Abstract
Objective:
To assess factors associated with trial participation in the context of a low-risk intervention intended to reduce adverse drug events in recently hospitalized older adults.
Design:
Mixed methods: analysis of data collected during enrollment efforts and focus groups.
Setting:
A large, multi-specialty group practice.
Participants:
Individuals ≥50 years old recently discharged from the hospital and prescribed at least one high-risk medication were eligible for the trial. Enrollees, decliners, and their caregivers were eligible to participate in focus groups.
Measurements:
Reasons for declining to participate during the initial invitation as well as reasons for not providing consent were recorded. Focus groups were conducted with eligible individuals to explore reasons for enrolling or declining. We conducted multivariable logistic regression to compare characteristics (including sex, age, healthcare proxy, number and type of medications, visiting nurse services, reason for admission, and length of hospital stay) of those who enrolled to those who did not enroll.
Results:
Of 3606 individuals determined eligible, 3147 (87%) declined, 98 (3%) verbally consented to participate but did not complete written consent, and 361 (10%) provided written consent and were considered enrolled. Individuals ≥80 years-of-age (OR 0.44, CI 0.30, 0.65) and those with visiting nurse services (OR 0.64, CI 0.48, 0.85) were least likely to enroll. Among those who provided a reason for declining (2473), the most common was the belief they did not need additional medication assistance (18%). Another 332 (11%) declined because they were receiving visiting nurse services.
Conclusion:
Recruiting older adults recently discharged from the hospital to participate in trials of low-risk, system-level interventions is challenging and may under-enroll the oldest individuals and those potentially at the highest risk for adverse events, limiting generalizability of study findings. Alternative study designs may be more effective than individually randomized trials in assessing low-risk, system-level interventions.
Keywords: Clinical Trials, Care Transitions, Medication, Health Systems Intervention
INTRODUCTION
The importance of adequate inclusion of appropriate populations in clinical studies was highlighted in the recent Inclusion Across the Lifespan Policy of the National Institutes of Health.1 Enrolling older persons in clinical trials means that patients with greater burdens of multimorbidity, polypharmacy, and functional and social challenges will be included in studies, leading to a more heterogeneous study population and findings that are more generalizable. However, recruiting older, more complex patients into trials is inherently more difficult, costly, and time-consuming.
We conducted a randomized trial of a low-risk intervention providing in-home pharmacist support to older adults during the immediate post-hospital discharge period to reduce adverse drug events. The trial was intended as an evaluation of a system-level practice which would be readily implemented by healthcare systems. However, the trial individually randomized subjects and required obtaining written informed consent from all participants.
To determine the potential value of the intervention at the system level, it was critical to enroll a representative sample of older adults, including individuals likely to be at the greatest risk. We recognized the potential difficulties in recruiting participants during this period,2 when they are tired, adjusting to being home, and often dealing with new medications, medical conditions, psychological and physical symptoms, and pain. To better understand factors which influence recently hospitalized older adults to participate in such a trial, we examined the responses of older adults to an invitation that was offered within four days of hospital discharge, conducted focus groups with eligible individuals including enrollees, decliners, and their caregivers, and assessed the potential generalizability of the trial’s results based on the proportion of important sub-groups who fully enrolled in the trial.
METHODS
Overview
The present study was conducted in the context of a clinical trial examining the impact of a clinical pharmacist-based intervention to patients who had been recently discharged from the hospital and prescribed medications in three selected high-risk categories (anticoagulants, diabetes agents [insulin and oral agents], and/or opioids). Recruitment for the trial occurred between June 2016 and September 2018. The trial was designed with individual-level recruitment and randomization. Components of the intervention included: (1) in-home assessment by a clinical pharmacist (within four days of discharge from the hospital); (2) use of evidence-based medication safety tools and resources targeted to patients on high-risk medications and their caregivers; (3) communication with the primary care team via the electronic health record (EHR) regarding issues of concern relevant to medication safety; and (4) a follow-up telephone call by the pharmacist to the patient and/or caregiver 14 days after the home visit. The in-home visit by the study pharmacist consisted of three components: (1) medication review; (2) observation of medication organization and administration; and (3) in-depth patient and caregiver discussions about challenges to safe medication use.
Control group participants were mailed print materials with information on the high-risk medications relevant to them.
The initial recruitment goal was 500 participants. This study was approved by the University of Massachusetts Medical School Institutional Review Board. The study was registered on ClinicalTrials.gov, NCT02781662.3
Eligibility Criteria
Eligibility criteria included individuals discharged from the hospital who were patients of primary care physicians in the participating multispecialty medical group, prescribed at least one high-risk medication (anticoagulants, diabetes agents [insulin and oral agents], and/or opioids), and who met at least one of the following additional criteria, indicating they may be at higher risk for adverse drug events: (1) prescribed ≥2 high-risk medications; (2) low health literacy; (3) poor (self-reported) medication adherence; (4) had a proxy or reported having a caregiver; or (5) prescribed ≥7 different medications. Individuals were not eligible if they were: (1) discharged to hospice; (2) hospitalized for a psychiatric condition; (3) discharged to a skilled nursing facility, rehabilitation hospital, or nursing home; or (4) non-English speaking. Initially, only patients age 65 years or older were considered eligible for participation.
After eight months of lower than expected recruitment, eligibility criteria relevant to patient age were relaxed from age 65 and older to age 50 and older, and the recruitment period was extended from one to two years to increase the number of potentially eligible individuals to recruit. Additionally, we made changes to the study protocol in an attempt to increase the percentage of those recruited among those deemed eligible. We expanded the window of time from 2 to 3 days post-hospital discharge available to reach a patient for the purpose of recruitment. In addition, we increased the number of recruitment calls made to each potential participant each day, and when a patient was reached we began to invoke the name of their primary care provider as being supportive of our efforts.
Recruitment for the Trial
Recruitment calls for potentially eligible individuals were initiated as soon as possible after hospital discharge to enable scheduling for a pharmacist in-home visit by the fourth day post-discharge. Calls were placed from the medical group so that the medical group name and number would appear on caller ID. Study staff members were either experienced recruiters or trained and evaluated by experienced recruiters prior to recruiting independently. Staff introduced the study, determined eligibility, and invited eligible individuals to participate. Participants were offered a $25 gift card.
If an individual provided verbal consent, he or she was randomized so that the home visit could be scheduled immediately, but the individual was not considered enrolled until written informed consent was obtained. Written consent was sought during the study visit for the intervention group and via mail for the control group, with the gift card attached to the consent form.
All of those who were eligible and declined to enroll were asked to provide a reason. Study staff also recorded reasons for not providing a signed consent form (if known) following randomization. All reasons were captured as text. A coding scheme was developed by the study team to facilitate categorization and analysis.
Focus Groups
In response to low recruitment numbers, focus groups were conducted approximately one year after the start of recruitment to explore reasons for accepting or declining to participate in the trial. Participants who had enrolled in the trial were invited via telephone to participate in a focus group at the time of the final contact (45 days after enrollment). Individuals who had declined to participate in the trial were asked about their willingness to participate in a focus group at a later time. Those who agreed were sent an invitation letter via mail with instructions to call the study team if interested. Caregivers of individuals eligible for the trial were also eligible to participate. Focus group participants were offered $75. Sessions lasted approximately 90 minutes, were audio recorded, and professionally transcribed. Directed content analysis4 was used to identify key themes and create a coding scheme. Once the coding scheme was finalized, all transcripts were coded by one member of the study staff, with code assignment reviewed by a second member.
Of the 147 individuals who were mailed an invitation to take part in the focus groups, 24 (16%) participated. Three caregivers also participated. The five focus groups involved 27 participants: 9 who enrolled in the trial, 15 who declined to participate, and 3 caregivers. Of the focus group participants, 17 were female, 11 were ≤65 years-of-age, 12 were 65-79 years-of-age, and 4 were ≥80 years-of-age.
Analysis
We conducted multivariable logistic regression to compare the characteristics of those who enrolled in the study to eligible individuals who did not enroll to assess potential issues of generalizability. These characteristics included: sex; age; whether a healthcare proxy was listed in the EHR; medications (number and type); whether a visiting nurse was assigned; and information about the recent hospitalization including reason for admission, whether admitted through the emergency room, and length of stay. All statistical analyses were conducted using SAS statistical software, version 9.4 (Cary, NC). We also summarized reasons for declining during the initial telephone invitation, reasons for not completing the consent form, and focus group participants’ perspectives on reasons for enrolling and declining.
RESULTS
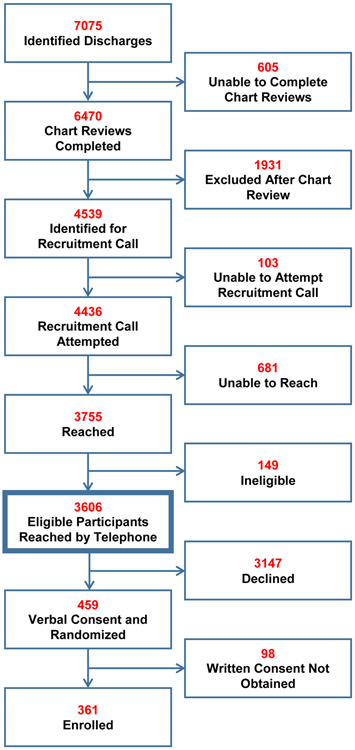
As detailed in Figure 1, a total of 7,075 patients were identified as potentially eligible for the trial. Chart reviews were not conducted on 605 potentially eligible patients due to a variety of reasons, including documentation of a “do not call for research” in the patient record, staff availability, and delay of data transfer to ascertain eligibility. Study staff conducted chart reviews on 6,470 patients and identified 4,539 patients as eligible for recruitment calls. Of the 3,755 patients reached by telephone, 3,606 were found to be eligible. Of these, 459 gave verbal consent and were randomized (230 intervention, 229 control); 361 individuals (180 intervention, 181 control) completed written consent and were enrolled in the study. Thus, approximately 10% of the 3,606 confirmed eligible patients were ultimately enrolled in the study.
Figure 1. Process of Recruitment, Consent, and Enrollment.
This flow diagram presents efforts to recruit older adults recently discharged from the hospital on three selected high-risk medications.
Table 1 presents the characteristics of individuals who were reached and determined to be eligible (N=3,606), the characteristics of those who ultimately enrolled (N=361), and the results of the logistic regression analysis comparing enrolled participants to those who did not enroll. Several sub-groups were underrepresented among the final enrollees. Potential participants ≥80 years-of-age were less likely to enroll as compared to those ages 65-69 (odds ratio (OR) 0.44; 95% confidence interval (CI) 0.30, 0.65). Those with visiting nurse services were less likely to enroll (OR 0.64; CI 0.48, 0.85) than those without such services. Potential participants whose reason for admission to the hospital was for a medical procedure were less likely to enroll (OR 0.41; CI 0.17, 0.99) compared with those hospitalized for a surgical procedure. All potential participants were taking one or more of the three high-risk medications; within this eligible group, those prescribed an anticoagulant were more likely to enroll than those taking the other high-risk medications (OR 1.45; CI 1.04, 2.02). In addition, those taking seven or more prescribed medications were more likely to enroll (OR 1.59; CI 1.02, 2.47) than those prescribed fewer than seven medications.
Table 1.
Demographic and Clinical Characteristics of Eligible Individuals Reached by Telephone
| Total Determined Eligible (N=3606) |
Not Enrolled (N=3245) |
Enrolled (N=361) |
Enrolled vs. Not Enrolled Adjusted odds ratios and 95% confidence intervals |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | OR* | 95% CI | |||
| Sex | ||||||||||
| Female | 1788 | 50% | 1611 | 50% | 177 | 49% | 1.02 | 0.81, 1.27 | ||
| Male | 1818 | 50% | 1634 | 50% | 184 | 51% | Ref | |||
| Age | ||||||||||
| 50-54 | 187 | 5% | 165 | 5% | 22 | 6% | 0.99 | 0.59, 1.66 | ||
| 55-59 | 321 | 9% | 279 | 9% | 42 | 12% | 1.08 | 0.71, 1.63 | ||
| 60-64 | 392 | 11% | 336 | 10% | 56 | 16% | 1.15 | 0.79, 1.69 | ||
| 65-69 | 607 | 17% | 534 | 17% | 73 | 20% | Ref | |||
| 70-74 | 580 | 16% | 517 | 16% | 63 | 17% | 0.90 | 0.63, 1.30 | ||
| 75-79 | 554 | 15% | 498 | 15% | 56 | 16% | 0.84 | 0.58, 1.22 | ||
| 80+ | 965 | 27% | 916 | 28% | 49 | 14% | 0.44 | 0.30, 0.65 | ||
| Proxy Listed in EHR | 77 | 2% | 71 | 2% | 6 | 2% | 1.11 | 0.47, 2.62 | ||
| Prescribed >1 High-Risk Medication | 1722 | 48% | 1527 | 47% | 195 | 54% | 0.89 | 0.62, 1.27 | ||
| Specific High-Risk Medications | ||||||||||
| Anticoagulant | 1705 | 47% | 1521 | 47% | 184 | 51% | 1.45 | 1.04, 2.02 | ||
| Anti-Diabetic | 1345 | 37% | 1210 | 37% | 135 | 37% | 1.33 | 0.91, 1.93 | ||
| Opioid | 1978 | 55% | 1755 | 54% | 223 | 62% | 1.14 | 0.81, 1.61 | ||
| Prescribed ≥7 Medications of Any Kind | 3267 | 91% | 2933 | 90% | 334 | 93% | 1.59 | 1.02, 2.47 | ||
| Has VNA Services | 1966 | 55% | 1779 | 55% | 187 | 52% | 0.64 | 0.48, 0.85 | ||
| Reason for Admission | ||||||||||
| Medical | 2329 | 65% | 2131 | 66% | 198 | 55% | 0.78 | 0.50, 1.20 | ||
| Surgical1 | 716 | 20% | 628 | 19% | 88 | 24% | Ref | |||
| Orthopedic2 | 448 | 12% | 379 | 12% | 69 | 19% | 1.08 | 0.71, 1.64 | ||
| Medical Procedure | 113 | 3% | 107 | 33% | 6 | 2% | 0.41 | 0.17, 0.99 | ||
| Admitted Via ER | 2491 | 69% | 2278 | 70% | 213 | 59% | 0.85 | 0.56, 1.29 | ||
| Average Length of Stay (Days) | 2.85 | N/A | 2.86 | NA | 2.73 | N/A | 1.00 | 0.98, 1.02 | ||
Adjusted for all variables in table.
Examples of surgical admissions include appendectomy, laminectomy, spinal stenosis surgey, aortic valve replacement, cardiac surgey, hernias if admitted to surgical service, AAA repair, and planned PCI
Examples of orthopedic admissions include total knee or hip replacement, ankle or knee surgery, and meniscus repair/surgery
Reasons for Declining to Participate
A total of 3,147 eligible participants declined the initial telephone invitation to participate in the trial. Reasons for declining are summarized in Table 2. There were 674 individuals who did not provide a reason for declining; of those who did provide a reason, common reasons included being too busy or not feeling well enough (e.g. being too sick or too tired) to participate. Study staff explained to eligible participants that the trial focused on medication safety, and many participants expressed that they did not feel the study would be useful for them either because they already had all the information they needed, already received the support they needed, had been on the same medications for a long time, or were on very few medications. Relatively uncommon reasons for declining included privacy concerns, unwillingness to invite a pharmacist into the home due to a living situation (e.g., an aggressive dog), or an unwillingness to participate once they learned the group to which they had been randomized. Reasons for declining differed for those ≥80 years-of-age, with older individuals more likely to state that they did not need the intervention due to having a visiting nurse (16% compared to 8%) or having a caregiver or spouse who took care of their medications (15% compared to 6%).
Table 2.
Reasons for Declining Participation in the Clinical Trial
| Reasons | N = 3147 | |
|---|---|---|
| Medication-Related | n | % |
| Patient indicated they have been taking the same medications for a long time | 193 | 6% |
| Patient indicated they were on very few medications | 138 | 4% |
| Patient indicated their medications would be changing | 11 | <1% |
| Other Support/Resources | ||
| Patient thought they had all the information necessary to manage their medications, including receiving clear instructions from the hospital | 551 | 18% |
| Patient receives Visiting Nurse or other home services and did not think other services were necessary | 332 | 11% |
| Patient receives assistance managing their medications from a caregiver/spouse | 259 | 8% |
| Patient wanted to speak only with their healthcare provider about their medications or to speak with their healthcare provider prior to deciding to enroll | 75 | 2% |
| Research-Related | ||
| Patient indicated they did not think the study would be useful to them | 303 | 10% |
| Patient did not want the pharmacist visit | 89 | 3% |
| Patient indicated concerns about providing consent (e.g., concerns about privacy and who would have access to the data) | 39 | 1% |
| Patient did not want to be assigned to the control arm (wanted the pharmacist visit, not just the informational mailing) | 8 | <1% |
| General/Other | ||
| Patient declined participation due to life circumstances or health issues (e.g., too sick, too busy, moving, grieving, etc.) | 434 | 14% |
| Patient declined due to other circumstances or conditions (e.g., cognitive issues or hearing impairment) | 17 | 1% |
| Patient indicated they would soon be re-hospitalized | 9 | <1% |
| Patient provided another reason for declining | 15 | <1% |
| Not Interested/No Specific Reason Given | 674 | 18% |
A total of 98 individuals verbally consented during the initial telephone invitation but were not ultimately enrolled; reasons are summarized in Supplementary Table S1. Reasons for Not Returning the Consent Form. Many potential participants were too busy or too sick to complete the visit with the pharmacist within the required timeframe or to send back written consent (N=12). Others did not complete the consent form due to concerns about the informed consent process (e.g. privacy concerns about sharing their medical record) (N=12) or because they did not want to be randomized to a certain group (N=4). Some individuals were not enrolled due to changes in eligibility, such as admission to a skilled nursing facility or readmission to the hospital, prior to completing the consent process (N=8).
Focus Group Results
Reasons discussed by focus group participants generally corresponded to reasons expressed by those who declined over the telephone. One reason identified by focus group participants not explicitly noted by those who declined the initial invitation was timing of the invitation relative to leaving the hospital; focus group participants indicated that if the request had come later they may have been more likely to say yes (N=3). A second unique finding from the focus groups was that many participants worried about the authenticity of the invitation, suspecting that the call was a “scam” (N=6).
The most common motivation noted for enrolling in the trial by focus group participants was that the study might result in benefits for the participant or others in the future. One focus group participant stated “I’ve got an appreciation for how important these kinds of studies are to help inform policy decision making in health care…You need to collect this information and understand what’s happening with your patients so that you can make better decisions about how to serve them.” Other reasons included the opportunity to voice their opinions, the benefit of “company” that a home visit would offer, and valuing research.
Further details on focus group participants’ reasons for deciding to enroll or decline participating in the trial can be found in Supplementary Table S2. Focus Group Reasons for Enrolling or Declining.
DISCUSSION
In this study of a low-risk intervention to reduce adverse drug events in recently hospitalized older adults, we found a recruitment rate of 10%. Reasons offered for declining were most often related to a perceived lack of need for the intervention being studied as well as being too sick or too tired to participate at this stage in their recovery.
The findings reported here may have implications for future trials of low-risk, system-level interventions directed at older adults. With the increased recognition that interventions often have heterogeneous effects among patients of various ages and with differing burdens of comorbidities and functional impairment, there has been an increased focus on inclusion of older adults in clinical trials.1,5 However, achieving requisite sample sizes in this segment of the population is likely to be a lengthy and expensive process – a process that should be recognized and accommodated in study plans. As the need for increased inclusion of older adults in trials of medical interventions has been emphasized, there have been calls for funding agencies and reviewers to recognize the need for longer timelines and increased funding.6,7
We found that some important sub-groups were particularly difficult to recruit, including those ≥80 years-of-age. The absence of segments of the population who may be at greatest need for a tested intervention and whose medical and functional conditions are likely to impact its effectiveness undermines the external validity of a trial’s results.8,9,10,11,12,13
The focus of this trial was a low-risk intervention intended for implementation at the level of the health care delivery system. The challenges we encountered in recruitment may suggest that few patients would accept the intervention if it became a standard component of post-discharge care. If true, that would substantially decrease its potential value at a population level. However, willingness to participate in a randomized trial may bear little relationship to patients’ interest in and ability to allow a pharmacist to visit their home if woven into usual post-hospital discharge care processes. The use of an individually randomized trial, with a rigorous and extensive two-step informed consent process to determine the potential value of this low-risk health system intervention adversely impacted our ability to answer this important question.
There are alternative study designs that may be of use in assessing low-risk, health care system-level interventions. Pragmatic trials set in multiple medical practice sites with randomization at the site level have been suggested to enhance external validity.14 If designed and managed well, this design can estimate the effectiveness of an intervention in actual practice. Decisions about the use of multi-site pragmatic trials vs. individually randomized trials would ideally be based on thoughtful consideration of the need for balance between internal and external validity.15
Our study corroborates the findings of other researchers, who have pointed out the difficulty of recruiting older adults into trials.2,6,16,17,18 Similar studies of interventions conducted immediately post-hospital discharge achieved higher rates of recruitment (30-40%), but initiated their recruitment efforts earlier during the patient’s hospital stay.19,20 Our recruitment rate of 10% to achieve an enrolled population of 361 individuals over the course of approximately two years reflects the substantial amount of time and sustained resources required to carry out a trial within older adult populations recently discharged from the hospital. This has implications both for planning future trials targeting older adults, and for informing healthcare systems’ decisions on whether to implement these interventions based upon perceived cost to the system and benefit to older adults.
Recruitment challenges and the low yields for studies of low-risk health system interventions are nothing new. Lessons learned from our own experience include being certain to have an excess pool of potential participants to account for lower than expected enrollment yields and to be prepared to modify eligibility criteria (e.g., age) to increase the pool if needed. However, making the decision to relax age-related eligibility criteria has the risk of diminishing power by reducing the overall outcome rate, given the more favorable characteristics of younger participants.16 Second, early interaction with and education of the target population of potential participants is likely to be beneficial. In the case of our study, interaction with patients prior to hospital discharge may have substantially enhanced our recruitment efforts. This would have provided an opportunity to highlight general issues about medication safety that have the potential to occur during the post-hospitalization period. In addition, we should have sought input from patients and family caregivers earlier, which would have provided insight into recruitment challenges and possible solutions before beginning to recruit and enroll participants. Finally, the additional step of requiring formal written consent, following having obtained verbal consent, was a logistical challenge requiring mailings to and subsequent returns of the forms by control subjects. Being able to eliminate this mandated, time-consuming, and burdensome step for older patients recently discharged from the hospital, and still not feeling well, may have been beneficial to this low-risk intervention.
For older adults, the transition from hospital to home is a high-risk period for adverse drug events, functional decline, and hospital readmission, especially for those taking high-risk medications.21,22,23,24,25,26,27,28,29,30 While this timeframe provides a critical, targeted window for an intervention to improve outcomes post-hospitalization, researchers planning randomized trials to improve this transition may need creative approaches to successfully recruit older adults immediately after hospital discharge, even for low-risk interventions.
Supplementary Material
Supplementary Table S1. Reasons for Not Returning the Consent Form. This table lists patients’ reasons for not enrolling in the study after initially consenting over the telephone.
Supplementary Table S2. Focus Group Reasons for Enrolling or Declining. This table presents focus group participants’ reasons for enrolling or declining to enroll in the study as a series of representative quotes.
ACKNOWLEDGEMENTS
This work was supported by the Agency for Healthcare Research and Quality (18HS023774).
Footnotes
Presentations:
1. Anzuoni K. A Trial to Improve Medication Safety in Older Adults: Recruitment Challenges Have Generalizability Implications. Poster session presented at: The Gerontological Society of America (GSA) 2019 Annual Scientific Meeting; November 13-17, 2019; Austin, TX.
2. Anzuoni K. Recruitment Challenges in Trials of Low Risk Health Care Delivery System Interventions: Improving Medication Safety Post-Discharge in Older Adults. Poster session presented at: The 2019 Health Care Systems Research Network Conference (HCSRN); April 8- 10, 2019; Portland, OR.
Conflict of Interest: (1) Dr. Gurwitz serves on the Pharmacy and Therapeutics Committee of United Healthcare. (2) Dr. Kapoor has received research grant support from Pfizer through its Independent grants for learning and change funding mechanism and from Bristol-Myers Squibb through a competitive review of responses to request for Independent Medical Education proposals. More recently, he has received research grant support through a competitive process adjudicated and funded by the alliance which is formed by both Pfizer and Bristol-Myers Squibb. He has also been awarded a grant by Pfizer to examine conversations between patients and providers.
Sponsor’s Role: The sponsor had no role in the design, methods, data collections, analysis, or preparation of this paper.
REFERENCES
- 1.Bernard MA, Clayton JA, Lauer MS. Inclusion Across the Lifespan: NIH Policy for Clinical Research. Jama. 2018;320(15):1535–1536. [DOI] [PubMed] [Google Scholar]
- 2.Ferguson GT, Beck B, Clerisme-Beaty E, et al. Recruiting Patients After Hospital Discharge for Acute Exacerbation of COPD: Challenges and Lessons Learned. Chronic obstructive pulmonary diseases (Miami, Fla). 2017;4(4):265–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Improving Safety After Hospitalization in Older Persons on High-Risk Medications. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02781662?term=improving+safety+after+hospitalization&draw=2&rank=1. Published May 24, 2016. Updated September 25, 2019 Accessed April 15, 2020. [Google Scholar]
- 4.Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qualitative health research. 2005;15(9):1277–1288. [DOI] [PubMed] [Google Scholar]
- 5.Vaughan CP, Dale W, Allore HG, et al. AGS Report on Engagement Related to the NIH Inclusion Across the Lifespan Policy. Journal of the American Geriatrics Society. 2019;67(2):211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knechel NA. The challenges of enrolling older adults into intervention studies. The Yale journal of biology and medicine. 2013;86(1):41–47. [PMC free article] [PubMed] [Google Scholar]
- 7.Witham MD, McMurdo ME. How to get older people included in clinical studies. Drugs & aging. 2007;24(3):187–196. [DOI] [PubMed] [Google Scholar]
- 8.Ackerman B, Schmid I, Rudolph KE, et al. Implementing statistical methods for generalizing randomized trial findings to a target population. Addict Behav. 2018;25:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kennedy-Martin T, Curtis S, Faries D, Robinson S, Johnston J. A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials. 2015;16:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen TQ, Ackerman B, Schmid I, Cole SR, Stuart EA. Sensitivity analyses for effect modifiers not observed in the target population when generalizing treatment effects from a randomized controlled trial: Assumptions, models, effect scales, data scenarios, and implementation details. PloS one. 2018;13(12):e0208795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pearl J, Bareinboim E. Note on "Generalizability of Study Results". Epidemiology (Cambridge, Mass). 2019;30(2):186–188. [DOI] [PubMed] [Google Scholar]
- 12.Rothwell PM. External validity of randomised controlled trials: "to whom do the results of this trial apply?". Lancet (London, England). 2005;365(9453):82–93. [DOI] [PubMed] [Google Scholar]
- 13.Westreich D, Edwards JK, Lesko CR, Cole SR, Stuart EA. Target Validity and the Hierarchy of Study Designs. American journal of epidemiology. 2019;188(2):438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuidgeest MGP, Goetz I, Groenwold RHH, Irving E, van Thiel G, Grobbee DE. Series: Pragmatic trials and real world evidence: Paper 1. Introduction. Journal of clinical epidemiology. 2017;88:7–13. [DOI] [PubMed] [Google Scholar]
- 15.Godwin M, Ruhland L, Casson I, et al. Pragmatic controlled clinical trials in primary care: the struggle between external and internal validity. BMC medical research methodology. 2003;3:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gill TM, McGloin JM, Latham NK, et al. Screening, Recruitment, and Baseline Characteristics for the Strategies to Reduce Injuries and Develop Confidence in Elders (STRIDE) Study. The journals of gerontology Series A, Biological sciences and medical sciences. 2018;73(11):1495–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMurdo ME, Roberts H, Parker S, et al. Improving recruitment of older people to research through good practice. Age and ageing. 2011;40(6):659–665. [DOI] [PubMed] [Google Scholar]
- 18.Ridda I, MacIntyre CR, Lindley RI, Tan TC. Difficulties in recruiting older people in clinical trials: an examination of barriers and solutions. Vaccine. 2010;28(4):901–906. [DOI] [PubMed] [Google Scholar]
- 19.Deer RR, Goodlett SM, Fisher SR, et al. A Randomized Controlled Pilot Trial of Interventions to Improve Functional Recovery After Hospitalization in Older Adults: Feasibility and Adherence. The journals of gerontology Series A, Biological sciences and medical sciences. 2018;73(2):187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flink M, Brandberg C, Ekstedt M. Why patients decline participation in an intervention to reduce re-hospitalization through patient activation: whom are we missing? Trials. 2019;20(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brennan JJ, Chan TC, Killeen JP, Castillo EM. Inpatient Readmissions and Emergency Department Visits within 30 Days of a Hospital Admission. The western journal of emergency medicine. 2015;16(7):1025–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. Jama. 2013;309(4):355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. Functional trajectories among older persons before and after critical illness. JAMA internal medicine. 2015;175(4):523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forster AJ, Clark HD, Menard A, et al. Adverse events among medical patients after discharge from hospital. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2004;170(3):345–349. [PMC free article] [PubMed] [Google Scholar]
- 25.Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Annals of internal medicine. 2003;138(3):161–167. [DOI] [PubMed] [Google Scholar]
- 26.Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. Adverse drug events occurring following hospital discharge. Journal of general internal medicine. 2005;20(4):317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanaan AO, Donovan JL, Duchin NP, et al. Adverse drug events after hospital discharge in older adults: types, severity, and involvement of Beers Criteria Medications. Journal of the American Geriatrics Society. 2013;61(11):1894–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mudge AM, McRae P, Hubbard RE, et al. Hospital-Associated Complications of Older People: A Proposed Multicomponent Outcome for Acute Care. Journal of the American Geriatrics Society. 2019;67(2):352–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Seben R, Reichardt LA, Aarden JJ, et al. The Course of Geriatric Syndromes in Acutely Hospitalized Older Adults: The Hospital-ADL Study. Journal of the American Medical Directors Association. 2019;20(2):152–158.e152. [DOI] [PubMed] [Google Scholar]
- 30.van Seben R, Reichardt LA, Essink DR, van Munster BC, Bosch JA, Buurman BM. "I Feel Worn Out, as if I Neglected Myself": Older Patients' Perspectives on Post-hospital Symptoms After Acute Hospitalization. The Gerontologist. 2019;59(2):315–326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Reasons for Not Returning the Consent Form. This table lists patients’ reasons for not enrolling in the study after initially consenting over the telephone.
Supplementary Table S2. Focus Group Reasons for Enrolling or Declining. This table presents focus group participants’ reasons for enrolling or declining to enroll in the study as a series of representative quotes.