Abstract
The growing interest in magnetic resonance imaging (MRI) for assessing regional lung function relies on the use of nuclear-spin hyperpolarized gas as a contrast agent. The long gas-phase lifetimes of hyperpolarized 129Xe make this inhalable contrast agent acceptable for clinical research today despite limitations such as high cost, low throughput of production and challenges of 129Xe imaging on clinical MRI scanners, which are normally equipped with proton detection only. We report on low-cost and high-throughput preparation of proton-hyperpolarized diethyl ether, which can be potentially employed for pulmonary imaging with a non-toxic, simple, and sensitive overall strategy using proton detection commonly available on all clinical MRI scanners. Diethyl ether is hyperpolarized by pairwise parahydrogen addition to vinyl ethyl ether and characterized by 1H NMR spectroscopy. Proton polarization levels exceeding 8% are achieved at near complete chemical conversion within seconds, causing the activation of radio amplification by stimulated emission radiation (RASER) throughout detection. Although gas-phase T1 relaxation of hyperpolarized diethyl ether (at partial pressure of 0.5 bar) is very efficient with T1 of ca. 1.2 second, we demonstrate that at low magnetic fields, the use of long-lived singlet states created via pairwise parahydrogen addition extends the relaxation decay by approximately 3-fold, paving the way to bioimaging applications and beyond.
Keywords: parahydrogen, hyperpolarization, diethyl ether, anesthetic, long-lived spin states
Graphical Abstract
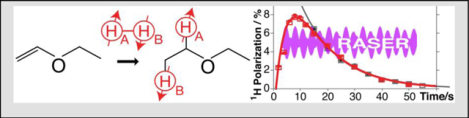
Diethyl ether anesthetic is NMR hyperpolarized by pairwise parahydrogen addition to vinyl ethyl ether. Proton polarization levels exceeding 8% are achieved at near complete chemical conversion within seconds. Although gas-phase T1 is ~1.2 s, the use of long-lived singlet states created in low magnetic field extends the relaxation decay by approximately 3-fold, paving the way to bioimaging applications and beyond.
Introduction
The degree of nuclear spin alignment with applied static magnetic field, termed nuclear spin polarization (P), is typically on the order of 10−5 for protons at clinically and physiologically relevant conditions. Because NMR detection sensitivity is directly proportional to P,[1] NMR spectroscopy and imaging are generally regarded as relatively low-sensitivity techniques. Thus, in vivo MRI is limited primarily to highly concentrated compounds such as water and lipids. However, NMR hyperpolarization techniques can transiently increase P to the order of unity resulting in corresponding gains in detection sensitivity.[2–5] Hyperpolarized (HP) compounds can be employed as injectable or inhalable contrast agents.[6, 7] To date, the two groups of HP contrast agents that have transitioned to clinical trials are HP 13C–labeled biomolecules (most notably [1-13C]-pyruvate)[8, 9] and 129Xe gas,[10–12] which can be employed for spectroscopic imaging of in vivo metabolism (e.g., glycolysis)[13, 14] and organ function (e.g., lung ventilation and perfusion).[10, 15–19] The key motivation of the biomedical HP community that has historically focused on 13C and 129Xe nuclei is the significantly longer lifetime of their HP states compared to those of protons. For example, in vivo T1 are on the order of 1 min (in favorable cases)[9] and 4–20 s[10, 20, 21] for 13C and 129Xe, respectively, versus 1–2 s for protons.
Despite some success and a number of ongoing clinical trials, there are several translational limitations that 13C and 129Xe HP contrast agents face to address critical medical needs. First and foremost, 13C and 129Xe detection is not available on most clinical MRI scanners, which are limited to proton detection only. Second, 13C and 129Xe have ~4-fold lower magnetic moments and ~4-fold lower resonance frequencies compared to those of protons, resulting in a factor of (~4)2 less NMR signal for 13C and 129Xe nuclei.[1] Besides, MRI spatial encoding also requires a factor of 42 more gradient power to achieve the same spatial resolution.[5, 22] Third, clinical-scale 13C and 129Xe hyperpolarization techniques have been demonstrated using dissolution Dynamic Nuclear Polarization (d-DNP)[23] and Spin-Exchange Optical Pumping (SEOP),[24] respectively, which have limited production throughput (approximately 4 doses per hour) and highly complex and expensive instrumentation ($0.5-$2M cost, circa 2020).[8, 23, 25–29] More affordable methods compared to clinical-scale d-DNP as well as new indirect detection schemes may eventually emerge for hyperpolarization of [1-13C]-pyruvate[30–33] and other agents,[34] although alternatives for HP 129Xe gas as an inhalable HP contrast agent are still rather limited.[35]
Levitt and co-workers have provided numerous examples of the existence of long-lived singlet states (LLS),[36–39] with TLLS values significantly greater than corresponding T1.[39] This new concept rekindled the interest in HP proton contrast agents,[40–44] yet no LLS have been translated to in vivo demonstration on biologically suitable carriers. Lately, we have demonstrated the existence of LLS in parahydrogen-hyperpolarized propane gas at low magnetic fields, i.e., in the strong coupling regime where the spin-spin coupling J between the protons of interest is greater than the difference between their chemical shifts.[45, 46] The LLS of HP propane was found immune to O2, indicating that it may be a useful contrast agent for pulmonary imaging applications[47]—efforts are in progress in our laboratory to demonstrate the utility of HP propane in a large animal model.
Here we report on a simple and fast approach to prepare HP diethyl ether (DE), the first anesthetic produced on a commercial scale. We employ parahydrogen-induced polarization (PHIP) for pairwise parahydrogen (p-H2) addition to ethyl vinyl ether (EVE) to form HP DE in the gas and liquid phases (Scheme 1). DE was on the World Health Organization (WHO) essential list of drugs until 2005.[48] The substrates EVE (a.k.a. vinamar) and divinyl ether (a.k.a. vinethene) have also been employed as inhalable anesthetics in clinical anesthesiology.[49] Although these ethers have been phased out in the US and other countries due to the availability of nonflammable alternatives (desflurane, isoflurane, sevoflurane, etc.), they remain approved for medical use as anesthetics in many countries[50] including Russia.
Scheme 1.
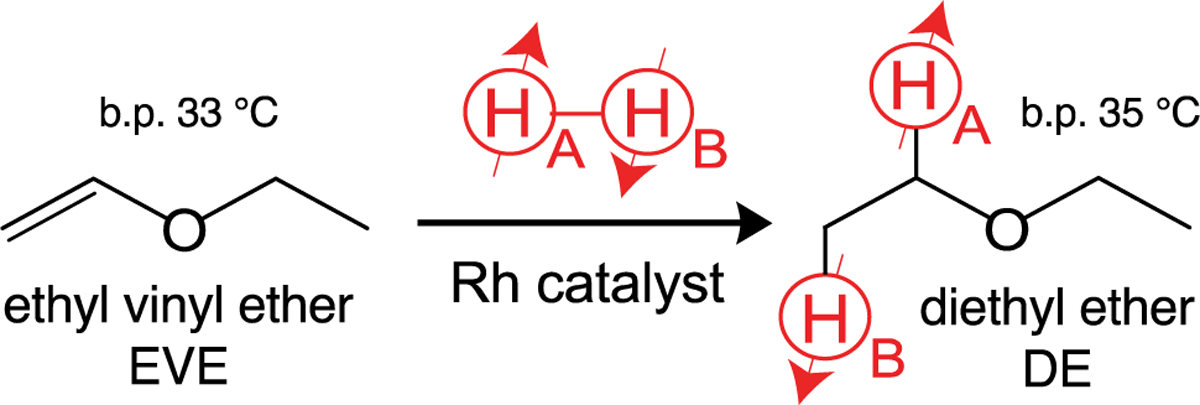
Pairwise parahydrogen addition to ethyl vinyl ether (EVE) to form HP diethyl ether (DE). Note the symmetry breaking of protons HA and HB.
Results and Discussion
Liquid-phase hyperpolarization studies were performed with solutions of ~200 mM of EVE (Sigma Aldrich, 99%) in CD3OD and 4 mM of rhodium catalyst (1,4-bis(diphenylphosphino)butane(1,5-cyclooctadiene) rhodium(I) tetrafluoroborate, STREM, 79255-71-3). 0.5 mL samples were placed in 5 mm NMR tubes under argon atmosphere. NMR tubes were pressurized with p-H2 at ~ 8 bar and heated at 80 °C for 30 seconds. p-H2 (enriched at >99%[51]) was bubbled through the solution at a flow rate of 150 standard cubic centimeters per minute (sccm). Pairwise p-H2 addition to EVE was performed in the Earth’s magnetic field followed by detection at 1.4 T via a benchtop NMR spectrometer (SpinSolve Carbon60, Magritek), corresponding to ALTADENA condition.[52] 1H NMR spectra of neat thermally polarized ethyl acetate-1-13C (EA-1-13C) were used as signal reference to evaluate polarization levels (Figure 1a,b). For each sample, the chemical conversion and residual DE concentration in CD3OD (liquid fraction) were evaluated from the thermal NMR spectra acquired before and after the reaction (Figure S1). 1H polarization levels were corrected accordingly (see calculations in the SI).
Figure 1.
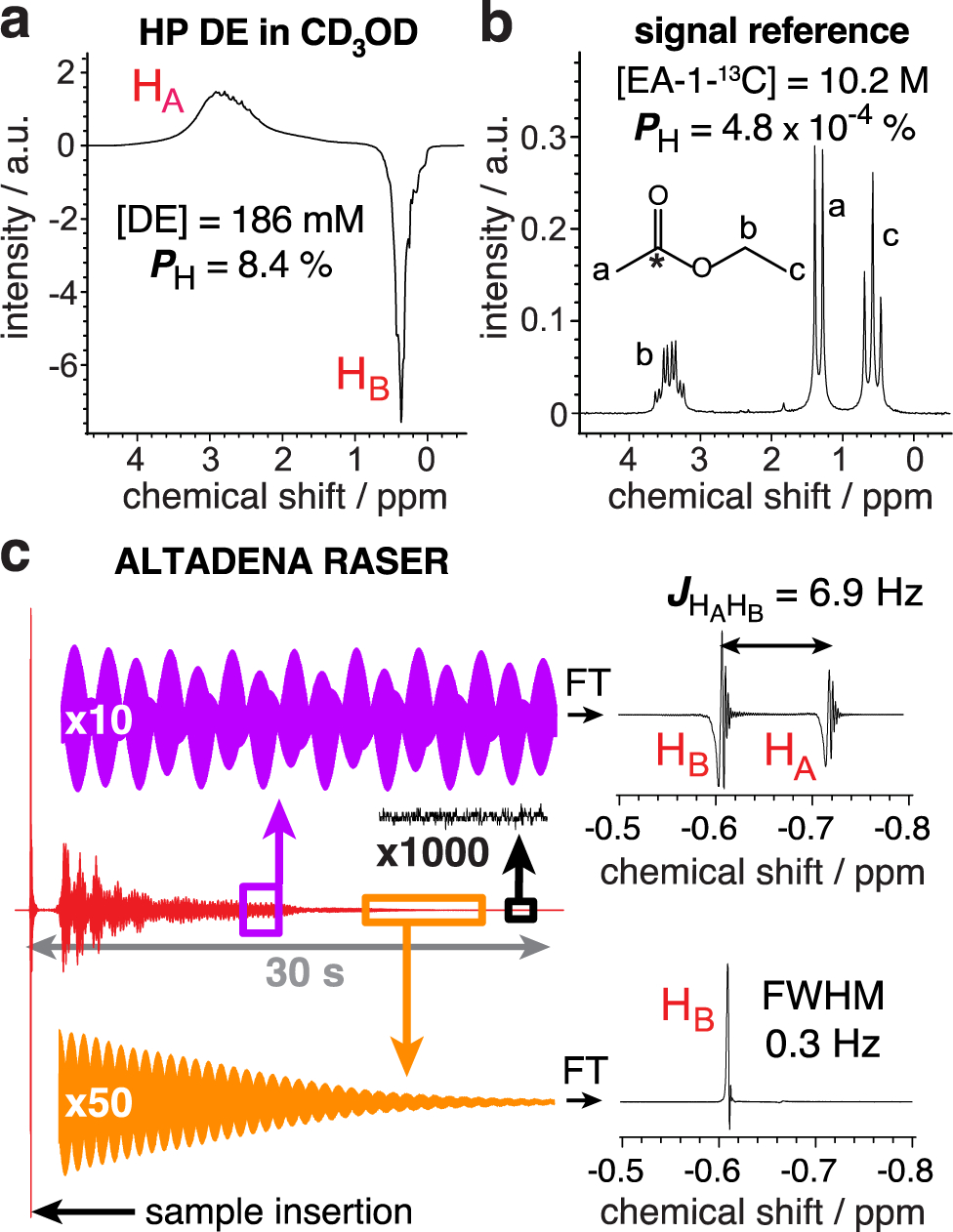
a) 1H NMR spectrum of HP DE in CD3OD solution acquired using 8° pulse angle with polarization of 8.4% after correction for evaporation and the Earth’s field relaxation. b) Corresponding NMR spectrum of neat thermally polarized ethyl-acetate-1-13C. c) ALTADENA RASER signal of HP DE recorded without excitation pulse (red), along with Fourier transform (FT) spectra of the regions outlined by purple (HB / HA two-mode RASER) and orange (HB single-mode RASER) boxes.
After cessation of p-H2 flow, the NMR sample was left for additional 15 seconds in the Earth’s magnetic field (~50 μT) before insertion in the 1.4 T spectrometer to collect NMR spectrum using 8° excitation pulse. This delay was necessary to avoid strong radio amplification by stimulated emission radiation (RASER, Figure 1c).[53, 54] This recently discovered phenomena, due to the coherent coupling of the inductive detector with the magnetization pool of HP proton spins, is manifested here by the appearance of non-linear effects such as multi-mode RASER with both HA and HB emitting (Figure 1c). RASER activity can last for more than 30 s in molecules hyperpolarized by p-H2,[55] preventing polarization level measurement immediately after the reaction completion. The observation of PHIP-RASER indicates the production of highly magnetized DE samples and may be useful in the context of future imaging studies. HP DE polarization levels were back-calculated to account for the relaxation losses (in the Earth’s magnetic field).
A maximum polarization of 8.4% was obtained in the liquid phase after bubbling p-H2 for 10 s (Figures 1a and 2a). At that time, the chemical conversion of EVE to DE was complete and more than 80% of DE remained in the liquid phase (Figure 2b). 1H polarization levels were fitted to:
| Eq. 1 |
to determine Pmax, the theoretical maximum polarization neglecting relaxation, and Tcat, the time constant for the catalytic reaction (or polarization build-up), while leaving TLLS and t0 fixed to 14 s and 1 s, respectively. These latter values correspond to independent measurements performed at 50 μT (Figure 2c). %Pmax of 12.8±0.6% and Tcat of 4.5±0.6 s were obtained, indicating that highly polarized DE can be prepared via PHIP with production speed significantly exceeding relaxation decay. %Pmax can be potentially further improved via the rational design of a PHIP catalyst; nevertheless, the polarization levels of HP DE and the speed of the reaction derived here are promising for in vivo applications. Moreover, the lifetime of HP state can be further extended at higher magnetic field: the T1 values are 29 ± 1 s and 24 ± 1 s for HA and HB protons at 1.4 T, respectively.
Figure 2.
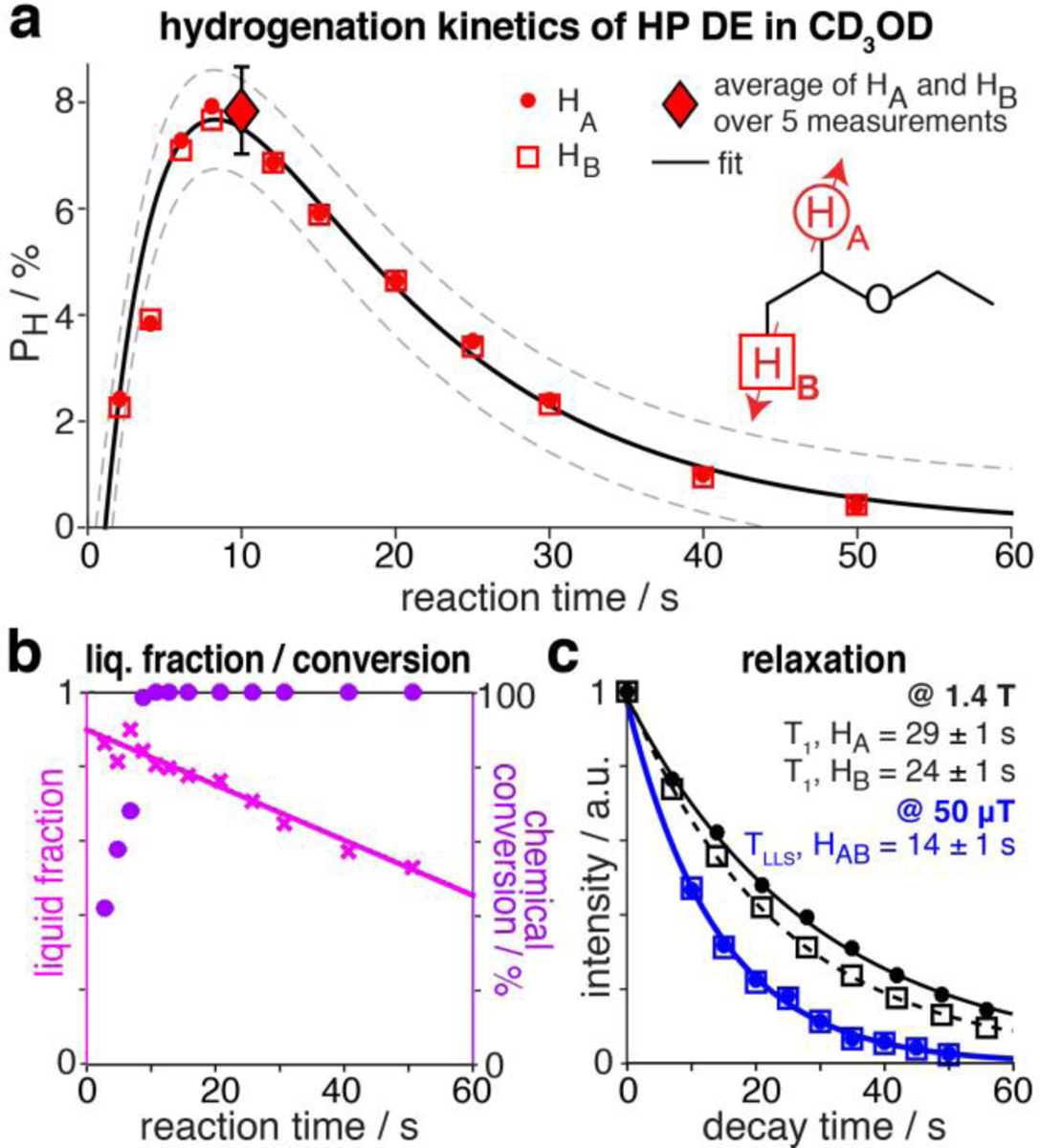
a) 1H polarization levels of HP DE in CD3OD as a function of reaction time in the Earth’s magnetic field (50 μT). Dashed lines indicate 95% confidence boundaries of the fit b) DE liquid fraction (pink crosses) and chemical conversion (magenta circles) of EVE to DE as a function of reaction time. c) Exponential decays of HP DE NMR signals at 1.4 T (black circles and squares) and 50 μT (blue circles and squares).
For gas-phase relaxation experiments, p-H2 was bubbled in a glass column filled with ~ 5–10 mL of neat EVE at a flow rate of 4000 sccm. The resulting gas mixture comprising p-H2 saturated with EVE vapor was directed through a Rh/TiO2 catalytic reactor (300 mg of Rh/TiO2 in 42 g of Cu particles) maintained at 170 °C for heterogeneous pairwise p-H2 addition[56] (Figure S2), and collected at 3.3 bar and ~35 °C in (i) a 5 mm NMR tube for NMR detection using 1.4 T benchtop NMR device (Figure 3a), (ii) a 17 mL phantom sphere for NMR detection with a 47.5 mT Kea2-based NMR spectrometer (Magritek, New Zealand) (Figure S2). The concentration of DE in the gas phase (22 mM) was evaluated at 1.4 T against reference spectra of neat ethyl acetate. Inversion recovery experiments with thermally polarized DE vapor yielded T1 = 1.21 ± 0.03 s at 1.4 T (Figure 3c), a typical value under these low partial pressure conditions (0.5 bar).[57] Even though the LLS of HP DE is NMR invisible at 47.5 mT, it can be converted into observable magnetization by singlet-to-triplet conversion with the spin-lock induced crossing (SLIC) radio-frequency pulse sequence (Figure 3b).[58]
Figure 3.
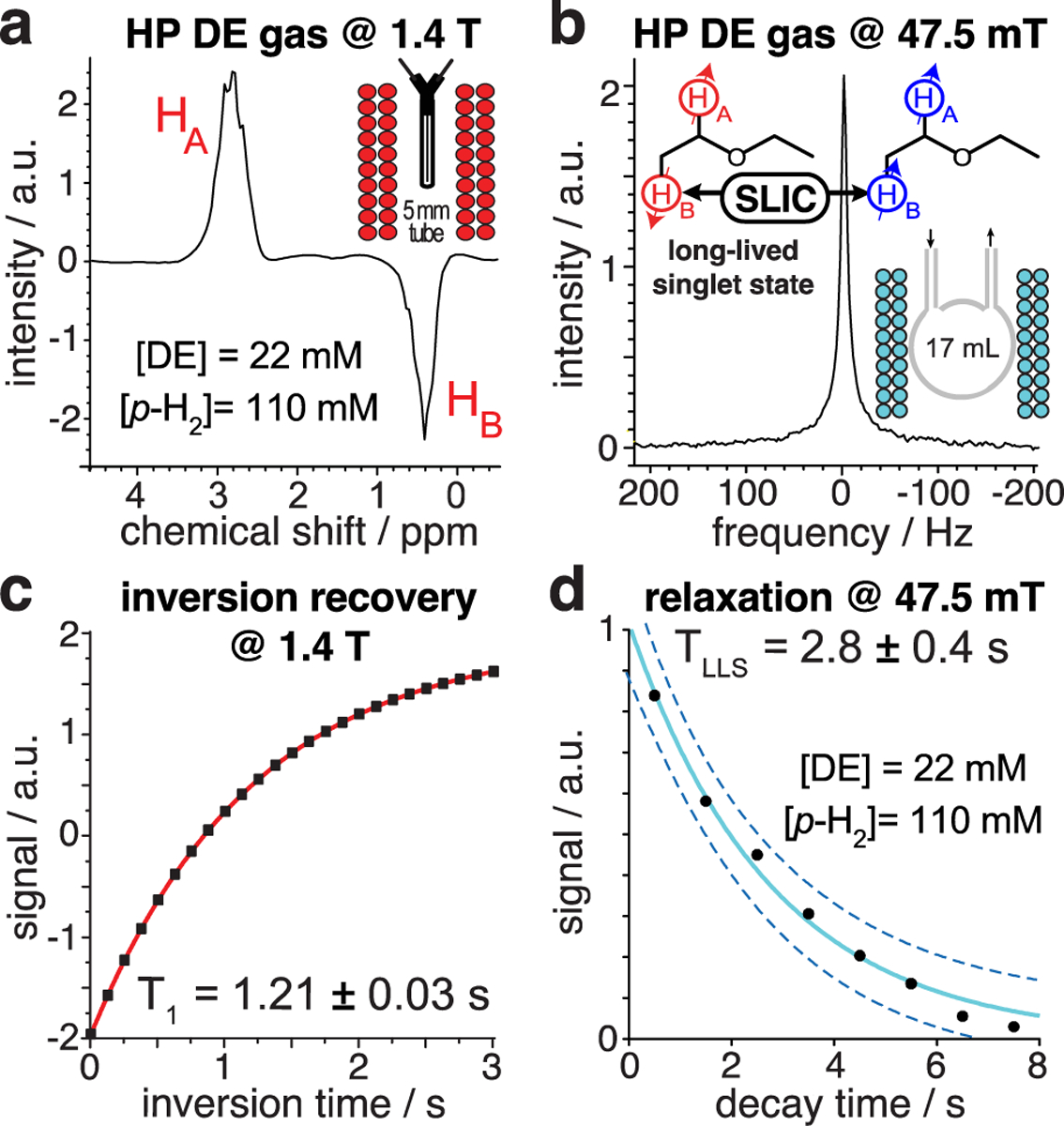
a) 1H spectrum at 1.4 T of 22 mM [DE] / 110 mM [p-H2] at 3.3 bar total pressure. b) 1H spectrum at 47.5 mT of 22 mM [DE] / 110 mM [p-H2] at 3.3 bar using SLIC. c) Inversion recovery of thermal DE vapor at 1.4 T. d) Exponential decay of LLS of HP DE at 47.5 mT. Not the NMR spectra are not calibrated with respect to the chemical shift (ppm) reference.
SLIC was optimized by tuning pulse amplitude, power, duration and frequency in the gas and liquid phases (Figures S3 and S4). TLLS was determined by applying a “partial” (200 ms duration) SLIC pulse every second to a batch of HP DE vapor and recording the produced signal—corresponding data points were employed for mono-exponential fitting (Figure 3d), yielding TLLS = 2.8 ± 0.4 s. The “partial” SLIC approach is known to induce an underestimation of the TLLS value, because some fraction of the magnetization is lost in each SLIC transformation.[57] Comparative partial SLIC experiments on HP DE in CD3OD suggest correcting the measured TLLS by a factor of 1.4 in our case (Figure S4). Our estimate is TLLS = 4.0 ± 0.7 s for HP DE at 0.5 bar partial pressure, i.e., ~ 3 times greater than the corresponding T1 at high field—in line with the overall trends of LLS lifetime in HP propane.[46, 57]
Conclusion
In summary, hyperpolarization of DE anesthetic was successfully achieved. High levels of proton polarization (>8%) were obtained on two hydrogen sites along with 100% conversion of the EVE precursor—the produced magnetization was sufficiently high for inducing RASER activity. We also report on the existence of LLS in gas phase HP DE under clinically relevant conditions. Alternative precursor divinyl ether could be potentially employed to double the payload of p-H2-derived polarization. All three compounds: DE, EVE and divinyl ether have very safe toxicity profile, which, combined with the ease and scalability of HP DE preparation and existence of long-lived states in the gas phase, bode well for future bioimaging applications—especially for functional 3D pulmonary imaging in a manner similar to that with HP 129Xe. The flammability of HP DE should be addressed in the context of potential biomedical use as an inhalable contrast agent: for example, through the use of small inhalation doses or by capturing the exhaled gas with carbon filters. Alternatively, the use of partially fluorinated PHIP precursors (e.g., fluroxene) can possibly mitigate some or all flammability issues.
Supplementary Material
Acknowledgements
This work was supported by the National Science Foundation under grant CHE-1904780, National Heart, Lung, and Blood Institute under grant R21HL154032, and by DOD CDMRP under grant W81XWH-15-1-0271. The Russian team thanks the Russian Foundation for Basic Research (Grants 17-54-33037, 19-53-12013, 19-33-60045) and the Russian Ministry of Science and Higher Education (Grant AAAA-A16-116121510087-5). V.I.B., I.V.K. and L.M.K. thank the Russian Science Foundation (Grant 19-13-00172) for the support of hydrogenation with parahydrogen.
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- [1].Hoult DI, Richards RE, J. Magn. Reson 1976, 24, 71–85. [DOI] [PubMed] [Google Scholar]
- [2].Nikolaou P, Goodson BM, Chekmenev EY, Chem. Eur. J 2015, 21, 3156–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Goodson BM, Whiting N, Coffey AM, Nikolaou P, Shi F, Gust BM, Gemeinhardt ME, Shchepin RV, Skinner JG, Birchall JR, Barlow MJ, Chekmenev EY, Emagres 2015, 4, 797–810. [Google Scholar]
- [4].Kovtunov KV, Pokochueva EV, Salnikov OG, Cousin S, Kurzbach D, Vuichoud B, Jannin S, Chekmenev EY, Goodson BM, Barskiy DA, Koptyug IV, Chem. Asian J 2018, 13, 1857–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kurhanewicz J, Vigneron DB, Brindle K, Chekmenev EY, Comment A, Cunningham CH, DeBerardinis RJ, Green GG, Leach MO, Rajan SS, Rizi RR, Ross BD, Warren WS, Malloy CR, Neoplasia 2011, 13, 81–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Golman K, Ardenkjær-Larsen JH, Petersson JS, Månsson S, Leunbach I, Proc. Natl. Acad. Sci. U. S. A 2003, 100, 10435–10439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Golman K, in’t Zandt R, Thaning M, Proc. Natl. Acad. Sci. U. S. A 2006, 103, 11270–11275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ardenkjaer-Larsen JH, J. Magn. Reson 2016, 264, 3–12. [DOI] [PubMed] [Google Scholar]
- [9].Kurhanewicz J, Vigneron DB, Ardenkjaer-Larsen JH, Bankson JA, Brindle K, Cunningham CH, Gallagher FA, Keshari KR, Kjaer A, Laustsen C, Mankoff DA, Merritt ME, Nelson SJ, Pauly JM, Lee P, Ronen S, Tyler DJ, Rajan SS, Spielman DM, Wald L, Zhang X, Malloy CR, Rizi R, Neoplasia 2019, 21, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mugler JP, Altes TA, J. Magn. Reson. Imaging 2013, 37, 313–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Goodson BM, J. Magn. Reson 2002, 155, 157–216. [DOI] [PubMed] [Google Scholar]
- [12].Driehuys B, Martinez-Jimenez S, Cleveland ZI, Metz GM, Beaver DM, Nouls JC, Kaushik SS, Firszt R, Willis C, Kelly KT, Wolber J, Kraft M, McAdams HP, Radiology 2012, 262, 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nelson SJ, Kurhanewicz J, Vigneron DB, Larson PEZ, Harzstark AL, Ferrone M, van Criekinge M, Chang JW, Bok R, Park I, Reed G, Carvajal L, Small EJ, Munster P, Weinberg VK, Ardenkjaer-Larsen JH, Chen AP, Hurd RE, Odegardstuen LI, Robb FJ, Tropp J, Murray JA, Sci. Transl. Med 2013, 5, 198ra108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Brindle KM, J. Am. Chem. Soc 2015, 137, 6418–6427. [DOI] [PubMed] [Google Scholar]
- [15].Walkup LL, Woods JC, NMR Biomed. 2014, 27, 1429–1438. [DOI] [PubMed] [Google Scholar]
- [16].He M, Robertson SH, Kaushik SS, Freeman MS, Virgincar RS, Davies J, Stiles J, Foster WM, McAdams HP, Driehuys B, Magn. Reson. Imaging 2015, 33, 877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Branca RT, He T, Zhang L, Floyd CS, Freeman M, White C, Burant A, Proc. Natl. Acad. Sci. U. S. A 2014, 111, 18001–18006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Barskiy DA, Coffey AM, Nikolaou P, Mikhaylov DM, Goodson BM, Branca RT, Lu GJ, Shapiro MG, Telkki V-V, Zhivonitko VV, Koptyug IV, Salnikov OG, Kovtunov KV, Bukhtiyarov VI, Rosen MS, Barlow MJ, Safavi S, Hall IP, Schröder L, Chekmenev EY, Chem. Eur. J 2017, 23, 725–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rao MR, Stewart NJ, Griffiths PD, Norquay G, Wild JM, Radiology 2018, 286, 659–665. [DOI] [PubMed] [Google Scholar]
- [20].Albert MS, Schepkin VD, Budinger TF, Journal of Computer Assisted Tomography 1995, 19, 975–978. [DOI] [PubMed] [Google Scholar]
- [21].Choquet P, Hyacinthe J-N, Duhamel G, Grillon E, Leviel J-L, Constantinesco A, Ziegler A, Magn. Reson. Med 2003, 49, 1014–1018. [DOI] [PubMed] [Google Scholar]
- [22].Coffey AM, Kovtunov KV, Barskiy D, Koptyug IV, Shchepin RV, Waddell KW, He P, Groome KA, Best QA, Shi F, Goodson BM, Chekmenev EY, Anal. Chem 2014, 86, 9042–9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ardenkjaer-Larsen JH, Leach AM, Clarke N, Urbahn J, Anderson D, Skloss TW, NMR Biomed. 2011, 24, 927–932. [DOI] [PubMed] [Google Scholar]
- [24].Walker TG, J. Phys. Conf. Ser 2011, 294, 012001. [Google Scholar]
- [25].Ruset IC, Ketel S, Hersman FW, Phys. Rev. Lett 2006, 96, 053002. [DOI] [PubMed] [Google Scholar]
- [26].Zook AL, Adhyaru BB, Bowers CR, J. Magn. Reson 2002, 159, 175–182. [DOI] [PubMed] [Google Scholar]
- [27].Nikolaou P, Coffey AM, Walkup LL, Gust BM, Whiting N, Newton H, Barcus S, Muradyan I, Dabaghyan M, Moroz GD, Rosen M, Patz S, Barlow MJ, Chekmenev EY, Goodson BM, Proc. Natl. Acad. Sci. U. S. A 2013, 110, 14150–14155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Driehuys B, Cates GD, Miron E, Sauer K, Walter DK, Happer W, Appl. Phys. Lett 1996, 69, 1668–1670. [Google Scholar]
- [29].Norquay G, Collier GJ, Rao M, Stewart NJ, Wild JM, Phys. Rev. Lett 2018, 121, 153201. [DOI] [PubMed] [Google Scholar]
- [30].Chukanov NV, Salnikov OG, Shchepin RV, Kovtunov KV, Koptyug IV, Chekmenev EY, ACS Omega 2018, 3, 6673–6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Iali W, Roy SS, Tickner BJ, Ahwal F, Kennerley AJ, Duckett SB, Angew. Chem. Int. Ed 2019, 58, 10271–10275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Reineri F, Boi T, Aime S, Nat. Commun 2015, 6, 5858. [DOI] [PubMed] [Google Scholar]
- [33].Cavallari E, Carrera C, Sorge M, Bonne G, Muchir A, Aime S, Reineri F, Sci. Rep 2018, 8, 8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hövener J-B, Pravdivtsev AN, Kidd B, Bowers CR, Glöggler S, Kovtunov KV, Plaumann M, Katz-Brull R, Buckenmaier K, Jerschow A, Reineri F, Theis T, Shchepin RV, Wagner S, Bhattacharya P, Zacharias NM, Chekmenev EY, Angew. Chem. Int. Ed 2018, 57, 11140–11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lilburn DML, Pavlovskaya GE, Meersmann T, J. Magn. Reson 2013, 229, 173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Carravetta M, Johannessen OG, Levitt MH, Phys. Rev. Lett 2004, 92, 153003. [DOI] [PubMed] [Google Scholar]
- [37].Carravetta M, Levitt MH, J. Am. Chem. Soc 2004, 126, 6228–6229. [DOI] [PubMed] [Google Scholar]
- [38].Pileio G, Levitt MH, J. Chem. Phys 2009, 130, 214501. [DOI] [PubMed] [Google Scholar]
- [39].Levitt MH, J. Magn. Reson 2019, 306, 69–74. [DOI] [PubMed] [Google Scholar]
- [40].Franzoni MB, Buljubasich L, Spiess HW, Munnemann K, J. Am. Chem. Soc 2012, 134, 10393–10396. [DOI] [PubMed] [Google Scholar]
- [41].Franzoni MB, Graafen D, Buljubasich L, Schreiber LM, Spiess HW, Munnemann K, Phys. Chem. Chem. Phys 2013, 15, 17233–17239. [DOI] [PubMed] [Google Scholar]
- [42].Graafen D, Franzoni MB, Schreiber LM, Spiess HW, Münnemann K, J. Magn. Reson 2016, 262, 68–72. [DOI] [PubMed] [Google Scholar]
- [43].Zhang YN, Soon PC, Jerschow A, Canary JW, Angew. Chem. Int. Ed 2014, 53, 3396–3399. [DOI] [PubMed] [Google Scholar]
- [44].Zhang Y, Duan X, Soon PC, Sychrovský V, Canary JW, Jerschow A, ChemPhysChem 2016, 17, 2967–2971. [DOI] [PubMed] [Google Scholar]
- [45].Kovtunov KV, Truong ML, Barskiy DA, Koptyug IV, Coffey AM, Waddell KW, Chekmenev EY, Chem. Eur. J 2014, 20, 14629–14632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Barskiy DA, Salnikov OG, Romanov AS, Feldman MA, Coffey AM, Kovtunov KV, Koptyug IV, Chekmenev EY, J. Magn. Reson 2017, 276, 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kovtunov KV, Truong ML, Barskiy DA, Salnikov OG, Bukhtiyarov VI, Coffey AM, Waddell KW, Koptyug IV, Chekmenev EY, J. Phys. Chem. C 2014, 118, 28234–28243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Essential Medicines WHO Model List (revised April 2003) (13th ed.), World Health Organization, Geneva, Switzerland, 2003. [Google Scholar]
- [49].Slater HM, Can. Anaes. J 1957, 4, 5–12. [DOI] [PubMed] [Google Scholar]
- [50].Chang CY, Goldstein E, Agarwal N, Swan KG, BMC Anesthesiol 2015, 15, 149–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Feng B, Coffey AM, Colon RD, Chekmenev EY, Waddell KW, J. Magn. Reson 2012, 214, 258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Pravica MG, Weitekamp DP, Chem. Phys. Lett 1988, 145, 255–258. [Google Scholar]
- [53].Suefke M, Lehmkuhl S, Liebisch A, Blumich B, Appelt S, Nat. Phys 2017, 13, 568–572. [Google Scholar]
- [54].Appelt S, Kentner A, Lehmkuhl S, Blümich B, Prog. Nucl. Mag. Res. Spectrosc 2019, 114–115, 1–32. [DOI] [PubMed] [Google Scholar]
- [55].Joalland B, Ariyasingha NM, Lehmkuhl S, Theis T, Appelt S, Chekmenev EY, Angew. Chem. Int. Ed 2020, 132, 8732–8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Salnikov OG, Nikolaou P, Ariyasingha NM, Kovtunov KV, Koptyug IV, Chekmenev EY, Anal. Chem 2019, 91, 4741–4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ariyasingha NM, Salnikov OG, Kovtunov KV, Kovtunova LM, Bukhtiyarov VI, Goodson BM, Rosen MS, Koptyug IV, Gelovani JG, Chekmenev EY, J. Phys. Chem. C 2019, 18, 11734–11744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].DeVience SJ, Walsworth RL, Rosen MS, Phys. Rev. Lett 2013, 111, 173002-173001-173005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


