Abstract
Objective.
Osteoarthritis (OA) is a common cause of joint pain and disability, and effective treatments are lacking. Extracellular adenosine has anti-inflammatory effects and can prevent and treat OA in animal models. Ticagrelor and clopidogrel are both used in patients with coronary artery disease, but only ticagrelor increases extracellular adenosine. The aim of this study was to determine whether treatment with ticagrelor was associated with a lower risk of OA.
Methods.
We conducted a 1:2 propensity score matching analysis using the Optum Clinformatics™ Data Mart from 2011 to 2017. We included patients who received either ticagrelor or clopidogrel for at least 90 days and excluded those with a prior diagnosis of OA or inflammatory arthritis. OA was identified using International Classification of Diseases codes. The primary outcome was the time to diagnosis of OA after treatment with ticagrelor versus clopidogrel.
Results.
Our propensity score matched cohort consisted of 7,007 ticagrelor-treated patients and 14,014 clopidogrel-treated patients, with a median number of days on treatment of 287 and 284 respectively. For both groups, the mean age was 64 years, and 73% of the patients were male. Multivariate Cox-regression analysis estimated a hazard ratio of 0.71 (95% CI 0.64–0.79, p<0.001) for developing OA after treatment with ticagrelor compared to clopidogrel.
Conclusion.
Treatment with ticagrelor was associated with a 29% lower risk of developing OA compared to clopidogrel over five years of follow-up. We hypothesize that the reduction in OA seen in patients who received ticagrelor may in part be due to increased extracellular adenosine.
Keywords: Osteoarthritis, Adenosine, Ticagrelor, Clopidogrel, Arthritis
INTRODUCTION
Osteoarthritis (OA) is a major cause of joint pain and disability, affecting roughly 27 million adults in the United States, and poses a significant economic burden [1–3]. The knees, hips, hands, and spine are most commonly affected, leading to joint deformity and dysfunction. OA is no longer viewed simply as degeneration in the joints, but rather a disease that is driven by low-grade inflammation and complex interactions between genes and the environment [4, 5]. There are no effective treatments to prevent the onset or progression of OA, and novel therapies are needed [6, 7].
Preclinical models of OA have provided important insights about disease pathogenesis and potential pathways for therapeutic intervention. One such pathway involves adenosine, which is produced endogenously from adenosine triphosphate hydrolysis and serves as an important regulator of inflammation [8]. Extracellular adenosine has been shown to reduce inflammatory responses and ameliorate OA in mouse and rat models [9, 10]. These findings suggest that extracellular adenosine may play an important role in reducing cartilage inflammation and damage, and that it might serve as a therapeutic target for preventing and treating OA in humans.
Ticagrelor and clopidogrel are antiplatelets agents used to treat patients with coronary artery disease. They both work by antagonizing the binding of adenosine diphosphate to the P2Y12 receptor on platelets, thereby reducing platelet aggregation [11]. Ticagrelor was approved by the Food and Drug Administration in 2011 and clopidogrel in 1997.
Ticagrelor differs from clopidogrel in that ticagrelor secondarily increases extracellular adenosine by inhibiting the nucleoside transporter ENT1, which prevents intracellular uptake of adenosine [8, 12–14]. Unlike ticagrelor, clopidogrel does not increase extracellular adenosine concentrations, as it does not antagonize ENT1 [12]. Based on this difference, we hypothesized that ticagrelor, but not clopidogrel, would be protective against the development of OA in humans.
This study aims to investigate if patients who received ticagrelor for at least 90 days had a lower risk of developing OA compared to those who received clopidogrel for at least 90 days, using propensity score matching. The Optum Clinformatics™ Data Mart was used to determine the time to diagnosis of OA after treatment with ticagrelor versus clopidogrel.
MATERIALS AND METHODS
Data source and cohort definitions.
Data for this study were obtained from Optum Clinformatics™ Data Mart. This database is comprised of administrative health claims from a large national managed care company affiliated with Optum and Medicare Advantage Part D members. It includes 15–18 million annual covered lives in the United States and contains de-identified clinical information abstracted from medical records, pharmacy, medical claims, and mortality data. The population is geographically diverse and spans all 50 states.
We used data from January 1, 2011 to December 31, 2017. We included patients who had received prescriptions for either ticagrelor or clopidogrel for at least 90 days based on their brand name or generic name coding. We defined “time zero” (or, the index date) as 90 days after the start of either ticagrelor or clopidogrel. All patients who were included had at least 365 days of continuous enrollment prior to starting treatment with ticagrelor or clopidogrel.
We excluded patients who had either (a) a prior diagnosis of OA or any inflammatory arthritis, defined as having at least two claims for OA or inflammatory arthritis separated by 7 days or more with International Classification of Disease (ICD) codes 9 or 10, or (b) a Common Procedural Technology (CPT) code for hip or knee arthroplasty in the 365 days prior to starting treatment or during the first 90 days of treatment with ticagrelor or clopidogrel (Supplementary Table 1). Patients who received both ticagrelor and clopidogrel were excluded (n = 15,470). We also excluded patients with more than one episode of treatment with ticagrelor or clopidogrel (n = 107,166), defined as the start of a second prescription of either ticagrelor or clopidogrel > 120 days from the prior. Study patients were followed from 90 days after starting treatment with either ticagrelor or clopidogrel until they were newly diagnosed with OA, no longer present in the Optum database, or until the end of the follow-up period (December 31, 2017).
Data collection.
The study design is illustrated in Supplementary Figure 1. We collected baseline covariates, including demographics (age, sex, race/ethnicity, education level, and household income), and comorbidities, which were identified by scanning all diagnosis codes in the 365 days prior to the start of treatment with ticagrelor or clopidogrel. This data was used to calculate the Charlson comorbidity score [15].
We also calculated the days exposed to treatment at the time of censoring (Supplementary Figure 2). Specifically, for those subjects who were censored while on treatment with ticagrelor or clopidogrel (after time zero but before the treatment ended), days exposed to treatment were calculated as the number of days between time zero and the censoring date. For patients who were censored at some point after the treatment ended or at the end of the follow-up period, the days exposed to treatment at the time of censoring were defined as the total number of treatment days after time zero.
Outcomes.
The primary outcome was the time to diagnosis of OA after at least 90 days of treatment with ticagrelor versus clopidogrel. Patients were defined as having OA if they had at least two medical claims with an ICD-9 or ICD-10 code for OA separated by 7 days or more (Supplementary Table 1) [16–18]. The secondary outcomes were the diagnosis of OA subtypes (hand, knee, or hip) and the occurrence of hip or knee arthroplasty.
Statistical methods.
A 1:2 propensity score matched cohort of patients who received ticagrelor versus clopidogrel was created using the following variables: age, sex, race/ethnicity, Charlson comorbidity score, days exposed to treatment (at the time of censoring), and the duration of enrollment after treatment initiation. Each patient who received ticagrelor was matched to two patients who received clopidogrel. Standardized mean difference (SMD), a measure of balance between the two groups after propensity score matching, was calculated using the method implemented in the ‘tableone’ package in R (Table 1 and 2). We considered estimates of SMD that were less than 0.2 to be a negligible difference.
Table 1.
Baseline characteristics of patients before and after 1:2 propensity score matching
| Before PS-matching | After PS-matching |
|||||
|---|---|---|---|---|---|---|
| Ticagrelor (n = 7007) |
Clopidogrel (n = 112940) |
SMD | Ticagrelor (n = 7007) |
Clopidogrel (n = 14014) |
SMD |
|
| Age in years, mean (SD) | 63.8 (11.7) | 69.2 (11.8) | 0.461 | 63.8 (11.7) | 63.9 (12.6) | 0.011 |
| Gender, n (%) | 0.239 | 0.006 | ||||
| Female | 1897 (27.1) | 43161 (38.2) | 1897 (27.1) | 3834 (27.4) | ||
| Male | 5108 (72.9) | 69745 (61.8) | 5108 (72.9) | 10176 (72.6) | ||
| Unknown | 2 (0.0) | 34 (0.0) | 2 (0.0) | 4 (0.0) | ||
| Race, n (%) | 0.070 | 0.009 | ||||
| White | 4493 (64.1) | 72643 (64.3) | 4493 (64.1) | 9004 (64.3) | ||
| Black | 537 (7.7) | 10185 (9.0) | 537 (7.7) | 1069 (7.6) | ||
| Asian | 225 (3.2) | 3048 (2.7) | 225 (3.2) | 435 (3.1) | ||
| Hispanic | 612 (8.7) | 10442 (9.2) | 612 (8.7) | 1225 (8.7) | ||
| Unknown | 1140 (16.3) | 16622 (14.7) | 1140 (16.3) | 2281 (16.3) | ||
| Education, n (%) | 0.105 | 0.097 | ||||
| Less than 12th grade | 31 (0.4) | 816 (0.7) | 31 (0.4) | 100 (0.7) | ||
| High school diploma | 2125 (30.3) | 37779 (33.5) | 2125 (30.3) | 4721 (33.7) | ||
| Less than bachelor’s degree | 3830 (54.7) | 60991 (54.0) | 3830 (54.7) | 7448 (53.1) | ||
| Bachelor’s degree or higher | 1004 (14.3) | 12973 (11.5) | 1004 (14.3) | 1695 (12.1) | ||
| Unknown | 17 (0.2) | 381 (0.3) | 17 (0.2) | 50 (0.4) | ||
| Household income range, n (%) | 0.254 | 0.158 | ||||
| < $40K | 1361 (19.4) | 29346 (26.0) | 1361 (19.4) | 3140 (22.4) | ||
| $40K–$49K | 427 (6.1) | 8782 (7.8) | 427 (6.1) | 1050 (7.5) | ||
| $50K–$59K | 471 (6.7) | 9325 (8.3) | 471 (6.7) | 1083 (7.7) | ||
| $60K–$74K | 684 (9.8) | 12032 (10.7) | 684 (9.8) | 1464 (10.4) | ||
| $75K–$99K | 1045 (14.9) | 15429 (13.7) | 1045 (14.9) | 1967 (14.0) | ||
| ≥ $100K | 1997 (28.5) | 21830 (19.3) | 1997 (28.5) | 3140 (22.4) | ||
| Unknown | 1022 (14.6) | 16196 (14.3) | 1022 (14.6) | 2170 (15.5) | ||
| Charlson comorbidity score, n (%) | 0.227 | 0.030 | ||||
| 0 | 3410 (48.7) | 45068 (39.9) | 3410 (48.7) | 6819 (48.7) | ||
| 1–2 | 2247 (32.1) | 36343 (32.2) | 2247 (32.1) | 4397 (31.4) | ||
| 3–4 | 973 (13.9) | 21482 (19.0) | 973 (13.9) | 2000 (14.3) | ||
| 5–6 | 271 (3.9) | 7260 (6.4) | 271 (3.9) | 606 (4.3) | ||
| > 6 | 106 (1.5) | 2787 (2.5) | 106 (1.5) | 192 (1.4) | ||
PS = propensity score; SMD = standardized mean difference; SD = standard deviation.
Table 2.
Treatment characteristics before and after propensity score matching
| Before PS-matching | After PS-matching | |||||
|---|---|---|---|---|---|---|
| Ticagrelor (n = 7007) |
Clopidogrel (n = 112940) |
SMD |
Ticagrelor (n = 7007) |
Clopidogrel (n = 14014) |
SMD |
|
| Days treated, median (IQR) | 287 (186, 400) | 392 (231, 783) | 0.491 | 287 (186, 400) | 284 (186, 436) | 0.043 |
| 90–180 days, n (%) | 1649 (23.5) | 19340 (17.1) | 1649 (23.5) | 3220 (23.0) | ||
| 181–365 days, n (%) | 3273 (46.7) | 33575 (29.7) | 3273 (46.7) | 6344 (45.3) | ||
| > 365 days, n (%) | 2085 (29.8) | 60025 (53.1) | 2085 (29.8) | 4450 (31.8) | ||
| Year treatment started, n (%) | 0.842 | 0.580 | ||||
| 2011 | 11 (0.2) | 14718 (13.0) | 11 (0.2) | 1340 (9.6) | ||
| 2012 | 255 (3.6) | 15776 (14.0) | 255 (3.6) | 1492 (10.6) | ||
| 2013 | 726 (10.4) | 17130 (15.2) | 726 (10.4) | 1715 (12.2) | ||
| 2014 | 809 (11.5) | 15564 (13.8) | 809 (11.5) | 1642 (11.7) | ||
| 2015 | 1144 (16.3) | 16806 (14.9) | 1144 (16.3) | 1935 (13.8) | ||
| 2016 | 1701 (24.3) | 17383 (15.4) | 1701 (24.3) | 2601 (18.6) | ||
| 2017 | 2361 (33.7) | 15563 (13.8) | 2361 (33.7) | 3289 (23.5) | ||
| Days enrolled after 90 days of treatment, median (IQR) | 433 (243, 788) | 733 (363, 1294) | 0.600 | 433 (243, 788) | 408 (259, 746) | 0.019 |
PS = propensity score; SMD = standardized mean difference; IQR = interquartile range; OA = osteoarthritis.
Logistic regression analysis was used to estimate the propensity score. Participants were matched on the logit of the propensity score using calipers of width equal to 0.2 of the pooled estimate of the common standard deviation of the logits of the propensity scores [19]. This caliper width has been found to result in optimal estimation of absolute and relative risk differences in a variety of settings [20, 21]. Missing data for the categorical variables are reported in Table 1 as “Unknown.” For the continuous variables, there were no missing data.
We used a multivariate Cox-regression model to estimate the hazard ratios (HR) of the primary and secondary outcomes comparing ticagrelor to clopidogrel with and without adjustment for patient characteristics (age, sex, race/ethnicity, Charlson comorbidity score, days exposed to treatment at the time of censoring, and duration of enrollment after treatment initiation) (Table 3 and Supplementary Table 2).
Table 3.
Incidence of osteoarthritis in patients who received ticagrelor versus clopidogrel
| Before PS-matching | After PS-matching | |||
|---|---|---|---|---|
| Treatment | Ticagrelor (n = 7007) |
Clopidogrel (n = 112940) |
Ticagrelor (n = 7007) |
Clopidogrel (n = 14014) |
| Patients diagnosed with OA, n (%) | 534 (7.6) | 17844 (15.8) | 534 (7.6) | 1429 (10.2) |
| Person-years | 8505 | 209396 | 8505 | 16013 |
| IR (95% CI)* | 62.8 (57.7–68.4) | 85.2 (84.0–86.5) | 62.8 (57.7–68.4) | 89.2 (84.7–93.4) |
| HR (95% CI)† | 0.68 (0.63–0.74) | 1.0 | 0.71 (0.64–0.79) | 1.0 |
PS = propensity score; OA = osteoarthritis;
IR = incidence rate per 1,000 person-years;
HR = adjusted hazard ratio; 95% CI = 95% confidence interval. Incidence rate was estimated with Poisson regression of log link.
Incidence rates and the 95% confidence intervals for each group were estimated with Poisson regression with a log link (Table 3). Incidence rate was reported as the number of events per 1,000 person years.
Kaplan-Meier curves were created to report the time to diagnosis of OA or inflammatory arthritis among patients who received ticagrelor versus clopidogrel, and log-rank test was performed to compare the distributions between the two groups (Figure 2 and Supplementary Figure 3).
Figure 2.
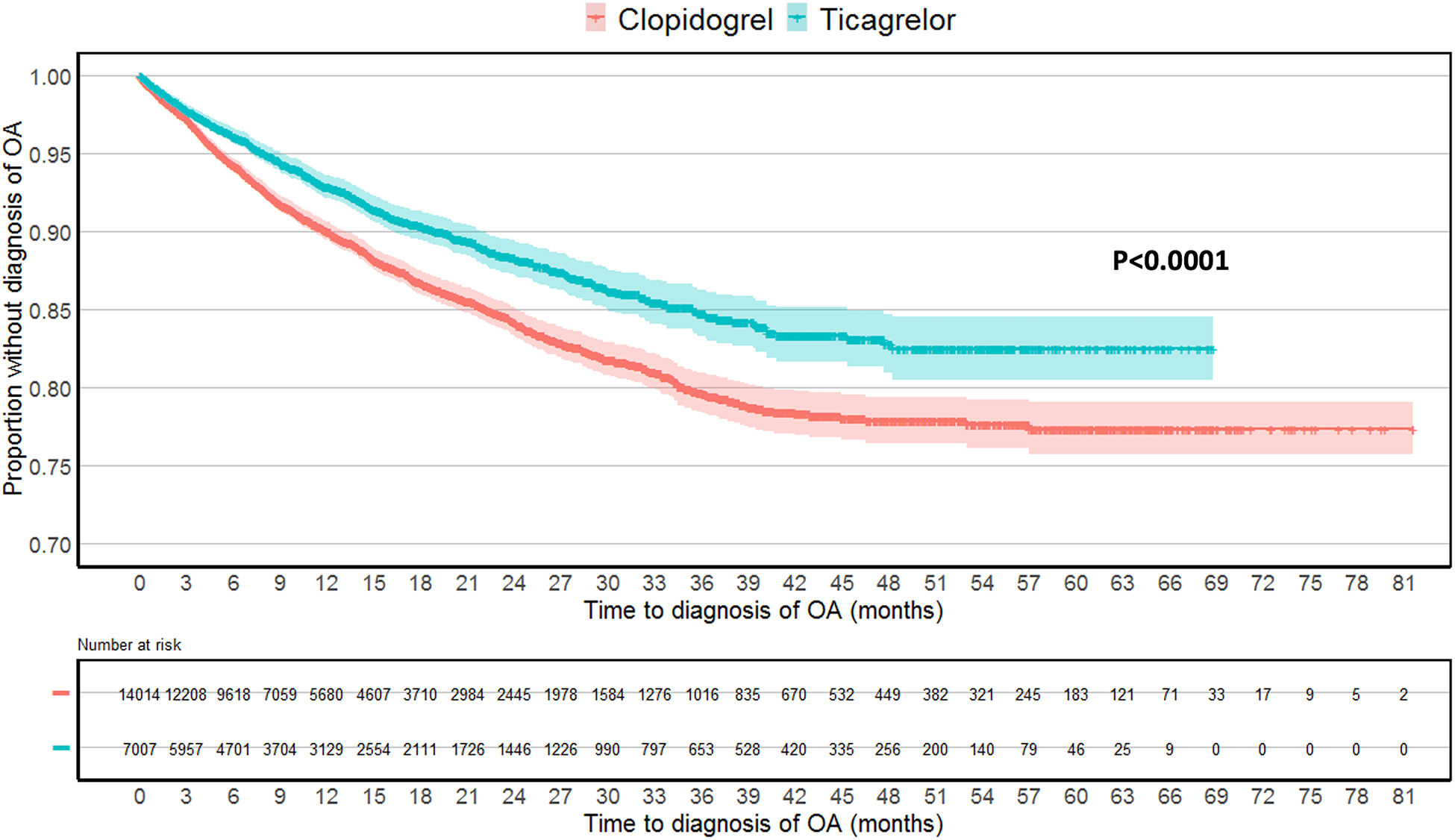
Kaplan-Meier plot of time to osteoarthritis diagnosis in the propensity score matched cohorts. Log-rank test was used to compare the two survival distributions.
Sensitivity analysis was performed with the entire cohort of eligible patients before propensity score matching, with and without adjusting for the confounding variables (Table 3, Supplementary Table 2, Supplementary Figure 3).
Analyses were conducted using SAS, version 9.4, and Charlson comorbidity scores were calculated using the ‘icd’ package implemented in R3.4.3. Type I error was controlled at 0.05. The Stanford University Institutional Review Board exempted this study from review since this is a de-identified database (Federal regulation 45 CFR 46.102).
RESULTS
We identified 119,947 patients with no known diagnosis of OA, inflammatory arthritis, or prior arthroplasty. Of these, 7,007 patients received ticagrelor for at least 90 days, and 112,940 patients received clopidogrel for at least 90 days (Figure 1). Prior to matching, patients who received ticagrelor were younger (mean age 63.8 years versus 69.2 years), more likely to be male (72.9% versus 61.8%), had a lower Charlson comorbidity score (48.7% with a score of 0 versus 39.9%), fewer days on treatment (median duration 287 days versus 392 days), and shorter duration of enrollment after 90 days of treatment (median of 433 days versus 733 days) (Table 1 and Table 2).
Figure 1.
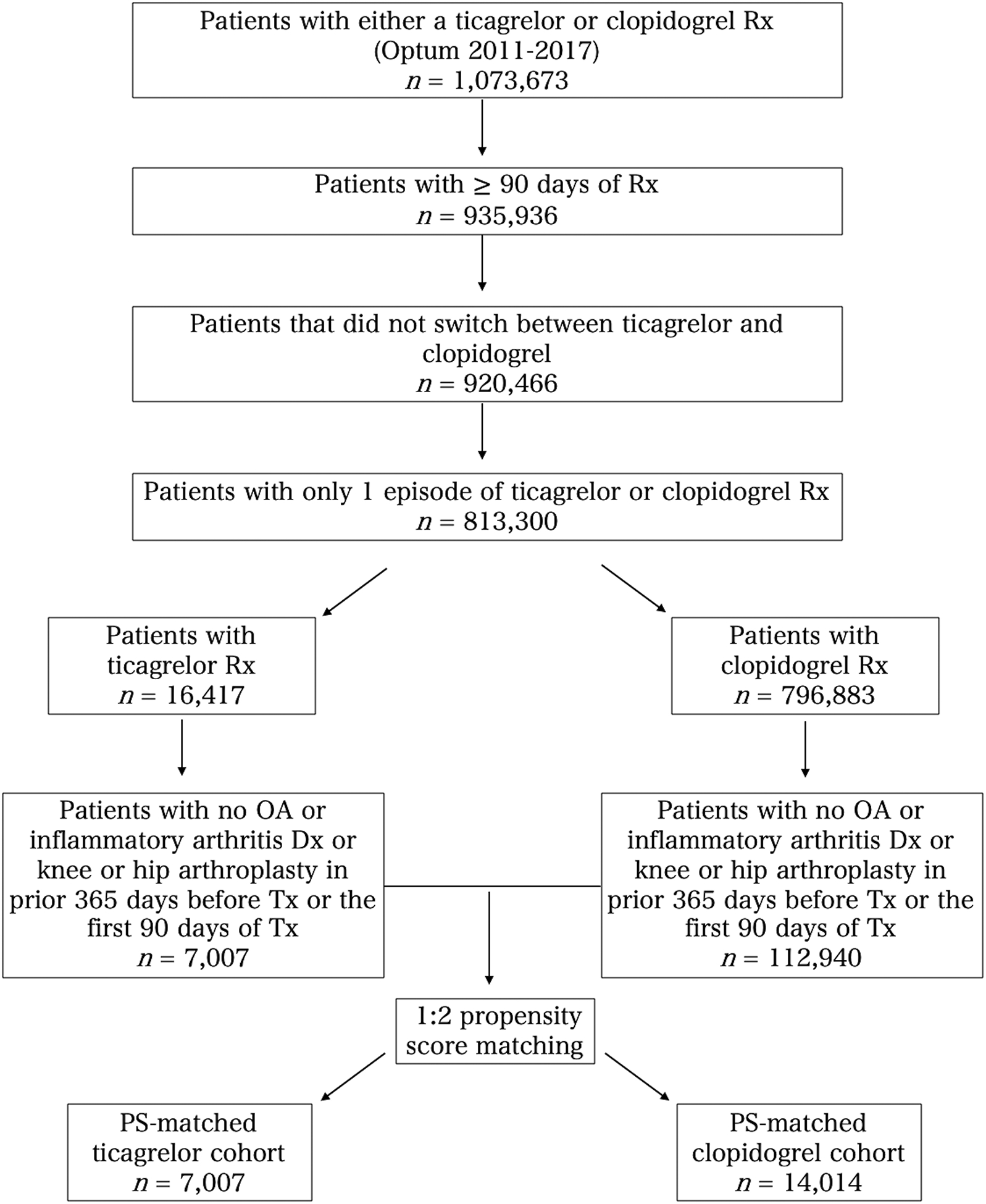
Flow chart of study cohort selection. Rx = prescription; OA = osteoarthritis; Dx = diagnosis; Tx = treatment; PS = propensity score.
After propensity score matching, 7,007 patients who received ticagrelor and 14,014 patients who received clopidogrel were included (Figure 1). Tables 1 and 2 compare the characteristics of patients in the two groups.
Primary outcome.
After propensity score matching, the incidence rate of OA diagnosis for patients who received ticagrelor was 62.8 per 1,000 person years, compared to 89.2 per 1,000 person years for patients who received clopidogrel (Table 3). In the adjusted analysis, patients who received ticagrelor were 29% less likely to develop OA compared to those who received clopidogrel (HR 0.71 [95% CI, 0.64–0.79], p<0.001) (Table 3).
The Kaplan-Meier plot demonstrated that patients who received ticagrelor were less likely to be diagnosed with OA over time, with a visual separation of the curves by 6 months from time zero (p<0.0001 with log-rank test), and this persisted at 5 years after the index date (Figure 2).
Secondary outcomes.
Among patients who received ticagrelor compared to clopidogrel, there was a lower risk of developing OA of the hands (HR 0.71 [95% CI, 0.53–0.96], p=0.025) and knees (HR 0.769 [95% CI, 0.58–0.83], p<0.001) but not hips (HR 0.72 [95% CI, 0.34–1.53], p=0.394) (Supplementary Table 2). The number of patients undergoing hip or knee arthroplasty was small in both groups (23 patients in the ticagrelor group versus 49 patients in the clopidogrel group), and no significant difference in the risk of joint arthroplasty between the two groups was observed (HR 0.90 [95% CI, 0.55–1.49], p=0.690) (Supplementary Table 3).
Sensitivity analysis.
The results from the unadjusted and adjusted analyses for the entire cohort of eligible patients was similar before and after propensity score matching (Table 3, Supplementary Figure 3). The sensitivity analysis also demonstrated a significantly reduced risk of hand and knee OA among patients treated with ticagrelor compared to clopidogrel (Table 3, Supplementary Table 2, Supplementary Figure 3).
DISCUSSION
In this large cohort of patients, we found that patients who received ticagrelor were 29% less likely to develop OA compared to those who received clopidogrel. OA-free survival was significantly higher in the ticagrelor group compared to the clopidogrel group, which became evident by month 6 and persisted until the end of follow-up at five years. Ticagrelor appeared to reduce the risk of all OA subtypes, with significant reductions in hand and knee OA. There was no difference in the risk of undergoing knee or hip arthroplasty between the ticagrelor and clopidogrel groups, however the event rate was too low to draw a meaningful conclusion.
We postulate that the potential underlying biologic mechanism explaining these findings is an increase in extracellular adenosine caused by ticagrelor, which does not occur with clopidogrel. In mouse models, it has been demonstrated that mice lacking the A2A adenosine receptor, which is responsible for transmitting the anti-inflammatory effects of adenosine, develop spontaneous OA as they age [9, 22]. In rat models of post-traumatic OA, intra-articular treatment with liposomal adenosine ameliorates disease [10]. Prior work has shown that methotrexate, a commonly used disease modifying anti-rheumatic drug for the treatment of rheumatoid arthritis (RA), in part exerts its anti-inflammatory effect by increasing extracellular adenosine [23]. There is minimal data evaluating this pathway in humans with OA, however preliminary data from The Pain Reduction with Oral Methotrexate in knee Osteoarthritis (PROMOTE) trial suggests methotrexate may improve OA symptoms as well [24].
In our study, patients were treated with ticagrelor for a median of 287 days. We believe that even this relatively short treatment period may provide protection against developing OA over the subsequent years. It is not clear how long this effect may last, as the follow-up period for this study ended at five years, with a relatively small number of patients at risk after three years.
This study has several limitations. First, this is a retrospective study using claims data, and there may be residual or unmeasured confounders. We used propensity score matching to balance the covariates and adjusted for baseline characteristics, including age, sex, race/ethnicity, Charlson comorbidity score, days on treatment, and the duration of follow-up after treatment. Second, we did not have data on important variables such as obesity (a known risk factor for OA and cardiovascular disease), body mass index, history of trauma to the joints, or level of physical activity. Although these variables are risk factors for OA, they would not affect the decision to receive ticagrelor or clopidogrel, and hence are not potential confounders. Third, since the majority of patients who have cardiovascular disease (and are therefore more likely to receive treatment with ticagrelor or clopidogrel) are older, this study may have excluded patients who developed OA at a young age. If OA that presents at a young age has a different pathobiology, we cannot comment on the potential effectiveness of ticagrelor in such cases. Fourth, with the use of ICD and CPT codes there is a risk of misclassification of variables and outcomes, however we believe this is likely to be non-differential between the two groups. Fifth, we were unable to account for the competing risk of death due to limitations of our dataset. However, we do not expect this to differ by treatment group after propensity score matching. Lastly, we cannot determine the degree of medication compliance in either group.
This study has several strengths. We used a large claims database covering patients in a wide geographic area in the United States. We chose clopidogrel-treated patients as a comparator group because they are inherently similar to ticagrelor-treated patients, thus minimizing the impact of measured confounding. We had longitudinal follow-up for up to five years. We were able to ask a question and evaluate a pathway in humans that is unlikely to be assessed with an interventional study due to the possibility of adverse events.
In this large, nationwide cohort of patients treated with either ticagrelor or clopidogrel, we found a 29% decreased risk of developing OA in patients who received ticagrelor. We believe the lower incidence of OA among patients who received ticagrelor may be due to an increase in extracellular adenosine—an effect not seen with clopidogrel. Our findings suggest that the development of drugs that increase extracellular adenosine concentrations by inhibiting the nucleoside transporter ENT1, or through other mechanisms, might prevent or treat OA in humans. Ticagrelor carries a risk of bleeding due to its antiplatelet effects, and thus we hope that data from this study will inform future research to identify safer therapies for OA that can similarly increase extracellular adenosine.
Supplementary Material
Acknowledgments:
We thank John and Jacque Jarve for their generous support.
Funding Sources: Matthew Baker received support for this work from the KL2 component of the Stanford Clinical and Translational Science Award to Spectrum (NIH KL2 TR 001083).
Footnotes
Conflicts of Interest: None
REFERENCES
- 1.Lawrence RC, et al. , Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum, 2008. 58(1): p. 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glyn-Jones S, et al. , Osteoarthritis. Lancet, 2015. 386(9991): p. 376–87. [DOI] [PubMed] [Google Scholar]
- 3.Bijlsma JW, Berenbaum F, and Lafeber FP, Osteoarthritis: an update with relevance for clinical practice. Lancet, 2011. 377(9783): p. 2115–26. [DOI] [PubMed] [Google Scholar]
- 4.Berenbaum F, et al. , Modern-day environmental factors in the pathogenesis of osteoarthritis. Nat Rev Rheumatol, 2018. 14(11): p. 674–681. [DOI] [PubMed] [Google Scholar]
- 5.Robinson WH, et al. , Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol, 2016. 12(10): p. 580–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McAlindon TE and Bannuru RR, Osteoarthritis in 2017: Latest advances in the management of knee OA. Nat Rev Rheumatol, 2018. 14(2): p. 73–74. [DOI] [PubMed] [Google Scholar]
- 7.Ghouri A and Conaghan PG, Prospects for Therapies in Osteoarthritis. Calcif Tissue Int, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cattaneo M, Schulz R, and Nylander S, Adenosine-mediated effects of ticagrelor: evidence and potential clinical relevance. J Am Coll Cardiol, 2014. 63(23): p. 2503–2509. [DOI] [PubMed] [Google Scholar]
- 9.Corciulo C, et al. , Endogenous adenosine maintains cartilage homeostasis and exogenous adenosine inhibits osteoarthritis progression. Nat Commun, 2017. 8: p. 15019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X, et al. , Adenosine-Functionalized Biodegradable PLA-b-PEG Nanoparticles Ameliorate Osteoarthritis in Rats. Sci Rep, 2019. 9(1): p. 7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurbel PA, et al. , Combination Antiplatelet and Oral Anticoagulant Therapy in Patients With Coronary and Peripheral Artery Disease. Circulation, 2019. 139(18): p. 2170–2185. [DOI] [PubMed] [Google Scholar]
- 12.Li X, et al. , Ticagrelor Compared with Clopidogrel Increased Adenosine and Cyclic Adenosine Monophosphate Plasma Concentration in Acute Coronary Syndrome Patients. Basic Clin Pharmacol Toxicol, 2017. 120(6): p. 610–614. [DOI] [PubMed] [Google Scholar]
- 13.Mediero A, et al. , Ticagrelor regulates osteoblast and osteoclast function and promotes bone formation in vivo via an adenosine-dependent mechanism. FASEB J, 2016. 30(11): p. 3887–3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonello L, et al. , Ticagrelor increases adenosine plasma concentration in patients with an acute coronary syndrome. J Am Coll Cardiol, 2014. 63(9): p. 872–7. [DOI] [PubMed] [Google Scholar]
- 15.Quan H, et al. , Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol, 2011. 173(6): p. 676–82. [DOI] [PubMed] [Google Scholar]
- 16.Funck-Brentano T, et al. , Causal Factors for Knee, Hip, and Hand Osteoarthritis: A Mendelian Randomization Study in the UK Biobank. Arthritis Rheumatol, 2019. 71(10): p. 1634–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hindy G, et al. , Cardiometabolic Polygenic Risk Scores and Osteoarthritis Outcomes: A Mendelian Randomization Study Using Data From the Malmo Diet and Cancer Study and the UK Biobank. Arthritis Rheumatol, 2019. 71(6): p. 925–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarmanova A, et al. , Statin use and risk of joint replacement due to osteoarthritis and rheumatoid arthritis: a propensity-score matched longitudinal cohort study. Rheumatology (Oxford), 2020. [DOI] [PubMed] [Google Scholar]
- 19.Rosenbaum PR and Rubin DB, Constructing a Control Group Using Multivariate Matched Sampling Methods That Incorporate the Propensity Score. The American Statistician, 1985. 39(1): p. 33–38. [Google Scholar]
- 20.Austin PC, Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat, 2011. 10(2): p. 150–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Austin PC, The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med, 2014. 33(7): p. 1242–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bekisz JM, et al. , The Role of Adenosine Receptor Activation in Attenuating Cartilaginous Inflammation. Inflammation, 2018. 41(4): p. 1135–1141. [DOI] [PubMed] [Google Scholar]
- 23.Brown PM, Pratt AG, and Isaacs JD, Mechanism of action of methotrexate in rheumatoid arthritis, and the search for biomarkers. Nat Rev Rheumatol, 2016. 12(12): p. 731–742. [DOI] [PubMed] [Google Scholar]
- 24.Conaghan PG, et al. , Therapeutic options for targeting inflammatory osteoarthritis pain. Nat Rev Rheumatol, 2019. 15(6): p. 355–363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


