Dear editor,
The World Health Organization (WHO) declared the Coronavirus disease 2019 (COVID-19) a pandemic on March 11th, 2020. At the time of writing (Nov 30, 2020), the causative virus SARS-CoV-2 has resulted in >62 million confirmed cases worldwide, with over 1453,000 fatalities (https://covid19.who.int/). Although detection of SARS-CoV-2 has become the top priority for patients with pneumonia, co-infection with other common pathogens should not be neglected as co-infection is often associated with a greater risk of complications, particularly secondary bacterial pneumonia. Several case reports and a large-scale surveillance study on various respiratory pathogens via PCR-based methods targeting a limited number of known pathogens have revealed high rates of microbial co-infection, such as co-infection of SARS-CoV-2 and Influenza virus, Dengue virus or Mycobacterium tuberculosis1, 2, 3. The more informative metagenomic next-generation sequencing (mNGS) approach, particularly meta-transcriptome sequencing, is unbiased for characterizing the total infectome within patients. In this journal, researchers previously reported SARS-CoV-2 and co-infections detected in 8 COVID-19 patients by metagenomics4. Besides that, only few studies with limited sample size have been reported about using metagenomics to analyze co-infections in COVID-19 cases5 , 6.
Herein, we performed the total transcriptome sequencing of upper respiratory tract samples (nasopharynx swabs and sputum) from 162 PCR-confirmed COVID-19 cases from 12 cities in Shandong province, China. RNA sequencing libraries were constructed using MGIEasy mRNA Library Preparation protocol (MGI) after ribosomal RNA (rRNA) was depleted. 162 libraries were obtained in total and subsequently 100 bp and 150 bp paired-end sequencing of the RNA libraries were performed on the MGISEQ-2000RS platform (MGI). The remaining sequencing reads after mapping to the human genome were compared against the non-redundant nucleotide (nt) database using blastn to identify potential viruses. For a broader microbe discovery, we utilized MetaPhlan27, which covers ∼1 million unique clade-specific marker genes from bacterial, archaeal and eukaryotic reference genomes.
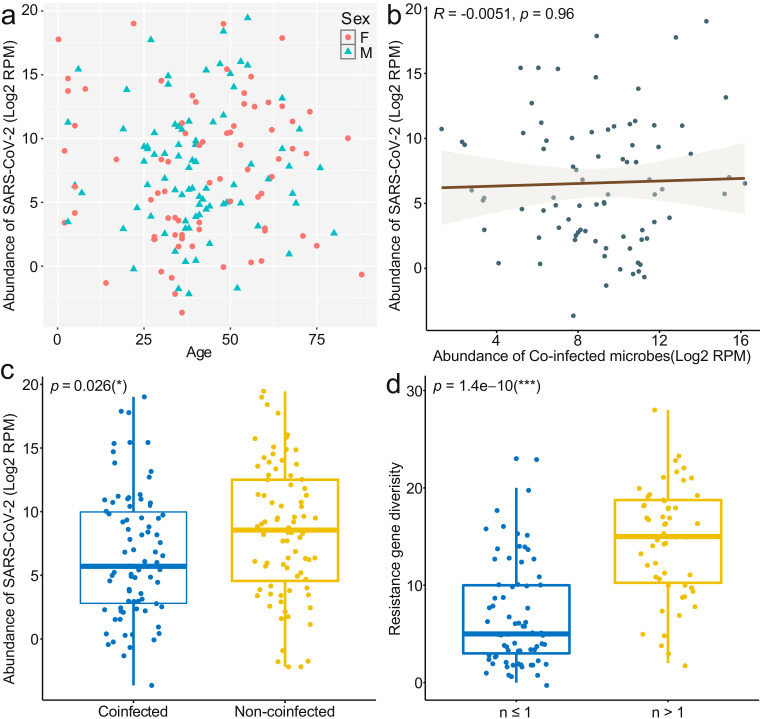
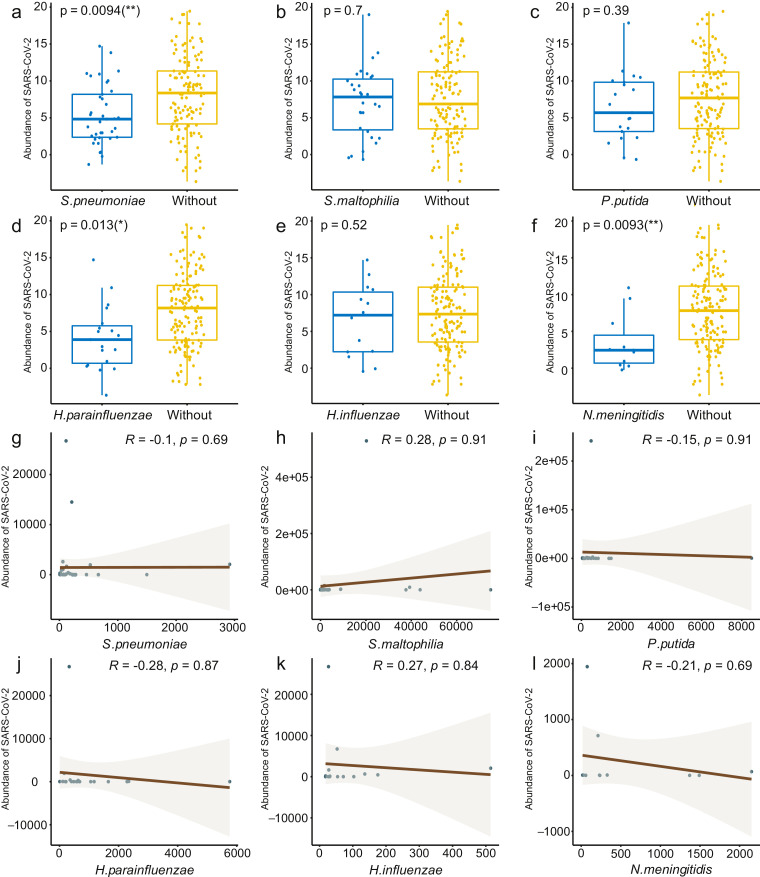
Reads mapping to SARS-CoV-2 were found in all samples, with the absolute read numbers from 14 to 115,299,054, and the number of Reads Per Million (RPM) ranging from 0.07 to 717,538.52. Overall, 82 out of the 162 SARS-CoV-2 cases (50.62%) were co-infected by at least one additional potentially pathogenic microbe (Table 1 ). Among these, 42 cases (25.93%) were co-infected with one pathogen, including viruses (n = 9), bacteria (n = 33). The remaining 40 cases (24.69%) were co-infected with two or more pathogens, including multiple virus co-infection (n = 1), virus and bacteria co-infections (n = 4), virus, bacteria and fungi co-infections (n = 1), and multiple bacterial co-infections (n = 34). Meanwhile, the abundance of SARS-CoV-2 was lower in samples co-infected with at least one microbe (p < 0.05) compared to that of the samples without co-infections (Fig S1c). Further analysis also showed a significantly lower abundance of SARS-CoV-2 in samples with Streptococcus pneumoniae, Haemophilus parainfluenzae and Neisseria meningitidis (p < 0.05) (Fig S2a, 2d, 2f). However, we did not find a positive correlation between associated factors (age or sex of the patients, variety or abundance of the co-infected microbes) and the abundance of SARS-CoV-2 (Fig S1a-b and Fig S2g-l).
Table 1.
Summary of co-infections of SARS-CoV-2 and other microbes.
| SARS-CoV-2 | Co-infecting microbes | No. of cases | Percent |
|---|---|---|---|
| + | Virus | 9 | 1.85% |
| + | Multiple viruses | 1 | 0.62% |
| + | Virus + Bacteria | 4 | 2.47% |
| + | Virus + Bacteria + Fungi | 1 | 0.62% |
| + | One bacterium | 33 | 20.37% |
| + | Multiple bacteria | 34 | 20.99% |
| Total | 82 | 50.62% |
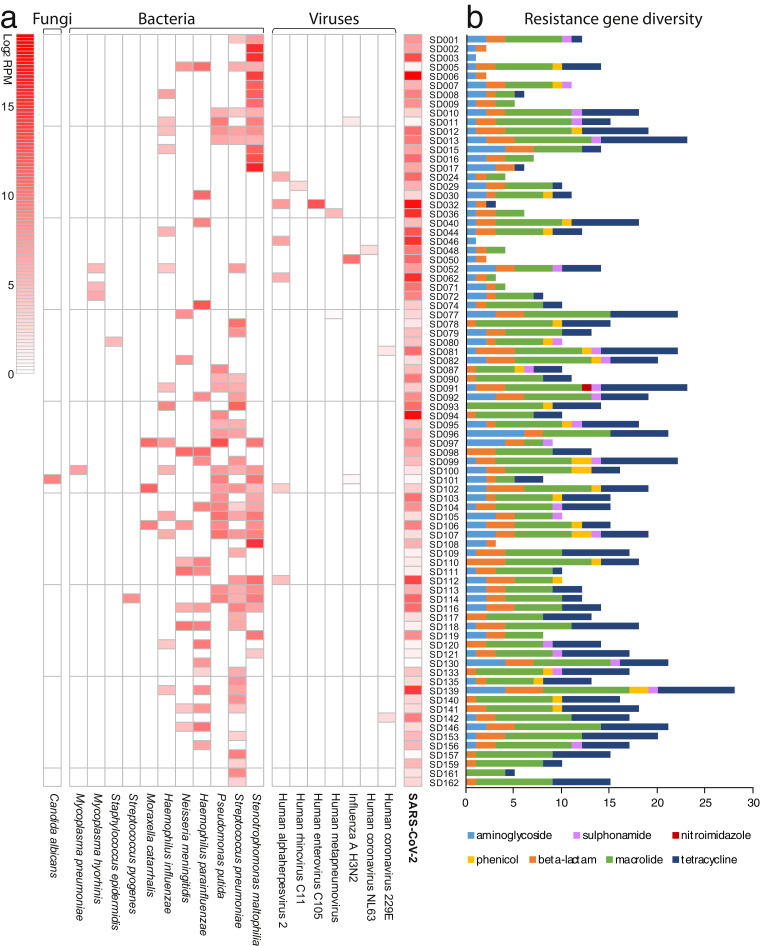
We identified 7 viruses with potential pathogenicity in 15 of the 162 (9.26%) COVID-19 cases. Human alphaherpesvirus 2 was the most frequently detected virus (n = 6), followed by Human H3N2 influenza virus (n = 3), Human coronavirus 229E (n = 2), Human metapneumovirus (n = 2), and Human coronavirus NL63 (n = 1) (Fig 1 a, Table S1). In addition, two rare respiratory viruses, Human rhinovirus C11 (n = 1, RPM: 6.84) first reported in 2020 and Human enterovirus C105 (n = 1, RPM: 6969.31) first reported in 2019 in China, were also identified (Fig 1a, Table S1), sharing 91% and 96% nucleotide identity to known reference viruses, respectively. Human enterovirus C105 has been associated with acute flaccid paralysis in children and respiratory tract infection in teenagers8. Our results revealed a relative low co-infection rate of other respiratory viruses with SARS-CoV-2: 9.26% (15/162) versus 20.7% (24/116) in a previous report9.
Fig. 1.
Co-infecting microbes with SARS-CoV-2 and the diversity of ARGs in each sample. (a) Heatmap showing the abundance of different microbes within each sample, normalized to the number of unique reads mapped per million input reads (RPM) and log2 transformed. (b) Diversity of resistance genes observed in each sample, colored by the drug class to which these genes confer resistance. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
High rates of co-infection of common, but important pathogenic or opportunistic bacteria were also detected (Fig 1a), including S. pneumoniae (n = 37), Stenotrophomonas maltophilia (n = 31), Pseudomonas putida (n = 21), H. parainfluenzae (n = 19), Haemophilus influenzae (n = 14), N. meningitidis (n = 11), Moraxella catarrhalis (n = 3), Streptococcus pyogenes (n = 1), Streptococcus epidermidis (n = 1), as well as two species of mycoplasma: Mycoplasma hyorhinis (n = 3) and Mycoplasma pneumoniae (n = 1) (Fig 1a). Importantly, all bacterial infections were confirmed by PCR using species-specific primers. As a commensal microbe in the respiratory tract of pigs and rarely seen in humans, M. hyorhinis was unexpected identified in three cases, although it was present at a relatively low abundance (39–79 RPM). Overall, 72 of the 162 SARS-CoV-2 patients (44.44%) possessed at least one additional bacterium. One species of fungi, Candida albicans (n = 1, RPM: 600) was identified (Fig 1a). Our results highlighted more potential co-infections with bacteria (44.44%, 72/162) than with viruses (9.26%, 15/162) or fungi (0.62, 1/162), in contrast to a recent study10.
We further analyzed the expression of 52 unique antibiotic resistance genes (ARGs) associated with phenotypic resistance to seven classes of antibiotics: aminoglycoside, nitroimidazole, sulphonamide, phenicol, tetracycline, beta-lactam and macrolide (Fig. 1b). The number of ARGs varied sharply from 0 to 28 among the 162 SARS-CoV-2 cases. Regarding those with concurrent infections (n = 82), most presented resistance to aminoglycosides (66/82), beta-lactam (78/82), macrolide (74/82) and tetracycline (63/82), whereas a small number of samples possessed genes resistant to nitroimidazole (1/82), phenical (26/82) and sulphonamide (24/82) (Fig 1b). Unsurprisingly, a greater diversity of resistance genes was identified in samples with more bacteria (p < 0.05) (Fig S1d).
In sum, our mNGS analysis did not reveal high co-infection rates of other respiratory viruses (such as influenza viruses), mycoplasma and fungi with SARS-CoV-2. However, we did document high rates of co-infection between SARS-CoV-2 and multiple bacteria with numerous ARGs. Although the potential influence of co-infections of SARS-CoV-2 and other microbes in disease progress and severity remains poorly understood, the treatment of bacterial infection might provide clinical benefit for COVID-19 in cases where therapeutics are available.
Ethics statements
This study was approved by the ethics committee of the Shandong First Medical University & Shandong Academy of Medical Sciences. The research-related information was used anonymously. The whole research was supervised by the Health Commission of Shandong Province. All human-related sample processing and sequencing were performed in accordance with relevant guidelines and regulations of the Shandong Provincial CDC.
Author contributions
Conceived the project: W.S., D.K., P.H.; collected samples: T.L., N.N., H.Z., S.R., P.H.; performed laboratory work: H.Z., C.L., T.H., J.L.; analysed the data: H.Z., C.L., T.H., W.C., H.W., S.F., O.G.P., W.S.; wrote the paper: H.Z., C.L., E.C.H., W.S. All authors have read and approved the final manuscript.
Fig. S1.
Statistical analysis of the correlation between different factors and the abundance of SARS-CoV-2 and Statistical analysis of the diversity of ARGs. (a) Correlation between age/sex of the patients and the abundance of SARS-CoV-2. (b) Correlation between the abundance co-infected microbes and SARS-CoV-2. Correlation coefficient between groups were assessed with Spearman's rank correlation test. (c) Comparisons of the abundance of SARS-CoV-2 between groups of co-infected and without coinfections. (d) Comparisons of the diversity of ARGs between samples with less than or equal to one bacteria and samples with more than one. Differences between groups were assessed with a Wilcoxon test.
Fig. S2.
Comparisons of the abundance of SARS-CoV-2 between samples co-infected with different bacteria and samples without the counterpart bacterium. (a)S. pneumoniae, (b) S. maltophilia, (c) P. putida, (d) H. parainfluenzae, (e) H. influenzae and (f) N. meningitidis. Differences between groups were assessed with a Wilcoxon test. (g) S. pneumoniae, (h) S. maltophilia, (i) P. putida, (j) H. parainfluenzae, (k) H. influenzae and (l) N. meningitidis. Correlation coefficient between groups were assessed with Spearman's rank correlation test.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Acknowledgments
This work was supported by Key research and development project of Shandong province (Grant No. 2020SFXGFY01 and 2020SFXGFY08), and the Academic Promotion Programme of Shandong First Medical University (2019QL006). W.F.S is supported by the Taishan Scholars Programme of Shandong Province (ts201511056). E.C.H. is supported by an Australian Research Council Australian Laureate Fellowship (FL170100022).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2020.12.004.
Appendix. Supplementary materials
References
- 1.Cuadrado-Payán E. SARS-CoV-2 and influenza virus co-infection. Lancet. 2020;395(10236):e84. doi: 10.1016/S0140-6736(20)31052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saddique Arbab. et al. Emergence of co-infection of COVID-19 and dengue: a serious public health threat. J Infect. 2020 doi: 10.1016/j.jinf.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar Rohit. et al. COVID-19 and TB co-infection - ’Finishing touch” in perfect recipe to ’severity’ or ‘death’-journal of infection. J Infect. 2020;81(3):e39–e40. doi: 10.1016/j.jinf.2020.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thanh Tran Tan. SARS-CoV-2 and co-infections detection in nasopharyngeal throat swabs of COVID-19 patients by metagenomics. J Infect. 2020;81(2):e175–e177. doi: 10.1016/j.jinf.2020.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L. et al. RNA based mNGS approach identifies a novel human coronavirus from two individual pneumonia cases in 2019 Wuhan outbreak. Emerg Microbes Infect. 2020;9(1):313–319. doi: 10.1080/22221751.2020.1725399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peddu V. Metagenomic analysis reveals clinical SARS-CoV-2 infection and bacterial or viral superinfection and colonization. Clin Chem. 2020;66(7):966–972. doi: 10.1093/clinchem/hvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Truong D.T. et al. MetaPhlAn2 for enhanced metagenomic taxonomic profiling. Nat Methods. 2015;12(10):902–903. doi: 10.1038/nmeth.3589. [DOI] [PubMed] [Google Scholar]
- 8.Horner L.M. et al. Acute Flaccid Paralysis Associated with Novel Enterovirus C105. Emerg Infect Dis. 2015;21(10):1858–1860. doi: 10.3201/eid2110.150759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim D. et al. Rates of Co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA. 2020;323(20):2085–2086. doi: 10.1001/jama.2020.6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H.C. et al. Metatranscriptomic characterization of COVID-19 identified a host transcriptional classifier associated with immune signaling. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa663. ciaa663. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.