Abstract
Collagen XI is a fibril-forming collagen that regulates collagen fibrillogenesis. Collagen XI is normally associated with collagen II-containing tissues such as cartilage, but it also is expressed broadly during development in collagen I-containing tissues, including tendons. The goals of this study are to define the roles of collagen XI in regulation of tendon fibrillar structure and the relationship to function. A conditional Col11a1-null mouse model was created to permit the spatial and temporal manipulation of Col11a1 expression. We hypothesize that collagen XI functions to regulate fibril assembly, organization and, therefore, tendon function. Previous work using cho mice with ablated Col11a1 alleles supported roles for collagen XI in tendon fibril assembly. Homozygous cho/cho mice have a perinatal lethal phenotype that limited the studies. To circumvent this, a conditional Col11a1flox/flox mouse model was created where exon 3 was flanked with loxP sites. Breeding with Scleraxis-Cre (Scx-Cre) mice yielded a tendon-specific Col11a1-null mouse line, Col11a1Δten/Δten. Col11a1flox/flox mice had no phenotype compared to wild type C57BL/6 mice and other control mice, e.g., Col11a1flox/flox and Scx-Cre. Col11a1flox/flox mice expressed Col11a1 mRNA at levels comparable to wild type and Scx-Cre mice. In contrast, in Col11a1Δten/Δten mice, Col11a1 mRNA expression decreased to baseline in flexor digitorum longus tendons (FDL). Collagen XI protein expression was absent in Col11a1Δten/Δten FDLs, and at ~50% in Col11a1+/Δten compared to controls. Phenotypically, Col11a1Δten/Δten mice had significantly decreased body weights (p < 0.001), grip strengths (p < 0.001), and with age developed gait impairment becoming hypomobile. In the absence of Col11a1, the tendon collagen fibrillar matrix was abnormal when analyzed using transmission electron microscopy. Reducing Col11a1 and, therefore collagen XI content, resulted in abnormal fibril structure, loss of normal fibril diameter control with a significant shift to small diameters and disrupted parallel alignment of fibrils. These alterations in matrix structure were observed in developing (day 4), maturing (day 30) and mature (day 60) mice. Altering the time of knockdown using inducible I-Col11a1−/− mice indicated that the primary regulatory foci for collagen XI was in development. In mature Col11a1Δten/Δten FDLs a significant decrease in the biomechanical properties was observed. The decrease in maximum stress and modulus suggest that fundamental differences in the material properties in the absence of Col11a1 expression underlie the mechanical deficiencies. These data demonstrate an essential role for collagen XI in regulation of tendon fibril assembly and organization occurring primarily during development.
Keywords: Collagen XI, Col11a1, Tendon, Collagen fibrillogenesis, Tendon structure, Tendon biomechanics, Conditional mouse model
Introduction
The hierarchical assembly of collagen into connective tissues during development, growth and maturation determines tissue structure and function [1–3]. Disruption of this assembly results in tissue dysfunction [4] and often underlies connective tissue diseases [5,6]. In addition, mechanical influences such as tension on fibrils has been shown to influence degradation with varying effects at different hierarchal levels [7].
Tendon structure and function is dependent upon the regulated assembly of collagen I into fibrils and higher order structures. Collagen I is the major fibril-forming collagen in tendons and ligaments [1–3]. The regulatory fibril-forming collagens XI and V are essential in the regulation of initial fibril assembly and fiber organization, despite being quantitatively minor components in tendons [1,3]. Collagen XI is expressed during development, but is virtually absent in mature tendons [8] In contrast, collagen V is expressed throughout development and maturation, as well as in mature tendon [8]. Mutations in COL11A1 result in Stickler syndrome type II [9] while mutations in collagen V define classic Ehlers Danlos Syndrome (EDS) [10]. Both diseases are generalized connective tissue disorders with broad tissue involvement and patients with these disorders have abnormal regulation of collagen fibril assembly. Both clinical phenotypes include joint laxity involving tendons and ligaments [11]. This supports roles for these collagens in establishing tendon structure and function. COL11A1 and COL11A2 genes have been linked to tendinopathy [12,13] supporting critical roles for this collagen in tendon biology.
Collagens V and XI are fibril-forming collagens, but they form a subclass, termed regulatory fibril-forming collagens [1,14,15]. These collagens are very homologous and can be considered different molecular forms of a single collagen type [1,16–18]. The major isoforms in tendon, are [α1(V)]2α2(V) and α1(XI)α2(XI)α3(XI) [8]. Collagen V has multiple isoforms including: [α1(V)]2α2(V), [α1(V)]3, and α1(V)α2(V)α3(V) while collagen XI has a single isoform α1(XI)α2(XI)α3(XI) [16]. However, hybrid molecules containing both collagen V and XI gene products, such as [α1(XI)]2α2(V), and α1(XI)α2(V)α3(XI) have been described [18–20]. Additional variation results from alternative splicing. The N-terminus of the α1 (XI) chain of collagen XI undergoes alternative splicing resulting in multiple variants [18,21,22]. The alternatively spliced forms differ in the acidic or basic domains that may alter interactions with other components of the extracellular matrix resulting in tissue-specific functions [23]. For example, in the embryonic tendon a basic exon is preferentially utilized that may influence interactions with extracellular matrix proteins including small leucine rich proteoglycans [21].
These collagens are characterized by partial processing of their N-propeptide domain. The N-terminal domain is composed of a variable domain and a proline/arginine rich protein domain. Processing removes the proline/arginine rich domain, but retains a hinge region, short collagenous (COL2) domain, and variable domains [16,24]. These regulatory fibril-forming collagens co-assemble with collagens I and II as heterotypic fibrils with the N-terminal regulatory domain on the fibril surface [1,25,26]. Assembly of heterotypic fibrils is closely associated with cell surfaces, and interactions with tenocytes would permit cellular control of fibril deposition and organization [27].
Collagen XI is not generally considered a component of the tendon extracellular matrix. Collagens XI and V are generally associated with collagen II- and I-containing tissues respectively [15]. However, collagen XI is broadly localized during development in a variety of tissues including tendons, [28–30] while collagen V also is found in cartilage and other collagen II-containing tissues [20,31]. In tissues primarily containing collagen I, collagen V has been shown to be a critical regulator of fibrillogenesis; determining fibril structure, fiber organization and functional properties in a variety of tissues including tendons and ligaments [8,32–34] as well as skin [35–37] and cornea [38,39]. Collagen XI has comparable regulatory roles in regulating the assembly of collagen II-containing fibrils in cartilage [26,40]. Our previous analyses in the tendon provided unexpected results suggesting that regulation of tendon fibril nucleation and initial assembly involved coordinate interactions of both collagens V and XI. In addition, collagen XI was shown to interact with collagen I to regulate fibril assembly in vitro [17]. These studies strongly support regulatory roles for collagen XI in tendon.
Our previous work demonstrated a role for collagen XI in the tendon [8]. A transcript analysis demonstrated expression of Col5a1, Col5a2, Col11a1, Col11a2 and Col11a3 in the tendon. In addition, the α1(V) and α1(XI) chains were present. These data suggested that the ubiquitous forms of collagen XI, α1(XI)α2(XI)α3(XI) and collagen V, [α1(V)]2α2(V) were both expressed during tendon development and maturation. However, there was a differential expression of collagens XI and V. The analyses indicated comparable expression of the collagen V and XI genes during development and early post-natal stages. In mature tendons, collagen XI expression was virtually absent while collagen V expression was decreased, but constant. Immuno-localization in postnatal tendons demonstrated that collagens V and XI were not co-localized suggesting independent regulatory functions rather than redundant functions. Hybrid collagen V/XI molecules were not were not supported with a lack of co-localization. Studies utilizing mouse models where collagen V and/or XI expression was manipulated during development suggested a key role for collagen XI in establishing tendon structure. In addition, synergistic roles are supported by the analyses [8]. However, these previous studies were limited by the perinatal lethal phenotype in the collagen XI-null (cho/cho) mice [41]. To address this limitation, we have developed a conditional Col11a1-null mouse model and targeted it to the tendon using Scleraxis-Cre (Col11a1Δten /Δten). Using this novel model where Col11a1 was knocked out in tendons supports an essential role for collagen XI in the regulation of early fibril assembly and fiber organization in tendons processes that are critical in the acquisition of mechanical function.
Results
Generation of a tendon-targeted Col11a1 conditional knockout mouse model
To analyze the role(s) of collagen XI in establishing and maintaining tendon structure and function, a tendon-targeted Col11a1 conditional knockout mouse model was created. Col11a1 was chosen since its knockout would preclude the formation of the collagen XI trimer (α1(XI)α2(XI)α3(XI)). In addition, the 2 known collagen V/XI hybrid molecules ([α1(XI)]2α2(V), and α1(XI)α2(V)α3(XI)) both contain the α1(XI) chain. Therefore, the knockout of Col11a1 would preclude the assemble of these molecular forms as well. The α1(XI) chain has 3 major alternatively spliced forms and several minor ones. These alternatively spliced variants involve the N terminal domain that is retained after processing. Exon 3 is present in all the splice variants. Therefore, targeting exon 3 ensures the knockout of α1(XI) and precludes transcription/translation of an alternatively spliced α1(XI) isoform. Deletion of exon 3 results in a skip from exon 2 to exon 4 where the resulting transcript is out of frame. This transcript codes for a 102 amino acid peptide before the first of several stop codons. This peptide that includes the signal sequence coded by exons 1 and 2 is susceptible to nonsense mediated decay with no expression of the Col11a1 transcript. This strategy does not allow for the targeting of a specific collagen XI isoform, but rather will knock out all collagen XI isoforms.
The strategy utilized was similar to that in our published work (Fig. 1) [34]. Targeted ES cells were obtained after electroporation of the Col11a1 targeting vector into mouse embryonic stem (ES) cells. ES cells containing the targeted Col11a1 allele were identified by the absence of the negative selection marker DTA, the presence of LacZ, and orientation was confirmed by 3’ and 5’ gene and vector specific sequence using short-range and long-range PCR (Fig. 2A). The successfully targeted ES cells were then injected into wild type blastocysts, resulting in chimeric mice. The chimeric mice were backcrossed with wild type mice to produce the germline transmitted mice with the targeted allele Col11a1+/ta. Breeding Col11a1+/ta mice with FLPe mice resulted in the conditional knockout mice Col11a1flox/flox, where the FRT flanked neo sequences were excised. Mice at each different stage were characterized using PCR that amplified specific element sequences (Fig. 2B). This demonstrated that a conditional Col11a1flox/flox knockout was obtained.
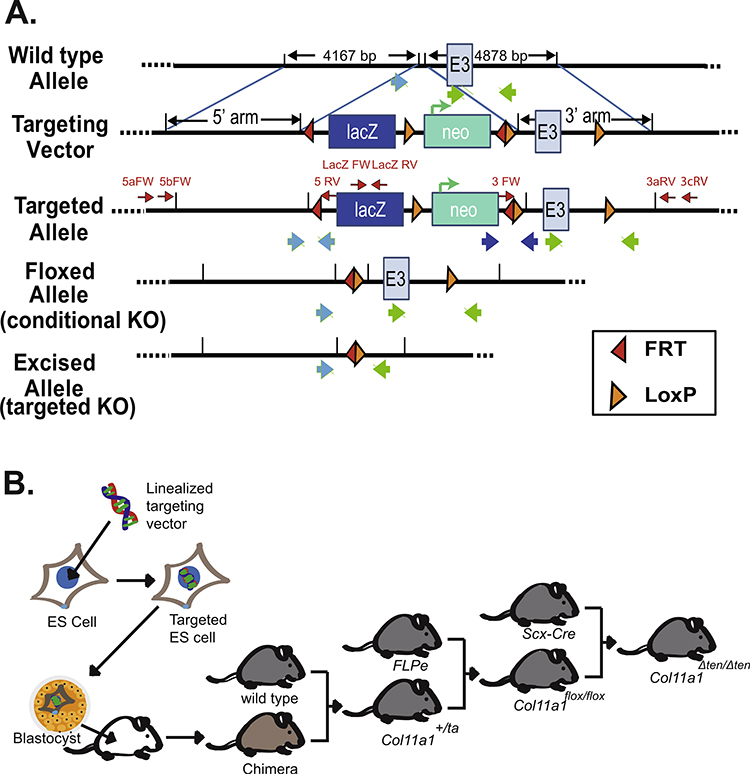
Fig. 1.
Strategy for creating tendon targeted Col11a1 conditional knockout mice. (A) Schematic diagram showing the wild-type allele, targeting vector, targeted allele, and floxed allele as well as the excised allele after Cre recombination of Col11a1 gene. The Col11a1 targeting vector was obtained from the KOMP Repository (project ID: CSD80258). Exon 3 of Col11a1 is flanked with LoxP sites and the Neo cassette is flanked with FRT sites. Location of primers for LacZ, as well as 3’ and 5’ primers used to determine insert orientation are shown (red arrows). The large blue and green arrows indicate the location of the primers used to identify specific alleles. (B) Strategy for gene targeting of embryonic stem cells and generation of tendon-targeted Col11a1 conditional-knockout mice. Linearized Col11a1 targeting vector was electroporeated into ES cells. Blastocysts were injected with targeted ES cells yielding chimeric mice. The chimeric mice were crossed with wild type mice yielding F1 progeny with the targeted allele (Col11a1+/ta). Breeding of Col11a1+/ta with FLPe mice resulted in excision of FRT flanked sequences, removal of the Neo cassette and creation of conditional knockout mice (Col11a1flox/flox). These mice were bred with mice expressing ScxCre to target the knockout to tendons (Col11a1Δten/Δten).
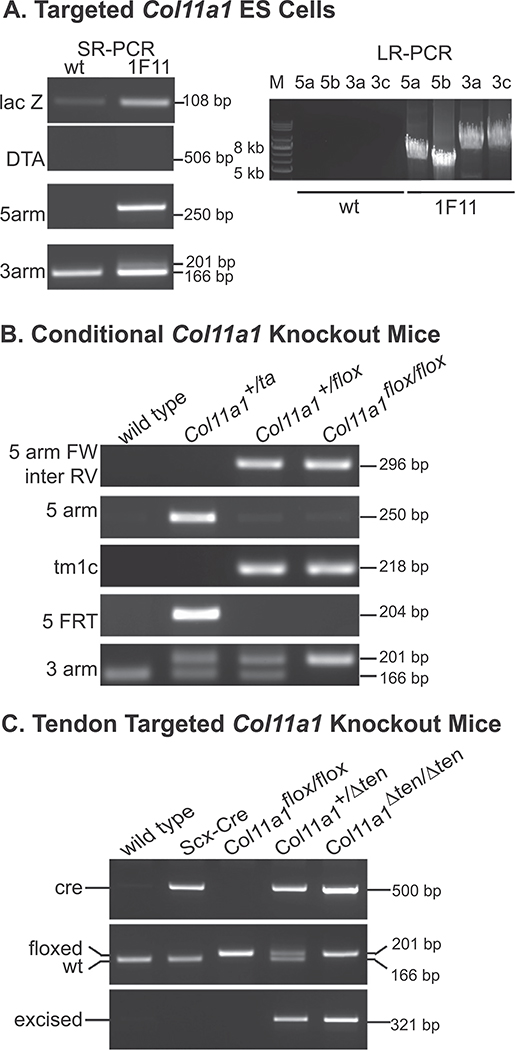
Fig. 2.
Analysis of tendon targeted Col11a1 knockout mice. (A) Characterization of the targeted Col11a1 ES cells using short-range PCR (left panel) and long-range PCR (right panel). Clone 1F11 is the targeted ES cell clone injected into blastocysts. (B) Genotyping of the targeted allele, and floxed alleles in different stages of creating the conditional Col11a1 knock-out mice. (C) Genotyping of the Scleraxis-Cre mice (Scx-Cre), heterozygous (Col11a1+/Δten) and homozygous (Col11a1Δten/Δten) tendon targeted Col11a1 knockout mice.
To target the knockout to tendons the Col11a1flox/flox mice were bred with Scx-Cre mice. Scleraxis is widely expressed in early embryonic development, but with maturation becomes specific for tendons and ligaments. The promoter region utilized to drive Cre expression has been shown to be specific for tendons and ligaments [42–44]. In our previous work using double reporter mice, it was demonstrated that Cre excision occurred only in tendons and ligaments with other limb tissue showing no excision [39]. Genotyping analysis of these mice was carried out using Cre primers and specific primers at the junction of the 3’ arm and targeting sequence. The excision of the Col11a1 exon 3 was identified in tendon-targeted Col11a1 conditional knockout Col11a1+/Δten and Col11a1Δten /Δten mice, but as expected, not in parental Scx-Cre mice or Col11a1flox/flox mice (Fig. 2C). The results confirmed a knockout of Col11a1 expression in the FDL tendon.
Col11a1 gene and protein expression is knocked out in FDLs of Col11a1Δten /Δten mice
The tendon-targeted FDLs were Col11a1 null after Scx-Cre- mediated exon 3 excision. Since in tendon Col11a1 is only highly expressed in development and perinatal periods, FDLs from day 14 mice were analyzed using qPCR (Fig. 3A). Expression of Col11a1 mRNA in control, wild type and conditional control mice was compared to heterozygous (Col11a1+ /Δten) and homozygous Col11a1Δten /Δten mice using qPCR with primers from the C terminal non-helical region. Expression of Col11a1 was not different in the 2 control groups. In contrast, the expression was at baseline in the Col11a1Δten /Δten mice. Compared to the control group, expression of Col11a1 mRNA was reduced by approximately 50% in the heterozygous mice, Col11a1+/Δten mice (Fig. 3A). Primers from the helical region gave comparable results (data not shown).
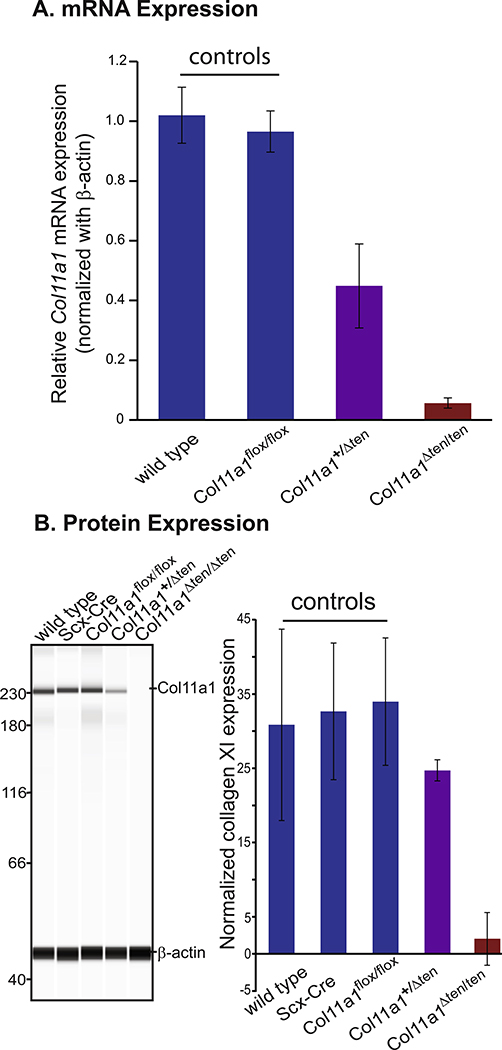
Fig. 3.
Knockout of Col11a1 mRNA and protein expression in the Col11a1 tendon targeted conditional mice. (A) Quantitative real-time PCR shows comparable expression of Col11a1 mRNA in the wild type and Col11a1flox/flox controls. Col11a1 mRNA expression in Col11a1Δten/Δten FDLs is reduced to baseline while expression in the Col11a1+/Δten FDLs was ~50% of control values. Day 14 wild-type (n=3), Col11a1flox/flox (n=5), Col11a1+/Δten (n=8), Col11a1flox/flox (n=7) mice for each genotype. (B) Western blot analysis of Col11a1 protein (α1(XI)) content in control; wild type (n=3), Scx-Cre (n=4), Col11a1flox/flox (n=7) mice was done using Wes automated western blotting. The Col11a1+/Δten (n=3), and Col11a1Δten/Δten (n=4) FDL contained reduced and virtually no α1(XI) reactivity relative to controls. All 3 control mice contained comparable amounts of α1(XI). Day 4 mice. Left panel shows a representative image and the right panel presents the quantitation results from all mice.
The α1 chain of collagen XI in tendon-targeted Col11a1 knockout mice was analyzed immunochemically. The α1(XI) chain was analyzed in day 4 FDL tendons using a WES simple western blotting system. The α1(XI) chain was present at comparable levels in the control group; wild type, Scx-Cre and Col11a1flox/flox mice. In contrast, the α1(XI) chain was present at lower levels in Col11a1+/Δten mice and not detectable in Col11a1Δten /Δten mice (Fig. 3B). Taken together the gene and protein expression data show a knockout of the α1(XI) chain in the Col11a1Δten /Δten mice. Since the α1(XI) chain is present in the collagen XI trimer and collagen V/XI hybrid molecules, the data support a knockout of collagen XI and its isoforms.
Altered fibril structure and organization in Col11a1Δten /Δten FDLs
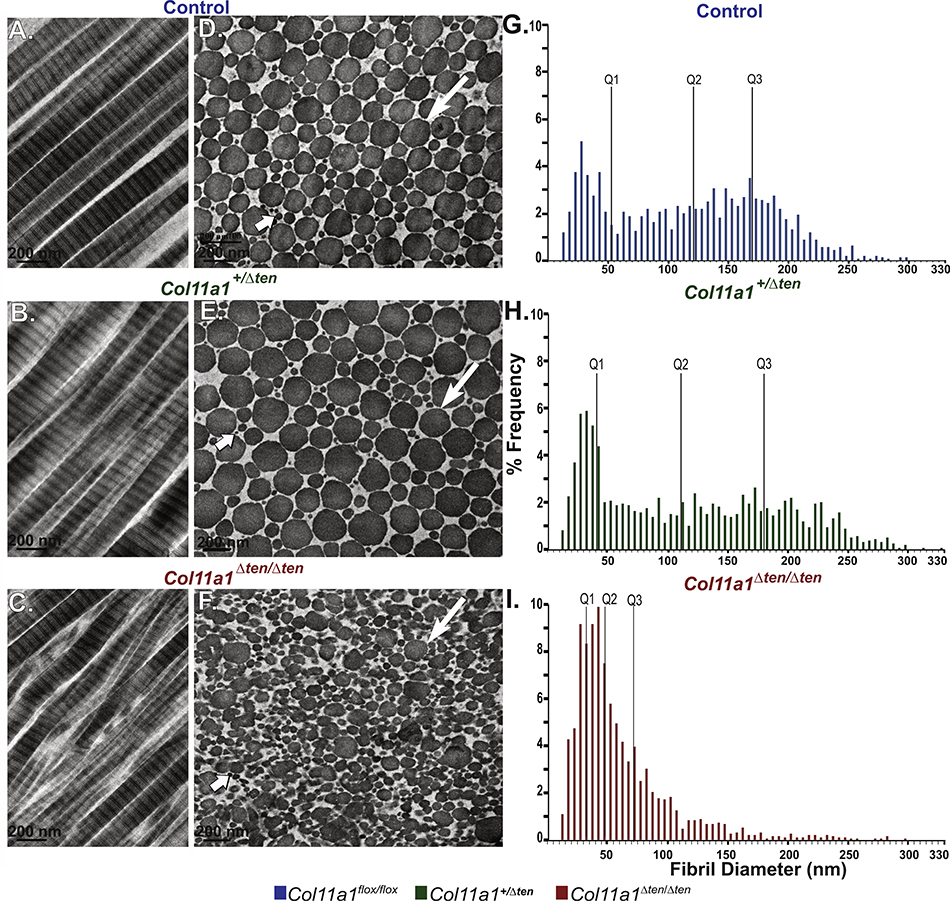
FDLs from maturing male mice at day 30 were analyzed using transmission electron microscopy in control tendons with normal Col11a1 expression and Col11a1+/Δten AS as well as Col11a1Δten /Δten tendons with reduced and no expression respectively (Fig. 4). The control FDLs contained well organized uniaxial fibrils with heterogeneous fibril diameters. In longitudinal sections fibrils were linear with parallel fibril alignment. In transverse sections, the fibrils had regular, roughly circular cross-sectional profiles with a broad distribution of sizes (Fig. 4A,D). In contrast, Col11a1Δten /Δten FDLs lacked linearity and parallel fibril alignment in longitudinal sections. In cross sections the contrast with the control tendons was striking. The regular fibril packing seen in the control was lost. There was a substantially larger percentage of smaller, heterogeneous fibrils compared to the control tendons. In addition, the fibrils assembled in the absence of Col11a1 expression displayed aberrant fibril structures with structurally abnormal fibrils with irregular fibril profiles in the cross sections (Fig. 4C,F). There was a virtual absence of larger fibrils characteristic of the mature FDL in the absence of Col11a1 expression. In Col11a1+/Δten FDLs, with reduced collagen Col11a1 expression, the fibril phenotype was mild. There was some disruption of longitudinal organization and in cross section irregular fibril profiles were common (Fig. 4B,E). However, fibril structure and organization were more comparable to the control tendons than the Col11a1Δten /Δten tendons. In all genotypes, cross sections of large diameter fibrils often displayed surface bumps consistent with lateral fusion of small diameter fibrils. However, Col11a1Δten /Δten tendons showed evidence of poorly regulated lateral fibril fusion in both longitudinal and cross sections. A quantitative analysis of fibril diameter distributions was performed on the 3 genotypes (Fig. 4G–I). Both the Col11a1+/Δten and Col11a1Δten/Δten distributions are significantly different from the control distribution and from each other (p<0.0001, Kolmogorov-Smimov Test). In the control and Col11a1+/Δten while statistically different, the fibril distributions are very comparable. Both are broad multimodal distributions with a well-defined subpopulation of small diameter fibrils. The haploinsufficient distribution has a larger representation of larger diameter fibrils. In contrast, the Col11a1Δten /Δten distribution is strikingly different from the other 2 genotypes. The small diameter fibril distribution is over-represented with greater than 75 percent of the fibril diameters under 100 nm compared to less than 50 percent in the other distributions. The rest of the distribution is poorly developed suggesting an aberrant regulation of lateral fibril growth in the absence of Col11a1 expression. The data are consistent with dose dependent regulation of fibril structure and organization in the tendon. Comparable results were observed in female 30 day FDLs (Supplemental Fig. 4).
Fig. 4.
Abnormal fibril structure in FDLs from tendon targeted Col11a1 knockout mice. (A-C) Transmission electron microscopy demonstrates a disruption in the parallel fibril alignment in FDLs in Col11a1Δten/Δten mice compared to wild type mice while fibril alignment in Col11a1+/Δten FDLs is partially disrupted. (D-F) Col11a1Δten/Δten FDLs have a larger percentage of smaller, more heterogeneous and structurally abnormal fibrils compared with wild types controls. In contrast, haploinsufficient Col11a1+/Δten FDLs show only a mild disruption of fibril structure. (G-I) All 3 distributions are significantly different from one another (p<0.001 K-S test). There is a striking difference between the Col11a1Δten/Δten and the other 2 distributions. The fibril diameter distribution is significantly shifted to the smaller diameter fibrils with the larger diameter subpopulation reduced to a minor shoulder in the Col11a1Δten/Δten mice compared to the wild type mice. The wild type and Col11a1+/Δten distributions are very similar with the reduction in Col11a1 associated with a mild phenotype with an increase in large diameter fibrils relative to the control. Day 30 males, n=2 for each genotype.
Phenotype of tendon-targeted Col11a1Δten /Δten mouse models
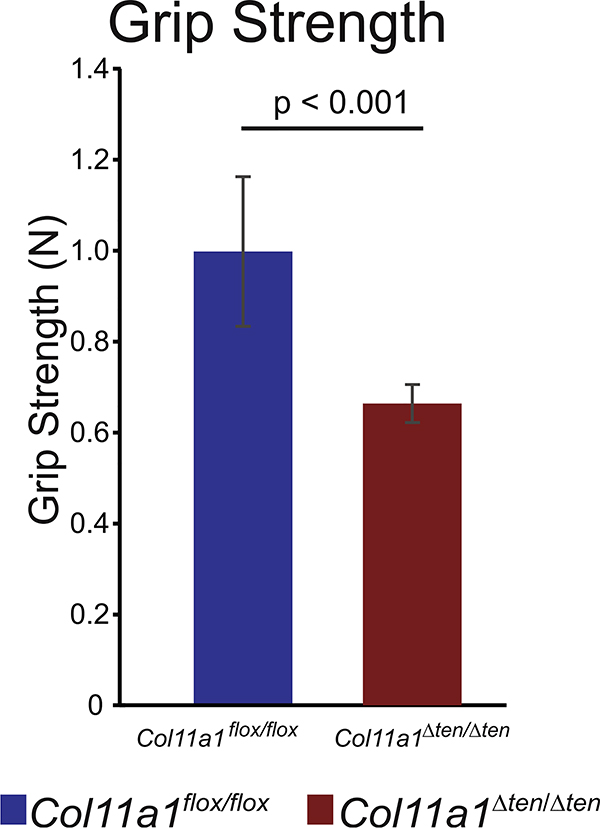
The Col11a1Δten /Δten mice were viable and fertile. However, they were not optimal breeders. This may be due to the stress and difficulty accessing food and water because of impaired mobility. The Col11a1Δten /Δten mice were reluctant to move around their cages. In addition, there was excessive joint laxity in the Col11a1Δten /Δten mice compared with controls. The Col11a1Δten /Δten mice were smaller than control mice. The average body weight of male day 60 Col11a1Δten /Δten mice was significantly smaller (p<0.0001) than that of the control, Col11a1flox/flox mice. The mean body weights were 27.72 ± 1.40g (n=9) and 24.22 ± 1.14 g (n=15) for control and Col11a1Δten /Δten mice, respectively. As the Col11a1Δten /Δten mice aged they developed gait impairment seen as dragging of their hind limbs and becoming hypomobile. Euthanasia had to be performed at this stage. To evaluate musculoskeletal and motor function in the Col11a1Δten /Δten mouse model, grip strength tests were conducted on the fore-limbs of day 60 male Col11a1Δten /Δten mice and Col11a1flox/flox control mice. Significant impairment of function was observed with the Col11a1Δten /Δten mice being weaker than the control mice (Fig. 5). The mean grip strengths were significantly different (p<0.0001). The value for control mice was 0.998 ± 0.165N, n=9 (0.224 ± 0.037 lb), and 0.664 ± 0.042N, n=9 (0.149 ± 0.009 lb) in the Col11a1Δten /Δten mice.
Fig. 5.
Tendon targeted Col11a1 knockout mice are weaker. Fore-limb grip strength is significantly decreased in Col11a1Δten/Δten mice (n=9) compared to Col11a1flox/flox control mice (n=8). Day 60 male mice.
Reduction in biomechanical properties in Col11a1Δten /Δten FDLs
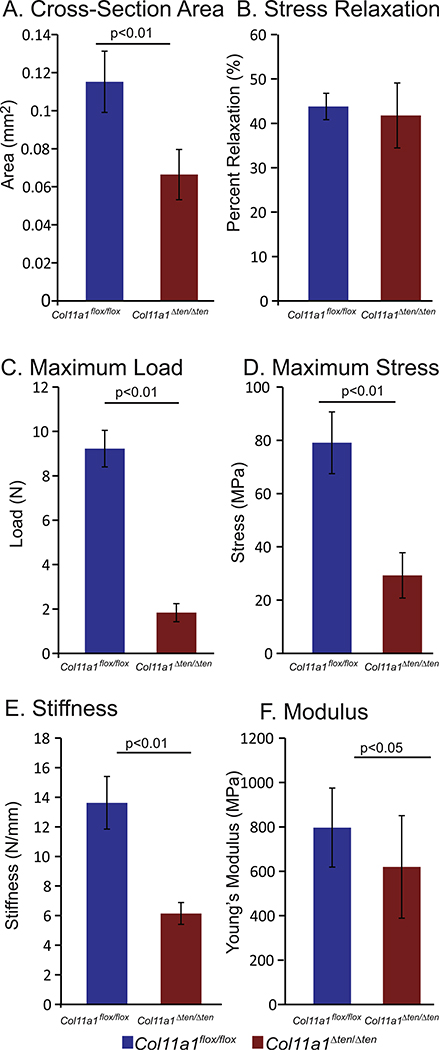
The FDL tendons from Col11a1Δten /Δten day 60 male mice demonstrated significant alterations in their biomechanical properties. The Col11a1Δten /Δten FDLs were smaller and weaker compared to the control Col11a1flox/ flox FDLs. A significant decrease in cross-sectional area was observed compared to the control tendons (Fig. 6A). Maximum load and maximum stress were both significantly reduced in the Col11a1Δten /Δten FDLs compared to controls (Fig. 6C,D) as was stiffness, and modulus (Fig. 6E, F). These data indicate that the absence of Col11a1 expression in the FDL significantly reduces the biomechanical properties of these tendons. Decreases in maximum stress and modulus suggest that fundamental changes in the material properties of Col11a1Δten /Δten and control FDL tendons underlie the observed mechanical deficiencies. In contrast, no differences were observed in tendon stress relaxation (Fig. 6B). This viscoelastic (time-dependent) response was comparable in Col11a1Δten /Δten and control Col11a1flox/ flox tendons. This suggests that Col11a1 expression does not play a substantial role in modulating fibril sliding or crosslinking properties, that have been previously correlated with changes in the viscoelastic response of tendons [45,46].
Fig. 6.
Altered biomechanical properties in FDLs in tendon targeted Col11a1 knockout mice. FDL tendons from tendon-targeted Col11a1Δten/Δten null mice demonstrated significant alterations in the biomechanical properties. The Col11a1Δten/Δten FDLs were smaller, weaker, and stiffer compared to the wild type controls. (A) A significant decrease in cross-sectional area was observed. In addition, (B) maximum load, (C) maximum stress, (D) stiffness, and (E) modulus were significantly reduced in the Col11a1Δten/Δten mice compared to controls. Decreases in maximum stress and modulus suggest that fundamental differences in the material properties of Col11a1Δten/Δten and control FDL tendons underlie the mechanical deficiencies. Day 60 male Col11a1flox/flox (n=12) and Col11a1Δten/ Δten (n=15) male mice.
Col11a1Δten /Δten FDLs have abnormal fibril structure at all ages primarily due to regulatory effects in development
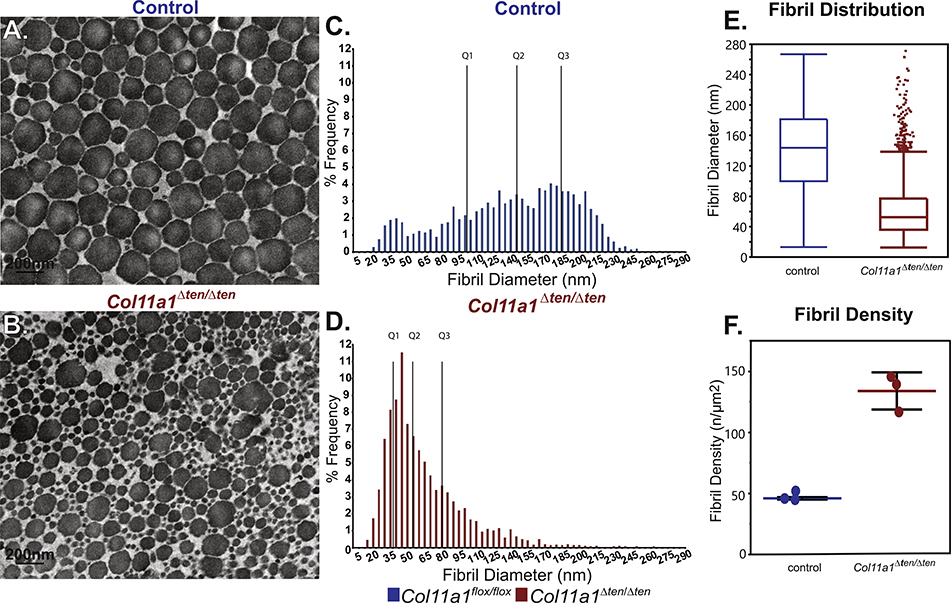
An analysis of fibril structure was done in control and Col11a1Δten /Δten FDLs in mature (day 60) and developing (day 4) FDLs to define critical regulatory stages. In the mature FDL, the absence Col11a1 expression resulted in a shift to smaller diameter fibrils when compared to controls (Fig. 7A–D). In addition to the shift to small diameter fibrils there was aberrant fibril structure in the absence of Col11a1 expression. Overall, these results are a logical extension based on the day 30, maturing tendon data (Fig. 4). In the absence of Col11a1 expression, the diameter distribution is dominated by two distinct subpopulations, small diameter fibrils and a smaller population of large diameter fibrils (Fig. 7E). Fibril density was significantly higher in Col11a1Δten /Δten compared to control tendons (Fig. 7F). This is consistent with the larger number of small diameter fibrils observed in the absence of Col11a1 expression.
Fig. 7.
The absence of Col11a1 expression results in abnormal FDL fibrils in mature Col11a1Δten/Δten mice. FDLs from mature male Col11a1Δten/Δten mice (day 60) have a severe disruption in collagen fibril structure and diameter distributions compared to control tendons. (A,B) Electron microscopic analysis demonstrates a substantial increase in small diameter fibrils as well as overall smaller diameters in the FDLs from Col11a1Δten/Δten compared to control FDLs. (C-E) The fibril distributions from wild type and Col11a1Δten/Δten tendons are significantly different (KS p<0.0001). In the Col11a1Δten/Δten mice there is a significant shift to small diameter fibrils and a virtual absence of large diameter fibrils characteristic of the mature FDL. The mature day 60 mice show the same trends as seen in maturing day 30 mice (Fig. 4). (F) Fibril density is significantly increased in the Col11a1Δten/Δten FDLs compared to controls, consistent with the significant shift to small diameter fibrils observed in this genotype. (n=3 mice for each genotype).
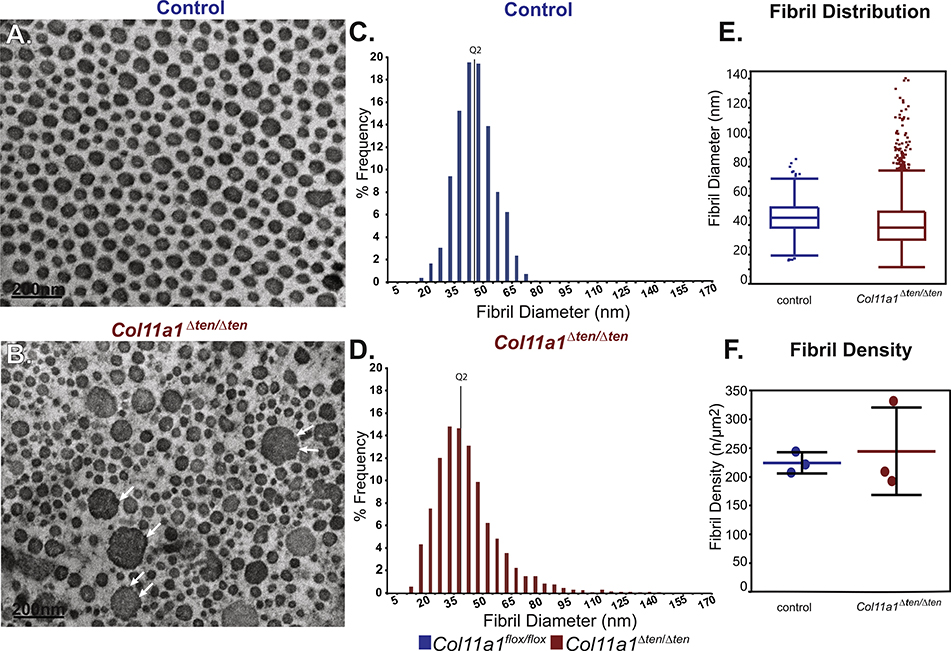
Collagen XI genes are expressed in early stages of tendon development while expression was minimal in mature FDLs [8], therefore, the effect of its absence was evaluated in day 4 developing FDLs (Fig. 8). Col11a1Δten /Δten tendons had a significant disruption in fibril packing and organization with a subpopulation of very large fibrils. This subpopulation of large diameter fibrils demonstrated evidence of lateral fibril fusion suggesting that they arose from fusion of the small diameter fibrils seen in the controls at this developmental stage. The overall disruption in fibril packing observed in the absence of Col11a1 expression further supports abnormal lateral fibril growth. Overall, at day 4, fibril structure was severely abnormal in the absence of Col11a1 expression (Fig. 8A,B). In the control tendons, fibrils are small with a unimodal distribution. In contrast, Col11a1Δten /Δten fibrils have a broader distribution with a distinct right shoulder with larger diameter fibrils. The median diameter is smaller compared with fibrils from control tendons (Fig. 8C–E). Fibril density in Col11a1Δten /Δten FDLs was more heterogenous compared to controls (Fig. 8F), consistent with the fibril disorganization observed in this genotype. These data suggest a primary regulatory role in tendon development and the perinatal period.
Fig. 8.
Expression of Col11a1 regulates early steps of fibrillogenesis. (A,B) Electron microscopic analysis demonstrates smaller and more heterogeneous fibrils in the FDLs from developing Col11a1Δten/Δten mice compared with the wild type mice at day 4. The large diameter fibrils seen in Col11a1Δten/Δten showed evidence of fusion with small diameter fibrils (arrows) suggesting their formation was a result of unregulated lateral fibril growth. (C-E) The diameter distributions from wild type and Col11a1Δten/Δten FDLs are significantly different (KS p<0.0001). The fibril diameter distributions shows a substantial increase in small diameter fibrils and the broader distribution of fibril diameters in the Col11a1Δten/Δten FDLs compared to controls. (F) Fibril density in Col11a1Δten/Δten FDLs is similar to the controls, but considerably more variable consistent with the presence of a distinct subpopulation of very large diameter fibrils not seen in the control tendons. (n=3 mice for each genotype).
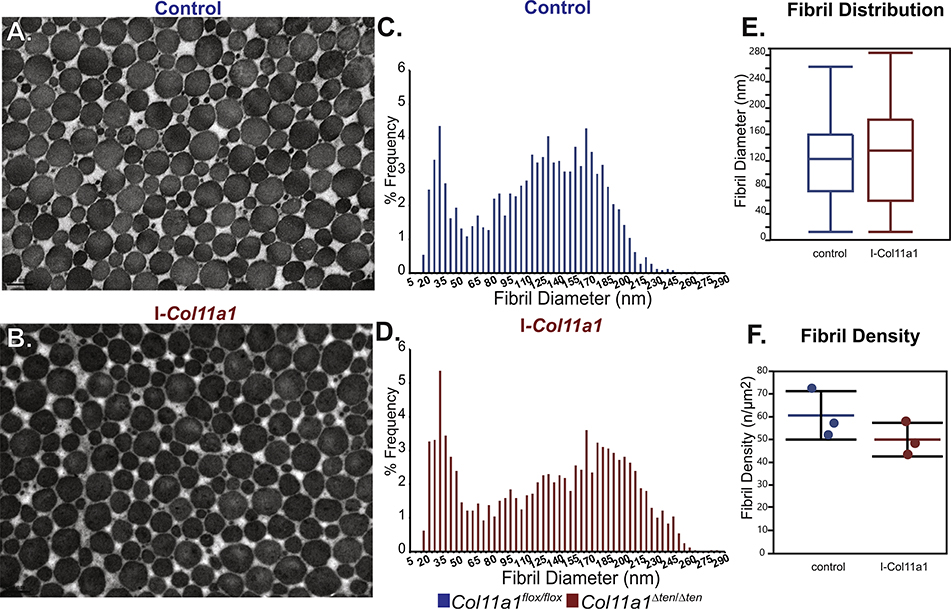
To determine whether the regulatory dysfunction during tendon development resulted in the mature phenotype of if there was a continued regulatory influence of Col11a1 expression during tendon maturation we utilized an inducible Col11a1 knockdown mouse model (I-Col11a1−/−). Tendons were allowed to develop normally and then knockdown of Col11a1 was induced during maturation (day 25) and the FDLs analyzed in mature tendons at day 60 (Fig. 9). Collagen fibril structure was comparable in both I-Col11a1−/− FDLs with an induced knockdown of Col11a1 during maturation, and control FDLs. Both had heterogeneous populations of fibrils with regular circular profiles (Fig. 9A,B). Fibril diameter distributions were comparable with both having a bimodal distribution with a narrow subpopulation of small diameter fibrils and a subpopulation of larger diameter fibrils with a broad distribution. Fibril densities were not significantly different in the induced and control tendons (Fig. 9C–F). The larger diameter subpopulation was marginally broader with a modest increase in largest diameter fibrils in the absence of Col11a1 expression. Overall, these data indicate a critical regulatory role for Col11a1 expression during the development of tendon structure and function. Disruption of regulation during this period is sufficient to continue to exert an influence on tendon structure and function in the mature tendon, however, continued minor regulatory influences of Col11a1 expression during maturation cannot be excluded.
Fig. 9.
Induced knockdown Col11a1 in the mature tendon does not affect fibril structure. Knockdown of Col11a1 was induced using tamoxifen once daily for 3 days (i.p 10mg/100g body weight) beginning at day 25 and fibril structure analyzed at day 60. This approach allows the FDLs to develop as in wild type through development and into maturation. Knockdown is during maturation and mature FDLs are analyzed. (A,B) Electron microscopic analysis shows no significant effect on the fibril diameter and organization. Fibrils from both genotype have normal circular profiles. (C-E) The diameter distributions from wild type and I-Col11a1−/− FDLs are both bimodal and very similar. The larger diameter subpopulation is shifted modestly toward larger diameter in the I-Col11a1−/− compared to wild type FDLs. (F) Fibril density in I-Col11a1−/− FDLs is decreased, consistent with the increase in diameter observed. (n=3 mice for each genotype).
Discussion
The overall goals of this study were to define the roles of collagen XI in regulation of tendon fibrillar structure and organization as well as the relationship to function. We hypothesize that collagen XI functions during developmental stages to regulate tendon fibril assembly, and organization. In addition, it is likely that the primary regulatory mechanism is confined to development and early perinatal stages based on the expression pattern of Col11a1 in the FDL [8]. However, little is known about regulation involving collagen XI in the mature tendon. To address these goals a conditional Col11a1-null mouse model was created. The α1(XI) chain is required for assembly of the major collagen XI isoform, α1(XI)α2(XI)α3(XI). In addition, it is required for assembly of the described collagen V/XI hybrid trimers. Therefore, we anticipate that all collagen XI isoforms would be knocked out. This approach allows an analysis of the functional roles of collagen XI in the tendon but will not address roles for specific isoforms. Our previous work indicated that the major collagen XI isoform (α1(XI)α2(XI)α3(XI)) was present in the FDL.[8] However, collagen V is more prominent in tendons and the presence of collagen V/XI hybrid isoforms was not addressed. Therefore, definition of the different isoforms in tendons and their individual roles will require further analysis.
Our previous work was done using the cho/cho mouse model [8]. This mouse is described as being null (cho/cho) or haploinsufficient (+/cho) for collagen XI [40,41]. The cho mutation results in a loss of the α1(XI) chain and as a result none of the collagen XI isoforms can assemble. Similar to the data presented here, the +/cho tendons had a mild disruption in fibril structure compared to controls in both embryonic and mature tendons. In contrast, as shown here, cho/cho embryonic tendons had a more severe phenotype with aberrant fibril structure, altered diameter regulation, disorganized fibrils and a reduced number of fibrils compared to wild type controls [8]. However, the cho/cho mouse model is perinatal lethal and mature null mice are not available for these studies [41]. Our conditional Col11a1 knockout model circumvents this limitation. The conditional mice (Col11a1flox/ flox) are comparable to wild type mice in gross phenotype and collagen XI expression. We created 2 derivative models, one that was targeted to tendons and ligaments using Scx-Cre and a second TM inducible model. Both of these mouse lines avoid the perinatal lethal phenotype. These Col11a1-null mouse models permit the spatial or temporal manipulation of collagen XI expression required for these studies. Tendon-targeted Col11a1Δten/Δten mice also allowed the spatial targeting of the mutation to tendons. This circumvents potential secondary influences on the tendons such as those due to alterations in cartilage, bone and muscle. The inducible knockdown mice (I-Col11a1−/−) allow for temporal control of Col11a1 knockdown. This permits normal development in the presence of collagen XI with the knockdown occurring at maturity thus isolating the effect(s) to this specific time.
Collagen XI is a fibril-forming collagen found in collagen II containing tissues like cartilage and vitreous where it has been shown to regulate collagen fibril assembly [26,40]. Generally, collagen XI has not been considered to be a major factor in collagen I-containing tissues such as tendons, ligaments, cornea, and skin. However, it is widely distributed in embryonic tissues including tendons [28–30]. Also, collagen XI has been shown to interact with collagen I influencing fibril assembly in in vitro studies [17]. Using Col11a1-null mouse models, we demonstrate a significantly regulatory role for collagen XI in the tendon. Given that the α1(XI)α2(XI)α3(XI) isoform is expressed in FDLs, it is likely that this form has a role. However, as discussed above, independent or synergistic roles with other collagen XI isoforms cannot be excluded.
In the absence of Col11a1 expression and therefor collagen XI, mature tendons have a significant disruption in fibril phenotype with a relative absence of large diameter fibrils compared to wild type mice. The heterozygous mice more closely resembled the wild type mice suggesting that the reduced collagen XI expression was sufficient for regulation of fibrillogenesis. This is consistent with in vitro fibrillogenesis assays that demonstrated decreased fibril diameter as collagen XI concentration was increased relative to collagen I [17]. This result implicates collagen XI as a critical regulator of tendon fibril assembly. Collagen V has also been shown to be a major regulator of the fibril assembly in collagen I containing tissues where it initiates/nucleates the fibril assembly process in association with the fibroblast surface [27,35,36,39]. It also has a key role in regulation of tendon fibril assembly [34]. Collagens V and XI are closely related and can be considered different isoforms of the same collagen type [16,17]. These data suggest that collagens XI and V both have regulatory roles in the tendon. Our work is consistent with an independent role for collagen XI. This work and previously published studies [8] suggest different, but coordinate roles for collagens XI and V in the tendon. The coordinate roles require further study to be fully elucidated.
Interestingly, consistent with Col11a1 expression restricted to developmental and perinatal periods [8], we demonstrate that the absence of collagen XI leads to a more severe regulatory dysfunction in developing compared to mature tendons. In contrast, the situation is reversed in the absence of collagen V with developing tendons having a very mild phenotype [34]. These data suggest a restricted time frame for collagen XI regulation during the establishment of tendon extracellular matrix architecture. To address this, the FDL was allowed to develop normally in the presence of Col11a1 expression until day 25 and then during the final stages of maturation induced a knockdown of Col11a1 expression. When we analyzed mature tendons at day 60, we found fibril structure and organization comparable, but not identical in the knockdown and the control tendons. This indicates that the major regulatory role of collagen XI is restricted to periods when tendon structure is being established coincident with the period of collagen XI expression [8]. However, the developing phenotype is not lost, diluted out, in the day 60 mature FDL. This suggests that the tendon matrices assembled in the presence or absence of collagen XI continue to guide assembly in maturing tendons. Collagen V regulated fibril assembly is dominant during this period and has only a minor function during development [34]. However, fibril structure, organization and tendon mechanical properties are all abnormal in mature, day 60 FDLs from the Col11a1Δten /Δten mice. The knockout of Col11a1 results in the knockout of the major collagen XI isoform as well as collagen V/XI hybrid molecules. It is possible that these isoforms could have differential regulatory roles, and this requires further evaluation. However, the data clearly support a critical regulatory role for collagen XI.
Our studies also demonstrated a disorganization of fibril alignment in the absence of Col11a1 expression. Both collagens XI and V are associated with the tenocyte surface and do not co-localize [8,27]. It is possible that the association of collagen XI with the tenocyte surface allows the tenocytes to position the assembling fibrils in the developing matrix. This control would be lost in the absence of collagen XI resulting in disorganized fibril deposition. The presence of collagen XI in the early stages of tendon development suggest a key role is establishing tendon fibrillar matrix architecture. It is possible that the collagen XI-directed deposition of fibrils provides a template for further matrix assembly. The abnormal assembly of a template may explain the continued influence of collagen XI seen in the mature tendon. In addition, the lack of co-localization of collagens XI and V at the tenocyte surface,[8] suggests separate, non-redundant functions for these collagens. One might speculate that collagen XI nucleates fibril assembly and that there is a strong affinity of collagen XI for the tenocyte surface resulting in a slow release of these fibrils into the developing matrix forming a stable template. In contrast, [2] collagen V would be rapidly released into the matrix with the collagen XI-directed template responsible for their organization. In mature tendon where collagen V is the regulator, the matrix architecture is established there would be no further need for a template. In addition, the data suggested an alteration in fibril structure consistent with dysfunctional regulation of lateral fibril fusion. This may result directly from the absence of charged α1(XI) domains on the surface of heterotypic tendon fibrils. Alternatively, the lack of specific charges domains may alter the interaction of molecules such as decorin, fibromodulin known to regulate lateral fibril growth [1,2]. In addition to altered fibril structure, changes in fibril-associated molecules can result in altered growth factor availability with resulting changes in tenocyte behavior that would influence tissue function [47]. In addition, collagen V/XI fragments can bind to specific growth factors including FGF-2 [48]. This suggest specific sites can bind these factors and sequester or present them during physiological and pathological processes. The different alternatively spliced domains vary in charge and charge density. This may provide a mechanism for differential regulation in tissues, developmental stages, and after injury.
Finally, collagen XI underlies a human congenital disorder with a broad spectrum of connective tissue defects. Stickler syndrome type II is caused by mutations in COL11A1. It is characterized by skeletal and joint abnormalities specifically hypermobility [11,49] among other features. Joint hypermobility involves defects in supporting tendons and ligaments. Interestingly, in affected children and young adults, joints are often loose and very flexible, however, the joints become less flexible with age. This is consistent with the structural phenotype in our mouse model. Joint hypermobility may involve contributions from both affected cartilages and tendons. However, the reduction in structural and material tendon mechanical properties, i.e., decreased strength and stiffness supports tendon involvement. It has previously been postulated that a bimodal distribution of collagen fibril diameters is necessary for optimal tendon mechanical resilience [50]. A near complete loss of larger diameter fibrils, and therefore the bimodal distribution, was observed in the Col11a1Δten/Δten tendons, that might explain the substantial loss of mechanical integrity in these tendons. The tendon targeted Col11a1 null mouse model excludes cartilage involvement. In addition, the improvement in hypermobility from childhood to adult is consistent with a critical role for collagen XI in early tendon development and lesser role in the mature tendon. This is consistent with the definition of the primary regulatory foci of collagen XI being during tendon development in the FDLs. Stickler syndrome type II is a heterozygous condition and our Col11a1 mouse is predicted to be an excellent experimental model for this human congenital disorder. The conditional model can be utilized in its heterozygous and/or homozygous state to isolate the contributions of different tissues, define the temporal requirements for Col11a1 expression and to probe the mechanisms underlying the pathobiology.
In conclusion, unique conditional Col11a1 mouse models were created that permit the spatial and temporal targeting of tissues and times. Utilizing these mice, it was demonstrated that Col11a1 expression has a critical regulatory function in tendon development but has little to no direct regulatory function in mature tendons. However, the mature Col11a1Δten/Δten mice had a severe disruption of fibril structure and organization associated with a severe loss of mechanical function suggesting a continued indirect role for Col11a1 expression in tendon matrix assembly. This work suggests that the lack of the α1(XI) chain results in the absence of α1(XI)α2(XI)α3(XI) and a collagen XI null model. However, the influence of the Col11a1 knockout on other isoforms and hybrid collagen V/XI molecules requires investigation.
Experimental procedures
The work described was approved by the University of South Florida and the University of Pennsylvania Institutional Animal Care and Use Committees. These studies were performed using male and female mice. The tendon-targeted (Scx-Cre) collagen XI knockout mouse model (Col11a1Δten/Δten) is in a C57/BL6 Charles River background. Control mice were wild type, Col11a1-flox/flox, and Scx-Cre all in a C57/BL6 Charles River background.
Development of tendon-targeted Col11a1 knockout mouse model
The strategy for generating conditional Col11a1 knockout Col11a1flox/flox mice is presented in Fig. 1. A promoter-driven knockout first targeting vector was obtained from the KOMP Repository (University of California at Davis, project ID: CSD80258). In this targeting vector, Col11a1 exon 3 was flanked by loxP elements. Briefly, the targeting vector was linearized and electroporated into V6.5 129 Sv/J mouse embryonic stem (ES) cells. The ES cells were cloned and screened for appropriate targeting as previously described [39]. After selection, ES cells containing the targeted Col11a1 allele were identified by the absence of the negative selection marker DTA, the presence of LacZ, and orientation was confirmed using 3’ and 5’ gene and vector specific sequence using short-range and long range PCR. The successfully targeted ES cell clones were karyotyped and appropriate clones were injected into wild type C57 BL/6-Albino blastocysts, resulting in chimeric mice. The chimeric mice were backcrossed with wild type C57 BL/6 mice to produce the germline transmitted mice with the targeted allele Col11a1+/ta. The Col11a1+/ta mice were bred with FLPe mice (B6; SJL-Tg(ACTFLPe) 9205Dym/J, Jackson Labs) to excise the FRT flanked neo and lacZ sequences. The resulting offspring were cross bred with C57BL/6 (Charles River) mice for 6 generations and then inter-crossed resulting in the conditional knockout mice, Col11a1flox/flox. Mice at each different stage were characterized using PCR that amplified specific element sequences. To target the Col11a1 knockout to tendons, the Col11a1flox/flox mice were bred with Scleraxis-Cre mice (Scx-Cre) mice [34]. The Scx-Cre mice were a gift from Dr. Ronen Schweitzer, Oregon Health and Science University. Genotyping analysis of tendon-targeted Col11a1 conditional knockout mouse was carried out using Cre primers and specific primers at the junction of the 3’ arm and targeting sequence. The primers for the genotyping and characterization of these mice are listed in Table 1.
Table 1:
Primers for selection and characterization of Col11a1 targeted ES cells and conditional mice.
| Reaction name | Primer name | Col11a1 primer sequence | Size | Detect | Function |
|---|---|---|---|---|---|
| LacZ Insertion | LacZ FW LacZ RV |
ATCACGACGCGCTGTATC ACATCGGGCAAATAATATCG |
108 bp | targeted vector targeted allele |
Vector characterization ES cell selection |
| FRT | 5FRT_F | AGGCGCATAACGATACCACGAT | 204 bp | targeted vector | Vector characterization |
| 5FRT_R | CCACAACGGGTTCTTCTGTT | targeted allele | ES cell selection | ||
| DTA negative selection | DTA-5 DTA-3 |
AGGGAAGGCTGAGCACTACA CATTCTGCACGCTTCAAAAG |
506 bp | targeted vector | Vector characterization ES cell selection |
| 5 arm | 5 arm FW 5 arm RV |
TTCAGTGGCATGGTTTTCAA CCAACCCCTTCCTCCTACAT |
250 bp | targeted vector targeted allele |
ES cell selection targeted mice section |
| 3 arm | exon1 FW 3 arm RV |
TCAGCCAACTGAACAACGAC CATCCTCTTCTCCTGCCTTG |
166 bp 203 bp |
wild type, targeted, and floxed alleles | genotyping wild type, targeted, and floxed alleles |
| 5’ insertion/ orientation* | 5a FW 5b FW 5 RV |
CCATAGGTGATCACAAGAGTTTGC GAATAATGCCAGAGTGAACACACC CACAACGGGTTCTTCTGTTAGTCC |
6299 bp 5397 bp |
targeted allele targeted allele |
ES cell selection ES cell selection |
| 3’ insertion/ orientation* | 3 FW 3a RV 3c RV |
CACACCTCCCCCTGAACCTGAAAC CTTGGAATCAGGGAGGAGAAAAAG CACCCCAGTGACCACATCTTTAGC |
7736 bp 8312 bp |
targeted allele targeted allele |
ES cell selection ES cell selection |
| tm1c | tm1c_F tm1c_R |
AAGGCGCATAACGATACCAC CCGCCTACTGCGACTATAGAGA |
218 bp | floxed allele | floxed mice selection |
| Scx Cre | ra47 ra48 |
GCAGAACCTGAAGATGTTCGC ACACCAGAGACGGAAATCCATC |
500 bp | Cre transgene | Cre mice selection |
| Rosa Cre | wt forw wt rev mut rev |
AAAGTCGCTCTGAGTTGTTAT GGAGCGGGAGAAATGGATATG CCTGATCCTGGCAATTTCG |
825 bp 8312 bp |
Rosa Cre allele wild type |
TM-induced Cre mice genotyping |
| Cre excised | 5 arm FW 3 arm RV |
TTCAGTGGCATGGTTTTCAA CATCCTCTTCTCCTGCCTTG |
321 bp | Cre-excised allele | Cre-excision characterization |
PCR products are ~5–9kb. Long range PCR (LRPCR) will be performed (Invitrogen SequalPrep LRPCR kit) between a universal primer in the 5’ or 3’ arm of the targeting cassette and 2 different genomic specific primers in the region upstream or downstream of the construct.
A bitransgenic inducible Col11a1-null (I-Col11a1−/−) mouse model also was created for use in these studies. A tamoxifen (TM) inducible Col11a1-null mouse model (I-Col11a1−/−) was generated by breeding the conditional Col11a1flox/flox mice with knockin TM-inducible Cre mice (B6.129-Gt(ROSA) 26Sortm1(cre/ERT2)Tyj/J, Jackson Labs) as previously described [51]. Two male Cre+/+/Col11a1flox/flox mice (I-Col11a1−/−) were injected i.p with TM (Sigma, St. Louis) at 10 mg/100g body weight starting at day 25 and continued once daily for three days. Efficient Cre excision is obtained after induction with TM using this protocol [51].The mice were euthanized at day 60 and processed for electron microscopy.
RT-PCR
Samples of FDL tendons were removed from wild type, Col11a1flox/flox Col11a1Δten/Δten, and Col11a1+/Δten mice at day 14. The tissue samples were cut into small pieces and lysed in QIAzol reagent (Qiagen, Germantown, MD), the crude total RNA was cleanup with the RNeasy MinElute Cleanup Kit (Qiagen, Germantown, MD). The resulting RNA underwent reverse transcription with the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). This was followed by real time PCR using SYBR Green PCR master mix (Thermo Fisher Scientific) in a StepOnePlus Real Time PCR system (Applied Biosystems). The resulting Col11a1 mRNA expression levels were normalized with β-actin. Primers from the C terminal non-helical region were used for the real time PCR reactions. They are Col11a1 FW: GACCAGAAGACACACTGAAAGCA, Col11a1 RV: TCCATGCCATCTGAGTAGTCAAGA; β-actin FW: AGATGACCCAGATCATGTTTGAGA, β-actin RV: CACAGCCTGGATGGCTACGT. Primers from the helical region also were used and they are: Col11a1 FW2: CTGGTCATCCTGGGAAAGAA; Col11a1 RV2: AGCCCTTGAGACCTCTGACA.
Immuno-blots
Collagen XI content was analyzed immuno-chemically using a Wes™ automated western blotting system (ProteinSimple, San Jose, CA). FDL samples were dissected from wild type, Scx-Cre, Col11a1+/Δten, and Col11a1Δten/Δten mice at day 4. Developing mice were used to minimize the effects of crosslinking and collagen extraction. In addition, the use of young healthy mice should minimize the potential effects of non-specific proteolysis on the susceptible variable domain. Individual mice (n=3–8) were used for each genotype. Two FDLs from each mouse were cut into small pieces and protein was extracted using an extraction buffer composed of 50 mM Tris-HCl, pH 6.8, 1% SDS with proteinase inhibitor cocktail (ThermoFisher Scientific). Equal amounts (1.5 mg) of denatured protein samples were loaded into single designated wells of Wes Separation 12–230 kDa 25 Capillary Cartridges, 1:50 diluted rabbit anti-mouse Col XI antibody (300A) and the Wes anti-rabbit detection module was used for collagen XI detection. Quantification by densitometry was performed using the area of the targeted protein (α1(XI)) and normalized to total protein amount, which was analyzed by loading an equal amount of protein to a separate capillary cartridge and detected with the Wes total protein detection module. All Wes reagents (separation module and detection modules) were purchased from ProteinSimple and the Wes assay was carried out following the manufacturer’s instructions. Data analyses were performed using the Compass Software (ProteinSimple).
Antibody
A Rabbit anti-mouse α1(XI) antibody was customer produced by Zymed Laboratory Inc, CA. Peptide (C)YGTMEPYQTETPRR-amide conjugated KLH was used to immunize rabbits, antisera was affinity purified against the peptide and eluted with 3M KSCN. The peptide (Ms #309–322) is coded within exon 7 and located in the α1(XI) N terminal nonhelical region.
Grip strength
Grip strength was evaluated in Col11a1flox/flox control and Col11a1Δten/Δten knockout male mice at day 60. A grip strength meter (San Diego Instrument, San Diego, CA) was used to record the peak force each mouse exerts in grasping a grip placed at their fore limb. The mouse was held by the tail and lowered toward the grip strength platform until it grasped the grip with its forepaws. The mouse was then pulled steadily by the tail away from the rod until the grip was broken. The force applied to the grip just before the animal loses its grip was recorded as the peak tension. In each genotype 8–9 mice were tested. Ten measurements from each mouse were recorded and the average force was used to represent the grip strength for individual mice.
Transmission electron microscopy
Samples of FDL taken from wild type, Col11a1Δten/Δten, and Col11a1+/Δten mice at day 4, day 30 and day 60 were examined using transmission electron microscopy as previously described [52–54]. The tendons were fixed in 4% paraformaldehyde, 2.5% glutaraldehyde, 0.1 M sodium cacodylate, pH 7.4, with 8.0 mM CaCl2 followed by post-fixation with 1% osmium tetroxide. Both transverse and longitudinal sections were stained with 2% aqueous uranyl acetate and examined at 80 kV using a JEOL 1400 transmission electron microscope. An Orius wide-field CCD camera with a resolution of 3648 × 2672 and magnification of 60,000X was used to capture the images. Images from the tendon midsubstance were masked and transferred to an RM Biometrics-Bioquant Image Analysis System for analysis. Fibril diameter analyses were done from transverse section images. All fibrils within a predetermined region of interest (ROI) on the digitized image were measured. Non-overlapping ROIs were placed in the central tendon based on fibril orientation (i.e., cross section) and absence of cells. Diameters were measured along the minor axis. For measurement of fibril density, the total number of fibrils within the ROI was normalized for area. Statistical analysis of differences in the diameter distributions was done using the Kolmogorov–Smirnov (K-S) test.
Mechanical testing
Male mice, Col11a1flox/ flox (n=12) and Col11a1Δten/Δten, (n=15) were euthanized at 60 days. FDL tendons were removed from the ventral aspect of the mouse foot and cleaned free of excess soft tissue. Mechanical testing was done as previously described [33,34,55,56]. Verhoeff’s stain lines were placed 2.5 mm apart within the mid-substance to track the strain optically. Cross-sectional area of the tendon was then measured with a custom built optical device [57]. Each end of the FDL tendon was glued to sandpaper and the gauge length is approximately 5 mm for mechanical testing. The tendon was then placed in custom made grips and a custom holding fixture was secured to the grips.
Samples were placed in phosphate buffered saline bath at room temperature and loaded in a tensile testing system (model 5542, Instron, Norwood, MA). To determine biomechanical properties, tensile testing along the long axis of the tendon was performed with the following protocol. For ACH and SST, the mechanical testing protocol involved a preload to 0.02 N; 10 cycles of preconditioning (0.02–0.04 N at 1% strain/s); rest for 300 s; stress relaxation at 5% strain (ramp rate of 5%/s) followed by a 600 s hold; a return to zero-displacement, 60 s hold, and ramp to failure at 0.1%/s [33]. For FDL, the mechanical testing protocol involved a preload to 0.01 N; 10 cycles of preconditioning (0.01–0.02 N at 1% strain/s); rest for 300 s; stress relaxation at 5% strain (ramp rate of 5%/s) followed by a 600 s hold; a return to zero-displacement, 60 s hold, and ramp to failure at 0.5%/s. A 10 N load cell was used with a resolution of 0.01 N. During the testing, images were obtained with a digital camera (Basler, Exton, PA) every 5 s for optical strain analysis. Maximum stress was calculated as the maximum force divided by cross sectional area. Maximum force and maximum stress were only calculated for samples that experienced physiological failure within the midsubstance or insertion site and failures that occurred at the grips were excluded. A custom Matlab program (Matlab R2017a, Natick, MA) was used to optically track strain lines to quantify stiffness and regional modulus in the linear region of the mechanical test [58].
Shapiro–Wilk tests were performed to verify normality of the data sets. Independent-sample t-tests (two-tailed) were used to determine statistical significance between the control and Col11a1Δten/Δten groups for normally distributed data sets. Mann-Whitney U tests were used for non-normal data sets. Statistical evaluation for realignment of collagen fiber organization used a two-way ANOVA. Any significant main effects were followed by one-way ANOVAs with Dunnett post-hoc tests to compare circular variance (VAR) at 1% strain to VAR at every other strain level. Significance was set at p≤0.05 and trends at p≤0.1.
Supplementary Material
Acknowledgments
Funding
This study was supported by NIH/NIAMS grants AR044745, AR073231, and NIH/NIAMS grant AR050950 supporting the Penn Center for Musculoskeletal Disorders.
Footnotes
Declaration of Competing Interest
The authors declare no competing interests.
Supplementary materials
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.matbio.2020.09.001.
References
- [1].Birk DE, Bruckner P, Collagens, suprastructures and collagen fibril assembly, in: Mecham RP (Ed.), The Extracellular Matrix: an Overview, Springer, NY, 2011, pp. 77–115. [Google Scholar]
- [2].Zhang G, Young BB, Ezura Y, Favata M, Soslowsky LJ, Chakravarti S, Birk DE, Development of tendon structure and function: regulation of collagen fibrillogenesis, J. Musculoskelet. Neuronal Interact. 5 (1) (2005) 5–21. [PubMed] [Google Scholar]
- [3].Mienaltowski MJ, Birk DE, Structure, physiology, and biochemistry of collagens, Adv. Exp. Med. Biol. 802 (2014) 5–29. [DOI] [PubMed] [Google Scholar]
- [4].Walraven M, Hinz B, Therapeutic approaches to control tissue repair and fibrosis: Extracellular matrix as a game changer, Matrix Biol. 71–72 (2018) 205–224. [DOI] [PubMed] [Google Scholar]
- [5].Karamanos NK, Theocharis AD, Neill T, Iozzo RV, Matrix modeling and remodeling: a biological interplay regulating tissue homeostasis and diseases, Matrix Biol. 75–76 (2019) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Iozzo RV, Gubbiotti MA, Extracellular matrix: the driving force of mammalian diseases, Matrix Biol. 71–72 (2018) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Saini K, Cho S, Dooling LJ, Discher DE, Tension in fibrils suppresses their enzymatic degradation a molecular mechanism for ‘use it or lose it’, Matrix Biol. 85–86 (2020) 34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wenstrup RJ, Smith SM, Florer JB, Zhang G, Beason DP, Seegmiller RE, Soslowsky LJ, Birk DE, Regulation of collagen fibril nucleation and initial fibril assembly involves coordinate interactions with collagens V and XI in developing tendon, J. Biol. Chem. 286 (23) (2011) 20455–20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Symoens S, Syx D, Malfait F, Callewaert B, De Backer J, Vanakker O, Coucke P, De Paepe A, Comprehensive molecular analysis demonstrates type V collagen mutations in over 90% of patients with classic EDS and allows to refine diagnostic criteria, Hum Mutat 33 (10) (2012) 1485–1493. [DOI] [PubMed] [Google Scholar]
- [10].Majava M, Hoornaert KP, Bartholdi D, Bouma MC, Bouman K, Carrera M, Devriendt K, Hurst J, Kitsos G, Niedrist D, Petersen MB, Shears D, Stolte-Dijkstra I, Van Hagen JM, Ala-Kokko L, Mannikko M, Mortier GR, A report on 10 new patients with heterozygous mutations in the COL11A1 gene and a review of genotype-phenotype correlations in type XI collagenopathies, Am. J. Med. Genet. A 143A (3) (2007) 258–264. [DOI] [PubMed] [Google Scholar]
- [11].Cattalini M, Khubchandani R, Cimaz R, When flexibility is not necessarily a virtue: a review of hypermobility syndromes and chronic or recurrent musculoskeletal pain in children, Pediatric Rheumatol. Online J 13 (1) (2015) 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Saunders CJ, Jalali Sefid Dashti M, Gamieldien J, Semantic interrogation of a multi knowledge domain ontological model of tendinopathy identifies four strong candidate risk genes, Sci Rep 6 (2016) 19820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hay M, Patricios J, Collins R, Branfield A, Cook J, Handley CJ, September AV, Posthumus M, Collins M, Association of type XI collagen genes with chronic Achilles tendinopathy in independent populations from South Africa and Australia, Br J Sports Med 47 (9) (2013) 569–574. [DOI] [PubMed] [Google Scholar]
- [14].Smith SM, Birk DE, Focus on molecules: collagens V and XI, Exp. Eye Res. 98 (2012) 105–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Birk DE, Bruckner P, Collagen suprastructures, Top Curr Chem 247 (2005) 185–205. [Google Scholar]
- [16].Hoffman GG, Branam AM, Huang G, Pelegri F, Cole WG, Wenstrup RM, Greenspan DS, Characterization of the six zebrafish clade B fibrillar procollagen genes, with evidence for evolutionarily conserved alternative splicing within the pro-alpha1(V) C-propeptide, Matrix Biol. 29 (4) (2010) 261–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hansen U, Bruckner P, Macromolecular specificity of collagen fibrillogenesis: fibrils of collagens I and XI contain a heterotypic alloyed core and a collagen I sheath, J. Biol. Chem. 278 (39) (2003) 37352–37359. [DOI] [PubMed] [Google Scholar]
- [18].Wu JJ, Weis MA, Kim LS, Carter BG, Eyre DR, Differences in chain usage and cross-linking specificities of cartilage type V/XI collagen isoforms with age and tissue, J. Biol. Chem. 284 (9) (2009) 5539–5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kleman JP, Hartmann DJ, Ramirez F, van der Rest M, The human rhabdomyosarcoma cell line A204 lays down a highly insoluble matrix composed mainly of alpha 1 type-XI and alpha 2 type-V collagen chains, Eur J Biochem 210 (1) (1992) 329–335. [DOI] [PubMed] [Google Scholar]
- [20].Mayne R, Brewton RG, Mayne PM, Baker JR, Isolation and characterization of the chains of type V/type XI collagen present in bovine vitreous, J. Biol. Chem. 268 (13) (1993) 9381–9386. [PubMed] [Google Scholar]
- [21].Zhidkova NI, Justice SK, Mayne R, Alternative mRNA processing occurs in the variable region of the pro-alpha 1 (XI) and pro-alpha 2(XI) collagen chains, J. Biol. Chem. 270 (16) (1995) 9486–9493. [DOI] [PubMed] [Google Scholar]
- [22].Moradi-Ameli M, de Chassey B, Farjanel J, van der Rest M, Different splice variants of cartilage alpha1 (XI) collagen chain undergo uniform amino-terminal processing, Matrix Biol. 17 (5) (1998) 393–396. [DOI] [PubMed] [Google Scholar]
- [23].Warner LR, Brown RJ, Yingst SM, Oxford JT, Isoform-specific heparan sulfate binding within the amino-terminal noncollagenous domain of collagen alpha1(XI), J. Biol. Chem. 281 (51) (2006) 39507–39516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gregory KE, Oxford JT, Chen Y, Gambee JE, Gygi SP, Aebersold R, Neame PJ, Mechling DE, Bachinger HP, Morris NP, Structural organization of distinct domains within the non-collagenous N-terminal region of collagen type XI, J. Biol. Chem. 275 (15) (2000) 11498–11506. [DOI] [PubMed] [Google Scholar]
- [25].Linsenmayer TF, Gibney E, Igoe F, Gordon MK, Fitch JM, Fessler LI, Birk DE, Type V collagen: molecular structure and fibrillar organization of the chicken alpha 1(V) NH2-terminal domain, a putative regulator of corneal fibrillogenesis, J Cell Biol 121 (5) (1993) 1181–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Blaschke UK, Eikenberry EF, Hulmes DJ, Galla HJ, Bruckner P, Collagen XI nucleates self-assembly and limits lateral growth of cartilage fibrils, J. Biol. Chem. 275 (14) (2000) 10370–10378. [DOI] [PubMed] [Google Scholar]
- [27].Smith SM, Zhang G, Birk DE, Collagen V localizes to pericellular sites during tendon collagen fibrillogenesis, Matrix Biol. 33 (2014) 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bernard M, Yoshioka H, Rodriguez E, Van der Rest M, Kimura T, Ninomiya Y, Olsen BR, Ramirez F, Cloning and sequencing of pro-alpha 1 (XI) collagen cDNA demonstrates that type XI belongs to the fibrillar class of collagens and reveals that the expression of the gene is not restricted to cartilagenous tissue, J. Biol. Chem. 263 (32) (1988) 17159–17166. [PubMed] [Google Scholar]
- [29].Lincoln J, Florer JB, Deutsch GH, Wenstrup RJ, Yutzey KE, ColVa1 and ColXIa1 are required for myocardial morphogenesis and heart valve development, Dev Dyn 235 (12) (2006) 3295–3305. [DOI] [PubMed] [Google Scholar]
- [30].Yoshioka H, Iyama K, Inoguchi K, Khaleduzzaman M, Ninomiya Y, Ramirez F, Developmental pattern of expression of the mouse alpha 1 (XI) collagen gene (Col11a1), Dev Dyn 204 (1) (1995) 41–47. [DOI] [PubMed] [Google Scholar]
- [31].Bos KJ, Holmes DF, Kadler KE, McLeod D, Morris NP, Bishop PN, Axial structure of the heterotypic collagen fibrils of vitreous humour and cartilage, J. Mol. Biol. 306 (5) (2001) 1011–1022. [DOI] [PubMed] [Google Scholar]
- [32].Connizzo BK, Adams SM, Adams T, Birk DE, Soslowsky LJ, Collagen V expression is crucial in regional development of the supraspinatus tendon, J. Ortho. Res. 34 (12) (2016) 2154–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Connizzo BK, Freedman BR, Fried JH, Sun M, Birk DE, Soslowsky LJ, Regulatory role of collagen V in establishing mechanical properties of tendons and ligaments is tissue dependent, J. Ortho. Res. 33 (6) (2015) 882–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sun M, Connizzo BK, Adams SM, Freedman BR, Wenstrup RJ, Soslowsky LJ, Birk DE, Targeted deletion of collagen V in tendons and ligaments results in a classic Ehlers-Danlos syndrome joint phenotype, Am. J. Pathol. 185 (5) (2015) 1436–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wenstrup RJ, Florer JB, Brunskill EW, Bell SM, Chervoneva I, Birk DE, Type V collagen controls the initiation of collagen fibril assembly, J. Biol. Chem. 279 (51) (2004) 53331–53337. [DOI] [PubMed] [Google Scholar]
- [36].Wenstrup RJ, Florer JB, Cole WG, Willing MC, Birk DE, Reduced type I collagen utilization: a pathogenic mechanism in COL5A1 haplo-insufficient Ehlers-Danlos syndrome, J. Cell. Biochem. 92 (1) (2004) 113–124. [DOI] [PubMed] [Google Scholar]
- [37].Wenstrup RJ, Florer JB, Davidson JM, Phillips CL, Pfeiffer BJ, Menezes DW, Chervoneva I, Birk DE, Murine model of the Ehlers-Danlos syndrome. col5a1 haploinsufficiency disrupts collagen fibril assembly at multiple stages, J. Biol. Chem. 281 (18) (2006) 12888–12895. [DOI] [PubMed] [Google Scholar]
- [38].Segev F, Heon E, Cole WG, Wenstrup RJ, Young F, Slomovic AR, Rootman DS, Whitaker-Menezes D, Chervoneva I, Birk DE, Structural abnormalities of the cornea and lid resulting from collagen V mutations, Invest. Ophthalmol. Vis. Sci. 47 (2) (2006) 565–573. [DOI] [PubMed] [Google Scholar]
- [39].Sun M, Chen S, Adams SM, Florer JB, Liu H, Kao WW, Wenstrup RJ, Birk DE, Collagen V is a dominant regulator of collagen fibrillogenesis: dysfunctional regulation of structure and function in a corneal-stroma-specific Col5a1-null mouse model, J Cell Sci 124 (Pt 23) (2011) 4096–4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Li Y, Lacerda DA, Warman ML, Beier DR, Yoshioka H, Ninomiya Y, Oxford JT, Morris NP, Andrikopoulos K, Ramirez F, et al. , A fibrillar collagen gene, Col11a1, is essential for skeletal morphogenesis, Cell 80 (3) (1995) 423–430. [DOI] [PubMed] [Google Scholar]
- [41].Seegmiller R, Fraser FC, Sheldon H, A new chondrodystrophic mutant in mice. Electron microscopy of normal and abnormal chondrogenesis, J Cell Biol 48 (3) (1971) 580–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Murchison ND, Price BA, Conner DA, Keene DR, Olson EN, Tabin CJ, Schweitzer R, Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons, Development 134 (14) (2007) 2697–2708. [DOI] [PubMed] [Google Scholar]
- [43].Pryce BA, Brent AE, Murchison ND, Tabin CJ, Schweitzer R, Generation of transgenic tendon reporters, ScxGFP and ScxAP, using regulatory elements of the scleraxis gene, Dev Dyn 236 (6) (2007) 1677–1682. [DOI] [PubMed] [Google Scholar]
- [44].Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin CJ, Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments, Development 128 (19) (2001) 3855–3866. [DOI] [PubMed] [Google Scholar]
- [45].Dourte LM, Pathmanathan L, Jawad AF, Iozzo RV, Mienaltowski MJ, Birk DE, Soslowsky LJ, Influence of decorin on the mechanical, compositional, and structural properties of the mouse patellar tendon, J Biomech Eng 134 (3) (2012) 031005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Connizzo BK, Han L, Birk DE, Soslowsky LJ, Collagen V-heterozygous and -null supraspinatus tendons exhibit altered dynamic mechanical behaviour at multiple hierarchical scales, Interface Focus 6 (1) (2016) 20150043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Schonborn K, Willenborg S, Schulz JN, Imhof T, Eming SA, Quondamatteo F, Brinckmann J, Niehoff A, Paulsson M, Koch M, Eckes B, Krieg T, Role of collagen XII in skin homeostasis and repair, Matrix Biol. (2020) In press, doi: 10.1016/j.matbio.2020.08.002. [DOI] [PubMed] [Google Scholar]
- [48].Jia T, Vaganay E, Carpentier G, Coudert P, Guzman-Gonzales V, Manuel R, Eymin B, Coll JL, Ruggiero F, A collagen Valpha1-derived fragment inhibits FGF-2 induced-angiogenesis by modulating endothelial cells plasticity through its heparin-binding site, Matrix Biol. (2020) In press, doi: 10.1016/j.matbio.2020.07.001. [DOI] [PubMed] [Google Scholar]
- [49].Poulson AV, Hooymans JM, Richards AJ, Bearcroft P, Murthy R, Baguley DM, Scott JD, Snead MP, Clinical features of type 2 Stickler syndrome, J Med Genet 41 (8) (2004) e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Goh KL, Holmes DF, Lu Y, Purslow PP, Kadler KE, Bechet D, Wess TJ, Bimodal collagen fibril diameter distributions direct age-related variations in tendon resilience and resistance to rupture, J Appl Physiol (1985) 113 (6) (2012) 878–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Robinson KA, Sun M, Barnum CE, Weiss SN, Huegel J, Shetye SS, Lin L, Saez D, Adams SM, Iozzo RV, Soslowsky LJ, Birk DE, Decorin and biglycan are necessary for maintaining collagen fibril structure, fiber realignment, and mechanical properties of mature tendons, Matrix Biol. 64 (2017) 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Birk DE, Zycband EI, Winkelmann DA, Trelstad RL, Collagen fibrillogenesis in situ: fibril segments are intermediates in matrix assembly, Proc Natl Acad Sci U S A 86 (12) (1989) 4549–4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Trelstad RL, Birk DE, The fibroblast in morphogenesis and fibrosis: cell topography and surface-related functions, Ciba Found Symp 114 (1985) 4–19. [DOI] [PubMed] [Google Scholar]
- [54].Birk DE, Trelstad RL, Extracellular compartments in matrix morphogenesis: collagen fibril, bundle, and lamellar formation by corneal fibroblasts, J Cell Biol 99 (6) (1984) 2024–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Izu Y, Ansorge HL, Zhang G, Soslowsky LJ, Bonaldo P, Chu ML, Birk DE, Dysfunctional tendon collagen fibrillogenesis in collagen VI null mice, Matrix Biol. 30 (1) (2011) 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ansorge HL, Adams S, Birk DE, Soslowsky LJ, Mechanical, compositional, and structural properties of the post-natal mouse Achilles tendon, Ann Biomed Eng 39 (7) (2011) 1904–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Peltz CD, Perry SM, Getz CL, Soslowsky LJ, Mechanical properties of the long-head of the biceps tendon are altered in the presence of rotator cuff tears in a rat model, J. Ortho. Res. 27 (3) (2009) 416–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Derwin KA, Soslowsky LJ, Green WD, Elder SH, A new optical system for the determination of deformations and strains: calibration characteristics and experimental results, J Biomech 27 (10) (1994) 1277–1285. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.