Abstract
Purpose
To obtain high-sensitivity CEST maps by exploiting the spatiotemporal correlation between CEST images.
Methods
A post-processing method accomplished by multilinear singular value decomposition (MLSVD) was used to enhance the CEST signal-to-noise ratio (SNR) by exploiting the correlation between the Z-spectrum for each voxel and the low-rank property of the overall CEST data. The performance of this method was evaluated using creatine CEST (CrCEST) in ischemic mouse brain at 11.7 T. Then, MLSVD CEST was applied to obtain Cr, amide, and amine CEST maps of the ischemic mouse brain to demonstrate its general applications.
Results
Complex-valued Gaussian noise was added to CEST k-space data to mimic low SNR situation. MLSVD CEST analysis was able to suppress the noise, recover the degraded CEST peak, and provide better CrCEST quality compared to the smoothing and singular value decomposition (SVD) based denoising methods. High-resolution Cr, amide, and amine CEST maps of an ischemic stroke using MLSVD CEST suggest that CrCEST is also a sensitive pH mapping method, and a wide range of pH changes can be detected by combing CrCEST with amine CEST at high magnetic fields.
Conclusions
MLSVD CEST provides a simple and efficient way to improve the SNR of CEST images.
Keywords: Chemical exchange saturation transfer (CEST), Creatine (Cr), Amide, Amine, Ischemic stroke, pH Mapping, Polynomial and Lorentzian line-shape fitting (PLOF), Singular value decomposition (SVD), Multilinear singular value decomposition (MLSVD)
Introduction
Chemical exchange saturation transfer (CEST) MRI is a versatile technique that can significantly enhance the sensitivity of detecting low concentrations of proteins and metabolites through their exchangeable protons (1–8). Nowadays, this technique has been successfully applied to detecting proteins (9–11) and Nuclear Overhauser Enhancement (NOE) (12–16) in tissues, as well as various endogenous metabolites, such as glycogen (17), glutamate (18), glycosaminoglycan (12), creatine (19–23), and phosphocreatine (19,24–26). However, due to the strong scaled-down effect produced by the magnetization transfer contrast (MTC) induced by the macromolecules, CEST contrasts in tissues are significantly reduced compared to the CEST signal in solutions (27–30), which leads to a diminished signal-to-noise ratio (SNR) and challenges the accuracy of CEST quantification. Many efforts have been devoted to optimizing certain saturation parameters (e.g., saturation power and length) to increase CEST contrasts in tissues (30–33), but improvements following this strategy have been limited. Reducing the noise and oscillations in the Z-spectrum is an alternative way to improve the CEST SNR. For example, some of the noise and oscillations in the Z-spectrum are introduced through subject physiological motions or MRI instability, which could be solved using motion insensitive MRI methods, such as the self-gated technique UTE-CEST (13), or using navigators to correct frequency fluctuations (34,35). Some post-processing methods have also been proposed to reduce the noise and oscillations in the Z-spectrum by exploiting the redundancy of CEST images between different saturation offsets, such as singular value decomposition (SVD) (36,37) and principal component analysis (PCA) (38–40).
Recently, one powerful reconstruction method, called Spectroscopic Imaging by exploiting spatiospectral CorrElation (SPICE) (41,42) and one post-processing tensor factorization denoising method (43), have been developed in the magnetic resonance spectroscopy (MRS) community by making use of a property known as the partial separability of spectroscopic signals. This property indicates that high-dimensional spectroscopic signals can be well represented by a very low-dimensional subspace, and that the SNR of MRS can be dramatically improved without sacrificing accuracy. Noted that this partial separability property is also exploited in SVD and PCA methods implemented by two-dimensional tensor decomposition (36–40). Inspired by these techniques, here we proposed a higher-order tensor decomposition for better exploiting the partial separability of CEST images and improving the SNR of CEST contrasts. The higher-order tensor decomposition was accomplished using multilinear singular value decomposition (MLSVD) (44). The performance of the MLSVD CEST was compared with that of other denoising methods in CEST MRI. For the application and validation of the method, MLSVD CEST was applied to obtain high-resolution creatine (Cr), amide and amine CEST images from mouse brains using an ischemic stroke model. The wide range of Cr, amide, and amine CEST intensities, due to the pH drop in the infarct and penumbra zones (11,45), forms an excellent model to test the efficiency and accuracy of MLSVD CEST.
Methods
MLSVD CEST Theory
The purpose of MLSVD CEST is to remove noise in the Z-spectrum using a tensor decomposition-based low-rank denoising method. Without losing generality, the following derivation assumes the acquisition of a series of 2-dimensional (2D) images with different frequency offsets, whereas the processing of a series of 3-dimensional (3D) images with different frequency offsets can be performed in complete accordance with the procedures hereafter.
For 2D images with different frequency offsets, the acquired CEST data can be stored in a 3D array matrix M with dimensions x, y, and n. The variables x and y refer to the number of pixels in the two spatial domains, and n stands for the number of frequency offsets adopted in the Z-spectrum (Fig. 1b). The acquired data M can be written in the following form using MLSVD (44):
| (1) |
where U1, U2, and U3 are orthonormal matrices, and S refers to the core tensor. MLSVD was accomplished by the Tensorlab v3.0 toolbox (https://www.tensorlab.net/). Due to the spatiospectral correlation of CEST data, M can be approximately represented by a low-rank model with reduced noise level.(41,42,46). The rank numbers xtrun, ytrun, and ntrun were adopted to truncate the orthonormal matrices and core tensor, and the denoising CEST data could then be obtained using the following equation:
| (2) |
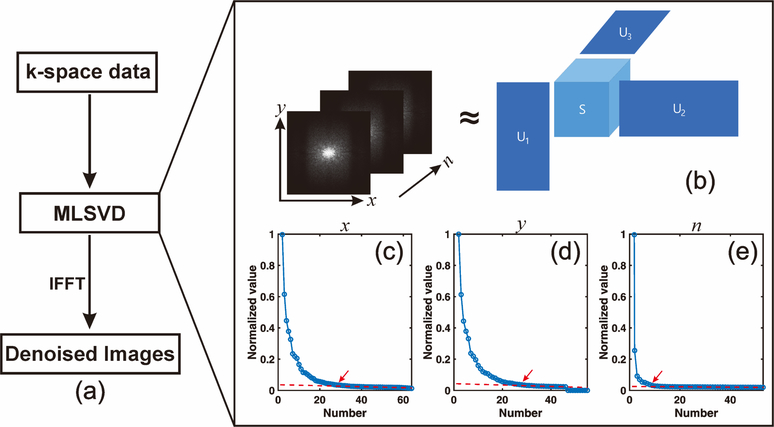
Fig. 1.
(a) Flowchart of MLSVD denoising. (b) Schematic diagram of truncated multilinear singular value decomposition of k-space data at different frequency offsets n. (c~d) The multilinear singular values along different dimensions (two spatial, one frequency offset). The rank number at the corner indicated by the red arrow was selected to truncate the orthonormal matrices and core tensor.
The truncated rank numbers were set to the corner of multilinear singular value L-curve along different dimensions using the following steps. First, the last part (40% in this study) of the plot of multilinear singular value versus rank number was selected to fit a linear reference line. Second, the multilinear singular values were subtracted from this linear reference line, and the first index for which the difference was smaller than a certain threshold (0.02 in this study) was selected as the truncated rank number.
Animal Preparation
The procedures on mice were approved by the Johns Hopkins University Animal Care and Use Committee. The transient middle cerebral artery occlusion (MCAO) stroke model was performed on three C57BL/6 male mice (age: 5 months). The mice were anesthetized initially with 4% isoflurane followed by 2% isoflurane during surgery. An incision was made at the midline of the neck, and the common carotid artery and external carotid artery were ligated with silk suture. A filament was inserted into the external carotid artery and threaded forward into the internal carotid artery until the tip occluded the origin of the MCA. An MCA occlusion period of 60 min was applied, followed by reperfusion. The MRI experiments were performed approximately one hour after surgery.
MRI Experiments
All MRI experiments were performed on a horizontal bore 11.7 T Bruker Biospec system (Bruker, Ettlingen, Germany). For the animal studies, a 72 mm quadrature volume resonator was used as a transmitter, and a four-element (2 × 2) phased array cryoprobe was used as a receiver. The animals were anesthetized using 2% isoflurane in medical air, followed by 1% to 1.5% isoflurane for maintenance during the MRI scan. The mouse head was positioned using a bite bar and two ear pins. During the MRI scan, the mice were placed on a water-heated animal bed equipped with temperature and respiratory controls. Respiratory rates were monitored via a pressure sensor (SAII, Stony Brook, NY, USA) and maintained at 30 to 50 breaths per minute. The B0 field over the image slice was adjusted using field-mapping and second-order shimming.
All the CEST experiments were recorded using continuous-wave CEST experiments. MR images were acquired using a Turbo Spin Echo (TSE) sequence with TE = 18 ms, TR=5 s, TSE factor = 20, slice thickness = 1 mm, a matrix size of 64 × 64, and a resolution of 0.25 × 0.25 mm2. Different saturation powers and saturation lengths were used for Cr, amine, and amide CEST MRIs (i.e., 2 μT and 1s for Cr, 3 μT and 0.4 s for amine, 1 μT and 3 s for amide), since previous studies had already demonstrated that the maximum amide CEST is achieved with 1 μT saturation power in a high field (47). Although the maximum CrCEST guanidinium signal occurs at around 1.5 μT (30), 2 μT was chosen to minimize the competing protein guanidinium signal (22). Similar to CrCEST, the maximum amine CEST was achieved with 1.5 to 2 μT saturation power (30); however, a high saturation power (3 μT) was also used for the amine CEST to suppress contaminations from slow or intermediate-exchanging protons (30). The saturation lengths were chosen based on the discovery that the optimal saturation length for in vivo CEST signals is determined by R1ρ relaxation time (30). The R1 relaxation of the mouse brain was measured with the variable TR RARE (RAREVTR) (TR = 5.5, 3.0, 1.5, 0.8, 0.5, 0.3 s), with identical image slices as those used in the CEST studies.
CEST mapping using polynomial and Lorentzian line-shape fitting (PLOF)
The PLOF method for extracting a CEST signal with a discernible peak has been well described previously (22,24,26,30). Briefly, the normalized saturation signal at each frequency offset is given by (48–51):
| (3) |
| (4) |
Where Zss is the steady-state Z-spectral intensity, R1 is the longitudinal relaxation rate of water, tsat is the saturation time, and θ = tan−1ω1/Δ is the tilt angle of the effective magnetization with respect to the Z-axis induced by a saturation pulse with nutation frequency ω1 at frequency offset Δ. R1ρ is the water relaxation rate under the saturation pulse, which includes contributions from the effective water relaxation Reff and an apparent relaxation term Rback related to all saturation transfer processes other than the detected CEST signal (Rexch) in tissue (50):
| (5) |
where Reff = cos2θR1 + sin2θR2, is the measured longitudinal relaxation rate of water in the rotating frame without additional solution components. The observed CEST signal ΔZ is given by:
| (6) |
The CEST peak (Rexch) and broad background signal (Rback) in Z-spectrum can be represented as a Lorentzian function and a polynomial function, respectively:
| (7) |
| (8) |
where w is the peak full-width-at-half-maximum (FWHM) of the Lorentzian line-shape. is the true apparent relaxation rate contribution of the CEST peak. Δexch is the chemical shift offset of the CEST peak relative to the water signal; Cn terms are the zero to M-order polynomial coefficients. The initial chemical shift offsets δini for Cr, amide and amine were set to 1.95 ppm, 3.6 ppm and 2.7 ppm with respect to water, respectively. The Z-spectral range of [δini − 1, δini + 1 ] (ppm) was used for PLOF. For Cr and amide, the Z-spectral range between [δini − 0.4, δini + 0.4 ] (ppm) was excluded from the background fitting. For amine, the Z-spectral range between [δini − 0.6, δini + 0.6 ] (ppm) was excluded from the background fitting, which was determined by the optimization shown in Supporting Information Figure S3.
Results
Validation of MLSVD CEST
The schematic diagram of MLSVD CEST is shown in Fig. 1(b). The original CrCEST maps were used as a reference, and complex valued Gaussian noise was added to CEST k-space data to mimic the low SNR situation. In MLSVD CEST, the noise of the CEST data was suppressed by truncating the orthonormal matrices and core signal intensity tensor. As shown in Figs. 1b–d, the multilinear singular values decayed dramatically with the increase of rank number, especially along the offset dimension. The rank numbers around the corner of the L-curve, indicated by the red arrow, were chosen for truncation, which were 30, 30, and 10 for x, y and n dimensions, respectively. From the difference maps shown in Supporting Information Fig. S1, it can be seen that the adopted truncated rank numbers could efficiently remove noise while maintaining useful information such as image contrasts.
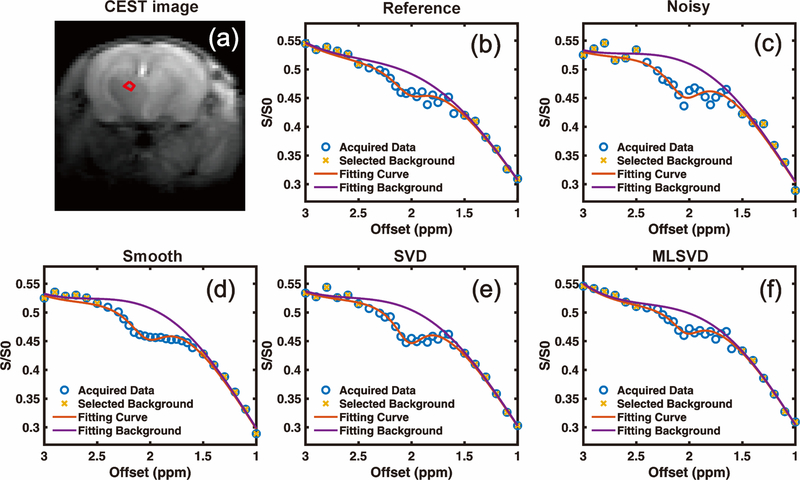
The original Z-spectrum from a small ROI before noise addition is plotted in Fig. 2a for reference. The Z-spectrum with noise added is plotted in Fig. 2c. The Z-spectra obtained by the smooth method via the Matlab build-in function smooth with a span number of three and the SVD method (40) from the same CEST images with added noise are illustrated in Figs. 2d and 2e, respectively. The choice of truncated rank number for the SVD method is illustrated in Supporting Information Fig. S2. These extracted Z-spectra were fitted using the PLOF method following Eqs. 6–8. Without additional denoising, the CEST peak at 1.95 ppm was indiscernible and could not be well fitted with PLOF, as shown in Fig. 2(c). From Fig. 2 (d & e), both smooth and SVD methods can reduce the oscillation induced by additional noise, however, the restored CEST contrast (i.e. 5.56% for smooth and 5.82% for SVD) was clearly larger than the original before noise addition (3.83%). From the results shown in Fig. 2(f), it is clear that MLSVD CEST can well suppress the noise and recover the degraded CEST peak. The CEST contrast obtained by MLSVD CEST (i.e. 3.66 %) was closest to the reference.
Fig. 2.
Effect of different denoising methods on the Z-spectrum. The noisy images were generated by adding complex valued Gaussian noise to CEST k-space data. (a) M0 image at Δω = 200 ppm. The ROI circumscribed by the red circle was selected for extracting a Z-spectrum. (b) Reference Z-spectrum before adding noise. (c) Z-spectrum extracted from noisy images without denoising. Z-spectrum obtained after applying (d) smooth function, (e) SVD and (f) MLSVD.
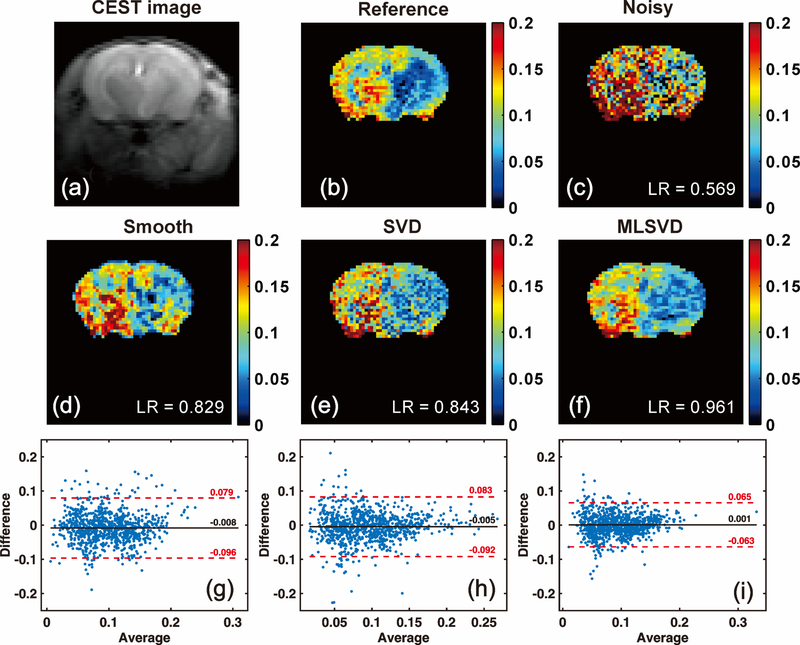
To further evaluate the performance of different denoising methods, CrCEST maps (e.g. the maps) were calculated and displayed in Fig. 3. Due to the lower pH values in the ischemic regions, a clearly reduced Cr CEST contrast was observed on the ipsilateral side of the mouse brain. However, it is more difficult to distinguish the ischemic regions in the CrCEST with added noise, as shown in Fig. 3(c). All these three methods yielded much improved CrCEST maps compared to that without denoising, in which the ischemic region could be well distinguished. To provide an objective evaluation, we calculated the voxel-by-voxel linear regression (LR) between the denoising results and the reference. The Bland-Altman plots between the reference and the denoising results are also given in Fig. 3(g~i). From the LR scores and Bland-Altman plots, it is clear that MLSVD CEST yielded the best CrCEST map among the three denoising methods.
Fig. 3.
Cr maps obtained by PLOF with different denoising methods. (a) M0 image at Δω = 200 ppm. (b) Reference map without adding noise. (c) map determined from Z-spectra with additional random noise imposed. The denoising maps obtained by (d) the smooth function, (e) the SVD method and (f) the MLSVD approach before applying PLOF. The linear regression (LR) between reference and denoising map was calculated by Matlab built-in function regress. Bland-Altman plots between the reference and the denoising results obtained by (g) the smooth function, (h) SVD and (i) MLSVD, respectively.
Cr, amide, and amine maps with MLSVD CEST
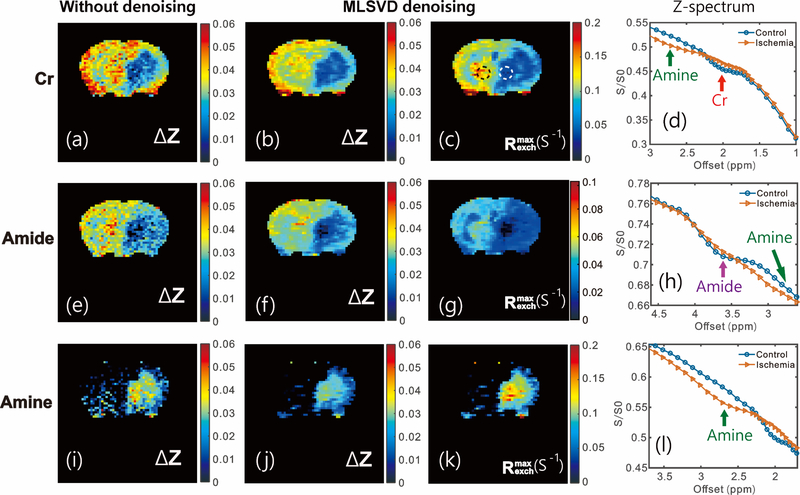
We further applied MLSVD CEST to obtain Cr, amide, and amine CEST maps in vivo in the mouse MCAO stroke model and the results are plotted in Fig. 4. Overall, the image quality improved significantly after MLSVD processing (Figs. 4a, b, e, f, i, j). The ischemic regions are more clearly visible after MLSVD for all proton types. As both Cr and amide CEST show distinguishable peaks in the Z-spectra at physiological pH values, as shown in Figs. 4d and h, respectively, their CEST signals could be extracted via PLOF, and the maps are plotted in Figs. 4a–c, e–g. Both the Cr and amide contrasts show strong pH dependence, as indicated by the much-reduced Cr and slightly-reduced amide CEST values in the ischemic zone and confirmed by the Z-spectra in the main ischemic region in which both Cr and amide CEST peaks are barely observable (Figs. 4d and h). The CrCEST effect (ΔZ = 0.036 ± 0.05) was slightly higher than the amide CEST (ΔZ = 0.03 ± 0.06) peak for this MRI field and saturation power, as shown by the ΔZ values in the contralateral regions (Figs. 4b and f). For the amine CEST, there was no resonance visible in the spectrum at physiological pH (Fig. 4l). However, a peak could be clearly seen at lower pH (52) that could be extracted via the PLOF method. The amine-weighted image is significantly enhanced at low pH values (Fig. 4i–k), which is opposite to the Cr and amide CEST contrasts, indicating that the slowed-down exchange places the proton exchange rate in a more appropriate exchange regime for CEST detection.
Fig. 4.
Typical ΔZ CEST maps obtained from the PLOF method for Cr (a,b), amide (e,f) and amine (i,j) protons. CEST maps obtained before (a,e,i) and after (b,f,j) applying the MLSVD method. The MLSVD CEST maps for Cr (c), amide (g) and amine (k) are shown together with the Z-spectra extracted from the main ischemic region (white dashed circle) and a contralateral control region (black dash circle) for Cr (2 μT and 1s) (d), amide (1 μT and 3s ) (h) and amine (3 μT and 0.4s)(1), respectively.
Discussion
In this paper, we tested a tensor decomposition- based low-rank denoising method, dubbed MLSVD CEST, to improve the SNR of the Z-spectrum and yield high-sensitivity CEST maps of mouse brains. By selecting appropriate truncation numbers in the spatial and offset domains, we demonstrated that the MLSVD denoising algorithm could reduce noise significantly while maintaining CEST contrasts. This is critical for CEST applications, considering that the majority of them have a magnitude on the order of only a few percents of the water signal.
One-dimensional denoising methods, such as the smooth function, remove noise voxel-by-voxel by averaging the Z-spectrum over certain range; however, for Z-spectra with low CEST contrast and high noise levels, such methods will suppress both noise and CEST contrast, leading to greatly underestimated quantification results (Fig. 2d and Fig. 3d). SVD and PCA methods utilize the spectral correlation between all voxels throughout the entire image to reduce noise levels (36–40). Compared to SVD and PCA methods, MLSVD CEST can exploit the additional spectral correlation between all the rows and columns of the CEST images, and hence yield better noise reduction performance, as demonstrated in Figs. 2 and 3. MLSVD and SVD retain optimal performance when the noise is Gaussian distribution, such as the noise in complex k-space. Noted that MLSVD and SVD can also be applied to the magnitude image domain when the raw data or complex image space data is unavailable. In that case, the performance of MLSVD and SVD may be further improved by applying it in more suitable domains or by adapting the noise model to fit magnitude Rician noise (53) as demonstrated in (54).
MLSVD CEST can be applied to other CEST contrasts, as demonstrated in the amide and amine CEST images (Fig. 4). The CEST signal at 1.95 ppm is a mixture of slow-exchanging protein and intermediate-exchanging Cr guanidinium protons, as recently shown in a knockout mouse model (22) and an ex vivo study using homogenous rat brain tissue (23). Previous studies have demonstrated that the 1.95 ppm signal is dominated by guanidinium protons (22) with low saturation powers (< 0.6 μT), and the exchange rate increases with lower pH values across the physical pH range (55,56). A similar pH dependence was observed for urea (57). On the other hand, the exchange rate of the Cr guanidinium protons decreased significantly with lower pH values, as confirmed by phantom studies (58). The current pH-dependent CrCEST results confirm our previous result that, with a saturation power of 2 μT, Cr dominates the CEST signal at 1.95 ppm with a contribution of around 66% (30). Our data also indicate that CrCEST can be applied to mapping pH in the brain at high fields, similar to amide CEST. However, one needs to be cautious in the interpretation, because the Cr concentration will increase in ischemic regions due to the conversion of phosphocreatine into Cr (59,60). Under the current acquisition conditions, we were also able to observe the amine peaks from mobile proteins at lower pH values, with a center frequency at around 2.7 ppm. The amine CEST showed potential for sensitive pH mapping in low pH ranges, since the amine CEST increases strongly, but in this case due to the much-reduced exchange rates at the low pH values leading to better detection by moving from the fast to intermediate exchange regime. Recently amine-based CEST mapping (29) has also been suggested for tumor pH mapping (61,62).
The measurement of T2 is not necessary for the calculation of Reff due to the high magnetic field applied in this study, which leads to large frequency offsets. The saturation powers used for the creatine (at offset 1.95 ppm), amine (at 2.7 ppm) and amide (at 3.6 ppm) CEST experiments were 2μT, 3 μT, and 1 μT, respectively. According to the equation θ = tan−1ω1/Δ, this corresponds to sin2θ terms for creatine, amine, and amide CEST of 0.0076, 0.0089 and 0.0006, respectively. Therefore, the Reff term can be simplified as Reff = cos2θR1 + sin2θR2 ≈ R1 in this study. However, the T2 measurement may become important at lower MRI fields at which the sin2θ term can not be neglected anymore.
Conclusion
MLSVD CEST was developed to provide a simple and efficient way to improve the SNR of CEST images. The method outperformed conventional smoothing and SVD methods. High-resolution Cr, amide, and amine MLSVD CEST maps could be acquired on a mouse model of ischemic stroke, demonstrating that CrCEST, amide CEST and amine CEST are sensitive pH mapping methods at 11.7T. We expect that maps for a wide range of pH values can be achieved by combining CrCEST and amine CEST at high magnetic fields.
Supplementary Material
Supporting Information Figure S1. (a) Noisy CEST images. (b) Denoised CEST images using MLSVD. (c) The difference map between noisy and denoised images.
Supporting Information Figure S2. The singular value distribution obtained by SVD method. The truncated rank number was set to 10.
Supporting Information Figure S3. (a) ΔZ of amine CEST peak obtained by PLOF as a function of different peak ranges. (b) Representative PLOF result for amine CEST signal with a peak range of 1.2 ppm.
Acknowledgment
Grant support from NIH: P41EB015909, P41EB015032, R01EB019934, R03NS109664, R21AG065794, P50AG05146 and DOD W81XWH-18-1-0797.
Footnotes
Data Availability Statement
The code that supports the findings of this study is openly available at https://github.com/LinChenMRI/MLSVD-CEST.git.
References
- 1.Ward K, Aletras A, Balaban RS. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). J Magn Reson 2000;143(1):79–87. [DOI] [PubMed] [Google Scholar]
- 2.Van Zijl PC, Yadav NN. Chemical exchange saturation transfer (CEST): what is in a name and what isn’t? Magn Reson Med 2011;65(4):927–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou J, van Zijl PCM. Chemical exchange saturation transfer imaging and spectroscopy. Progress in Nuclear Magnetic Resonance Spectroscopy 2006;48(2–3):109–136. [Google Scholar]
- 4.Woessner DE, Zhang S, Merritt ME, Sherry AD. Numerical solution of the Bloch equations provides insights into the optimum design of PARACEST agents for MRI. Magn Reson Med 2005;53(4):790–799. [DOI] [PubMed] [Google Scholar]
- 5.Liu G, Song X, Chan KW, McMahon MT. Nuts and bolts of chemical exchange saturation transfer MRI. NMR Biomed 2013;26(7):810–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aime S, Castelli DD, Crich SG, Gianolio E, Terreno E. Pushing the sensitivity envelope of lanthanide-based magnetic resonance imaging (MRI) contrast agents for molecular imaging applications. Accounts Chem Res 2009;42(7):822–831. [DOI] [PubMed] [Google Scholar]
- 7.van Zijl PC, Lam WW, Xu J, Knutsson L, Stanisz GJ. Magnetization transfer contrast and chemical exchange saturation transfer MRI. Features and analysis of the field-dependent saturation spectrum. NeuroImage 2018;168:222–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones KM, Pollard AC, Pagel MD. Clinical applications of chemical exchange saturation transfer (CEST) MRI. J Magn Reson Imaging 2018;47(1):11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou J, Tryggestad E, Wen Z, Lal B, Zhou T, Grossman R, Wang S, Yan K, Fu DX, Ford E, Tyler B, Blakeley J, Laterra J, van Zijl PC. Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides. Nat Med 2011;17(1):130–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou J, Payen JF, Wilson DA, Traystman RJ, van Zijl PC. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med 2003;9(8):1085–1090. [DOI] [PubMed] [Google Scholar]
- 11.Sun PZ, Zhou J, Sun W, Huang J, van Zijl PCM. Detection of the ischemic penumbra using pH-weighted MRI. J Cereb Blood Flow Metab 2006;27(6):1129–1136. [DOI] [PubMed] [Google Scholar]
- 12.Ling W, Regatte RR, Navon G, Jerschow A. Assessment of glycosaminoglycan concentration in vivo by chemical exchange-dependent saturation transfer (gagCEST). Proc Natl Acad Sci USA 2008;105(7):2266–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L, Wei Z, Chan KWY, Cai S, Liu G, Lu H, Wong PC, van Zijl PCM, Li T, Xu J. Protein aggregation linked to Alzheimer’s disease revealed by saturation transfer MRI. Neuroimage 2018;188:380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang XY, Wang F, Jin T, Xu J, Xie J, Gochberg DF, Gore JC, Zu Z. MR imaging of a novel NOE-mediated magnetization transfer with water in rat brain at 9.4 T. Magn Reson Med 2017;78(2):588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paech D, Zaiss M, Meissner JE, Windschuh J, Wiestler B, Bachert P, Neumann JO, Kickingereder P, Schlemmer HP, Wick W, Nagel AM, Heiland S, Ladd ME, Bendszus M, Radbruch A. Nuclear overhauser enhancement mediated chemical exchange saturation transfer imaging at 7 Tesla in glioblastoma patients. PLoS One 2014;9(8):e104181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaiss M, Kunz P, Goerke S, Radbruch A, Bachert P. MR imaging of protein folding in vitro employing nuclear-Overhauser-mediated saturation transfer. NMR Biomed 2013;26(12):1815–1822. [DOI] [PubMed] [Google Scholar]
- 17.van Zijl PC, Jones CK, Ren J, Malloy CR, Sherry AD. MRI detection of glycogen in vivo by using chemical exchange saturation transfer imaging (glycoCEST). Proc Natl Acad Sci U S A 2007;104(11):4359–4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai K, Haris M, Singh A, Kogan F, Greenberg JH, Hariharan H, Detre JA, Reddy R. Magnetic resonance imaging of glutamate. Nat Med 2012;18(2):302–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haris M, Nanga RP, Singh A, Cai K, Kogan F, Hariharan H, Reddy R. Exchange rates of creatine kinase metabolites: feasibility of imaging creatine by chemical exchange saturation transfer MRI. NMR Biomed 2012;25(11):1305–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haris M, Singh A, Cai K, Kogan F, McGarvey J, Debrosse C, Zsido GA, Witschey WR, Koomalsingh K, Pilla JJ, Chirinos JA, Ferrari VA, Gorman JH, Hariharan H, Gorman RC, Reddy R. A technique for in vivo mapping of myocardial creatine kinase metabolism. Nat Med 2014;20(2):209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai K, Singh A, Poptani H, Li W, Yang S, Lu Y, Hariharan H, Zhou XJ, Reddy R. CEST signal at 2ppm (CEST@2ppm) from Z-spectral fitting correlates with creatine distribution in brain tumor. NMR Biomed 2015;28(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen L, Zeng H, Xu X, Yadav NN, Cai S, Puts NA, Barker PB, Li T, Weiss RG, van Zijl PCM, Xu J. Investigation of the contribution of total creatine to the CEST Z-spectrum of brain using a knockout mouse model. NMR Biomed 2017;30(12):e3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang XY, Xie J, Wang F, Lin EC, Xu J, Gochberg DF, Gore JC, Zu Z. Assignment of the molecular origins of CEST signals at 2 ppm in rat brain. Magn Reson Med 2017;78(3):881–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen L, Barker PB, Weiss RG, van Zijl PCM, Xu J. Creatine and phosphocreatine mapping of mouse skeletal muscle by a polynomial and Lorentzian line-shape fitting CEST method. Magn Reson Med 2019;81(1):69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung JJ, Jin T, Lee JH, Kim SG. Chemical exchange saturation transfer imaging of phosphocreatine in the muscle. Magn Reson Med 2019;81(6):3476–3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L, Schar M, Chan KWY, Huang J, Wei Z, Lu H, Qin Q, Weiss RG, van Zijl PCM, Xu J. In vivo imaging of phosphocreatine with artificial neural networks. Nat Commun 2020;11(1):1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desmond KL, Stanisz GJ. Understanding quantitative pulsed CEST in the presence of MT. Magn Reson Med 2012;67(4):979–990. [DOI] [PubMed] [Google Scholar]
- 28.Zaiss M, Zu Z, Xu J, Schuenke P, Gochberg DF, Gore JC, Ladd ME, Bachert P. A combined analytical solution for chemical exchange saturation transfer and semi-solid magnetization transfer. NMR Biomed 2015;28(2):217–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin T, Wang P, Zong X, Kim S-G. Magnetic resonance imaging of the Amine–Proton EXchange (APEX) dependent contrast. NeuroImage 2012;59(2):1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen L, Wei Z, Cai S, Li Y, Liu G, Lu H, Weiss RG, van Zijl PCM, Xu J. High-resolution creatine mapping of mouse brain at 11.7 T using non-steady-state chemical exchange saturation transfer. NMR Biomed 2019:e4168. [DOI] [PubMed] [Google Scholar]
- 31.Sun PZ, Wang E, Cheung JS, Zhang X, Benner T, Sorensen AG. Simulation and optimization of pulsed radio frequency irradiation scheme for chemical exchange saturation transfer (CEST) MRI-demonstration of pH-weighted pulsed-amide proton CEST MRI in an animal model of acute cerebral ischemia. Magn Reson Med 2011;66(4):1042–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun PZ, Benner T, Kumar A, Sorensen AG. Investigation of optimizing and translating pH-sensitive pulsed-chemical exchange saturation transfer (CEST) imaging to a 3T clinical scanner. Magn Reson Med 2008;60(4):834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zu Z, Li K, Janve VA, Does MD, Gochberg DF. Optimizing pulsed-chemical exchange saturation transfer imaging sequences. Magn Reson Med 2011;66(4):1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu R, Zhang H, Niu W, Lai C, Ding Q, Chen W, Liang S, Zhou J, Wu D, Zhang Y. Improved chemical exchange saturation transfer imaging with real-time frequency drift correction. Magn Reson Med 2019;81(5):2915–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Windschuh J, Zaiss M, Ehses P, Lee JS, Jerschow A, Regatte RR. Assessment of frequency drift on CEST MRI and dynamic correction: application to gagCEST at 7 T. Magn Reson Med 2019;81(1):573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wech T, Kostler H. Robust motion correction in CEST imaging exploiting low-rank approximation of the z-spectrum. Magn Reson Med 2018;80(5):1979–1988. [DOI] [PubMed] [Google Scholar]
- 37.Dopfert J, Witte C, Kunth M, Schroder L. Sensitivity enhancement of (Hyper-)CEST image series by exploiting redundancies in the spectral domain. Contrast Media Mol Imaging 2014;9(1):100–107. [DOI] [PubMed] [Google Scholar]
- 38.Breitling J, Deshmane A, Goerke S, Korzowski A, Herz K, Ladd ME, Scheffler K, Bachert P, Zaiss M. Adaptive denoising for chemical exchange saturation transfer MR imaging. NMR Biomed 2019;32(11):e4133. [DOI] [PubMed] [Google Scholar]
- 39.Bie C, Liang Y, Zhang L, Zhao Y, Chen Y, Zhang X, He X, Song X. Motion correction of chemical exchange saturation transfer MRI series using robust principal component analysis (RPCA) and PCA. Quant Imaging Med Surg 2019;9(10):1697–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Breitling J, Deshmane A, Goerke S, Korzowski A, Herz K, Ladd ME, Scheffler K, Bachert P, Zaiss M. Adaptive denoising for chemical exchange saturation transfer MR imaging. NMR Biomed 2019:e4133. [DOI] [PubMed] [Google Scholar]
- 41.Lam F, Liang ZP. A subspace approach to high-resolution spectroscopic imaging. Magn Reson Med 2014;71(4):1349–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lam F, Ma C, Clifford B, Johnson CL, Liang ZP. High-resolution (1) H-MRSI of the brain using SPICE: Data acquisition and image reconstruction. Magn Reson Med 2016;76(4):1059–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brender JR, Kishimoto S, Merkle H, Reed G, Hurd RE, Chen AP, Ardenkjaer-Larsen JH, Munasinghe J, Saito K, Seki T, Oshima N, Yamamoto K, Choyke PL, Mitchell J, Krishna MC. Dynamic Imaging of Glucose and Lactate Metabolism by 13C-MRS without Hyperpolarization. Scientific Reports 2019;9(1):3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Lathauwer L, De Moor B, Vandewalle J. A multilinear singular value decomposition. SIAM journal on Matrix Analysis and Applications 2000;21(4):1253–1278. [Google Scholar]
- 45.Zhou J, van Zijl PC. Defining an Acidosis-Based Ischemic Penumbra from pH-Weighted MRI. Translational Stroke Research 2011;3(1):76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma C, Clifford B, Liu Y, Gu Y, Lam F, Yu X, Liang ZP. High-resolution dynamic (31) P-MRSI using a low-rank tensor model. Magn Reson Med 2017;78(2):419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jin T, Wang P, Zong X, Kim S-G. MR imaging of the amide-proton transfer effect and the pH-insensitive nuclear overhauser effect at 9.4 T. Magn Reson Med 2013;69(3):760–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trott O, Palmer AG. R 1ρ relaxation outside of the fast-exchange limit. J Magn Reson 2002;154(1):157–160. [DOI] [PubMed] [Google Scholar]
- 49.Jin T, Autio J, Obata T, Kim SG. Spin-locking versus chemical exchange saturation transfer MRI for investigating chemical exchange process between water and labile metabolite protons. Magn Reson Med 2011;65(5):1448–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zaiss M, Bachert P. Chemical exchange saturation transfer (CEST) and MR Z - spectroscopy in vivo : a review of theoretical approaches and methods. Phys Med Biol 2013;58(22):R221. [DOI] [PubMed] [Google Scholar]
- 51.Zaiss M, Bachert P. Exchange-dependent relaxation in the rotating frame for slow and intermediate exchange -- modeling off-resonant spin-lock and chemical exchange saturation transfer. NMR Biomed 2013;26(5):507–518. [DOI] [PubMed] [Google Scholar]
- 52.Zong X, Wang P, Kim SG, Jin T. Sensitivity and source of amine-proton exchange and amide-proton transfer magnetic resonance imaging in cerebral ischemia. Magn Reson Med 2014;71(1):118–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gudbjartsson H, Patz S. The rician distribution of noisy mri data. Magn Reson Med 1995;34(6):910–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lam F, Babacan SD, Haldar JP, Weiner MW, Schuff N, Liang Z-P. Denoising diffusion-weighted magnitude MR images using rank and edge constraints. Magn Reson Med 2014;71(3):1272–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jin T, Wang P, Hitchens TK, Kim SG. Enhancing sensitivity of pH-weighted MRI with combination of amide and guanidyl CEST. NeuroImage 2017;157:341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou IY, Lu D, Ji Y, Wu L, Wang E, Cheung JS, Zhang XA, Sun PZ. Determination of multipool contributions to endogenous amide proton transfer effects in global ischemia with high spectral resolution in vivo chemical exchange saturation transfer MRI. Magn Reson Med 2019;81(1):645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zeng H, Xu J, Yadav NN, McMahon MT, Harden B, Frueh D, van Zijl PC. (15)N Heteronuclear Chemical Exchange Saturation Transfer MRI. J Am Chem Soc 2016;138(35):11136–11139. [DOI] [PubMed] [Google Scholar]
- 58.Rerich E, Zaiss M, Korzowski A, Ladd ME, Bachert P. Relaxation-compensated CEST-MRI at 7 T for mapping of creatine content and pH--preliminary application in human muscle tissue in vivo. NMR Biomed 2015;28(11):1402–1412. [DOI] [PubMed] [Google Scholar]
- 59.Crockard HA, Gadian DG, Frackowiak RSJ, Proctor E, Allen K, Williams SR, Russell RWR. Acute Cerebral-Ischemia - Concurrent Changes in Cerebral Blood-Flow, Energy Metabolites, Ph, and Lactate Measured with Hydrogen Clearance and P-31 and H-1 Nuclear-Magnetic-Resonance Spectroscopy .2. Changes during Ischemia. J Cerebr Blood F Met 1987;7(4):394–402. [DOI] [PubMed] [Google Scholar]
- 60.Taylor JM, Zhu XH, Zhang Y, Chen W. Dynamic correlations between hemodynamic, metabolic, and neuronal responses to acute whole-brain ischemia. NMR Biomed 2015;28(11):1357–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Y-L, Yao J, Chakhoyan A, Raymond C, Salamon N, Liau LM, Nghiemphu PL, Lai A, Pope WB, Nguyen N. Association between tumor acidity and hypervascularity in human gliomas using pH-weighted amine chemical exchange saturation transfer echo-planar imaging and dynamic susceptibility contrast perfusion MRI at 3T. American Journal of Neuroradiology 2019;40(6):979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harris RJ, Yao J, Chakhoyan A, Raymond C, Leu K, Liau LM, Nghiemphu PL, Lai A, Salamon N, Pope WB. Simultaneous p H-sensitive and oxygen-sensitive MRI of human gliomas at 3 T using multi-echo amine proton chemical exchange saturation transfer spinand-gradient echo echo-planar imaging (CEST-SAGE-EPI). Magn Reson Med 2018;80(5):1962–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure S1. (a) Noisy CEST images. (b) Denoised CEST images using MLSVD. (c) The difference map between noisy and denoised images.
Supporting Information Figure S2. The singular value distribution obtained by SVD method. The truncated rank number was set to 10.
Supporting Information Figure S3. (a) ΔZ of amine CEST peak obtained by PLOF as a function of different peak ranges. (b) Representative PLOF result for amine CEST signal with a peak range of 1.2 ppm.