Abstract
Objectives
To clarify which rheumatoid arthritis (RA) patients benefit most from the anti-receptor activator of nuclear factor-κB ligand antibody denosumab to reduce the progression of joint destruction.
Methods
We pooled patient data from the 12-month, double-blind, placebo-controlled DRIVE (phase II) and DESIRABLE (phase III) studies. In DRIVE, concomitant treatment was limited to methotrexate, salazosulfapyridine and bucillamine. In DESIRABLE, patients could receive any disease-modifying antirheumatic drug. RA patients were randomised to denosumab 60 mg every 6 months (Q6M), every 3 months (Q3M) or placebo. Efficacy was assessed by van der Heijde-modified total Sharp score (mTSS), bone erosion score (ES) and joint space narrowing score (JSNS). Change in mTSS was assessed in subgroups stratified by risk factors for radiographic damage if the interaction factor was significant.
Results
The pooled analysis included 909 patients. Denosumab reduced worsening of mTSS (mean (SD)) at 12 months in the Q6M (0.88 (3.30), p=0.0024) and Q3M (0.66 (2.16), p=0.0002) groups versus placebo (1.50 (3.73)). This reduction in mTSS progression was due to the change in ES (Q6M, 0.44 (1.89), p=0.0006; Q3M, 0.20 (0.86), p<0.0001) versus placebo (0.98 (2.54)); no effect was observed on JSNS. Anti-cyclic citrullinated peptide (CCP) antibodies, glucocorticoid use and baseline ES showed a significant interaction. Denosumab was particularly effective in patients who were anti-CCP antibody positive (p<0.05). Changes in mTSS versus placebo were observed in all denosumab dose groups, regardless of glucocorticoid use and baseline ES.
Conclusions
Denosumab broadly reduced the progression of joint destruction in RA patients with risk factors for radiographic damage such as especially anti-CCP antibody positivity.
Keywords: Rheumatoid Arthritis, DMARDs (biologic), Treatment
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic disease characterised by persistent synovitis, systemic inflammation and joint destruction. Although the exact aetiology of RA remains unknown, the development of biological disease-modifying anti-rheumatic drugs (bDMARDs) for RA has markedly improved treatment outcomes. Despite the advantages of these agents, the percentage of patients with RA treated with these drugs was reported to be only 20–30% in Japan.1 The main reasons for these low percentages include: (1) not all patients respond to current bDMARDs; (2) some patients experience loss of drug efficacy; (3) risk of serious adverse drug reactions, including immunosuppression and infections and (4) high treatment cost.2–5
In joints affected by RA, osteoclasts play a critical role in the inflammatory response that causes bone erosion. Dysregulation of the bone remodelling process—normally regulated by osteoblasts—results in excessive activation and maturation of osteoclasts.6–9 Activation of osteoclast precursors is mediated via the receptor activator of nuclear factor-κB ligand (RANKL), a key mediator of osteoclast formation, differentiation and survival.10–12
It has been reported that patients with increased inflammation are likely to present more marked joint destruction. However, in some cases, joint destruction progresses even without marked inflammation.13 For such patients, denosumab is expected to have a suppressive effect on the progression of joint destruction.
Denosumab, a fully human monoclonal antibody (IgG2 subclass) that inhibits bone resorption by inhibiting RANKL,2 12 has been proven to prevent the progression of joint destruction, although it has no effect on cartilage and does not improve RA disease activity.14–17 Given the prohibitive high financial cost of existing biological products, denosumab has the added advantage of a lower cost of treatment compared with these existing biological products.
Previous phase II (DRIVE)17 18 and phase III (DESIRABLE)13 studies demonstrated that denosumab reduced the progression of joint destruction in Japanese patients with RA. Identifying the patient subpopulation in which denosumab is most effective is important in the clinical setting. For bDMARDs, the impacts of baseline swollen joint count (SJC), tender joint count (TJC), C reactive protein (CRP), erythrocyte sedimentation rate (ESR), rheumatoid factor (RF) and anti-cyclic citrullinated peptide (CCP) antibodies have previously been evaluated,19 20 and clear prognostic factors have been established. However, there are no reports on the effects of baseline characteristics on the efficacy of denosumab; there are only preliminary results of the DRIVE study.18
The present study aimed to evaluate the effect of denosumab on joint destruction in subgroups of RA patients with bone destruction risk factors and to identify prognostic background factors associated with the efficacy of denosumab.
METHODS
Study design and patients
This study was a pooled analysis of Japanese patients diagnosed with RA from the phase II (DRIVE)17 and phase III (DESIRABLE)13 studies.
The DRIVE study was a 12-month, multicentre, randomised, double-blind, placebo-controlled, phase II study of denosumab to validate its safety and effect on bone erosion in RA patients taking methotrexate (MTX). The DESIRABLE study was a 12-month, double-blind, randomised, placebo-controlled, phase III parallel-group study of denosumab to evaluate its inhibitory effect on the progression of joint destruction. Although the DESIRABLE study also included a 24-month open-label extension, the present analysis only includes the results from the initial 12-month double-blind phase. The DRIVE and DESIRABLE studies used similar patient eligibility criteria, with the main difference being that in the DRIVE study, only MTX, salazosulfapyridine and bucillamine were permitted for concomitant use. In contrast, all anti-rheumatic drugs, other than biological products and tofacitinib, were permitted in the DESIRABLE study. Additionally, stratification for randomisation was by steroid use and with/without RF in the DRIVE study and by steroid use in the DESIRABLE study. The eligibility criteria and design for both of these studies have been described previously.13 17 Briefly, patients were ambulatory outpatients, aged 20 years or older (in DRIVE, age was up to 75 years), with RA fulfilling the American College of Rheumatology criteria,21 disease duration between 6 months and less than 5 years, use of DMARDs for at least 8 weeks that could be continued throughout the study, at least six swollen joints out of 58 counted in DRIVE and 58 counted in DESIRABLE, and radiographic evidence of bone erosion in the hands and feet or those who met any of the following at screening: CRP ≥1.0 mg/dL and positive for anti-CCP antibodies, CRP ≥1.0 mg/dL and positive for RF (RF >20 IU/mL in DRIVE), ESR ≥28 mm/hour and positive for anti-CCP antibodies, or ESR ≥28 mm/hour and positive for RF (RF >20 IU/mL in DRIVE). The main exclusion criteria were almost identical between both trials, except that in DESIRABLE, patients treated with tofacitinib for RA within 4 weeks of enrolment were excluded. In DRIVE, flares were managed either with oral treatment with corticosteroids 10 mg/day or intraarticular treatment with corticosteroids or hyaluronic acid in joints other than those being assessed by the van der Heijde modified-total Sharp score (mTSS); non-steroidal anti-inflammatory drugs were also permitted. In DESIRABLE, non-steroidal anti-inflammatory drugs or oral treatment with corticosteroids within 10 mg/day prednisone equivalent were used for flare management. Baseline demographics and characteristics of patients in the DRIVE and DESIRABLE studies were similar.
The whole group and subgroup analyses used data pooled from the aforementioned trials of Japanese patients with RA treated with denosumab (60 mg every 6 months (Q6M) or 60 mg every 3 months (Q3M)) or placebo. All patients continued treatment with conventional synthetic DMARDs (including MTX), calcium and vitamin D supplements throughout the studies. The use of bisphosphonates and/or oral glucocorticoids ≥10 mg/day was prohibited during the study.
The mTSS, the modified Sharp erosion score (ES) and the joint space narrowing score (JSNS) were assessed for all patients using the modified Sharp van der Heijde method.22 This score is based on a radiographic assessment of hands and feet. The changes from baseline in mTSS, ES and JSNS at 12 months were assessed in the pooled analysis of all patients (whole group analysis). Changes from baseline in mTSS were assessed in the subgroup analyses, which stratified patients by risk factors for radiographic damage. The subgroups analysed were: baseline CRP (<1 mg/dL, ≥1 mg/dL); baseline ESR (<28 mm/hour, ≥28 mm/hour); RF status (negative, positive); anti-CCP antibodies (negative, positive); baseline TJC (≤2, 3–5, ≥6); baseline SJC (<10, ≥10); glucocorticoid use (absence, presence); baseline mTSS (<6.5, ≥6.5); and baseline ES (<3.5, ≥3.5). A further subgroup analysis was also conducted, in which anti-CCP antibody-positive patients were classified according to titre (high/low). A high titre was defined as an antibody titre exceeding the baseline value by threefold and a low titre was defined as an antibody titre within threefold of baseline.
Ethical approval was given by the institutional review boards of all participating sites in the DRIVE and the DESIRABLE studies, and these were conducted in accordance with the Declaration of Helsinki principles. All included patients provided written informed consent.
Statistical analysis
Comparisons of each denosumab group with placebo to determine changes from baseline were performed for the total group using the van Elteren stratified rank test adjusted for study and baseline glucocorticoid use. This analysis method was used for each of the changes in the radiographic scores (mTSS, ES and JSNS). Subsequently, comparisons were performed in the same manner using the van Elteren stratified rank test for each subgroup.
As a post hoc analysis, the treatment-by-subgroup interaction was tested using an ANCOVA model including treatment, subgroup and treatment-by-subgroup interaction. If the p value was <0.10, the interaction was considered significant.
Missing values were imputed using linear extrapolation/interpolation as described previously.13 A p value of <0.05 was considered statistically significant. The statistical analyses were performed using SAS Software, Version 9.2, Cary, NC, USA.
Patient and public involvement
Patients and the public were not involved in the design of the various phases of the trials.
RESULTS
Baseline characteristics
A total of 909 patients were included in the present pooled analysis (Q6M, 302; Q3M, 301 and placebo, 306) from the DRIVE and DESIRABLE studies. Baseline demographics and characteristics are shown in table 1. No differences in baseline values or patient characteristics were identified among the three groups (Q6M, Q3M and placebo). However, the Q3M group included slightly less women than the Q6M and placebo groups (70.8% vs 77.2% vs 79.4%, respectively). The mean age was approximately 56 years in all three groups with a mean RA disease duration between 2.1 and 2.2 years.
Table 1.
Baseline patient demographics and characteristics
| Pooled analysis | DRIVE study | DESIRABLE study | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Denosumab | Denosumab | Denosumab | |||||||
| Placebo (N=306) |
Q6M (N=302) |
Q3M (N=301) |
Placebo (N=88) |
Q6M (N=85) |
Q3M (N=82) |
Placebo (N=218) |
Q6M (N=217) |
Q3M (N=219) |
|
| Female, n (%) | 243 (79.4) | 233 (77.2) | 213 (70.8) | 76 (86.4) | 65 (76.5) | 59 (72.0) | 167 (76.6) | 168 (77.4) | 154 (70.3) |
| Age (years) | 56.2±11.4 | 57.0±11.9 | 56.5±12.2 | 57.0±10.6 | 54.4±10.6 | 52.0±11.7 | 55.8±11.7 | 58.1±12.3 | 58.2±12.0 |
| RA disease duration (years) | 2.1±1.3 | 2.2±1.3 | 2.2±1.3 | 2.3±1.3 | 2.2±1.3 | 2.3±1.3 | 2.1±1.3 | 2.2±1.3 | 2.2±1.3 |
| CRP (mg/dL) | 0.47±0.84 | 0.61±1.16 | 0.53±1.07 | 0.75±1.24 | 0.52±0.92 | 0.61±1.17 | 0.36±057 | 0.65±1.25 | 0.50±1.03 |
| ESR (mm/hour) | 21.7±18.6 | 24.0±21.3 | 22.6±18.8 | 27.8±23.6 | 22.0±18.0 | 24.0±19.9 | 19.2±15.6 | 24.8±22.4 | 22.1±18.4 |
| RF status, n (%) | 197 (64.4) | 199 (65.9) | 184 (61.1) | 60 (68.2) | 59 (69.4) | 56 (68.3) | 137 (62.8) | 140 (64.5) | 128 (58.4) |
| Anti-CCP antibodies, n (%) | 211 (69.0) | 228 (75.5) | 213 (70.8) | 66 (75.0) | 70 (82.4) | 64 (78.0) | 145 (66.5) | 158 (72.8) | 149 (68.0) |
| Tender joint count (0–68) | 7.6±7.6 | 7.6±8.0 | 7.6±7.8 | 9.9±9.7 | 8.0±7.4 | 8.2±7.3 | 6.6±6.4 | 7.5±8.2 | 7.3±8.0 |
| Swollen joint count (0–66) | 9.7±4.9 | 9.3±4.7 | 9.4±4.4 | 10.5±5.9 | 8.9±4.2 | 10.5±4.6 | 9.4±4.4 | 9.5±4.9 | 9.0±4.3 |
| Glucocorticoid use, n (%) | 106 (34.6) | 109 (36.1) | 106 (35.2) | 37 (42.0) | 36 (42.4) | 37 (45.1) | 69 (31.7) | 73 (33.6) | 68 (31.1) |
| MTX use, n (%) | 278 (90.8) | 261 (86.4) | 271 (90.0) | 88 (100.0) | 85 (100.0) | 82 (100.0) | 190 (87.2) | 176 (81.1) | 189 (86.3) |
| Modified total Sharp score (0–448) | 13.26±22.18 | 14.66±20.41 | 13.77±17.89 | 13.56±24.03 | 11.43±14.48 | 10.02±14.03 | 13.14±21.44 | 15.92±22.21 | 15.17±18.97 |
| Modified Sharp erosion score (0–280) | 6.57±10.50 | 7.21±9.51 | 6.83±8.77 | 6.61±10.35 | 6.39±7.77 | 5.95±6.75 | 6.55±10.58 | 7.53±10.11 | 7.16±9.41 |
| Modified Sharp JSNS (0–168) | 6.69±12.64 | 7.45±12.59 | 6.94±10.31 | 6.94±14.29 | 5.04±8.31 | 4.07±8.06 | 6.59±11.94 | 8.39±13.82 | 8.01±10.86 |
Values are shown as mean±SD unless otherwise indicated.
N=number of patients who received at least one dose of investigational product and had a baseline value and at least one post-baseline measurement with radiographs.
CCP, cyclic citrullinated peptide; CRP, C reactive protein; ESR, erythrocyte sedimentation rate; JSNS, joint space narrowing score; MTX, methotrexate; Q3M, denosumab 60 mg every 3 months; Q6M, denosumab 60 mg every 6 months; RA, rheumatoid arthritis; RF, rheumatoid factor.
Changes in mTSS, ES and JSNS in the total group
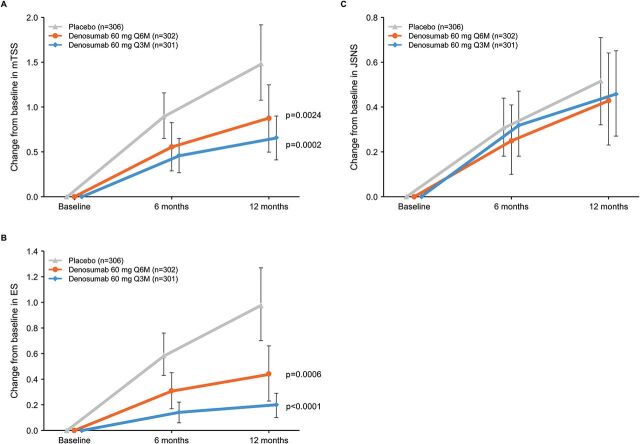
In the Q6M and Q3M versus placebo groups at 12 months, the pooled analysis showed that denosumab significantly reduced the worsening of mTSS (mean scores (SD): 0.88 (3.30), p=0.0024 and 0.66 (2.16), p=0.0002 vs 1.50 (3.73), respectively) and ES (mean scores (SD): 0.44 (1.89), p=0.0006 and 0.20 (0.86), p<0.0001 vs 0.98 (2.54), respectively) (figure 1A, B). In contrast, denosumab had no effect on JSNS versus placebo in either treatment arm (both p>0.05) (figure 1C).
Figure 1.
Change from baseline in mTSS (A), ES (B) and JSNS (C) in the total group for up to 12 months. ES, bone erosion score; JSNS, joint space narrowing score; mTSS, modified total Sharp score; Q3M, denosumab 60 mg every 3 months; Q6M, denosumab 60 mg every 6 months. Data are means with 95% CIs.
Interaction analyses
The results of the post hoc analysis to evaluate treatment-by-subgroup interaction are shown in table 2. With p values of <0.10, glucocorticoid use, the presence/absence of anti-CCP antibodies and baseline ES (p=0.075, p=0.029 and p=0.063, respectively) were considered to be significant factors in the Q3M group.
Table 2.
P values for treatment-by-subgroup interaction analyses
| Q6M | Q3M | |
|---|---|---|
| Swollen joint count | 0.402 | 0.553 |
| Tender joint count | 0.979 | 0.696 |
| CRP | 0.815 | 0.946 |
| ESR | 0.692 | 0.696 |
| Glucocorticoid use | 0.174 | 0.075 |
| RF status | 0.266 | 0.454 |
| Anti-CCP antibodies | 0.193 | 0.029 |
| Baseline mTSS | 0.587 | 0.154 |
| Baseline ES | 0.241 | 0.063 |
CCP, cyclic citrullinated peptide; CRP, C reactive protein; ES, bone erosion score; ESR, erythrocyte sedimentation rate; mTSS, modified total Sharp score; Q3M, denosumab 60 mg every 3 months; Q6M, denosumab 60 mg every 6 months; RF, rheumatoid factor.
Changes in mTSS in subgroup analyses
In terms of change in mTSS at 12 months, baseline patient characteristics such as sex, age and duration of RA did not influence the effectiveness of denosumab. The results of the subgroup analyses are provided in online supplemental table S1.
rmdopen-2020-001249supp001.pdf (87.6KB, pdf)
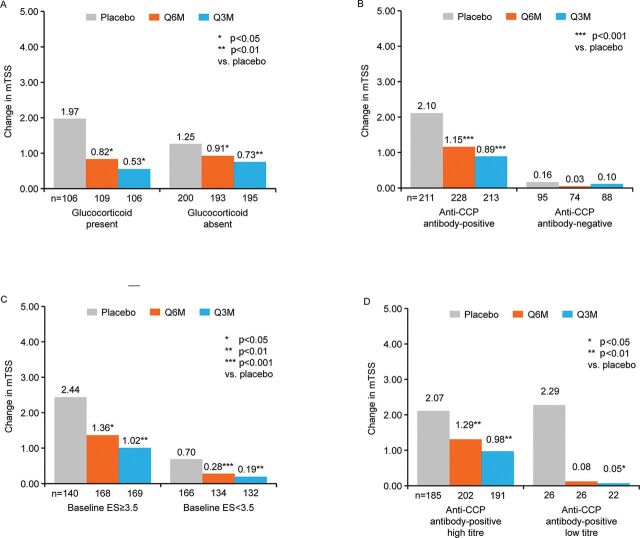
Change in mTSS by the three factors found to be significant in the interaction analyses (glucocorticoid use, anti-CCP antibody status and baseline ES) are shown in figure 2A, B, C. Among patients who received placebo, a greater change in mTSS was observed in patients with glucocorticoid use than in those who were not taking glucocorticoids at baseline (figure 2A). Significant reductions in changes in mTSS were observed in both denosumab dose groups versus placebo, regardless of glucocorticoid use (mean (SD) for Q6M and Q3M vs placebo: presence of glucocorticoid use 0.82 (3.25), p=0.0169 and 0.53 (1.47), p=0.0123 vs 1.97 (4.78), respectively; absence of glucocorticoid use 0.91 (3.34), p=0.0445 and 0.73 (2.46), p=0.0046 vs 1.25 (3.01), respectively). A greater change in mTSS was observed in patients who were positive for the anti-CCP antibody than in those who were negative for it at baseline (figure 2B). In patients stratified by positive/negative anti-CCP antibody, denosumab Q6M and Q3M versus placebo effectively reduced worsening of mTSS in the anti-CCP antibody-positive group (mean (SD): 1.15 (3.69), p=0.0008 and 0.89 (2.49), p<0.0001 vs 2.10 (4.33), respectively). However, in the anti-CCP antibody-negative group, changes in mTSS were marginal in the placebo group, and the statistically significant difference in the change in mTSS between denosumab Q6M and Q3M versus placebo was not observed (mean (SD): 0.03 (1.26) and 0.10 (0.77) vs 0.16 (0.82), respectively). Among patients who received placebo, a greater change in mTSS was observed in patients with baseline ES ≥3.5 vs those with baseline ES <3.5 (figure 2C). Significant reductions in changes in mTSS were observed in both denosumab dose groups versus placebo based on baseline ES (mean (SD) for Q6M and Q3M versus placebo: baseline ES ≥3.5 1.36 (4.16), p=0.0345 and 1.02 (2.77), p=0.0034 vs 2.44 (4.99), respectively; baseline ES <3.5 0.28 (1.49), p=0.0007 and 0.19 (0.70), p=0.0012 vs 0.70 (1.83), respectively).
Figure 2.
Change in mTSS by glucocorticoid status (A), anti-CCP antibody status (B), baseline ES (C) and anti-CCP antibody-positive titres (high/low) (D) of the total group by pooled analysis for up to 12 months. CCP, cyclic citrullinated peptide; ES, bone erosion score; mTSS, modified total Sharp score; Q3M, denosumab 60 mg every 3 months; Q6M, denosumab 60 mg every 6 months. Data are means with 95% CIs. *p<0.05; **p<0.01; ***p<0.001 vs placebo.
Results of a further subgroup analysis which classified the anti-CCP antibody-positive group according to titre (high/low) are shown in figure 2D. Among patients who received placebo, results were similar regardless of antibody titre. In patients receiving denosumab, mTSS worsening was reduced in both the high-titre subgroup (mean (SD) for Q6M and Q3M vs placebo: 1.29 (3.84), p=0.006 and 0.98 (2.57), p=0.001 vs 2.07 (4.32), respectively) and in the low-titre subgroup (mean (SD) for Q6M and Q3M vs placebo: 0.08 (1.88), p=0.08 and 0.05 (1.34), p=0.02 vs 2.29 (4.48), respectively).
DISCUSSION
Bone erosion and joint destruction are characteristic features of RA and are associated with dysregulation of the RANKL-mediated bone remodelling process. The development of bDMARDS has increased RA treatment options, but patient response is variable.23 Therefore, identifying markers and predictors of response is of interest. Denosumab is a RANKL inhibitor with proven inhibition of joint destruction in RA.14–17 However, only preliminary results have been published on the impact of baseline characteristics on denosumab efficacy.18
The present study evaluated the effect of denosumab on progressive joint destruction in patients with RA and in subgroups of patients pooled from the DRIVE and DESIRABLE studies.
In this pooled analysis, denosumab significantly reduced the progression of mTSS from baseline to 12 months compared with placebo, mainly due to ES reduction, but had no effect on JSNS, which is consistent with results from the previously reported phase II and III studies.13 17
The subgroups analysed were based on factors associated with a poor prognosis for bone destruction, which included CRP, ESR, RF, anti-CCP antibodies, baseline joint damage and TJC, as described in our recent publication evaluating bone erosion in the placebo-treated patients from the DRIVE and DESIRABLE studies.24 Glucocorticoid use and SJC subgroups were also included as these have previously been reported as parameters associated with bone destruction risk.25–28 The pooled analysis showed that most subgroups with specific baseline disease activity markers associated with bone erosion progression showed consistent efficacy of denosumab with respect to mTSS and ES. However, there were differences among subgroups depending on the presence or absence of some prognostic baseline factors.
Regarding the treatment-by-subgroup interaction analysis, denosumab demonstrated an effect in both of the glucocorticoid use (absence, presence) and baseline ES (<3.5, ≥3.5) subgroups, and its efficacy on mTSS was similar among both subgroups; however, progression in the placebo group was higher in patients using glucocorticoids and those with baseline ES ≥3.5. A statistical interaction was also seen in patients with the presence of anti-CCP antibodies.
The changes in mTSS were remarkably greater in patients with anti-CCP antibody-positive status. In contrast, in patients with anti-CCP antibody-negative status, there were almost no changes in mTSS in any groups, including the placebo group. Anti-CCP antibody levels have been established as a predictor of radiological progression of RA.26 29–32 However, patients with higher levels of anti-CCP antibody were more likely to develop radiographic progression33 and denosumab demonstrated significant inhibition of progression of joint destruction in patients with high anti-CCP antibody levels in this study. Based on these results, denosumab is expected to reduce the progression of joint destruction in all patients with RA who are positive for anti-CCP antibodies.
In patients with early RA, glucocorticoid given in addition to other treatments substantially reduced the rate of radiological progression.34 In terms of glucocorticoid use and baseline ES, the progression of joint destruction in the placebo groups was notably greater in patients with glucocorticoid use and those with baseline ES ≥3.5 than in patients without glucocorticoid treatment and with baseline ES <3.5 in this study. However, when denosumab was added, the progression was significantly reduced in both the Q6M and Q3M groups. Therefore, in patients using glucocorticoids and with a baseline ES ≥3.5, mTSS progression was higher in the placebo group, but denosumab effectively reduced progression, regardless of glucocorticoid use and baseline ES.
This study has several limitations. First, the DRIVE and DESIRABLE studies were not completely designed based on the ‘treat-to-target’ principle; their objectives were to evaluate the efficacy and safety of denosumab.16 18 This study, therefore, included patients who had partially controlled disease activity (the symptoms were manageable with conventional synthetic DMARDs). Second, the number of patients was relatively small in some subgroups, potentially limiting the detection of significant differences in subgroups. Third, because the duration of treatment was 1 year in both studies, the effectiveness of long-term treatment, that is, >1 year, cannot be evaluated until the results from the long-term extension phase of the DESIRABLE study become available (NCT01973569).
In conclusion, 12-month treatment with denosumab reduced the progression of joint destruction in Japanese patients with RA based on pooled data from the DRIVE and DESIRABLE studies. These results indicate that denosumab broadly reduces the progression of joint destruction in RA patients with risk factors for radiographic damage but is especially effective in patients who are anti-CCP antibody positive.
Key messages.
What is already known about this subject?
Previous phase II and III clinical studies demonstrated that denosumab reduces the progression of joint destruction in patients with RA; however, identifying the patient subpopulation in which denosumab is most effective is important in the clinical setting.
What does this study add?
Using pooled data from the phase II and III clinical trials, 12-month treatment with denosumab reduced the progression of joint destruction in patients with RA; these results indicate that denosumab broadly reduces the progression of joint destruction in patients with risk factors for radiographic damage, especially positivity for anti-CCP antibodies.
How might this impact on clinical practice?
This study indicates that in future clinical practice, RA patients who are anti-CCP antibody positive may be the best candidates for denosumab treatment.
Acknowledgments
This work was previously presented as a poster at the American College of Rheumatology (ACR/ARHP) Annual meeting (November 3–8, 2017, San Diego, CA). The published abstract is available at the following URL: https://acrabstracts.org/abstract/consistent-inhibition-of-joint-destruction-by-denosumab-in-important-subgroups-of-japanese-patients-with-rheumatoid-arthritis-pooled-analysis-of-phase-2-and-3-studies/. The authors thank all investigators of the studies, and J Ludovic Croxford, PhD, and Ingrid de Ruiter, MBChB, MPH, PhD, of Edanz Medical Writing for providing medical writing services.
Footnotes
Contributors: YT, SS, NI, HY and TT provided substantial contributions to the study conception and design. TY diagnosed the oral adverse events. ST diagnosed the atypical femoral fracture events. TO and TN were involved in the design and conduct of the study and in collecting data. NO was involved in the design of the study and data analysis. DvdH supervised scoring of the radiographs. All authors interpreted the data. All authors discussed and agreed on the content of the manuscript before submission.
Funding: This study was funded by Daiichi Sankyo Co., Ltd.
Competing interests: YT has received speaking fees and/or honoraria from Daiichi Sankyo, Astellas, Chugai, Eli Lilly, Pfizer, AbbVie, YL Biologics, Bristol-Myers Squibb, Takeda, Mitsubishi Tanabe, Novartis, Eisai, Janssen and Teijin; and has received research grants from Asahi Kasei Pharma, Mitsubishi Tanabe, Chugai, Takeda, Sanofi, Bristol-Myers Squibb, UCB, Daiichi Sankyo, Eisai and Ono. SS has received grant/research support from Chugai and Daiichi Sankyo; and has served on speakers’ bureaus for Asahi Kasei Pharma, Astellas, Daiichi Sankyo, Takeda, Chugai, Eisai, Pfizer, Eli Lilly and Ono. NI has received grant/research support from AbbVie GK, Asahi Kasei Corp, Astellas, Bristol-Myers Squibb, Chugai, Daiichi Sankyo, Eisai, Eli Lilly, Kaken, Medical Corporation Sanjikai, Medical Corporation Toukoukai, Mitsubishi Tanabe, Otsuka, Pfizer and Pfizer Japan, Takeda, Taisho Pharma and Zimmer Biomet GK; speakers’ bureau fees from Astellas, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, Pfizer and Taisho Pharma; and consulting fees or other remuneration from Ono. HY has received grant/research support from Astellas, AbbVie, Bristol-Myers Squibb, Kaken, UCB, Ono, Ayumi, Eisai, Daiichi Sankyo, Nippon Boehringer Ingelheim, Novartis, Taisho Pharma, Takeda, Mitsubishi Tanabe, Chugai, Teijin, Torii, Nippon Shinyaku, Pfizer and YL Biologics; and speakers’ bureau fees from Pfizer, YL biologics, Takeda, Teijin and Bristol-Myers Squibb. TY has received Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT #17H04377); and has acted as a consultant for Daiichi Sankyo. ST has received grant/research support from KYOCERA Corp and Asahi Kasei Corp; consulting fees from Amgen Astellas BioPharma K.K., KYOCERA Corp, Daiichi Sankyo and Pfizer; and speakers’ bureau fees from Asahi Kasei Corp, Astellas, Ayumi, Eisai, Ono, Daiichi Sankyo, Taisho Pharma, Mitsubishi Tanabe, Chugai, Teijin, Eli Lilly, Hisamitsu, Pfizer and Bristol–Myers Squibb. TO, TN and NO report full-time employment at Daiichi Sankyo. HG has received consulting fees or other remuneration from Daiichi Sankyo, Pfizer, Amgen, Bioclinica, Eli Lilly, Janssen, Servier, Novartis, Takeda, Merck, Biomarin, Clementia, Agnovos and Regeneron. DvdH has acted as a consultant for AbbVie, Amgen, Astellas, AstraZeneca, Bristol–Myers Squibb, Boehringer Ingelheim, Celgene, Cyxone, Daiichi Sankyo, Eisai, Eli Lilly, Galapagos, Gilead, GlaxoSmithKline, Janssen, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi, Takeda and UCB and is an employee of Imaging Rheumatology BV. TT has received grant/research support from Astellas, Chugai, Daiichi Sankyo, Takeda, AbbVie GK, Asahi Kasei Pharma, Mitsubishi Tanabe, Pfizer, Eisai, Nippon Kayaku, Novartis and Ayumi; speakers’ bureau fees from AbbVie GK, Bristol-Myers Squibb, Chugai, Mitsubishi Tanabe, Pfizer, Astellas, Daiichi Sankyo, Eisai, Sanofi, Teijin, Takeda and Novartis; and consulting fees from AstraZeneca, Eli Lilly, Novartis, Mitsubishi Tanabe, AbbVie GK, Nippon Kayaku, Janssen, Astellas, Taiho, Chugai, Taisho Pharma, GlaxoSmithKline and UCB.
Patient consent for publication: Not required.
Ethical approval: The study was approved by the institutional review boards (DRIVE study: Rheumatology, Hokkaido Medical Center for Rheumatic Diseases Ethics Committee and DESIRABLE study: Sunagawa City Medical Center Ethics Committee) of all participating sites and was conducted in accordance with the principles of the Declaration of Helsinki. This trial has been registered with JapicCTI-101 263 (DRIVE study) and ClinicalTrials.gov, number NCT01973569 (DESIRABLE).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: De-identified individual participant data and applicable supporting clinical trial documents (protocol, statistical analysis plan and clinical study report) may be available upon request, to qualified scientific and medical researchers for the purpose of conducting legitimate research, by applying to URL: https://vivli.org/. Details on data sharing criteria and the procedure for requesting access can be found at this URL: https://vivli.org/ourmember/daiichi-sankyo/.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
REFERENCES
- 1. Mahlich JC, Sruamsiri R. Treatment patterns of rheumatoid arthritis in Japanese hospitals and predictors of the initiation of biologic agents. Curr Med Res Opin 2017;33:101–7 10.1080/03007995.2016.1239191. [DOI] [PubMed] [Google Scholar]
- 2. Tanaka S, Tanaka Y, Ishiguro N, et al. RANKL: a therapeutic target for bone destruction in rheumatoid arthritis. Mod Rheumatol 2018;28:9–16 10.1080/14397595.2017.1369491. [DOI] [PubMed] [Google Scholar]
- 3. Ramiro S, Sepriano A, Chatzidionysiou K, et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2016 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis 2017;76:1101–36 10.1136/annrheumdis-2016-210708. [DOI] [PubMed] [Google Scholar]
- 4. Komano Y, Tanaka M, Nanki T, et al. Incidence and risk factors for serious infection in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitors: a report from the registry of Japanese rheumatoid arthritis patients for longterm safety. J Rheumatol 2011;38:1258–64 10.3899/jrheum.101009. [DOI] [PubMed] [Google Scholar]
- 5. Singh JA, Cameron C, Noorbaloochi S, et al. Risk of serious infection in biological treatment of patients with rheumatoid arthritis: a systematic review and meta-analysis. Lancet 2015;386:258–65 10.1016/S0140-6736(14)61704-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schett G, Gravallese E. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat Rev Rheumatol 2012;8:656–64 10.1038/nrrheum.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tanaka Y. Clinical immunity in bone and joints. J Bone Miner Metab 2019;37:2–8 10.1007/s00774-018-0965-5. [DOI] [PubMed] [Google Scholar]
- 8. Braun T, Zwerina J, Rantapää-Dahlqvist S. Positive regulators of osteoclastogenesis and bone resorption in rheumatoid arthritis. Arthritis Res Ther 2011;13:235 10.1186/ar3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Redlich K, Smolen J. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat Rev Drug Discov 2012;11:234–50 10.1038/nrd3669. [DOI] [PubMed] [Google Scholar]
- 10. Meednu N, Zhang H, Owen T, et al. Production of RANKL by memory B cells: a link between B cells and bone erosion in rheumatoid arthritis. Arthritis Rheumatol 2016;68:805–16 10.1002/art.39489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chiu YG, Ritchlin CT. Denosumab: targeting the RANKL pathway to treat rheumatoid arthritis. Expert Opin Biol Ther 2017;17:119–28 10.1080/14712598.2017.1263614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tanaka Y, Ohira T. Mechanisms and therapeutic targets for bone damage in rheumatoid arthritis, in particular the RANK-RANKL system. Curr Opin Pharmacol 2018;40:110–19 10.1016/j.coph.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 13. Takeuchi T, Tanaka Y, Soen S, et al. Effects of an anti-RANKL antibody, denosumab, on joint structural damage in patients with rheumatoid arthritis treated with csDMARDs (DESIRABLE study): a randomized, double-blind, placebo-controlled phase 3 trial. Ann Rheum Dis 2019;78:899–907 10.1136/annrheumdis-2018-214827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cohen SB, Dore RK, Lane NE, et al. Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum 2008;58:1299–309 10.1002/art.23417. [DOI] [PubMed] [Google Scholar]
- 15. Deodhar A, Dore RK, Mandel D, et al. Denosumab-mediated increase in hand bone mineral density associated with decreased progression of bone erosion in rheumatoid arthritis patients. Arthritis Care Res 2010;62:569–74 10.1002/acr.20004. [DOI] [PubMed] [Google Scholar]
- 16. Dore RK, Cohen SB, Lane NE, et al. Effects of denosumab on bone mineral density and bone turnover in patients with rheumatoid arthritis receiving concurrent glucocorticoids or bisphosphonates. Ann Rheum Dis 2010;69:872–5 10.1136/ard.2009.112920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Takeuchi T, Tanaka Y, Ishiguro N, et al. Effect of denosumab on Japanese patients with rheumatoid arthritis: a dose: Response study of AMG 162 (Denosumab) in patients with rheumatoid arthritis on methotrexate to validate inhibitory effect on bone erosion (DRIVE)—a 12-month, multicentre, randomised, double-blind, placebo-controlled, phase II clinical trial. Ann Rheum Dis 2016;75:983–90 10.1136/annrheumdis-2015-208052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ishiguro N, Tanaka Y, Yamanaka H, et al. Efficacy of denosumab on bone destruction in prognostic subgroups of Japanese rheumatoid arthritis patients in the phase II DRIVE study. Rheumatology (Oxford) 2019;58:997–1005 10.1093/rheumatology/key416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lv Q, Yin Y, Li X, et al. The status of rheumatoid factor and anti-cyclic citrullinated peptide antibody are not associated with the effect of anti-tnfa agent treatment in patients with rheumatoid arthritis: a meta-analysis. PLoS One 2014;9:e89442 10.1371/journal.pone.0089442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Castrejón I, Dougados M, Combe B, et al. Prediction of remission in a french early arthritis cohort by RAPID3 and other core data set measures, but not by the absence of rheumatoid factor, anticitrullinated protein antibodies, or radiographic erosions. J Rheumatol 2016;43:1285–91 10.3899/jrheum.141586. [DOI] [PubMed] [Google Scholar]
- 21. Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 22. van der Heijde D. How to read radiographs according to the sharp/van der Heijde method. J Rheumatol 2000;27:261–3. [PubMed] [Google Scholar]
- 23. Narváez J, Magallares B, Díaz Torné C, et al. Predictive factors for induction of remission in patients with active rheumatoid arthritis treated with tocilizumab in clinical practice. Semin Arthritis Rheum 2016;45:386–90 10.1016/j.semarthrit.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 24. Takeuchi T, Soen S, Ishiguro N, et al. Predictors of new bone erosion in rheumatoid arthritis patients receiving conventional synthetic disease-modifying antirheumatic drugs: analysis of data from the DRIVE and DESIRABLE studies. Mod Rheumatol 2020;1–8. 10.1080/14397595.2019.1703484 [DOI] [PubMed] [Google Scholar]
- 25. Kirwan JR, Bijlsma JWJ, Boers M, et al. Effects of glucocorticoids on radiological progression in rheumatoid arthritis (Review). Cochrane Database Syst Rev 2007;1:CD006356 10.1002/14651858.CD006356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dixey J, Solymossy C, Young A. Is it possible to predict radiological damage in early rheumatoid arthritis (RA)? A report on the occurrence, progression, and prognostic factors of radiological erosions over the first 3 years in 866 patients from the early RA study (ERAS). J Rheumatol Suppl 2004;69:48–54. [PubMed] [Google Scholar]
- 27. Visser K, Goekoop-Ruiterman YP, de Vries-bouwstra JK, et al. A matrix risk model for the prediction of rapid radiographic progression in patients with rheumatoid arthritis receiving different dynamic treatment strategies: post hoc analyses from the BeSt study. Ann Rheum Dis 2010;69:1333–7 10.1136/ard.2009.121160. [DOI] [PubMed] [Google Scholar]
- 28. Vastesaeger N, Xu S, Aletaha D, et al. A pilot risk model for the prediction of rapid radiographic progression in rheumatoid arthritis. Rheumatology (Oxford) 2009;48:1114–21 10.1093/rheumatology/kep155. [DOI] [PubMed] [Google Scholar]
- 29. Katchamart W, Koolvisoot A, Aromdee E, et al. Associations of rheumatoid factor and anti‑citrullinated peptide antibody with disease progression and treatment outcomes in patients with rheumatoid arthritis. Rheumatol Int 2015;35:1693–9 10.1007/s00296-015-3271-8. [DOI] [PubMed] [Google Scholar]
- 30. Rantapää-Dahlqvist S, De Jong BAW, Berglin E, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum 2003;48:2741–9 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 31. Nishimura K, Sugiyama D, Kogata Y, et al. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Int Med 2007;146:797–808 10.7326/0003-4819-146-11-200706050-00008. [DOI] [PubMed] [Google Scholar]
- 32. Forslind K, Ahlmén M, Eberhardt K, et al. Prediction of radiological outcome in early rheumatoid arthritis in clinical practice: role of antibodies to citrullinated peptides (anti-CPP). Ann Rheum Dis 2004;63:1090–5 10.1136/ard.2003.014233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Syversen SW, Gaarder PI, Goll GL, et al. High anti-cyclic citrullinated peptide levels and an algorithm of four variables predict radiographic progression in patients with rheumatoid arthritis: results from a 10-year longitudinal study. Ann Rheum Dis 2008;67:212–7 10.1136/ard.2006.068247. [DOI] [PubMed] [Google Scholar]
- 34. John R. Kirwan, and the Arthritis and Rheumatism Council Low-dose Glucocorticoid Study Group. The effect of glucocorticoids on joint destruction in rheumatoid arthritis. N Engl J Med 1995;333:142–7 10.1056/NEJM199507203330302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2020-001249supp001.pdf (87.6KB, pdf)