Abstract
Background
Pneumonia is the leading infection-related cause of death. The use of simple clinical criteria and contemporary epidemiology to identify patients at high risk of nosocomial pneumonia should enhance prevention efforts and facilitate development of new treatments in clinical trials.
Research Question
What are the clinical criteria and contemporary epidemiology trends that are helpful in the identification of patients at high risk of nosocomial pneumonia?
Study Design and Methods
Within the ICUs of 28 US hospitals, we conducted a prospective cohort study among adults who had been hospitalized >48 hours and were considered high risk for pneumonia (defined as treatment with invasive or noninvasive ventilatory support or high levels of supplemental oxygen). We estimated the proportion of high-risk patients who experienced the development of nosocomial pneumonia. Using multivariable logistic regression, we identified patient characteristics and treatment exposures that are associated with increased risk of pneumonia development during the ICU admission.
Results
Between February 6, 2016, and October 7, 2016, 4,613 high-risk patients were enrolled. Among 1,464 high-risk patients (32%) who were treated for possible nosocomial pneumonia, 537 (37%) met the study pneumonia definition. Among high-risk patients, a multivariable logistic model was developed to identify key patient characteristics and treatment exposures that are associated with increased risk of nosocomial pneumonia development (c-statistic, 0.709; 95% CI, 0.686-0.731). Key factors associated with increased odds of nosocomial pneumonia included an admission diagnosis of trauma or cerebrovascular accident, receipt of enteral nutrition, documented aspiration risk, and receipt of systemic antibacterials within the preceding 90 days.
Interpretation
Treatment for nosocomial pneumonia is common among patients in the ICU who are receiving high levels of respiratory support, yet more than one-half of patients who are treated do not fulfill standard diagnostic criteria for pneumonia. Application of simple clinical criteria may improve the feasibility of clinical trials of pneumonia prevention and treatment by facilitating prospective identification of patients at highest risk.
Key Words: ICU, nosocomial, pneumonia
Abbreviations: FDA, Food and Drug Administration; HABP, hospital-acquired bacterial pneumonia; VABP, ventilator-associated bacterial pneumonia
FOR EDITORIAL COMMENT, SEE PAGE 2245
Hospital-acquired bacterial pneumonia (HABP) and ventilator-associated bacterial pneumonia (VABP) are the most common nosocomial infections and the leading reasons for antibiotic prescriptions in the ICU.1,2 HABP/VABP development is associated with high mortality rates and substantial short- and long-term morbidity.3,4 Delayed effective antimicrobial therapy is associated with worse outcomes, so clinicians are compelled to treat promptly when HABP/VABP is suspected. Nevertheless, diagnosing HABP/VABP is inexact because diagnosis is based on a constellation of symptoms and clinical signs that are not sufficiently predictive of pneumonia.5, 6, 7 HABP/VABP management is further complicated by frequent infection with multidrug-resistant pathogens, by few available antibiotics with demonstrated efficacy in HABP/VABP treatment, and by a limited pipeline of new antibiotics that are undergoing evaluation in clinical trials.8,9
The low level of HABP/VABP antimicrobial development is a multifaceted problem that is driven, in part, by poor clinical trial feasibility, due to low enrollment.10, 11, 12 Poor enrollment itself is a complex issue in which the relative contributions of changing HABP/VABP prevalence and high screening failure rates are unknown. Estimates of HABP/VABP prevalence are highly variable because consensus definitions are lacking and because there is variability in interpretation of some criteria, such as the chest radiograph.13 Epidemiologic definitions of HABP/VABP likely underestimate the true frequency of antibiotic prescribing for suspected nosocomial pneumonia in modern clinical practice. Furthermore, historic estimates of HABP/VABP burden may not capture the impact of recent VABP prevention efforts and implementation of ventilator-associated event monitoring and reporting.14,15
Improved understanding of contemporary HABP/VABP incidence with a definition used in clinical trials may inform the design of more feasible trials. Evaluating a risk for HABP/VABP that is associated with patient characteristics and treatment exposures may help to identify those patients at highest risk for disease acquisition, ultimately promoting the study of new treatments and prevention efforts by facilitating the conduct of efficient clinical studies that are focused on the patients who are most likely to benefit, while decreasing harm in those less likely to benefit.16
With the use of a large multicenter cohort of prospectively identified patients and a standard definition of HABP/VABP outlined in United States Food and Drug Administration (FDA) draft guidance to industry,17 the Clinical Trials Transformation Initiative HABP/VABP studies team designed the Prospective Identification of Pneumonia in Hospitalized Patients in the ICU study that (1) defined the contemporary incidence of HABP/VABP among patients who are at high-risk for this infection and (2) identified demographic factors, comorbid conditions, and treatment exposures that are associated with the increased risk of HABP/VABP development during ICU admission.
Methods
Study Design
We conducted a multicenter, prospective, observational cohort study in ICUs of 28 United States hospitals. Enrolling sites comprised a diverse group of both community and tertiary academic medical centers with a median size of 727 inpatient beds (range, 252-1,394). All eligible adults who were admitted to participating ICUs were screened for the presence of predefined risk factors for HABP/VABP development (e-Appendix 1, e-Fig 1). Patients who were considered high risk for HABP/VABP development (defined as receiving invasive mechanical ventilation, noninvasive ventilation, or treatment with at least 50% fraction of inspired supplemental oxygen via high-flow, high-humidity nasal cannula, aerosol mask, partial or non-rebreather mask for a minimum of 12 hours within any 24-hour period in the preceding 7 days) were enrolled and prospectively followed for the development of signs or symptoms of possible pneumonia throughout their ICU course (e-Supplementary Methods).
Adults ≥18 years old who were admitted to participating ICUs were eligible for enrollment if hospitalized for >48 hours or within 7 days of discharge from acute or chronic care facilities. Patients were excluded if pregnant or currently breastfeeding, currently receiving treatment for lung cancer or metastatic cancers with lung involvement, currently receiving comfort measures only, or previously treated for suspected pneumonia while enrolled in the study. The study protocol was approved, and a waiver of informed consent was granted by Copernicus Group, an independent review board (CTTI_001, DCR2-15-710), or the institutional review board of the participating institution, when required.
Baseline demographics and treatment exposures were recorded for all patients at enrollment. High-risk patients were followed daily for the development of clinical signs or symptoms of possible pneumonia or receipt of antibiotics to treat possible pneumonia. Antibiotic exposures and results of clinically obtained microbiologic testing were recorded for all patients who received antibiotics for possible pneumonia.
Definitions
The high-risk population was defined as patients who were receiving high levels of respiratory support but were lacking study diagnostic criteria for pneumonia at the time of enrollment. The treated population was defined as the subset of high-risk patients who were receiving antibiotics for possible pneumonia, defined by documentation of antibiotic indications for pneumonia or undifferentiated sepsis for which pneumonia was considered a possible cause in the medical record, during their ICU course. The HABP/VABP population included only the subset of treated patients fulfilling the study HABP/VABP definition, which required at least one criterion to be present from each diagnostic domain including radiographic criteria, respiratory signs and symptoms, systemic inflammation, and timing of symptom onset. The study HABP/VABP definition was consistent with that used in treatment guidelines and developed from inclusion criteria in antibacterial drug treatment trials for HABP/VABP outlined in FDA draft guidance for industry (e-Supplementary Methods).3,17
Microbiologic Testing
Clinically obtained microbiologic testing results were recorded in the case report form. No specific microbiologic testing or procedures were mandated by the study protocol. For positive microbiologic results, the organism name and reported antibiotic susceptibilities were recorded. Extended spectrum beta-lactamase production was captured when identified by each site’s standard reporting protocol.
Outcomes
The primary outcome was the rate of study-defined HABP/VABP diagnosis in patients in the ICU who met the predetermined high-risk criteria. The key secondary outcome was determination of risk factors associated with HABP/VABP development in patients in the ICU who met prespecified high-risk criteria.
Statistical Analysis
All analyses were performed in predefined study populations. Patient characteristics were summarized as frequency and percentages for categoric variables and as medians with 25th and 75th percentiles for continuous variables. The cumulative percentage of patients who had experienced VABP or HABP before study completion (due for ICU discharge, transition to comfort measures, or death) was graphed as a function of time since high risk criteria were met. We performed risk modeling using multivariable logistic regression models and assessed relationships between 38 baseline risk factors and HABP/VABP development.
The aim of developing the multivariable logistic regression model was to identify patient characteristics and treatment exposures that were associated with increased risk for HABP/VABP development during the ICU course at the time the patient might be screened for enrollment in a HABP/VABP clinical trial. Patients who met the study definition of HABP/VABP at the time of enrollment were excluded from the model. Final predictors were identified with the use of clinical guidance and a backward variable selection process at the .1 level of significance for model retention. These predictors were confirmed independently with a forward variable selection process. Collinearity was assessed by calculation of the phi coefficient between prespecified covariates identified by clinical guidance as most likely to be associated. In a sensitivity analysis among the subset of high-risk patients who received >48 hours of invasive mechanical ventilation, we evaluated whether these predictors were also associated specifically with development of VABP. Discriminatory capacity of the multivariable models was assessed with the c-statistic. Calibration for each model was assessed graphically to display the level of agreement between observed and predicted rates of HABP/VABP and VABP respectively, by decile of risk. The out-of-sample performance of each model was evaluated with the use of internal validation by estimating the optimism-corrected c-statistic with 200 bootstrap samples. All analyses were performed using SAS software (version 9.4; SAS Institute Inc, Cary, NC).
Results
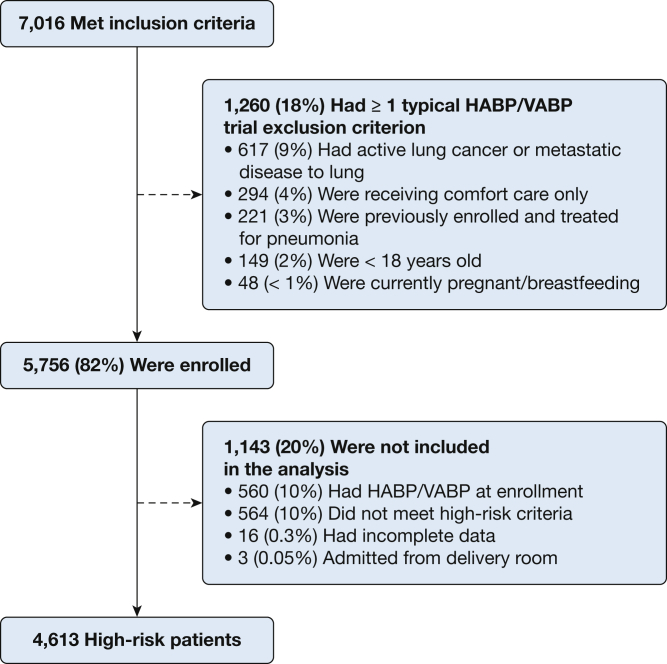
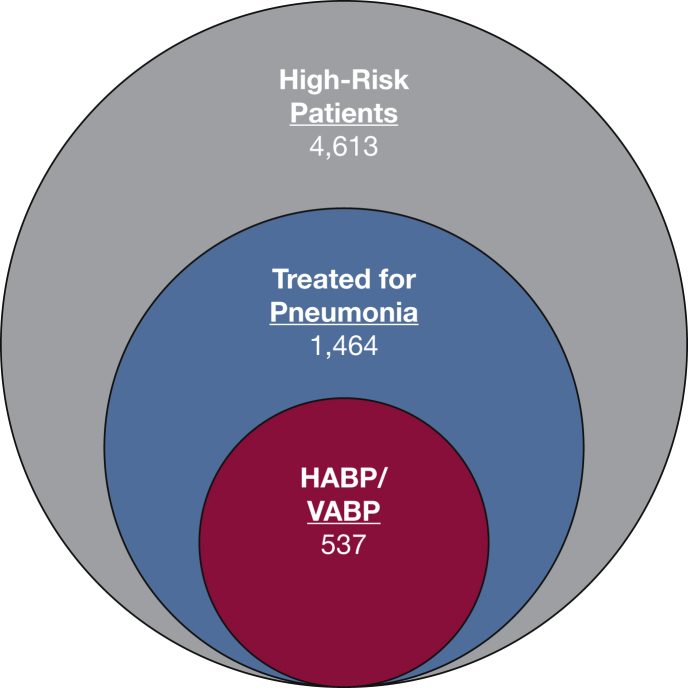
Between February 6, 2016, and October 7, 2016, the study enrolled 5,756 patients who were in the ICU; 4,613 of them (80%) had high-risk factors for HABP/VABP development at enrollment and met study inclusion/exclusion criteria (Fig 1). Of the 4,613 enrolled high-risk patients, 537 (12%) met the study HABP/VABP definition over a median follow up of 7 days (Fig 2). Among 1,464 of the high-risk patients (32%) who were treated for possible pneumonia during their ICU course, 927 patients, comprising 63% of the treated population, did not fulfill at least one domain of HABP/VABP diagnostic inclusion criteria recommended in FDA draft guidance (e-Table 1). Of 1,464 treated high-risk patients, 1,181 (81%) were prescribed antibiotics for an indication of pneumonia, and 523 (44%) met the study HABP/VABP definition. Among 283 high-risk patients (19%) who were treated with antibiotics for an indication of undifferentiated sepsis (for which pneumonia was being evaluated as a potential cause) or for which no antibiotic indication was recorded, 14 patients (5%) met the study definition for HABP/VABP.
Figure 1.
Screening, eligibility, and enrollment of patients who are at risk for nosocomial pneumonia. HABP = hospital-acquired bacterial pneumonia; VABP = ventilator-associated bacterial pneumonia.
Figure 2.
Study outcome for high-risk patients. Of 4,613 enrolled high-risk patients, 1,464 (32%) were treated for possible pneumonia during their ICU course; of these, 537 patients (37%) met the study HABP/VABP definition over a median follow-up time of 7 days. See Figure 1 legend for expansion of abbreviations.
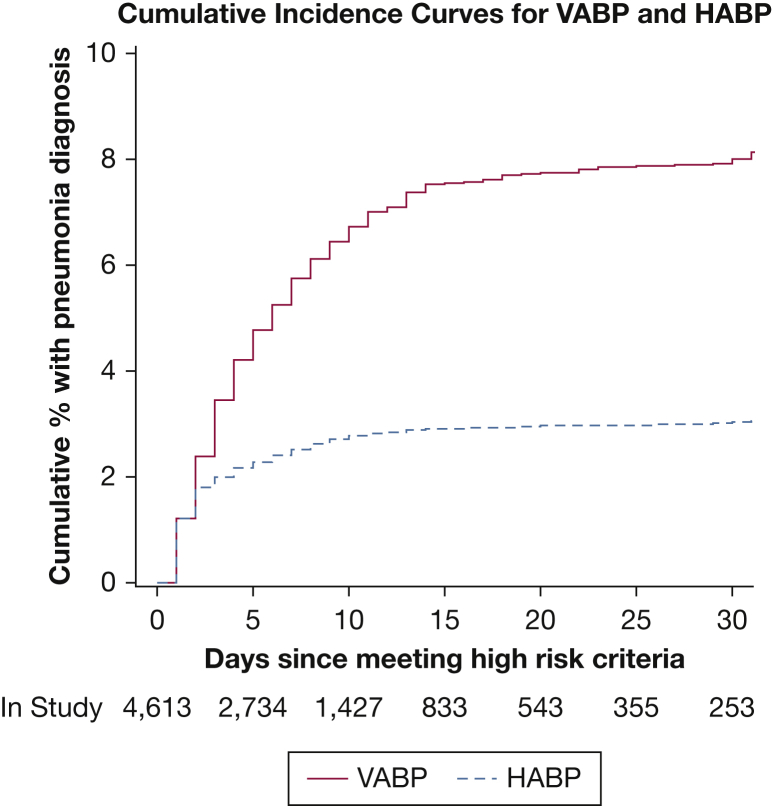
Characteristics were similar in high-risk, treated, HABP, and VABP populations, including age, ICU type, hospital and ICU length-of-stay, and type of respiratory support (Table 1). In the HABP/VABP population, 502 of 537 patients (93%) were receiving invasive mechanical ventilation at the time of pneumonia diagnosis, including 108 patients (20%) with ventilated HABP (<48 hours of invasive mechanical ventilation at time of diagnosis) and 394 patients (73%) with VABP. The median duration of mechanical ventilation for high-risk patients who subsequently experienced the development of VABP was 8 days (interquartile range, 5-14) (Fig 3).
Table 1.
Characteristics of Key Study Populations
| Characteristic | High-Risk Patients (n = 4,613) | Treated Patients (n = 1,464) | Patients With HABP (n = 143) | Patients With VABP (n = 394) |
|---|---|---|---|---|
| Demographicsa | ||||
| Age, median (IQR), y | 61.0 (50.0-70.0) | 60.0 (49.0-70.0) | 63.0 (55.0-74.0) | 58.0 (45.0-69.0) |
| Female sex, No. (%) | 2,058 (44.6) | 599 (40.9) | 51 (35.7) | 159 (40.4) |
| BMI, median (IQR), kg/m2 | 28.9 (24.1-35.0) | 28.5 (23.8-34.8) | 26.1 (22.1-31.6) | 29.4 (25.1-35.1) |
| Hospital length of stay, median (IQR), d | 4.0 (3.0-8.0) | 5.0 (3.0-9.0) | 6.0 (3.0-10.0) | 5.0 (3.0-9.0) |
| ICU length of stay, median (IQR), d | 3.0 (2.0-5.0) | 4.0 (2.0-6.0) | 4.0 (2.0-7.0) | 3.5 (2.0-6.0) |
| APACHE II Score,b median (IQR) | … | … | 19.0 (15.0-27.0) | 23.0 (17.0-28.0) |
| Treatment exposures,c No. (%) | ||||
| Invasive mechanical ventilation | 3,908 (84.7) | 1,316 (89.9) | 108 (75.5) | 394 (100) |
| Noninvasive mechanical ventilation | 751 (16.3) | 258 (17.6) | 36 (25.2) | 42 (10.7) |
| Enteral nutrition | 3,035 (65.8) | 1,149 (78.5) | 98 (68.5) | 357 (90.6) |
| Vasopressor/inotropic therapy | 2,211 (47.9) | 722 (49.3) | 70 (49.0) | 226 (57.4) |
| Biologic agents, current hospitalization | 169 (3.7) | 57 (3.9) | 3 (2.1) | 21 (5.3) |
| Corticosteroids, current hospitalization | 589 (12.8) | 226 (15.4) | 32 (22.4) | 54 (13.7) |
| PPI/H-2 blocker, current hospitalization | 3,475 (75.3) | 1,185 (80.9) | 114 (79.7) | 332 (84.3) |
| Blood product transfusion, prior 7 d | 1,062 (23.0) | 332 (22.7) | 33 (23.1) | 132 (33.5) |
| Systemic antibacterials, prior 90 d | 2,832 (61.4) | 1,020 (69.7) | 108 (75.5) | 275 (69.8) |
| Mechanical circulatory support | 220 (4.8) | 69 (4.7) | 4 (2.8) | 29 (7.4) |
| Massive volume resuscitation | 532 (11.5) | 174 (11.9) | 12 (8.4) | 61 (15.5) |
| Active medical problems,c No. (%) | ||||
| Acute respiratory distress syndrome | 686 (14.9) | 332 (22.7) | 36 (25.2) | 66 (16.8) |
| Acute kidney injury | 1,078 (23.4) | 410 (28.0) | 32 (22.4) | 88 (22.3) |
| Chronic kidney disease | 541 (11.7) | 173 (11.8) | 13 (9.1) | 45 (11.4) |
| End stage renal disease | 270 (5.9) | 70 (4.8) | 8 (5.6) | 15 (3.8) |
| Aspiration risk | 605 (13.1) | 325 (22.2) | 31 (21.7) | 100 (25.4) |
| Autoimmune disorder | 194 (4.2) | 68 (4.6) | 7 (4.9) | 21 (5.3) |
| Chemotherapy, prior 30 days | 139 (3.0) | 55 (3.8) | 7 (4.9) | 13 (3.3) |
| Diabetes mellitus | 1,304 (28.3) | 393 (26.8) | 24 (16.8) | 91 (23.1) |
| Immunocompromised | 545 (11.8) | 170 (11.6) | 23 (16.1) | 38 (9.6) |
| Chronic respiratory failure | 129 (2.8) | 39 (2.7) | 4 (2.8) | 10 (2.5) |
| Congestive heart failure, NYHA class IV | 141 (3.3) | 41 (3.1) | 3 (2.4) | 6 (1.7) |
| Cirrhosis or GI bleeding | 467 (10.1) | 150 (10.2) | 16 (11.2) | 40 (10.2) |
| Cerebrovascular accident | 400 (8.7) | 162 (11.1) | 14 (9.8) | 46 (11.7) |
| Substance abuse | 1,289 (27.9) | 422 (28.8) | 34 (23.8) | 115 (29.2) |
| HIV infection | 54 (1.2) | 10 (0.7) | 2 (1.4) | 3 (0.8) |
| Delirium or altered mental status | 1,276 (27.7) | 455 (31.1) | 40 (28.0) | 112 (28.4) |
| Seizures | 417 (9.0) | 163 (11.1) | 6 (4.2) | 42 (10.7) |
| COPD | 804 (17.4) | 262 (17.9) | 25 (17.5) | 52 (13.2) |
| Myocardial infarction | 337 (7.3) | 115 (7.9) | 11 (7.7) | 24 (6.1) |
| Chronic dialysis (any type) | 490 (10.6) | 145 (9.9) | 14 (9.8) | 35 (8.9) |
| ICU type, No. (%) | ||||
| Medical | 2,468 (53.5) | 837 (57.2) | 84 (58.7) | 188 (47.7) |
| Surgical/trauma | 852 (18.5) | 215 (14.7) | 22 (15.4) | 97 (24.6) |
| Cardiac/cardiac surgery | 769 (16.7) | 194 (13.3) | 18 (12.6) | 50 (12.7) |
| Neurosciences | 350 (7.6) | 139 (9.5) | 9 (6.3) | 42 (10.7) |
| Mixed | 174 (3.8) | 79 (5.4) | 10 (7.0) | 17 (4.3) |
| ICU admission source, No. (%) | ||||
| ED | 2,729 (59.2) | 926 (63.3) | 97 (67.8) | 225 (57.1) |
| Skilled nursing, long-term acute care | 177 (3.8) | 69 (4.7) | 11 (7.7) | 18 (4.6) |
| Scheduled procedure | 488 (10.6) | 79 (5.4) | 8 (5.6) | 26 (6.6) |
| No procedure; clinic or direct admission | 812 (17.6) | 282 (19.3) | 18 (12.6) | 83 (21.1) |
| Other | 407 (8.8) | 108 (7.4) | 9 (6.3) | 42 (10.7) |
| ICU admission diagnosis, No. (%) | ||||
| Acute hypercapnic respiratory failure | 233 (5.1) | 77 (5.3) | 4 (2.8) | 13 (3.3) |
| Acute hypoxemic respiratory failure | 893 (19.4) | 348 (23.8) | 40 (28.0) | 69 (17.5) |
| Acute myocardial infarction | 124 (2.7) | 41 (2.8) | 6 (4.2) | 7 (1.8) |
| Acute renal failure or severe electrolyte abnormality | 45 (1.0) | 12 (0.8) | 1 (0.7) | 2 (0.5) |
| Altered mental status | 337 (7.3) | 118 (8.1) | 10 (7.0) | 23 (5.8) |
| Cardiogenic shock | 86 (1.9) | 32 (2.2) | 2 (1.4) | 11 (2.8) |
| Cerebrovascular accident | 191 (4.1) | 70 (4.8) | 7 (4.9) | 23 (5.8) |
| Hemorrhagic shock or severe hemorrhage | 94 (2.0) | 27 (1.8) | 0 (0.0) | 11 (2.8) |
| Other hypovolemic shock | 17 (0.4) | 3 (0.2) | 1 (0.7) | 1 (0.3) |
| Planned postoperative ICU admission | 475 (10.3) | 82 (5.6) | 9 (6.3) | 32 (8.1) |
| Sepsis or septic shock | 337 (7.3) | 99 (6.8) | 12 (8.4) | 23 (5.8) |
| Shock | 41 (0.9) | 11 (0.8) | 1 (0.7) | 3 (0.8) |
| Frequent/refractory seizures | 94 (2.0) | 39 (2.7) | 1 (0.7) | 14 (3.6) |
| Trauma | 275 (6.0) | 101 (6.9) | 10 (7.0) | 60 (15.2) |
| Other | 1,371 (29.7) | 404 (27.6) | 39 (27.3) | 102 (25.9) |
APACHE = acute physiology and chronic health evaluation; HABP = hospital-acquired bacterial pneumonia; H2 = histamine blocker; IQR = interquartile range; PPI = proton pump inhibitor; VABP = ventilator-associated bacterial pneumonia
Characteristics recorded at the time of high-risk population enrollment.
Some variables that are required for APACHE II score calculation were recorded only when pneumonia diagnosis was confirmed.
Characteristics recorded when pneumonia diagnosis confirmed or upon ICU discharge (for patients not developing HABP/VABP).
Figure 3.
Cumulative incidence of nosocomial pneumonia for high-risk patients. See Figure 1 legend for expansion of abbreviations.
The multivariable logistic regression model was developed with the use of 4,613 high-risk patients. Key patient characteristics and treatment exposures that were associated with increased odds of pneumonia (meeting the study HABP/VABP definition) included an ICU admission diagnosis of trauma or cerebrovascular accident, the receipt of enteral nutrition, documented aspiration risk, and the receipt of systemic antibacterials within the preceding 90 days (Table 2). Collinearity that would impact stability of the multivariable model was not identified. The HABP/VABP logistic regression model demonstrated moderate discriminatory capacity and calibration (c-statistic, 0.709; 95% CI, 0.686-0.731) (e-Fig 2). The multivariable model yielded out-of-sample discrimination with an optimism-corrected c-statistic of 0.693 (95% CI, 0.670-0.715). The multivariable model was also evaluated in 3,712 of 4,613 patients (80%) at high risk for the development of VABP (exposure to invasive mechanical ventilation >48 hours) and demonstrated similar discriminatory capacity and calibration (c-statistic, 0.698; 95% CI, 0.671-0.726), optimism-corrected c-statistic 0.677 (95% CI, 0.650-0.705) (e-Table 2, e-Fig 3).
Table 2.
High-Risk Patient Characteristic and Treatment Exposure Associations With Pneumonia Development
| Factor | Type 3 Wald Chi-Square |
Beta Coefficient |
Adjusted OR (95% CI) | P Value |
|---|---|---|---|---|
| ICU admission diagnosis | 53.10 | … | … | … |
| Acute hypercapnic respiratory failure | … | −0.31 | 0.73 (0.38-1.39) | .336 |
| Acute hypoxemic respiratory failure | … | 0.13 | 1.14 (0.74-1.76) | .552 |
| Acute myocardial infarction | … | 0.12 | 1.12 (0.55-2.28) | .749 |
| Altered mental status or seizures | … | −0.06 | 0.94 (0.57-1.55) | .815 |
| Cerebrovascular accident | … | 0.51 | 1.67 (0.95-2.94) | .073 |
| Sepsis or septic shock | … | −0.12 | 0.88 (0.52-1.49) | .646 |
| Trauma | … | 1.16 | 3.19 (1.96-5.20) | <.001 |
| Shock (excluding septic shock) | … | 0.06 | 1.06 (0.62-1.83) | .822 |
| Other | … | 0.10 | 1.11 (0.73-1.68) | .629 |
| Planned postoperative ICU admission | … | … | Reference | … |
| Enteral nutrition | 41.26 | 0.87 | 2.38 (1.83-3.11) | <.001 |
| Aspiration risk | 39.18 | 0.74 | 2.10 (1.66-2.65) | <.001 |
| Systemic antibacterials within 90 d | 16.78 | 0.44 | 1.56 (1.26-1.92) | <.001 |
| Admission source | 13.53 | … | … | |
| Skilled nursing, long-term acute care | … | 0.60 | 1.82 (1.17-2.82) | .007 |
| No procedure; clinic or direct admission | … | 0.19 | 1.20 (0.93-1.55) | .152 |
| Scheduled procedure | … | −0.37 | 0.69 (0.45-1.06) | .089 |
| Other | … | 0.14 | 1.15 (0.83-1.61) | .396 |
| ED | … | … | Reference | … |
| Diabetes mellitus | 6.44 | −0.29 | 0.75 (0.59−0.94) | .011 |
| Invasive mechanical ventilation | 5.96 | 0.49 | 1.63 (1.10-2.40) | .015 |
| Noninvasive mechanical ventilation | 4.57 | 0.30 | 1.35 (1.03-1.78) | .032 |
| Proton pump inhibitor therapy/histamine blocker therapy | 4.36 | 0.27 | 1.30 (1.02-1.67) | .037 |
| Blood product transfusion in the last 7 d | 3.80 | 0.21 | 1.24 (1.00-1.53) | .051 |
| Corticosteroids at current hospitalization | 2.96 | 0.23 | 1.26 (0.97-1.65) | .086 |
| Female sex | 2.70 | −0.16 | 0.85 (0.70-1.03) | .101 |
| ICU length of stay per 1-day increase, d | 2.31 | 0.01 | 1.01 (1.00-1.03) | .128 |
Characteristics and treatment exposures were recorded at time of high-risk population enrollment; 4,613 patients were included in the analysis. Risk factors were selected with the use of backward selection with α = .1 for model inclusion and clinical expertise. C-statistic: 0.709 (95% CI, 0.686−0.731).
Microbiologic testing was collected and recorded in 477 of 537 patients (89%) who fulfilled study HABP/VABP criteria. A bacterial pathogen was identified from at least one source in 306 of 477 of tested patients (64%) (e-Figs 4 and 5). Staphylococcus aureus (102/477 patients [21%]) and Pseudomonas aeruginosa (52/477 patients [11%]) were the most frequently isolated bacterial pathogens among tested patients with HABP/VABP (e-Figs 6 and 7). Enterobacteriaceae were identified in 116 of 477 patients (24%) who were tested HABP/VABP. Extended spectrum beta-lactamase-producing bacteria were reported in 13 of 477 patients (3%) and carbapenem-resistant organisms in 3 of 477 patients (<1%) who were tested HABP/VABP.
Discussion
This large, contemporary, prospective cohort study made two pivotal observations. First, treatment for nosocomial pneumonia is common; 32% of prospectively identified high-risk patients received antibiotics for possible HABP/VABP, and 12% of these high-risk patients met case definitions for HABP/VABP that were consistent with FDA draft guidance for sponsors who were conducting interventional trials.17 Second, we were able to identify common patient characteristics and treatment exposures that were associated with increased odds of HABP/VABP development among prospectively identified high-risk patients. Identification of these risk associations, in combination with the high-risk criteria we used in this study, may help focus future prevention efforts, inform the design of more efficient clinical trials, and facilitate innovative enrollment strategies such as early screening or consent of patients at high-risk for the development of HABP/VABP.
Because this study was developed to inform design of more efficient clinical trials, we used a HABP/VABP definition that was consistent with recommended clinical trial inclusion criteria in FDA draft guidance.17 Although national surveillance data suggest a decreasing incidence of nosocomial pneumonia, this study demonstrates that HABP and VABP remain common nosocomial infections.18 The higher rates of pneumonia that were observed in this study may be due partially to the use of a HABP/VABP definition similar to that recommended in clinical practice guidelines, rather than an epidemiologic definition.3,19,20 To minimize risk of underestimating HABP/VABP among high-risk patients who were treated with antibiotics for unclear indications, we included patients who were prescribed antibiotics for undifferentiated sepsis if pneumonia was considered a possible cause. Even if high-risk patients who were treated with antibiotics for a clinical indication of undifferentiated sepsis were excluded, 26% of the high-risk population was treated with antibiotics for a clinical indication of pneumonia, and only 44% of these patients ultimately met the study HABP/VABP definition; this discrepancy highlights diagnostic uncertainty in the management of HABP/VABP and the urgent need for new tools to improve the accuracy and consistency of HABP/VABP diagnosis.13,21
Discordance between treatment and diagnostic confirmation may reflect clinicians’ reluctance to base treatment decisions on imprecise chest radiography, insensitive HABP/VABP diagnostic criteria, or variability within treatment practices.22, 23, 24 Though impossible to confidently evaluate within the design of this study, the frequency of antibiotic prescribing for clinical syndromes not fulfilling the study HABP/VABP definition also raises concern for antibiotic overprescription in this high-risk population. Such concerns emphasize the need for prospective evaluation of patient-centered outcomes that are associated with antibacterial exposure in the management of suspected HABP/VABP with the use of the criteria of increasing stringency, particularly because receiving antibiotics is itself a risk factor for the development of pneumonia, carries the risk of adverse events, and may preclude eligibility for HABP/VABP trial enrollment.25 Nevertheless, this study does provide evidence that patients in the ICU who receive high levels of respiratory support frequently do receive antibiotics for HABP/VABP and fulfill recommended inclusion criteria for enrollment in antibacterial drug trials.
A key result of this study was the identification of common characteristics in patient and treatment exposures that are associated with increased odds of HABP/VABP development. Our model identified several clinical characteristics and potentially modifiable risk factors (receipt of systemic antibacterials within the preceding 90 days or antacid medications during the current hospitalization) that previously were associated with increased odds of HABP/VABP.26, 27, 28, 29 The findings from this large prospective cohort validate previous risk associations and may also inform the future development of a more comprehensive HABP/VABP risk prediction tool used to design efficient clinical trial enrollment strategies or effectively steward costly or higher risk-prevention strategies that cannot be practically or safely implemented universally. Development of a comprehensive risk prediction tool could complement real-time monitoring systems to identify effectively patients who are experiencing the development of nosocomial pneumonia as early and efficiently as possible.30 Prospective identification of patients at high-risk for HABP/VABP, with the use of the high-risk criteria that were used in this study, potentially enhanced by more comprehensive risk prediction tools, may also help focus clinical trial screening efforts on patients at highest risk, facilitating enrollment in more efficient clinical trials and furthering evaluation of early informed consent trial designs whereby patients or their surrogates may be approached about enrollment in HABP/VABP clinical trials before the development of nosocomial pneumonia.31
Limitations
This study has some limitations. First, because only US adult ICUs were included, our study may not be generalizable to other populations; therefore, our findings have been evaluated in a PICU cohort, and analysis of data from a European cohort is ongoing (e-Table 3).32 Second, candidate risk factors for HABP/VABP were evaluated only in patients who met prespecified high-risk criteria, so the odds of pneumonia could not be evaluated in patients who did not receive high levels of respiratory support and were presumably at lower risk for the development of HABP/VABP. Third, because this study was conducted only in patients in the ICU, 85% of whom received invasive mechanical ventilation during their ICU course, nonventilated HABP is underrepresented. Epidemiologic studies suggest that HABP is increasingly common and accounts for the majority of nosocomial pneumonias.33,34 The clinical characteristics and treatment exposures that were associated with increased odds of HABP/VABP in our study may not have similar associations with nonventilated HABP, especially HABP that develops outside the ICU setting. Because this study was developed to inform the design of efficient HABP/VABP clinical trials in the ICU setting, we evaluated risk associated with a combined HABP/VABP end point. We did not observe significant differences in the prevalence of candidate risk factors between HABP and VABP populations or in the performance of the multivariable model when we evaluated only the subgroup of patients who were at high risk for VABP, but this does not diminish the fact that HABP and VABP are distinct clinical entities and an evaluation of risk factors for nonventilated HABP would require a broader inclusion of hospitalized patients outside the ICU. Fourth, because some variables that are required to calculate standard severity of illness scores were not collected on study enrollment, we could not include these patient characteristics in the multivariable model. Finally, although the proportion of cases with a bacterial pathogen detected (64%) was consistent with prior studies, we could not estimate accurately the burden of nosocomial pneumonia that was associated with viral pathogens that have been associated with nosocomial pneumonia in several single-center studies.8,35,36
Interpretation
In conclusion, the burden of HABP and VABP among critically ill patients is substantial. Treatment for possible nosocomial pneumonia is exceedingly common among patients who receive high levels of respiratory support; however, most of these patients do not fulfill standard clinical definitions of HABP/VABP. Prospective identification of patients at high-risk for HABP/VABP with the use of simple clinical criteria may facilitate that conduct of innovative and efficient clinical trials to promote the development of optimal preventive, diagnostic, and treatment strategies to improve management of this disease.
Acknowledgments
Author contributions: S. P. B. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. S. P. B and A. C. contributed to the conception and design of the study, the data analysis, the data interpretation, the manuscript drafting, and the critical revision of the manuscript. S. B. C., J. F., J. H. P., H. K. D., D. R., and P. T. contributed to the conception and design of the study, data interpretation, the manuscript drafting, and the critical revision of the manuscript. M. J. Z., M. S., M. H. K., M. J. D., B. A. K., A. C. B., C. G., J. S., and P. G. contributed to the data interpretation, the manuscript drafting, and the critical revision of the manuscript. J.-L. S. and K. C. contributed to data analysis, the data interpretation, the manuscript drafting, and the critical revision of the manuscript. V. G. F. contributed to the conception and design of the study, the supervision, data interpretation, the manuscript drafting, and the critical revision of the manuscript. T. L. H. contributed to the conception and design of the study, the supervision, data acquisition, analysis and interpretation, the manuscript drafting, and the critical revision of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: S. B. C. reports salary support from the US FDA grant R18FD005292 and CTTI membership fees. M. J. Z. reports serving on an adjudication committee for ContraFect and receiving research grants from Genentech, Medimune, and Merck. M. S. reports consulting for Curetis GmBH, Paratek Pharmaceuticals, and Cutis Pharma, receiving a grant from Cubist Pharmaceuticals Inc (Now Merck and Co), receiving research support from Astra Zeneca Pharmaceuticals LP, Cempra Inc, Aradigm Corp, Cubist Pharmaceuticals Inc (Now Merck and Co), Synthetic Biologics Inc, Deibopharm International SA, Bayer HealthCare AG, Theravance Inc, Seres Therapeutics Inc, Rempex, Vela Diagnostics, AM-Pharma, Abbott Molecular Inc, Gilead Sciences Inc, Merck and Co, NeuMoDx Molecular, Nabriva Therapeutics AG, Sanofi Pasteur Inc, Diasorin Molecular, Curetis GmbH, Pfizer Inc, Cidara Therapeutics Inc, Shire, ContraFect, Aridis Pharmaceuticals Inc, Epigenomics Inc, Genentech Inc, Finch Therapeutics, MedImmune, Janssen Research and Development LLC, The Medicines Company, Summit Therapeutics, Iterum Therapeutics International, outside the submitted work; in addition, he has a patent Methods of diagnosing increased risk of developing MRSA or CA-MRSA issued, and a patent DETECTING AND TREATING MRSA and SSI pending. M. H. K. reports support from the Barnes-Jewish Hospital Foundation. M. J. D. reports funding from the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number KL2TR002346. P. T. reports salary support from the US FDA grant R18FD005292 and CTTI membership fees. V. G. F. reports serving as chair of the V710 Scientific Advisory Committee (Merck); has received grant support from Cerexa/Actavis/Allergan, Pfizer, Advanced Liquid Logics, NIH, MedImmune, Basilea Pharmaceutica, Karius, ContraFect, Regeneron Pharmaceuticals, and Genentech; has NIH Small Business Technology Transfer/Small Business Innovation Research grants pending with Affinergy, Locus, and Medical Surface; has been a consultant for Achaogen, Astellas Pharma, Arsanis, Affinergy, Basilea Pharmaceutica, Bayer, Cerexa Inc, ContraFect, Cubist, Debiopharm, Durata Therapeutics, Grifols, Genentech, MedImmune, Merck, the Medicines Company, Pfizer, Novartis, NovaDigm Therapeutics Inc, Theravance Biopharma, and XBiotech; has received honoraria from Theravance Biopharma and Green Cross; and has a patent pending in sepsis diagnostics. T. L. H. reports consulting for Basilea Pharmaceutica (ceftobiprole), Genentech (immunotherapeutic), Motif Bio (iclaprim), and Theravance (telavancin). None declared (S. P. B., A. C., J. F., J. H. P., B. A. K., H. K. D., A. C. B., C. G., D. R., J.-L. S., K. C., J. S., and P. G.).
Role of sponsors: The FDA and CTTI membership fees funded the study; members of the both the FDA and CTTI were part of the study team. FDA and CTTI authors contributed to the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Other contributions: The authors would like to thank the PROPHETIC study investigators and coordinators for conducting the study, the CTTI HABP/VABP studies project team (https://www.ctti-clinicaltrials.org/projects/habpvabp-studies) for contributions to the concept and design of the study, and Erin Campbell for editorial support. Views expressed in this manuscript do not necessarily reflect the official policies of the Department of Health and Human Services; nor does any mention of trade names, commercial practices, or organization imply endorsement by the United States Government.
Additional information: The e-Appendix, e-Figures, and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This study was funded by the Food and Drug Administration through grant R18FD005292 and partially funded by pooled membership fees from CTTI’s member organizations (https://www.ctti-clinicaltrials.org/membership); V. G. F. was supported by mid-career mentoring award K24-AI093969 from the National Institutes of Health.
Supplementary Data
References
- 1.Magill S.S., O’Leary E., Janelle S.J. Changes in prevalence of health care-associated infections in U.S. hospitals. N Engl J Med. 2018;379(18):1732–1744. doi: 10.1056/NEJMoa1801550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vincent J.L., Bihari D.J., Suter P.M. The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. EPIC International Advisory Committee. JAMA. 1995;274(8):639–644. [PubMed] [Google Scholar]
- 3.Kalil A.C., Metersky M.L., Klompas M. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melsen W.G., Rovers M.M., Groenwold R.H. Attributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studies. Lancet Infect Dis. 2013;13(8):665–671. doi: 10.1016/S1473-3099(13)70081-1. [DOI] [PubMed] [Google Scholar]
- 5.Klompas M. Does this patient have ventilator-associated pneumonia? JAMA. 2007;297(14):1583–1593. doi: 10.1001/jama.297.14.1583. [DOI] [PubMed] [Google Scholar]
- 6.Kuti E.L., Patel A.A., Coleman C.I. Impact of inappropriate antibiotic therapy on mortality in patients with ventilator-associated pneumonia and blood stream infection: a meta-analysis. J Crit Care. 2008;23(1):91–100. doi: 10.1016/j.jcrc.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Muscedere J.G., Shorr A.F., Jiang X., Day A., Heyland D.K., Canadian Critical Care Trials Group The adequacy of timely empiric antibiotic therapy for ventilator-associated pneumonia: an important determinant of outcome. J Crit Care. 2012;27(3):322.e7–322.e14. doi: 10.1016/j.jcrc.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Jones R.N. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis. 2010;51(suppl 1):S81–S87. doi: 10.1086/653053. [DOI] [PubMed] [Google Scholar]
- 9.Kinch M.S., Patridge E., Plummer M., Hoyer D. An analysis of FDA-approved drugs for infectious disease: antibacterial agents. Drug Discov Today. 2014;19(9):1283–1287. doi: 10.1016/j.drudis.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Barriere S.L. Challenges in the design and conduct of clinical trials for hospital-acquired pneumonia and ventilator-associated pneumonia: an industry perspective. Clin Infect Dis. 2010;51(suppl 1):S4–S9. doi: 10.1086/653033. [DOI] [PubMed] [Google Scholar]
- 11.Bettiol E., Wetherington J.D., Schmitt N., Harbarth S., COMBACTE Consortium Challenges and solutions for clinical development of new antibacterial agents: results of a survey among pharmaceutical industry professionals. Antimicrob Agents Chemother. 2015;59(7):3695–3699. doi: 10.1128/AAC.00638-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stergiopoulos S., Calvert S.B., Brown C.A. Cost drivers of a hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia phase 3 clinical trial. Clin Infect Dis. 2018;66(1):72–80. doi: 10.1093/cid/cix726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ego A., Preiser J.C., Vincent J.L. Impact of diagnostic criteria on the incidence of ventilator-associated pneumonia. Chest. 2015;147(2):347–355. doi: 10.1378/chest.14-0610. [DOI] [PubMed] [Google Scholar]
- 14.Klompas M., Anderson D., Trick W. The preventability of ventilator-associated events: the CDC Prevention Epicenters Wake Up and Breathe Collaborative. Am J Respir Crit Care Med. 2015;191(3):292–301. doi: 10.1164/rccm.201407-1394OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magill S.S., Li Q., Gross C., Dudeck M., Allen-Bridson K., Edwards J.R. Incidence and characteristics of ventilator-associated events reported to the National Healthcare Safety Network in 2014. Crit Care Med. 2016;44(12):2154–2162. doi: 10.1097/CCM.0000000000001871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knirsch C., Alemayehu D., Botgros R. Improving conduct and feasibility of clinical trials to evaluate antibacterial drugs to treat hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia: recommendations of the Clinical Trials Transformation Initiative Antibacterial Drug Development Project Team. Clin Infect Dis. 2016;63(suppl 2):S29–S36. doi: 10.1093/cid/ciw258. [DOI] [PubMed] [Google Scholar]
- 17.US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research Guidance for industry. Hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia: developing drugs for treatment (draft guidance, revision 2). Food and Drug Administration web site. https://www.fda.gov/downloads/drugs/guidances/ucm234907.pdf Published May 2014. Accessed July 20, 2018.
- 18.Dudeck M.A., Weiner L.M., Allen-Bridson K. National Healthcare Safety Network (NHSN) report, data summary for 2012, device-associated module. Am J Infect Control. 2013;41(12):1148–1166. doi: 10.1016/j.ajic.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y., Eldridge N., Metersky M.L. National trends in patient safety for four common conditions, 2005-2011. N Engl J Med. 2014;370(4):341–351. doi: 10.1056/NEJMsa1300991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skrupky L.P., McConnell K., Dallas J., Kollef M.H. A comparison of ventilator-associated pneumonia rates as identified according to the National Healthcare Safety Network and American College of Chest Physicians criteria. Crit Care Med. 2012;40(1):281–284. doi: 10.1097/CCM.0b013e31822d7913. [DOI] [PubMed] [Google Scholar]
- 21.Kollef M.H., Burnham C.D. Ventilator-associated pneumonia: the role of emerging diagnostic technologies. Semin Respir Crit Care Med. 2017;38(3):253–263. doi: 10.1055/s-0037-1599224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wunderink R.G., Woldenberg L.S., Zeiss J., Day C.M., Ciemins J., Lacher D.A. The radiologic diagnosis of autopsy-proven ventilator-associated pneumonia. Chest. 1992;101(2):458–463. doi: 10.1378/chest.101.2.458. [DOI] [PubMed] [Google Scholar]
- 23.Martin-Loeches I., Povoa P., Rodriguez A. Incidence and prognosis of ventilator-associated tracheobronchitis (TAVeM): a multicentre, prospective, observational study. Lancet Respir Med. 2015;3(11):859–868. doi: 10.1016/S2213-2600(15)00326-4. [DOI] [PubMed] [Google Scholar]
- 24.Wunderink R.G. Ventilator-associated tracheobronchitis: public-reporting scam or important clinical infection? Chest. 2011;139(3):485–488. doi: 10.1378/chest.10-2641. [DOI] [PubMed] [Google Scholar]
- 25.Tamma P.D., Avdic E., Li D.X., Dzintars K., Cosgrove S.E. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med. 2017;177(9):1308–1315. doi: 10.1001/jamainternmed.2017.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chastre J., Trouillet J.L., Vuagnat A. Nosocomial pneumonia in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 1998;157(4):1165–1172. doi: 10.1164/ajrccm.157.4.9708057. [DOI] [PubMed] [Google Scholar]
- 27.Kollef M.H. Ventilator-associated pneumonia: s multivariate analysis. JAMA. 1993;270(16):1965–1970. [PubMed] [Google Scholar]
- 28.Collard H.R., Saint S., Matthay M.A. Prevention of ventilator-associated pneumonia: an evidence-based systematic review. Ann Intern Med. 2003;138(6):494–501. doi: 10.7326/0003-4819-138-6-200303180-00015. [DOI] [PubMed] [Google Scholar]
- 29.Kollef M.H. Prevention of hospital-associated pneumonia and ventilator-associated pneumonia. Crit Care Med. 2004;32(6):1396–1405. doi: 10.1097/01.ccm.0000128569.09113.fb. [DOI] [PubMed] [Google Scholar]
- 30.Shenoy E.S., Rosenthal E.S., Shao Y.P. Real-time, automated detection of ventilator-associated events: avoiding missed detections, misclassifications, and false detections due to human error. Infect Control Hosp Epidemiol. 2018;39(7):826–833. doi: 10.1017/ice.2018.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corneli A., Perry B., Collyar D. Assessment of the perceived acceptability of an early enrollment strategy using advance consent in health care-associated pneumonia. JAMA Netw Open. 2018;1(8) doi: 10.1001/jamanetworkopen.2018.5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ericson JE, McGuire J, Michaels MG, et al. Hospital-acquired pneumonia and ventilator-associated pneumonia in children: a prospective natural history and case-control study. Ped Infect Dis J. 2018;39(8):658-664. [DOI] [PMC free article] [PubMed]
- 33.Corrado R.E., Lee D., Lucero D.E., Varma J.K., Vora N.M. Burden of adult community-acquired, health-care-associated, hospital-acquired, and ventilator-associated pneumonia: New York City, 2010 to 2014. Chest. 2017;152(5):930–942. doi: 10.1016/j.chest.2017.04.162. [DOI] [PubMed] [Google Scholar]
- 34.Giuliano K.K., Baker D., Quinn B. The epidemiology of nonventilator hospital-acquired pneumonia in the United States. Am J Infect Control. 2018;46(3):322–327. doi: 10.1016/j.ajic.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Shorr A.F., Fisher K., Micek S.T., Kollef M.H. The burden of viruses in pneumonia associated with acute respiratory failure: an underappreciated issue. Chest. 2018;154(1):84–90. doi: 10.1016/j.chest.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Shorr A.F., Zilberberg M.D., Micek S.T., Kollef M.H. Viruses are prevalent in non-ventilated hospital-acquired pneumonia. Respir Med. 2017;122:76–80. doi: 10.1016/j.rmed.2016.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.