Abstract
Objective
To assess satisfaction with the effectiveness and tolerability of treatments in patients with rheumatoid arthritis (RA).
Methods
Patients from the RABBIT register, starting a biological (b) or targeted synthetic (ts) disease-modifying antirheumatic drug (DMARD), or a conventional synthetic (cs)DMARD treatment after ≥1 csDMARD failure, were included. Treatment satisfaction was measured after 1 year of treatment in four categories and binarised for analysis. Logistic regression models were performed to calculate ORs for factors associated with treatment satisfaction.
Results
Data of 10 646 patients (74% women, mean 58 years) were analysed. At baseline, 55% of the patients were satisfied with the efficacy and 68% with the tolerability of their previously given treatments. After 1 year, 85% of the patients were satisfied with treatment effectiveness and 90% with tolerability. Baseline satisfaction (OR 2.98, 95% CI 2.58 to 3.44), seropositivity (OR 1.36, 95% CI 1.17 to 1.57), reduction of DAS28 (OR 1.38, 95% CI 1.31 to 1.46) and pain (OR 1.26, 95% CI 1.22 to 1.31), and the improvement of physical capacity (OR 1.22, 95% CI 1.17 to 1.29) were positively associated with treatment satisfaction at follow-up while glucocorticoids (GCs) >5 mg/day, depression, fibromyalgia, obesity, prior bDMARDs and therapy changes were negatively associated. The impact of GC on satisfaction was dose-dependent, becoming strongest for GC >15 mg (OR 0.24, 95% CI 0.16 to 0.34). A 5 mg/day reduction within 12 months was positively associated with satisfaction regarding efficacy (OR 1.19, 95% CI 1.11 to 1.27) and tolerability (OR 1.11, 95% CI 1.03 to 1.21).
Conclusion
Most patients were satisfied with their treatment’s effectiveness and tolerability after 1 year of treatment. Tapering GCs was positively associated with the improvement of patients’ satisfaction.
Keywords: Arthritis, Rheumatoid, Biological Therapy, Patient Reported Outcome Measures
Key messages.
What is already known about this subject?
Mostly qualitative studies have revealed that patients with RA are often highly satisfied with their therapy but reluctant to change therapies due to concerns about side effects.
What does this study add?
In a real-life prospective biological register, satisfaction with treatment is high.
Depression, obesity and fibromyalgia are associated with reduced treatment satisfaction, while seropositivity, reduction of disease activity and pain as well as the improvement of physical capacity are associated with increased treatment satisfaction.
The lower the glucocorticoid dose, the more satisfied the patients are with their treatment.
How might this impact on clinical practice?
Among factors associated with patient satisfaction, physicians have the most influence on GC therapy.
Beyond the concern for treatment satisfaction, patients with the key comorbidities depression, fibromyalgia and obesity may particularly benefit from addressing their limited well-being when discussing treatment options.
INTRODUCTION
Patient satisfaction with their pharmacological therapy is increasingly considered an important outcome in rheumatoid arthritis (RA). Due to the increasing number of highly effective medications, many patients with RA today achieve a significant improvement in symptoms, joint function and quality of life.1 Current treatment recommendations have included a shared decision between patients and physicians as an overarching principle.2 Within the framework of a shared decision-making process between the patient and the rheumatologist, satisfaction has a decisive influence on the course of therapy, contributing to treatment adherence and continuation.3 Conversely, a successful shared decision-making process may also have positive effects on the patient’s treatment satisfaction.4 Side effects and application mode are crucial factors for satisfaction with the tolerability of disease-modifying antirheumatic drugs (DMARDs) and have been investigated for different biological (b)DMARDs.5 6 Evidence from mostly qualitative studies found high patient satisfaction with their RA therapy with reluctance to change therapies due to concerns about side effects.1 Satisfaction with the effectiveness of a therapy, on the other hand, has not yet been investigated frequently. Overall, only very few quantitative studies with large patient numbers exist, which have reported on treatment satisfaction.
For many years, the German biological register RABBIT has been collecting data on the effectiveness and safety of therapy in patients with RA. Patient satisfaction on treatment efficacy and tolerability has been assessed since 2009. The aim of this analysis was to investigate the factors exerting a potential influence on the patient satisfaction with the effectiveness and tolerability of treatments in patients with RA and to quantify the strength of their association.
PATIENTS AND METHODS
Patients
The German biological register RABBIT is a prospective longitudinally followed cohort of patients with RA that are included with a new start of biological, biosimilar or targeted synthetic (ts) DMARDs, or with a conventional synthetic (cs)DMARD treatment after at least one prior csDMARD therapy. They are subsequently observed for a minimum of 5 and up to 10 years, irrespective of treatment changes. At the time of enrolment, after 3 and 6 months and then every 6 months during the time of observation, information is collected from rheumatologists and patients including demographics, clinical status including joint counts, treatment, laboratory tests and patient-reported outcomes. Between January 2009 and April 2019, a total of 12 129 patients have been enrolled in RABBIT. For this analysis, we included 10 646 of the patients who had ≥1 follow-up and ≥12 months of observation time. The patients’ assessment of satisfaction with their therapy after 12 months of observation was analysed as the outcome. All patients provided written informed consent before enrolment. The RABBIT study received approval by the Ethics Committee of Charité—Universitätsmedizin Berlin. This research was conducted in agreement with the Declaration of Helsinki.
Data collection
Age and age at disease onset were measured in years, and smoking was assessed as current, former or never. Disease activity was recorded by the Disease Activity Score in 28 joints-erythrocyte sedimentation rate (DAS28-ESR), using the ESR and further including tender and swollen joint scores and patients global health. Global health, pain, fatigue and sleep disturbances were assessed on numeric rating scales from 0 (no) to 10 (worst outcome). Physical capacity was assessed by the Hannover Functional Ability Questionnaire (0–100) with 100 representing full capacity. Seropositivity was defined as the presence of rheumatoid factor (RF) and/or autoimmune anticitrullinated protein antibodies (ACPA). Comorbidity was recorded by the rheumatologist as present or not. Satisfaction with the applied treatment was measured in four categories (very satisfied, rather satisfied, rather unsatisfied and very unsatisfied).4 Separate questions were used to assess satisfaction with treatment effectiveness and with tolerability. Analogous to other studies, the variables were binarised for analysis.7 8 b/tsDMARD treatments prescribed at the time of enrolment were etanercept, infliximab, adalimumab, certolizumab, golimumab, rituximab, abatacept, tocilizumab, sarilumab, baricitinib and tofacitinib. csDMARDs included methotrexate, leflunomide, sulfasalazine or hydroxychloroquine, among others. Concomitant therapies with glucocorticoids (GCs) were recorded in milligrams per day.
Statistical analysis
Descriptive statistics were used to compare satisfied with dissatisfied patients. To avoid a bias due to missing data (online supplemental table 1), missing values are predicted by regression models. Ten imputations of each missing value were performed employing fully conditional specification,9 logistic regression was performed in each data set and results were combined to calculate overall estimates. Logistic regression combined with multiple imputation of missing values was performed to calculate ORs for factors that might have an influence on treatment satisfaction regarding effectiveness and tolerability after 1 year of treatment. Parameters included were selected by clinical relevance and findings in the literature.4 ORs were considered statistically significant for p-values < 0.05.
rmdopen-2020-001290supp001.pdf (168.5KB, pdf)
Alternative models were calculated using the individual components of the DAS28-ESR (swollen joint count, tender joint count, ESR and patient global assessment) instead of the composite score, as well as the reduction in GC dose within 1 year instead of the absolute GC dose at 6–12 months. As RF and ACPA are clearly correlated (85% of the patients have the same status), we did not include them separately in the models.
RESULTS
Patient characteristics
The data of 10 646 patients from the RABBIT cohort were analysed (mean 57.6 years, 74.3% women). At baseline, 55% of the patients were satisfied, while 45% were unsatisfied with the effectiveness of their previously given therapy. Patients already satisfied at baseline on average had less disease activity, less pain, less tender joints and better physical function than dissatisfied patients. They received GCs less often (53% vs 61%) and with a lower mean GC dose (6.7 vs 7.4 mg/day), and they received less often tumour necrosis factor-alpha inhibitors and other b/tsDMARDs than patients not satisfied at baseline (table 1). Regarding satisfaction with tolerability (68%), less pronounced differences in disease activity were observed between satisfied and dissatisfied patients.
Table 1.
Baseline patient characteristics
| Effectiveness | Tolerability | |||
|---|---|---|---|---|
| Satisfied n=5822 (54.7%) |
Unsatisfied n=4824 (45.3%) |
Satisfied n=7273 (68.3%) |
Unsatisfied n=3373 (31.7%) |
|
| Age, years | 58.3±12.6 | 56.7±12.7 | 57.7±12.8 | 57.3±12.4 |
| Female sex | 4273 (73.4%) | 3639 (75.4%) | 5270 (72.5%) | 2642 (78.3%) |
| Disease duration, years | 9.1 (8.6) | 9 (8.7) | 9.3±8.7 | 8.5±8.5 |
| Seropositivity (RF and/or ACPA positive) | 4244 (72.9%) | 3461 (71.6%) | 5338 (73.4%) | 2358 (69.9%) |
| Current smoker | 1600 (27.5%) | 1369 (28.4%) | 1957 (26.9%) | 839 (24.9%) |
| DAS28-ESR <3.2 | 899 (15.4%) | 361 (7.5%) | 939 (12.9%) | 321 (9.5%) |
| 3.2≤ DAS28-ESR <5.1 | 2961 (50.9%) | 2181 (45.4%) | 3595 (48.4%) | 1558 (46.2%) |
| DAS28-ESR ≥5.1 | 1962 (33.7%) | 2272 (47.1%) | 2739 (37.7%) | 1495 (44.3%) |
| Swollen joint count | 4.7±4.1 | 5.5±4.7 | 5.0±4.3 | 5.2±4.5 |
| Tender joint count | 6.8±6.0 | 8.5±6.9 | 7.3±6.2 | 8.3±6.9 |
| Patient global health assessment | 5.1±2.1 | 6.4±1.9 | 5.5±2.1 | 6.2±1.9 |
| Erythrocyte sedimentation rate (mm) | 27.5±20.5 | 29.2±21.7 | 28.4±21.1 | 28.0±20.8 |
| Pain (0–10 scale) | 5.2±2.3 | 6.2±2.0 | 5.5±2.3 | 6.3±2.1 |
| Percentage of full physical function | 70.7±22.3 | 61.8±22.6 | 68.5±22.7 | 62.7±22.8 |
| Number of previous bDMARDs | 0.4±0.8 | 0.5±0.9 | 0.4±0.9 | 0.4±0.9 |
| Number of previous csDMARDs | 1.9±1.0 | 2.2±1.1 | 2.0±1.1 | 1.2±1.1 |
| Current TNFi therapy | 2320 (39.9%) | 2433 (50.5%) | 3119 (42.9%) | 1634 (48.5%) |
| Current other b/tsDMARD therapy | 1336 (22.9%) | 1256 (26.0%) | 1789 (24.6%) | 803 (23.8%) |
| Glucocorticoids (last 6 months) | 3071 (52.8%) | 2934 (60.8%) | 4031 (55.4%) | 1974 (58.5%) |
| Glucocorticoid dose, mg/day (last 6 months) | 6.7±3.3 | 7.4±3.7 | 6.8±3.3 | 7.5±3.8 |
Numbers are expressed as mean±SD or frequencies (n (%)). Frequencies may be rounded due to multiple imputation of missing values.
ACPA, autoimmune anticitrullinated protein antibodies; bDMARDs, biological disease-modifying antirheumatic drugs; csDMARS; conventional synthetic disease-modifying antirheumatic drugs; DAS28-ESR, Disease Activity Score in 28 joints-erythrocyte sedimentation rate; RF, rheumatoid factor; tsDMARD, targeted synthetic disease-modifying antirheumatic drug; TNFi, tumour necrosis factor-alpha inhibitor.
After 1 year of observation, 85% of the patients were satisfied with the effectiveness and 90% with the tolerability of their treatment. A total of 643 patients (11.1%) who were satisfied at baseline were unsatisfied with efficacy at follow-up, 551 (7.6%) were unsatisfied regarding tolerability. In 2432 patients, ≥1 DMARD therapy was changed during the follow-up. About 35% of the patients still received >5 mg GC/day at 6–12 months. Improvements in clinical outcomes were achieved by the following percentages of patients: 60% DAS28 reduction by ≥1 point, 63% pain reduction by ≥1 point on a 0–10 scale, 28% increase in physical capacity by ≥10 points, 52% fatigue reduction by ≥1 point and 49% sleeping disorder reduction by ≥1 point.
Factors associated with treatment satisfaction
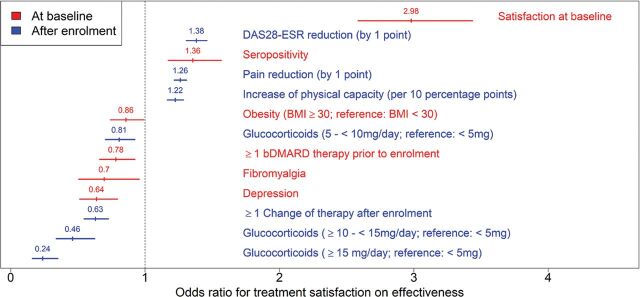
Satisfaction with the prior treatment at baseline had the largest positive association with treatment satisfaction concerning effectiveness after 1 year (OR 2.98, 95% CI 2.58 to 3.44). Further factors positively associated were seropositivity (OR 1.36, 95% CI 1.17 to 1.57), reduction of DAS28-ESR (OR 1.38, 95% CI 1.31 to 1.46) and pain (OR 1.26, 95% CI 1.22 to 1.31) as well as the improvement of physical function at follow-up (OR 1.22, 95% CI 1.17 to 1.29) (figure 1).
Figure 1.
Factors significantly associated with satisfaction with efficacy of treatment after 1 year of treatment. Shown are adjusted ORs with 95% CIs. bDMARD, biological disease-modifying antirheumatic drug; BMI, body mass index; DAS28-ESR, Disease Activity Score in 28 joint-erythrocyte sedimentation rate.
The factor with the greatest negative influence on satisfaction with treatment was a concomitant therapy with >5 mg GC/day (table 1). Here, the risk of not achieving treatment satisfaction increased dose-dependently: the chance of achieving satisfaction when receiving a GC dose of ≥15 mg/day (OR 0.24, 95% CI 0.16 to 0.35) was only half of the chance to do so while receiving a GC dose of 10 to <15 mg/day (OR 0.46, 95% CI 0.34 to 0.62), which in turn is only about half of the chance to do so while receiving a GC dose of 5 to <10 mg/day (OR 0.81, 95% CI 0.71 to 0.92). Further factors negatively associated with treatment satisfaction were treatment changes after enrolment (OR 0.63, 95% CI 0.55 to 0.73), depression (OR 0.64, 95% CI 0.51 to 0.80), fibromyalgia (OR 0.70, 95% CI 0.51 to 0.96), prior bDMARD therapies (OR 0.78, 95% CI 0.66 to 0.92) and obesity (OR 0.86, 95% CI 0.74 to 0.99). Not associated with treatment satisfaction were sex, age at onset, smoking status, current b/tsDMARD therapy and other comorbidities. Analysis of satisfaction with tolerability showed less pronounced effects (figure 2). Besides comparable factors to the analyses on effectiveness, the reduction of fatigue by 1 point was additionally associated with satisfaction with tolerability (table 2).
Figure 2.
Factors significantly associated with satisfaction with tolerability of treatment after 1 year of treatment. Shown are adjusted ORs with 95% CIs. DAS28-ESR, Disease Activity Score in 28 joints-erythrocyte sedimentation rate.
Table 2.
Results from logistic regression models
| Effectiveness | Tolerability | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Baseline parameter | ||||||
| Satisfaction at baseline | 2.98 | 2.58 to 3.44 | <0.0001 | 2.40 | 2.03 to 2.83 | <0.0001 |
| Seropositivity | 1.36 | 1.17 to 1.57 | <0.0001 | 1.22 | 1.04 to 1.43 | 0.0129 |
| ≥1 bDMARD prior to enrolment | 0.78 | 0.66 to 0.92 | 0.0042 | 0.97 | 0.79 to 1.21 | 0.8134 |
| Depression | 0.64 | 0.51 to 0.80 | <0.0001 | 0.54 | 0.43 to 0.68 | <0.0001 |
| Fibromyalgia | 0.70 | 0.51 to 0.96 | 0.0257 | 0.79 | 0.56 to 1.10 | 0.1656 |
| Obesity (BMI ≥30 kg/m2, ref. <30 kg/m2) | 0.86 | 0.74 to 0.99 | 0.0377 | 0.93 | 0.78 to 1.11 | 0.4485 |
| Heart failure | 1.23 | 0.77 to 1.98 | 0.3884 | 0.92 | 0.58 to 1.45 | 0.7151 |
| Diabetes | 0.93 | 0.74 to 1.17 | 0.5468 | 0.87 | 0.70 to 1.08 | 0.2023 |
| Chronic kidney disease | 0.97 | 0.71 to 1.32 | 0.8467 | 1.05 | 0.71 to 1.55 | 0.8145 |
| Malignant neoplasm | 1.02 | 0.71 to1.45 | 0.9249 | 0.93 | 0.64 to 1.35 | 0.7041 |
| Degenerative spine disease | 0.93 | 0.76 to 1.12 | 0.4309 | 0.87 | 0.71 to 1.06 | 0.1611 |
| Osteoarthritis | 0.90 | 0.75 to 1.07 | 0.2416 | 0.94 | 0.78 to 1.13 | 0.4801 |
| Osteoporosis | 0.93 | 0.77 to 1.12 | 0.4491 | 0.98 | 0.79 to 1.22 | 0.8527 |
| Age at onset (10 years) | 1.04 | 0.98 to 1.10 | 0.1792 | 1.00 | 0.94 to 1.06 | 0.9362 |
| Female sex | 1.08 | 0.92 to 1.28 | 0.3344 | 1.12 | 0.93 to 1.36 | 0.2372 |
| Former smoking | 0.91 | 0.76 to 1.08 | 0.2702 | 0.96 | 0.79 to 1.16 | 0.6685 |
| Current smoking | 0.96 | 0.80 to 1.15 | 0.6586 | 0.99 | 0.83 to 1.19 | 0.9421 |
| No information on smoking | 1.12 | 0.77 to 1.62 | 0.5517 | 0.92 | 0.65 to 1.30 | 0.6398 |
| Parameter after 12 months | ||||||
| DAS28-ESR reduction by 1 point | 1.38 | 1.31 to 1.46 | <0.0001 | 1.31 | 1.24 to 1.39 | <0.0001 |
| Pain reduction by 1 point | 1.26 | 1.22 to 1.31 | <0.0001 | 1.13 | 1.09 to 1.17 | <0.0001 |
| Increase in physical capacity by 10 points | 1.22 | 1.17 to 1.29 | <0.0001 | 1.11 | 1.05 to 1.17 | 0.0004 |
| Fatigue reduction by 1 point | 1.02 | 0.98 to 1.05 | 0.3601 | 1.04 | 1.00 to 1.08 | 0.0303 |
| Sleeping disorder reduction by 1 point | 1.02 | 0.99 to 1.05 | 0.3188 | 1.03 | 1.00 to 1.06 | 0.0632 |
| Glucocorticoids 5 to <10 mg/day, ref. <5 mg | 0.81 | 0.71 to 0.92 | 0.0019 | 0.86 | 0.74 to 1.00 | 0.0439 |
| Glucocorticoids 10 to <15 mg/day, ref. <5 mg | 0.46 | 0.34 to 0.62 | <0.0001 | 0.50 | 0.37 to 0.67 | <0.0001 |
| Glucocorticoids ≥15 mg/day, ref. <5 mg | 0.24 | 0.16 to 0.35 | <0.0001 | 0.46 | 0.30 to 0.70 | 0.0004 |
| ≥1 change of therapy after enrolment | 0.63 | 0.55 to 0.73 | <0.0001 | 0.65 | 0.55 to 0.76 | <0.0001 |
| b/tsDMARD therapy | 1.14 | 0.98 to 1.34 | 0.0982 | 1.18 | 1.00 to 1.39 | 0.0504 |
Effects statistically significant at level α=0.05 are marked in bold.
bDMARD, biological disease-modifying antirheumatic drug; BMI, body mass index; DAS28-ESR, Disease Activity Score in 28 joints-erythrocyte sedimentation rate; tsDMARD, targeted synthetic disease-modifying antirheumatic drug.
When the absolute GC dose at 6–12 months in the model was replaced by the achieved GC reduction within 12 months, a reduction by 5 mg/day was positively associated with treatment satisfaction, in terms of both efficacy (OR 1.19, 95% CI 1.11 to 1.27) and tolerability (OR 1.11, 95% CI 1.03 to 1.21) (online supplemental table 2).
Among the components of DAS28-ESR, a reduction of the patient-reported global health by 1 point had a greater influence on the achievement of treatment satisfaction with effectiveness than a reduction of ESR by 10 mm and of the tender and swollen joint count by one joint (online supplemental table 3). Results for satisfaction with tolerability were similar.
DISCUSSION
The majority of patients included in the German biological register RABBIT were satisfied with their treatment’s effectiveness and tolerability after 1 year of treatment. At baseline, the satisfied patients were already doing better on all outcomes than those not satisfied. As outcomes improved during subsequent treatment, so did satisfaction increase, up to 85% (concerning effectiveness) or 90% (concerning tolerability) overall after 12 months which is a really pleasing result. The high patient satisfaction in treated RA is in line with former evidence1 6 but much higher than in the current data of a US web-based survey.10 This study used the Treatment Satisfaction Questionnaire for Medication (TSQM) as an instrument for measuring satisfaction11 resulting in only 26% of the responders being satisfied with their RA therapy (67% reporting to receive bDMARDs). Besides a more specific assessment of satisfaction, this cross-sectional survey represented an unselected outpatient collective with self-reported RA.10 Patients enrolled in RABBIT are all in rheumatology care, closely monitored and familiar with the use of questionnaires which may explain differences in the results.
Patients already satisfied with their prior treatment at baseline were most likely to remain satisfied with their treatment after 1 year, which may be due to an overall higher well-being at baseline compared to other patients regarding, for example, disease activity. Another reason could be an overall more positive attitude of the patient to medication and illness, about which no data are ascertained in RABBIT. Due to a high satisfaction at baseline, especially with regard to tolerability, the effects of other parameters are not as pronounced. A decrease in disease activity and pain as well as improvement in function are direct indicators of treatment effectiveness and therefore comprehensible factors for a higher patient satisfaction.
The positive association with seropositivity is worth a discussion. Apart from abatacept and rituximab, current evidence does not suggest that seropositivity is a marker for better treatment response in terms of retention, response or achievement of low disease activity.12–14 The more important it is to recognise that in our cohort, seropositivity was significantly associated with higher treatment satisfaction after 1 year of therapy. Comparable to treatment delay in early seronegative RA15 treatment, reluctance may also be present in established RA not least because seropositivity is regarded as a poor prognostic factor allowing for more aggressive treatment strategies.2 In the German National Database, only half as many seronegative as seropositive patients with long-standing RA and comparable disease activity are treated with bDMARDs.16 Less efficient therapy may well be an explanation for a lower satisfaction in seronegative patients. The association of the patient global assessment with a lower remission rate in seronegative RA in the study of Coffey et al 17 supports these assumptions.
A further and largely modifiable factor of influence is concomitant GC therapy. Ongoing GC doses above 5 mg/day affect one-third of patients within this cohort. As the negative influence of GC increases notably with the dose, tapering GC remains an important goal also in the interest of the patients’ satisfaction on therapy. Even patients who have had their dose reduced may still not achieve satisfaction if their absolute dose continues to be high. However, in spite of the adjustments the model performs, it cannot be ruled out that treatment satisfaction is affected rather by the need for GC therapy, particularly the need for high dosages, than by GC therapy itself. In any case, if GCs are still required for a patient, other study results suggest that early intervention by addressing the patient concerns may be helpful.18 Giving patients the opportunity to express their concerns may improve their overall treatment satisfaction.5
Depression, obesity and fibromyalgia are comorbid conditions in RA, which are often associated with limitations in physical activity and emotional well-being. From a clinical view, it is not surprising that patients with these comorbidities are less satisfied with their therapy. It should also be considered in clinical trials to use satisfaction as a secondary outcome. With a large prospective patient collective, our results confirm cross-sectional data of a Japanese survey4 that depression is independently associated with treatment dissatisfaction.
Limitations and strengths
Satisfaction was assessed using a Likert scale rather than a validated scoring instrument such as the TSQM.11 In a longitudinal register, it is not feasible to have too many questionnaires filled in. Separate questions on effectiveness and tolerability allowed the differentiation of both outcomes, but in the end, results did not differ substantially. Longitudinal follow-up in the RABBIT register with a very low dropout rate enables consideration of treatment changes, dose alterations and all available treatment options in RA in a large patient collective, enabling robust results on the analyses performed.
We conclude from our data that among the factors that have a significant impact on patient satisfaction, physicians have the most influence on GC therapy. Limited well-being due to depression, fibromyalgia or obesity can also be addressed when discussing treatment options with the patient, although this naturally goes beyond the concern for treatment satisfaction.
Acknowledgments
The authors acknowledge the invaluable contributions of all participating consultant rheumatologists and their patients. In particular, the authors would like to thank those rheumatologists who enrolled the highest numbers of patients: Kaufmann J, Klopsch T, Eisterhues C, Braun J, Schwarze I, Liebhaber A, Krause A, Rockwitz K, Zinke S, Kneitz C, Möbius C, Ständer E, Tony H, Berger S, Gräßler A, Kühne C, Remstedt S, Ochs W, Wilden E, Bohl-Bühler M, Wassenberg S, Kellner H, Burmester G, Haas F, Richter C, Röser M, Bruckner A, Balzer S, Fricke-Wagner H, Bergerhausen H, Harmuth W, Wiesmüller G, Lebender S, Bussmann A, Hamann F, Stille C, Feuchtenberger M, Edelmann E, Tremel H, Krummel-Lorenz B, Körber H, Krüger K, Meier L, Kapelle A, Müller L, Thiele A, Schmitt-Haendle M, Prothmann U, Pick D, Karberg K, Brandt H, Weiß K, Kekow J, Seifert A, Müller-Ladner U, Manger K, Baumann C, Krause D, Aringer M, Worsch M, Roßbach A, Zänker M, Streibl H, Backhaus M, Richter C, Schulze-Koops H, Herzberg C, Grünke M, Heel N, Herzer P, Heel N, Reck A, Wiesent F, Dahmen G, Blank N, Max R, Eidner T, Dockhorn R, Zeh G, Winkler K, Menne H, von Hinüber U, Demary W, Sörensen H, Schneider M, Gause A, Bruns A, Bielecke C, Marycz T, Häckel B, Alliger K, Euler H, Gause A, Moosig F, Iking-Konert C, Häntsch J and Boldemann R. The authors also acknowledge the significant contributions of Peter Herzer, Munich, Bernhard Manger, Erlangen, and Matthias Schneider, Düsseldorf, as members of the advisory board.
Footnotes
Contributors: All authors have given a substantial contribution to one or more of the following aspects of the manuscript: conception and design, acquisition, analysis and interpretation of the data, drafting and revising the manuscript.
Funding: RABBIT is supported by a joint, unconditional grant from AbbVie, Amgen, Bristol-Myers Squibb, Celltrion, Fresenius Kabi, Hexal, Lilly, MSD Sharp & Dohme, Mylan, Pfizer, Roche, Samsung Bioepis, Sanofi-Aventis and UCB. Role of the funding source: the principal investigators and their team had full academic freedom in study design and conduct, data analysis and publication of results. These stipulations were delineated in their contract with the sponsors. For the purpose of information, all funding companies received the manuscript 30 days prior to submission. Publication of this article was not contingent on their approval. The data interpretation, drafting, critical revision and approval of the final manuscript were performed solely by the authors.
Competing interests: AS has received speaking fees from Bristol-Myers Squibb, MSD Sharp & Dome, Pfizer and Roche. AZ has received speaking fees from AbbVie, Janssen, Pfizer, Roche and Sanofi-Aventis. All other authors have declared no competing interests.
Patient consent for publication: Not required.
Ethics approval: Ethical approval was obtained from the Ethics Committee of the Charité-Universitätsmedizin Berlin, Berlin.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: No additional data are available.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
REFERENCES
- 1. Barton JL. Patient preferences and satisfaction in the treatment of rheumatoid arthritis with biologic therapy. Patient Prefer Adherence 2009;3:335–44. 10.2147/PPA.S5835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smolen JS, Landewe RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020. 10.1136/annrheumdis-2019-216655 [DOI] [PubMed] [Google Scholar]
- 3. Bartlett SJ, De Leon E, Orbai AM, et al. Patient-reported outcomes in RA care improve patient communication, decision-making, satisfaction and confidence: qualitative results. Rheumatology (Oxford) 2019. 10.1093/rheumatology/kez506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mahlich J, Schaede U, Sruamsiri R. Shared decision-making and patient satisfaction in Japanese rheumatoid arthritis patients: a new “preference fit” framework for treatment assessment. Rheumatol Ther 2019;6:269–83. 10.1007/s40744-019-0156-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kelly A, Tymms K, Tunnicliffe DJ, et al. Patients’ attitudes and experiences of disease-modifying antirheumatic drugs in rheumatoid arthritis and spondyloarthritis: a qualitative synthesis. Arthritis Care Res (Hoboken) 2018;70:525–32. 10.1002/acr.23329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Calvo-Alen J, Vela P, Bustabad S, et al. Satisfaction, fulfillment of expectations and adherence to subcutaneous biological drugs in patients with rheumatoid arthritis: ARCO study. Rheumatol Clin 2018. 10.1016/j.reuma.2018.02.015 [DOI] [PubMed] [Google Scholar]
- 7. Kjeken I, Dagfinrud H, Mowinckel P, et al. Rheumatology care: involvement in medical decisions, received information, satisfaction with care, and unmet health care needs in patients with rheumatoid arthritis and ankylosing spondylitis. Arthritis Rheum 2006;55:394–401. 10.1002/art.21985 [DOI] [PubMed] [Google Scholar]
- 8. Wolfe F, Michaud K. Resistance of rheumatoid arthritis patients to changing therapy: discordance between disease activity and patients’ treatment choices. Arthritis Rheum 2007;56:2135–42. 10.1002/art.22719 [DOI] [PubMed] [Google Scholar]
- 9. van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 2007;16:219–42. 10.1177/0962280206074463 [DOI] [PubMed] [Google Scholar]
- 10. Radawski C, Genovese MC, Hauber B, et al. Patient perceptions of unmet medical need in rheumatoid arthritis: a cross-sectional survey in the USA. Rheumatol Ther 2019;6:461–71. 10.1007/s40744-019-00168-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Atkinson MJ, Kumar R, Cappelleri JC, et al. Hierarchical construct validity of the treatment satisfaction questionnaire for medication (TSQM version II) among outpatient pharmacy consumers. Value Health 2005;8:S9–S24. 10.1111/j.1524-4733.2005.00066.x [DOI] [PubMed] [Google Scholar]
- 12. Baganz L, Richter A, Albrecht K, et al. Are prognostic factors adequately selected to guide treatment decisions in patients with rheumatoid arthritis? A collaborative analysis from three observational cohorts. Semin Arthritis Rheum 2019;48:976–82. 10.1016/j.semarthrit.2018.09.003 [DOI] [PubMed] [Google Scholar]
- 13. Choi S, Lee KH. Clinical management of seronegative and seropositive rheumatoid arthritis: a comparative study. PLoS One 2018;13:e0195550 10.1371/journal.pone.0195550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nordberg LB, Lillegraven S, Aga AB, et al. Comparing the disease course of patients with seronegative and seropositive rheumatoid arthritis fulfilling the 2010 ACR/EULAR classification criteria in a treat-to-target setting: 2-year data from the ARCTIC trial. RMD Open 2018;4:e000752 10.1136/rmdopen-2018-000752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lukas C, Mary J, Debandt M, et al. Predictors of good response to conventional synthetic DMARDs in early seronegative rheumatoid arthritis: data from the ESPOIR cohort. Arthritis Res Ther 2019;21:243 10.1186/s13075-019-2020-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Albrecht K, Huscher D, Eidner T, et al. Medical treatment of rheumatoid arthritis in 2014: current data from the German Collaborative Arthritis Centers. Z Rheumatol 2017;76:50–7. 10.1007/s00393-016-0156-5 [DOI] [PubMed] [Google Scholar]
- 17. Coffey CM, Crowson CS, Myasoedova E, et al. Evidence of diagnostic and treatment delay in seronegative rheumatoid arthritis: missing the window of opportunity. Mayo Clin Proc 2019;94:2241–8. 10.1016/j.mayocp.2019.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meyfroidt S, Van der Elst K, De Cock D, et al. Patient experiences with intensive combination-treatment strategies with glucocorticoids for early rheumatoid arthritis. Patient Educ Couns 2015;98:384–90. 10.1016/j.pec.2014.11.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2020-001290supp001.pdf (168.5KB, pdf)