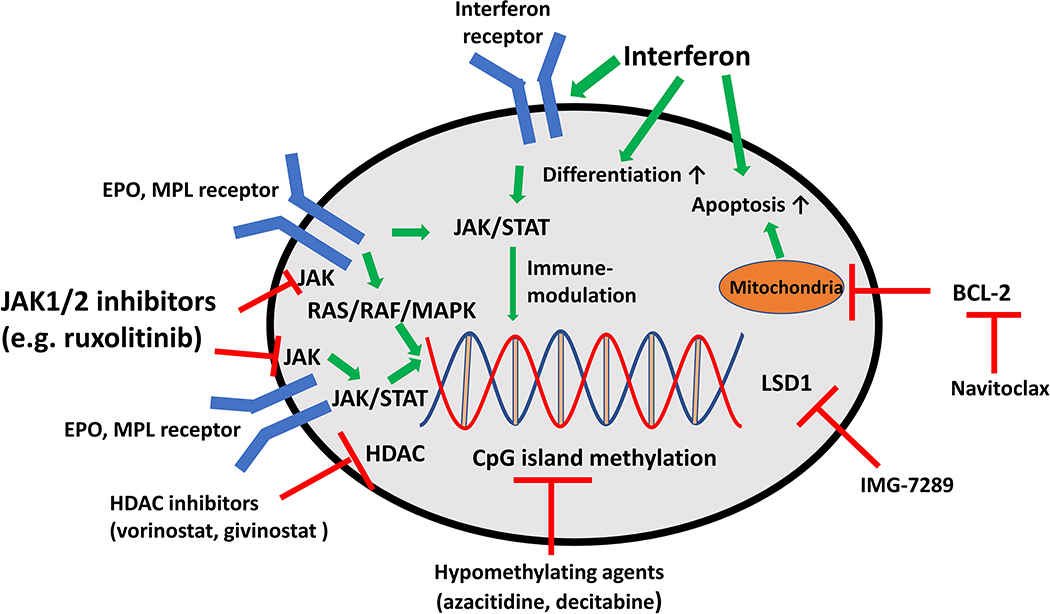
Elevated levels of pro-inflammatory cytokines such as IL-1, IL-6, tumor necrosis factor (TNF)-α, and IFN-γ have been reported in various studies of ET and PV patients and associated with adverse survival [20,23,24]. Those cytokines are also produced by non-malignant cells within the bone marrow microenvironment and stimulate the proliferation of the mutant MPN clone [25]. Additionally, reactive oxygen species have been associated with genomic instability and DNA damage in JAK2 V617F-positive MPNs [26]. Finally, dysregulations in regulatory T-cells, increased expression of myeloid-derived suppressor cells, and upregulation of the inhibitory immune checkpoint receptor programmed death receptor ligand (PD-L1) has been reported and might be contributing to the immune evasion and proliferation of MPN clones [20,27,28]. This preclinical evidence has led to the development of various immunomodulatory treatment strategies with promising results. Figure 1 provides an overview of their mechanism of action. Excellent and more detailed reviews on the underlying immune pathobiology of MPNs have been published recently [18,20,29].
Figure 1: Mechanisms of action of novel therapies in ET and PV.
JAK kinases are associated with and mediate signaling via EPO and MPL receptors. Mutated JAK2 and CALR activate cell proliferation via the JAK-STAT and RAS-RAF-MAPK pathways leading to proliferation of hematopoietic cells. JAK-STAT pathway activation can be inhibited by the JAK1/2 inhibitor ruxolitinib at the level of the EPO and MPL receptor. IFNs have been associated with a variety of mechanisms of action which are not fully elucidated to date. IFN has been associated with immunomodulatory effects, stimulation of HSC differentiation and apoptosis. Epigenetic processes such as DNA methylation and histone (de)acetylation can be targeted by HDAC inhibitors (e.g. vorinostat and givinostat), the LSD1 inhibitor IMG-7289, and hypomethylating agents. Navitoclax inhibits anti-apoptotic signaling via BCL-2 and has been successfully tested in myelofibrosis patients but not ET or PV.
4. Interferon
IFN-α has been used since the 1980’s in various formulations for the treatment of ET and PV [30,31]. Although a disease-modifying potential has been suggested by prolonged treatment-free remissions, reversal of bone marrow histopathological abnormalities, and more recently by a reduction in JAK2 V617F allele burden, the widespread use of IFN has been hampered by the substantial burden on patients in terms of adverse events and parenteral administration [32–35]. IFN-α has been shown to have anti-proliferative, pro-apoptotic, and immunomodulatory effects on hematopoietic progenitor and immune cells in the bone marrow by activation of the JAK-STAT pathway [20,30,31]. Besides a broader immunomodulatory effect leading to the upregulation of tumor antigen presentation and restoration of immune surveillance, IFN has been shown to preferentially act on JAK2 V617F mutated clones inducing proliferation and differentiation of hematopoietic progenitor cells [30,36–38].
A recent meta-analysis showed an overall response rate (ORR), primarily defined as a composite of peripheral blood count improvements, of 80.6% (95% confidence interval [CI]: 76.6–84.1%) and 76.7% (95% CI: 67.4–84.0%) for ET and PV patients, respectively, with complete hematologic responses (CHR) seen in 59.0% (95% CI: 51.5%−66.1%) and 48.5% (95% CI: 37.8–59.4%) of ET and PV patients, respectively [39]. Especially with the introduction of pegylated (peg)-IFN formulations and more recently ropegylated IFN, which is administered only every other week, tolerability of IFN has been improved. The recently published, randomized, open-label phase III PROUD-PV trial and its extension phase CONTINUATION-PV compared ropeginterferon alfa-2b with HU in 257 patients with early stage PV [40]. While ropeginterferon alfa-2b failed to achieve non-inferiority compared with HU in terms of the composite primary endpoint of CHR and spleen size normalization at 12 months (ropeginterferon alfa-2b: 21% vs 28% for HU; 95% CI for difference in response: −17.23 – 4.09; p=0.23), ropeginterferon alfa-2b was superior to standard therapy (97% HU) after 36 months of follow up in terms of CHR and reduction in symptom burden (ropeginterferon alfa-2b: 53% vs 38% for standard therapy; p=0.044) [40]. Based on those results ropeginterferon alfa-2b has been approved by the European Medicines Agency for the treatment of PV patients without symptomatic splenomegaly. Similarly promising preliminary results have been published from the open label, randomized phase III trial MPN-RC 112 (NCT01259856) which compared treatment with HU and pegylated IFN (peg-IFN) alfa-2a in 168 patients with high risk ET and PV [41]. There was no statistically significant difference between peg-IFN and HU in terms of ORR, defined as a composite of partial response (PR) and complete response (CR), after 12 months (HU: 69.8% vs peg-IFN: 78%; p=0.22) or 24 months of treatment (HU: 88% vs peg-IFN: 91%). However among the subset of patients who were eligible to receive 24 months of treatment, this difference reached statistical significance favoring peg-IFN after 24 months of therapy (HU: 40.7% vs peg-IFN: 59.6%; p=0.04) [41]. However, grade 3 or higher adverse events were more common in the peg-IFN group (27.5% vs 46.3%) and no differences in quality of life were observed [41,42]. The potential benefit of peg-IFN has also been shown in single-arm studies in patients refractory or intolerant to HU (NCT01259856) and in patients with splanchnic vein thrombosis [43,44]. A randomized phase III trial comparing ropeginterferon alfa-2b with anagrelide in ET patients with suboptimal or failed response to HU is ongoing and has started recruiting patients (NCT04285086). Outcomes of selected clinical trials are shown in Table 1.
Table 1:
Results from selected studies of IFN in ET and PV patients
| Author | Reference | Year of publication | Treatment and treatment schedule | Phase | Study population | Outcomes |
|---|---|---|---|---|---|---|
| Gisslinger et al. | [100] | 2015 | Ropeginterferon α-2b 50–540 mg every 2 weeks (mean dose 263 μg every 2 weeks) | I/II | 51 PV patients (100% JAK2 V617F) | 90% cumulative overall response (47% CHR; 21% CMR) |
| Gisslinger et al. | [40] | 2020 |
PROUD-PV: ropeginterferon alfa-2b 50–100 μg vs HU CONTINUATION-PV: ropeginterferon alfa-2b 50–100 μg vs best available therapy |
III |
PROUD-PV: ropeginterferon alfa-2b (127 patients) vs HU (130 patients) CONTINUATION-PV: ropeginterferon alfa-2b (95 patients) vs HU (76 patients) |
PROUD-PV: 1.) ropeginterferon: 21% composite primary endpoint (CHR + normal spleen size); 43% CHR alone 2.) Hydroxyurea: 28% composite primary endpoint; 46% CHR alone CONTINUATION-PV: 1.) ropeginterferon: 71% CHR alone 2.) BAT: 51% CHR alone |
| Knudsen et al. | [101] | 2018 | Patients ≤ 60 years randomized (I:I) to peg-IFNα-2a or peg-IFNα-2b: patients > 60 years randomized (I:I:I) to peg-IFNα-2a, peg-IFNα-2b or HU | III | 90 PV patients without prior cytoreductive therapy | 1.) ORR: 68% (13/19) for HU, 42% (14/33) for peg-IFNα ≤ 60 years and 39% (13/33) for peg-IFNα > 60 years. 2.) PMR: 21% (4/19) of HU; 24% (7/29) of peg-IFNα treated patients ≤ 60 years; 18% (6/33) peg-IFNα > 60 years |
| Masarova et al. | [102] | 2017 | Peg-IFN-α-2a 90–450 μg/week | II | 40 ET, 43 PV patients | 1.) ET: ORR: 80% (CHR: 73%; PHR: 3%) 2.) PV: ORR: 84% (CHR: 77%; PHR: 7%) |
| Mascarenhas et al. | [41] | 2018 | Peg-IFN-α-2a vs HU | III | 168 ET/PV patients (82 peg-IFN, 86 HU) | 1.) 12 months: ORR 69.8% for HU vs 78% for peg-IFN 2.) 24 months: ORR 40.7%% for HU vs 59.6% for peg-IFN |
| Verger et al. | [46] | 2015 | Peg-IFN-α 230–496 μg/month (mean dose) | Prospective cohort study | 31 ET patients (100% CALR mutation) | ORR: 100% (CHR: 77%, PHR: 23%) |
| Yacoub et al. | [43] | 2019 | Peg-IFN α-2a 45–180 μg/week | II | 65 ET, 50 PV patients; refractory/intolerant of HU | 1.) ET: ORR: 69% (CHR: 43%; PHR: 26%) 2.) PV: ORR: 60% (CHR: 22%; PHR: 38%) |
BAT – best available therapy; CALR – calreticulin; CHR – complete hematologic response; CMR – complete molecular response; ET – essential thrombocythemia; HU – hydroxyurea; JAK2 – janus kinase 2; ORR – overall response rate; PEG – pegylated; PHR – partial hematologic remission; PMR – partial molecular response; PV – polycythemia vera
A major challenge remains the absence of validated biomarkers predictive of a higher likelihood of response to IFN. While Yacoub et al. found that response rates were higher in ET patients with a CALR mutation and lower in the setting of ASXL1 and TP53 mutations, additional studies are needed to further delineate the influence of co-mutations and other predictive biomarkers [43]. Similarly, TET2-mutated clones appear less susceptible to elimination by IFN [45,46]. Furthermore, the role of IFN in the treatment landscape of ET and PV is likely going to evolve and frontline treatment with IFN could be an alternative to HU in selected patient populations .To date, IFN is already the preferred treatment option in pregnant patients due to the teratogenic potential of HU and data supporting the safety of IFN in pregnant ET and PV patients [51,52]. Additionally, given the disease-modifying effect of IFN, a time-limited therapy followed by extended periods of treatment-free remission seems possible, which could be especially attractive for younger patients. As data on the leukemogenic effects of HU are highly controversial, using this as a major factor in treatment selection is likely not justified[47–50]. However, additional studies with longer follow up to determine the duration of hematologic responses and the effect on thrombotic complications are needed.
Although available data from ET and PV patients are limited, the combination of the JAK1/2 inhibitor ruxolitinib with peg-IFN in PV (n=32 patients) or MF (n=18) patients who had been previously treated with peg-IFN-α2 has shown synergistic effects with better tolerability and improvements in bone marrow histology and JAK2 V617F allele burden [53]. Mechanistically, ruxolitinib could enhance tolerability of IFN by down-regulation of cytokine production, while IFN is acting on hematopoietic stem and progenitor cells [20,53]. Given that both ruxolitinib and IFN have shown safety and efficacy in PV patients, such a combination could be a promising strategy in patients especially in the second-line setting.
A limitation and source of potential bias in studies using IFN and comparing it to oral medications is the unblinded design of both phase III trials comparing IFN to HU or best available therapy [40,41]. While objective endpoints such as spleen size reduction and normalization of cell counts should be less affected by an open-label design, subjective, patient-reported outcomes such as symptom burden might be subject to bias.
5. JAK inhibitors – ruxolitinib and beyond
Janus kinases (JAK1, JAK2, JAK3, and TYK2) are essential regulators of the proliferation, survival, and differentiation of hematopoietic cells [54]. Especially, JAK2, which associates with the receptors for erythropoietin, thrombopoietin, and granulocyte colony-stimulating factor, is fundamental to signaling via those receptors and activates various signaling pathways including STAT, MAPK, PI3K, and mTOR [54]. With the identification of JAK2 V617F mutations as the most common mutation in ET and PV and as a driver of clonal proliferation, several JAK inhibitors have been developed [54]. Additionally, JAK inhibitors exert immunomodulatory effects via inhibition of the JAK-STAT pathway leading to reductions in proinflammatory cytokine levels [20,25]. This immunomodulatory effect is also evident in the benefit of ruxolitinib in the treatment of graft-versus-host disease and is likely responsible for the improvement in MPN-related symptom burden [55,56].
The JAK1/2 inhibitor ruxolitinib has shown significant improvements in symptom burden, spleen size, and also overall survival in randomized phase III trials in patients with MF [55,57]. In PV patients, ruxolitinib has been shown to be superior to best available therapy (including 59% HU, 11.6% IFN) in patients refractory to or intolerant of HU. In the RESPONSE trial (NCT01243944), 222 PV patients with splenomegaly who had been previously treated with HU were randomized to ruxolitinib or standard therapy with statistically significantly higher rates for the primary outcome of both hematocrit control and ≥35% spleen size reduction (20.9% vs 0.9%; p<0.001) in the ruxolitinib group [58]. Several secondary outcomes including hematocrit control (60% vs 20%), CHR (24% vs 9%), ≥50% symptom reduction (49% vs 5%) and ≥35% reduction in spleen size (38% vs 1%) also appeared to be superior in ruxolitinib-treated patients [58]. Similar results have been reported from the phase III RESPONSE-2 study (NCT02038036) that enrolled HU-intolerant or -resistant PV patients without splenomegaly and randomized them to either ruxolitinib or best available therapy (49% HU, 13% IFN) [59]. Patients treated with ruxolitinib had statistically significantly higher odds of achieving hematocrit control (62% vs 19%; p<0.0001) [59]. Post-hoc analyses comparing the patients treated with ruxolitinib and IFN showed similar results favoring ruxolitinib in terms of hematocrit control (RESPONSE: ruxolitinib: 60% vs IFN: 23%; RESPONSE-2: ruxolitinib: 62% vs IFN: 15%) [60]. Furthermore, patients who crossed over to the ruxolitinib arm after treatment with IFN experienced hematologic and spleen size responses in 62% of cases [60]. Limitations of the RESPONSE trials potentially leading to bias include the non-blinded treatment assignment, the fact that the majority of patients in the standard therapy arm received HU despite known refractoriness or intolerance, and the higher rates of treatment discontinuation in the standard therapy arm [4,58,59].
The randomized double-blind phase III RELIEF trial that randomized patients on stable dose HU to either continue HU or switch to ruxolitinib did not show a statistically significant difference in the primary endpoint of ≥50% symptom improvement (odds ratio 1.82; 95% CI: 0.82–4.04; p=0.139) [61]. Therefore, the ELN expert panel is recommending IFN as the preferred second-line therapy in PV patients [4]. Table 2 summarizes selected clinical trials of ruxolitinib in ET and PV.
Table 2:
Results from selected studies of JAK inhibitors in ET and PV patients
| Author | Reference | Year of publication | Treatment and treatment schedule | Phase | Study population | Outcomes |
|---|---|---|---|---|---|---|
| Vannucchi et al. | [58] | 2015 | Ruxolitinib vs standard therapy | III | Phlebotomy-dependent PV patients with splenomegaly and resistance or intolerance to HU | Primary endpoint (hematocrit control + ≥35% reduction in spleen size): 21% in ruxolitinib vs 1% in standard therapy (p<0.001) |
| Passamonti et al. | [59] | 2017 | Ruxolitinib vs BAT | IIIb | PV patients with resistance or intolerance to HU | Hematocrit control: 62% of ruxolitinib-treated patients vs 19% of BAT-treated patients (odds ratio 7.28 [95% CI 3.43–15.45]; p<0.0001) |
| Mesa et al. | [61] | 2017 | Patients on stable dose of HU randomized to continue HU or switch to ruxolitinib | IIIb | Symtpomatic PV patients on stable dose of HU | ≥50% reduction in MPN-related symptoms: 43.4% vs. 29.6% of ruxolitinib‐ and HU-treated patients, respectively (odds ratio, 1.82; 95% CI 0.82–4.04; p = 0.139) |
| Sorensen et al. | [53] | 2019 | Ruxolitinib + low-dose peg-IFN-α-2a | II | PV (n=32) and myelofibrosis (n-18) intolerant or refractory to peg-IFN-α-2a | PV patients: 31% remission (9% CR); 85% CHR MF patients: 44% remission (28% CR); 75% MF |
BAT – best available therapy; CALR – calreticulin; CHR – complete hematologic response; CR – complete response; ET – essential thrombocythemia; HU – hydroxyurea; JAK2 – janus kinase 2; MF - myelofibrosis; PEG – pegylated; PHR – partial hematologic remission; PV – polycythemia vera
Data on ruxolitinib in ET are scarce. In the randomized phase II MAJIC trial (ISRCTN61925716), 110 high-risk ET patients resistant to or intolerant of HU were treated with ruxolitinib or best available therapy (HU 71%; anagrelide 48%, IFN 40%) [62]. There was no statistically significant difference in CR rate after 1 year (46.6% with ruxolitinib vs 44.2% with best available therapy; p=0.40) and no statistically significant difference in rates of leukemic transformation, thrombotic or hemorrhagic complications at 2 years of follow up [62]. However, compared to best available therapy ruxolitinib led to a significantly greater maximum percentage reduction in the total symptom score (TSS; median reduction 32% with ruxolitinib vs 0% with best available therapy; p=0.03), mean TSS and pruritus subscore [62]. Grade 3/4 anemia (19% vs 0%) and thrombocytopenia (6.9% vs 0%) were more common in the ruxolitinib arm [62]. Similar to observations from PV patients in the RESPONSE trial, rates of complete or partial molecular response in ET patients treated with ruxolitinib were low [62,63]. Ruxolitinib in combination with the BCL-2 inhibitor navitoclax has also shown promising preliminary results in MF patients and is currently being studied in patients with ET and PV (NCT04041050) [64].
While several other JAK inhibitors such as fedratinib, pacritinib, and momelotinib have been successfully tested in MF patients with fedratinib being approved by the United States Food and Drug Administration, none of those agents has been tested in ET or PV patients thus far [65–68].
6. Novel agents, combination therapies, and future directions
Table 3 provides an overview of active clinical trials in ET and PV patients. With a better understanding of the immunobiology of MPNs, novel combination therapies have been developed but primarily tested in MF patients [20,69]. For example, combinations of ruxolitinib with the immunomodulators lenalidomide and thalidomide have been evaluated in small clinical trials with evidence of efficacy but especially the lenalidomide-ruxolitinib combination is limited by substantial myelosuppression [20,70]. Conversely, low-doses of thalidomide in combination with prednisone and androgens (danazol, stanozolol) have been shown to ameliorate the anemia and thrombocytopenia associated with the underlying disease and/or ruxolitinib treatment in retrospective studies in MF patients [71,72]. Similarly, data showing the increased expression of PD-L1 in MPN patients, could provide the scientific rationale for the use of anti-PD-1/PD-L1 antibodies [27,28]. However, no such trials are currently ongoing in ET or PV.
Table 3:
Ongoing clinical trials in ET and PV patients
| Drug | Phase | NCT | Patient characteristics | Intervention |
|---|---|---|---|---|
| Essential thrombocythemia | ||||
| Interferon | III | NCT04285086 | ET patients with resistance or intolerance to HU | Ropeginterferon alfa-2b vs anagrelide |
| II | NCT00452023 | ET and PV | Peg-IFN alfa-2a | |
| IV | NCT04226950 | Children (<18 years) with ET | Peg-IFN alfa-2b vs. IFN alfa | |
| Ruxolitinib | II | NCT03123588 | ET patients with resistance or intolerance to HU | Ruxolitinib + placebo vs anagrelide + placebo |
| II/III | NCT02962388 | ET patients with resistance or intolerance to HU | Ruxolitinib vs best available therapy (anagrelide, IFN alfa/peg-IFN alfa) | |
| II | NCT02577926 | High-risk ET or PV (>60 years or prior thromboembolic event) | Ruxolitinib vs BAT | |
| Navitoclax (BCL2 inhibitor) | I | NCT04041050 | Myeloproliferative neoplasms | Navitoclax alone or in combination with ruxolitinib (only after progression to myelofibrosis) |
| Givinostat (HDAC inhibitor) | II | NCT01761968 | JAK2 V617F-positive Myeloproliferative Neoplasms | Givinostat |
| IMG-7289 (LSD1 inhibitor) | II | NCT04081220 | ET patients with resistance or intolerance to hydroxyurea | LSD1 inhibitor IMG-7289 |
| II | NCT04262141 | ET and PV patients who failed at least one line of therapy | LSD1 inhibitor IMG-7289 | |
| II | NCT04254978 | ET and PV patients who failed at least one line of therapy | LSD1 inhibitor IMG-7289 | |
| Peptide vaccines | I | NCT03566446 | CALR-mutant myeloproliferative neoplasms | CALR Exon 9 mutant peptide vaccine |
| I/II | NCT04051307 | ET and PV | PD-L1 and Aginase1 peptide vaccines | |
| Polycythemia vera | ||||
| Interferon | II | NCT03003325 | Low-risk PV (i.e. age 18–60 years; no prior cardiovascular PV-related event) | Aspirin + phlebotomy +/− ropeginterferon alfa-2b |
| II | NCT04182100 | PV patients with limitations of standard therapy | Aspirin + phlebotomy + ropeginterferon alfa-2b | |
| Ruxolitinib | III | NCT04116502 | High risk PV (WBC >11 × 109/L + age >60 years, prior thrombosis or hemorrhage, or platelet count >1000 × 109/L) | Ruxolitinib vs hydroxyurea or IFN |
| KRT-232 (MDM2 inhibitor) | II | NCT03669965 | Part A: PV patients with/without splenomegaly; resistant or intolerant to HU or prior IFN Part B: PV patients with splenomegaly; resistant or intolerant to HU |
Part A: KRT-232 Part B: KRT-232 or ruxoltinib |
| PTG-300 (hepcidin mimetic) | II | NCT04057040 | Phlebotomy-dependent PV patients | PTG-300 vs placebo |
BAT – best available therapy; BCL2 – B-cell lymphoma 2; CALR – calreticulin; ET – essential thrombocythemia; HDAC – histone deacetylase; HU – hydroxyurea; IFN – interferon; JAK2 – janus kinase 2; LSD1 – lysine-specific demethylase 1A; PD-L1 – programmed death ligand-1; PEG – pegylated; PHR – partial hematologic remission; PV – polycythemia vera
Gene expression is tightly regulated by DNA methylation and demethylation as well as histone acetylation and deacetylation with aberrant DNA methylation having been documented in MPN patients [1]. Notably, those aberrant DNA methylation patterns were enriched in genes associated with inflammatory signaling pathways such as NF-κB [73]. While hypomethylating agents (HMA) and histone deacetylase inhibitors (HDAC) have been studied extensively in patients with AML and myelodysplastic syndromes [74], data on their use in ET and PV patients is very limited. In a phase II study of 63 patients (19 with ET, 44 with PV) 35% of patients treated with the HDAC inhibitor vorinostat achieved a hematologic response with 65% of patients experiencing a reduction in JAK2 V617F allele burden. However, 28 out of 63 patients (44.4%) of patients discontinued the study early due to side effects [75]. Similar results have been reported for another HDAC inhibitor givinostat as well [76].
The lysine-specific demethylase 1A (LSD1) inhibitor IMG-7289 (bomedemstat) has been successfully tested in a phase II trial in MF patients [77]. As preclinical evidence suggests that treatment with LSD1 inhibitors can reduce thrombopoiesis, modulate inflammatory signaling, improve bone marrow fibrosis and improve survival in mice, several clinical trials are currently testing IMG-7289 in ET patients [78].
Telomeres are repetitive DNA sequences that shorten with each cell division thereby limiting the replicative potential of cells [79]. Telomere shortening has been shown to correlate with JAK2 V617F allele burden in PV and MF patients and could potentially contribute to genomic instability [1,80]. Conversely, telomerase activity is essential to maintain telomere length in rapidly dividing cells and has been documented in various cancers [81]. Following in-vitro studies showing that the telomerase inhibitor imetelstat was able to inhibit megakaryocyte proliferation in cells from ET patients, a subsequent phase II study (NCT01243073) showed hematologic responses in 100% (CHR 89%) and molecular responses in a majority of patients [82]. Additional in-vitro studies showed that imetelstat is specifically targeting neoplastic megakaryocytes while sparing normal megarkaryocytes [83,84]. Despite those promising results, no clinical trials using imetelstat in ET or PV patients are currently active.
Advances in genetic testing will likely enable a more nuanced approach to risk stratification and potentially treatment of ET and PV patients [85,86]. For example, noncanonical variants in JAK2 and MPL have been described in “triple-negative” (i.e. no detectable JAK2 V617F, CALR, MPL mutation) cases of ET and PV [87,88]. Similarly, mutations observed in genes involved in epigenetic and splicing regulation as well as TP53 and RAS are observed in MPNs as well [29]. However, their prognostic relevance is uncertain and warrants further studies. In ET and PV patients, variants in SH2B3, SF3B1, U2AF1, TP53, IDH2, EZH2 and ASXL1, SRSF2, IDH2 had been identified as adverse prognostic factors, respectively [91]. While this has led to the development of novel risk stratification systems in both MF (e.g. MIPSS-70) and ET and PV (MIPSS-ET and MIPSS-PV) that include genetic testing results [92–94], additional validation studies especially in ET and PV are needed. The impact of co-mutations in genes involved in epigenetic and splicing regulation as well as genes involved in signaling processes such as TP53 and RAS has recently been described by Grinfeld et al. [6]. While their random effects modelling showed that age and male sex were associated with adverse prognosis, no clear association of certain genetic abnormalities with leukemic transformation or survival outcomes were identified [6]. Although their model appeared to have a superior performance compared to other models such as the International Prognostic Score for ET, additional external validation is required prior to routine use [6,95]. Beyond prognostication, comprehensive genetic testing might also be able to identify biomarkers predictive of a higher likelihood of response to novel therapies such as imetelstat or IFN.
Finally, several novel agents a such as the bromodomain and extra-terminal motif (BET) inhibitor CPI-0610, the smac-mimetic LCL-161, the murine double minute 2 (MDM2) inhibitor KRT-232, or the recombinant human pentraxin-2 molecule PRM-151 have been studied in patients with MF with promising preliminary results [96–99]. However, among these agents only KRT-232 is currently being tested in clinical trials in PV patients (NCT03669965) but could represent additional therapeutic options in the future.
7. Conclusion
Advances in both the understanding of the immunology of MPN pathogenesis and the molecular landscape have enabled novel (e.g. JAK inhibitors) or the renaissance of older medications (e.g. IFN) as well as a more individualized treatment for ET and PV patients. Especially IFN appears to have a disease-modifying potential as suggested by reductions in mutant allele burden which might translate into prolonged treatment-free remission. Additional research to identify biomarkers predictive of a higher or lower response rate to a certain therapy as well as a more nuanced and individualized risk stratification are needed.
8. Expert opinion
Although the prognosis of patients with ET and PV compares favorably to other myeloid neoplasms, the life-expectancy is reduced compared to age- and sex-matched controls primarily due to thromboembolic complications and progression to MF and AML. While treatment has focused primarily on the mitigation of thrombosis risk by means of phlebotomy and use of low-dose aspirin as well as cytoreduction with HU in high-risk patients, none of those therapies has a disease-modifying effect and can be associated with burdensome adverse events. Additionally, for patients intolerant of or refractory to HU, therapeutic options are very limited.
More recently, the JAK inhibitor ruxolitinib has been shown to be superior to best available therapy in PV patients intolerant of or refractory to HU. However, molecular response rates were low and ruxolitinib is associated with a higher risk of infections, skin malignancies, cytopenias, and financial toxicities for the patients and the health care system. Furthermore, the landmark phase III trial has been limited by the fact that HU – at a lower dose - was the most common agent used in the best available therapy group and its non-blinded design. In contrast to ruxolitinib, IFN formulations have been shown to have disease-modifying potential as evidenced by complete molecular responses, changes in bone marrow histopathology, and prolonged treatment-free remissions. Especially, the novel ropegylated IFN version which appears better tolerated and is administered only at 2-week intervals, which should promote patient adherence to therapy, could become an attractive option for younger – especially if pregnancy is being considered – patients with PV. However, extended follow up is needed to determine whether IFN is able to reduce thromboembolic events and whether the molecular responses seen translate into a lower rate of progression to MF or AML. Additional research is also needed in ET patients and the results of the ongoing phase III trial (NCT04285086) comparing ropegylated IFN to anagrelide in HU-intolerant or refractory patients will be very informative. Finally, the combination of ruxolitinib and IFN has been suggested to have synergistic effects in terms of treatment efficacy and in reducing IFN-associated side effects.
While the field of MPNs has recently seen the publication of several randomized phase III trials, especially novel agents and combination therapies have mostly been tested in open-label, single-arm studies and even for randomized phase III trials limitations related to trial design exist. For example, both phase III trials on IFN for the treatment of ET and PV used an open-label design primarily due to the route of administration, which introduces the risk of bias especially for subjective, patient-reported outcomes such as symptom burden. Similarly, the RESPONSE trials that compared ruxolitinib with best available therapy had an open-label design. As the RELIEF study comparing HU and ruxolitinib employed a double-blinded design, cross-study comparisons are limited. While placebo injections would be technically possible and ethical in our opinion, it might reduce the attractiveness of such a trial to patients and limit enrollment. Given that ET and PV are already rare diseases, large international collaborations are necessary to meet accrual goals, which becomes even more important if dedicated studies in clinically or genetically-defined patient subsets are planned.
With advances in molecular diagnostics treatment of ET and PV patients is likely going to become increasingly individualized both in terms of risk stratification and treatment selection. While risk stratification tools incorporating genetic information such as the MIPSS-ET and MIPSS-PV have been developed, additional validation studies are needed. Additionally, genetic profiling could serve as a biomarker to predict response to treatments such as IFN. However, as documented by studies in AML and MDS, the genetic landscape is likely more complicated especially in older patients who can harbor other small clonal populations as part of clonal hematopoiesis of indeterminate potential (CHIP) and additional longitudinal studies to evaluate the prognostic impact are needed. The impact of co-occurring mutations will be helpful to further elucidate the processes leading to progression to MF and AML.
In summary, HU will likely remain the most frequently used therapy for most high-risk ET and PV patients but several alternatives such as ropegylated IFN, ruxolitinib, or novel agents (e.g. HDAC inhibitors, imetelstat) are promising. We are also about to enter a new era of genetically-driven and individualized prognostication and treatment approaches, but additional validation is needed at this time prior to routine use.
Article highlights box.
While hydroxyurea remains the most frequently used agent for cytoreduction in high-risk ET and PV patients, ropegylated interferon and the JAK1/2 inhibitor ruxolitinib have shown promising results and garnered regulatory approval
Advances in molecular testing enable a more individualized approach to prognostication and treatment selection
Promising novel agents in clinical testing include the histone deacetylase inhibitor givinostat, the telomerase inhibitor imetelstat, and the LSD-1 inhibitor IMG-7289
Acknowledgments
Amer Zeidan is a Leukemia and Lymphoma Society Scholar in Clinical Research and is also supported by a National Cancer Institute (NCI) Cancer Clinical Investigator Team Leadership Award (CCITLA). Research reported in this publication was supported by the NCI of the National Institutes of Health under Award Number P30 CA016359. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding
This paper was not funded.
Declaration of interest
AM Zeidan received research funding (institutional) from Celgene, Acceleron, Abbvie, Novartis, Otsuka, Pfizer, Medimmune/AstraZeneca, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, and ADC Therapeutics; had a consultancy with and received honoraria from AbbVie, Otsuka, Pfizer, Celgene, Jazz, Ariad, Incyte, Agios, Boehringer-Ingelheim, Novartis, Acceleron, Astellas, Daiichi Sankyo, Cardinal Health, Seattle Genetics, BeyondSpring, Trovagene, Ionis, Epizyme, Amgen, Tyme, Janssen, and Takeda and received travel support for meetings from Pfizer, Novartis, and Trovagene. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Footnotes
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- 1.Spivak JL. Myeloproliferative Neoplasms. N Engl J Med. 2017;376(22):2168–2181. [DOI] [PubMed] [Google Scholar]
- 2.Tefferi A, Pardanani A. Essential Thrombocythemia. N Engl J Med. 2019;381(22):2135–2144. [DOI] [PubMed] [Google Scholar]
- 3.Shallis RM, Wang R, Davidoff A, et al. Epidemiology of the classical myeloproliferative neoplasms: The four corners of an expansive and complex map. Blood Rev. 2020. 2020/05/22/:100706. [DOI] [PubMed] [Google Scholar]
- 4.Barbui T, Tefferi A, Vannucchi AM, et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia. 2018;32(5):1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tefferi A, Vannucchi AM, Barbui T. Essential thrombocythemia treatment algorithm 2018. Blood Cancer J. 2018. January 10;8(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grinfeld J, Nangalia J, Baxter EJ, et al. Classification and Personalized Prognosis in Myeloproliferative Neoplasms. N Engl J Med. 2018. October 11;379(15):1416–1430.** This landmark study describes the influence of specific genetic alterations on the prognosis of patients with myeloproliferative neoplasms
- 7.Guglielmelli P, Vannucchi AM. Current management strategies for polycythemia vera and essential thrombocythemia. Blood Rev. 2020. 2020/06/03/:100714. [DOI] [PubMed] [Google Scholar]
- 8.Network NCC. NCCN Guidelines Version 1.2020: Myeloproliferative neoplasms 2020. [cited 2020 7/31/2020]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/mpn.pdf
- 9.Szuber N, Mudireddy M, Nicolosi M, et al. 3023 Mayo Clinic Patients With Myeloproliferative Neoplasms: Risk-Stratified Comparison of Survival and Outcomes Data Among Disease Subgroups. Mayo Clin Proc. 2019. April;94(4):599–610. [DOI] [PubMed] [Google Scholar]
- 10.Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013. 2013/09/01;27(9):1874–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbui T, Finazzi G, Carobbio A, et al. Development and validation of an International Prognostic Score of thrombosis in World Health Organization–essential thrombocythemia (IPSET-thrombosis). Blood. 2012;120(26):5128–5133. [DOI] [PubMed] [Google Scholar]
- 12.Barbui T, Masciulli A, Marfisi MR, et al. White blood cell counts and thrombosis in polycythemia vera: a subanalysis of the CYTO-PV study. Blood. 2015. July 23;126(4):560–1. [DOI] [PubMed] [Google Scholar]
- 13.Ronner L, Podoltsev N, Gotlib J, et al. Persistent leukocytosis in polycythemia vera is associated with disease evolution but not thrombosis. Blood. 2020. May 7;135(19):1696–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rumi E, Pietra D, Ferretti V, et al. JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood. 2014. March 6;123(10):1544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spivak JL. How I treat polycythemia vera. Blood. 2019;134(4):341–352. [DOI] [PubMed] [Google Scholar]
- 16.Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013. January 3;368(1):22–33. [DOI] [PubMed] [Google Scholar]
- 17.Harrison CN, Campbell PJ, Buck G, et al. Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia. N Engl J Med. 2005. July 7;353(1):33–45. [DOI] [PubMed] [Google Scholar]
- 18.Koschmieder S, Chatain N. Role of inflammation in the biology of myeloproliferative neoplasms. Blood Rev. 2020. 2020/05/30/:100711. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez-Meira A, Buck G, Clark S-A, et al. Unravelling Intratumoral Heterogeneity through High-Sensitivity Single-Cell Mutational Analysis and Parallel RNA Sequencing. Mol Cell. 2019. 2019/03/21/;73(6):1292–1305.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masarova L, Bose P, Verstovsek S. The Rationale for Immunotherapy in Myeloproliferative Neoplasms. Curr Hematol Malig Rep. 2019. 2019/08/01;14(4):310–327.** This is a very detailed review of the immunology of myeloproliferative neoplasms and treatment options targeting the aberrant immune response
- 21.Rampal R, Al-Shahrour F, Abdel-Wahab O, et al. Integrated genomic analysis illustrates the central role of JAK-STAT pathway activation in myeloproliferative neoplasm pathogenesis. Blood. 2014;123(22):e123–e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plo I, Nakatake M, Malivert L, et al. JAK2 stimulates homologous recombination and genetic instability: potential implication in the heterogeneity of myeloproliferative disorders. Blood. 2008;112(4):1402–1412. [DOI] [PubMed] [Google Scholar]
- 23.Vaidya R, Gangat N, Jimma T, et al. Plasma cytokines in polycythemia vera: phenotypic correlates, prognostic relevance, and comparison with myelofibrosis. Am J Hematol. 2012. November;87(11):1003–5. [DOI] [PubMed] [Google Scholar]
- 24.Pourcelot E, Trocme C, Mondet J, et al. Cytokine profiles in polycythemia vera and essential thrombocythemia patients: Clinical implications. Exp Hematol. 2014. 2014/05/01/;42(5):360–368. [DOI] [PubMed] [Google Scholar]
- 25.Kleppe M, Kwak M, Koppikar P, et al. JAK-STAT pathway activation in malignant and nonmalignant cells contributes to MPN pathogenesis and therapeutic response. Cancer Discov. 2015. March;5(3):316–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marty C, Lacout C, Droin N, et al. A role for reactive oxygen species in JAK2V617F myeloproliferative neoplasm progression. Leukemia. 2013. 2013/11/01;27(11):2187–2195. [DOI] [PubMed] [Google Scholar]
- 27.Prestipino A, Emhardt AJ, Aumann K, et al. Oncogenic JAK2V617F causes PD-L1 expression, mediating immune escape in myeloproliferative neoplasms. Sci Transl Med. 2018;10(429):eaam7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sørensen AL, Bjørn ME, Riley CH, et al. B-cell frequencies and immunoregulatory phenotypes in myeloproliferative neoplasms: Influence of ruxolitinib, interferon-α2, or combination treatment. Eur J Haematol. 2019;103(4):351–361. [DOI] [PubMed] [Google Scholar]
- 29.Vainchenker W, Kralovics R. Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood. 2017;129(6):667–679. [DOI] [PubMed] [Google Scholar]
- 30.How J, Hobbs G. Use of Interferon Alfa in the Treatment of Myeloproliferative Neoplasms: Perspectives and Review of the Literature. Cancers (Basel). 2020;12(7):1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiladjian J-J, Mesa RA, Hoffman R. The renaissance of interferon therapy for the treatment of myeloid malignancies. Blood. 2011;117(18):4706–4715. [DOI] [PubMed] [Google Scholar]
- 32.Kiladjian JJ, Cassinat B, Chevret S, et al. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood. 2008. October 15;112(8):3065–72. [DOI] [PubMed] [Google Scholar]
- 33.Quintas-Cardama A, Abdel-Wahab O, Manshouri T, et al. Molecular analysis of patients with polycythemia vera or essential thrombocythemia receiving pegylated interferon alpha-2a. Blood. 2013. August 8;122(6):893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stauffer Larsen T, Iversen KF, Hansen E, et al. Long term molecular responses in a cohort of Danish patients with essential thrombocythemia, polycythemia vera and myelofibrosis treated with recombinant interferon alpha. Leuk Res. 2013. September;37(9):1041–5. [DOI] [PubMed] [Google Scholar]
- 35.Gisslinger H, Chott A, Scheithauer W, et al. Interferon in essential thrombocythaemia. British Journal of Haematology. 1991. October;79 Suppl 1:42–7. [DOI] [PubMed] [Google Scholar]
- 36.Lu M, Zhang W, Li Y, et al. Interferon-α targets JAK2V617F-positive hematopoietic progenitor cells and acts through the p38 MAPK pathway. Exp Hematol. 2010. 2010/06/01/;38(6):472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Essers MAG, Offner S, Blanco-Bose WE, et al. IFNα activates dormant haematopoietic stem cells in vivo. Nature. 2009. 2009/04/01;458(7240):904–908. [DOI] [PubMed] [Google Scholar]
- 38.Mullally A, Bruedigam C, Poveromo L, et al. Depletion of Jak2V617F myeloproliferative neoplasm-propagating stem cells by interferon-α in a murine model of polycythemia vera. Blood. 2013;121(18):3692–3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bewersdorf JP, Giri S, Wang R, et al. Interferon alpha therapy in essential thrombocythemia and polycythemia vera-a systematic review and meta-analysis. Leukemia. 2020. September 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gisslinger H, Klade C, Georgiev P, et al. Ropeginterferon alfa-2b versus standard therapy for polycythaemia vera (PROUD-PV and CONTINUATION-PV): a randomised, non-inferiority, phase 3 trial and its extension study. Lancet Haematol. 2020. March;7(3):e196–e208.* This study showed compared ropegylated interferon to hydroxyurea for the frontline treatment of PV patients leading to the approval of ropegylated interferon in this setting.
- 41.Mascarenhas J, Kosiorek HE, Prchal JT, et al. Results of the Myeloproliferative Neoplasms - Research Consortium (MPN-RC) 112 Randomized Trial of Pegylated Interferon Alfa-2a (PEG) Versus Hydroxyurea (HU) Therapy for the Treatment of High Risk Polycythemia Vera (PV) and High Risk Essential Thrombocythemia (ET). Blood. 2018;132(Supplement 1):577–577.29954751 [Google Scholar]
- 42.Mesa RA, Kosiorek HE, Mascarenhas J, et al. Impact on MPN Symptoms and Quality of Life of Front Line Pegylated Interferon Alpha-2a Vs. Hydroxyurea in High Risk Polycythemia Vera and Essential Thrombocythemia: Results of Myeloproliferative Disorders Research Consortium (MPD-RC) 112 Global Phase III Trial. Blood. 2018;132(Supplement 1):3032–3032. [Google Scholar]
- 43.Yacoub A, Mascarenhas J, Kosiorek H, et al. Pegylated interferon alfa-2a for polycythemia vera or essential thrombocythemia resistant or intolerant to hydroxyurea. Blood. 2019. October 31;134(18):1498–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mascarenhas J, Kosiorek H, Prchal J, et al. A prospective evaluation of pegylated interferon alfa-2a therapy in patients with polycythemia vera and essential thrombocythemia with a prior splanchnic vein thrombosis. Leukemia. 2019;33(12):2974–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kiladjian JJ, Massé A, Cassinat B, et al. Clonal analysis of erythroid progenitors suggests that pegylated interferon α−2a treatment targets JAK2V617F clones without affecting TET2 mutant cells. Leukemia. 2010. 2010/08/01;24(8):1519–1523. [DOI] [PubMed] [Google Scholar]
- 46.Verger E, Cassinat B, Chauveau A, et al. Clinical and molecular response to interferon-alpha therapy in essential thrombocythemia patients with CALR mutations. Blood. 2015;126(24):2585–91. [DOI] [PubMed] [Google Scholar]
- 47.Sterkers Y, Preudhomme C, Lai JL, et al. Acute myeloid leukemia and myelodysplastic syndromes following essential thrombocythemia treated with hydroxyurea: high proportion of cases with 17p deletion. Blood. 1998. January 15;91(2):616–22. [PubMed] [Google Scholar]
- 48.Kiladjian JJ, Rain JD, Bernard JF, et al. Long-term incidence of hematological evolution in three French prospective studies of hydroxyurea and pipobroman in polycythemia vera and essential thrombocythemia. Semin Thromb Hemost. 2006. June;32(4 Pt 2):417–21. [DOI] [PubMed] [Google Scholar]
- 49.Beauverd Y, Radia D, Cargo C, et al. Pegylated interferon alpha-2a for essential thrombocythemia during pregnancy: outcome and safety. A case series. Haematologica. 2016. May;101(5):e182–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Björkholm M, Derolf AR, Hultcrantz M, et al. Treatment-related risk factors for transformation to acute myeloid leukemia and myelodysplastic syndromes in myeloproliferative neoplasms. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(17):2410–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robinson SE, Harrison CN. How we manage Philadelphia-negative myeloproliferative neoplasms in pregnancy. Br J Haematol. 2020. May;189(4):625–634. [DOI] [PubMed] [Google Scholar]
- 52.Maze D, Kazi S, Gupta V, et al. Association of Treatments for Myeloproliferative Neoplasms During Pregnancy With Birth Rates and Maternal Outcomes: A Systematic Review and Meta-analysis. JAMA Netw Open. 2019. October 2;2(10):e1912666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sorensen AL, Mikkelsen SU, Knudsen TA, et al. Ruxolitinib and interferon-alpha2 combination therapy for patients with polycythemia vera or myelofibrosis: a phase II study. Haematologica. 2019. December 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vainchenker W, Leroy E, Gilles L, et al. JAK inhibitors for the treatment of myeloproliferative neoplasms and other disorders. F1000Res. 2018;7:82–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012. March 1;366(9):799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeiser R, von Bubnoff N, Butler J, et al. Ruxolitinib for Glucocorticoid-Refractory Acute Graft-versus-Host Disease. N Engl J Med. 2020;382(19):1800–1810. [DOI] [PubMed] [Google Scholar]
- 57.Verstovsek S, Gotlib J, Mesa RA, et al. Long-term survival in patients treated with ruxolitinib for myelofibrosis: COMFORT-I and -II pooled analyses. J Hematol Oncol. 2017. September 29;10(1):156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vannucchi AM, Kiladjian JJ, Griesshammer M, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med. 2015. January 29;372(5):426–35.* This study showed the efficacy of ruxolitinib in hydroxyurea-intolerant or -refractory PV patients leading to the approval of ruxolitinib in this setting.
- 59.Passamonti F, Griesshammer M, Palandri F, et al. Ruxolitinib for the treatment of inadequately controlled polycythaemia vera without splenomegaly (RESPONSE-2): a randomised, open-label, phase 3b study. The Lancet Oncology. 2017. 2017/01/01/;18(1):88–99. [DOI] [PubMed] [Google Scholar]
- 60.Kiladjian JJ, Guglielmelli P, Griesshammer M, et al. Efficacy and safety of ruxolitinib after and versus interferon use in the RESPONSE studies. Ann Hematol. 2018. April;97(4):617–627. [DOI] [PubMed] [Google Scholar]
- 61.Mesa R, Vannucchi AM, Yacoub A, et al. The efficacy and safety of continued hydroxycarbamide therapy versus switching to ruxolitinib in patients with polycythaemia vera: a randomized, double-blind, double-dummy, symptom study (RELIEF). Br J Haematol. 2017;176(1):76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harrison CN, Mead AJ, Panchal A, et al. Ruxolitinib vs best available therapy for ET intolerant or resistant to hydroxycarbamide. Blood. 2017. October 26;130(17):1889–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vannucchi AM, Verstovsek S, Guglielmelli P, et al. Ruxolitinib reduces JAK2 p.V617F allele burden in patients with polycythemia vera enrolled in the RESPONSE study. Ann Hematol. 2017. 2017/07/01;96(7):1113–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harrison CN, Garcia JS, Mesa RA, et al. Results from a Phase 2 Study of Navitoclax in Combination with Ruxolitinib in Patients with Primary or Secondary Myelofibrosis. Blood. 2019;134(Supplement_1):671–671. [Google Scholar]
- 65.Harrison CN, Vannucchi AM, Platzbecker U, et al. Momelotinib versus best available therapy in patients with myelofibrosis previously treated with ruxolitinib (SIMPLIFY 2): a randomised, open-label, phase 3 trial. Lancet Haematol. 2018. February;5(2):e73–e81. [DOI] [PubMed] [Google Scholar]
- 66.Bewersdorf JP, Jaszczur SM, Afifi S, et al. Beyond Ruxolitinib: Fedratinib and Other Emergent Treatment Options for Myelofibrosis. Cancer Manag Res. 2019;11:10777–10790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pardanani A, Harrison C, Cortes JE, et al. Safety and Efficacy of Fedratinib in Patients With Primary or Secondary Myelofibrosis: A Randomized Clinical Trial. JAMA Oncology. 2015;1(5):643–651. [DOI] [PubMed] [Google Scholar]
- 68.Harrison CN, Gerds AT, Kiladjian J-J, et al. Pacifica: A Randomized, Controlled Phase 3 Study of Pacritinib Vs. Physician’s Choice in Patients with Primary Myelofibrosis, Post Polycythemia Vera Myelofibrosis, or Post Essential Thrombocytopenia Myelofibrosis with Severe Thrombocytopenia (Platelet Count <50,000/mL). Blood. 2019;134(Supplement_1):4175–4175. [Google Scholar]
- 69.Vannucchi AM, Harrison CN. Emerging treatments for classical myeloproliferative neoplasms. Blood. 2017. February 9;129(6):693–703. [DOI] [PubMed] [Google Scholar]
- 70.Daver N, Cortes J, Newberry K, et al. Ruxolitinib in combination with lenalidomide as therapy for patients with myelofibrosis. Haematologica. 2015. August;100(8):1058–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luo X, Xu Z, Li B, et al. Thalidomide plus prednisone with or without danazol therapy in myelofibrosis: a retrospective analysis of incidence and durability of anemia response. Blood Cancer J. 2018. January 15;8(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Duan M, Zhou D. Improvement of the hematologic toxicities of ruxolitinib in patients with MPN-associated myelofibrosis using a combination of thalidomide, stanozolol and prednisone. Hematology. 2019. December;24(1):516–520. [DOI] [PubMed] [Google Scholar]
- 73.Pérez C, Pascual M, Martín-Subero JI, et al. Aberrant DNA methylation profile of chronic and transformed classic Philadelphia-negative myeloproliferative neoplasms. Haematologica. 2013;98(9):1414–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bewersdorf JP, Shallis R, Stahl M, et al. Epigenetic therapy combinations in acute myeloid leukemia: what are the options? Ther Adv Hematol. 2019;10:2040620718816698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Andersen CL, McMullin MF, Ejerblad E, et al. A phase II study of vorinostat (MK-0683) in patients with polycythaemia vera and essential thrombocythaemia. Br J Haematol. 2013;162(4):498–508. [DOI] [PubMed] [Google Scholar]
- 76.Finazzi G, Vannucchi AM, Martinelli V, et al. A phase II study of Givinostat in combination with hydroxycarbamide in patients with polycythaemia vera unresponsive to hydroxycarbamide monotherapy. Br J Haematol. 2013. June;161(5):688–94. [DOI] [PubMed] [Google Scholar]
- 77.Pettit K, Gerds AT, Yacoub A, et al. A Phase 2a Study of the LSD1 Inhibitor Img-7289 (bomedemstat) for the Treatment of Myelofibrosis. Blood. 2019;134(Supplement_1):556–556. [Google Scholar]
- 78.Jutzi JS, Kleppe M, Dias J, et al. LSD1 Inhibition Prolongs Survival in Mouse Models of MPN by Selectively Targeting the Disease Clone. HemaSphere. 2018;2(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Calado RT, Young NS. Telomere diseases. The New England journal of medicine. 2009;361(24):2353–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ruella M, Salmoiraghi S, Risso A, et al. Telomere shortening in Ph-negative chronic myeloproliferative neoplasms: a biological marker of polycythemia vera and myelofibrosis, regardless of hydroxycarbamide therapy. Exp Hematol. 2013. July;41(7):627–34. [DOI] [PubMed] [Google Scholar]
- 81.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011. March 4;144(5):646–74. [DOI] [PubMed] [Google Scholar]
- 82.Baerlocher GM, Oppliger Leibundgut E, Ottmann OG, et al. Telomerase Inhibitor Imetelstat in Patients with Essential Thrombocythemia. N Engl J Med. 2015;373(10):920–928. [DOI] [PubMed] [Google Scholar]
- 83.Baerlocher GM, Haubitz M, Braschler TR, et al. Imetelstat inhibits growth of megakaryocyte colony-forming units from patients with essential thrombocythemia. Blood Adv. 2019. November 26;3(22):3724–3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mosoyan G, Kraus T, Ye F, et al. Imetelstat, a telomerase inhibitor, differentially affects normal and malignant megakaryopoiesis. Leukemia. 2017;31(11):2458–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grinfeld J Prognostic models in the myeloproliferative neoplasms. Blood Rev. 2020. 2020/05/30/:100713. [DOI] [PubMed] [Google Scholar]
- 86.Debureaux PE, Cassinat B, Soret-Dulphy J, et al. Molecular profiling and risk classification of patients with myeloproliferative neoplasms and splanchnic vein thromboses. Blood Adv. 2020. August 11;4(15):3708–3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cabagnols X, Favale F, Pasquier F, et al. Presence of atypical thrombopoietin receptor (MPL) mutations in triple-negative essential thrombocythemia patients. Blood. 2016. January 21;127(3):333–42. [DOI] [PubMed] [Google Scholar]
- 88.Milosevic Feenstra JD, Nivarthi H, Gisslinger H, et al. Whole-exome sequencing identifies novel MPL and JAK2 mutations in triple-negative myeloproliferative neoplasms. Blood. 2016. January 21;127(3):325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vannucchi AM, Lasho TL, Guglielmelli P, et al. Mutations and prognosis in primary myelofibrosis. Leukemia. 2013. September;27(9):1861–9. [DOI] [PubMed] [Google Scholar]
- 90.Guglielmelli P, Lasho TL, Rotunno G, et al. The number of prognostically detrimental mutations and prognosis in primary myelofibrosis: an international study of 797 patients. Leukemia. 2014. September;28(9):1804–10. [DOI] [PubMed] [Google Scholar]
- 91.Tefferi A, Lasho TL, Guglielmelli P, et al. Targeted deep sequencing in polycythemia vera and essential thrombocythemia. Blood Adv. 2016. November 29;1(1):21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guglielmelli P, Lasho TL, Rotunno G, et al. MIPSS70: Mutation-Enhanced International Prognostic Score System for Transplantation-Age Patients With Primary Myelofibrosis. J Clin Oncol. 2018;36(4):310–318. [DOI] [PubMed] [Google Scholar]
- 93.Tefferi A, Guglielmelli P, Nicolosi M, et al. GIPSS: genetically inspired prognostic scoring system for primary myelofibrosis. Leukemia. 2018. 2018/07/01;32(7):1631–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tefferi A, Guglielmelli P, Lasho TL, et al. Mutation-Enhanced International Prognostic Systems for Essential Thrombocythemia (MIPSS-ET) and Polycythemia Vera (MIPSS-PV). Blood. 2018;132(Supplement 1):578–578. [Google Scholar]
- 95.Passamonti F, Thiele J, Girodon F, et al. A prognostic model to predict survival in 867 World Health Organization-defined essential thrombocythemia at diagnosis: a study by the International Working Group on Myelofibrosis Research and Treatment. Blood. 2012. August 9;120(6):1197–201. [DOI] [PubMed] [Google Scholar]
- 96.Mascarenhas J, Kremyanskaya M, Hoffman R, et al. MANIFEST, a Phase 2 Study of CPI-0610, a Bromodomain and Extraterminal Domain Inhibitor (BETi), As Monotherapy or “Add-on” to Ruxolitinib, in Patients with Refractory or Intolerant Advanced Myelofibrosis. Blood. 2019;134(Supplement_1):670–670. [Google Scholar]
- 97.Pemmaraju N, Carter BZ, Kantarjian HM, et al. LCL161, an Oral Smac Mimetic/IAP Antagonist for Patients with Myelofibrosis (MF): Novel Translational Findings Among Long-Term Responders in a Phase 2 Clinical Trial. Blood. 2018;132(Supplement 1):687–687. [Google Scholar]
- 98.Al-Ali HK, García Delgado R, Lange A, et al. KRT‑232, A FIRST‑IN‑CLASS, MURINE DOUBLE MINUTE 2 INHIBITOR (MDM2I), FOR MYELOFIBROSIS (MF) RELAPSED OR REFRACTORY (R/R) TO JANUS‑ASSOCIATED KINASE INHIBITOR (JAKI) TREATMENT (TX). EHA Library 295035; S215 2020. [Google Scholar]
- 99.Verstovsek S, Hasserjian RP, Pozdnyakova O, et al. PRM-151 in Myelofibrosis: Efficacy and Safety in an Open Label Extension Study. Blood. 2018;132(Supplement 1):686–686. [Google Scholar]
- 100.Gisslinger H, Zagrijtschuk O, Buxhofer-Ausch V, et al. Ropeginterferon alfa-2b, a novel IFNalpha-2b, induces high response rates with low toxicity in patients with polycythemia vera. Blood. 2015. October 08;126(15):1762–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Knudsen TA, Hansen DL, Ocias LF, et al. Long-Term Efficacy and Safety of Recombinant Interferon Alpha-2 Vs. Hydroxyurea in Polycythemia Vera: Preliminary Results from the Three-Year Analysis of the Daliah Trial - a Randomized Controlled Phase III Clinical Trial. Blood. 2018;132(Supplement 1):580–580. [Google Scholar]
- 102.Masarova L, Patel KP, Newberry KJ, et al. Pegylated interferon alfa-2a in patients with essential thrombocythaemia or polycythaemia vera: a post-hoc, median 83 month follow-up of an open-label, phase 2 trial [Clinical Trial]. The Lancet Haematology. 2017. April;4(4):e165–e175. [DOI] [PMC free article] [PubMed] [Google Scholar]