ABSTRACT
Our goal was to evaluate the utility of diffusion tensor imaging (DTI) for predicting future cognitive decline in mild cognitive impairment (MCI) in conjunction with AD biomarkers (amyloid PET and AD signature neurodegeneration) in 132 MCI individuals ≥60 years old with structural MRI, DTI, amyloid PET, and at least one clinical follow-up. We used mixed effect models to evaluate the prognostic ability of fractional anisotropy of genu of the corpus callosum (FA-Genu), as a cerebrovascular disease (CVD) marker, for predicting cognitive decline along with AD biomarkers. We contrasted the value of white matter hyperintensities, a traditional CVD marker as well as FA in the hippocampal cingulum bundle (FA-HCB) with the FA-Genu models. FA-Genu significantly predicted cognitive decline even after accounting for AD biomarkers. WMH was not associated with cognitive decline in the model with WMH and FA-Genu. DTI specifically FA-Genu provides unique complementary information to AD biomarkers and has significant utility for prediction of cognitive decline in MCI.
Keywords: Diffusion tensor imaging, mild cognitive impairment, cerebrovascular disease, genu of corpus callosum
1. INTRODUCTION
Mild Cognitive Impairment (MCI) is often a prodrome of dementia(Petersen, 2009) with a progression rate of 10–15% per year (Bruscoli and Lovestone, 2004; Petersen et al., 2001) and many of those with MCI who progress to AD dementia have elevated levels of Aβ (Quigley, Colloby and O’Brien, 2011). Because a significant proportion of variability in progression to dementia from MCI is not explained by brain Aβ levels, there is a need for additional biomarkers to aid in the prediction of future cognitive decline in MCI.
Diffusion tensor imaging (DTI) is a promising marker for cerebrovascular disease (CVD)(Tu et al., 2017; Williams et al., 2017). Studies have shown that reduction of fractional anisotropy (FA) or directionality and increase in overall mean diffusivity (MD) are associated with cognitive decline (Croall et al., 2017; Tuladhar et al., 2015). We recently reported that the FA of the genu of the corpus callosum (FA-Genu) is a useful biomarker of CVD because the loss of microstructural integrity in the genu was associated with worsening of systemic vascular health and cerebrovascular injury even after accounting for AD pathologies (Vemuri et al., 2018). This was supported by several studies that have found disconnection of the white matter (WM) tracts in MCI in the context of CVD (Catani and ffytche, 2005; O’Sullivan et al., 2001). Our primary aim was to test if poor WM microstructural health, as captured by FA-Genu, was predictive of faster cognitive decline and to evaluate its clinical utility in the presence of AD biomarkers (amyloid and neurodegeneration).
Using DTI, we investigated the interrelationships between FA-Genu, amyloid pathology, neurodegeneration (cortical thickness in AD signature regions) and cognitive decline among MCI participants, aged 60 years and older, and enrolled in the population-based Mayo Clinic Study of Aging (MCSA). In addition, we also compared the effectiveness of DTI measures from a different region, FA in the hippocampal cingulum bundle (FA-HCB), a tract that has been known to be vulnerable to AD pathology.
2. MATERIALS AND METHODS
2.1. Selection of Participants
The MCSA was designed to investigate the epidemiology of MCI and risk factors for MCI and dementia among the residents of Olmsted County Minnesota. The MCSA sample population was enumerated from the Olmsted county population using the Rochester Epidemiology Project (REP) medical records -linkage system (Rocca et al., 2012; St Sauver et al., 2012). The MCI participants were diagnosed based on the published consensus criteria (Petersen et al., 2010) that were described previously. In the current analyses, we included participants ≥60 years of age who had a diagnosis of MCI at the time of their structural MRI, DTI, FLAIR-MRI, and amyloid PET scans that had sufficient quality and had detailed neuropsychology evaluation at the imaging visit, and at least one clinical follow-up.
Standard protocol approvals, registrations, and patient consents:
The study was approved by the Mayo Clinic and Olmsted Medical Center institutional review board and written informed consent was obtained from all participants.
2.2. Imaging Biomarkers
All MRI scans were obtained on a 3T GE scanner with an eight channel phase array coil (GE, Milwaukee, WI).
2.2.1. DTI acquisition and processing:
DTI acquisition was performed using a single-shot echo-planar imaging sequence with an isotropic resolution of 2.7 mm. The DTI data consisted of 46 images for each set with 41 diffusion-encoding gradient directions (b=1000 s/mm2) and 5 non diffusion-weighted images (b=0 s/mm2). The diffusion data were preprocessed using the in-house developed pipeline. Briefly, the images were skull stripped (Reid et al., 2018), denoised (Veraart et al., 2016), corrected for head motion and eddy current distortion (Andersson et al., 2016), Gibbs ringing (Kellner et al., 2016), and then Rician noise bias correction was performed (Koay et al., 2009). Diffusion tensors were then fitted by nonlinearly minimizing the squared residuals (Garyfallidis et al., 2014), and FA and MD maps were calculated from the tensors. Advanced normalization tools –Symmetric Normalization (ANTS-SyN) (Avants et al., 2011) was used to non-linearly register FA image of the JHU “Eve” atlas to each participant’s FA image (Oishi et al., 2009) and the median values of FA in each region were obtained. The atlas was slightly modified by fusing the left and right portions of structures spanning the left-right mid-plane, such as the genu and pons.
2.2.2. Neurodegeneration Assessment from structural scans:
The cortical thickness measurements were performed from MPRAGE scans using the FreeSurfer version v5.3, and estimated the average cortical thickness of AD signature regions including the entorhinal cortex, inferior temporal, middle temporal and fusiform gyrus. A derived single measure was used as the surrogate marker of neurodegeneration (Schwarz et al., 2016).
2.2.3. Amyloid Assessment from PET scans:
The detailed descriptions of acquisition, processing and summary measure calculation for amyloid PET scans were published previously (Jack et al., 2017). A cortical global Pittsburgh compound B positron emission tomography (PiBPET) standardized uptake value ratio (SUVR) was computed by calculating the median uptake over voxels in the prefrontal, orbitofrontal, parietal, temporal, anterior cingulate, and posterior cingulate/precuneus ROI values for each participant normalized by the cerebellar crus grey matter of the in house atlas modified from (Lopresti et al., 2005) and used as a global amyloid load measure.
2.2.4. Cerebrovascular Pathology Assessment from FLAIR scans:
The acquisition and analysis of FLAIR-MRI images on the study participants were described in detail by Graff-Radford et al (Graff-Radford et al., 2019). Briefly, all subjects MPRAGE and FLAIR images were co-registered to template space using Statistical parametric Mapping. Then FLAIR images were masked using the segmented WM mask from MPRAGE image to remove the non-brain tissue and voxels other than WMH. WMH voxels on FLAIR images were segmented using semi-automated clustering technique as described in (Graff-Radford et al., 2019). These custom made masks were further visually inspected and manually edited by the trained analysts to exclude the artifacts from the WMH volume. We used the WMH fraction as ratio of WMH to the total intracranial volume as our outcome measure.
2.3. Cognitive Performance Measures
A detailed neuropsychological evaluation was performed on all MCSA participants as described previously (Petersen et al., 2010; Roberts et al., 2008). We assessed four cognitive domains using nine tests: executive (Trail Making Test Part B and Wechsler Adult Intelligence Scale–Revised (WAIS-R) Digit Symbol), language (Boston Naming Test and category fluency), Memory (Wechsler Memory Scale–Revised (WMS-R) Logical Memory-II (delayed recall), WMS-R Visual Reproduction-II (delayed recall), Auditory Learning Verbal Test delayed recall) and Visuospatial performance (WAIS-R Picture Completion and WAIS-R Block Design). In this study, we used a global cognitive function standard score that was derived from the z-transformation of the average of the four standardized cognitive domain scores: memory, language, attention/executive, and visuo-spatial function (Vemuri et al., 2014).
2.4. Statistical Analysis
All statistical analyses were performed using R statistical software, version 3.4.2 (RFoundation).
2.4.1. Evaluation of the DTI biomarker and education/occupation variables for predicting global cognition
We examined the effect of each of the DTI markers (FA-Genu) in the primary models and FA-HCB in the sensitivity analyses) and AD markers on longitudinal global cognitive performance using linear mixed effect models. We fit three separate linear mixed effect models for the DTI marker with global cognition as the dependent variable, and follow-up time, age, sex, education/occupation and various imaging biomarkers as fixed predictors. In the first model, we only used the DTI marker, in the second model the DTI marker in conjunction with amyloid and in the third model the DTI marker in conjunction with amyloid and neurodegeneration. In these models we considered all the main effects as well as the interactions of interest with time. We also tested for interactions of age, sex and education/occupation with the DTI marker. The models were fit with random intercepts and slopes. We did not test for three way interaction because of limited power. Among the three separate models for our predictors of interest, we compared the goodness-of-fit using Akaike information criteria (AIC). Lower AIC suggests greater prediction of variance in the data. The non-significant terms were removed from the model one at time, accounting for nested effects, until the final parsimonious models were reached. All models were tested and controlled for within-subject autocorrelation of the repeated measures.
The final parsimonious model included all main effects as well as all significant two-way interactions of potential predictors with time. We tested specifically for: interactions of the DTI imaging biomarker, other imaging biomarkers (PIB and cortical thickness), education/occupation, age and time. The global amyloid values used in the model were log transformed. A significant interaction of predictor with time would estimate the rate of change of global cognition over time based on the values of predictor variables.
2.4.2. Assessing the utility of DTI biomarker in conjunction with WMH
We estimated the added value of DTI marker for prediction of longitudinal cognition in MCI after accounting for WMH. The WMH was analyzed as percentage of total intracranial volume (TIV) in the model and values were log transformed. We fitted linear mixed effect models with global cognition as the outcome variable and with age and time as fixed effects. The final parsimonious model included all significant interactions of imaging biomarkers with time, main effects nested within those interactions, and other significant main effects.
3. RESULTS
The demographics, APOE4 status, intellectual enrichment variables, cognitive measures, vascular and AD biomarker values are provided in Table 1. As depicted in Table 1, out of 132 MCI participants, 51 (39%) were APOE4 positive and 73 (55%) amyloid-positive. Participants had a mean education level of 13.8 years and an education/occupation mean score of 11.7. The baseline global cognition z-scores were normally distributed among the participants. The mean follow-up time was 4.05 years (SD).
Table 1.
Characteristics table of MCI subjects with the mean (SD) listed for the continuous variables and count (%) for the categorical variables.
| Characteristic | All Subjects n = 132 |
|---|---|
| Demographics | |
| Age, yrs | 77.3 (7.4) |
| Males, no. (%) | 78 (59%) |
| APOE, no. (%) | 51 (39%) |
| Intellectual Enrichment | |
| Education/Occupation | 11.7 (2.7) |
| Education, yrs | 13.8 (2.9) |
| Cognitive Measures | |
| MMSE | 25.8 (1.8) |
| Global z-score | −1.58 (0.97) |
| Memory z-score | −1.56 (1.06) |
| Language z-score | − 1.29 (1.20) |
| Attention z-score | −1.40 (1.44) |
| Visualspatial z-score | −0.79 (1.06) |
| PiB, Neurodegeneration, and Follow-up | |
| PIB SUVr | 1.83 (0.55) |
| PIB+, no. (%) | 73 (55%) |
| Neurodegeneration, mm | 2.73 (0.21) |
| Neurodegeneration+, no. (%) | 93 (72%) |
| Follow-up, yrs | 4.05 (2.16) |
| Dementia Progression, no. (%) | 19 (14%) |
| Vascular Markers | |
| Cardio Metabolic Condition | 2.71 (1.47) |
| FA-Genu | 0.57 (0.06) |
| FA-HCB | 0.45 (0.04) |
| WMH/Total Intracranial Volume | 0.014 (0.011) |
3.1. FA-Genu for prediction of longitudinal cognitive decline
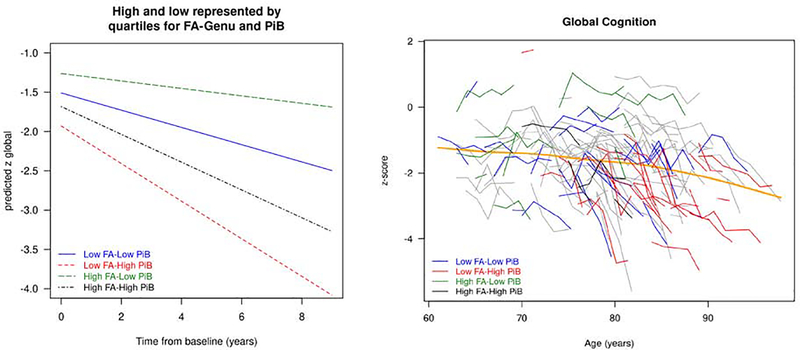
The results of the three linear mixed effect models: 1) FA-Genu alone; 2) FA-Genu and PIB combined; and 3) FA-Genu, PIB and cortical thickness are presented in Table 2. In all three models with FA-Genu, higher education/occupation scores were associated with better baseline cognitive performance (p<0.01). In the model of FA-Genu alone, age was a predictor of baseline cognitive performance and had a faster rate of cognitive decline. Sex was not a significant predictor of cognition in any of the models and dropped out from further analysis. In model 3, all three biomarkers (FA-Genu, PIB, and cortical thickness) significantly predicted the rate of cognitive decline (p<0.05). The significant interaction between FA-Genu and time indicated that MCI participants with higher FA declined more slowly over time. In the third model with all three imaging predictors, the AIC was lower providing evidence that including all imaging predictors improved prediction of longitudinal cognition. A plot of predicted global cognition categorized by the imaging biomarkers and a spaghetti plot of global cognition as a function of age are shown in Figures 1. In the left panel, we illustrated the predicted cognition z-score trajectories by FA-Genu and PIB values maintaining education/occupation as constant. We found that the participants with low FA-Genu and high PIB declined fastest among the groups.
Table 2:
Mixed models evaluating the utility of the Genu FA (FA-Genu) in predicting cognitive performance
| Models | Variable | Coefficient (s.e.) | p-value |
|---|---|---|---|
|
FA-Genu AIC 973.9088 |
Intercept | −2.118 (1.625) | 0.19 |
| Time | −0.068 (0.267) | 0.80 | |
| Baseline age | −0.028 (0.013) | 0.04 | |
| Education/occupation | 0.097 (0.032) | 0.003 | |
| FA-Genu | 2.730 (1.726) | 0.12 | |
| FA-Genu × time | 0.748 (0.289) | 0.01 | |
| Baseline age × time | −0.006 (0.002) | 0.002 | |
|
FA-Genu+ PIB AIC 940.3542 |
Intercept | −4.050 (1.003) | <0.001 |
| Time | −0.517 (0.152) | <0.001 | |
| Education/occupation | 0.077 (0.030) | 0.011 | |
| FA-Genu | 3.607 (1.557) | 0.022 | |
| PiB | −0.803 (0.306) | 0.01 | |
| FA-Genu × time | 0.904 (0.255) | <0.001 | |
| PiB × time | −0.246 (0.049) | <0.001 | |
|
FA-Genu+ PIB + Cortical thickness AIC 900.9365 |
Intercept | −5.543 (1.401) | <0.001 |
| Time | −1.193 (0.222) | <0.001 | |
| Education/occupation | 0.090 (0.029) | 0.003 | |
| FA-Genu | 2.833 (1.605) | 0.08 | |
| PiB | −0.738 (0.315) | 0.021 | |
| Cortical thickness | 0.650 (0.452) | 0.15 | |
| FA-Genu × time | 0.528 (0.268) | 0.049 | |
| PiB × time | −0.164 (0.052) | 0.002 | |
| Cortical thickness × time | 0.307 (0.076) | <0.001 |
Figure 1:
(Left) The predicted global cognitive trajectories for a 78 year old participant by time from baseline for a given FA-Genu and amyloid level. In the graph, low and high were defined by 25th and 75th percentiles. (Right) Spaghetti plot of global cognition as a function of age.
3.2. FA-HCB for prediction of longitudinal cognitive decline
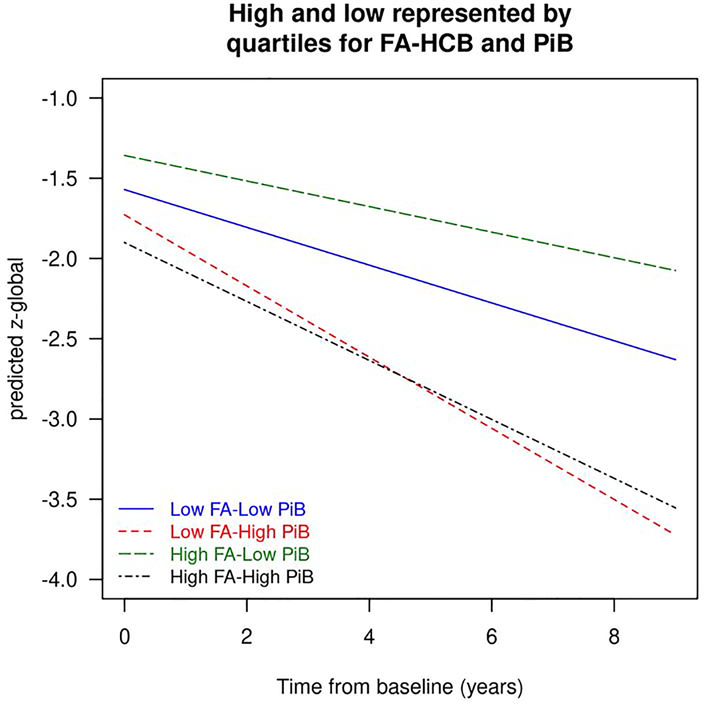
The results of the linear mixed effect models of FA-HCB, which were conducted as part of our sensitivity analyses, are presented in Table 3. In all three models of FA-HCB, education/occupation was an important component as in the FA-Genu models. Older age significantly predicted faster rate of annual cognitive decline in models with FA-HCB alone and model 2 (FA-HCB+ with PIB). FA-HCB had significant interactions with sex and PIB in model 2. FA-HCB predicted cognitive decline (interaction with time) only in models 1 and 2. In the third model (FA-HCB, PIB and cortical thickness), only PIB and cortical thickness but not FA-HCB significantly predicted the longitudinal rate of cognitive decline. However there were significant interactions of FA-HCB with PIB and FA-HCB with baseline age in model 3. The predicted cognition z-score trajectories by the FA-HCB and PiB levels maintaining education/occupation as constant are illustrated in Figure 2. We found that although the participants with low FA-HCB and high PIB declined fastest among the groups, the FA-HCB interacts with PIB. The Comparison of these results with FA-Genu suggests that FA-Genu is a better DTI marker than FA-HCB which may be in the pathway of the AD biomarker cascade.
Table 3:
Mixed models evaluating the utility of the hippocampal cingulum bundle FA (FA-HCB) in predicting cognitive performance
| Models | Variable | Coefficient (s.e.) | p-value |
|---|---|---|---|
|
FA-HCB AIC 968.5585 |
Intercept | 8.694 (5.222) | 0.10 |
| Time | 0.008 (0.260 | 0.98 | |
| Baseline age | −0.029 (0.013) | 0.028 | |
| Education/occupation | −0.844 (0.429) | 0.051 | |
| FA-HCB | −20.270 (11.221) | 0.073 | |
| FA-HCB × time | 1.002 (0.413) | 0.016 | |
| FA-HCB × education/occupation | 2.083 (0.946) | 0.03 | |
| Baseline age × time | −0.008 (0.002) | <0.001 | |
|
FA-HCB + PIB AIC 949.1162 |
Intercept | −2.306 (2.795) | 0.41 |
| Time | −0.046 (0.243) | 0.85 | |
| Baseline age | −0.021 (0.014) | 0.12 | |
| Male | −5.078 (2.156) | 0.02 | |
| Education/occupation | 0.078 (0.031) | 0.014 | |
| FA-HCB | 4.021 (5.477) | 0.46 | |
| PiB | 6.545 (3.458) | 0.061 | |
| FA-HCB × time | 0.823 (0.388) | 0.034 | |
| FA-HCB × male | 11.395 (4.773) | 0.019 | |
| FA-HCB × PiB | −15.960 (7.759) | 0.042 | |
| PiB × time | −0.199 (0.056) | <0.001 | |
| Baseline age × time | −0.005 (0.002) | 0.026 | |
|
FA-HCB+ PIB + Cortical thickness AIC 899.9934 |
Intercept | 21.230 (11.670 | 0.07 |
| Time | −1.074 (0.215) | <0.001 | |
| Baseline age | −0.412 (0.163) | 0.013 | |
| Education/occupation | 0.089 (0.030) | 0.003 | |
| FA-HCB | −49.805 (25.514) | 0.053 | |
| PiB | 9.937 (3.896) | 0.012 | |
| Cortical thickness | 0.252 (0.476) | 0.60 | |
| FA-HCB × baseline age | 0.868 (0.360) | 0.017 | |
| FA-HCB × PiB | −23.645 (8.760) | 0.008 | |
| PiB × time | −0.155 (0.054) | 0.004 | |
| Cortical thickness × time | 0.371 (0.072) | <0.001 | |
| FA-HCB × time | 0.568 (0.383) | 0.14 |
Figure 2:
The predicted global cognitive trajectories for a 78 year old participant by time from baseline for a given FA-HCB and amyloid level. In the graph, low and high were defined by 25th and 75th percentiles.
3.2. DTI association with WMH
The linear mixed model results with both DTI marker and WMH after adjusting for age, time from baseline, and education/occupation score results in a simplified model as shown in Table 4 because WMH drops out due to non-significant effect. Therefore only the DTI marker, FA-Genu (p=0.01) but not WMH, significantly predicted longitudinal cognition.
Table 4:
Mixed models evaluating the utility of the Genu FA (FA-Genu) in conjunction with WMH predicting cognitive performance
| Model | Variable | Coefficient | S.E | P-value |
|---|---|---|---|---|
|
FA-Genu + WMH AIC 995.102 |
Intercept | −2.462 | 1.694 | 0.147 |
| Time | −0.110 | 0.285 | 0.699 | |
| Baseline age | −0.026 | 0.014 | 0.066 | |
| Education/occupation | 0.103 | 0.034 | 0.003 | |
| FA-Genu | 2.231 | 1.866 | 0.234 | |
| WMH | −0.091 | 0.136 | 0.506 | |
| FA-Genu × time | 0.727 | 0.296 | 0.014 | |
| Baseline age × time | −0.006 | 0.002 | 0.011 | |
| WMH × time | −0.011 | 0.018 | 0.543 | |
| Educ/occ × time | −0.005 | 0.005 | 0.372 |
4. DISCUSSION
The present study aimed to examine the utility of DTI imaging for predicting cognitive decline in MCI individuals over 60 years of age in conjunction with brain amyloidosis and neurodegeneration. The major findings of this population based study are as follows:(1) FA-Genu was significantly associated with cognitive rate of decline in MCI individuals even after adjusting for information provided by amyloid deposition and neurodegeneration; (2) FA-HCB was not significantly associated with cognitive rate of decline after adjusting for amyloid deposition and neurodegeneration; and (3) only FA-Genu and not WMH was associated with cognitive decline when the model contained both WMH and FA-Genu.
DTI is a promising biomarker for studying the integrity of WM in MCI. Here, we found a significant association between WM structural integrity, measured by FA-Genu, and cognitive decline among MCI participants. Importantly, this association was independent of PIB and cortical thickness, supporting its utility as a biomarker in MCI.
The well-defined mechanism of cognitive impairment is that of cortical disconnection (Catani and ffytche, 2005; Lamar et al., 2008) which has been suggested to be influenced by CVD (Croall et al., 2017; Tu et al., 2017; Tuladhar et al., 2015). The CC is the largest WM tract in the brain and plays a crucial role in interhemispheric communication. The loss of these connections can disrupt connectivity of several cortical regions and recognized in age related cognitive declines (O’Sullivan et al., 2001). These WM changes can therefore be the underlying cause of widespread cognitive impairment in CVD characterized by predominant dysfunction in attention, psychomotor speed, and executive function.
Several lines of evidence indicate the relationship between frontal lobe damage and vascular disease (Knopman et al., 2015; Tullberg et al., 2004) suggesting that the genu of the CC is the most susceptible region to vascular disease effects. Further, FA-Genu was found to capture early changes due to systemic vascular health which lends itself as a useful CVD biomarker in MCI (Vemuri et al., 2018). As reported in prior studies (Croall et al., 2017; Vemuri et al., 2018), FA is a sensitive marker of CVD. We did not investigate MD here because it measures overall water diffusion and may be less specific to subtle microstructure WM abnormalities due to CSF contamination (CSFC). The contribution of partial volume effect due to CSFC has been found more problematic in brain structures with close proximity of ventricles such as the fornix and the genu of CC (Concha et al., 2005; Jones and Cercignani, 2010). Additionally, a greater CSFC for the diffusivity measurements than FA was demonstrated previously in the fornix of the older adults (Metzler-Baddeley et al., 2012) and genu of CC in the MCI subjects (Berlot et al., 2014).
Multiple studies have documented CC atrophy in clinically diagnosed MCI and AD dementia (Elahi et al., 2015; L. Wang et al., 2009), and some have reported predominant atrophy in the anterior part (Thomann et al., 2006; Zhu et al., 2012). However, the CC changes in MCI across studies have not been consistent (Thomann et al., 2006; H. Wang and Su, 2006). A previous Rotterdam Study in MCI patients with CVD found microstructural damage in the genu, internal and external capsule, and periventricular WM, but in those without CVD, deterioration was found along the hippocampal tract (Papma et al., 2014) suggesting that the anterior changes may be more specific to CVD. A consistent DTI change in the genu was also demonstrated in patients with subcortical vascular dementia (Chen et al., 2009) further supporting our use of FA-Genu.
One would argue against using a specific tract instead of global DTI measures. For example, data from the participants of Atherosclerosis Risk in Communities Study (ARIC) showed an association between overall DTI measures with cognitive decline, incident MCI, dementia and mortality longitudinally (Power et al., 2019). The same was true in a recent Harvard Aging Brain Study where a global FA measure significantly predicted longitudinal cognitive decline even after adjusting for amyloid level (Rabin et al., 2019). However we specifically chose FA-Genu because other limbic tracts such as hippocampal cingulum and fornix have been observed in preclinical AD (Chao et al., 2013; Gold et al., 2014; Jacobs et al., 2018; Rieckmann et al., 2016), suggesting its relationship with amyloid pathology. The sensitivity analyses we conducted in Table 3 support associations between HCB and AD pathophysiology. With amyloidosis and neurodegeneration in the model (model 3), we did not find any significant interaction between FA-HCB and time. The observed interaction between FA-HCB and PiB suggests that CGH may be a part of the AD pathway (Jacobs et al., 2018). More importantly, measuring FA-Genu we are able to capture the independent effect of vascular disease. We also performed sensitivity analyses using cognitive subdomains which indicated variability in associations across the domains as expected due to the heterogeneity seen in etiology of MCI (supplementary table).
Evaluating conventional CVD measures like WMH is important to determine the utility of DTI over traditional measures. Although the modifiable cardiovascular risk factors such as hypertension, diabetes and BMI are identified in association with WM integrity declines (Wassenaar et al., 2019), measurement of WMH provides the extent of CVD damage. While WMH alone was predictive of cognitive decline, we found that WMH was not predictive of cognitive decline in the combined linear mixed model with FA-Genu in the model. These findings support the independent utility of FA-Genu for predicting cognitive performance in MCI patients aged 60 years and older as it may be able to capture more subtle microstructural damage.
The present study has some strengths and limitations. A major strength is the availability of amyloid PET, MRI, and longitudinal cognitive outcomes in a population based sample. There are several limitations. We relied on simple and robust measures (genu FA and global cognitive- z score) to study the relationship between DTI metrics and longitudinal cognitive decline after accounting for AD biomarkers. The small sample size to detect subtle effects such as those with WMH may limit the findings of the study. Highly educated individuals in the cohort may have an influence on the generalizability of this study. There is some regional variability in the etiology of WMH as shown recently (Al-Janabi et al., 2018; Graff-Radford et al., 2019; Weaver et al., 2019) but we only considered global WMH in this work and not regional WMH because global WMH is the typically used CVD measure in most aging and dementia studies. Additionally, newer biophysical modelling methods that may allow for robust metrics such as NODDI (Neurite Orientation Dispersion and Density Imaging) and Free Water Elimination (FWE) may be helpful in providing better metrics of WM integrity and will be part of future investigations. In contrast to DTI, these techniques could reliably account for the partial volume effects by CSFC and therefore provide better sensitivity even in the asymptomatic/preclinical stages.
5. CONCLUSIONS:
Our findings illustrate the complex interplay between brain structure, brain function, and cognitive decline in MCI. Greater DTI measures (indicator of brain reserve or structure) predicted slower decline over time. Accounting for brain structure (neurodegeneration and WM integrity) and amyloid strengthen our findings. DTI has significant utility for prediction of cognitive decline in MCI from population-based sample and will provide complementary information to AD biomarkers.
Supplementary Material
Supplementary Table: The final mixed models evaluating the utility of the Genu FA in predicting domain specific cognitive performance
Highlights:
DTI changes in the genu of the corpus callosum predicts cognitive decline in MCI.
Poor white matter health provided prognostic information beyond Alzheimer’s disease biomarkers.
DTI measure in the genu provided greater prognostic information than the WMH.
Acknowledgments
We thank all the study participants and staff in the Mayo Clinic Study of Aging, Mayo Alzheimer’s Disease Research Center, and Aging Dementia Imaging Research laboratory at the Mayo Clinic for making this study possible. We gratefully acknowledge the support of NVIDIA Corporation for the donation of the Quadro P5000 GPU used in this research.
2. Funding and Disclosures
This work was supported by NIH grants R01 NS097495 (PI: Vemuri), R01 AG056366 (PI: Vemuri), U01 AG006786 (PI: Petersen), P50 AG016574 (PI: Petersen), R37 AG011378 (PI: Jack), R01 AG041851 (PIs: Jack and Knopman); R01 AG034676 (Rochester Epidemiology Project PI: Rocca); the Gerald and Henrietta Rauenhorst Foundation grant, the Millis Family, the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Foundation, Alzheimer’s Association (Zenith Fellows Award), Liston Award, Elsie and Marvin Dekelboum Family Foundation, Schuler Foundation, and Opus building NIH grant C06 RR018898 Dr. Knopman reported serving on a data safety monitoring board for the DIAN study, serving on a Data Safety monitoring Board for a tau therapeutic for Biogen, but receives no personal compensation, and serving as an investigator in a clinical trials sponsored by Lilly Pharmaceuticals and the University of Southern California, and receiving research support from the National Institutes of Health (NIH) outside the submitted work. Dr. Graff-Radford reported receiving research support from the National Institute on Aging outside the submitted work. Dr. Mielke reported receiving research support from the NIH, Department of Defense, and unrestricted research grants from Biogen outside the submitted work. Dr. Jack reported consulting for Eli Lilly, serving on an independent data monitoring board for Roche, and serving as a speaker for Eisai but receives no personal compensation from any commercial entity; he also reported receiving research support from the NIH and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Clinic. Dr. Petersen reported receiving consulting fees from Hoffman-La Roche Inc, Merck Inc, Genentech Inc, Biogen Inc, GE Healthcare, and Eisai Inc. outside the submitted work. Dr. Vemuri reported receiving grants from the NIH during the conduct of the study.
3. The data contained in the manuscript being submitted have not been previously published, have not been submitted elsewhere and will not be submitted elsewhere while under consideration at Neurobiology of Aging.
4. Standard protocol approvals, registrations, and patient consents: The study was approved by the Mayo Clinic and Olmsted Medical Center institutional review board and written informed consent was obtained from all participants.
5. All authors have reviewed the contents of the manuscript being submitted, approve of its contents and validate the accuracy of the data.
Footnotes
1. Disclosure: The authors report no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Janabi OM, Brown CA, Bahrani AA, Abner EL, Barber JM, Gold BT, … Jicha GA (2018). Distinct White Matter Changes Associated with Cerebrospinal Fluid Amyloid-β1–42 and Hypertension. J Alzheimers Dis, 66(3), 1095–1104. doi: 10.3233/jad-180663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JLR, Graham MS, Zsoldos E, & Sotiropoulos SN (2016). Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. Neuroimage, 141, 556–572. doi: 10.1016/j.neuroimage.2016.06.058 [DOI] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, & Gee JC (2011). A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage, 54(3), 2033–2044. doi: 10.1016/j.neuroimage.2010.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlot R, Metzler-Baddeley C, Jones DK, & O’Sullivan MJ (2014). CSF contamination contributes to apparent microstructural alterations in mild cognitive impairment. Neuroimage, 92(100), 27–35. doi: 10.1016/j.neuroimage.2014.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruscoli M, & Lovestone S (2004). Is MCI really just early dementia? A systematic review of conversion studies. Int Psychogeriatr, 16(2), 129–140. [DOI] [PubMed] [Google Scholar]
- Catani M, & ffytche DH (2005). The rises and falls of disconnection syndromes. Brain, 128(Pt 10), 2224–2239. doi: 10.1093/brain/awh622 [DOI] [PubMed] [Google Scholar]
- Chao LL, Decarli C, Kriger S, Truran D, Zhang Y, Laxamana J, … Weiner MW (2013). Associations between white matter hyperintensities and beta amyloid on integrity of projection, association, and limbic fiber tracts measured with diffusion tensor MRI. PLoS One, 8(6), e65175. doi: 10.1371/journal.pone.0065175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TF, Lin CC, Chen YF, Liu HM, Hua MS, Huang YC, & Chiu MJ (2009). Diffusion tensor changes in patients with amnesic mild cognitive impairment and various dementias. Psychiatry Res, 173(1), 15–21. doi: 10.1016/j.pscychresns.2008.09.002 [DOI] [PubMed] [Google Scholar]
- Concha L, Gross DW, & Beaulieu C (2005). Diffusion tensor tractography of the limbic system. AJNR Am J Neuroradiol, 26(9), 2267–2274. [PMC free article] [PubMed] [Google Scholar]
- Croall ID, Lohner V, Moynihan B, Khan U, Hassan A, O’Brien JT, … Markus HS (2017). Using DTI to assess white matter microstructure in cerebral small vessel disease (SVD) in multicentre studies. Clin Sci (Lond), 131(12), 1361–1373. doi: 10.1042/cs20170146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elahi S, Bachman AH, Lee SH, Sidtis JJ, & Ardekani BA (2015). Corpus callosum atrophy rate in mild cognitive impairment and prodromal Alzheimer’s disease. J Alzheimers Dis, 45(3), 921–931. doi: 10.3233/jad-142631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garyfallidis E, Brett M, Amirbekian B, Rokem A, van der Walt S, Descoteaux M, & Nimmo-Smith I (2014). Dipy, a library for the analysis of diffusion MRI data. Front Neuroinform, 8, 8. doi: 10.3389/fninf.2014.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BT, Zhu Z, Brown CA, Andersen AH, LaDu MJ, Tai L, … Smith CD (2014). White matter integrity is associated with cerebrospinal fluid markers of Alzheimer’s disease in normal adults. Neurobiol Aging, 35(10), 2263–2271. doi: 10.1016/j.neurobiolaging.2014.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff-Radford J, Arenaza-Urquijo EM, Knopman DS, Schwarz CG, Brown RD, Rabinstein AA, … Vemuri P (2019). White matter hyperintensities: relationship to amyloid and tau burden. Brain, 142(8), 2483–2491. doi: 10.1093/brain/awz162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr., Wiste HJ, Weigand SD, Therneau TM, Lowe VJ, Knopman DS, … Petersen RC (2017). Defining imaging biomarker cut points for brain aging and Alzheimer’s disease. Alzheimers Dement, 13(3), 205–216. doi: 10.1016/j.jalz.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs HIL, Hedden T, Schultz AP, Sepulcre J, Perea RD, Amariglio RE, … Johnson KA (2018). Structural tract alterations predict downstream tau accumulation in amyloid-positive older individuals. Nat Neurosci, 21(3), 424–431. doi: 10.1038/s41593-018-0070-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, & Cercignani M (2010). Twenty-five pitfalls in the analysis of diffusion MRI data. NMR Biomed, 23(7), 803–820. doi: 10.1002/nbm.1543 [DOI] [PubMed] [Google Scholar]
- Kellner E, Dhital B, Kiselev VG, & Reisert M (2016). Gibbs-ringing artifact removal based on local subvoxel-shifts. Magn Reson Med, 76(5), 1574–1581. doi: 10.1002/mrm.26054 [DOI] [PubMed] [Google Scholar]
- Knopman DS, Griswold ME, Lirette ST, Gottesman RF, Kantarci K, Sharrett AR, … Mosley TH Jr. (2015). Vascular imaging abnormalities and cognition: mediation by cortical volume in nondemented individuals: atherosclerosis risk in communities-neurocognitive study. Stroke, 46(2), 433–440. doi: 10.1161/strokeaha.114.007847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koay CG, Ozarslan E, & Basser PJ (2009). A signal transformational framework for breaking the noise floor and its applications in MRI. J Magn Reson, 197(2), 108–119. doi: 10.1016/j.jmr.2008.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamar M, Catani M, Price CC, Heilman KM, & Libon DJ (2008). The impact of region-specific leukoaraiosis on working memory deficits in dementia. Neuropsychologia, 46(10), 2597–2601. doi: 10.1016/j.neuropsychologia.2008.04.007 [DOI] [PubMed] [Google Scholar]
- Lopresti BJ, Klunk WE, Mathis CA, Hoge JA, Ziolko SK, Lu X, … Price JC (2005). Simplified quantification of Pittsburgh Compound B amyloid imaging PET studies: a comparative analysis. J Nucl Med, 46(12), 1959–1972. [PubMed] [Google Scholar]
- Metzler-Baddeley C, O’Sullivan MJ, Bells S, Pasternak O, & Jones DK (2012). How and how not to correct for CSF-contamination in diffusion MRI. Neuroimage, 59(2), 1394–1403. doi: 10.1016/j.neuroimage.2011.08.043 [DOI] [PubMed] [Google Scholar]
- O’Sullivan M, Summers PE, Jones DK, Jarosz JM, Williams SC, & Markus HS (2001). Normal-appearing white matter in ischemic leukoaraiosis: a diffusion tensor MRI study. Neurology, 57(12), 2307–2310. doi: 10.1212/wnl.57.12.2307 [DOI] [PubMed] [Google Scholar]
- Oishi K, Faria A, Jiang H, Li X, Akhter K, Zhang J, … Mori S (2009). Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: application to normal elderly and Alzheimer’s disease participants. Neuroimage, 46(2), 486–499. doi: 10.1016/j.neuroimage.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papma JM, de Groot M, de Koning I, Mattace-Raso FU, van der Lugt A, Vernooij MW, … Smits M (2014). Cerebral small vessel disease affects white matter microstructure in mild cognitive impairment. Hum Brain Mapp, 35(6), 2836–2851. doi: 10.1002/hbm.22370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC (2009). Early diagnosis of Alzheimer’s disease: is MCI too late? Curr Alzheimer Res, 6(4), 324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, … Winblad B (2001). Current concepts in mild cognitive impairment. Arch Neurol, 58(12), 1985–1992. doi: 10.1001/archneur.58.12.1985 [DOI] [PubMed] [Google Scholar]
- Petersen RC, Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz VS, … Rocca WA (2010). Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology, 75(10), 889–897. doi: 10.1212/WNL.0b013e3181f11d85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power MC, Su D, Wu A, Reid RI, Jack CR, Knopman DS, … Mosley TH (2019). Association of white matter microstructural integrity with cognition and dementia. Neurobiol Aging, 83, 63–72. doi: 10.1016/j.neurobiolaging.2019.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley H, Colloby SJ, & O’Brien JT (2011). PET imaging of brain amyloid in dementia: a review. Int J Geriatr Psychiatry, 26(10), 991–999. doi: 10.1002/gps.2640 [DOI] [PubMed] [Google Scholar]
- Rabin JS, Perea RD, Buckley RF, Neal TE, Buckner RL, Johnson KA, … Hedden T (2019). Global White Matter Diffusion Characteristics Predict Longitudinal Cognitive Change Independently of Amyloid Status in Clinically Normal Older Adults. Cereb Cortex, 29(3), 1251–1262. doi: 10.1093/cercor/bhy031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid RI, Nedelska Z, Schwarz CG, Ward C, Jack CR,. (2018). Diffusion specific segmentation: skull stripping with diffusion MRI data alone (F. G. In: Kaden E, Ning L, Tax C, Veraart J, eds Ed.): Cham: Springer. [Google Scholar]
- Rieckmann A, Van Dijk KR, Sperling RA, Johnson KA, Buckner RL, & Hedden T (2016). Accelerated decline in white matter integrity in clinically normal individuals at risk for Alzheimer’s disease. Neurobiol Aging, 42, 177–188. doi: 10.1016/j.neurobiolaging.2016.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, … Rocca WA (2008). The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology, 30(1), 58–69. doi: 10.1159/000115751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, & Melton LJ 3rd. (2012). History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc, 87(12), 1202–1213. doi: 10.1016/j.mayocp.2012.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz CG, Gunter JL, Wiste HJ, Przybelski SA, Weigand SD, Ward CP, … Jack CR Jr. (2016). A large-scale comparison of cortical thickness and volume methods for measuring Alzheimer’s disease severity. Neuroimage Clin, 11, 802–812. doi: 10.1016/j.nicl.2016.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Pankratz JJ, Brue SM, & Rocca WA (2012). Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol, 41(6), 1614–1624. doi: 10.1093/ije/dys195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomann PA, Wustenberg T, Pantel J, Essig M, & Schroder J (2006). Structural changes of the corpus callosum in mild cognitive impairment and Alzheimer’s disease. Dement Geriatr Cogn Disord, 21(4), 215–220. doi: 10.1159/000090971 [DOI] [PubMed] [Google Scholar]
- Tu MC, Lo CP, Huang CF, Hsu YH, Huang WH, Deng JF, & Lee YC (2017). Effectiveness of diffusion tensor imaging in differentiating early-stage subcortical ischemic vascular disease, Alzheimer’s disease and normal ageing. PLoS One, 12(4), e0175143. doi: 10.1371/journal.pone.0175143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuladhar AM, van Norden AG, de Laat KF, Zwiers MP, van Dijk EJ, Norris DG, & de Leeuw FE (2015). White matter integrity in small vessel disease is related to cognition. Neuroimage Clin, 7, 518–524. doi: 10.1016/j.nicl.2015.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullberg M, Fletcher E, DeCarli C, Mungas D, Reed BR, Harvey DJ, … Jagust WJ (2004). White matter lesions impair frontal lobe function regardless of their location. Neurology, 63(2), 246–253. doi: 10.1212/01.wnl.0000130530.55104.b5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri P, Lesnick TG, Przybelski SA, Graff-Radford J, Reid RI, Lowe VJ, … Jack CR Jr. (2018). Development of a cerebrovascular magnetic resonance imaging biomarker for cognitive aging. Ann Neurol, 84(5), 705–716. doi: 10.1002/ana.25346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri P, Lesnick TG, Przybelski SA, Machulda M, Knopman DS, Mielke MM, … Jack CR Jr. (2014). Association of lifetime intellectual enrichment with cognitive decline in the older population. JAMA Neurol, 71(8), 1017–1024. doi: 10.1001/jamaneurol.2014.963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veraart J, Novikov DS, Christiaens D, Ades-Aron B, Sijbers J, & Fieremans E (2016). Denoising of diffusion MRI using random matrix theory. Neuroimage, 142, 394–406. doi: 10.1016/j.neuroimage.2016.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, & Su MY (2006). Regional pattern of increased water diffusivity in hippocampus and corpus callosum in mild cognitive impairment. Dement Geriatr Cogn Disord, 22(3), 223–229. doi: 10.1159/000094934 [DOI] [PubMed] [Google Scholar]
- Wang L, Goldstein FC, Veledar E, Levey AI, Lah JJ, Meltzer CC, … Mao H (2009). Alterations in cortical thickness and white matter integrity in mild cognitive impairment measured by whole-brain cortical thickness mapping and diffusion tensor imaging. AJNR Am J Neuroradiol, 30(5), 893–899. doi: 10.3174/ajnr.A1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassenaar TM, Yaffe K, van der Werf YD, & Sexton CE (2019). Associations between modifiable risk factors and white matter of the aging brain: insights from diffusion tensor imaging studies. Neurobiol Aging, 80, 56–70. doi: 10.1016/j.neurobiolaging.2019.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver NA, Doeven T, Barkhof F, Biesbroek JM, Groeneveld ON, Kuijf HJ, … Biessels GJ (2019). Cerebral amyloid burden is associated with white matter hyperintensity location in specific posterior white matter regions. Neurobiol Aging, 84, 225–234. doi: 10.1016/j.neurobiolaging.2019.08.001 [DOI] [PubMed] [Google Scholar]
- Williams OA, Zeestraten EA, Benjamin P, Lambert C, Lawrence AJ, Mackinnon AD, … Barrick TR (2017). Diffusion tensor image segmentation of the cerebrum provides a single measure of cerebral small vessel disease severity related to cognitive change. Neuroimage Clin, 16, 330–342. doi: 10.1016/j.nicl.2017.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Gao W, Wang X, Shi C, & Lin Z (2012). Progression of corpus callosum atrophy in early stage of Alzheimer’s disease: MRI based study. Acad Radiol, 19(5), 512–517. doi: 10.1016/j.acra.2012.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table: The final mixed models evaluating the utility of the Genu FA in predicting domain specific cognitive performance