Abstract
Mucin secretion from conjunctival goblet cells forms the tear film mucin layer and requires regulation to function properly. Maresin 1 (MaR1) is a specialized proresolving mediator produced during the resolution of inflammation. We determined if MaR1 stimulates mucin secretion and signaling pathways used. Cultured rat conjunctival goblet cells were used to measure the increase in intracellular Ca2+ ([Ca2+]i) concentration and mucin secretion. MaR1-increased [Ca2+]i and secretion were blocked by inhibitors of phospholipase C, protein kinase C, Ca2+/calmodulin-dependent protein kinase II, and extracellular-regulated kinase 1/2. MaR1 added before addition of histamine counterregulated histamine-stimulated increase in [Ca2+]i and secretion. We conclude that MaR1 likely has two actions in conjunctival goblet cells: first, maintaining optimal tear film mucin levels by increasing [Ca2+]i and stimulating mucin secretion in health and, second, attenuating the increase in [Ca2+]I and overproduction of mucin secretion by counterregulating the effect of histamine as occurs in ocular allergy.
Keywords: conjunctiva, goblet cell, signal transduction, specialized proresolving mediator (SPM)
1 |. INTRODUCTION
The ocular surface consists of the cornea and conjunctiva, the apical layer of which is stratified nonkeratinized epithelium. Present within the conjunctival epithelium are mucin-producing goblet cells that are essential for the protection of the ocular surface (Hodges & Dartt, 2013). The mucin secreted from the goblet cells is an essential component of the tear film and functions to clear allergens, pathogens, and other potentially damaging substances from the ocular surface and to lubricate the surface (Mantelli & Argueso, 2008). The goblet cells have an important role in maintaining the health of the ocular surface by ensuring a balanced amount of mucin secretion under normal, physiologic conditions. In disease, both mucin overproduction and deficiency can lead to diseases of the ocular surface (Dartt, 2004). Inflammatory diseases such as allergic conjunctivitis, keratoconjunctivitis sicca (dry eye), Sjögren’s syndrome, and bacterial and viral conjunctivitis can cause alterations in mucin homeostasis. These alterations in the normally balanced mucin secretion due to inflammatory disease can result in reduced quality of life and impairment of vision (Barabino, Labetoulle, Rolando, & Messmer, 2016; Mantelli & Argueso, 2008; Palmares et al., 2010; Uchino & Schaumberg, 2013). Treatment options for inflammatory diseases are limited and often ineffective for many patients.
The resolution of disease-induced inflammation is an active process with a complex time- and cell-dependent interaction between epithelial cells and inflammatory cells with proinflammatory and proresolving mediators (Serhan & Petasis, 2011). During resolution of the inflammatory process, a complex program of biochemical mediator production and leucocyte influx work to actively terminate the inflammatory process (Serhan & Savill, 2005). Immune and epithelial cells biosynthesize lipid compounds called specialized proresolving mediators (SPMs), which have both an antiinflammatory and novel proresolving actions (Dalli & Serhan, 2016; Serhan, Dalli, Colas, Winkler, & Chiang, 2015). Macrophages are important immune cells in the inflammatory regulation process and biosynthesize specific family of SPMs, termed maresins (macrophage mediators in the resolution of inflammation, MaR; Serhan et al., 2009) Maresin 1 (MaR1,) is the first MaR discovered and is produced by macrophages when stimulated by efferocytosis of polymorphonuclear neutrophils (PMNs) or PMN microparticles (Dalli & Serhan, 2016). MaR are biosynthesized from the precursor ω−3 fatty acid docosahexaenoic acid (DHA) known to be important in ocular physiology (Dartt & Masli, 2014; Hodges, Li, Shatos, Serhan, & Dartt, 2016; Li et al., 2013; Lippestad, Hodges, Utheim, Serhan, & Dartt, 2017; Saban et al., 2019) and their production is increased as M1 macrophages are converted to M2 macrophages (Serhan et al., 2015). The complete structure of MaR1 is established and confirmed by total organic synthesis, and its structure portends proresolving actions (Serhan et al., 2012). MaR1 can then interact with PMNs and macrophages via specific receptors including the recently discovered leucine-rich-repeat-containing G protein-coupled receptor 6 (LGR6) that is responsible for MaR1 proresolving actions (Chiang, Libreros, Norris, de la Rosa, & Serhan, 2019; Colas et al., 2016; Gu et al., 2018). MaR1 limits PMN tissue infiltration during the inflammatory response, which helps to prevent and reduce local tissue damage, contributes to the resolution of inflammation, increases regeneration, and is nociceptive (Serhan et al., 2009). Many of the target tissues of MaR1 in which MaR1 functions have yet to be identified and are of interest.
The purpose of this study was to determine if MaR1 interacts with rat conjunctival goblet cells and regulates the secretory function of these cells under physiological regulation and during inflammatory disease. One of the main stimulatory signals for high molecular weight glycoprotein secretion that includes mucins is an increase in the intracellular Ca2+ concentration ([Ca2+]i). We used inhibitors of receptors, enzymes, and downstream molecules of different intracellular pathways to determine if MaR1 increases [Ca2+]i and stimulates mucin secretion and to identify the signaling pathways used. In addition, because MaR1 is thought to be a central proresolving mediator during inflammatory diseases, we investigated the actions of MaR1 on increase in [Ca2+]i and secretion stimulated by the allergic, proinflammatory mediator, histamine.
2 |. MATERIALS AND METHODS
2.1 |. Materials
Roswell Park Memorial Institute (RPMI) 1640 cell culture medium, penicillin/streptomycin, and l-glutamine were purchased from Lonza (Walkerville, IL). Fetal bovine serum (FBS) was from Atlanta Biologicals (Norcross, GA). Ulex europaeus agglutinin I (UEA-1), histamine, carbachol (Cch), aristolochic acid (AA), 2-aminoethoxydiphenyl borate (2-APB), 1-butanol (1-but), and t-butanol (t-but) were obtained from Sigma-Aldrich (St. Louis, MO). MaR1 (7R,14S-dihydroxy-4Z,8E,10E,12Z,16Z,19Z-docosahexaenoic acid) was from Cayman Chemical (Ann Arbor, MI). MaR1 was stored in an ethanol solution at −80°C as supplied by the manufacturer. The solution was diluted immediately before use in Krebs–Ringer bicarbonate buffer with 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (KRB-HEPES, 119 mM NaCl, 4.8 mM KCl, 1.0 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25 mM NaHCO3, 10 mM HEPES, and 5.5 mM glucose [pH7.40–7.45]) to the desired concentrations and added to the cells.
VIP, U73122, U73343, KN92, and KN93 were purchased from Tocris Bioscience (Ellisville, MO). Fura-2-acetoxymethyl ester (Fura-2/AM) and 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA/AM) were purchased from Life Technologies (Grand Island, NY). UO126 was ordered from R&D Systems. Lipoxin A4 (LXA4), H89, thapsigargin, and RO-317549 were ordered from EMD Millipore (Billerica, MA). All inhibitors were initially diluted and stored in dimethyl sulfoxide at −20°C. Immediately before use, the inhibitors were diluted to desired concentrations in KRB-HEPES and added to the cells.
2.2 |. Animals
Four-to-eight-week-old male albino Sprague–Dawley rats (Taconic Farms, Germantown, NY) weighing between 125 and 150 g were anesthetized with CO2 for 5 min, decapitated, and the bulbar and forniceal conjunctival epithelia removed from both eyes. All experiments were in accordance with the US National Research Council’s Guide for the Care and Use of Laboratory Animals, the US Public Health Service’s Policy on Humane Care and Use of Laboratory Animals, and Guide for the Care and Use of Laboratory Animals and were approved by the Schepens Eye Research Institute Animal Care and Use of Committee.
2.3 |. Cell culture
The conjunctival tissue was dissected and placed in six-well plates with 0.5 ml RPMI 1640 medium supplemented with 10% FBS, 2 mM l-glutamine, and 100 mg/ml penicillin-streptomycin. RPMI medium was changed every second day. Cultured goblet cells were identified periodically by staining with anti-cytokeratin 7, anti-MUC5AC, and the lectin UEA-1 directly conjugated to a fluorophore. The cells were trypsinized and transferred to glass bottom dishes for Ca2+ experiments or 24-well plates for secretion experiments as described previously (Hayashi et al., 2012; Hodges et al., 2017, 2016; Li, Carozza, Shatos, Hodges, & Dartt, 2012; Li et al., 2013; Li, Jiao, Shatos, Hodges, & Dartt, 2013; Lippestad et al., 2017).
2.4 |. Measurement of high molecular weight glycoprotein secretion
Cultured rat conjunctival goblet cells were trypsinized and transferred to 24-well plates. The cells were serum-starved in RPMI 1640 media containing 0.35% bovine serum album (BSA) for 2 hr. MaR1 (10−10–10−7 M) was then added alone or the cells were incubated with inhibitors for 30 min, and then stimulated with MaR1 (10−9 M), histamine (10−5 M) or the cholinergic agonist Cch (10−4 M) for 2 hr. As an index of mucin secretion, the amount of goblet cell high molecular weight glycoprotein (HMWG) secretion was measured using the lectin UEA-1 in an enzyme-linked lectin assay (Hayashi et al., 2012; Hodges et al., 2017, 2016; Li et al., 2012; Li, Hodges, et al., 2013; Li, Jiao, et al., 2013; Lippestad et al., 2017). Cells were homogenized and the amount of protein was determined using the Bradford assay. Glycoprotein secretion was standardized to amount of protein in each condition and is shown as fold increase above basal (which was set to 1).
2.5 |. Measurement of [Ca2+]i
Cultured rat conjunctival goblet cells were trypsinized and transferred to 35-mm glass bottom dishes and incubated at 37°C overnight. The cells were then incubated at 37°C for 1 hr with KRB-HEPES containing 0.5% BSA, 0.5 μM of Fura-2/AM, 250 μM sulfinpyrazone, and 8 μM pluronic acid F127. [Ca2+]i was measured with a ratio imaging system (InCytIm2; Intracellular Imaging, Cincinnati, OH) using wavelengths of 340 and 380 nm and an emission wavelength of 505 nm. A minimum of 10 goblet cells were selected and the Ca2+ response was followed for ~2 min.
MaR1 was either added alone or cells were pretreated with inhibitors before addition of either MaR1or a positive control. The inhibitors were added 30 min before MaR1 except 1-but, t-but, and thapsigargin were added 15 min before MaR1. Change in peak [Ca2+]i was calculated by subtracting the average basal [Ca2+]i from the peak [Ca2+]i.
2.6 |. Measurement of cyclic adenosine monophosphate level
Goblet cells seeded in 24-well plates were grown to 75% confluence. All cells were incubated with 3-isobutyl-1-methylxanthine 10−3 M for 40 min total. MaR1 (10−8) M was added for 40 min. VIP (10−8 M) added for 5 min was the positive control. Cells were lysed in 0.1 M HCl. Total cell cyclic adenosine monophosphate (cAMP) was assayed by direct cAMP enzyme-linked immunosorbent assay kit following the manufacturer’s instructions (Enzo Life Sciences, Farmingdale, NY). The acetylation protocol was used to increase sensitivity. Total cell protein was determined by the Bradford assay, and the cellular cAMP was normalized to total protein. cAMP levels are presented in real numbers.
2.7 |. Statistical analysis
Data are expressed as mean ± standard error of the mean. Data were analyzed by either Student’s t-test or one-way analysis of variance followed by the Tukey test. A p < .05 was considered significant.
3 |. RESULTS
3.1 |. MaR1 increases high molecular weight glycoprotein secretion in rat conjunctival goblet cells
Other SPMs including LXA4, resolvin D1 (RvD1), and resolvin E1 (RvE1) stimulate HMWG secretion, our index of mucin secretion, in rat conjunctival goblet cells (Hodges et al., 2017; Li et al., 2013; Lippestad et al., 2017; Lippestad, Hodges, Utheim, Serhan, & Dartt, 2018). To determine if MaR1 increases HMWG secretion, goblet cells were incubated with MaR1 (10−10–10−7 M) and secretion measured. The cholinergic agonist Cch 10−4 M was used as a positive control (Hayashi et al., 2012; Rios et al., 1999). MaR1 at 10−10 M and 10−9 M increased HMWG secretion significantly above basal by 2.0 ± 0.1 fold and 2.4 ± 0.4 fold above basal (Figure 1; n = 3), respectively. The two highest concentrations of MaR1 (10−8 and 10−7 M) did not stimulate secretion. Cch significantly increased mucin secretion 2.2 ± 0.2 fold above basal (n = 3). Thus, MaR1 stimulates HMGC secretion with a peak at 10−9 M.
FIGURE 1.
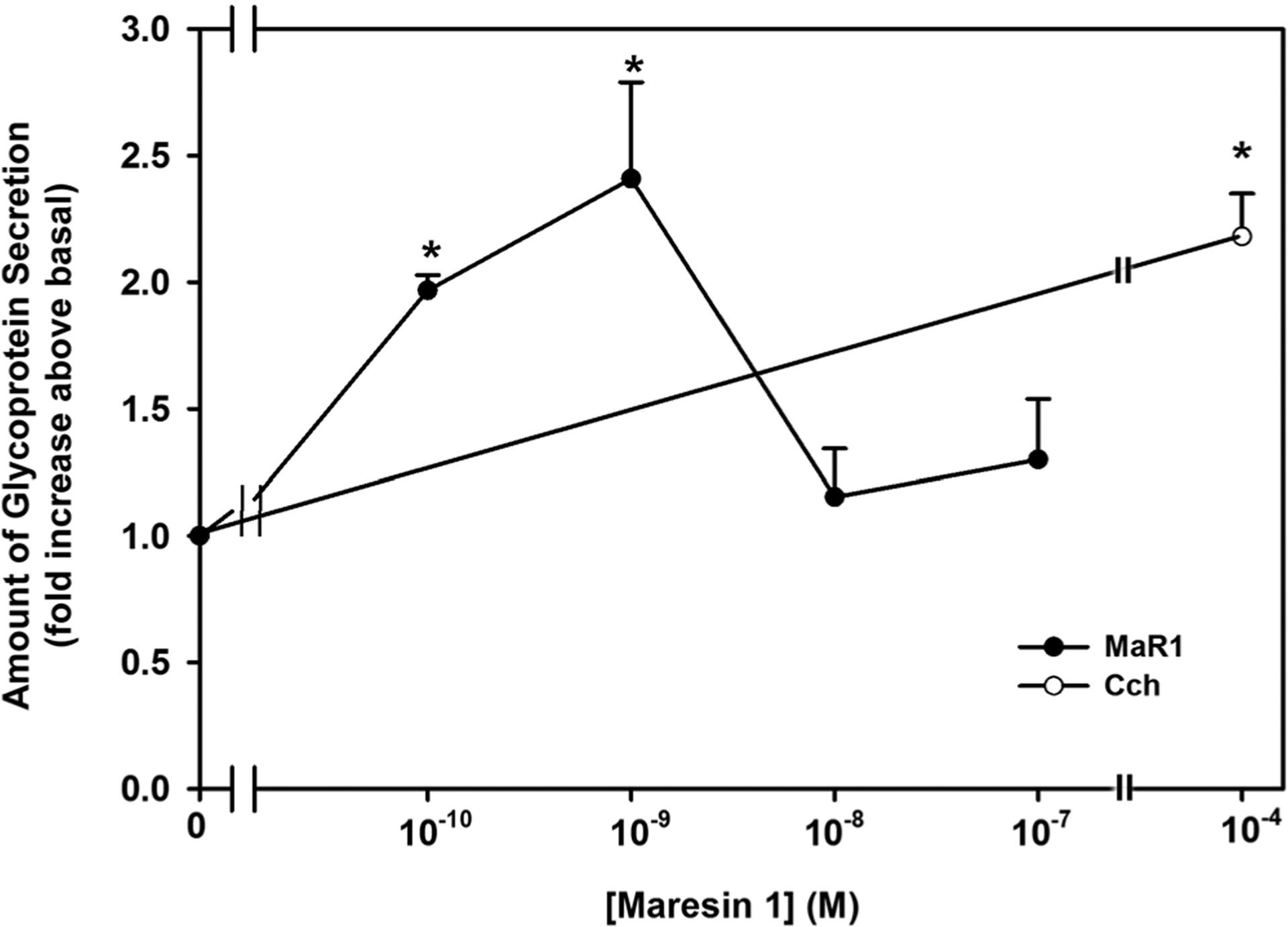
Maresin 1 (MaR1) stimulates glycoconjugate secretion. Rat conjunctival goblet cells were stimulated with either MaR1 (10−10–10−8 M) or carbachol (Cch, 10−4 M) for 2 hr. High molecular weight glycoprotein secretion was measured. Data are mean ± SEM from three experiments. *Significance above basal. SEM, standard error of the mean
3.2 |. MaR1 increases [Ca2+]i in rat conjunctival goblet cells
In addition to stimulating secretion, the SPMs LXA4, RvD1, and RvE1 also increase [Ca2+]i in rat conjunctival goblet cells (Hodges et al., 2017; Li et al., 2013; Lippestad et al., 2018, 2017). To determine if MaR1 also increases [Ca2+]i, cultured goblet cells were incubated in fura-2/AM for 1 hr and stimulated with MaR1 (10−10–10−7 M). Each concentration of MaR1 statistically significantly increased [Ca2+]i (Figure 2a–c; n = 6). MaR1-stimulated increase in [Ca2+]i was 178.2 ± 68.4 nM at 10−10 M, 120.5 ± 29.0 nM at 10−9 M, 220.3 ± 37.8 at 10−8 M, and 160.1 ± 27.4 nM at 10−7 M (Figure 2c). The maximum increase in peak [Ca2+]i occurred at MaR1 10−8 M, thus this was the concentration of MaR1 used in subsequent experiments. In the cells from the same animals, histamine, the positive control, increased [Ca2+]i by 305.7 ± 66.8. MaR1 increases [Ca2+]i in conjunctival goblet cells.
FIGURE 2.
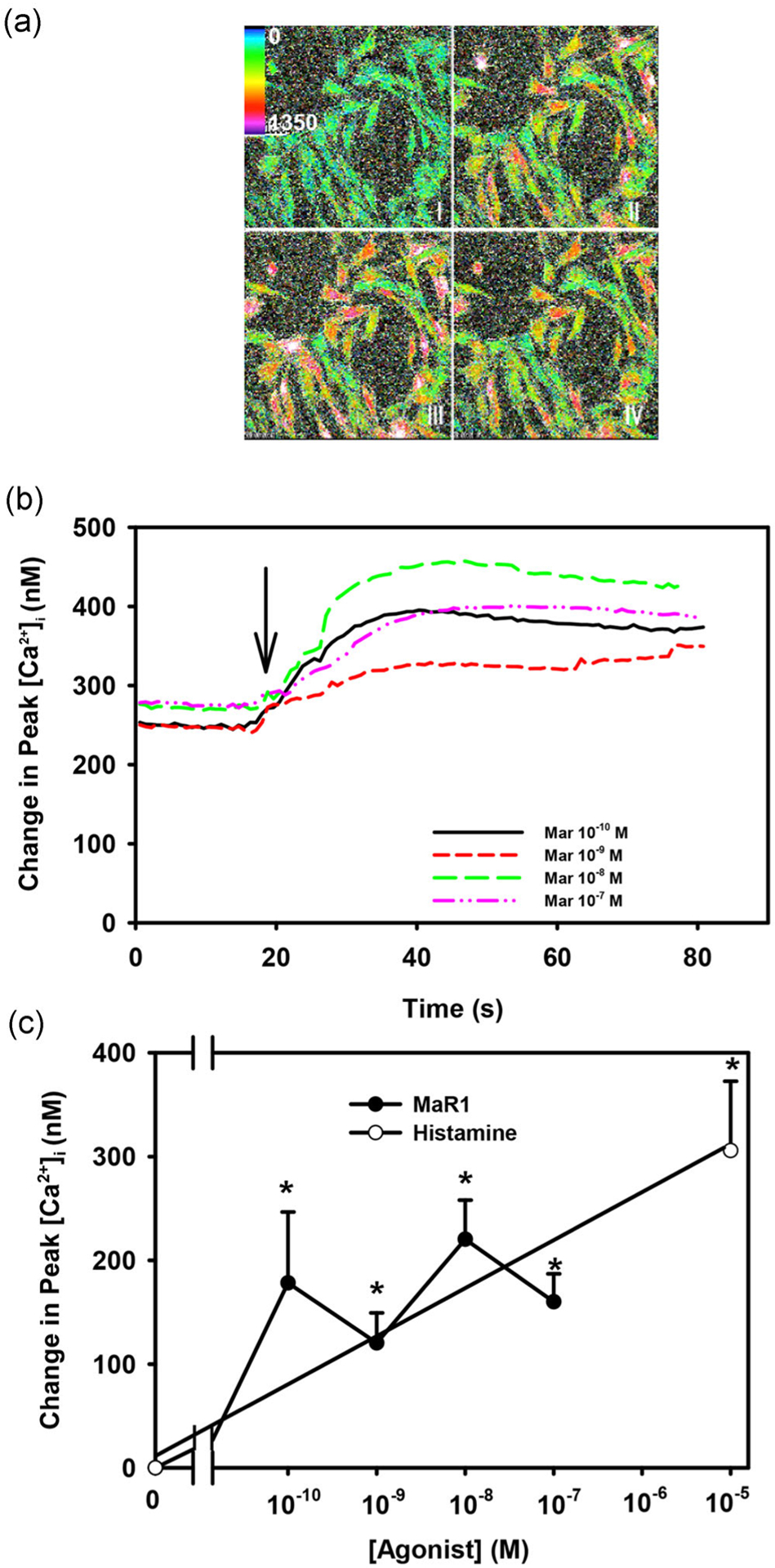
Maresin 1 (MaR1) stimulates an increase in [Ca2+]i. Pseudocolor images of rat conjunctival goblet cells stimulated with MaR1 (10−8 M) (a) at t = 0, 30, 50, and 80 s.Changes in [Ca2+]i over time with different concentrations of MaR1 are shown in (b). Change in peak [Ca2+]i is shown in (c). Data are mean ± SEM from six experiments. *Significance above basal. Arrow indicates addition of MaR1. [Ca2+]i, intracellular Ca2+; SEM, standard error of the mean
3.3 |. MaR1-stimulated increase high molecular weight glycoprotein secretion is dependent upon [Ca2+]i in rat conjunctival goblet cells
To determine if MaR1 utilizes the increase in [Ca2+]i to stimulate HMWG secretion, rat conjunctival goblet cells were loaded with the intracellular calcium chelator BAPTA. First, to determine the appropriate concentration of BAPTA ester (BAPTA/AM) to use to chelate [Ca2+]i, cells were preincubated for 30 min with BAPTA/AM (10−4 M) and MaR1-stimulated increase in [Ca2+]i measured. MaR1 (10−8 M) increased [Ca2+]i by 243.7 ± 57.5 nM (Figure S1; n = 5). When these cells were preincubated with BAPTA/AM, the response was significantly reduced to 82.8 ± 11.1 nM, a decrease of ~66%. Similar results were obtained when cells were stimulated with LXA4.
To determine if HMWG secretion is altered by chelation of Ca2+i, cells were incubated with BAPTA/AM (10−4 M) and MaR1-stimulated HMWG secretion was measured. MaR1 (10−9 M) stimulated secretion by 1.6 ± 0.04 fold above basal (Figure 3; n = 3). Incubation with BAPTA/AM significantly decreased this response and was 0.91 ± 0.14 fold of basal (Figure 3). Cch-stimulated increase in secretion was also significantly decreased and was below basal levels when incubated with BAPTA/AM. These data indicate that the MaR1 increase in [Ca2+]i stimulates goblet cell secretion.
FIGURE 3.
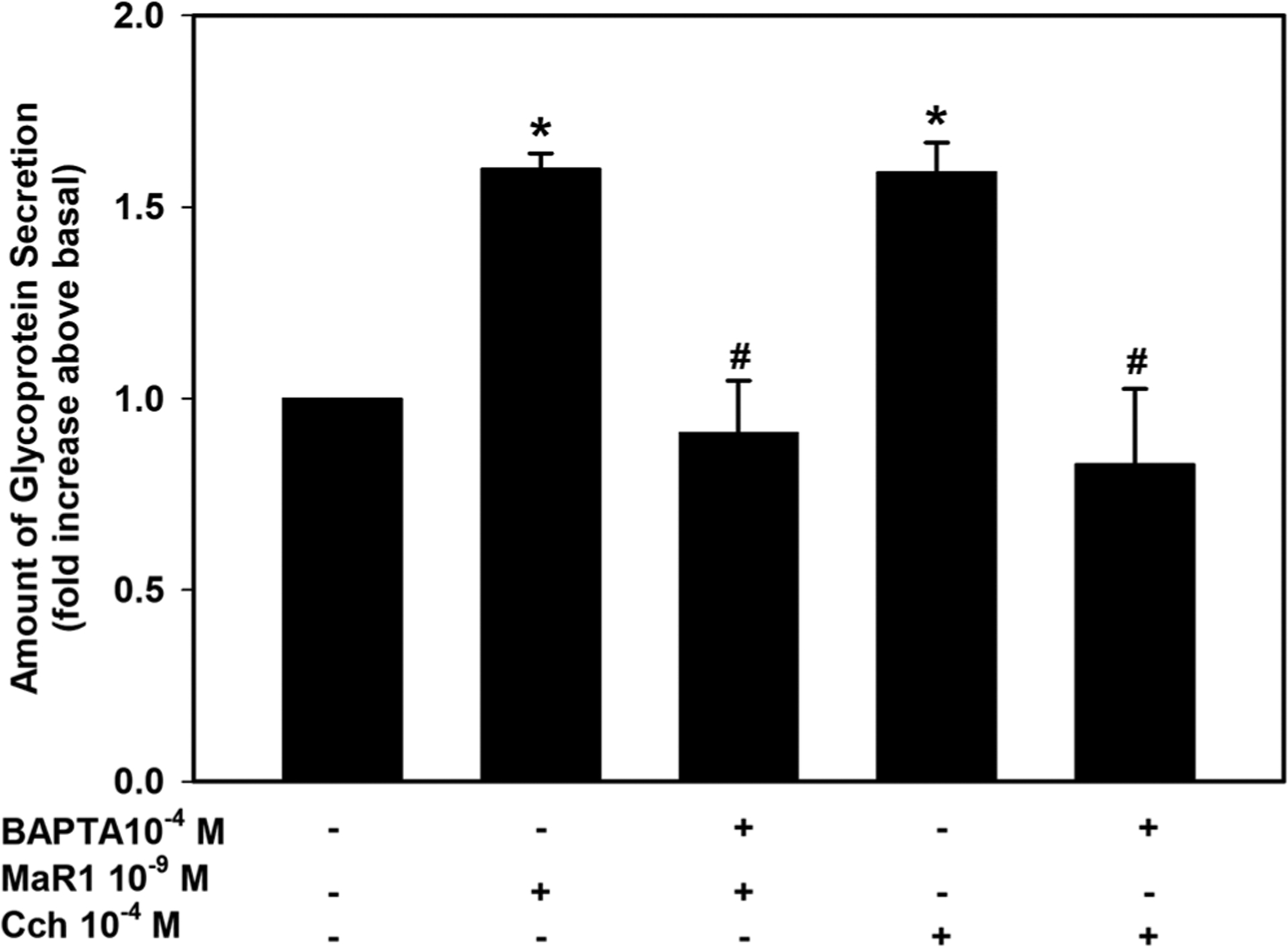
Inhibition of [Ca2+]i blocks maresin 1 (MaR1)-stimulated high molecular weight glycoprotein secretion. Rat conjunctival goblet cells were incubated with the Ca2+ chelator BAPTA/AM and secretion measured for 2 hr in response to MaR1 (10−9 M) or the cholinergic agonist carbachol (Cch, 10−4 M). Data are mean ± SEM from three experiments. *significance above basal. BAPTA, 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid; SEM, standard error of the mean
3.4 |. MaR1 activates the phospholipase C pathway to increase [Ca2+]i and stimulate high molecular weight glycoprotein secretion in cultured rat goblet cells
The phospholipase C (PLC) pathway regulates cellular events such as exocytosis, fluid secretion, contraction, metabolism, proliferation, ion channel opening, and many other cellular processes (Berridge, 2009). The association between the PLC pathway and intracellular Ca2+ is well established (Hokin, 1966; Michell, 1975). To determine if MaR1 uses the PLC pathway to increase [Ca2+]i, goblet cells were incubated with the PLC inhibitor U73122 (10−7 M) or the inactive control U73343 (10−7 M) for 30 min before MaR1 (10−8 M) stimulation. MaR1 significantly stimulated [Ca2+]i to a peak value of 213.5 ± 19.4 nM (Figure 4a; n = 3). The active inhibitor U73122 caused a significant decrease in stimulated [Ca2+]i to a peak of 55.4 ± 5.7 nM. Incubation with the inactive control U73343 caused an increase in [Ca2+]i by a maximum of 134.4 ± 31.1 nM which is unchanged from MaR1 alone. Cch, which is known to activate PLC in goblet cells (Dartt et al., 2000), also significantly increased [Ca2+]i which was blocked by U73122, but not U73343 (Figure 4a).
FIGURE 4.
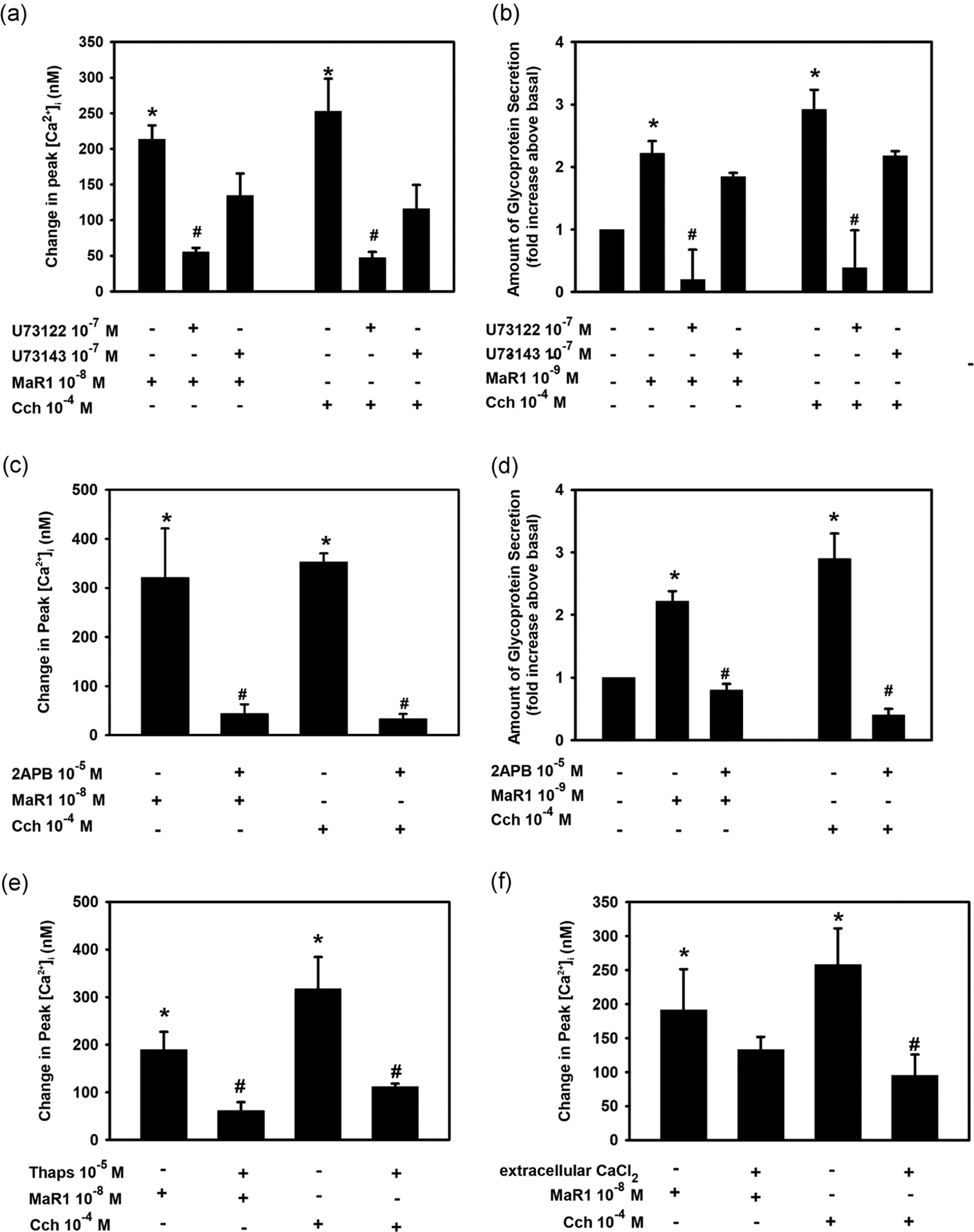
Inhibition of phospholipase C (PLC) blocks maresin 1 (MaR1)-stimulated increase in [Ca2+]i. Goblet cells were incubated with the PLC inhibitor U73122 (10−7 M), its negative control U73143 (10−7 M, a and b) or (c and d) IP3 receptor inhibitor 2-APB (10−5 M) for 30 min and stimulated with MaR1 at 10−8 M for [Ca2+]i or 10−9 M for secretion or carbachol (Cch, 10−4 M). (a) and (c) show the change in peak [Ca2+]i while (b) and (d) show high molecular weight glycoprotein secretion. (e) Rat conjunctival goblet cells were preincubated with the SERCA-inhibitor thapsigargin (10−5 M) for 15 min and stimulated with either MaR1 (10−8 M) or carbachol (Cch, 10−4 M). (f) Goblet cells were incubated with KRB with or without CaCl2 and stimulated with MaR1 (10−8 M) or Cch (10−4 M). (e and f) The change in peak [Ca2+]i. Data are mean ± SEM of three (a–e) or five (f) experiments. *Significance above basal. #Significant difference from MaR1 or Cch alone. 2-APB, 2-aminoethoxydiphenyl borate; [Ca2+]i, intracellular Ca2+; KRB, Krebs–Ringer bicarbonate buffer; SEM, standard error of the mean; SERCA, sarco/endoplasmic reticulum
Similar to the increase in [Ca2+]i, both the MaR1- and Cch-stimulated increase in secretion was significantly blocked by U73122 but not U73343 (Figure 4b; n = 3). This suggests that MaR1 activates PLC to increase [Ca2+]i and stimulate secretion.
3.5 |. MaR1 releases Ca2+ from intracellular stores to increase [Ca2+]i and stimulate high molecular weight glycoprotein secretion in cultured rat goblet cells
It is well known that the PLC pathway generates inositol trisphosphate (IP3) that binds to IP3 receptors on the membrane of an intracellular organelle, probably endoplasmic reticulum that stores intracellular Ca2+ (Williamson, 1986). This binding causes a release of Ca2+ from the intracellular stores into the cytoplasm to increase [Ca2+]i. To determine if MaR1 increases IP3 to raise the [Ca2+]i, rat conjunctival goblet cells were incubated with the IP3 receptor inhibitor 2-APB (10−5 M) for 30 min before adding MaR1 (10−8 M). MaR1 significantly increased [Ca2+]i by 321.0 ± 100.3 nM (Figure 4c; n = 3). In the presence of 2-APB, the response was 44.2 ± 18.9 nM, a significant decrease from MaR1 alone. The positive control, Cch significantly increased [Ca2+]i that was blocked by preincubation with 2-APB.
The effect of 2-APB on glycoconjugate secretion was then explored. MaR1-increased secretion 2.2 ± 0.2 fold increase above basal. Treatment with 2-APB significantly decreased MaR1-stimulated response to 0.8 ± 0.1 fold above basal (Figure 4d; n = 3). Cch increased secretion 2.9 ± 0.4 fold above basal, which was also significantly inhibited by 2-APB. These data indicate that MaR1 generates IP3 to increase [Ca2+]i and stimulate secretion.
To investigate further MaR1’s effect on the release of calcium from intracellular stores, we used the sarco/endoplasmic reticulum Ca2+-ATPase inhibitor thapsigargin. Thapsigargin depletes intracellular Ca2+ stores by preventing the reuptake of Ca2+ that passively leaks out. If MaR1 uses this store, addition of MaR1 after thapsigargin will not increase [Ca2+]i. Rat conjunctival goblet cells incubated with MaR1 alone stimulated an increase in [Ca2+]i to a maximum of 189.6 ± 36.7 nM (Figure 4e; n = 3). When cells were treated with thapsigargin (10−5 M) for 15 min, then stimulated with MaR1 (10−8 M), the peak response to MaR1 was decreased to 61.9 ± 6.5 nM. Cch-stimulated increase in [Ca2+]i, the positive control, was also blocked by incubation with thapsigargin (Figure 4e). This is an additional support that MaR1 releases Ca2+ from intracellular stores.
3.6 |. MaR1 does not activate influx of extracellular Ca2+
To determine if the MaR1-stimulated increase in [Ca2+]i is dependent on the influx of extracellular Ca2+, goblet cells were incubated in KRB buffer with and without 1.0 mM CaCl2. In the presence of extracellular Ca2+, MaR1 (10−8 M) significantly increased [Ca2+]i by 191.5 ± 60.0 nM (Figure 4f; n = 5). When extracellular CaCl2 was removed, the increase in [Ca2+]i stimulated by MaR1 was 133.3 ± 41.7 nM, which was not a significant decrease. In contrast, Cch-stimulated increase in [Ca2+]i was significantly decreased by the removal of extracellular Ca2+ (Figure 4f). This suggests that MaR1 is mainly dependent on intracellular calcium stores, and not influx of extracellular calcium to increase [Ca2+]i.
3.7 |. MaR1 activates protein kinase C to increase [Ca2+]i and stimulate HMWG secretion in cultured rat goblet cells
Protein kinase C (PKC) was discovered by Nishizuka (1984). This enzyme can be activated by the PLC pathway, which generates diacylglycerol that in turn activates PKC. To determine if MaR1 stimulates PKC activity to increase [Ca2+]i, rat conjunctival goblet cells were treated with the PKC inhibitor RO-317549 for 30 min, then stimulated with MaR1 (10−8 M). MaR1 significantly increased [Ca2+]i to a peak of 191.6 ± 6.1 nM (Figure 5a; n = 4). Incubation of MaR1 with RO-317549 (10−7 M) caused a significantly decreased response of 118.3 ± 25.9 nM. Cch was again used as a positive control as it is known to activate PKC in conjunctival goblet cells (Dartt et al., 2000). Cch-stimulated increase in [Ca2+]i was significantly reduced after incubation with RO-317549 (Figure 5a). Thus, MaR1 activates PKC to increase [Ca2+]i
FIGURE 5.
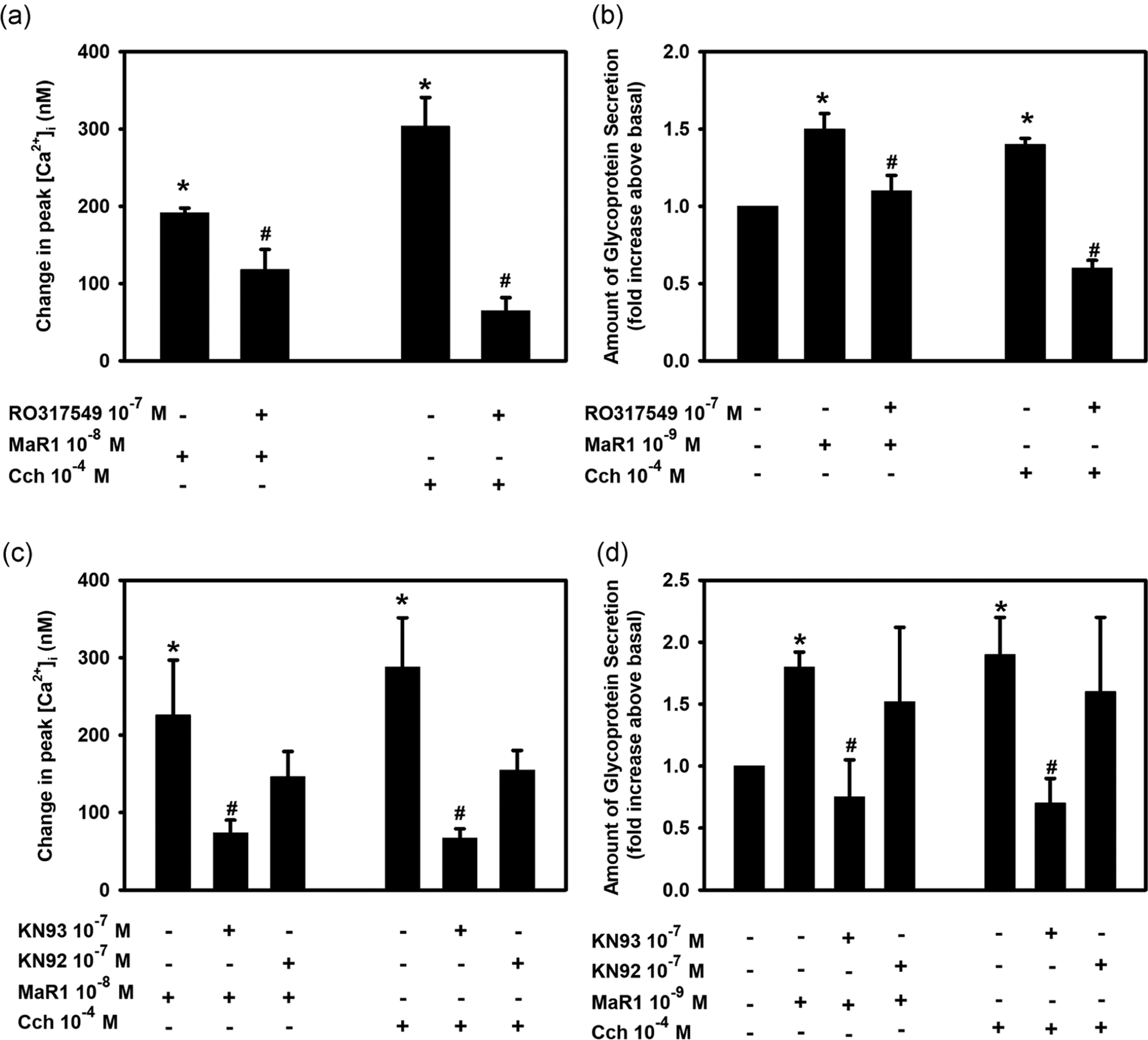
Inhibition of protein kinase C (PKC) or calcium calmodulin-dependent kinase II (CaMK) blocks maresin 1 (MaR1)-stimulated increase in [Ca2+]i and high molecular weight glycoprotein secretion. Rat conjunctival goblet cells were incubated with the PKC inhibitor RO-317549 (10−7 M) or the CaMKII inhibitor KN93 (10−7 M) and its inactive control KN92 (10−7 M) for 30 min, then stimulated with MaR1 at 10−8 M for [Ca2+]i or 10−9 M for secretion or carbachol (Cch, 10−4 M). The change in peak [Ca2+]i (a and c) and secretion (b and d) are shown. Data are mean ± SEM from four (a), three (b), five (c), and three (d) experiments. *Significance above basal. #Significance from maresin 1 alone. [Ca2+]i, intracellular Ca2+; SEM, standard error of the mean
The effect of RO-317549 on MaR1-stimulated increase in glycoconjugate secretion was investigated. MaR1 increased secretion 1.5 ± 0.1 fold above basal (Figure 5b; n = 3), and was significantly blocked by RO-317549 to 1.1 ± 0.1 fold above basal. Cch-stimulated increase in secretion was also significantly blocked by RO-317549 (Figure 5b). These results demonstrate that MaR1 activates PKC as a component of the PLC signaling pathway to increase [Ca2+]i and stimulate secretion.
3.8 |. MaR1 utilizes calcium/calmodulin-dependent protein kinase II to increase [Ca2+]i and stimulate secretion in cultured rat goblet cells
In addition to PLC, other SPMs such as LXA4 and RVD1 use calcium/calmodulin-dependent protein kinase II (CaMKII) to increase [Ca2+]i (Hodges et al., 2017; Lippestad et al., 2017). To investigate if MaR1 also uses CaMKII to increase [Ca2+]i, we used the CaMKII inhibitor KN93 and its inactive control KN92. MaR1 (10−8 M) significantly increased [Ca2+]i by 219.2 ± 58.3 nM (Figure 5c; n = 5). Incubation with KN93 (10−7 M) before addition of MaR1 (10−8 M) caused a significantly inhibited response of 85.3 ± 17.9 nM. Preincubation with the inactive compound KN92 (10−7 M) caused a MaR1-stimulated response of 137.2 ± 28.2 nM (Figure 5c), which is not a significant decrease. These data indicate that MaR1 uses CamKII to increase [Ca2+]i. Cch was again used as a positive control, and Cch significantly stimulated [Ca2+]i (Figure 5c). The Cch-stimulated response was significantly blocked by KN93, but not KN92. Similar to the increase in [Ca2+]i, MaR1-stimulated secretion was inhibited by KN93, but not KN92 (Figure 5d; n = 3). MaR1 alone significantly stimulated secretion 1.8 ± 0.1 fold above basal, and was completely blocked to 0.8 ± 0.3 fold by KN93, but not KN92. Cch-stimulated increase in secretion was also blocked by KN93, but not KN92. These data indicate that MaR1 activates CaMKII to increase [Ca2+]i and stimulate secretion.
3.9 |. MaR1 does not activate phospholipase D nor phospholipase A2 to increase [Ca2+]i in rat conjunctival goblet cells but uses phospholipase D to stimulate HMWG secretion
In addition to PLC, other phospholipases, namely phospholipase D (PLD) and phospholipase A2 (PLA2) can be activated by SPMs (Berridge, 2009; Li et al., 2013). To explore if MaR1 uses PLD to increase [Ca2+]i, cells were incubated with the PLD inhibitor 1-but (0.3%) and the inactive control t-but (0.3%). The cells were incubated with the active or the inactive compound for 15 min before MaR1 stimulation. MaR1 (10−8 M) alone increased [Ca2+]i by 247.0 ± 82.2 nM (Figure 6a; n = 4). Incubation with 1-but did not have a significant effect on MaR1-stimulated increase in [Ca2+]i and was 159.2 ± 45.3 nM. Similarly, treatment with the inactive control t-but did not significantly alter the MaR1 response and was 122.3 ± 41.1 nM. In contrast, the increase in [Ca2+]i stimulated by the control agonist Cch was significantly decreased by 1-but, but not t-but.
FIGURE 6.
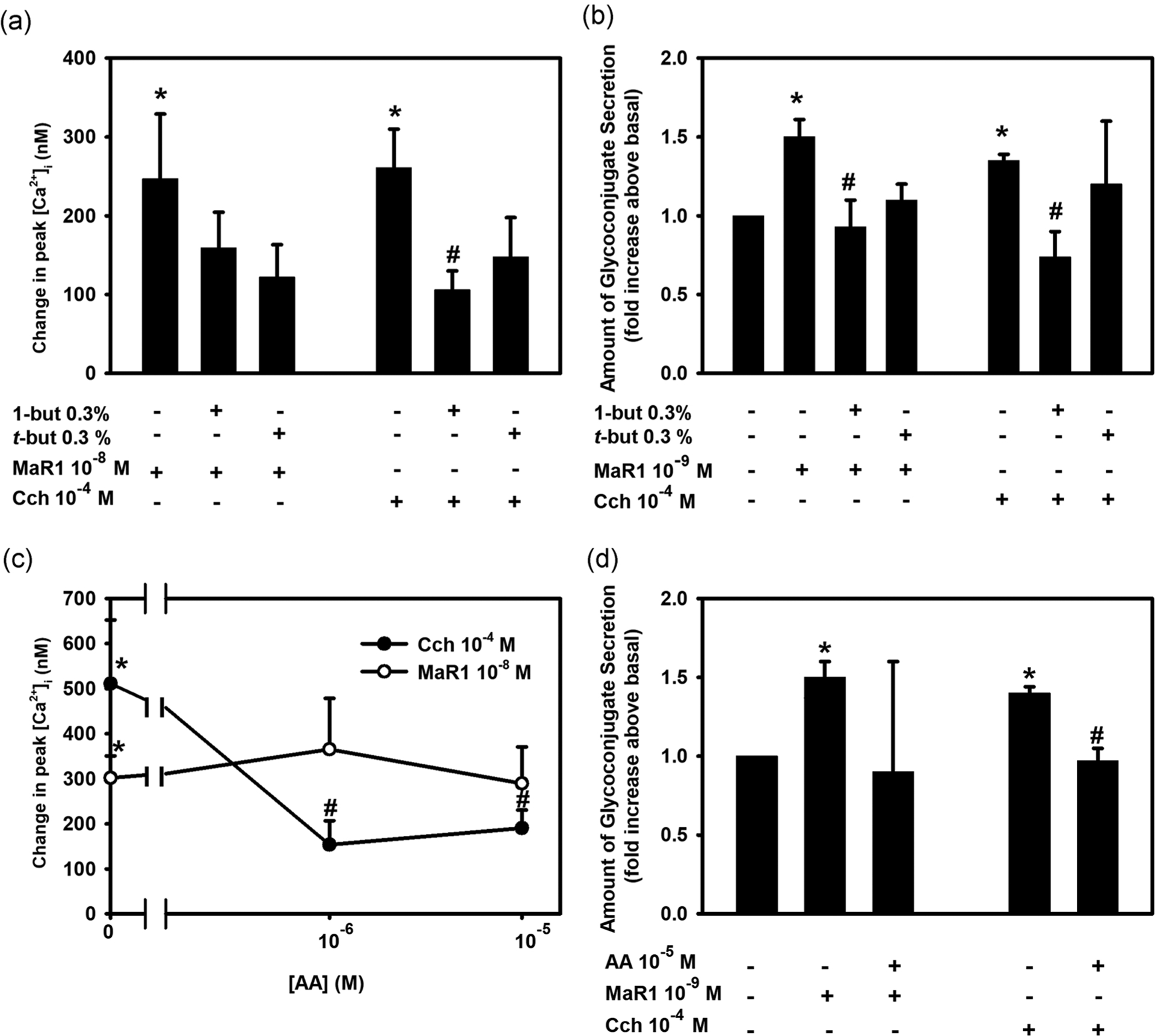
Effect of Inhibition of phospholipase D (PLD) and phospholipase A2 (PLA2) on maresin 1 (MaR1)-stimulated increase in [Ca2+]i and high molecular weight glycoprotein secretion. Rat conjunctival goblet cells were preincubated with the PLD inhibitor 0.3% 1-butanol or the inactive control 0.3% t-butanol for 15 min and stimulated with MaR1 at 10−8 M for [Ca2+]i or 10−9 M for secretion or carbachol (Cch, 10−4 M). Changes in peak [Ca2+]i are shown in (a) and secretion shown in (b). Goblet cells were incubated with the PLA2 inhibitor aristolochic acid (AA; 10−6–10−5 M) for 30 min and stimulated with MaR1 at 10−8 M for [Ca2+]i or 10−9 M for secretion or Cch (10−4 M). Data show changes in peak [Ca2+]i (c) while secretion is shown in (d). Data are mean ± SEM of four (a), three (b), eight (c), and three (d) experiments. *Significance from basal. #Significance from MaR1 alone. [Ca2+]i, intracellular Ca2+; SEM, standard error of the mean
To explore the role of PLD in glycoconjugate secretion, goblet cells were incubated with 1-but or t-but before stimulation with MaR1. MaR1 increased secretion 1.5 ± 0.1 fold above basal (Figure 6b; n = 3). 1-But, but not t-but, significantly inhibited MaR1-stimulated secretion. This is similar to the response obtained with the positive control, Cch. These data indicate that MaR1 does not utilize PLD to increase [Ca2+]i but does use it to stimulate secretion.
PLA2 plays an important role in phospholipid metabolism to activate the inflammatory process (Dennis, 1994). To determine if MaR1 is dependent on PLA2 to increase [Ca2+]i, we used the PLA2 inhibitor AA. Rat conjunctival goblet cells were incubated for 30 min with AA, then stimulated with MaR1. MaR1 (10−8 M) increased [Ca2+]i to 301.80 ± 48.92 nM (Figure 6c; n = 8). When treated with AA at 10−6 and 10−5 M, MaR1-stimulated response was unaltered at 365.33 ± 112.77 and 289.42 ± 81.31 nM. In contrast, the increase in [Ca2+]i stimulated by Cch, used as a control, was significantly decreased by AA at both concentrations.
Similar to MaR1-stimulated increase in [Ca2+]i, MaR1-stimulated increase in glycoconjugate secretion was not altered by incubation with AA. MaR1 alone increased secretion 1.5 ± 0.1 fold above basal (Figure 6d; n = 3). This secretion was unchanged after incubation with AA. The Cch-stimulated increase in secretion was significantly decreased in the presence of AA. These results indicate that MaR1 does not activate PLA2 to increase [Ca2+]]i or stimulate HMWG secretion.
3.10 |. MaR1 activates extracellular-regulated protein kinase 1/2 to stimulate an increase in [Ca2+]i and HMWG secretion
To determine if MaR1 uses extracellular-regulated protein kinase 1/2 (ERK 1/2) to increase [Ca2+]i and stimulate secretion, rat conjunctival goblet cells were incubated with the ERK-inhibitor UO126 (10−8–10−6 M) for 30 min. MaR1 (10−8 M) increased [Ca2+]i with a peak increase of 171.1 ± 27.6 nM (Figure 7a,b; n = 3). UO126 significantly decreased MaR1-stimulated increase in [Ca2+]i to 71.0 ± 22.3, 73.5 ± 3.0, and 82.1 ± 20.5 nM at 10−8, 10−7, and 10−6 M, respectively. Cch (10−4 M), the positive control, increased [Ca2+]i to 221.1 ± 47.2 nM. Cch-stimulated response was significantly blocked by UO126 at 10−6 M and was 62.8 ± 19.4 nM. U0126 (10−6 M) also significantly decreased MaR1-stimulated HMWG secretion from 2.2 ± 0.2 to 1.2 ± 0.2 fold above basal (Figure 7c; n = 3). Similar results were obtained with the positive control Cch. These results indicate that MaR1 activates ERK1/2 to increase both [Ca2+]i and HMWG secretion.
FIGURE 7.
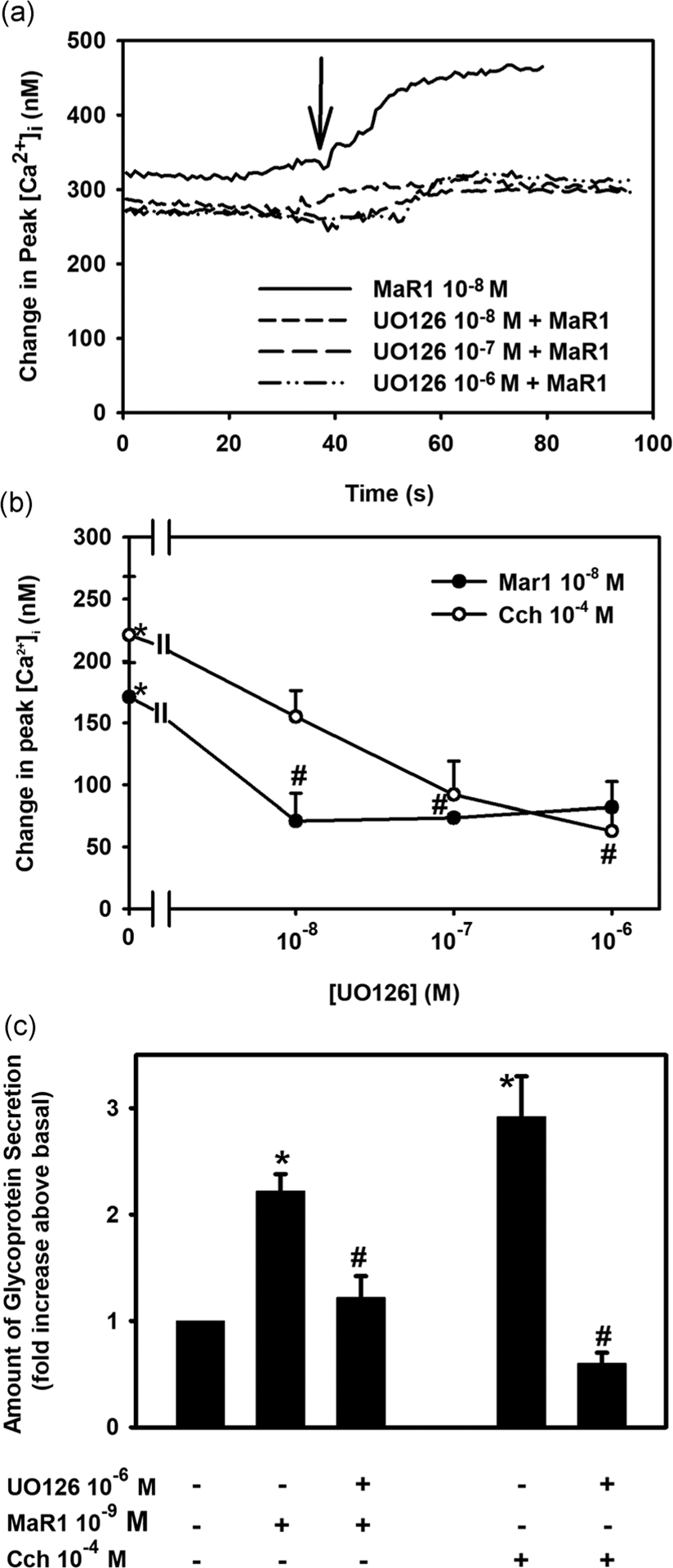
Inhibition of extracellular-regulated kinase 1/2 (ERK 1/2) blocks maresin 1 (MaR1)-stimulated increase in [Ca2+]i and high molecular weight glycoprotein secretion. Goblet cells were preincubated with the ERK 1/2 inhibitor UO126 (10−8–10−6 M) for 30 min and stimulated with MaR1 at 10−8 M for [Ca2+]i or 10−9 M for secretion or carbachol (Cch, 10−5 M). Changes in [Ca2+]i over time are shown in (a). Changes in peak [Ca2+]i are shown in (b). Secretion is shown in (c). Data are mean ± SEM of three experiments. *Significance above basal. #Significance from MaR1 or Cch. Arrow indicates addition of MaR1. [Ca2+]i, intracellular Ca2+; SEM, standard error of the mean
3.11 |. MaR1 does not use cAMP-dependent protein kinase A to increase [Ca2+]i or stimulate HMWG secretion
The classical cAMP pathway involves the activation of Gαs, which activates adenylyl cyclase to catalyze ATP to cAMP. cAMP activates protein kinase A (PKA). To determine if MaR1 uses PKA, rat conjunctival goblet cells were incubated with the PKA inhibitor H89 for 30 min before stimulation with MaR1. MaR1 (10−8 M) caused an increase in [Ca2+]i with a peak of 132.5 ± 20.6 nM (Figures 8a and 8c; n = 4). MaR1 (10−8 M) incubated with H89 caused a peak in [Ca2+]i of 122.4 ± 45.0, 151.9 ± 29.9, and 173.83 ± 50.5 nM at H89 10−7, 10−6, and 10−5 M, respectively. None of these values was altered from the response to MaR1 alone. VIP (10−8 M) was used as the positive control. VIP increased [Ca2+]i that was significantly decreased by H89 at 10−5 M. These findings suggest that MaR1 does not activate cAMP-dependent PKA to increase [Ca2+]i.
FIGURE 8.
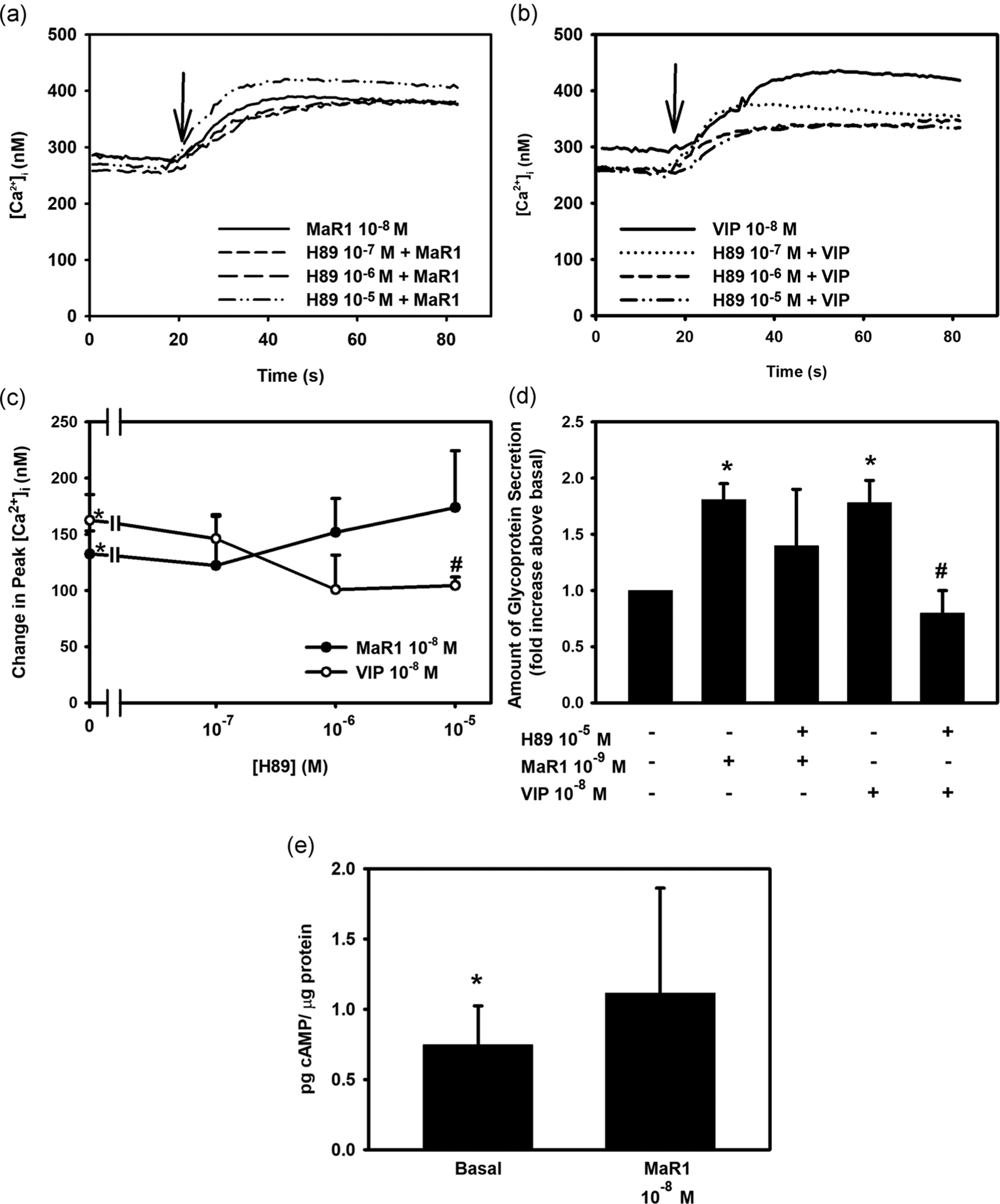
Inhibition of the cAMP-dependent protein kinase A (PKA) does not block maresin 1 (MaR1)-stimulated increase in [Ca2+]i, high molecular weight glycoprotein secretion, and MaR1 does not increase (cAMP). Goblet cells were preincubated with the cAMP-dependent PKA inhibitor H89 at 10−7–10−5 M concentrations for 30 min and stimulated with MaR1 at 10−8 M for [Ca2+]i and cAMP levels or 10−9 M for secretion or VIP (10−8 M). Changes in [Ca2+]i over time is shown in (a) and (b), while changes in peak [Ca2+]i are shown in (c). Secretion is shown in (d). The amount of cAMP in goblet cells is shown in (e). Data are mean ± SEM of four (a–c), seven (d), and five (e) experiments. *Significance above basal. #Significance from MaR1 or VIP alone. Arrow indicates addition of MaR1 or VIP. cAMP, cyclic adenosine monophosphate; [Ca2+]i, intracellular Ca2+; SEM, standard error of the mean
To examine the dependency of MaR1 on PKA to stimulate secretion, rat conjunctival goblet cells were treated with H89 (10−5 M) for 30 min before addition of MaR1. MaR1 increased secretion by 1.8 ± 0.14 fold above basal (Figure 8d; n = 7). Incubation with H89 did not significantly block MaR1-stimulated secretion and was 1.4 ± 0.5 fold above basal. VIP was used as a positive control and VIP-stimulated secretion was blocked by H89.
To confirm these results, the effect of MaR1 on cAMP levels on rat conjunctival goblet cells was determined. The basal level of cAMP was 0.75 ± 0.28 pg cAMP/μg protein (Figure 8e; n = 5). MaR1 caused an increase to 1.11 ± 0.20 pg cAMP/μg protein, which was not significantly different from basal. VIP was used as a positive control. VIP caused an increase to 2.20 ± 0.45 pg cAMP/μg protein, significantly different from basal (data not shown). This suggests that MaR1, in contrast to VIP, does not alter the level of cAMP and does not activate PKA to increase [Ca2+]i or stimulate secretion in conjunctival goblet cells.
3.12 |. Increase in [Ca2+]i and the stimulation of HMWG secretion by histamine is counterregulated by MaR1 in rat conjunctival goblet cells
Histamine is a major mediator contributing to the symptoms of allergic conjunctivitis (del Cuvillo et al., 2009). To examine the effect of MaR1 on histamine stimulation in rat conjunctival goblet cells, the cells were treated with MaR1 (10−8 M) for 30 min before stimulation with histamine (10−5 M). Histamine caused an increase in [Ca2+]i with a peak of 265.0 ± 45.6 nM (Figure 9a,b; n = 8). When preincubated with MaR1 (10−8 M), the peak in [Ca2+]i was significantly reduced to 84.0 ± 18.8 nM.
FIGURE 9.
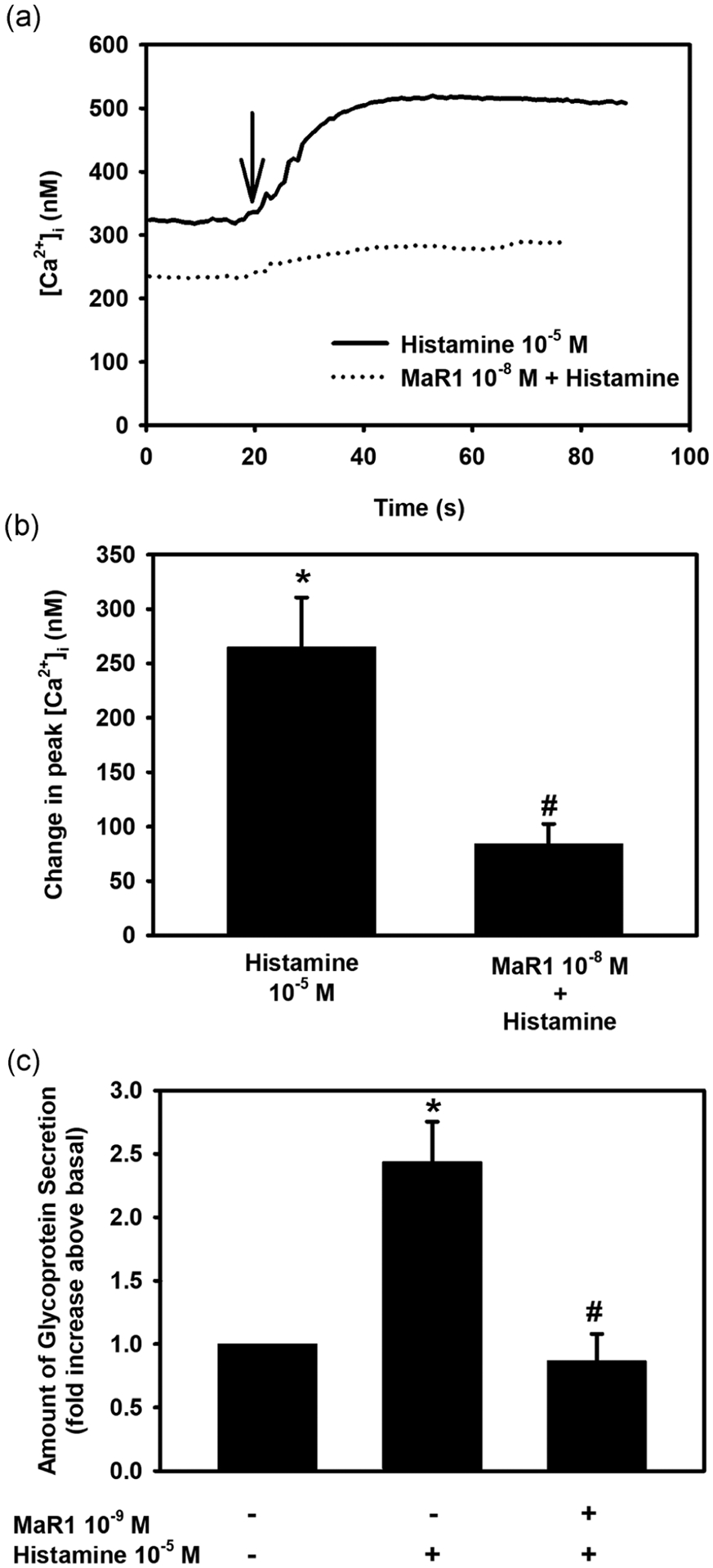
Maresin 1 (Mar1) counterregulates histamine-stimulated increase in [Ca2+]i and high molecular weight glycoprotein secretion. Rat conjunctival goblet cells were preincubated with MaR1 at 10−8 M for [Ca2+]i or 10−9 M for secretion for 30 min, then stimulated with histamine (10−5 M). Changes in [Ca2+]i over time are shown in (a) and changes in peak [Ca2+]i are shown in (b). Secretion is shown in (c). Data are mean ± SEM of eight (a and b) and four (c) experiments. *Significance from basal. #Significance between histamine alone. [Ca2+]i, intracellular Ca2+; SEM, standard error of the mean
To determine the effect of MaR1 on histamine-stimulated secretion, rat conjunctival goblet cells were incubated with MaR1 (10−9 M) for 30 min before stimulation with histamine (10−5 M). Histamine caused a secretory response above basal of 2.4 ± 0.32 (Figure 9c; n = 4). When incubated with MaR1 (10−9 M), the histamine-induced secretory response was decreased to 0.87 ± 0.21. Thus, MaR1 counterregulates the stimulatory effect of histamine on [Ca2+]i and secretion.
4 |. DISCUSSION
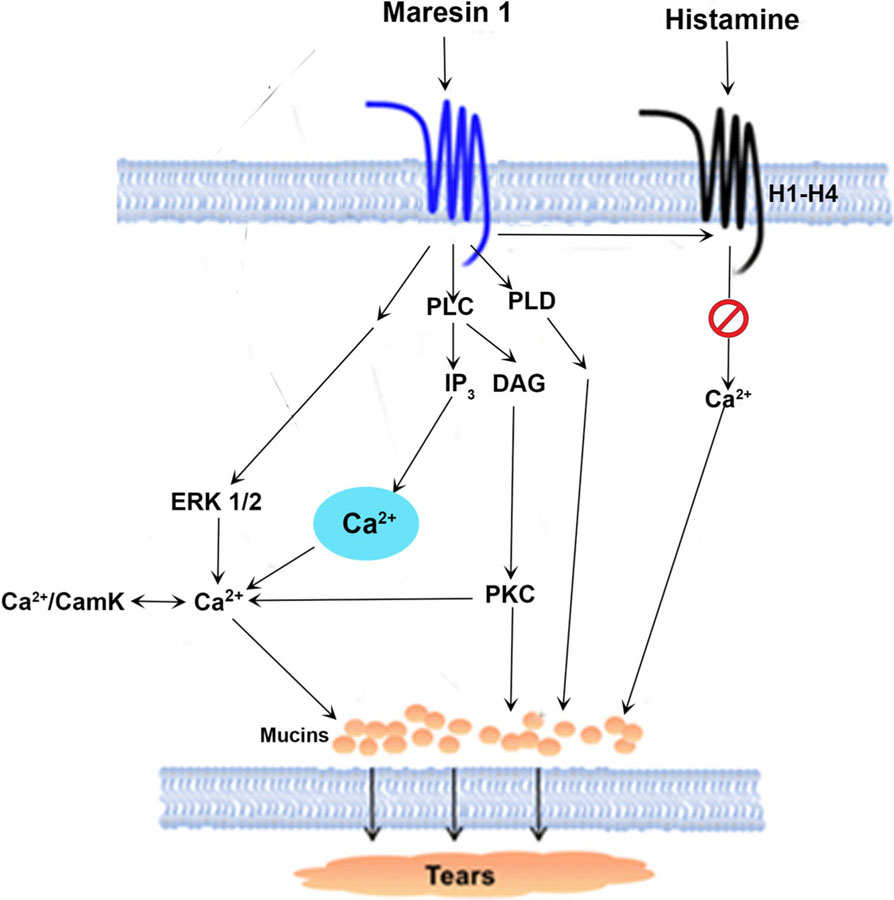
In this study, we found that in rat conjunctival goblet cells MaR1 increases [Ca2+]i and stimulates secretion. The increase in [Ca2+]i and secretion occurs through the induction of PLC and its downstream effectors, namely IP3, PKC, ERK 1/2, and Ca2+/CaMK (Figure 10). MaR1 does not utilize PLA2 to stimulate an increase [Ca2+]i or secretion. Interestingly stimulation of PLD activity by MaR1, leads to an increase in secretion only. MaR1 neither increases cAMP nor activates PKA to stimulate [Ca2+]i or secretion. Similar to other SPMs such as LXA4, RvD1, and RvE1, MaR1 regulates [Ca2+]i and stimulates secretion in conjunctival goblet cells which likely maintain optimal mucin layer on the ocular surface under physiological conditions (Hodges et al., 2016; Lippestad et al., 2017, 2018).
FIGURE 10.
Schematic diagram of signaling pathways activated by maresin 1 (MaR1). MaR1 activates the pathways of phospholipase (PL)C and D. PLC generates diacylglycerol (DAG) and inositol trisphosphate (IP3). Ca2+/CamKII, calcium/calmodulin-dependent protein kinase II; ERK 1/2, extracellular-regulated protein kinase 1/2; PKC, protein kinase C
MaR1 stimulates conjunctival goblet cells similarly to LXA4 and RvD1; however, the pathways used by the three SPMs differ. Similar to MaR1, both LXA4 and RvD1 use the PLC pathway including using Ca2+/CaMKII to increase [Ca2+]i and stimulate secretion. In contrast to MaR1, LXA4 and RvD1 also use PLD and PLA2 to increase [Ca2+]i and stimulate secretion (Hodges et al., 2017; Lippestad et al., 2017). Thus, the signaling pathways activated appear to be SPM-specific.
MaR1 uses the PLD pathway to stimulate glycoconjugate secretion, but not to increase [Ca2+]i. The signaling pathways activated by PLD are complex and can vary between agonists and cell type. There are two types of mammalian PLD, PLD1 and PLD2 (Gomez-Cambronero, 2014) and can interact with many other proteins. For example, PKC enhances the lipase activity of PLD1 and while PLD2 and PLCγ directly interact in an epidermal growth factor (EGF)-dependent manner. This interaction also links PLD to the mitogen-activated protein kinase and Ras/ERK pathways. In conjunctival goblet cells, MaR1 could activate PLD by this EGF-dependent pathway as MaR1 activates PKC and ERK1/2 to stimulate secretion.
MaR1 is mainly dependent on the activation of the PLC pathway to increase [Ca2+]i and stimulates secretion. All steps of the PLC pathway for which inhibitors were used in the present study were blocked indicated by a decrease in [Ca2+]i, and in secretion. These steps included the activation of PLC, interaction of IP3 with its receptor on intracellular Ca2+ stores, release of intracellular Ca2+ stores, and activation of PKC. Furthermore, activation of PLC is a widely used mechanism for stimulating secretion by exocytosis.
MaR1 does not activate PKA to stimulate secretion. Herein, we used the PKA inhibitor H89 that did not alter MaR1-stimulated increase in [Ca2+]i and secretion. In addition, we measured cAMP levels that were not increased by MaR1. In contrast, Chiang et al. (2019) demonstrated that MaR1 activates the human LGR6 and the activation of this receptor-stimulated ERK1/2 and cAMP activity. In a work by Chiang et al. (2019), the actions of MaR1 were tested on macrophages, neutrophils (PMNs), fibroblasts, and in two epithelial cell lines CHO and HEK cells. In accordance with Chiang et al. (2019), MaR1 activated ERK 1/2 in the present study with rat conjunctival goblet cells. In contrast, MaR1 increased [Ca2+]i, but did not alter cAMP levels nor activate PKA. Possible reasons for these differences between the two studies are (a) primary epithelial cells in culture have different responses and intracellular signaling than immune cells or cell lines, for example, cell-type specificity. (b) Rat LGR6 is coupled to a different G protein than human LGR6, or. (c) In rat conjunctival goblet cells, LGR6 is coupled to a different G protein than in other cell types. In future studies, we plan to determine if in rat conjunctival goblet cells, the LGR6 receptor is present, and if MaR1 also acts via this receptor to increase [Ca2+]i and stimulate secretion.
SPMs from both ω−3 fatty acids and the ω−6 fatty acid arachidonic acid each increase [Ca2+]i and stimulate secretion from rat conjunctival goblet cells in the absence of disease. Thus RvD1, RvD2, and MaR1 derived from the ω−3 fatty acid DHA; RvE1 derived from precursor eicosapentaenoic acid (EPA); and LXA4 biosynthesized from arachidonic acid each interact with their receptors, activate specific signaling pathways, increase [Ca2+]i, and stimulate secretion (Botten et al., 2019; Hodges et al., 2017; Lippestad et al., 2017, 2018). The diversity of the origin of different SPMs supports the hypothesis that if precursors of one of the biosynthetic pathways is depleted, others can potentially take over to maintain homeostasis in conjunctival tissue. Thus, MaR1 is similar to other SPMs in that it has a direct action on goblet cells alone in the absence of disease and can play a functional role in ocular surface homeostasis by regulating the mucous layer of the tear film.
The ω−6 fatty acid arachidonic acid and the ω−3 fatty acids DHA and EPA, which are the precursors of the SPMs, are detectable in human tears (Walter et al., 2016). The finding of precursors of SPMs, including precursors of MaR1, in human tears, supports that MaR1 plays a role in maintaining tissue homeostasis and stabilizing the tear film.
In addition to the actions of MaR1 alone, MaR1 also has an effect in disease conditions. When an allergic type 1 hypersensitivity reaction is initiated, a complex cascade is activated, with both cells and mediators involved. Histamine is a major mediator contributing to the symptoms of allergic conjunctivitis (del Cuvillo et al., 2009). Histamine is released from mast cells and basophils to cause an inflammatory response with increased vascular permeability and vasodilation (Thurmond, Gelfand, & Dunford, 2008). In conjunctival goblet cells histamine binds to its receptors (H1–H4) to increase [Ca2+]i, mainly through the PLC pathway (Li et al., 2012). Histamine also stimulates secretion (Hayashi et al., 2012). We previously showed that RvD1 and LXA4 counterregulate the histamine receptors, thereby blocking histamine-stimulated increase in [Ca2+]i and secretion (Hodges et al., 2016; Li et al., 2013). When rat conjunctival goblet cells are incubated with MaR1 before histamine stimulation, the histamine-stimulated increase in [Ca2+]i and secretion are decreased. This suggests that MaR1 could be a candidate as novel treatment of allergic conjunctivitis.
Based on our findings, we conclude that MaR1 likely has two main functions; one in health and one in disease. First, during physiological conditions, MaR1 likely contributes to maintaining tissue homeostasis by activating the PLC pathway to increase [Ca2+]i, thereby regulating the amount of secretion from goblet cells. Second, during an ocular surface inflammatory disease, MaR1 likely stimulates the resolution of inflammation by inhibiting the overproduction of mucin by attenuating histamine-stimulated mucin secretion. Thus, based on these findings, MaR1 or its mimetic analogs could be a novel treatment for different inflammatory diseases spanning from allergic conjunctivitis to dry eye disease.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Marie Shatos and Dayu Li for their helpful assistance. This study was supported by the Norwegian Research Council to Markus V. Olsen and by the National Institute of Health R01 EY019470 to Darlene A. Dartt and R01GM038765 to Charles N. Serhan.
Funding information
Norwegian Research Council; National Eye Institute, Grant/Award Number: R01 EY019470; National Institute of General Medical Sciences, Grant/Award Number: R01GM038765
Footnotes
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- Barabino S, Labetoulle M, Rolando M, & Messmer EM (2016). Understanding symptoms and quality of life in patients with dry eye syndrome. The Ocular Surface, 14, 365–376. 10.1016/j.jtos.2016.04.005 [DOI] [PubMed] [Google Scholar]
- Berridge MJ (2009). Inositol trisphosphate and calcium signalling mechanisms. Biochimica et Biophysica Acta, 1793, 933–940. 10.1016/j.bbamcr.2008.10.005 [DOI] [PubMed] [Google Scholar]
- Botten N, Hodges RR, Li D, Bair JA, Shatos MA, Utheim TP, …Dartt DA (2019). Resolvin D2 elevates cAMP to increase intracellular [Ca(2+)] and stimulate secretion from conjunctival goblet cells. FASEB Journal, 33, 8468–8478. 10.1096/fj.201802467R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Libreros S, Norris PC, de la Rosa X, & Serhan CN (2019). Maresin 1 activates LGR6 receptor promoting phagocyte immunoresolvent functions. Journal of Clinical Investigation, 129, 5294–5311. 10.1172/JCI129448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colas RA, Dalli J, Chiang N, Vlasakov I, Sanger JM, Riley IR, & Serhan CN (2016). Identification and actions of the maresin 1 metabolome in infectious inflammation. Journal of Immunology, 197, 4444–4452. 10.4049/jimmunol.1600837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Cuvillo A, Sastre J, Montoro J, Jauregui I, Davila I, Ferrer M,…Valero A (2009). Allergic conjunctivitis and H1 antihistamines. Journal of Investigational Allergology and Clinical Immunology, 19(Suppl. 1), 11–18. [PubMed] [Google Scholar]
- Dalli J, & Serhan C (2016). Macrophage proresolving mediators-the When and Where. Microbiology Spectrum, 4, 367–383. 10.1128/microbiolspec.MCHD-0001-2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartt DA (2004). Control of mucin production by ocular surface epithelial cells. Experimental Eye Research, 78, 173–185. [DOI] [PubMed] [Google Scholar]
- Dartt DA, & Masli S (2014). Conjunctival epithelial and goblet cell function in chronic inflammation and ocular allergic inflammation. Current Opinion in Allergy and Clinical Immunology, 14, 464–470. 10.1097/ACI.0000000000000098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartt DA, Rios JD, Kanno H, Rawe IM, Zieske JD, Ralda N, …Zoukhri D (2000). Regulation of conjunctival goblet cell secretion by Ca(2+)and protein kinase C. Experimental Eye Research, 71, 619–628. 10.1006/exer.2000.0915 [DOI] [PubMed] [Google Scholar]
- Dennis EA (1994). Diversity of group types, regulation, and function of phospholipase A2. Journal of Biological Chemistry, 269, 13057–13060. [PubMed] [Google Scholar]
- Gomez-Cambronero J (2014). Phospholipase D in cell signaling: From a myriad of cell functions to cancer growth and metastasis. Journal of Biological Chemistry, 289, 22557–22566. 10.1074/jbc.R114.574152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Luo L, Wang Q, Yan S, Lin J, Li D, … Jin SW (2018). Maresin 1 attenuates mitochondrial dysfunction through the ALX/cAMP/ROS pathway in the cecal ligation and puncture mouse model and sepsis patients. Laboratory Investigation, 98, 715–733. 10.1038/s41374-018-0031-x [DOI] [PubMed] [Google Scholar]
- Hayashi D, Li D, Hayashi C, Shatos M, Hodges RR, & Dartt DA (2012). Role of histamine and its receptor subtypes in stimulation of conjunctival goblet cell secretion. Investigative Ophthalmology and Visual Science, 53, 2993–3003. 10.1167/iovs.11-8748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges RR, & Dartt DA (2013). Tear film mucins: Front line defenders of the ocular surface; comparison with airway and gastrointestinal tract mucins. Experimental Eye Research, 117, 62–78. 10.1016/j.exer.2013.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges RR, Li D, Shatos MA, Bair JA, Lippestad M, Serhan CN, & Dartt DA (2017). Lipoxin A4 activates ALX/FPR2 receptor to regulate conjunctival goblet cell secretion. Mucosal Immunology, 10, 46–57. 10.1038/mi.2016.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges RR, Li D, Shatos MA, Serhan CN, & Dartt DA (2016). Lipoxin A4 counterregulates histamine-stimulated glycoconjugate secretion in conjunctival goblet cells. Scientific Reports, 6, 36124 10.1038/srep36124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokin LE (1966). Effects of calcium omission on acetylcholine-stimulated amylase secretion and phospholipid synthesis in pigeon pancreas slices. Biochimica et Biophysica Acta, 115, 219–221. [DOI] [PubMed] [Google Scholar]
- Li D, Carozza RB, Shatos MA, Hodges RR, & Dartt DA (2012). Effect of histamine on Ca(2+)-dependent signaling pathways in rat conjunctival goblet cells. Investigative Ophthalmology and Visual Science, 53, 6928–6938. 10.1167/iovs.12-10163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Hodges RR, Jiao J, Carozza RB, Shatos MA, Chiang N, …Dartt DA (2013). Resolvin D1 and aspirin-triggered resolvin D1 regulate histamine-stimulated conjunctival goblet cell secretion. Mucosal Immunology, 6, 1119–1130. 10.1038/mi.2013.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Jiao J, Shatos MA, Hodges RR, & Dartt DA (2013). Effect of VIP on intracellular [Ca2+], extracellular regulated kinase 1/2, and secretion in cultured rat conjunctival goblet cells. Investigative Ophthalmology and Visual Science, 54, 2872–2884. 10.1167/iovs.12-11264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippestad M, Hodges RR, Utheim TP, Serhan CN, & Dartt DA (2017). Resolvin D1 increases mucin secretion in cultured rat conjunctival goblet cells via multiple signaling pathways. Investigative Ophthalmology and Visual Science, 58, 4530–4544. 10.1167/iovs.17-21914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippestad M, Hodges RR, Utheim TP, Serhan CN, & Dartt DA (2018). Signaling pathways activated by resolvin E1 to stimulate mucin secretion and increase intracellular Ca(2+) in cultured rat conjunctival goblet cells. Experimental Eye Research, 173, 64–72. 10.1016/j.exer.2018.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantelli F, & Argueso P (2008). Functions of ocular surface mucins in health and disease. Current Opinion in Allergy and Clinical Immunology, 8, 477–483. 10.1097/ACI.0b013e32830e6b04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell RH (1975). Inositol phospholipids and cell surface receptor function. Biochimica et Biophysica Acta, 415, 81–47. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y (1984). The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature, 308, 693–698. [DOI] [PubMed] [Google Scholar]
- Palmares J, Delgado L, Cidade M, Quadrado MJ, Filipe HP, & Season Study Group (2010). Allergic conjunctivitis: A national cross-sectional study of clinical characteristics and quality of life. European Journal of Ophthalmology, 20, 257–264. [DOI] [PubMed] [Google Scholar]
- Rios JD, Zoukhri D, Rawe IM, Hodges RR, Zieske JD, & Dartt DA (1999). Immunolocalization of muscarinic and VIP receptor subtypes and their role in stimulating goblet cell secretion. Investigative Ophthalmology and Visual Science, 40, 1102–1111. [PubMed] [Google Scholar]
- Saban DR, Hodges RR, Mathew R, Reyes NJ, Yu C, Kaye R, … Dartt DA (2019). Resolvin D1 treatment on goblet cell mucin and immune responses in the chronic allergic eye disease (AED) model. Mucosal Immunology, 12, 145–153. 10.1038/s41385-018-0089-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Dalli J, Colas RA, Winkler JW, & Chiang N (2015). Protectins and maresins: New proresolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochimica et Biophysica Acta, 1851, 397–413. 10.1016/j.bbalip.2014.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ, … Petasis NA (2012). Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB Journal, 26, 1755–1765. 10.1096/fj.11-201442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, & Petasis NA (2011). Resolvins and protectins in inflammation resolution. Chemical Reviews, 111, 5922–5943. 10.1021/cr100396c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, & Savill J (2005). Resolution of inflammation: The beginning programs the end. Nature Immunology, 6, 1191–1197. 10.1038/ni1276 [DOI] [PubMed] [Google Scholar]
- Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, … Spite M (2009). Maresins: Novel macrophage mediators with potent antiinflammatory and proresolving actions. Journal of Experimental Medicine, 206, 15–23. 10.1084/jem.20081880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurmond RL, Gelfand EW, & Dunford PJ (2008). The role of histamine H1 and H4 receptors in allergic inflammation: The search for new antihistamines. Nature Reviews. Drug Discovery, 7, 41–53. 10.1038/nrd2465 [DOI] [PubMed] [Google Scholar]
- Uchino M, & Schaumberg DA (2013). Dry eye disease: Impact on quality of life and vision. Current Ophthalmology Reports, 1, 51–57. 10.1007/s40135-013-0009-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter SD, Gronert K, McClellan AL, Levitt RC, Sarantopoulos KD, & Galor A (2016). Omega-3 tear film lipids correlate with clinical measures of dry eye. Investigative Ophthalmology and Visual Science, 57, 2472–2478. 10.1167/iovs.16-19131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson JR (1986). Role of inositol lipid breakdown in the generation of intracellular signals. State of the art lecture. Hypertension, 8, II140–II156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.