Abstract
Ruppert–Prakash type reagents (TMSCF3, TMSC2F5, and TMSC3F7) are readily available, air-stable, and easy-to-handle fluoroalkyl sources. Herein, we describe a mild, copper-catalyzed cross-coupling of these fluoroalkyl nucleophiles with aryl and alkyl bromides to produce a diverse array of trifluoromethyl, pentafluoroethyl, and heptafluoropropyl adducts. This light-mediated transformation proceeds via a silyl-radical-mediated halogen atom abstraction pathway, which enables perfluoroalkylation of a broad range of organobromides of variable steric and electronic demand. The utility of the method is demonstrated through the late-stage functionalization of several drug analogues.
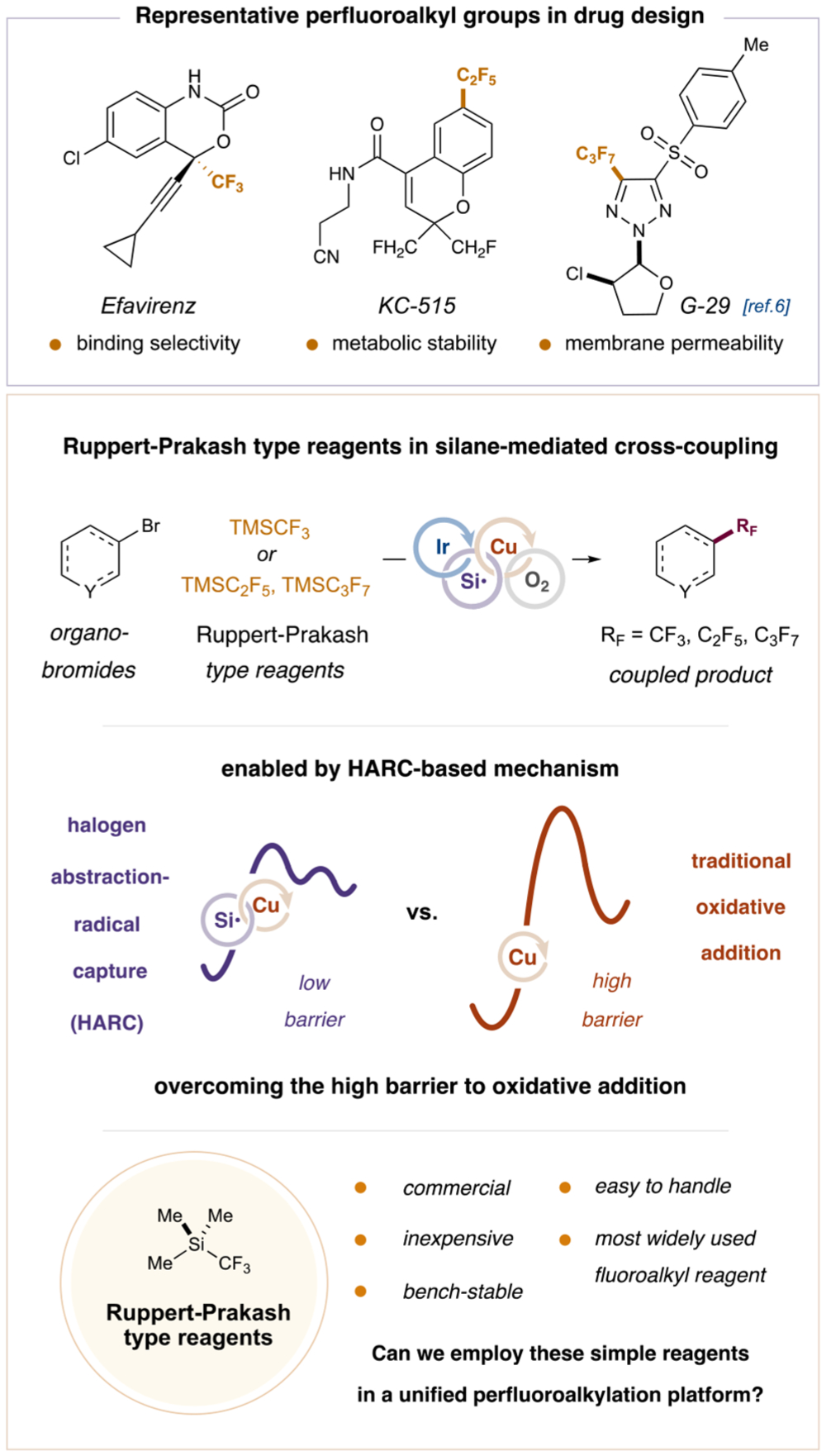
It is well understood that the incorporation of a fluoroalkyl group into a drug molecule can confer significant advantages, including enhanced protein binding selectivity, improved membrane permeability, and protection against in vivo metabolism.1 While the trifluoromethyl (CF3) group has been a major focus of medicinal chemistry, the relatively underexplored pentafluoroethyl (C2F5) moiety also possesses some distinct characteristics, such as increased steric demand, electronegativity, and lipophilicity,2 that render it an attractive alternative to the trifluoromethyl group. The potential utility of the C2F5 moiety is exemplified by its inclusion in many bioactive molecules, including the angiotensin II receptor antagonist DuP 532,3 the antibreast cancer drug Fulvestrant,4 and the antihypertensive K+ channel opener KC-515 (Figure 1).5,6
Figure 1.
Unified perfluoroalkylation of organobromides.
Modern approaches to the direct, selective introduction of small perfluoroalkyl groups into organic molecules typically involve transition-metal-mediated cross-coupling of organohalides with appropriate perfluoroalkyl reagents.7 While Ni-and Pd-based strategies are hindered by the high kinetic barrier to reductive elimination,8 Cu complexes have been shown to undergo facile reductive elimination of electronegative coupling partners from the high-valent metal center.9 This observation has inspired extensive efforts to develop protocols to achieve direct, copper-mediated perfluoroalkylation with haloarenes. However, the diminished capacity of Cu(I) to undergo oxidative addition with organohalides limits the utility of these methods, which are typically narrow in substrate scope and require high catalyst loadings,10,11 expensive copper-based perfluoroalkyl reagents,12,13 and elevated temperatures. Although they do not readily undergo two-electron oxidative addition with organohalides, copper salts can efficiently trap carbon-centered radicals,14 providing an alternate route to copper oxidative insertion via an open-shell mechanism. We recently harnessed this reactivity to develop a silyl radical-mediated halogen abstraction-radical capture (HARC) system.15,16 According to this dual copper-photoredox catalytic pathway, aryl and alkyl bromides are converted to intermediate radical species capable of forming aryl-Cu(III)-CF3 and alkyl-Cu(III)-CF complexes.17 These complexes readily undergo reductive elimination to afford the desired trifluoromethyl adducts.15a,b Recently, we sought to employ this general activation strategy, along with well-established aerobic copper chemistry, as a means to use perfluoroalkyl nucleophiles as coupling partners with a diverse range of organic bromides.
Trifluoromethyltrimethylsilane (TMSCF3) and its homologues TMSC2F5 and TMSC3F7—collectively known as the Ruppert–Prakash type reagents—are the most widely used perfluoroalkyl nucleophiles due to their commercial availability, bench stability, and operational convenience.18 Although it is possible to perfluoroalkylate aryl iodides using the Ruppert–Prakash reagents activated by fluoride anion,11 there are currently no general methods that directly employ these nucleophiles for the functionalization of more stable and readily accessible organobromides. We speculated that our recently developed HARC trifluoromethylation protocol might be expanded to accommodate nucleophilic Ruppert–Prakash type reagents, providing a unified approach for the direct introduction of CF3, C2F5, and C3F7 groups to a diverse array of aryl and alkyl frameworks. Herein, we disclose the successful implementation of this strategy and present a mild, convenient, and broadly applicable metallaphotoredox-catalyzed perfluoroalkylation of organobromides.
A proposed mechanism for the oxidative trifluoromethylation of organobromides is illustrated in Figure 2. Upon irradiation with visible light, excitation of photocatalyst [Ir(F-mppy)2(phen)](PF6) (1) to the long-lived triplet *IrIII complex 2 is expected. Given the oxidation potentials of the excited state 2 (E1/2red[*IrIII/IrII] = +0.83 V vs SCE in MeCN)19 and aminosilane 4 (Epa [4/4+•] = +0.66 V vs SCE in MeCN),20 we assume that a rapid SET event would generate a silicon-centered radical (5) after deprotonation by a suitable base and subsequent aza-radical Brook rearrangement. The nucleophilic silyl radical 5 can then abstract a bromine atom from organobromide 6 to form open-shell radical intermediate 7. Concurrently, Cu(I) species 8 would undergo ligand exchange with the nucleophilic trifluoromethyl source, likely in a base-mediated process, thereby generating ligated Cu(I)–CF3 complex 9, which can be subsequently oxidized by a terminal oxidant. The resultant Cu(II) species 10 can rapidly capture radical 7 to generate transient formal Cu(III)–CF3 complex 11, which would be expected to undergo facile reductive elimination to afford product 12 and regenerate the Cu(I) catalyst. Oxidative turnover of the photocatalytic cycle returns Ir(II) complex 3 to the ground-state photocatalyst 1.
Figure 2.
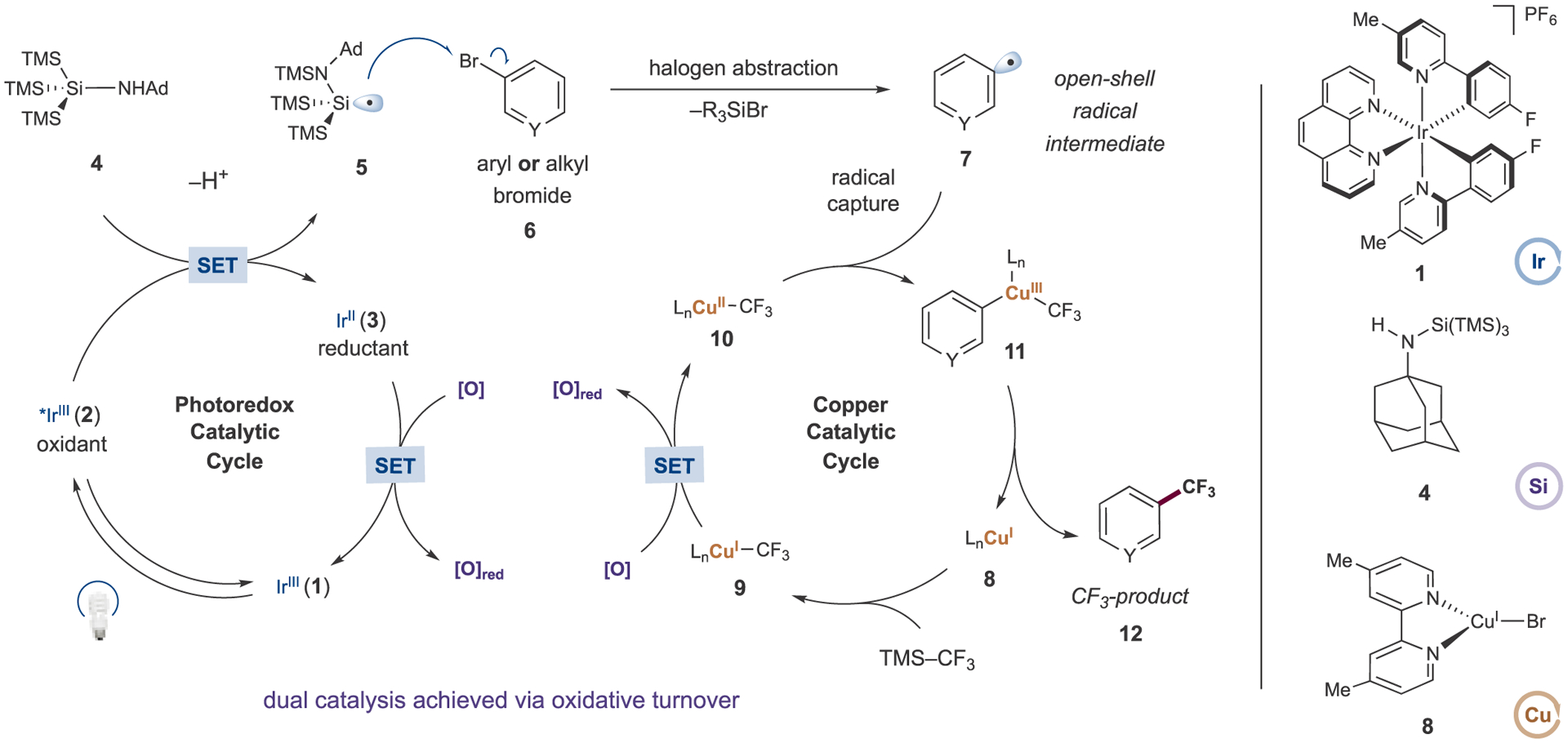
Proposed mechanism for the copper-catalyzed perfluoroalkylation of organobromides
At the outset, we evaluated the trifluoromethylation of aryl bromide 13 with the nucleophilic TMSCF3 reagent. Following an extensive survey of supersilane derivatives, catalysts, and ligands (see Supporting Information (SI) for details), we ultimately identified the optimized conditions outlined in Table 1, entry 1. Thus, the aminosilane reagent 4 facilitates formation of the desired product in 87% yield using a combination of photocatalyst 1 (0.5 mol %), 4,4′-dimethyl-2,2′-bipyridine ligand (25 mol %), and copper(I) bromide (20 mol %). The low oxidation potential of organosilane 4 permits generation of a silyl radical by photocatalyst 1 via SET under mild conditions; accordingly, this procedure can tolerate sensitive functional groups, such as aniline and tertiary alkyl amine, that are traditionally susceptible to oxidation. Interestingly, other silane reagents previously used in photo-redox cross-electrophile couplings (including supersilanol and supersilane)21 were ineffective (entries 2 and 3). Conveniently, the optimal conditions employ air as an oxidant, obviating the need for rigorous deoxygenation. Control experiments revealed that iridium photocatalyst, light, silane reagent, ligand, air, and copper catalyst were all necessary for product formation (entries 4–10).
Table 1.
Control Reactions of Optimized Conditionsa
 | ||
|---|---|---|
| entry | deviations | yieldb |
| 1 | none | 87% |
| 2 | TMS3SiH instead of 4 | 15% |
| 3 | TMS3SiOH instead of 4 | 35% |
| 4 | no silane source | 0% |
| 5 | no CuBr | 0% |
| 6 | no ligand | 0% |
| 7 | no light | 0% |
| 8 | no photocatalyst | 0% |
| 9 | N2 sparge | 0% |
| 10 | no base | 38% |
Performed with aminosilane reagent 4 (1.8 equiv), NaOAc (4 equiv), aryl bromide 13 (0.1 mmol), and TMSCF3 (3 equiv) in MeCN (0.1 M).
Yields determined by 19F NMR analysis using 1,4-difluorobenzene as internal standard. See SI for more details.
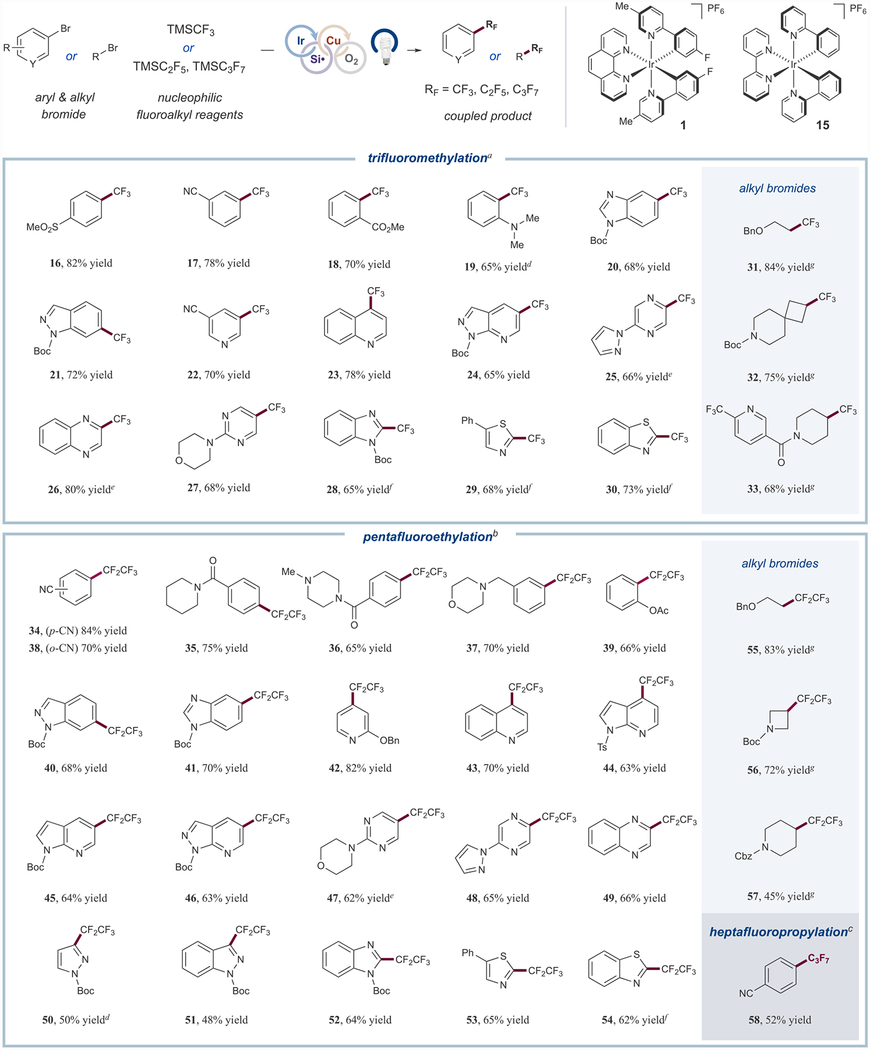
With optimized conditions in hand, we turned our attention to evaluating the scope of the aryl trifluoromethylation. As shown in Table 2, para- and meta-substituted aryl bromides bearing sulfone and nitrile groups generated trifluoromethyl adducts in high yields (16 and 17, 82% and 78% yield). Notably, the efficiency of the reaction was not impeded by the installation of ortho substituents on the aryl ring (18 and 19, 70% and 65% yield). Additionally, aryl bromides with fused cyclic motifs afforded the desired trifluoromethylarenes with good efficiency (20 and 21, 68% and 72% yield). Heteroaryl bromides were readily accommodated in the transformation: pyridine-derived substrates were functionalized in good yields (22–24, 65–78% yield), and multiple-nitrogen-bearing heteroarenes, such as pyrazines, quinoxalines, and pyrimidines, were readily converted into the corresponding trifluoromethylarenes (25–27, 66–80% yield). Five-membered heteroaryl bromides, including benzimidazoles, thiazoles, and benzothiazoles, were also found to serve as competent coupling partners (28–30, 65–73% yield). Consistent with the modular radical activation paradigm, we expected aliphatic bromides to be an amenable substrate class. Gratifyingly, trifluoromethylation of primary alkyl bromides was successfully achieved (31, 84% yield), while secondary cyclic alkyl bromides were likewise converted to their trifluoromethyl congeners in good yields (32 and 33, 75% and 68% yield). This broad substrate scope validates the utility of this transformation as a means to gain rapid access to analogs of drug-like molecules.
Table 2.
Scope Evaluation of Silane-Mediated Perfluoroalkylation of Alkyl and Aryl Bromides
|
Performed with photocatalyst 1 (0.5 mol %), CuBr (20 mol %), 4,4′-dimethyl-2,2′-bipyridine (25 mol %), aminosilane 4 (1.8 equiv), NaOAc (4 equiv), aryl or alkyl bromide (0.5 mmol), and TMSCF3 (3 equiv) in MeCN (0.2 M). Yields isolated unless otherwise noted.
Performed with photocatalyst 15 (0.5 mol %), CuBr (20 mol %), 4,4′-dimethyl-2,2′-bipyridine (25 mol %), aminosilane 4 (1.8 equiv), NaOAc (4 equiv), aryl or alkyl bromide (0.5 mmol), and TMSC2F5 (3 equiv) in MeCN (0.2 M).
Performed with photocatalyst 15 (0.5 mol %), [Cu(MeCN)4]BF4 (20 mol %), 4,4′-dimethyl-2,2′-bipyridine (25 mol %), aminosilane 4 (1.8 equiv), NaOAc (4 equiv), pyridine (2.0 equiv), aryl bromide (0.5 mmol), and TMSC3F7 (3 equiv) in MeCN (0.2 M).
Yields determined by 19F NMR using 1,4-difluorobenzene as internal standard.
25 mol % 4,4′-dimethoxy-2,2′-bipyridine as ligand.
25 mol % 1,10-phenanthroline as ligand.
25 mol % 2,2′-bipyridine as ligand. See SI for full experimental details.
Given the value of the C2F5 group in medicinal chemistry,2 we next investigated the extension of this reaction to the pentafluoroethylation of bromoarenes using the TMSC2F5 reagent. In initial experiments using the representative aryl bromide 13, the desired C2F5-bearing product was produced in only 48% yield (see Supporting Information for details). Changing the photocatalyst to the more reducing [Ir-(ppy)2bpy](PF6) (15) and prolonging the reaction time led to an increase in the overall reaction efficiency (68% yield). Interestingly, performing the reaction in the Integrated Photoreactor,22 which precisely controls light intensity and stirring rate, allowed further optimization to a reproducible 85% assay yield. This optimization of stirring rate and light intensity curtailed the formation of aryl radical-derived side products, including those generated through dehalogenation and copper-mediated aryl dimerization.
With optimal conditions for the pentafluoroethylation in hand, we next examined the scope with respect to the aryl bromide component. As shown in Table 2, para-substituted bromoarenes containing nitrile and amide groups generated the corresponding pentafluoroethyl adducts in good to excellent yields (34 and 35, 84% and 75% yield). As a useful demonstration of the mild conditions and broad functional group tolerance of this new coupling procedure, we found that aryl electrophiles bearing tertiary alkylamine groups, namely morpholine and piperazine motifs, generate the desired products, 36 and 37, in good yields (65% and 70%). Ortho-substitution on the aryl coupling partner was well accommodated, furnishing adducts 38 and 39 in 70% and 66% yield, respectively. Moreover, aryl bromides with fused cyclic motifs were converted to pentafluoroethylarenes 40 and 41 in good yields (68% and 70%). With respect to heteroaryl bromide substrates, we observed that pyridine-derived substrates—motifs commonly found in drug scaffolds—were readily accommodated (42–46, 63–82% yield). Multinitrogen-containing heterocycles, such as pyrimidines, pyrazines, and quinoxalines, participated in good yields (47–49, 62–66% yield). A wide range of five-membered heteroaryl bromides, including pyrazoles, indazoles, benzimidazoles, thiazoles, and benzothiazoles, underwent pentafluoroethylation in useful to good yields (50–54, 48–65% yield). Notably, primary and secondary alkyl bromides proved to be suitable substrates for the pentafluoroethylation protocol (55–57, 45–83% yield). Last, as a demonstration that this method can be extended to the installation of longer-chain perfluoroalkyl groups, heptafluoropropylation can also be accomplished using this new protocol to provide the corresponding perfluoropropylarene in useful efficiencies (58, 52% yield; see Figure S13 for additional heptafluoropropylated substrates). These results represent a novel and straightforward way to introduce varied perfluoroalkyl groups onto a diverse range of carbon electrophiles.
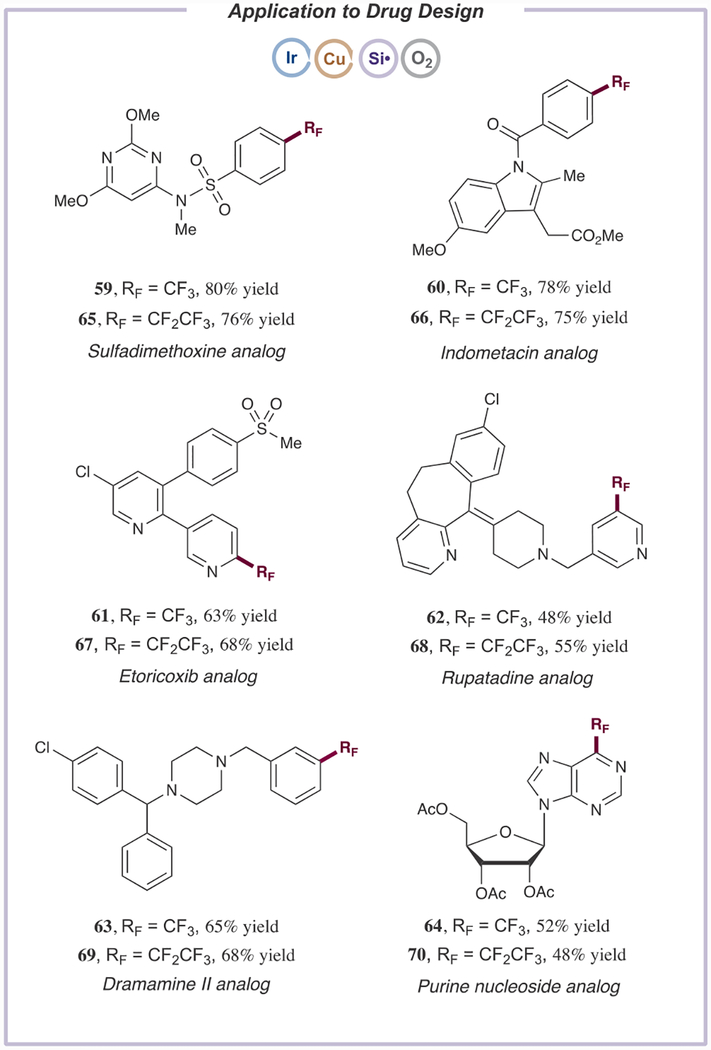
Given the pharmaceutical relevance of the CF3 and C2F5 functional motifs, we further sought to showcase our novel procedure by selectively appending CF3 and C2F5 groups onto several medicinal scaffolds. As shown in Table 3, aryl bromide progenitors of sulfadimethoxine, indometacin, dramamine II, rupatadine, etoricoxib, and a purine nucleoside were subjected to our standard trifluoromethylation conditions, generating the corresponding trifluoromethylated derivatives 59–64 (48–80% yield). Pentafluoroethylation of these complex medicinal agents similarly delivered the desired adducts 65–70 with good efficiency (48–76% yield). These results further highlight the real-world utility of this new perfluoroalkylation technology, and its ability to tolerate a wide range of medicinally relevant functional groups including sulfonamides, nitrogen heterocycles, styrenes, aryl chlorides, and tertiary amines.23
Table 3.
Application to Late-Stage Functionalizationa
|
All yields are isolated yields. See SI for experimental details.
In summary, we have developed a novel procedure by which to achieve nucleophilic perfluoroalkylation of a variety of aryl, heteroaryl, and alkyl bromides. This reaction circumvents the need for substrates to undergo challenging Cu-mediated oxidative additions by employing a silyl radical-mediated halogen abstraction-radical capture (HARC) pathway and common Ruppert–Prakash type nucleophiles as a convenient source of the perfluoroalkyl group. Given its operational simplicity and broad applicability to pharmaceutically relevant scaffolds, we expect this method to be widely adopted within the synthetic and medicinal chemistry communities.
Supplementary Material
ACKNOWLEDGMENTS
Financial support was provided by the National Institute of General Medical Sciences (NIGMS), the NIH (under Award R35GM134897-01), the Princeton Catalysis Initiative, and kind gifts from Merck, Janssen, BMS, Genentech, Celgene and Pfizer. X.B. is grateful for a postdoctoral fellowship from the Shanghai Institute of Organic Chemistry. We thank Yufan Liang, Ian Perry, Xiaheng Zhang, Wei Liu, and Vlad Bacauanu for their help in preparing this paper.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.0c09977.
Experimental procedures and spectral data (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/jacs.0c09977
The authors declare no competing financial interest.
Contributor Information
Xiangbo Zhao, Merck Center for Catalysis at Princeton University, Princeton, New Jersey 08544, United States.
David W. C. MacMillan, Merck Center for Catalysis at Princeton University, Princeton, New Jersey 08544, United States.
REFERENCES
- (1).For general reviews:; (a) Hagmann WK The many roles for fluorine in medicinal chemistry. J. Med. Chem 2008, 51, 4359. [DOI] [PubMed] [Google Scholar]; (b) Purser S; Moore PR; Swallow S; Gouverneur V Fluorine in medicinal chemistry. Chem. Soc. Rev 2008, 37, 320. [DOI] [PubMed] [Google Scholar]; (c) Müller K; Faeh C; Diederich F Fluorine in Pharmaceuticals: Looking beyond intuition. Science 2007, 317, 1881. [DOI] [PubMed] [Google Scholar]
- (2).(a) Prchalová E; Štêpánek O; Smrcek S; Kotora M Medicinal applications of perfluoroalkylated chain-containing compounds. Future Med. Chem 2014, 6, 1201. [DOI] [PubMed] [Google Scholar]; (b) Fujiwara T; O’Hagan D Successful fluorine-containing herbicide agrochemicals. J. Fluorine Chem 2014, 167, 16. [Google Scholar]
- (3).(a) Chiu AT; Carini DJ; Duncia JV; Leung KH; McCall DE; Price J; William A; Wong PC; Smith RD; Wexler RR; Timmermans PBMWM DuP 532: A second generation of nonpeptide angiotensin II receptor antagonists. Biochem. Biophys. Res. Commun 1991, 177, 209. [DOI] [PubMed] [Google Scholar]; (b) Pierce ME; Carini DJ; Huhn GF; Wells GJ; Arnett JF Practical synthesis and regioselective alkylation of methyl 4(5)-(pentafluoroethyl)-2-propylimidazole-5(4)-carboxylate to give DuP 532, a potent angiotensin II antagonist. J. Org. Chem 1993, 58, 4642. [Google Scholar]
- (4).(a) Curran M; Wiseman L Fulvestrant. Drugs 2001, 61, 807. [DOI] [PubMed] [Google Scholar]; (b) Vergote I; Abram P Fulvestrant, a new treatment option for advanced breast cancer: tolerability versus existing agents. Ann. Oncol 2006, 17, 200. [DOI] [PubMed] [Google Scholar]
- (5).(a) Taka N; Koga H; Sato H; Ishizawa T; Takahashi T; Imagawa J 6-substituted 2,2-bis(fluoromethyl)-benzopyran-4-carbox-amide K+ channel openers. Bioorg. Med. Chem 2000, 8, 1393. [DOI] [PubMed] [Google Scholar]; (b) Carmody M; Byrne B; Murphy B; Breen C; Lynch S; Flood E; Finnan S; Caffrey P Analysis and manipulation of amphotericin biosynthetic genes by means of modified phage KC-515 transduction techniques. Gene 2004, 343, 107. [DOI] [PubMed] [Google Scholar]
- (6).(a) Biliavska L; Pankivska Y; Povnitsa O; Zagorodnya S; Gudz G; Shermolovich Y Anti-Adenoviral Activity of 2-(3-Chlorotetrahydrofuran-2-yl)-4-Tosyl-5-(Perfluoropropyl)-1,2,3-Triazole. Medicina 2018, 54, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Saleh S; Aboul-Enein HY; Parhar R; Collison R; Al-Mohanna F Ca2+ dependent production of reactive oxygen metabolites by human neutrophils in response to fluorinated propranolol analogues. Biochem. Pharmacol 2001, 61, 517. [DOI] [PubMed] [Google Scholar]; (c) Zhang J; Didierlaurent S; Fortin M; Lefrançois D; Uridat E; Vevert JP Nonpeptide endothelin antagonists: from lower affinity pyrazol-5-ols to higher affinity pyrazole-5-carboxylic acids. Bioorg. Med. Chem. Lett 2000, 10, 1351. [DOI] [PubMed] [Google Scholar]
- (7).For general reviews:; (a) Chen P; Liu G Recent advances in transition-metal-catalyzed trifluoromethylation and related transformations. Synthesis 2013, 45, 2919. [Google Scholar]; (b) Alonso C; de Marigorta EM; Rubiales G; Palacios F Carbon trifluoromethylation reactions of hydrocarbon derivatives and heteroarenes. Chem. Rev 2015, 115, 1847. [DOI] [PubMed] [Google Scholar]; (c) Liang T; Neumann CN; Ritter T Introduction of fluorine and fluorine-containing functional groups. Angew. Chem., Int. Ed 2013, 52, 8214. [DOI] [PubMed] [Google Scholar]; (d) Tomashenko OA; Grushin VV Aromatic trifluoromethylation with metal complexes. Chem. Rev 2011, 111, 4475. [DOI] [PubMed] [Google Scholar]; (e) Amal Joseph PJ; Priyadarshini S Copper-mediated C–X functionalization of aryl halides. Org. Process Res. Dev 2017, 21, 1889 [Google Scholar]
- (8).(a) Dubinina GG; Brennessel WW; Miller JL; Vicic DA Exploring trifluoromethylation reactions at nickel: a structural and reactivity study. Organometallics 2008, 27, 3933. [Google Scholar]; (b) Cho EJ; Senecal TD; Kinzel T; Zhang Y; Watson DA; Buchwald SL The Palladium-Catalyzed Trifluoromethylation of Aryl Chlorides. Science 2010, 328, 1679. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ferguson DM; Bour JR; Canty AJ; Kampf JW; Sanford MS Stoichiometric and catalytic aryl–perfluoroalkyl coupling at tri-tert-butylphosphine palladium(II) complexes. J. Am. Chem. Soc 2017, 139, 11662. [DOI] [PubMed] [Google Scholar]
- (9).(a) Senecal TD; Parsons AT; Buchwald SL Room temperature aryl trifluoromethylation via copper-mediated oxidative cross-coupling. J. Org. Chem 2011, 76, 1174. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Liu T; Shen Q Copper-catalyzed trifluoromethylation of aryl and vinyl boronic acids with an electrophilic trifluoromethylating reagent. Org. Lett 2011, 13, 2342. [DOI] [PubMed] [Google Scholar]; (c) Ye Y; Sanford MS Merging visible-light photocatalysis and transition-metal catalysis in the copper-catalyzed trifluoromethylation of boronic acids with CF3I. J. Am. Chem. Soc 2012, 134, 9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).For examples of trifluoromethylation of aryl iodides:; (a) Chen Q-Y; Wu S-W Methyl fluorosulphonyldifluoroacetate; a new trifluoromethylating agent. J. Chem. Soc., Chem. Commun 1989, 11, 705. [Google Scholar]; (b) Schareina T; Wu XF; Zapf A; Cotte A; Gotta M; Beller M Towards a practical and efficient copper-catalyzed trifluoromethylation of aryl halides. Top. Catal 2012, 55, 426. [Google Scholar]; (d) Zhang CP; Wang ZL; Chen QY; Zhang CT; Gu YC; Xiao JC Copper-mediated trifluoromethylation of heteroaromatic compounds by trifluoromethyl sulfonium salts. Angew. Chem., Int. Ed 2011, 50, 1896. [DOI] [PubMed] [Google Scholar]
- (11).For examples of perfluoroalkylation of aryl iodides using Ruppert–Prakash type reagents:; (a) Urata H; Fuchikami T A novel and convenient method for trifluoromethylation of organic halides using CF3SiR’3/KF/Cu(I) system. Tetrahedron Lett. 1991, 32, 91. [Google Scholar]; (b) Oishi M; Kondo H; Amii H Aromatic trifluoromethylation catalytic in copper. Chem. Commun 2009, 14, 1909. [DOI] [PubMed] [Google Scholar]; (c) Weng ZQ; Lee R; Jia WG; Yuan YF; Wang WF; Feng X; Huang KW Cooperative Effect of Silver in Copper catalyzed trifluoromethylation of aryl iodides using Me3SiCF3. Organometallics 2011, 30, 3229. [Google Scholar]; (d) Xie QQ; Li LC; Zhu ZY; Zhang RY; Ni CF; Hu JB From C1 to C2: TMSCF3 as a precursor for pentafluoroethylation. Angew. Chem., Int. Ed 2018, 57, 13211. [DOI] [PubMed] [Google Scholar]
- (12).For examples of stoichiometric copper-based perfluoroalkyl reagents for perfluoroalkylation of aryl halides:; (a) Dubinina GG; Furutachi H; Vicic DA Active trifluoromethylating agents from well-defined copper(I)-CF3 complexes. J. Am. Chem. Soc 2008, 130, 8600. [DOI] [PubMed] [Google Scholar]; (b) Dubinina GG; Ogikubo J; Vicic DA Structure of bis(trifluoromethyl)cuprate and its role in trifluoromethylation reactions. Organometallics 2008, 27, 6233. [Google Scholar]; (c) Morimoto H; Tsubogo T; Litvinas ND; Hartwig JF A broadly applicable copper reagent for trifluoromethylations and perfluoroalkylations of aryl Iodides and bromides. Angew. Chem., Int. Ed 2011, 50, 3793. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Mormino MG; Fier PS; Hartwig JF Copper-mediated perfluoroalkylation of heteroaryl bromides with (Phen)CuRF. Org. Lett 2014, 16, 1744. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Tomashenko OA; Escudero-Adan EC; Belmonte MM; Grushin VV Simple, stable, and easily accessible well-defined CuCF3 aromatic trifluoromethylating agents. Angew. Chem., Int. Ed 2011, 50, 7655. [DOI] [PubMed] [Google Scholar]; (f) Lishchynskyi A; Grushin VV Cupration of C2F5H: isolation, structure, and synthetic applications of [K(DMF)2][(t-BuO)Cu(C2F5)]. Highly efficient pentafluoroethylation of unactivated aryl bromides. J. Am. Chem. Soc 2013, 135, 12584. [DOI] [PubMed] [Google Scholar]; (g) Zanardi A; Novikov MA; Martin E; Benet-Buchholz J; Grushin VV Direct cupration of fluoroform. J. Am. Chem. Soc 2011, 133, 20901. [DOI] [PubMed] [Google Scholar]; (h) Lishchynskyi A; Novikov MA; Martin E; Escudero-Adán EC; Novák P; Grushin VV Trifluoromethylation of aryl and heteroaryl halides with fluoroform-derived CuCF3: scope, limitations, and mechanistic features. J. Org. Chem 2013, 78, 11126. [DOI] [PubMed] [Google Scholar]; (i) Aikawa K; Nakamura Y; Yokota Y; Toya W; Mikami K Stable but reactive perfluoroalkylzinc reagents: application in ligand free copper-catalyzed perfluoroalkylation of aryl iodides. Chem. - Eur. J 2015, 21, 96. [DOI] [PubMed] [Google Scholar]; (j) Popov I; Lindeman S; Daugulis O Copper-catalyzed arylation of 1H-perfluoroalkanes. J. Am. Chem. Soc 2011, 133, 9286. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Serizawa H; Aikawa K; Mikami K Direct synthesis of pentafluoroethyl copper from pentafluoropropionate as an economical C2F5 source: application to pentafluoroethylation of arylboronic acids and aryl bromides. Org. Lett 2014, 16, 3456. [DOI] [PubMed] [Google Scholar]; (l) Serizawa H; Aikawa K; Mikami K Direct synthesis of a trifluoromethyl copper reagent from trifluoromethyl ketones: application to trifluoromethylation. Chem. - Eur. J 2013, 19, 17692. [DOI] [PubMed] [Google Scholar]; (m) Knauber T; Arikan F; Roschenthaler GV; Goossen LJ Copper-catalyzed trifluoromethylation of aryl iodides with potassium (trifluoromethyl)trimethoxyborate. Chem. - Eur. J 2011, 17, 2689. [DOI] [PubMed] [Google Scholar]; (n) Sugiishi T; Kawauchi D; Sato M; Sakai T; Amii H A convenient method for catalytic aromatic pentafluoroethylation using potassium (pentafluoroethyl)trimethoxyborate. Synthesis 2017, 49, 1874. [Google Scholar]; (o) Huang YJ; Ajitha MJ; Huang KW; Zhang ZX; Weng ZQ A class of effective decarboxylative perfluoroalkylating reagents: [(Phen)2Cu] (O2CRF). Dalton Trans 2016, 45, 8468. [DOI] [PubMed] [Google Scholar]; (p) Mestre J; Castillon S; Boutureira O Ligandless” pentafluoroethylation of unactivated (hetero)aryl and alkenyl halides enabled by the controlled self-condensation of TMSCF3-derived CuCF3. J. Org. Chem 2019, 84, 15087. [DOI] [PubMed] [Google Scholar]
- (13).For examples of trifluoromethylation of alkyl halides:; (a) Ambler BR; Zhu L; Altman RA Copper-catalyzed synthesis of trifluoro-ethylarenes from benzylic bromodifluoroacetates. J. Org. Chem 2015, 80, 8449. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Shen H; Liu Z; Zhang P; Tan X; Zhang Z; Li C Trifluoromethylation of alkyl radicals in aqueous solution. J. Am. Chem. Soc 2017, 139, 9843. [DOI] [PubMed] [Google Scholar]; (c) Chen Y; Ma G; Gong H Copper-catalyzed reductive trifluoromethylation of alkyl iodides with Togni’s reagent. Org. Lett 2018, 20, 4677. [DOI] [PubMed] [Google Scholar]
- (14).(a) Creutz SE; Lotito KJ; Fu GC; Peters JC Photoinduced Ullmann C–N coupling: demonstrating the viability of a radical pathway. Science 2012, 338, 647. [DOI] [PubMed] [Google Scholar]; (b) Johnson MW; Hannoun KI; Tan Y; Fu GC; Peters JC A mechanistic investigation of the photoinduced, copper-mediated cross-coupling of an aryl thiol with an aryl halide. Chem. Sci 2016, 7, 4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).(a) Le C; Chen TQ; Liang T; Zhang P; MacMillan DWC A radical approach to the copper oxidative addition problem: trifluoromethylation of bromoarenes. Science 2018, 360, 1010. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kornfilt DJP; MacMillan DWC Copper-catalyzed trifluoromethylation of alkyl bromides. J. Am. Chem. Soc 2019, 141, 6853. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lavagnino ML; Liang T; MacMillan DWC HARC as an open-shell strategy to bypass oxidative addition in Ullmann–Goldberg couplings. Proc. Natl. Acad. Sci. U. S. A 2020, 117, 21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).(a) Chatgilialoglu C Structural and Chemical Properties of Silyl Radicals. Chem. Rev 1995, 95, 1229. [Google Scholar]; (b) Chatgilialoglu C Organosilanes as radical-based reducing agents in synthesis. Acc. Chem. Res 1992, 25, 188. [Google Scholar]; (c) Chatgilialoglu C; Ferreri C; Landais Y; Timokhin VI Thirty years of (TMS)3SiH: A milestone in radical-based synthetic chemistry. Chem. Rev 2018, 118, 6516. [DOI] [PubMed] [Google Scholar]
- (17).For recent examples of isolated trifluoromethyl-copper(III) complexes:; (a) Paeth M; Tyndall SB; Chen LY; Hong JC; Carson WP; Liu XL; Sun XD; Liu J; Yang K; Hale EM; Tierney DL; Liu B; Cao Z; Cheng MJ; Goddard WA III; Liu W Csp3–Csp3 bond-forming reductive elimination from well-defined copper(III) complexes. J. Am. Chem. Soc 2019, 141, 3153. [DOI] [PubMed] [Google Scholar]; (b) Liu SS; Liu H; Liu S; Lu ZH; Lu CH; Leng XB; Lan Y; Shen QL C(sp3)-CF3 reductive elimination from a five-coordinate neutral copper(III) complex. J. Am. Chem. Soc 2020, 142, 9785. [DOI] [PubMed] [Google Scholar]; (c) Lu ZH; Liu H; Liu SH; Leng XB; Lan Y; Shen QL A key intermediate in copper mediated arene trifluoromethylation, [nBu4N][Cu(Ar)(CF3)3]: synthesis, characterization, and C(sp2)-CF3 reductive elimination. Angew. Chem., Int. Ed 2019, 58, 8510. [DOI] [PubMed] [Google Scholar]
- (18).(a) Ruppert I; Schlich K; Volbach W Die ersten CF3-substituierten organyl(chlor)silane. Tetrahedron Lett. 1984, 25, 2195. [Google Scholar]; (b) Prakash GKS; Krishnamurti R; Olah GA Fluoride-induced trifluoromethylation of carbonyl compounds with trifluoromethyltrimethylsilane (TMS-CF3). A trifluoromethide equivalent. J. Am. Chem. Soc 1989, 111, 393. [Google Scholar]; (c) Shibata N; Mizuta S; Kawai H Recent advances in enantioselective trifluoromethylation reactions. Tetrahedron: Asymmetry 2008, 19, 2633. [Google Scholar]
- (19).Goldsmith JI; Hudson WR; Lowry MS; Anderson TH; Bernhard S Discovery and high-throughput screening of heteroleptic iridium complexes for photoinduced hydrogen production. J. Am. Chem. Soc 2005, 127, 7502. [DOI] [PubMed] [Google Scholar]
- (20). See Supporting Information for cyclic voltammogram of aminosilane 4 in MeCN.; Sakai HA; Liu W; Le C; MacMillan DWC Cross-electrophile coupling of unactivated alkyl chlorides. J. Am. Chem. Soc 2020, 142, 11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).(a) Zhang P; Le CC; MacMillan DWC Silyl radical activation of alkyl halides in metallaphotoredox catalysis: a unique pathway for cross-electrophile coupling. J. Am. Chem. Soc 2016, 138, 8084. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Bacauanu V; Cardinal S; Yamauchi M; Kondo M; Fernandez DF; Remy R; MacMillan DWC Metallaphotoredox difluoromethylation of aryl bromides. Angew. Chem., Int. Ed 2018, 57, 12543. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Smith RT; Zhang X; Rincon JA; Agejas J; Mateos C; Barberis M; Garcia-Cerrada S; de Frutos O; MacMillan DWC Metallaphotoredox-catalyzed cross-electrophile Csp3-Csp3 coupling of aliphatic bromides. J. Am. Chem. Soc 2018, 140, 17433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Le CC; Wismer MK; Shi Z-C; Zhang R; Conway DV; Li G; Vachal P; Davies IW; MacMillan DWC A general small-scale reactor to enable standardization and acceleration of photocatalytic reactions. ACS Cent. Sci 2017, 3, 647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23). For additional examples and limitations of alkyl and aryl bromides substrates, see Supporting Information, Figure S14.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


