Abstract
Objectives
Several therapies are used for the treatment of rareautoinflammatory conditions like cryopyrin-associated periodic fever syndromes (CAPS), hyperimmunoglobulin Dsyndrome (HIDS)/mevalonate kinase deficiency (MKD) and tumour necrosis factor receptor-associated periodic syndrome (TRAPS). However, reviews reporting on treatment outcomes of these therapies are lacking.
Methods
A systematic literature review was conducted using Embase, MEDLINE, MEDLINE-In Process and Cochrane databases to identify the randomised/non-randomised controlled trials (RCTs/non-RCTs) and real-world observational studies of CAPS, HIDS/MKD and TRAPS published as full-texts (January 2000–September 2017) or conference abstracts (January 2014–September 2017). Studies with data for ≥1 biologic were included. Studies with <5 patients were excluded.
Results
Of the 3 342 retrieved publications, 72 studies were included (CAPS, n=43; HIDS/MKD, n=9; TRAPS, n=7; studies with ≥2 cohorts, n=13). Most studies were full-text (n=56), published after 2010 (n=56) and real-world observational studies (n=58). Among included studies, four were RCTs (canakinumab, n=2 (CAPS, n=1; HIDS/MKD and TRAPS, n=1); rilonacept, n=1 (in CAPS); simvastatin, n=1 (in HIDS/MKD)). Canakinumab and anakinra were the most commonly used therapies for CAPS and HIDS/MKD, whereas etanercept, canakinumab and anakinra were the most common for TRAPS. The available evidence suggested the efficacy or effectiveness of canakinumab and anakinra in CAPS, HIDS/MKD and TRAPS, and of etanercept in TRAPS; asingle RCT demonstrated the efficacy of rilonacept in CAPS.
Conclusions
Canakinumab, anakinra, etanercept and rilonacept were reported to be well tolerated; however, injection-site reactions were observed frequently with anakinra, rilonacept and etanercept. Data on the use of tocilizumab, infliximab and adalimumab in these conditions were limited; thus, further research is warranted.
Keywords: Fever Syndromes, Outcomes research, Treatment
INTRODUCTION
Hereditary periodic fever syndromes (HPFs) encompass a group of rare autoinflammatory diseases such as cryopyrin-associated periodic syndromes (CAPS), hyperimmunoglobulin D syndrome (HIDS), also known as mevalonate kinase deficiency (MKD) and tumour necrosis factor (TNF) receptor-associated periodic syndrome (TRAPS). 1 CAPS occurs because of gain-of-function mutations in the NLRP3 gene encoding cryopyrin, resulting in increased interleukin (IL)-1 secretion. CAPS manifests as three diseases varying in severity, from the least severe manifestation of familial cold autoinflammatory syndrome (FCAS), the mid-severity manifestation of Muckle–Wells syndrome (MWS), to the most severe manifestation of chronic infantile neurological cutaneous and articular syndrome (CINCA), which is also called neonatal-onset multisystem inflammatory disease (NOMID). 1 2 Symptoms of CAPS include cold-triggered episodes, urticaria-like rash, sensorineural hearing loss (in MWS and CINCA/NOMID), chronic meningitis (in CINCA/NOMID) and musculoskeletal manifestations (myalgia and arthralgia/arthritis). 1
HIDS is triggered by recessive mutations in the mevalonate kinase (MVK) gene leading to reduced or deficient activity of mevalonate kinase. 3 HIDS is characterised by fever, gastrointestinal manifestations, lymphadenopathy, hepatosplenomegaly, skin rash and mucosal ulcers. 3 Patients with MVK mutations having undetectable levels of mevalonate kinase develop a different disease called mevalonic aciduria (MA) which includes dysmorphic features, prenatal and postnatal growth retardation and ocular and neurological manifestations. 3 4 While both HIDS and MA can be grouped under the rubric of MKD from a genetic aetiological perspective, since MA is so rare, HIDS alone is the disease being referred to by the term MKD when used in the context of HPFs.
TRAPS is caused by dominant mutations in the TNFRSF1A gene encoding TNF receptor 1 and is characterised by abdominal pain, headache, peri-orbital manifestations, rash, pleuritic pain and lymphadenopathy. 5
The therapies for HPFs aim to control disease activity by suppressing inflammation. Only a few therapies are approved by the US Food and Drug administration (FDA) and European Medicines Agency (EMA). The IL-1 inhibitor anakinra is approved for CAPS (FDA approval only for CINCA/NOMID 6 and for CINCA/NOMID, MWS and FCAS by the EMA 7 ), canakinumab for CAPS, HIDS/MKD and TRAPS (by both FDA 8 and EMA 9 ) and rilonacept for CAPS (by FDA only). 10 The literature also reports the off-label use of conventional therapies such as non-steroidal anti-inflammatory drugs (NSAIDs) and corticosteroids in these patients. Biological therapies (eg, anti-IL-1) are often initiated when the disease is not controlled by conventional therapies. 11 Given that several therapies are being used for the treatment of these rare conditions, systematic searches/reviews reporting the outcomes of therapies are lacking. We conducted a systematic literature review (SLR) to collate and summarise the existing evidence on efficacy, effectiveness and safety of the current therapies for CAPS, HIDS/MKD and TRAPS.
METHODS
Data sources and searches
The SLR was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. 12 Comprehensive searches were conducted using the OVID platform in following databases: Embase (1996 to 6 October 2017); MEDLINE Epub ahead of print, In-process and other non-indexed citations (6 October 2017); MEDLINE without revisions (1996 to 6 October 2017); Evidence-Based Medicine (EBM) Reviews: Cochrane Database of Systematic Reviews (2005 to 6 October 2017); and EBM Reviews: Cochrane Central Register of Controlled Trials (6 October 2017).
Online supplementary table 1 provides the details of search strategy, which included both medical subject headings and free-text words for disease conditions (ie, CAPS [FCAS, MWS, CINCA/NOMID, FCU], HIDS/MKD, and TRAPS) and therapies/interventions. Search strategy also included terms for familial Mediterranean fever (FMF), the manuscript of which has been accepted for publication elsewhere. Searches were limited to English-language articles, published from January 2000 onwards for full-text publications, and from January 2014 to September 2017 for conference abstracts. A bibliographic search of relevant reviews was also performed to identify additional studies.
rmdopen-2020-001227supp001.pdf (1.3MB, pdf)
Study selection
Online supplementary table 2 presents the details of inclusion and exclusion criteria. Studies published as full-text publications or abstracts were included if they had ≥5 patients and reported the efficacy, effectiveness, and/or safety of therapies in patients with CAPS, HIDS/MKD or TRAPS. Both clinical trials (randomised (RCTs) or non-randomised (non-RCTs)) and prospective/retrospective observational studies providing real-world evidence were included. Two independent reviewers (R.G. and S.R.) screened all retrieved citations based on title and abstract as per predefined eligibility criteria; any discrepancies among them were resolved by a third independent reviewer (A.T.G.) by consensus after a discussion. Full-text publications were then screened, and those satisfying the inclusion criteria were included for data extraction. Multiple publications from the same study were linked.
Data extraction and quality assessment
Data extraction of the included studies was performed by one reviewer (R.G. or S.R., depending on the specific study). The quality check of data was performed by the second reviewer (S.R. or R.G.), with reconciliation of differences by the third reviewer (A.T.G.). Data were extracted into an extraction grid in Microsoft Excel for various parameters. Each included full-text publication was critically appraised for methodological quality, using the Cochrane risk of bias tool for RCTs 32 and the Newcastle-Ottawa Scale for non-RCTs and observational studies. 33 Data were analysed qualitatively, and results are reported as numbers and/or percentages.
RESULTS
The literature search yielded a total of 3 342 citations. After screening the titles and abstracts and then full-texts, 112 publications were included (online supplementary table 3). No additional studies were identified from the bibliographic search. Following the linking of multiple publications, 72 unique studies 13 15 17 21 23 29 34– 94 were included in this review (online supplementary figure 1).
Overview of studies
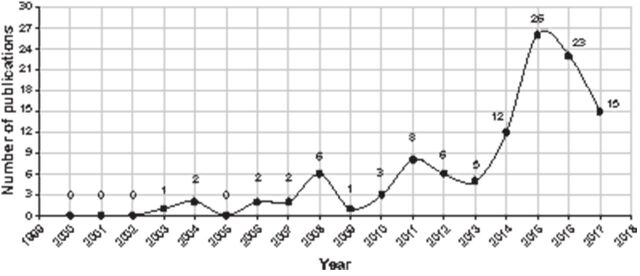
The included studies comprised 56 full-text publications and 16 conference abstracts. Most studies (n=56) were published after 2010 (figure 1). A large number of studies were from Europe (n=38) and the USA (n=9); 13 were multi-national studies. A majority of the studies (n=58) were prospective/retrospective observational studies. While 10 studies were open-label non-RCTs, four were double-blind, placebo-controlled RCTs.
Figure 1.
Growth in publication of CAPS, HIDS/MKD and TRAPS from the year 2000 to 2017.
CAPS, cryopyrin-associated periodic syndromes; HIDS, hyperimmunoglobulin D syndrome; MKD, mevalonate kinase deficiency; TRAPS, tumour necrosis factor receptor-associated periodic syndrome.
Patients
A majority of included studies had patients with CAPS (n=43), followed by HIDS/MKD (n=9) and TRAPS (n=7). Thirteen studies had cohorts with ≥2 conditions. Of the CAPS phenotypes, studies included only FCAS patients (n=3), only MWS patients (n=9), only CINCA/NOMID patients (n=4), MWS and CINCA/NOMID both (n=11) and a mix of all three phenotypes (n=16). Of note, two studies of HIDS/MKD had a few MA patients included.
Nineteen studies included exclusively or predominantly children (ie, >50% of patients). Similarly, 19 studies included exclusively or predominantly adult patients. Age across the included studies varied greatly, with patients as young as 44 days 35 to as old as 80 years. 16
Treatments and doses
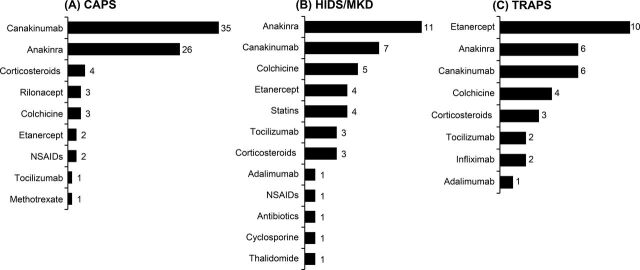
The most commonly used treatments in studies with CAPS patients were canakinumab (n=35) and anakinra (n=26). In HIDS/MKD studies, patients were often treated with anakinra (n=11) and canakinumab (n=7). Etanercept (n=10) was commonly used in studies for TRAPS patients, followed by anakinra (n=6) and canakinumab (n=6; figure 2A– C).
Figure 2.
An overview of treatments used in the included studies of CAPS, HIDS/MKD and TRAPS.
CAPS, cryopyrin-associated periodic syndromes; HIDS, hyperimmunoglobulin D syndrome; MKD, mevalonate kinase deficiency; NSAIDs, non-steroidal anti-inflammatory drugs; TRAPS, tumour necrosis factor receptor-associated periodic syndrome.
The dose of anakinra used in included studies was 100 mg or 1–5 mg/kg daily for CAPS, HIDS/MKD and TRAPS patients. Canakinumab was administered at 150 mg or 2 mg/kg every 8 weeks across all three indications. The dose of rilonacept given to patients with CAPS was 160 mg or 2.2 mg/kg weekly. 15 28 67 In the studies with TRAPS patients, 71 73 76 77 91 etanercept was administered at 25 mg or 0.4 mg/kg two times per week; dosing information in CAPS and HIDS/TRAPS studies was not reported. Dose for tocilizumab (ie, 8 mg/kg every 4 weeks) was provided in only one study with HIDS/MKD patients. 90
Outcomes and follow-up
Table 1 lists the outcomes assessed across the studies, which included clinical (n=62), biochemical markers (n=40) comprising acute-phase reactants (APRs) such as C reactive protein, erythrocyte sedimentation rate or serum amyloid A, safety (n=35), patient-reported outcomes (PROs) including health-related quality of life (HRQoL) measures (n=23). Twelve studies evaluated other clinical outcomes such as neurological related, hearing related, visual related, musculoskeletal related or pregnancy related. Thirteen studies provided information on switches between anti-IL-1 treatments. There was no remarkable difference in the clinical outcomes assessed across RCTs and non-RCTs or real-world observational studies, although the use of PROs varied greatly. HRQoL instruments such as Functional Assessment of Chronic Illness Therapy-Fatigue 14 and 36-item Short-Form Health Survey (SF-36) 14 27 28 were used in RCTs and non-RCTs only, whereas the measures like the Dermatology Life Quality Investigation, 64 RAND-36 Health Survey 94 and the TNO-AZL Adult Quality of Life (TAAQoL) 94 were used in the observational studies only.
Table 1.
List of outcomes reported across the included studies
| Outcomes | Number of studies |
|---|---|
| Clinical | 62 |
| Complete or partial response | 23 |
| Clinical remission | 10 |
| Symptoms resolution | 10 |
| Relapse | 7 |
| Time to development of attacks | 4 |
| Time to resolution of attacks | 4 |
| Clinical response | 3 |
| AIDAI | 3 |
| Reduction in attack frequency | 3 |
| Change in attack length | 2 |
| Clinical improvement | 1 |
| Biochemical markers | 40 |
| CRP | 34 |
| SAA | 26 |
| ESR | 17 |
| Biochemical remission/response | 2 |
| Haemoglobin | 2 |
| Platelet count | 2 |
| Mevalonic acid | 1 |
| Patient reported outcomes | 23 |
| PhGA | 17 |
| PtGA | 9 |
| HRQoL | |
| SF-36 | 3 |
| CHAQ/HAQ | 3 |
| CHQ-PF50 | 3 |
| FACIT-F | 1 |
| DLQI | 1 |
| RAND-36 | 1 |
| Not reported | 1 |
| Safety | 35 |
| Others | 12 |
| Hearing-related | 9 |
| Neurological | 5 |
| Visual | 4 |
| Pregnancy-related | 1 |
| Musculoskeletal | 1 |
| Switching between anti-IL-1 agents | 13 |
| From anakinra to canakinumab | 11 |
| Canakinumab to anakinra | 3 |
AIDAI, Auto-inflammatory Disease Activity Index; CHAQ, Child Health Assessment Questionnaire; CHQ-PF50, Child Health Assessment Questionnaire—Parent Form; CRP, C reactive protein; DLQI, Dermatology Life Quality Investigation; ESR, erythrocyte sedimentation rate; FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue; HRQoL, health-related quality of life; IL-1, interleukin 1; PhGA, Physician assessed Global assessment of disease; PtGA, patient/parent assessed Global assessment of disease; RAND-36, RAND-36 Health Survey; SAA, serum amyloid A; SF-36, 36-item Short-Form Health Survey.
A complete response (CR) and/or partial response (PR) was commonly evaluated in observational studies, but their definitions (online supplementary table 4) and follow-up durations differ remarkably. Based on the duration of follow-up, studies were categorised as short-term (up to 16 weeks or ≤4 months), mid-term (>4 months to 1 year) and long-term (>1 year). If a study reported CR/PR at more than one follow-up, it was considered in all the applicable categories.
Evidence on efficacy and effectiveness
CAPS
Canakinumab
Evidence for canakinumab in CAPS was available from one RCT, 13 14 five non-RCTs 21–23 25 35 and 29 observational studies. In the Phase III RCT in CAPS patients (MWS and NOMID/MWS-overlap, 9−74 years), 13 14 canakinumab demonstrated a rapid efficacy that was sustained up to 48 weeks, with 97% of patients having no/minimal disease per physician assessment (table 2). 13 14 The HRQoL assessed using SF-36 showed that all domain scores either approached or exceeded those of the general US population by week 8 and remained stable during canakinumab therapy. 14 The overall evidence of efficacy from five non-RCTs in different CAPS phenotypes (CINCA/NOMID, 23 both MWS and CINCA/NOMID, 21 25 and all three phenotypes combined), 24 35 age groups and study durations (2–3 years) indicated that canakinumab induced a rapid response; 100% of patients achieved a CR within 7 days of first dose, 25 with response maintained in 100% of patients up to 48 weeks 21 22 and 94% through 152 weeks. 35 95
Table 2.
Evidence of efficacy and safety from RCTs and non-RCTs of CAPS, HIDS/MKD and TRAPS
| Study, year, country | Study design | Population | Treatment(s) | N | Follow-up | Key findings | |
|---|---|---|---|---|---|---|---|
| Efficacy | Safety | ||||||
| RCTs | |||||||
| Lachmann et al 2009, 13 14 International | Phase III, DB, 3-parts (OL 8 W, DB 16 W, 24 OL Ext) | CAPS | Canakinumab, Placebo | 35 | 48 W | 8 W: CR‡, 34 (97%). 16 W DB: 15 (100%) Canakinumab-treated pts remained in remission, while 13/16 of placebo-treated pts (81%) had relapse. 24 W OL Ext: 30/31 (97%) had no or minimal disease activity by physician assessment. |
Canakinumab was well-tolerated up to 48 W. SAEs (2 patients; urosepsis in one patient, and vertigo in another). One patient discontinued because of an AE (vertigo). No deaths/life-threatening AE. >91% of patients reported no injection-site reactions; 4 patients had mild reactions, but no severe reactions. |
| Hoffman et al 2008, 15 16 U.S. | Two DB, Phase III sequential studies (24 W), followed by OL Ext (72 W) | CAPS | Rilonacept, Placebo | 101 | 96 W | Rilonacept vs Placebo: Study 1, n=47 (6 W): reduction in mean composite symptom score, 84% vs 13% (p<0.001); PhGA, PtGA, hsCRP and SAA reduced significantly with rilonacept vs placebo. Study 2, n=45 (24 W): Treatment effect continued. OL Ext, n=101 (44 from study 2 and 57 directly in OL) (72 W): Mean key symptom score reduced from 2.6 to 0. hsCRP and SAA normalised |
24 W: study 1 (rilonacept vs placebo), any AEs (74% vs 54%); injection-site reaction (48% vs 13%), upper RTI (26% vs 4%). Study 2 (rilonacept vs placebo), any AEs (68% vs 57%); injection-site reaction (36% vs 13%). One SAE (worsening sciatica). 72 W: Rilonacept, any AE (89%); erythema (32%), pruritus (13%), bruising (12%), swelling (11%), influenza, sinusitis (10% each). Nine SAEs reported by 7 patients: sinusitis and pneumococcal meningitis, coronary atherosclerosis, sciatica and arthritis, gastro-oesophageal reflux disease, cholelithiasis, and renal colic. |
| †De Benedetti et al 2016, 17– 20 International | Phase III, DB, 4 epoch (E1, 12 W screening; E2, 16 W DB; E3, 24 W WDL; E4, OL Ext 72 W | HIDS/MKD | Canakinumab, Placebo | 72 | 112 W | 16 W (E2): Canakinumab vs Placebo: clinical response
2
, 13/37 (35%) vs 2/35 (6%) (p=0.0020); inactive disease, 40%. 40 W (E3): 3/6 (50%) vs 1/7 (14%) (p=0.2168) |
16 W: AEs (canakinumab, 14 patients; placebo, 4 patients). Infections and infestations (canakinumab, 10 patients; placebo, 2 patients). SAEs (canakinumab, 0; placebo, 2 patients). 40 W: AE rate/100 PY (canakinumab, 1 121; placebo, 816.7). SAE rate/100 PY (canakinumab, 34.0; placebo, 42.7). |
| TRAPS | Canakinumab, Placebo | 46 | 112 W | 16 W (E2): Canakinumab vs Placebo: clinical response
2
, 10/22 (45%) vs 2/24 (8%) (p=0.0050); inactive disease, 46%. 40 W (E3): 3/4 (75%) vs 2/5 (40%) (p=0.3571) |
16 W: AEs (canakinumab, 9 pts; placebo, 3 patients). Infections and infestations (canakinumab, 2 patients; placebo, 2 patients). SAEs (canakinumab, 0; placebo, 0). 40 W: AE rate/100 PY (canakinumab, 747.1; placebo, 816.7). SAE rate/100 PY (canakinumab, 19.0; placebo, 42.7). |
||
| Simon et al 2004, 8 ‡ The Netherlands | DB, crossover (two TPs each 24 W and 4 W washout) |
HIDS/MKD | Simvastatin, Placebo | 6 | 52 W | Simvastatin vs Placebo: mean no. of febrile days per pts per 6 M, 16.7 vs 24.3 days; mean no. of fever attacks, 3.0 vs 3.8; mevalonic acid levels, 1.9 vs 4.9 mg/g creatinine (p<0.001) | No AEs in five patients. One patient reported flatulence, nightmares, tiredness and myalgias. |
| Non-RCTs | |||||||
| †Brogan et al 2015,‡ 6 9 ‡ International | Phase III, OL, MC (core study, 56 W; extension, 152 W) | CAPS (12 MWS, 4 CINCA/ NOMID, 1 FCAS) |
Canakinumab | 17 | 152 W | 56 W: CR
3
: 16 (94%); 4 pts subsequently relapsed, but all regained CR 152 W: CR 3 : 17 (100%); pts with no disease activity improved (24% to 65%) |
10 patients (59%) were suspected to have study-drug related AEs, with most common of diarrhoea, pneumonia, rhinitis and cough (3 patients each). SAEs, 8 patients (47.1%), with pneumonia as the most frequent SAE (12%). No deaths. |
| Imagawa et al 2013, 21 22 Japan | Phase III, OL (core, 24 W; Ext 22 M) | CAPS (7 MWS, CINCA/ NOMID 12) |
Canakinumab | 19 | 48 W | 24 W: CR
4
: 18 (95%); remission (relapse free): 14/18 (78%); pts with no disease activity improved (11% to 32%). 48 W: CR 4 : 19 (100%); remission: 18 (95%); pts with no disease activity, 11 (58%). |
48 W: Any AEs (100%). Most common AEs: upper RTI (73.7%), nasopharyngitis (52.6%), gastroenteritis (36.8%), sinusitis and stomatitis (each 26.3%). SAEs, 5 patients (26%): parvovirus infection and Epstein-Barr virus infection (n=1), pneumonia (n=1), sinoatrial block and headache (n=1), asthma (n=1) and appendicitis (n=1). No deaths. One patient discontinued (withdrew consent). |
| Sibley et al 2015, 23 U.S. | Phase I/II, OL | CAPS (CINCA/ NOMID) |
Canakinumab | 6 | 24 M | 6 M: inflammatory remission
5
: 4/6 (67%) 24 M: inflammatory remission 5 : 5/6 (83%) |
Infections (6 patients). One serious AE (abscess due to a methicillin-resistant Staphylococcus aureus infection). |
| Kuemmerle-Deschner et al 2011, 24 International | Phase III, OL, MC | CAPS | Canakinumab | 166 | 2 Y | CR‡ (Canakinumab-naïve pts): 85/109 (78%) (79 within D 8, and 5 within days 10 and 21). Of 141 pts with relapse assessment available, 90% did not relapse, their CRP/SAA levels normalised (<10 mg/L) | Any AEs (90.4%). Most common AEs were infections (65.7%) (mostly mild-to-moderate severity). SAEs, 18 patients (10.8%; mainly infections and were responsive to standard therapy). 92% of patients had no injection-site reactions and 8% had mild-to-moderate reactions. |
| Kuemmerle-Deschner et al 2011, 25 International | Phase II, OL | CAPS (MWS, CINCA/ NOMID) |
Canakinumab | 7 | _ | D 7: CR
6
, 100%; CRP and SAA normalised. Relapse, 6 (86%); median time to relapse, 49 days. CR 6 on retreatment, 4/6 (67%). |
Canakinumab was well-tolerated. Common AEs were upper RTI (71%), rash (57%), pharyngitis, nasopharyngitis, vomiting (43% each), diarrhoea, rhinitis, sleep disorder, cough and acne (29% each). SAE, 1 patient (vertigo). |
| Arostegui et al 2017, 26 Spain | Phase II, 3-part OL (6 M TP, 6 M WDL, 24 M TP) | HIDS/MKD | Canakinumab | 9 | 36 M | 6 M TP: CR 7 , 100%; median time to resolution, 3 days. Median no. of attacks decreased from 5 to 0 (p=0.009). 6 M WDL: relapse, 7 (78%); median time to relapse, 110 days. 24 M TP: No. of attacks/pts decreased significantly (p=0.008). | AEs, 9 patients (100%) (infections, 44% is most common). 14 SAEs occurred in 4 patients (8 SAEs occurred in same patient who had a complicated medical history; other SAEs were cellulitis in one patient, and hidradenitis suppurativa requiring hospitalisation in one patient). |
| Gattorno et al 2017, 27 Europe | Phase II, 3-part OL (6 M TP, 5 M WDL, 24 M TP) | Recurrent or chronic TRAPS | Canakinumab | 20 | ~2.5Y | D 15: CR
8
, 19 (95%); median time to clinical remission, 4 days. 5 M WD: relapse, 100%; median time to relapse, 91.5 days. Ext TP: all pts regained CR. CPS/SAA levels normalised by D15 and remained normal during TP |
Canakinumab was well tolerated. AEs, 20 pts (100%), with nasopharyngitis (60%), abdominal pain, headache, oropharyngeal pain (55% each) and fever (50%) as most common. SAEs reported in 7 patients: pericarditis, abdominal pain, diarrhoea, intestinal obstruction, vomiting, upper RTI, meniscus injury, hypertriglyceridaemia, hyperkalaemia, foot deformity and condition aggravated. |
| Bulua et al 2012, 6 ‡ U.S. | OL, single arm, dose-escalation | TRAPS | Etanercept | 15 | 10 Y | Etanercept two times per week (period 2) and 3 times weekly (period 3) was significantly associated with a dose-related attenuation in the total symptom score, decreasing by 36% (p=0.02) in period 2 and 66% (p<0.0001) in period 3. CRP, ESR and SAA levels were significantly lower during etanercept treatment | Etanercept was well-tolerated, with most AEs being local injection-site reactions. No SAEs reported. |
| Goldbach-Mansky et al 2008, 28 U.S. | Phase II, OL, pilot study | CAPS (FCAS) | Rilonacept | 5 | 24 M | Cold-induced clinical symptoms improved within days of RIL dose. ESR, hsCRP and SAA reduced statistically at doses of 100 mg. | Rilonacept was well-tolerated. No injection-site reactions reported. Infections occurred but resolved. No SAEs reported. |
| Goldbach-Mansky et al 2006, 29– 31 U.S. | Phase I/II, OL, single arm | CAPS | Anakinra | 43 | 5 Y | 6 M: all 18 pts had rapid response with disappearance of rash. Global diary scores and ESR, CRP and SAA reduced significantly (p<0.001). 3 and 5 Y: improvements sustained in diary scores, PtGA, PhGA, PtGA pain scores and inflammatory markers. |
6 M: No discontinuations. Injection-site reactions, 8 patients (44%). AEs included upper RTI (15 patients), UTI (2 patients), and a hospital admission for dehydration from non-bacterial diarrhoea (1 patient). 3 and 5 Y: AE rate was 7.7 AEs per patient per year. Anakinra had similar safety profiles in adults and children. Most common AEs were headache and arthralgia. 14 patients reported 24 SAEs: pneumonia, gastroenteritis, wound infection, post-lumbar puncture syndrome and macrophage activation syndrome. |
†Studies published as conference abstracts only.
‡CR: PhGA (no or minimal disease activity), no or minimal rash (skin disease) and CRP and SAA both <10 mg/L; 2 Clinical response: resolution of index flare at Day 15 and no new disease flare over 16 weeks of treatment; 3 CR: clinical response and normal CRP; 4 CR: PhGA (no or minimal), no or minimal skin disease, and serological remission (CRP and/or SAA <10 mg/L); 5 Inflammatory remission (global daily diary score (mean/week) ≤2 and normal CRP (≤10 mg/L)); 6 CR: PhGA (no or minimal disease activity), no or minimal rash, and normal CRP and/or SAA (<10 mg/L); 7 CR: PhGA (0 (no) or 1 (minimal) disease activity) and plasma CRP <10 mg/L; 8 CR: clinical remission (PhGA ≤1) and full serological remission (CRP and/or SAA <10 mg/L).
AE, adverse event; CAPS, cryopyrin-associated periodic syndromes; CINCA, chronic infantile neurological cutaneous and articular syndrome; CRP, C reactive protein; D, days; ESR, erythrocyte sedimentation rate; FCAS, familial cold autoinflammatory syndrome; HIDS, hyperimmunoglobulin D syndrome; hsCRP, high-sensitivity CRP; M, months; MC, multicentre; MKD, mevalonate kinase deficiency; MWS, Muckle–Wells syndrome; N, number of patients; NOMID, neonatal-onset multisystem inflammatory disease; OL, open-label; PhGA, physician assessed global assessment of the disease; PtGA, patient/parent assessed global assessment of the disease; PY, patient-years; RTI, respiratory tract infection; SAA, serum amyloid A; SAE, serious AE; TP, treatment period; TRAPS, tumour necrosis factor receptor-associated periodic syndrome; UTI, urinary tract infection; W, weeks; WDL, withdrawal; Y, years.
Ten observational studies reported the effectiveness of canakinumab in patients with different CAPS phenotypes and of various age groups. 34 39– 41 48 52 59 66 82 92 Among the studies reporting CR/PR, 93% of canakinumab-treated MWS patients achieved a CR at short-term follow-up. 59 Patients who achieved a CR with canakinumab ranged from 62% 48 to 93% 59 at mid-term follow-up, and 50% 34 52 to 100% 82 at long-term follow-up (online supplementary figure 2A–B). Other observational studies have also shown the effectiveness of canakinumab in improving the Disease Activity Score (DAS), 57 65 Autoinflammatory Disease Activity Index (AIDAI) score, 36 83 complete/partial resolution of symptoms, 38 45 88 improvement in physician/patient assessed PROs 36 65 85 and the Child Health Assessment Questionnaire (CHAQ). 65 Of the eight studies assessing other outcomes, 23 42 45 47 55– 57 65 treatment with canakinumab was associated with improvement or stabilisation in neurological outcomes (eg, migrainous headache), 23 42 45 hearing loss, 23 42 55– 57 65 vision outcomes (eg, uveitis, conjunctivitis) 23 47 and musculoskeletal outcomes. 23
Anakinra
One non-RCT 29 30 and 25 observational studies provided data for anakinra. In the non-RCT in CINCA/NOMID patients of mean age 11 years, anakinra showed a rapid response, with disappearance of rash. 29 The diary scores, APRs, PROs and CHAQ scores decreased significantly at 3 months, 29 and were maintained up to 6 months (table 2). 30
Eight observational studies reported CR/PR. 34 44 48 59 68 82 86 92 In a study with MWS patients, 67% of anakinra-treated patients achieved a CR at short-term follow-up and 75% at mid-term follow-up. 59 Patients who achieved a CR with anakinra ranged from 40% 34 to 100% 82 at long-term follow-up (online supplementary figure 2A–B). Other observational studies showed that anakinra was effective in improving AIDAI score 36 83 or DAS, 57 58 in attaining disease control, 73 clinical/biochemical remission, 84 85 complete/partial resolution of CAPS symptoms, 38 43 45 49 51 60 62 64 79 PROs, 36 58 79 85 CHQ-PF50 60 and DLQI. 64 Seven studies also demonstrated improvement or stabilisation with anakinra in neurological outcomes (eg, migrainous headache, papilledema), 45 60 62 hearing loss, 30 55– 57 60 62 and visual acuity. 30 62 A study by Chang et al showed that anakinra when administered during pregnancy in women with CAPS provided significant and persistent symptom relief, and prevented the long-term sequelae of CAPS. 50
Rilonacept
Data for the efficacy of rilonacept were available from three studies, including one RCT, 15 16 one non-RCT 28 and an observational study. 67 In a Phase III RCT comprising two sequential studies of 24 weeks in adults with CAPS (FCAS and MWS) 15 followed by a 72-week open-label treatment extension, 16 rilonacept significantly reduced (84%) the symptom score versus placebo (13%; table 2), 15 with reduction in the number of disease flare days, APRs and the limitations in patients’ daily activities. 15 16 In a non-RCT, all patients with FCAS responded immediately to rilonacept with reduction in cold-induced attacks and improvement in symptoms. 28 One study reported the significant reduction in APRs with rilonacept. 67
Etanercept
Two CINCA/NOMID patients in one study received etanercept with poor response. 68
Tocilizumab
One study reported the positive effect of tocilizumab in two patients with CAPS. 87
Others
The conventional treatments such as corticosteroids, colchicine, NSAIDs and methotrexate were also used in few studies of CAPS and provided either PR or no response (online supplementary figure 2A–B). 44 52 68 92
HIDS/MKD
Anakinra
Eleven observational studies 69 70 75 78 82 83 85 86 90 93 94 provided data on the effectiveness of anakinra in HIDS/MKD. Among the studies reporting CR/PR, 11% 70 to 30% 86 of anakinra-treated patients achieved a CR at mid-term follow-up, whereas 78% achieved a PR. 78 In two patients with MA, anakinra induced a PR in one patient, but no response in another patient. 70 At long-term follow-up, anakinra showed a 100% CR (online supplementary figure 3A–B). 82 Other observational studies revealed that anakinra decreased the AIDAI score, 83 and attained complete clinical response in 52% and functional status improvement in 81% of patients. 85
Canakinumab
One RCT, 17– 20 one non-RCT 26 and five observational studies 69 72 78 86 93 provided evidence for canakinumab in HIDS/MKD. In a pivotal Phase III RCT (CLUSTER trial) 17 with three cohorts of patients, including 72 patients with HIDS/MKD, patients achieving a clinical response (ie, resolution of index flare at Day 15 and no new disease flare over 16 weeks of treatment) at week 16 were significantly higher with canakinumab (35%) vs placebo (6%), 17 with 40% of canakinumab-treated patients achieving inactive disease (ie, AIDAI score <9). 18 The clinical response at week 40 was also numerically higher with canakinumab (50%) than placebo (14%; table 2). 19 The HRQoL assessments revealed that canakinumab treatment led to early clinically meaningful improvements in SF-12 Physical Component Summary (PCS), CHQ-PF50 Physical Subscale (PhS) and CHQ-PF50 Psychosocial Subscale (PsS) scores at week 5, which were sustained and increased to a large effect size by week 16. 20 In an open-label, 3-part non-RCT, all HIDS/MKD patients achieved a CR with canakinumab during 6-month treatment period. 26 Disease relapsed in 78% of patients during the canakinumab withdrawal period. Responses were regained on retreatment, with 89% of patients having excellent disease control at the end of the 24-month treatment extension period. 26 In the observational studies with long-term follow-up, 50% of canakinumab-treated patients reported a CR (online supplementary figure 3A–B). 86 Canakinumab was also shown to be effective in resolution of attacks. 72
Etanercept
Four observational studies provided evidence for etanercept, 69 85 93 94 with patients achieving a CR were 7%, 93 22% 69 and 31%. 94 Patients who reported a PR with etanercept were 22%, 69 39% 94 and 52% (online supplementary figure 3A–B). 93
Tocilizumab
In three studies with data for tocilizumab, 85 87 90 50% of patients achieved a CR in one study at mid-term follow-up (online supplementary figure 3A–B). 90 Another study reported the positive effects of tocilizumab in HIDS/MKD. 87 Tocilizumab also induced a complete clinical response and functional improvement. 85
Adalimumab
In one study, three patients received adalimumab, with one achieving good response, one PR and no effect in one patient. 69
Simvastatin/statins
In a single crossover RCT of simvastatin/placebo in HIDS/MKD patients, 83% of patients reported reduction in number of febrile days with simvastatin at 24 weeks, with significant decrease in urine mevalonic acid levels (table 2). 89 Besides this small RCT, statins were generally reported to be ineffective in most of the patients. 69 93 94
Others
In HIDS/MKD patients, treatment with colchicine alone was shown to be ineffective, 69 82 93 94 however, the combination of colchicine and prednisone showed 100% CR (online supplementary figure 3A–B). 76 NSAIDs 93 and corticosteroids 93 94 were shown to provide benefits in some patients. Cyclosporine, thalidomide and antibiotics used in one study were reported as ineffective. 94
TRAPS
Etanercept
One non-RCT 71 and nine observational studies 73– 77 82 85 91 92 provided evidence for etanercept in TRAPS. In the non-RCT, etanercept significantly attenuated the total symptoms score and reduced the symptoms frequency (table 2). 71 In the observational studies with long-term follow-up, 50% of patients achieved a CR with etanercept, 76 whereas 100% achieved a PR (online supplementary figure 4A–B). 75 82 Other observational studies revealed etanercept provided immediate response and good disease control in TRAPS patients, with resolution of fever and other clinical symptoms. 73 74 85 91
Canakinumab
One RCT, 17– 20 one non-RCT 27 and four observational studies 82 86– 88 comprised evidence for canakinumab. In the TRAPS cohort of CLUSTER trial described above, 17– 20 a significantly higher proportion of canakinumab-treated patients achieved the clinical response than placebo (45% vs 8%), 17 with 46% of patients achieving inactive disease at week 16. 18 The clinical response at week 40 was also numerically higher with canakinumab vs placebo (75% vs 40%; table 2). 19 In HRQoL assessments, canakinumab provided early clinically meaningful improvements in SF-12 PCS, CHQ-PF50 PhS and CHQ-PF50 PsS scores at week 5, which were sustained and increased in magnitude by week 16. 20 In the Phase II non-RCT, 19/20 patients (95%) with active recurrent/chronic TRAPS achieved a CR with canakinumab. 27 Disease relapsed in all patients during canakinumab withdrawal period, but similar responses were attained and sustained on retreatment with canakinumab. 27 In the observational studies, 100% of patients achieved a CR with canakinumab at mid- 86 and long-term follow-up (online supplementary figure 4A–B). 82 Other observational studies reported the positive effects of canakinumab with resolution of systemic manifestations and normal levels of APRs. 87 88
Anakinra
Six studies presented data for anakinra in TRAPS. 73 79 82 85 86 92 Among the studies reporting CR/PR, 33% of patients achieved a CR with anakinra at both short-term 86 and long-term follow-up (online supplementary figure 4A–B). 82 Other studies revealed TRAPS patients were successfully treated with anakinra 73 provided a complete clinical response and improvement in functional status, 85 controlled effectively both clinical and APRs and prevented disease relapse. 79
Tocilizumab
In two studies for tocilizumab, a PR was seen in one patient, 82 and positive effect was observed in two patients. 87
Infliximab
There were two studies for infliximab, but no clear effects/benefits reported. 75 85
Adalimumab
No clear effects/benefits were reported in one study for adalimumab. 85
Others
While colchicine alone showed a PR or no response, 76 82 92 the combination of colchicine and prednisone showed 100% CR. 76 With corticosteroids, 25% 82 to 49% 92 of patients achieved a CR and 67% 76 achieved a PR (online supplementary figure 4A–B). NSAIDs were also reported to be beneficial in some patients. 92
Evidence on safety
Table 2 represents safety outcomes from RCTs and non-RCTs. In the single non-RCT 29– 31 available for anakinra in CAPS patients, injection-site reactions (ISRs) were observed in 44% of patients at 6 weeks. 29 In the long-term follow-up of safety up to 5 years in 43 patients, the most common adverse events (AEs) reported with anakinra were headache (49%), arthralgia (42%), fever and upper respiratory tract infection (RTI; each 40%). 30 31 Pneumonia, gastroenteritis, wound infection, post-lumbar puncture syndrome and macrophage activation syndrome (MAS) were reported to be serious AEs (SAEs) with anakinra. 30 31 Two RCTs 13 17 and seven non-RCTs 21 23– 27 35 reported canakinumab safety outcomes. Canakinumab was reported as well tolerated by CAPS, HIDS/MKD and TRAPS patients. Most patients (>90%) did not report ISRs with canakinumab, 13 21 24 25 although infections, particularly RTIs were reported frequently (10–74% of patients). 17 21 23– 26 35 Common SAEs occurred with canakinumab included pneumonia, 21 24 26 vertigo, 13 25 upper RTI 24 27 and cellulitis. 24 26 In the CLUSTER trial, no new safety signals were observed for canakinumab in HIDS/MKD and TRAPS patients. 17 19 Several studies have demonstrated the long-term safety of canakinumab at 40 19 , 48 weeks, 14 22 2 years, 23 24 and up to 3 years. 26 27 For rilonacept, one RCT 15 16 and one non-RCT 28 provided information. Rilonacept was reported to be well tolerated by CAPS patients up to 96 weeks, 16 with ISRs and infections, headache, arthralgia and headache being the common AEs. 15 16 28 SAEs such as sinusitis and pneumococcal meningitis, coronary atherosclerosis, sciatica and arthritis, cholelithiasis, renal colic and gastro-oesophageal reflux disease were reported in one patient each. 16 The AE profile of rilonacept in pediatric patients in this study was similar to that in adults. 16 No AEs were reported in HIDS/MKD patients with simvastatin. 89 In the single non-RCT for etanercept in TRAPS patients, ISRs were common AEs and there were no SAEs reported in this study. 71
Table 3 shows safety outcomes from observational studies. In these real-world studies, canakinumab, anakinra, etanercept and tocilizumab 87 were reported to be well tolerated by CAPS, HIDS/MKD and TRAPS patients. ISRs occurred frequently with etanercept 74 77 and anakinra. 34 43 59 78 79 ISRs were generally not reported with canakinumab; common AEs included infections and infestations, 37 40 upper RTI, 34 59 SAEs included infection, 37 40 vertigo 59 and a musculoskeletal event. 40 One study by Eroglu et al reported MAS in two patients with anti-IL-1 therapy. 36 Common AEs with anakinra included upper RTI, 59 70 and SAEs included severe bronchitis in one study. 26 No SAEs reported in studies with etanercept. 74 77
Table 3.
Safety findings reported in observational studies of CAPS, HIDS/MKD and TRAPS
| Study, year, country (pub type) | Study design | Population | Treatment(s) | N | Safety findings |
|---|---|---|---|---|---|
| †Guerrero et al 2017, 52 Spain | Retrospective (Tertiary care hospital) | CAPS (MWS) | Canakinumab | 6 | Canakinumab was well-tolerated; injection-site reactions (1 patient). |
| Kuemmerle-Deschner et al 2016, 40 Germany | Prospective (Hospital) | CAPS (FCAS, MWS, CINCA/NOMID) | Canakinumab | 68 | AEs, 73 no. (infections and infestations, 45 no.; general and administrative site conditions, 16 no.); SAEs (2 no. (one infection and another musculoskeletal event)) |
| Anton et al 2015, 34 Spain | Retrospective (Cohort) | CAPS (FCAS, MWS) | Anakinra | 5 | Injection-site reactions (60%); no infections. |
| Canakinumab | 8 | No injection-site reactions; upper RTI (1 patient); acute appendicitis (1 patient). | |||
| Kuemmerle-Deschner et al 2013, 59 Germany | Prospective (Cohort) | CAPS (MWS) | Anakinra | 12 | Injection-site reaction (42%) (mild); upper RTI (33%); weight gain (≥5 kg). No SAEs. |
| Canakinumab | 14 | No injection-site reactions; upper RTI (29%); transient headache (14%). SAE, 1 patient (vertigo requiring hospitalisation) | |||
| Eroglu et al 2016, 36 Turkey | Prospective (Clinics) | CAPS | Anti-IL-1 (Anakinra, Canakinumab) | 14 | Macrophage activation syndrome (2 patients). No SAE requiring hospitalisation. |
| Mehar et al 2016, 43 Australia | Retrospective (Physician survey) | CAPS (FCAS, MWS, CINCA/NOMID) | Anakinra | 13 | Injection-site pain (54%). |
| Russo et al 2014, 65 England | Prospective (Clinics) | CAPS (MWS, CINCA/NOMID) | Canakinumab | 10 | Canakinumab was well-tolerated. No injection-site reactions. No serious infections. |
| Chang et al 2014, 50 U.S. | Retrospective (Patient survey) | CAPS (FCAS, CINCA/NOMID) | Anakinra | 9 | Anakinra was well-tolerated. |
| †Navarrete et al 2014, 61 Spain | Retrospective (Clinics) | CAPS (MWS) | Canakinumab | 10 | No AE by any patient. |
| Kuemmerle-Deschner et al 2011, 58 Germany | Prospective (OL) | CAPS (MWS) | Anakinra | 12 | Anakinra was well-tolerated. No SAEs. |
| Neven et al 2010, 62 Europe | Retrospective (Clinics) | CAPS (CINCA/NOMID) | Anakinra | 10 | Mild injection-site reactions. |
| †Hoffman et al 2016, 37 International | Prospective (Registry) | CAPS (FCAS, MWS, CINCA/NOMID) |
Canakinumab | 288 | IR/100 pyrs for overall AEs was 100.0 (FCAS, 78.1; MWS, 113.4; NOMID, 119.0). Most common AEs: infections and infestations (IR/100 pyrs, 39.1). SAEs, 86 patients (IR/100 pyrs, 16.3) (infections (IR/100 pyrs, 5.0)). Discontinuations, 22 pts (8%) (AEs, 5; poor efficacy and patient choice, 10; other reasons, 7). One death (due to metastatic rectal adenocarcinoma). |
| Rossi-Semerano et al 2015, 86 France | Retrospective (Physician survey) | HIDS | Anakinra | 10 | SAE, 1 patient (severe bronchitis) |
| Galeotti et al 2012, 78 France | Retrospective (Physician survey) | HIDS | Canakinumab | 6 | Injection-site reactions (1 patient), recurrent pharyngitis (1 patient) and transient hepatitis (1 patient). Overall, well-tolerated. |
| Anakinra | 9 | Injection-site reactions (4 patients), shivers and hypothermia (1 patient) and bacterial pneumonia (1 patient). Overall, well tolerated. | |||
| Bodar et al 2011, 70 The Netherlands | Prospective (Clinics) | HIDS | Anakinra | 11 | Injection-site reactions (2 patients), mild upper RTI (2 patients). |
| †Cakan et al 2017, 72 Turkey | Retrospective (Clinics) | CAPS (FCAS, CINCA/NOMID) | Canakinumab | 3 | No AEs observed under canakinumab treatment. |
| HIDS | Canakinumab | 2 | |||
| †Salugina et al 2017, 87 Russia | Retrospective (Clinics) | CAPS | Tocilizumab | 2 | Satisfactory tolerability of all treatments observed in all patients. |
| Canakinumab | 9 | ||||
| HIDS | Tocilizumab | 1 | |||
| TRAPS | Tocilizumab | 2 | |||
| Canakinumab | 4 | ||||
| †Salugina et al 2016, 88 Russia | Prospective (Clinics) | CAPS | Canakinumab | 8 | Canakinumab was satisfactorily tolerated. No SAEs reported. |
| TRAPS | 3 | ||||
| Cantarini et al 20106, 4 Italy | Retrospective (Case-series) | TRAPS | Etanercept | 7 | Injections-site reactions (2 patients). No SAEs observed. |
| Gattorno et al 2008, 79 Italy | Prospective | TRAPS | Anakinra | 5 | Injections-site reactions (all 5 patients). No SAEs observed. |
| Drewe et al 2003, 77 U.K. | Prospective (Case-series) | TRAPS | Etanercept | 7 | Injection-site reactions (1 patient), upper RTI (1 patient). No SAEs or hospital admissions reported. |
†Studies published as conference abstracts only.
AE, adverse event; CAPS, cryopyrin-associated periodic syndromes; CINCA, chronic infantile neurological cutaneous and articular syndrome; FCAS, familial cold autoinflammatory syndrome; HIDS, hyperimmunoglobulin D syndrome; MKD, mevalonate kinase deficiency; MWS, Muckle–Wells syndrome; NOMID, neonatal-onset multisystem inflammatory disease; RTI, respiratory tract infection; SAE, serious AE; TRAPS, TNF receptor-associated periodic syndrome; UTI, urinary tract infection.
N is number of patients who received a particular treatment.
Treatment switch
Information on switching between anti-IL-1 treatments was provided in 13 studies (11 studies with CAPS patients and 3 studies with HIDS/MKD). In CAPS studies, patients were switched from anakinra to canakinumab in 9 of the 11 studies, 34 36 38 45 57 59 65 67 86 and from canakinumab to anakinra in three studies. 39 41 45 The most common reasons patients switched from anakinra to canakinumab were insufficient response, inconvenience of daily injections, local reactions to anakinra and patient/parent preference (table 4). In three studies in which patients switched from canakinumab to anakinra, the main reasons included inadequate response, 45 and AE. 41 One patient was initially treated with canakinumab but changed to anakinra during pregnancy, and then restarted canakinumab following a successful pregnancy. 45
Table 4.
Studies reporting switch between anti-IL-1 treatments
| Study | Treatment switch | Patient | N | Reasons for switching |
|---|---|---|---|---|
| Eroglu et al 2016 36 | Anakinra to canakinumab | CAPS | 3 | Local reactions at injection site (n=2); pain at injection site and non-compliance (n=1) |
| Parker et al 2016 45 | Anakinra to canakinumab | CAPS | 13 | Due to canakinumab availability |
| Houx et al 2015 38 | Anakinra to canakinumab | CAPS | 48 | Personal convenience |
| Anton et al 2015 34 | Anakinra to canakinumab | CAPS | 5 | More convenient administration (n=4); severe local reactions to anakinra (n=1) |
| Rossi-Semerano et al 2015 86 | Anakinra to canakinumab | CAPS | 3 | Inefficacy or loss of efficacy, AE, persistent remission, or patient request |
| Russo et al 2014 65 | Anakinra to canakinumab | CAPS | 5 | Inadequate control of disease activity (n=5); poor compliance with daily injection of anakinra (n=3); patien/parent preference (n=2) |
| Kuemmerle-Deschner et al 2013 59 | Anakinra to canakinumab | CAPS (MWS) | 10 | Treatment failure (n=3); patient/parent preference (n=7) |
| Wittkowski et al 2011 67 | Anakinra to canakinumab | CAPS | 10 | Lack of efficacy or parent preference (n=10) |
| Kuemmerle-Deschner et al 2011 57 | Anakinra to canakinumab | CAPS (MWS) | 6 | Inconvenience of daily injection and secondary treatment failure (n=6) |
| Kone-Paut et al 2017 39 | Canakinumab to anakinra | CAPS (CINCA/NOMID) | 1 | Reason not reported |
| Parker et al 2016 45 | Canakinumab to anakinra | CAPS | 5 | One patient was initially treated with canakinumab but changed to anakinra during pregnancy, and then restarted canakinumab following a successful pregnancy. Incomplete response (n=3); for better response (n=1) |
| Lane et al 2015 41 | Canakinumab to anakinra | CAPS (CINCA/NOMID and MWS) | 2 | AEs |
| Ter Haar et al 2016 93 | Anakinra to canakinumab | HIDS/MKD | 2 | Inadequate efficacy (n=2) |
| Rossi-Semerano et al 2015 86 | Anakinra to canakinumab | HIDS/MKD | 4 | Inefficacy or loss of efficacy, AE or patient request |
| Galeotti et al 2012 78 | Anakinra to canakinumab | HIDS/MKD | 4 | More convenient dosing schedule and to avoid injection site reaction (n=4) |
N is the total number of patients who were switched from one treatment to another. n is the number of patients who reported the particular reason for switching.
AEs, adverse events; CAPS, cryopyrin-associated periodic syndromes; CINCA, chronic infantile neurological cutaneous and articular syndrome; HIDS, hyperimmunoglobulin D syndrome; MKD, mevalonate kinase deficiency; MWS, Muckle–Wells syndrome; NOMID, neonatal-onset multisystem inflammatory disease.
In all three HIDS/MKD studies with switching information, patients were switched from anakinra to canakinumab, with inadequate efficacy, AEs, more convenient dosing schedule of canakinumab, and patient preference being the main causes (table 4). 78 86 93
Quality of studies
All studies published as full-text articles were assessed for quality assessment. There was no or unclear risk of bias in the RCTs included based on Cochrane risk of bias tool. The quality score for observational studies ranged from 3 to 7 stars, with majority of studies having 5 stars on the Newcastle-Ottawa Scale. A higher number of stars indicates a better quality of study (online supplementary tables 5 and 6).
DISCUSSION
This SLR provides a comprehensive evidence for the efficacy, effectiveness and safety of therapies used for CAPS, HIDS/MKD and TRAPS, published in the last two decades. The extensive literature searches conducted allowed collation and comparison of evidence from 72 studies of various treatments, designs and geographies.
The present SLR made several notable observations. First, the strength of evidence on CAPS was stronger versus HIDS/MKD and TRAPS (51 vs 16−18 studies) suggesting that CAPS is better studied in comparison to the other two indications, which could partially be explained by the existence of validated criteria being available for the diagnosis of CAPS. 96 There has been a steady growth in the literature for these indications from 2000 to the present, with rapid growth in 2016 and 2017. Notably, more than half of included studies (44/72 studies) were published in the last 5 years. Fewer studies were performed in the USA compared with Europe (9 vs 38 studies) indicating that more studies are needed in the USA.
Since CAPS, HIDS/MKD and TRAPS are rare conditions and disease manifestations also vary greatly, many aspects of treatments are not standardised for optimal disease control and follow-up. Thus, we observed variability in the assessed outcomes. Although clinical outcomes were almost similar across studies, HRQoL measures varied greatly. HRQoL comprised only generic instruments, and disease-specific instruments in these rare conditions are lacking. We also observed a disparity in the definitions of CR and PR used across RCTs, non-RCTs and observational studies. The definition of CR used in RCTs/non-RCTs mainly included no/minimal disease activity as per physician assessment, no/minimal skin disease and full serological remission. 13 21 24– 27 However, the definitions of CR in observational studies varied widely, including the signs of active disease absent and APRs normalised, 44 66 68 69 86 92 93 physician assessed no/minimal disease activity and normalised APRs, 39 48 63 and good response. 76 82 94
The overall evidence indicated that in patients with HPFs, biological therapies have shown dramatic improvements in the outcomes, with a clear benefit of anti-IL-1 agents across the whole spectrum of CAPS, with patients of any age. Nonetheless, the evidence for HIDS/MKD and TRAPS is limited; anti-IL-1 agents appeared more effective than other biologics. Canakinumab is an approved therapy for CAPS (FDA-approved for FCAS and MWS, and EMA-approved for all subtypes), HIDS/MKD and TRAPS, 8 9 and its benefits were supported by evidence from the RCTs, non-RCT and numerous observational studies. Anakinra is approved for CAPS (CINCA/NOMID by the FDA and all CAPS subtypes by the EMA) and is not approved for HIDS/MKD and TRAPS, although a number of observational studies supported its use in HIDS/MKD and TRAPS. Anti-TNF-α agents (ie, etanercept) also showed some efficacy in patients with HIDS/MKD. 69 93 94 The benefits of etanercept were reported in some TRAPS patients, but its effect might decline over time. Very limited data are available on the efficacy of etanercept (in CAPS), tocilizumab, adalimumab and infliximab in these rare conditions. Of note, in all studies, the dose of anakinra and canakinumab (for CAPS, HIDS and TRAPS) and of etanercept (for TRAPS) did not differ between indications. Thus, the higher efficacy of these therapies observed versus each other or in a particular indication or study was not due to a specific change (eg, higher doses) in dose for that indication in different studies. The evidence on safety from all study designs indicated that canakinumab was well tolerated, with infections being the common AEs. Rilonacept (in CAPS), simvastatin (in HIDS/MKD), anakinra and etanercept (in all patients) were reported to be tolerable, but local ISRs occurred frequently with rilonacept, anakinra and etanercept. Besides this, it is noteworthy to mention that among anti-IL-1 agents, canakinumab appeared to be an acceptable treatment as indicated by the fact that more patients switched from anakinra to canakinumab due to what patients reported as a more convenient dosing schedule, less or no local reactions at injection sites, and high efficacy.
Regarding conventional treatments, a small body of evidence suggested the use of NSAIDs and corticosteroids in providing symptomatic relief during inflammatory attacks in CAPS, HIDS/MKD and TRAPS. Despite simvastatin being efficacious in significantly reducing the mevalonic acid levels in a small RCT, the statins were generally ineffective in HIDS/MKD. There was no evident effect of colchicine in CAPS, HIDS/MKD and TRAPS patients, but limited evidence indicated the effectiveness of colchicine and prednisone in combination.
The overall findings from this review show that only a few RCTs have been conducted in these rare conditions. Anti-IL-1 agents are commonly investigated; however, there is need for further research on the use of TNF-α and IL-6 inhibitors. In the guidelines that have been published on anti-IL-1 agents’ use in these autoinflammatory diseases, the recommendations were based on low-quality evidence and mainly on expert opinion. 11 Thus, well-designed prospective studies are needed to draw consistent conclusions about these biological therapies. Head-to-head comparative RCTs are needed to assess the superiority of therapies. We recommend a disease-specific instrument to measure the impact of these rare conditions on patients’ HRQoL. We also recommend a standard definition of both CR and PR that can be implemented across the studies irrespective of their designs.
There are certain limitations of this SLR. Firstly, only studies published in English were included. This may be considered a source of bias although most scientific articles are published in English. The majority of studies were observational studies which generally lack methodological rigour to make comparisons. 97 Nonetheless, they provide valuable insights on treatment practices and patient characteristics among patients in the real-world setting, and are considered to form a bridge from the results of RCTs to routine clinical practice. 97 Given the rarity of these conditions, only small numbers of patients received biological therapies in the real-world studies. The lack of standard definitions of CR and PR in observational studies limited the direct comparisons between therapies. Lastly, the full-text paper of an international, multicentre, Phase III trial (CLUSTER) 98 demonstrating the efficacy of canakinumab in patients with colchicine-resistant FMF, HIDS/MKD and TRAPS was not published up to the data collection period of this SLR (October 6, 2017). However, the efficacy and safety results for this trial were included in this SLR from multiple articles published as conference abstracts. 17– 20
CONCLUSIONS
This comprehensive review collating evidence from 72 studies of CAPS, HIDS/MKD and TRAPS indicated that canakinumab and anakinra were the most commonly used therapies for CAPS and HIDS/MKD, whereas etanercept, canakinumab and anakinra were the most common for TRAPS. The published evidence indicated the benefits of canakinumab and anakinra in CAPS, HIDS/MKD and TRAPS, rilonacept in CAPS, and etanercept in TRAPS. These therapies were reported to be well tolerated. This review recommends further research on TNF-α and IL-6 inhibitors; well-designed prospective studies and/or head-to-head comparison RCTs to enable comparisons; a disease-specific HRQoL instrument; and standard definitions of CR and PR that are implementable across all study designs.
Key messages.
What is already known about this subject?
Several biological and conventional therapies are used in the treatment of rare autoinflammatory diseases such as cryopyrin-associated periodic syndromes (CAPS), hyperimmunoglobulin D syndrome (HIDS)/mevalonate kinase deficiency (MKD) and TNF receptor-associated periodic syndrome (TRAPS). The systematic reviews reporting the outcomes of these therapies are, however, lacking.
What does this study add?
This review summarising evidence based on 72 studies reveals that interleukin (IL)-1 inhibitors canakinumab and anakinra are the most commonly used therapies for CAPS and HIDS/MKD, while etanercept, canakinumab and anakinra are the most common for TRAPS.
Evidence suggests the benefits of canakinumab and anakinra in CAPS, HIDS/MKD and TRAPS, rilonacept in CAPS, and etanercept in TRAPS. Safety findings indicate that these therapies are well tolerated.
How might this impact on clinical practice?
Evidence on the use of TNF-α (eg, adalimumab, infliximab) and IL-6 inhibitors (eg, tocilizumab) is very limited and further research is warranted.
Further research should focus on well-designed prospective studies and/or head-to-head comparison trials to enable comparisons across therapies; disease-specific instruments to measure quality of life; and standard definitions of complete and partial responses to assess the clinical outcomes.
What does this study add?
This review summarising evidence based on 72 studies reveals that interleukin (IL)-1 inhibitors canakinumab and anakinra are the most commonly used therapies for CAPS and HIDS/MKD, while etanercept, canakinumab and anakinra are the most common for TRAPS.
Evidence suggests the benefits of canakinumab and anakinra in CAPS, HIDS/MKD and TRAPS, rilonacept in CAPS, and etanercept in TRAPS. Safety findings indicate that these therapies are well tolerated.
How might this impact on clinical practice?
Evidence on the use of TNF-α (eg, adalimumab, infliximab) and IL-6 inhibitors (eg, tocilizumab) is very limited and further research is warranted.
Further research should focus on well-designed prospective studies and/or head-to-head comparison trials to enable comparisons across therapies; disease-specific instruments to measure quality of life; and standard definitions of complete and partial responses to assess the clinical outcomes.
Footnotes
Contributors: Conception and design: JBK-D, PH, KGL, RG, SR, ATG. Generation of data: RG, SR, ATG. Analysis and interpretation of data: PH, RG, SR, ATG. Drafting of the manuscript or revising it critically for important intellectual content: JBK-D, PH, KGL, RG, SR, ATG. Final approval of the submitted manuscript: JBK-D, PH, KGL, RG, SR, ATG. Some part of the work reported in this manuscript has been previously presented at the 2018 Annual Congress of the American College of Rheumatology and published as a conference abstract (Abstract number 1412).
Funding: This work was supported by Novartis Pharmaceuticals Corporation, USA.
Competing interests: JBK-D is an employee of University of Tuebingen, Germany, and received consultants/speakers fees from Novartis and SOBI pharmaceuticals and grant support from SOBI and Novartis. RG and ATG are employees of Novartis healthcare Pvt. Ltd., India. PH is an employee of Novartis Pharmaceuticals Corporation, USA. SR was an employee of Novartis at the time of conduct of this study and currently is an independent freelance consultant. KGL was an employee of Novartis at the time of conduct of this study and currently is an employee at Glenmark Pharmaceuticals, USA.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All data relevant to the study are included in the article or uploaded as supplementary information.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
REFERENCES
- 1. Lachmann HJ. Periodic fever syndromes. Best Pract Res Clin Rheumatol 2017;31:596–609. [DOI] [PubMed] [Google Scholar]
- 2. Marzano AV, Damiani G, Genovese G, et al. A dermatologic perspective on autoinflammatory diseases. Clin Exp Rheumatol 2018;36:32–8. [PubMed] [Google Scholar]
- 3. Zhang S. Natural history of mevalonate kinase deficiency: a literature review. Pediatr Rheumatol 2016;14:30. 10.1186/s12969-016-0091-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van der Burgh R, Ter Haar NM, Boes ML, et al. Mevalonate kinase deficiency, a metabolic autoinflammatory disease. Clin Immunol 2013;147:197–206. [DOI] [PubMed] [Google Scholar]
- 5. Lachmann HJ, Papa R, Gerhold K, et al. The phenotype of TNF receptor-associated autoinflammatory syndrome (TRAPS) at presentation: a series of 158 cases from the Eurofever/EUROTRAPS international registry. Ann Rheum Dis 2014;73:2160–7. 10.1136/annrheumdis-2013-204184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. U.S. Food and Drug Administration . Kineret (anakinra) full prescribing information . 2018. [Google Scholar]
- 7. European Medicines Agency . Kineret (anakinra) European public assessment reports summary . 2018. [Google Scholar]
- 8. U.S. Food and Drug Administration . Ilaris (canakinumab) full prescribing information . 2016. [Google Scholar]
- 9. European Medicines Agency . Ilaris (canakinumab) European public assessment reports summary . 2017. [Google Scholar]
- 10. U.S. Food and Drug Administration . Arcalyst (rilonacept) full prescribing information . 2008. [Google Scholar]
- 11. Ter Haar NM, Oswald M, Jeyaratnam J, et al. Recommendations for the management of autoinflammatory diseases. Ann Rheum Dis 2015;74:1636–44. 10.1136/annrheumdis-2015-207546 [DOI] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9, W64. [DOI] [PubMed] [Google Scholar]
- 13. Lachmann HJ, Kone-Paut I, Kuemmerle-Deschner JB, et al. Use of canakinumab in the cryopyrin-associated periodic syndrome. N Engl J Med 2009;360:2416–25. 10.1056/NEJMoa0810787 [DOI] [PubMed] [Google Scholar]
- 14. Kone-Paut I, Lachmann HJ, Kuemmerle-Deschner JB, et al. Sustained remission of symptoms and improved health-related quality of life in patients with cryopyrin-associated periodic syndrome treated with canakinumab: results of a double-blind placebo-controlled randomized withdrawal study. Arthritis Res Ther 2011;13:R202. 10.1186/ar3535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hoffman HM, Throne ML, Amar NJ, et al. Efficacy and safety of rilonacept (interleukin-1 Trap) in patients with cryopyrin-associated periodic syndromes: results from two sequential placebo-controlled studies. Arthritis Rheum 2008;58:2443–52. 10.1002/art.23687 [DOI] [PubMed] [Google Scholar]
- 16. Hoffman HM, Throne ML, Amar NJ, et al. Long-term efficacy and safety profile of rilonacept in the treatment of cryopyrin-associated periodic syndromes: results of a 72-week open-label extension study. Clin Ther 2012;34:2091–103. 10.1016/j.clinthera.2012.09.009 [DOI] [PubMed] [Google Scholar]
- 17. De Benedetti F, Anton J, Gattorno M, et al. A phase III pivotal um1brella trial of canakinumab in patients with autoinflammatory periodic fever syndromes (colchicine resistant FMF, HIDS/MKD and TRAPS). Ann Rheumatic Dis 2016;75:615–6. [Google Scholar]
- 18. Kone-Paut I, Hofer M, Benseler S, et al. Improvement of disease activity in patients with colchicine-resistant FMF, HIDS/MKD and traps assessed by autoinflammatory disease activity index (AIDAI): results from the cluster trial. Pediatr Rheumatol 2017;15:P176. [Google Scholar]
- 19. De Benedetti F, Frenkel J, Calvo I, et al. Efficacy and safety of canakinumab in patients with colchicine-resistant familial mediterranean fever, hyper-immunoglobulin D syndrome/mevalonate kinase deficiency and TNF receptor-associated periodic syndrome: 40 week results from the pivotal phase 3 umbrella cluster trial. Arthritis Rheum 2016;68:4369–71. [Google Scholar]
- 20. Lachmann H, Simon A, Anton J, et al. Canakinumab improves patient reported outcomes in patients with periodic fever syndromes. Ann Rheum Dis 2016;75:616.1–616. 10.1136/annrheumdis-2016-eular.3823 [DOI] [Google Scholar]
- 21. Imagawa T, Nishikomori R, Takada H, et al. Safety and efficacy of canakinumab in Japanese patients with phenotypes of cryopyrin-associated periodic syndrome as established in the first open-label, phase-3 pivotal study (24-week results). Clin Exp Rheumatol 2013;31:302–9. [PubMed] [Google Scholar]
- 22. Yokota S, Imagawa T, Nishikomori R, et al. Long-term safety and efficacy of canakinumab in cryopyrin-associated periodic syndrome: results from an open-label, phase III pivotal study in Japanese patients. Clin Exp Rheumatol 2017;35:19–26. [PubMed] [Google Scholar]
- 23. Sibley CH, Chioato A, Felix S, et al. A 24-month open-label study of canakinumab in neonatal-onset multisystem inflammatory disease. Ann Rheum Dis 2015;74:1714–19. 10.1136/annrheumdis-2013-204877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kuemmerle-Deschner JB, Hachulla E, Cartwright R, et al. Two-year results from an open-label, multicentre, phase III study evaluating the safety and efficacy of canakinumab in patients with cryopyrin-associated periodic syndrome across different severity phenotypes. Ann Rheum Dis 2011;70:2095–102. 10.1136/ard.2011.152728 [DOI] [PubMed] [Google Scholar]
- 25. Kuemmerle-Deschner JB, Ramos E, Blank N, et al. Canakinumab (ACZ885, a fully human IgG1 anti-IL-1beta mAb) induces sustained remission in pediatric patients with cryopyrin-associated periodic syndrome (CAPS). Arthritis Res Ther 2011;13:R34. 10.1186/ar3266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arostegui JI, Anton J, Calvo I, et al. Open-label, phase II study to assess the efficacy and safety of canakinumab treatment in active hyperimmunoglobulinemia D with periodic fever syndrome. Arthritis Rheumatol 2017;69:1679–88. 10.1002/art.40146 [DOI] [PubMed] [Google Scholar]
- 27. Gattorno M, Obici L, Cattalini M, et al. Canakinumab treatment for patients with active recurrent or chronic TNF receptor-associated periodic syndrome (TRAPS): an open-label, phase II study. Ann Rheum Dis 2017;76:173–8. 10.1136/annrheumdis-2015-209031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goldbach-Mansky R, Shroff SD, Wilson M, et al. A pilot study to evaluate the safety and efficacy of the long-acting interleukin-1 inhibitor rilonacept (interleukin-1 Trap) in patients with familial cold autoinflammatory syndrome. Arthritis Rheum 2008;58:2432–42. 10.1002/art.23620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goldbach-Mansky R, Dailey NJ, Canna SW, et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N Engl J Med 2006;355:581–92. 10.1056/NEJMoa055137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sibley CH, Plass N, Snow J, et al. Sustained response and prevention of damage progression in patients with neonatal-onset multisystem inflammatory disease treated with anakinra: a cohort study to determine three- and five-year outcomes. Arthritis Rheum 2012;64:2375–86. 10.1002/art.34409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kullenberg T, Lofqvist M, Leinonen M, et al. Long-term safety profile of anakinra in patients with severe cryopyrin-associated periodic syndromes. Rheumatology 2016;55:1499–506. 10.1093/rheumatology/kew208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 34. Anton J, Calvo I, Fernandez-Martin J, et al. Efficacy and safety of canakinumab in cryopyrin-associated periodic syndromes: results from a Spanish cohort. Clin Exp Rheumatol 2015;33:S67–71. [PubMed] [Google Scholar]
- 35. Brogan P, Hofer M, Kuemmerle-Deschner JB, et al. Efficacy and safety of canakinumab in patients with CAPS aged <24 months: results from an open-label, multicenter, Phase III trial. Arthritis Rheumatol 2015; 67: Abstract number 248. [Google Scholar]
- 36. Eroglu FK, Kasapcopur O, Besbas N, et al. Genetic and clinical features of cryopyrin-associated periodic syndromes in Turkish children. Clin Exp Rheumatol 2016;34:S115–S120. [PubMed] [Google Scholar]
- 37. Hoffman HM, Kuemmerle-Deschner JB, Hawkins PN, et al. Safety and efficacy of long-term canakinumab therapy in patients with caps: final results from beta-confident registry. Arthritis Rheumatol 2016;68:325–6. [Google Scholar]
- 38. Houx L, Hachulla E, Kone-Paut I, et al. Musculoskeletal symptoms in patients with cryopyrin-associated periodic syndromes: a large database study. Arthritis Rheumatol 2015;67:3027–36. 10.1002/art.39292 [DOI] [PubMed] [Google Scholar]
- 39. Kone-Paut I, Quartier P, Fain O, et al. Real-world experience and impact of canakinumab in cryopyrin-associated periodic syndrome: results from a French observational study. Arthritis Care Res 2017;69:903–11. 10.1002/acr.23083 [DOI] [PubMed] [Google Scholar]
- 40. Kuemmerle-Deschner JB, Hofer F, Endres T, et al. Real-life effectiveness of canakinumab in cryopyrin-associated periodic syndrome. Rheumatol 2016;55:689–96. 10.1093/rheumatology/kev416 [DOI] [PubMed] [Google Scholar]
- 41. Lane T, Williams RG, Rowczenio DM, et al. A decade of anti-IL-1 therapy in CAPS-a spectrum of efficacy in this spectrum of diseases. Pediatr Rheumatol 2015;13:O65. 10.1186/1546-0096-13-S1-O65 [DOI] [Google Scholar]
- 42. Mamoudjy N, Maurey H, Marie I, et al. Neurological outcome of patients with cryopyrin-associated periodic syndrome (CAPS). Orphanet J Rare Dis 2017;12:33. 10.1186/s13023-017-0589-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mehr S, Allen R, Boros C, et al. Cryopyrin-associated periodic syndrome in Australian children and adults: epidemiological, clinical and treatment characteristics. J Paediatr Child Health 2016;52:889–95. 10.1111/jpc.13270 [DOI] [PubMed] [Google Scholar]
- 44. Naselli A, Penco F, Cantarini L, et al. Clinical characteristics of patients carrying the Q703K variant of the NLRP3 gene: a 10-year multicentric national study. J Rheumatol 2016;43:1093–100. 10.3899/jrheum.150962 [DOI] [PubMed] [Google Scholar]
- 45. Parker T, Keddie S, Kidd D, et al. Neurology of the cryopyrin-associated periodic fever syndrome. Eur J Neurol 2016;23:1145–51. 10.1111/ene.12965 [DOI] [PubMed] [Google Scholar]
- 46. Saperia C, Kobric D, Barron C, et al. Autoinflammatory disease: presenting as urticaria. J Allergy Clin Immunol 2017;139:AB272. [Google Scholar]
- 47. Sobolewska B, Angermair E, Deuter C, et al. NLRP3 A439V mutation in a large family with cryopyrin-associated periodic syndrome: description of ophthalmologic symptoms in correlation with other organ symptoms. J Rheumatol 2016;43:1101–6. 10.3899/jrheum.150681 [DOI] [PubMed] [Google Scholar]
- 48. Caorsi R, Lepore L, Zulian F, et al. The schedule of administration of canakinumab in cryopyrin associated periodic syndrome is driven by the phenotype severity rather than the age. Arthritis Res Ther 2013;15:R33. 10.1186/ar4184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Caroli F, Pontillo A, D’Osualdo A, et al. Clinical and genetic characterization of Italian patients affected by CINCA syndrome. Rheumatol 2007;46:473–8. 10.1093/rheumatology/kel269 [DOI] [PubMed] [Google Scholar]
- 50. Chang Z, Spong CY, Jesus AA, et al. Anakinra use during pregnancy in patients with cryopyrin-associated periodic syndromes (CAPS). Arthritis Rheumatol 2014;66:3227–32. 10.1002/art.38811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gattorno M, Tassi S, Carta S, et al. Pattern of interleukin-1beta secretion in response to lipopolysaccharide and ATP before and after interleukin-1 blockade in patients with CIAS1 mutations. Arthritis Rheum 2007;56:3138–48. 10.1002/art.22842 [DOI] [PubMed] [Google Scholar]
- 52. Guerrero LJ, MacIas IC, Fernandez MDA, et al. Study of the use of canakinumab in Muckle–Wells syndrome in a tertiary hospital. Eur J Hosp Pharm 2017;24:A149. 10.1136/ejhpharm-2017-000640.327 [DOI] [Google Scholar]
- 53. Hoffman HM, Rosengren S, Boyle DL, et al. Prevention of cold-associated acute inflammation in familial cold autoinflammatory syndrome by interleukin-1 receptor antagonist. Lancet 2004;364:1779–85. 10.1016/S0140-6736(04)17401-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kilic SS, Cekic S, Arostegui J. CNS manifestations of patients with Muckle–Wells syndrome. Arthritis Rheumatol 2016;68:328–9. [Google Scholar]
- 55. Kuemmerle-Deschner JB, Koitschev A, Tyrrell PN, et al. Early detection of sensorineural hearing loss in Muckle-Wells-syndrome. Pediatr Rheumatol 2015;13:43. 10.1186/s12969-015-0041-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kuemmerle-Deschner JB, Koitschev A, Ummenhofer K, et al. Hearing loss in Muckle-Wells syndrome. Arthritis Rheum 2013;65:824–31. 10.1002/art.37810 [DOI] [PubMed] [Google Scholar]
- 57. Kuemmerle-Deschner JB, Lohse P, Koetter I, et al. NLRP3 E311K mutation in a large family with Muckle-Wells syndrome: description of a heterogeneous phenotype and response to treatment. Arthritis Res Ther 2011;13:R196. 10.1186/ar3526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kuemmerle-Deschner JB, Tyrrell PN, Koetter I, et al. Efficacy and safety of anakinra therapy in pediatric and adult patients with the autoinflammatory Muckle-Wells syndrome. Arthritis Rheum 2011;63:840–9. 10.1002/art.30149 [DOI] [PubMed] [Google Scholar]
- 59. Kuemmerle-Deschner JB, Wittkowski H, Tyrrell PN, et al. Treatment of Muckle-Wells syndrome: analysis of two IL-1-blocking regimens. Arthritis Res Ther 2013;15:R64. 10.1186/ar4237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lepore L, Paloni G, Caorsi R, et al. Follow-up and quality of life of patients with cryopyrin-associated periodic syndromes treated with Anakinra. J Pediatr 2010;157:310–15.e1. 10.1016/j.jpeds.2010.02.040 [DOI] [PubMed] [Google Scholar]
- 61. Moreira Navarrete V, Toyos Saenz De Miera FJ, Vargas Lebron C, et al. Canakinumab in clinical practice. Effectiveness in a cryopyrin-associated periodic syndrome single center cohort. Ann Rheum Dis 2014;73:1110.2–1110. 10.1136/annrheumdis-2014-eular.5712 [DOI] [Google Scholar]
- 62. Neven B, Marvillet I, Terrada C, et al. Long-term efficacy of the interleukin-1 receptor antagonist anakinra in ten patients with neonatal-onset multisystem inflammatory disease/chronic infantile neurologic, cutaneous, articular syndrome. Arthritis Rheum 2010;62:258–67. 10.1002/art.25057 [DOI] [PubMed] [Google Scholar]
- 63. Pastore S, Paloni G, Caorsi R, et al. Serum amyloid protein A concentration in cryopyrin-associated periodic syndromes patients treated with interleukin-1 beta antagonist. Clin Exp Rheumatol 2014;32:S63–6. [PubMed] [Google Scholar]
- 64. Ross JB, Finlayson LA, Klotz PJ, et al. Use of anakinra (Kineret) in the treatment of familial cold autoinflammatory syndrome with a 16-month follow-up. J Cutaneous Med Sur 2008;12:8–16. 10.2310/7750.2008.07050 [DOI] [PubMed] [Google Scholar]
- 65. Russo RA, Melo-Gomes S, Lachmann HJ, et al. Efficacy and safety of canakinumab therapy in paediatric patients with cryopyrin-associated periodic syndrome: a single-centre, real-world experience. Rheumatology 2014;53:665–70. 10.1093/rheumatology/ket415 [DOI] [PubMed] [Google Scholar]
- 66. Saperia C, McAuley P, Raffaghello J, et al. Clinical experiences with canakinumab as a treatment for autoinflammatory disorders. Pediatr Rheumatol 2015;13:P202. 10.1186/1546-0096-13-S1-P202 [DOI] [Google Scholar]
- 67. Wittkowski H, Kuemmerle-Deschner JB, Austermann J, et al. MRP8 and MRP14, phagocyte-specific danger signals, are sensitive biomarkers of disease activity in cryopyrin-associated periodic syndromes. Ann Rheum Dis 2011;70:2075–81. 10.1136/ard.2011.152496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Alenazi A, Al Sonbul A, Al Jumaah S, et al. A retrospective review of autoinflammatory diseases in Saudi children at a rheumatology clinic. Ann Saudi Med 2012;32:43–8. 10.5144/0256-4947.2012.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bader-Meunier B, Florkin B, Sibilia J, et al. Mevalonate kinase deficiency: a survey of 50 patients. Pediatrics 2011;128:e152–9 10.1542/peds.2010-3639. [DOI] [PubMed] [Google Scholar]
- 70. Bodar EJ, Kuijk LM, Drenth JP, et al. On-demand anakinra treatment is effective in mevalonate kinase deficiency. Ann Rheum Dis 2011;70:2155–8. 10.1136/ard.2011.149922 [DOI] [PubMed] [Google Scholar]
- 71. Bulua AC, Mogul DB, Aksentijevich I, et al. Efficacy of etanercept in the tumor necrosis factor receptor-associated periodic syndrome: a prospective, open-label, dose-escalation study. Arthritis Rheum 2012;64:908–13. 10.1002/art.33416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cakan M, Ayaz NA, Karadag JG, et al. Canakinumab experience from a pediatric rheumatology center in Istanbul. Pediatr Rheumatol 2017;15:B25. [Google Scholar]
- 73. Cantarini L, Lucherini OM, Brucato A, et al. Clues to detect tumor necrosis factor receptor-associated periodic syndrome (TRAPS) among patients with idiopathic recurrent acute pericarditis: results of a multicentre study. Clin Res Cardiol 2012;101:525–31. 10.1007/s00392-012-0422-8 [DOI] [PubMed] [Google Scholar]
- 74. Cantarini L, Rigante D, Lucherini OM, et al. Role of etanercept in the treatment of tumor necrosis factor receptor-associated periodic syndrome: personal experience and review of the literature. Int J Immunopathol Pharmacol 2010;23:701–7. 10.1177/039463201002300303 [DOI] [PubMed] [Google Scholar]
- 75. Cervinski M, Albert D. Periodic fever syndromes in an academic medical center. Arthritis Rheum 2014;66:S541. [Google Scholar]
- 76. Chandrakasan S, Chiwane S, Adams M, et al. Clinical and genetic profile of children with periodic fever syndromes from a single medical center in South East Michigan. J Clin Immunol 2014;34:104–13. 10.1007/s10875-013-9960-8 [DOI] [PubMed] [Google Scholar]
- 77. Drewe E, McDermott EM, Powell PT, et al. Prospective study of anti-tumour necrosis factor receptor superfamily 1B fusion protein, and case study of anti-tumour necrosis factor receptor superfamily 1A fusion protein, in tumour necrosis factor receptor associated periodic syndrome (TRAPS): clinical and laboratory findings in a series of seven patients. Rheumatology 2003;42:235–9. 10.1093/rheumatology/keg070 [DOI] [PubMed] [Google Scholar]
- 78. Galeotti C, Meinzer U, Quartier P, et al. Efficacy of interleukin-1-targeting drugs in mevalonate kinase deficiency. Rheumatology 2012;51:1855–9. 10.1093/rheumatology/kes097 [DOI] [PubMed] [Google Scholar]
- 79. Gattorno M, Pelagatti MA, Meini A, et al. Persistent efficacy of anakinra in patients with tumor necrosis factor receptor-associated periodic syndrome. Arthritis Rheum 2008;58:1516–20. 10.1002/art.23475 [DOI] [PubMed] [Google Scholar]
- 80. Gulez N, Sozeri B, Gulez P. Our experience of anti-interleukin 1 therapy. Pediatr Rheumatol 2015;13:P210. 10.1186/1546-0096-13-S1-P210 [DOI] [Google Scholar]
- 81. Havlicekova Z, Michnova Z, Hrubiskova K, et al. Autoinflammatory diseases in differential diagnosis of recurrent abdominal pain in the praxis of paediatric gastroenterologist in Central Europe. J Pediatr Gastroenterol Nutr 2017;64:309. [Google Scholar]
- 82. Hernandez-Rodriguez J, Ruiz-Ortiz E, Tome A, et al. Clinical and genetic characterization of the autoinflammatory diseases diagnosed in an adult reference center. Autoimmun Rev 2016;15:9–15. 10.1016/j.autrev.2015.08.008 [DOI] [PubMed] [Google Scholar]
- 83. Kozlova A, Kuzmenko N, Zimin S, et al. Characterization of a group of 6 patients with mevalonate kinase deficiency: symptoms and treatment with IL-1 inhibitors. Ann Rheum Dis 2016;75:395. 10.1136/annrheumdis-2016-eular.1677 [DOI] [Google Scholar]
- 84. Leslie KS, Lachmann HJ, Bruning E, et al. Phenotype, genotype, and sustained response to anakinra in 22 patients with autoinflammatory disease associated with CIAS-1/NALP3 mutations. Archives Dermatol 2006;142:1591–7. 10.1001/archderm.142.12.1591 [DOI] [PubMed] [Google Scholar]
- 85. Ozen S, Kuemmerle-Deschner JB, Cimaz R, et al. International retrospective chart review of treatment patterns in severe familial Mediterranean fever, tumor necrosis factor receptor-associated periodic syndrome, and mevalonate kinase deficiency/hyperimmunoglobulinemia D syndrome. Arthritis Care Res 2017;69:578–86. 10.1002/acr.23120.Epub 2017 Mar 3 [DOI] [PubMed] [Google Scholar]
- 86. Rossi-Semerano L, Fautrel B, Wendling D, et al. Tolerance and efficacy of off-label anti-interleukin-1 treatments in France: a nationwide survey. Orphanet J Rare Dis 2015;10:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Salugina SO, Fedorov ES, Kuzmina NN, et al. Monogenic autoinflammatory diseases in the practice of a rheumatologist in Russia. Pediatr Rheumatol 2017;15:P202. 10.1093/rheumatology/keu170 [DOI] [Google Scholar]
- 88. Salugina SO, Kaleda M, Fedorov E, et al. Experience with interleukin-1 inhibitor canakinumab in auto-inflammatory diseases and systemic juvenile idiopathic arthritis in clinical practice. Ann Rheum Dis 2016;75:622. 10.1136/annrheumdis-2016-eular.3889 [DOI] [Google Scholar]
- 89. Simon A, Drewe E, van der Meer JW, et al. Simvastatin treatment for inflammatory attacks of the hyperimmunoglobulinemia D and periodic fever syndrome. Clin Pharmacol Ther 2004;75:476–83. 10.1016/j.clpt.2004.01.012 [DOI] [PubMed] [Google Scholar]
- 90. Stoffels M, Jongekrijg J, Remijn T, et al. TLR2/TLR4-dependent exaggerated cytokine production in hyperimmunoglobulinaemia D and periodic fever syndrome. Rheumatology 2015;54:363–8. 10.1093/rheumatology/keu341 [DOI] [PubMed] [Google Scholar]
- 91. Stojanov S, Dejaco C, Lohse P, et al. Clinical and functional characterisation of a novel TNFRSF1A c.605T>A/V173D cleavage site mutation associated with tumour necrosis factor receptor-associated periodic fever syndrome (TRAPS), cardiovascular complications and excellent response to etanercept treatment. Ann Rheumatic Dis 2008;67:1292–8. 10.1136/ard.2007.079376 [DOI] [PubMed] [Google Scholar]
- 92. Ter Haar N, Lachmann H, Ozen S, et al. Treatment of autoinflammatory diseases: results from the Eurofever registry and a literature review. Ann Rheum Dis 2013;72:678–85. 10.1136/annrheumdis-2011-201268 [DOI] [PubMed] [Google Scholar]
- 93. Ter Haar NM, Jeyaratnam J, Lachmann HJ, et al. The phenotype and genotype of mevalonate kinase deficiency: a series of 114 cases from the Eurofever registry. Arthritis Rheumatol 2016;68:2795–805. 10.1002/art.39763 [DOI] [PubMed] [Google Scholar]
- 94. van der Hilst JC, Bodar EJ, Barron KS, et al. Long-term follow-up, clinical features, and quality of life in a series of 103 patients with hyperimmunoglobulinemia D syndrome. Medicine 2008;87:301–10. 10.1097/MD.0b013e318190cfb7 [DOI] [PubMed] [Google Scholar]
- 95. Brogan P, Hofer M, Kuemmerle-Deschner JB, et al. Efficacy and safety of canakinumab in patients aged one to six years with cryopyrin-associated periodic syndromes: results of an open-label, phase III extension study. Arthritis Rheumatol 2016;68:3106–8. [Google Scholar]
- 96. Kuemmerle-Deschner JB, Ozen S, Tyrrell PN, et al. Diagnostic criteria for cryopyrin-associated periodic syndrome (CAPS). Ann Rheum Dis 2017;76:942–7. 10.1136/annrheumdis-2016-209686 [DOI] [PubMed] [Google Scholar]
- 97. Cohen AT, Goto S, Schreiber K, et al. Why do we need observational studies of everyday patients in the real-life setting? Eur Heart J Suppl 2015;17:D2–D8. 10.1093/eurheartj/suv035 [DOI] [Google Scholar]
- 98. De Benedetti F, Gattorno M, Anton J, et al. Canakinumab for the treatment of autoinflammatory recurrent fever syndromes. N Engl J Med 2018;378:1908–19. 10.1056/NEJMoa1706314 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2020-001227supp001.pdf (1.3MB, pdf)