Abstract
Objective
PCDH19-related epilepsy is characterized by a distinctive pattern of X-linked inheritance, where heterozygous females exhibit seizures and hemizygous males are asymptomatic. A cellular interference mechanism resulting from the presence of both wild-type and mutant PCDH19 neurons in heterozygous patients or mosaic carriers of PCDH19 variants has been hypothesized. We aim to investigate seizure susceptibility and progression in the Pchd19 mouse model.
Methods
We assessed seizure susceptibility and progression in the Pcdh19 mouse model using three acute seizure-induction paradigms. We first induced focal, clonic seizures using the 6-Hz psychomotor test. Mice were stimulated with increasing current intensities and graded according to a modified Racine scale. We next induced generalized seizures using flurothyl or pentylenetetrazol (PTZ), both GABAA receptor function inhibitors, and recorded latencies to myoclonic and generalized tonic-clonic seizures.
Results
Pcdh19 knockout and heterozygous females displayed increased seizure susceptibility across all current intensities in the 6-Hz psychomotor test, and increased severity overall. They also exhibited shorter latencies to generalized seizures following flurothyl, but not PTZ, seizure induction. Hemizygous males showed comparable seizure incidence and severity to their wild-type male littermates across all paradigms tested.
Significance
The heightened susceptibility observed in Pcdh19 knockout females suggests additional mechanisms other than cellular interference are at play in PCDH19-related epilepsy. Further experiments are needed to understand the variability in seizure susceptibility so that this model can be best utilized toward development of future therapeutic strategies for PCDH19-related epilepsy.
Keywords: Protocadherin 19, Epilepsy, X-linked
1. Introduction
PCDH19 is a cell adhesion molecule belonging to the protocadherin subgroup within the cadherin superfamily. It is predominantly expressed in the nervous system. Heterozygous loss-of-function variants in the human X-linked gene PCDH19 lead to early infantile epileptic encephalopathy associated with varying degrees of intellectual disability and autistic features. PCDH19-related epilepsy shows an atypical pattern of inheritance where heterozygous females present with seizures while hemizygous carrier males are asymptomatic.1 Seizures occur in brief and reoccurring clusters that are predominantly focal in origin, although generalized seizures (tonic, myoclonic, absence) are also reported.2 PCDH19 has been identified as the second most clinically relevant gene in epilepsy.3 Yet, the pathogenic mechanism behind PCDH19-related epilepsy remains largely unknown.
A mechanism of cellular interference has been suggested, wherein the co-existence of neurons expressing wild-type (WT) or mutant PCDH19 disrupts cell-cell interactions.4 Recent cases of affected males with mosaicism for PCDH19 pathogenic variants 5–7 and a case of PCDH19-related epilepsy in a male with Klinefelter syndrome8 provide further support for the cellular interference hypothesis. Previous studies investigating PCDH19 function revealed that heterozygous, knockout, and hemizygous mice did not exhibit gross brain abnormalities or spontaneous seizures.9 Although Pcdh19 heterozygous females showed increased hyperactivity and a decreased fear response compared to their WT littermates, no differences were observed between Pcdh19 genotypes when behavioral phenotypes such as anxiety, social interaction behaviors, and spatial/working memory were assessed.10 No characterization of a seizure/epilepsy phenotype has been reported.
To address this issue, we investigated seizure susceptibility and progression in the Pchd19 mouse model. In particular, we characterized susceptibility to focal seizures using the 6-Hz psychomotor model, as well as susceptibility and progression to generalized seizures using flurothyl and PTZ.
2. Methods
2.1. Mice
All experiments were approved by the Northwestern University Animal Care and Use Committee in accordance with the NIH Guide for the Care and Use of Laboratory Animals and Animal Welfare Act. Animals were housed on a 14-hour light/10-hour dark schedule, with food and water available ad libitum. Pcdh19+/− and Pcdh19−/y mice (TF2108) on a mixed 129S5.C57BL/6 background were purchased from Taconic Biosciences (Cambridge City, IN) and crossed together to obtain Pcdh19+/− and Pcdh19−/− females, as well as Pcdh19+/y, and Pcdh19−/y males. Pcdh19+/y males were crossed with Pcdh19+/− female mice to also generate WT females. For each experiment, we used age-matched littermates from these 2 different mating setups. Genotyping was performed following vendor’s instructions. The study was done on mice aged 8 to 12 weeks.
2.2. Flurothyl seizure induction
Flurothyl seizure induction was performed as previously described.11 Briefly, mice were individually placed in a sealed induction chamber. Flurothyl (2,2,2-trifluroethyl ether, Sigma-Aldrich, Milwaukee, WI) was introduced by a syringe pump (KD Scientific, Holliston, MA, U.S.A.) at a constant rate of 20 μl/min. Latencies to the first myoclonic jerk (MJ) and generalized tonic-clonic seizure (GTCS) were recorded by reviewers blinded to the genotype following flurothyl induction. At the onset of GTCS, the mouse was immediately placed in a holding cage for recovery. All mice were euthanized at the end of the experimental session.
2.3. 6-Hz psychomotor seizure test
6-Hz psychomotor seizures were induced via auricular stimulation (0.2 ms pulse width at 6 Hz, 3 s duration). Mice were tested at current intensities of 16, 24, and 30 mA. Prior to stimulation, auricular electrodes were soaked in 0.9% saline solution to ensure complete electrical contact. Animals were manually restrained and delivered a single shock using an ECT unit (Ugo Basile; Comerio, Italy). Following 6-Hz stimulation, mice were observed for 2 minutes and scored by reviewers blinded to the genotype according to a modified Racine scale of seizure severity:
0 – No seizure
1 – Loss of posture associated with facial clonus and clonus of forelimbs and/or hindlimbs
2 – Grade 1 seizure followed by recovery of the righting reflex and low intensity bouncing
3 – Grade 1 and/or 2 features with recovery of the righting reflex followed by wild running and popcorning
Animals that did not resume normal exploratory behavior within 10 seconds following 6-Hz stimulation were not tested at higher current intensities.
2.4. Video-EEG recording and PTZ seizure induction
Mice were implanted with prefabricated EEG headmounts (Pinnacle Technology, Inc., Lawrence, KS, USA). Briefly, mice were anesthetized with isoflurane and placed in a stereotaxic frame (David Kopf Instruments, Tujunga, Canada). Headmounts with four stainless steel screws that served as cortical surface electrodes were affixed to the skull with dental cement (GC America, Alsip, IL, USA). Animals were subcutaneously administered 1 mg/kg buprenorphine SR as postoperative analgesic and single-housed for 7 days for recovery. Video EEG started with 1 hour of baseline recording followed by a single dose of PTZ (Sigma-Aldrich, St. Louis, MO, USA) administered intraperitoneally at 40 mg/kg. Video EEG data were collected for one additional 30 minutes post-treatment. Digitized data were acquired and analyzed with Sirenia software (Pinnacle Technology, Inc., Lawrence, KS, USA.) along with contemporaneous video recordings. Epileptiform activity was scored manually offline by a reviewer blinded to gender and genotype, according to a revised Racine scale12 as follows: −1, normal baseline; 0, whisker trembling; 1, sudden behavioral arrest; 2 facial jerking; 3, myoclonic jerks; 4, clonic seizure sitting; 5 clonic, tonic-clonic seizure, lying on belly; 6, clonic, tonic-clonic seizure with loss of posture and wild jumping; 7, tonic extension, possibly leading to respiratory arrest and death.
2.5. Statistical analysis
Statistical analyses were performed using GraphPad Prism 8 (GraphPad, La Jolla, California). Results are presented as mean ± standard deviation, unless mentioned otherwise. Outlier detection using the ROUT method (Q=1%) was used whenever the normality criterion was met. One-way analysis of variance (ANOVA) followed by the Tukey’s post hoc multiple comparisons test (females), or a two-tailed unpaired t-test (males), were used whenever normality and homoscedasticity criteria were met. Otherwise, the nonparametric Kruskal-Wallis test followed by the Dunn’s post hoc multiple comparisons test (females), or the Mann-Whitney test (males) were used. A P value < 0.05 was considered statistically significant.
The 6-Hz CC50 and 95% confidence intervals were determined using log-probit analysis.
3. Results
3.1. Pcdh19 female mice exhibit lower seizure thresholds and a more severe seizure phenotype following 6-Hz stimulation
We investigated susceptibility to focal seizures by comparing seizure incidence and severity between Pcdh19 mutant and WT littermates following 6-Hz stimulation (Fig. 1). The percentage of mice exhibiting psychomotor seizure behavior followed a current-dependent increase. Pchdh19+/− and Pcdh19−/− females displayed higher seizure incidence at each current intensity when compared with WT female mice. At 16 mA, 55% (11/20) of Pchdh19+/− and 77% (10/13) of Pcdh19−/− mice expressed seizures, compared with 22% (4/18) of WT females. At 24 mA, 95% of Pchdh19+/− and 100 % of Pcdh19−/− mice seized, compared with 44% of WT female mice. At 30 mA, 100% of Pchdh19+/− mice expressed seizures compared with 67% of their WT female littermates (Fig. 1A). Consequently, the median current level at which half of the mice seized (CC50) was significantly lower in heterozygous (CC50 = 15 mA; CI 95%: 13 to 17) and knockout females (CC50 = 13 mA; CI 95%: 11 to 16) when compared to WT female mice (CC50 = 24 mA; CI 95%: 21 to 27) (Fig. 1B). Pcdh19 female mice exhibited significantly more severe seizures than their WT littermates. Interestingly, at 16 mA, Pchdh19+/− females expressed a variable phenotype, with behavior ranging from no sign of seizure activity to advanced clonic seizures (wild running), while Pcdh19−/− females exhibited a significantly more robust seizure phenotype (wild running and popcorning). At 24 mA, both heterozygous and knockout females behaved comparably (Fig. 1C). Hemizygous males showed comparable seizure incidence and severity to their WT male littermates across all current levels (Fig. 1D, E, and F).
Figure 1.
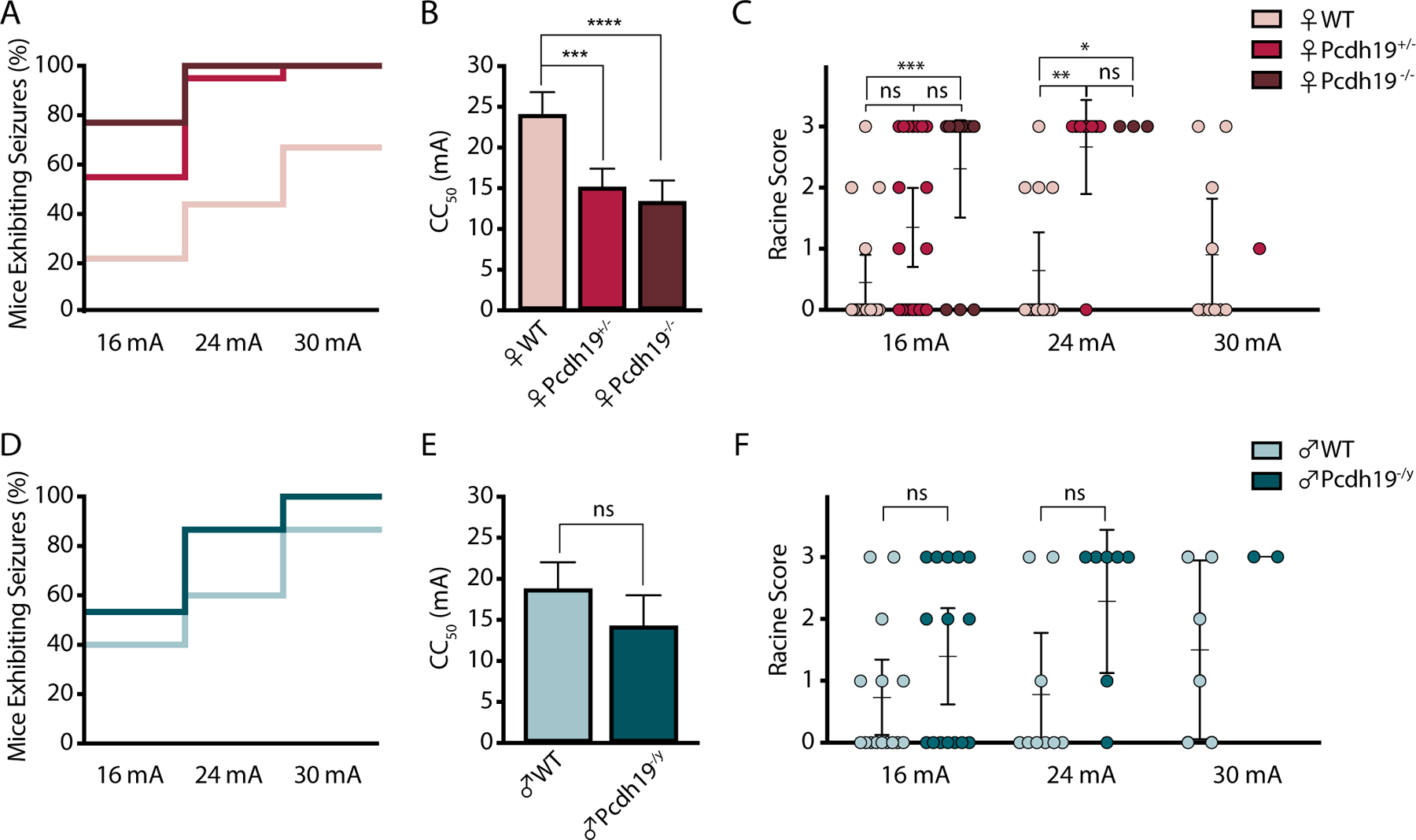
Pcdh19 female mice show increased susceptibility to 6-Hz seizures. A and D, Percentage of Pcdh19 female (A) or male (D) mice expressing seizures following stimulation with 16 mA, 24 mA, and 30 mA. At 16 mA, Female n = 18 (WT), 20 (Pcdh19+/−), 13 (Pcdh19−/−); Male n = 15 (WT), 15 (Pcdh19−/y). At 24 mA, Female n = 14 (WT), 9 (Pcdh19+/−), 3 (Pcdh19−/−); Male n = 9 (WT), 7 (Pcdh19−/y). At 30 mA, Female n = 10 (WT), 2 (Pcdh19+/−), 0 (Pcdh19−/−); Male n = 6 (WT), 2 (Pcdh19−/y). B and E, Average CC50 values based on Probit analysis for each genotype in Pcdh19 females (B) and males (E). *** P < 0.001, **** P < 0.0001, ns = not statistically significant. C and F, Racine scores at each current level in Pcdh19 females (C) and males (F). Female n = 18 (WT), 20 (Pcdh19+/−), 13 (Pcdh19−/−) (C). * P = 0.0204, ** P = 0.0015, *** P = 0.0008; Kruskal-Wallis ANOVA followed by Dunn’s test. Male n = 15 (WT), 15 (Pcdh19−/y). ns = not statistically significant; Mann-Whitney test. Data are represented as the mean with 95% confidence intervals.
3.2. Pcdh19 female mice show shorter latencies to flurothyl-, but not PTZ-induced generalized seizures.
We next assessed susceptibility to induced generalized seizures using the GABAA receptor function inhibitors flurothyl and PTZ. Mice exposed to flurothyl express a series of characteristic seizure behaviors, beginning with brief myoclonic jerks (MJ), which eventually progress to generalized tonic-clonic seizures (GTCS). The average latencies to MJ were comparable among all genotypes in both sexes (Fig. 2A, B). However, the average latency to GTCS was significantly reduced in heterozygous females (124 s; CI 95%: 107 to 141) when compared with their WT littermates (177 s; CI 95%: 128 to 226) (p = 0.0107) (Fig. 2A). Interestingly, knockout females also exhibited a decreased latency to GTCS (114 s; CI 95%: 105 to 124) when compared to WT mice (p =0.0033) (Fig. 2A). In contrast, no statistically significant differences were observed in latency to GTCS between hemizygous and WT males (latency to GTCS in WT males = 139 s; CI 95%: 99 to 179; latency to GTCS in Pcdh19−/y mice = 123 s; CI 95%: 92 to 154) (Fig. 2B).
Figure 2.
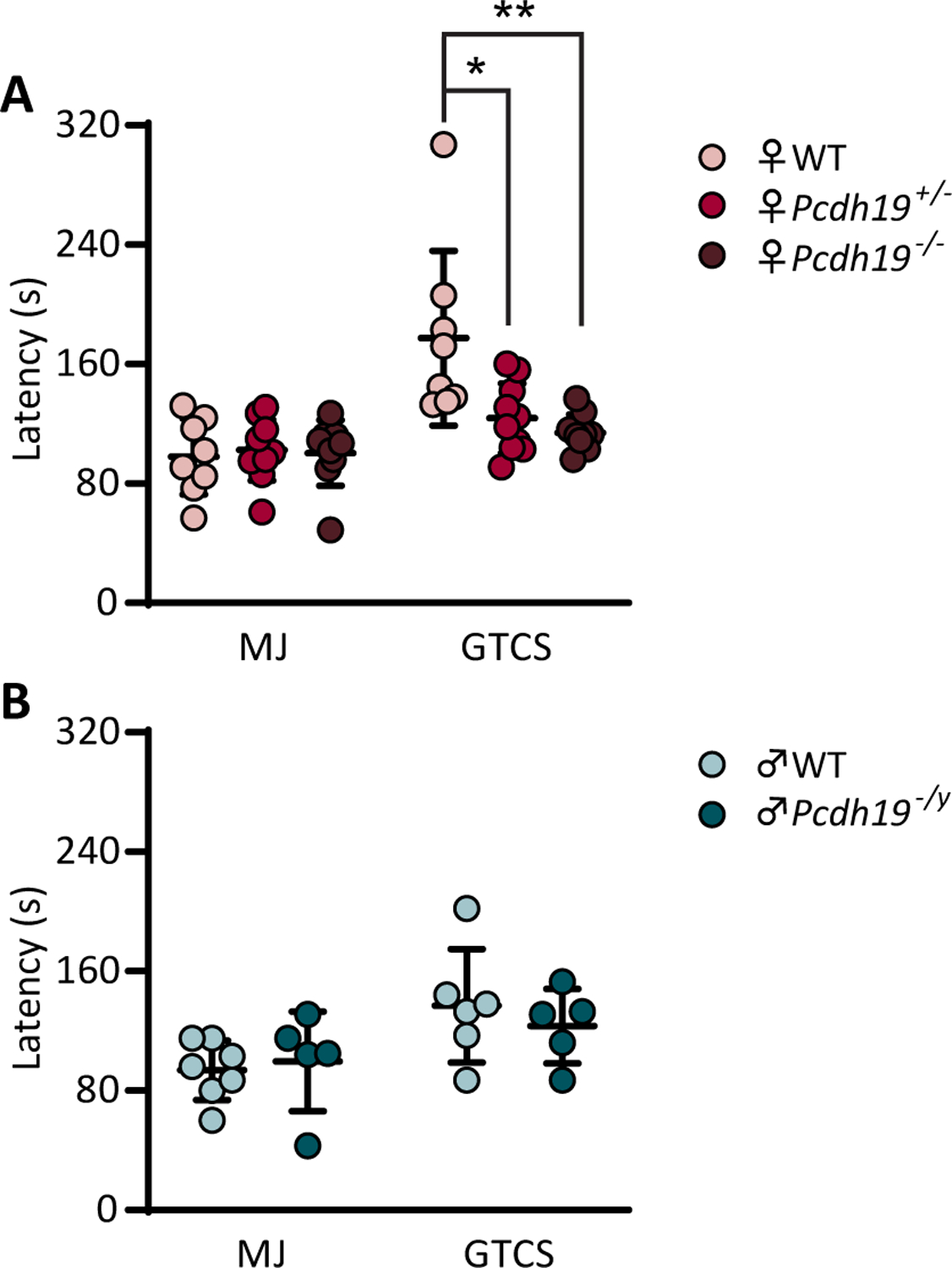
Pcdh19 female mice show lower seizure thresholds following flurothyl exposure. A and B, Latencies to the first myoclonic jerk (MJ) and generalized tonic-clonic seizure (GTCS) in Pcdh19 females (A) and males (B) Female n = 8 (WT), 10 (Pcdh19+/−), 9 (Pcdh19−/−). * P = 0.0107, ** P = 0.0033; Ordinary one-way ANOVA followed by Tukey’s test. Male n = 7 (WT), 5 (Pcdh19−/y). Unpaired t test (B). Data are represented as the mean ± standard deviation.
It has been previously reported that Pcdh19+/− mice showed altered network activity with increased number of spike-wave discharge (SWD) events when compared to their WT or Pcdh19−/− female littermates.4 SWD incidence on EEG accompanied by behavioral arrest are hallmarks of absence epilepsy. We thus performed video-EEG recordings to analyze spontaneous and induced seizure behavior in Pcdh19 mice (Fig. 3A). During baseline recording (Fig. 3B), all Pcdh19+/−mice exhibited SWD activity (Fig. 3D) concomitant with behavioral arrest, with 60% of Pcdh19+/− (6/10) expressing SWD episodes lasting longer than 3 s (Fig. 4A, F). In contrast, two thirds of WT females (4/6; 2/6 SWD < 3 s, 2/6 SWD > 3 s), and only 40 % of Pcdh19−/− mice (2/5; 1/5 SWD < 3 s, 1/5 SWD> 3 s) showed SWD. These patterns of SWD-expression were not hugely affected by PTZ injection (Fig. 4A). The 40 mg/kg PTZ dose did not induce generalized seizure in every mouse. 57% of WT females (4/7) reached the GTCS stage (Fig. 3G), which was systematically followed by clonic seizure episodes (Fig. 3E). Half (5/10), and 40% (2/5) of Pcdh19+/− and Pcdh19−/− mice, respectively, expressed GTCS. Interestingly, clonic seizure (CS) incidence did not depend on GTCS expression, with 70% (7/10) of Pcdh19+/− mice and 80% (4/5) of Pcdh19−/− mice exhibiting CS, compared to 57% of WT (4/7) (Fig. 4B). Average latencies to reach the first MJ (Fig. 3F) and GTCS were similar across all genotypes in both sexes (Fig. 4C, F), as well as seizure severity (Fig.4E, H). However, Pcdh19+/− mice expressed significantly less MJs (22 MJs; CI 95%: 10 to 33 MJs) during the 30 minutes post-PTZ injection, compared to WT (75 MJs; CI 95%: 27 to 123 MJs) and Pcdh19−/− females (82 MJs; CI 95%: 0 to 204 MJs) (Fig. 4D). No statistically significant differences were observed in the total number of MJs in 30 minutes post-PTZ injection between WT and hemizygous males (total number of MJs in WT males = 33; CI 95%: 0 to 68 MJs; total number of MJs in Pcdh19−/y mice = 69; CI 95%: 31 to 108 MJs) (Fig. 4G).
Figure 3.
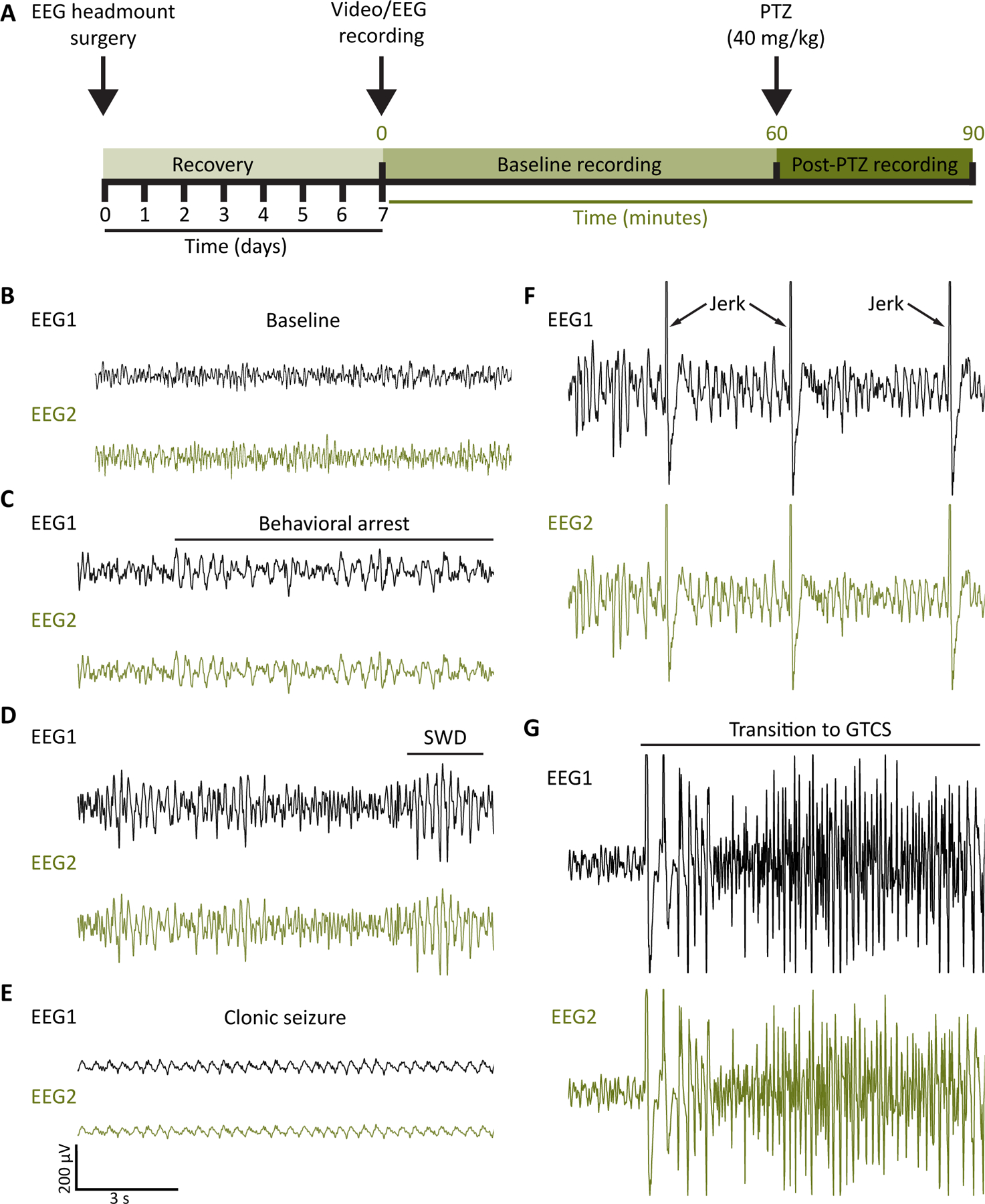
A, Schematic representation of the experimental setup for the video-EEG recording and PTZ administration experiment. B-G, Representative EEG recordings from a Pcdh19 mouse during baseline (B), and after PTZ injection (C-G). SWD: spike-wave discharge; GTCS: generalized tonic-clonic seizure.
Figure 4.
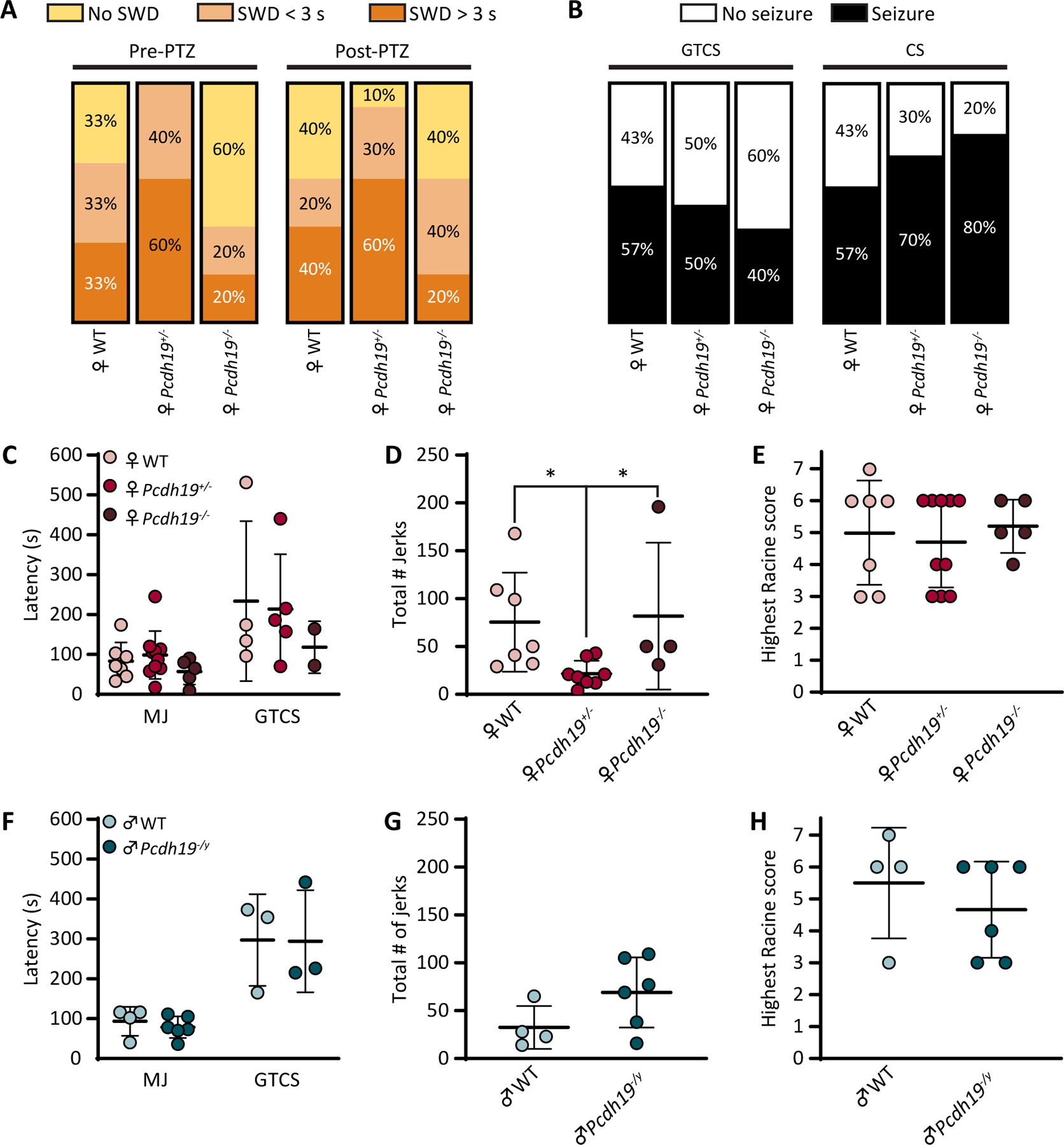
Seizure susceptibility and severity are not affected by loss of Pcdh19 following acute PTZ treatment. A, Percentages of Pcdh19 female mice expressing SWD during one-hour baseline recording and 30 minutes post-PTZ injection. B, Percentages of Pcdh19 female mice expressing generalized tonic-clonic seizures (GTCS), or clonic seizures (CS) following PTZ injection. Female n = 6 (WT), 10 (Pcdh19+/−), 5 (Pcdh19−/−). C and F, Latencies to the first MJ and GTCS following PTZ administration in Pcdh19 females (C) and males (F). D and G, Total number of jerks occurring during the first 30 minutes post-PTZ injection in Pcdh19 females (D) and males (G). E and H, Highest Racine score reached during the first 30 minutes post-PTZ injection in Pcdh19 females (E) and males (H). Female n = 7 (WT), 10 (Pcdh19+/−), 5 (Pcdh19−/−). * P = 0.0226 and 0.0446 for female WT vs. Pcdh19+/− and Pcdh19+/− vs. Pcdh19−/−, respectively; Kruskal-Wallis ANOVA followed by Dunn’s test. Male n = 4 (WT), 6 (Pcdh19−/y). Mann-Whitney test. Error bars indicate standard deviation.
4. Discussion
This study is the first to characterize seizure susceptibility in the Pcdh19 mouse model. We show that Pcdh19+/− mice have a higher SWD incidence, when compared to their WT and Pcdh19−/− female littermates, which is consistent with previous data.4 We also show that heterozygous female mice exhibit lower seizure thresholds and qualitatively more severe seizure phenotypes while hemizygous males behave comparably to WT males in the 6-Hz psychomotor and flurothyl seizure-induction tests. For these two paradigms, the seizure expression in Pcdh19+/− females and asymptomatic behavior in Pcdh19−/y males resembles the atypical pattern of inheritance observed in patients with PCDH19-related epilepsy. Interestingly, we also observe lower seizure thresholds and a more severe phenotype in knockout females. This heightened susceptibility somewhat challenges the cellular interference hypothesis, where knockout females are expected to behave comparably to WT females. Taken together, our data suggest that in addition to cellular interference, other mechanisms, possibly gender-related, may also play a role in the pathogenesis of PCDH19-related epilepsy. For example, differences in neurosteroid metabolism have been described in female patients with PCDH19-related epilepsy.13 Reduced blood levels of allopregnanolone is particularly relevant,14 as the neurosteroid is a positive modulator of GABAA receptor function, and has also been associated with anticonvulsive properties.15 Interestingly, besides assisting in homophilic cell binding and sorting 4, PCDH19 interacts with synaptic and extra-synaptic GABAA receptors, as well as actin cytoskeletal regulators 16, 17. Moreover, although the majority of mosaic males with pathogenic variants in PCDH19 develop epilepsy, there have been several reported cases of males with somatic mosaicism who are asymptomatic.18 Further investigation is needed to understand the broader role for PCDH19 in epileptogenesis and the cause of the phenotype variability observed in PCDH19-related epilepsy.
Contrary to flurothyl, we found that PTZ doesn’t affect seizure susceptibility or progression to GTCS in Pcdh19 mice. However, more Pcdh19 female mice present with CS. Why does exposing Pcdh19 mice to two GABAA receptor function inhibitors result in such different outcomes? It has been hypothesized that both convulsants inhibit GABAA receptor activity through different mechanisms. PTZ interacts with GABAA receptors at a domain comparable but distinct from the picrotoxin-binding site,19 while the flurothyl action site is suggested to be the anesthetic ethers-binding site.20 The drug delivery method matters too. Indeed, epileptogenic mechanisms following acute PTZ administration or PTZ kindling are different, as repetitive PTZ exposure may modify GABAA receptor subunit composition.21 As PCDH19 is known to interact with GABAA receptor α subunits,16 we don’t exclude that PTZ-kindled Pcdh19 mice could show differences in seizure susceptibility compared to the acute PTZ treatment performed in our study.
PCDH19 patients express seizures ranging from focal clustered seizures, often precipitated by fever,2 to generalized absence, tonic-clonic, and myoclonic seizures. However, the majority of patients outgrow their seizures over time.22 The characterization of seizure susceptibility presented in this study was performed on young adult mice. It would be interesting to assess seizure susceptibility in neonatal Pcdh19 mice using the febrile seizure model or convulsive drug injection during early postnatal development.
In summary, our study demonstrates that Pcdh19 mice seizure susceptibility varies with the seizure induction model used. Additional experiments are needed to understand the variability in seizure susceptibility seen with heterozygous and homozygous Pcdh19 female mice so that this model can be best utilized toward development of future therapeutic strategies for PCDH19-related epilepsy.
Supplementary Material
Key points:
Pcdh19+/− and Pcdh19−/− female mice show enhanced susceptibility to induced focal seizures.
Susceptibility to induced generalized seizure in Pcdh19+/− and Pcdh19−/− female mice varies with the induction model used.
Seizure expression in Pcdh19−/y male mice is comparable to their WT littermates, regardless of the seizure induction model used.
The heightened seizure susceptibility in Pcdh19+/− females and asymptomatic behavior in Pcdh19−/y males resembles the pattern of inheritance observed in PCDH19-epilepsy.
Pcdh19−/− females expressing heightened susceptibility suggests a broader role for PCDH19 in epileptogenesis.
Acknowledgments
This work was supported by NIH grant R00 NS089943 (AG-G). The authors thank Dr. Jennifer Kearney and Dr. Nicole Hawkins for assistance with the flurothyl and 6-Hz psychomotor seizure induction paradigms, as well as training and equipment sharing for the EEG-video recordings.
Footnotes
“This is the peer reviewed version of the following article: Rakotomamonjy J, Sabetfakhri NP, McDermott SL, Guemez-Gamboa A. Characterization of seizure susceptibility in Pcdh19 mice. Epilepsia. 2020;00:1–8, which has been published in final form https://doi.org/10.1111/epi.16675. This article may be used for non-commercial purposes in accordance with Wiley Terms and Conditions for Use of Self-Archived Versions.”
Conflict of interest statement
None of the authors has any conflict of interest to disclose.
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Dibbens LM, Tarpey PS, Hynes K, Bayly MA, Scheffer IE, Smith R, et al. X-linked protocadherin 19 mutations cause female-limited epilepsy and cognitive impairment Nature genetics. 2008. June;40:776–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marini C, Mei D, Parmeggiani L, Norci V, Calado E, Ferrari A, et al. Protocadherin 19 mutations in girls with infantile-onset epilepsy Neurology. 2010. August 17;75:646–653. [DOI] [PubMed] [Google Scholar]
- 3.Niazi R, Fanning EA, Depienne C, Sarmady M, Abou Tayoun AN. A mutation update for the PCDH19 gene causing early-onset epilepsy in females with an unusual expression pattern Human mutation. 2019. March;40:243–257. [DOI] [PubMed] [Google Scholar]
- 4.Pederick DT, Richards KL, Piltz SG, Kumar R, Mincheva-Tasheva S, Mandelstam SA, et al. Abnormal Cell Sorting Underlies the Unique X-Linked Inheritance of PCDH19 Epilepsy Neuron. 2018. January 3;97:59–66.e55. [DOI] [PubMed] [Google Scholar]
- 5.de Lange IM, Rump P, Neuteboom RF, Augustijn PB, Hodges K, Kistemaker AI, et al. Male patients affected by mosaic PCDH19 mutations: five new cases Neurogenetics. 2017. July;18:147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez D, Hsieh DT, Rohena L. Somatic Mosaicism of PCDH19 in a male with early infantile epileptic encephalopathy and review of the literature Am J Med Genet A. 2017. June;173:1625–1630. [DOI] [PubMed] [Google Scholar]
- 7.Terracciano A, Trivisano M, Cusmai R, De Palma L, Fusco L, Compagnucci C, et al. PCDH19-related epilepsy in two mosaic male patients Epilepsia. 2016. March;57:e51–55. [DOI] [PubMed] [Google Scholar]
- 8.Romasko EJ, DeChene ET, Balciuniene J, Akgumus GT, Helbig I, Tarpinian JM, et al. PCDH19-related epilepsy in a male with Klinefelter syndrome: Additional evidence supporting PCDH19 cellular interference disease mechanism Epilepsy Res 2018. September;145:89–92. [DOI] [PubMed] [Google Scholar]
- 9.Pederick DT, Homan CC, Jaehne EJ, Piltz SG, Haines BP, Baune BT, et al. Pcdh19 Loss-of-Function Increases Neuronal Migration In Vitro but is Dispensable for Brain Development in Mice Sci Rep 2016. May 31;6:26765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayashi S, Inoue Y, Hattori S, Kaneko M, Shioi G, Miyakawa T, et al. Loss of X-linked Protocadherin-19 differentially affects the behavior of heterozygous female and hemizygous male mice Scientific reports. 2017. July 19;7:5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin MS, Tang B, Papale LA, Yu FH, Catterall WA, Escayg A. The voltage-gated sodium channel Scn8a is a genetic modifier of severe myoclonic epilepsy of infancy Human molecular genetics. 2007. December 1;16:2892–2899. [DOI] [PubMed] [Google Scholar]
- 12.Van Erum J, Van Dam D, De Deyn PP. PTZ-induced seizures in mice require a revised Racine scale Epilepsy Behav 2019. June;95:51–55. [DOI] [PubMed] [Google Scholar]
- 13.Trivisano M, Lucchi C, Rustichelli C, Terracciano A, Cusmai R, Ubertini GM, et al. Reduced steroidogenesis in patients with PCDH19-female limited epilepsy Epilepsia. 2017. June;58:e91–e95. [DOI] [PubMed] [Google Scholar]
- 14.Tan C, Shard C, Ranieri E, Hynes K, Pham DH, Leach D, et al. Mutations of protocadherin 19 in female epilepsy (PCDH19-FE) lead to allopregnanolone deficiency Human molecular genetics. 2015. September 15;24:5250–5259. [DOI] [PubMed] [Google Scholar]
- 15.Biagini G, Panuccio G, Avoli M. Neurosteroids and epilepsy Curr Opin Neurol 2010. April;23:170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bassani S, Cwetsch AW, Gerosa L, Serratto GM, Folci A, Hall IF, et al. The female epilepsy protein PCDH19 is a new GABAAR-binding partner that regulates GABAergic transmission as well as migration and morphological maturation of hippocampal neurons Human molecular genetics. 2018. March 15;27:1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen B, Brinkmann K, Chen Z, Pak CW, Liao Y, Shi S, et al. The WAVE regulatory complex links diverse receptors to the actin cytoskeleton Cell. 2014. January 16;156:195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu A, Yang X, Yang X, Wu Q, Zhang J, Sun D, et al. Mosaicism and incomplete penetrance of PCDH19 mutations J Med Genet 2019. February;56:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang RQ, Bell-Horner CL, Dibas MI, Covey DF, Drewe JA, Dillon GH. Pentylenetetrazole-induced inhibition of recombinant gamma-aminobutyric acid type A (GABA(A)) receptors: Mechanism and site of action J Pharmacol Exp Ther 2001. September;298:986–995. [PubMed] [Google Scholar]
- 20.Krasowski MD. Differential modulatory actions of the volatile convulsant flurothyl and its anesthetic isomer at inhibitory ligand-gated ion channels Neuropharmacology. 2000;39:1168–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen SL, Sperling BB, Sanchez C. Anticonvulsant and antiepileptogenic effects of GABA(A) receptor ligands in pentylenetetrazole-kindled mice Prog Neuro-Psychoph 2004. January;28:105–113. [DOI] [PubMed] [Google Scholar]
- 22.Scheffer IE, Turner SJ, Dibbens LM, Bayly MA, Friend K, Hodgson B, et al. Epilepsy and mental retardation limited to females: an under-recognized disorder Brain. 2008. April;131:918–927. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


