Abstract
Objectives:
SLE is an autoimmune disease characterised by aberrant lymphocyte activation and autoantibody production. This study provides an in-depth preclinical and clinical characterisation of the treatment effect of cenerimod, a sphingosine-1-phosphate receptor type 1 (S1P1) modulator, in SLE.
Methods:
Cenerimod effect on lymphocyte numbers, organ pathology, inflammation, and survival was evaluated in the MRL/lpr lupus mouse model. Lymphocytes from healthy subjects and patients with SLE were assessed for cenerimod-induced S1P1 receptor internalisation. Lymphocyte subsets and inflammatory biomarkers were characterised in a 12-week phase 2 clinical study (NCT-02472795), where patients with SLE were treated with multiple doses of cenerimod or placebo.
Results:
In MRL/lpr mice treated with cenerimod, blood lymphocytes were reduced, leading to reduced immune infiltrates into tissue, and decreased tissue pathology, proteinuria, and inflammation, resulting in increased survival. Cenerimod was potent and efficacious in inducing S1P1 receptor internalisation in lymphocytes in both healthy subjects and patients with SLE. In patients with SLE, 12-week cenerimod treatment resulted in a dose-dependent reduction of blood lymphocytes, antibody-secreting cells (ASC), and plasma IFN-α.
Conclusion:
Cenerimod significantly ameliorated systemic and organ-specific pathology and inflammation in a mouse model of SLE. In lymphocytes from patients with SLE, the S1P1 receptor remained functional despite concomitant background medication. The preclinical lymphocyte reduction translated to patients with SLE and resulted in the normalisation of ASC and the reduction of IFN-associated biomarkers. The efficacy and safety of cenerimod is being further investigated in a long-term clinical study in patients with SLE (CARE; NCT-03742037).
Keywords: Systemic Lupus Erythematosus, Bcells, TCells, Treatment, Inflammation
INTRODUCTION
Both B and T lymphocytes have been implicated in playing a major role in the pathogenesis of systemic lupus erythematosus (SLE), a complex and heterogeneous autoimmune disease of unknown aetiology, characterised by the production of high titres of pathogenic autoantibodies, infiltrating lymphocytes and tissue damage across multiple organ systems.1
In SLE, T lymphocytes orchestrate pathogenesis by directly driving organ damage and additionally by supporting B lymphocyte activation and proliferation, thus enabling the maturation of autoantibody-producing B lymphocytes, so-called antibody-secreting cells (ASC). ASC represent proliferative plasmablasts and plasma cells which are the predominant effector B lymphocytes in pathogenic conditions of autoimmunity.2 3 Increased numbers of ASC correlate with various aspects of disease activity in patients with active SLE.4–6
Concomitant to the changes in immune lymphocyte populations observed in patients with SLE, the inflammatory environment in SLE is reminiscent of a chronic interferon (IFN)-driven immune response associated with viral infections. These include sustained expression of type I IFNs, often referred to as the ‘IFN signature’,7–9 and an increase in IFN-responsive chemokines such as CXCL9 and CXCL10. Additionally, increases in modulators associated with SLE pathology such as interleukin 6 (IL-6) and tumor necrosis factor (TNF) exacerbate the inflammatory environment in SLE.10
One key aspect of the inextricable network between immune lymphocytes and the inflammatory environment is the interaction between lymphocytes and chemokine gradients, underlying the tightly controlled process of immune lymphocyte migration, which is fundamental in driving an adaptive immune response. The sphingosine-1-phosphate (S1P) chemotactic gradient is the predominant factor for lymphocyte egress out of peripheral lymphoid organs into the lymphatic and vascular circulation via S1P/S1P1 receptor engagement on B and T lymphocytes.11 S1P1 receptor modulators are approved to treat multiple sclerosis and have shown clinical benefit in other diseases, providing evidence that this mode of action is of importance in autoimmune conditions.12–14
Therefore, cenerimod, a novel potent, selective and orally active S1P1 receptor modulator15 was evaluated for efficacy in the MRL/lpr mouse model of SLE and clinically in patients with SLE.
MATERIALS AND METHODS
Details of materials and methods are available in online supplemental section.
rmdopen-2020-001261supp001.pdf (151.1KB, pdf)
RESULTS
Cenerimod reduces circulating and organ-infiltrating lymphocytes, decreases proteinuria and increases survival in the MRL/lpr mouse model of SLE
The MRL/lpr lupus mouse model reflects autoimmunity and organ pathology associated with SLE16 and was therefore used to evaluate the preclinical efficacy of cenerimod. Seven-week-old MRL/lpr mice, an age at which B cell abnormalities are already detectable,17 were treated with vehicle or cenerimod food admix (figure 1A). At treatment week 11, 6/20 mice in the vehicle group had died, achieving the predefined endpoint of at least 20% morbidity/mortality in one group. At this time point, all cenerimod-treated mice were still alive (figure 1B). Analysis of cenerimod-treated animals revealed a significant decrease of peripheral CD19+ B lymphocytes (−78.9%), and CD4+ (−98.9%) and CD8+ (−90.4%) T lymphocytes when compared with vehicle control (figure 1C).
Figure 1.
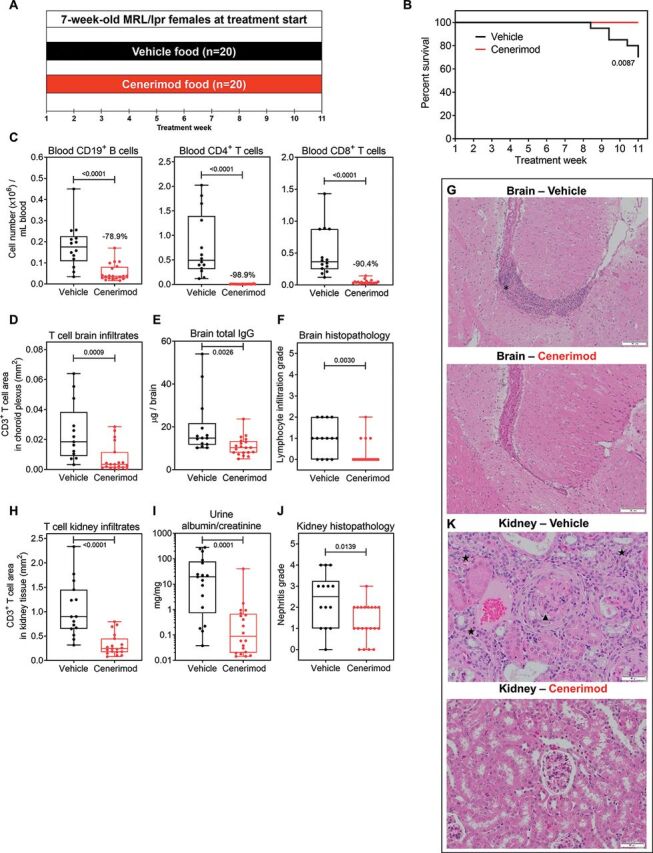
Cenerimod reduces circulating and organ-infiltrating lymphocytes, decreases proteinuria and increases survival in the MRL/lpr mouse model of SLE.
(A) Lupus study design in MRL/lpr female mice; mice were 7 weeks of age at treatment start (treatment week 1). Study was predefined to end when at least 20% morbidity/mortality was reached in one group, which was attained at treatment week 11. (B) Survival curve of vehicle-treated (n=20) versus cenerimod-treated (n=20) mice; Mantle–Cox test for comparisons between groups. (C) Blood B and T lymphocyte counts were quantified at end of treatment by flow cytometry (treatment week 11). Frequencies represent the specified lymphocyte subset decrease in the cenerimod- versus vehicle-treated group (median). (D) The total number of CD3+ T lymphocyte area (mm2) normalised against the total tissue area in the choroid plexus area of two brain levels was quantified using immunofluorescence analysis. (E) Total IgG in the brain was measured by ELISA. (F) Histopathological grade of lymphocyte infiltration in the right half brain of individual mice. The severity of lesions was graded using a 5-degree system: 0=normal, 1=minimal (focal cell infiltration), 2=slight (multifocal cell infiltration), 3=moderate, 4=marked and 5=severe. (G) Representative H&E sections from the right half brain morphologically assessed by bright field microscopy; lymphocyte infiltration in the tela choroidea (asterisk); scale bars=100 µm; objective 10×. (H) The total number of CD3+ T lymphocyte area (mm2) normalised against the total tissue area from a representative kidney section was quantified using immunofluorescence analysis. (I) Ratio of urine albumin to urine creatinine at treatment week 10. (J) Histopathological grade of nephritis in the right kidney of individual mice. The severity of lesions was graded using a 5-degree system: 0=normal (maximum inflammatory cell focus within the murine background), 1=minimal (pyelitis), 2=slight (tubulo-interstitial nephritis), 3=moderate (multifocal membranoproliferative glomerulonephritis (MPGN)), 4=marked (diffuse MPGN), and 5=severe. (K) Representative H&E sections from the kidney morphologically assessed by bright field microscopy; membranoproliferative glomerulonephritis (arrowhead) and chronic tubulo-interstitial nephritis (star); scale bars=50 µm; objective 20×. Each data point represents the measurement of individual mice; box and whisker plots, the whiskers indicate the minimum and maximum range; p values are shown when statically significant (Mann–Whitney test). SLE, systemic lupus erythematosus.
Brain and kidney pathology are commonly associated with severe complications in patients with SLE,10 aspects of which can be addressed in the MRL/lpr model. In the brain, cenerimod treatment led to a significant reduction of T cell infiltrates (figure 1D) and total IgG (figure 1E). Incidence of brain pathology was significantly decreased in cenerimod-treated (20%) compared with vehicle-treated mice (71%) (figure 1F), displaying reduced lymphocyte infiltrate in the choroid plexus and tela choroidea (figure 1G).
In the kidney, a significant reduction of T cell infiltrates (figure 1H) and a 25% decrease in kidney weight (online supplemental figure 1, p<0.0001) was observed in cenerimod-treated compared with vehicle-treated mice. Importantly, the decrease in urine albumin/creatinine ratio (figure 1I) was associated with reduced kidney pathology. The severity of histological damage (membranoproliferative glomerulonephritis associated with chronic tubulo-interstitial nephritis and pyelitis) was significantly lower in the cenerimod-treated group (median severity score of 1.0; IQR: 1.0–2.0) when compared with vehicle (median severity score of 2.5; IQR: 1.0–3.25) (figure 1J,K).
rmdopen-2020-001261supp002.pdf (121.9KB, pdf)
Cenerimod suppresses the systemic and local inflammatory environment in the MRL/lpr mouse model of SLE
Cenerimod treatment significantly reduced plasma anti-dsDNA and immunoglobulin (Ig) levels (figure 2A), and plasma IFN-associated, proinflammatory-associated and B cell-associated biomarkers (figure 2B). Importantly, the systemic effect of cenerimod was reflected in a statistically significant reduction of several of these disease relevant biomarkers in the brain (figure 2C).
Figure 2.
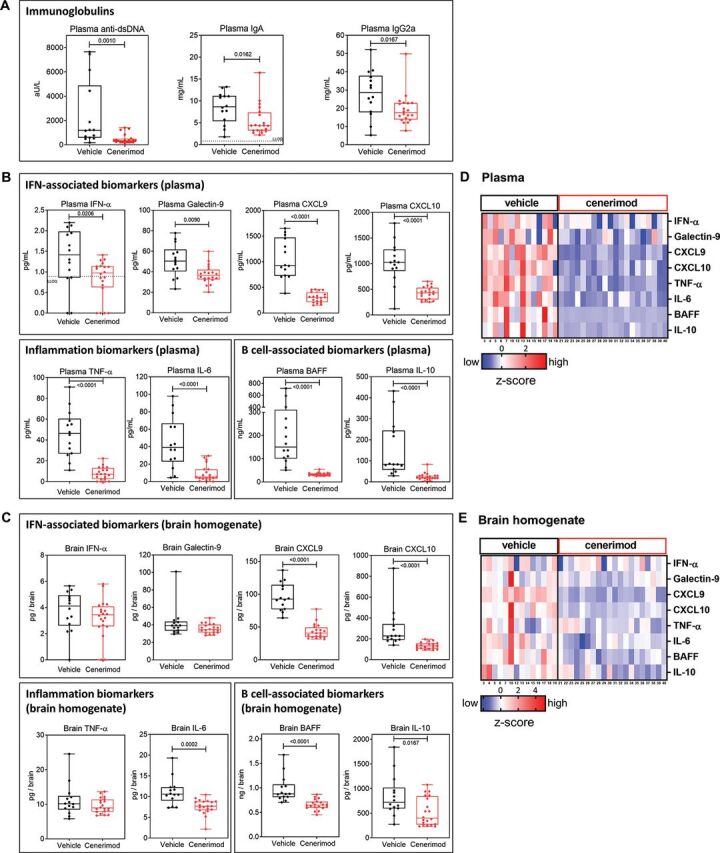
Cenerimod suppresses the systemic and local inflammatory environment in the MRL/lpr mouse model of SLE.
Disease relevant biomarkers in plasma and brain were measured at end of treatment (treatment week 11). (A) Plasma anti-double-stranded DNA (anti-dsDNA), plasma IgA and IgG2a antibody titres were measured with ELISA. (B, C) Biomarkers measured in plasma (B) and brain (C) representing IFN-associated (IFN-α, Galectin-9, CXCL9, CXCL10), inflammatory (TNF-α, IL-6) and B cell-associated biomarkers (B cell-activating factor (BAFF) and IL-10) using ELISA. (D, E) Heatmap plots of z-score normalised biomarker levels in plasma (D) and brain (E) from individual MRL/lpr mice at end of treatment (treatment week 11). Each vertical line represents z-scores from one individual mouse; numbers below heatmap plot represent individual mice. Missing numbers in vehicle group are from mice that died before treatment week 11; no mouse died in the cenerimod treatment group. Each data point represents the measurement of individual mice; box and whisker plots, the whiskers indicate the minimum and maximum range; p values are shown when statistically significant (Mann–Whitney test). IFN, interferon; LLOQ: lowest level of quantification; SLE, systemic lupus erythematosus; TNF, tumour necrosis factor.
In summary, cenerimod showed a consistent and pronounced suppression of the inflammatory environment in the MRL/lpr mouse model both systemically (figure 2D) and locally in brain tissue (figure 2E).
B and T lymphocytes in both healthy subjects and patients with SLE are responsive to cenerimod in vitro
In a non-interventional exploratory study of 10 healthy subjects and 10 patients with SLE (figure 3A), leucocyte populations were quantified (online supplemental figure 2A). Patients with SLE had decreased T lymphocytes, specifically central memory CD4+ and CD8+ T cells (online supplemental figure 2B), and plasmacytoid dendritic cells compared with healthy subjects while other populations including B lymphocytes were unchanged (online supplemental figure 2A).
Figure 3.
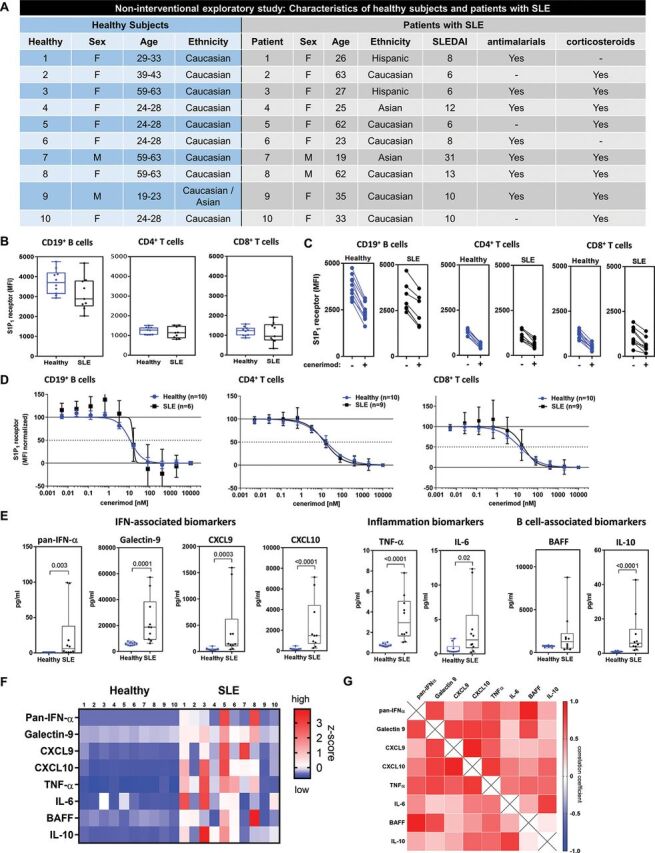
Lymphocytes in both healthy subjects and patients with SLE are responsive to cenerimod in vitro, and patients with SLE present with a heterogeneous inflammatory phenotype.
(A) In the non-interventional exploratory study, 10 patients with SLE (SLEDAI ≥6–31) and 10 matched healthy subjects were recruited. Patients with SLE were predominantly female (8/10), between 19 and 63 years old, and treated with antimalarials (7/10) and corticosteroids (7/10). (B) S1P1 receptor surface expression (MFI) on blood CD19+ B lymphocytes and CD4+ and CD8+ T lymphocytes isolated from healthy subjects and patients with SLE from the non-interventional exploratory study. Each data point represents the measurement of individual subjects; box and whisker plots, the whiskers indicate the minimum and maximum range. (C) S1P1 receptor surface expression (MFI) before (−) and following (+) incubation with cenerimod with lines indicating paired samples. (D) Mean concentration–response curves of min/max normalised S1P1 receptor surface expression levels for healthy subjects and patients with SLE. (E) Box and whisker plots of disease-relevant biomarkers in patients with SLE compared with healthy subjects (non-interventional exploratory study); the whiskers indicate the minimum and maximum range. P values are given (Mann–Whitney test). (F) Heatmap of z-score normalised biomarker levels for each individual subject in the non-interventional exploratory study. (G) Heatmap of Spearman rank correlation (r) of biomarkers in patients with SLE from the non-interventional exploratory study. Red represents positive and blue negative Spearman rank correlation. All p values are indicated where statistically significant (Mann–Whitney test). MFI, median fluorescence intensity; SLE, systemic lupus erythematosus.
rmdopen-2020-001261supp003.pdf (369.5KB, pdf)
Blood B (CD19+) and T lymphocytes (CD4+ and CD8+) were isolated and analysed for surface expression of S1P1 receptor using flow cytometry (online supplemental figure 2C). Mean surface expression of the S1P1 receptor was similar in healthy subjects and patients, and expression was threefold higher in B compared with T lymphocytes in all individuals (figure 3B). In vitro, cenerimod induced a consistent internalisation of the S1P1 receptor on B and T lymphocytes (figure 3C). Importantly, cenerimod had a similar potency (EC50, half-maximal effective concentration) on B (10.8 nM vs 15.3 nM), CD4+ T (16.5 nM vs 14 nM), and CD8+ T lymphocytes (13.2 nM vs 19.6 nM) from healthy subjects and patients (figure 3D). In summary, peripheral B and T lymphocytes in both healthy subjects and patients with SLE treated with standard of care were responsive to cenerimod-induced S1P1 receptor modulation.
Patients with SLE present with a heterogeneous inflammatory phenotype
Currently, no clinically approved biomarker exists to monitor SLE disease activity (monitoring), to predict disease progression (prognostic), or to capture disease heterogeneity. Therefore, a panel of known exploratory biomarkers were used to characterise patients with SLE. In the non-interventional exploratory study, these SLE-associated biomarkers were all increased in patients compared with healthy subjects (figure 3E). Specifically, type-1 IFN signature molecules such as pan-IFN-α,18 and Galectin-919 as well as the IFN-responsive chemokines CXCL920 and CXCL10,21 and the inflammation biomarkers TNF-α22 and IL-6,23 24 were statistically significantly elevated. In addition, B cell-activating factor (BAFF), a critical survival factor for mature B-lymphocytes and promoter of B-lymphocyte proliferation and differentiation, IgG class switching25 and anti-dsDNA production26 was increased. IL-10, a cytokine associated with B-lymphocyte hyperactivity and autoantibody production,27 28 was also statistically significantly increased (figure 3E). A heatmap of these plasma biomarkers demonstrated a heterogeneity in the inflammatory severity between patients. Within individual patients, the overall biomarker phenotype was consistent, ranging from broadly elevated to levels observed in healthy subjects (figure 3F). The correlation between the individual biomarkers was high in patients with SLE (figure 3G). The heterogeneity between patients was confirmed in a larger patient cohort (online supplemental figure 2D). Additionally, there was a positive correlation between the majority of individual biomarkers across the cohort (online supplemental figure 2E).
In the phase 2 SLE study, cenerimod treatment dose-dependently reduces blood B and T lymphocytes
In the phase 2 SLE study, patients with SLE were treated for 12 weeks with multiple doses of cenerimod (figure 4A).29 A dose-dependent reduction in blood CD19+ B, CD4+ and CD8+ T lymphocytes was observed following 12 weeks of cenerimod treatment compared with placebo control groups (figure 4B). The median reduction was most pronounced in CD4+ T (−94%) and B lymphocytes (−90%) compared with CD8+ T lymphocytes (−63%). A granular analysis of T lymphocytes revealed a dose-dependent reduction of all investigated subsets with the most pronounced effect on CD4+ T lymphocytes in general and central memory lymphocytes in particular (figure 4C).
Figure 4.
In the phase 2 SLE study, cenerimod treatment dose-dependently reduces blood B and T lymphocytes.
(A) In the phase 2 SLE study, 60 patients with SLE (modified PD set) from the phase 2 clinical study with cenerimod were included.29 In brief, the phase 2 SLE study part A consisted of a placebo group (n=11), 0.5 mg (n=12), 1 mg (n=10) and 2 mg cenerimod treatment groups (n=13); the phase 2 SLE study part B consisted of a placebo group (n=5) and 4 mg cenerimod treatment group (n=9). Treatment lasted for 12 weeks. (B) Enumeration of blood CD19+ B lymphocytes and CD4+ and CD8+ T lymphocytes from patients with SLE treated with cenerimod or placebo from the phase 2 SLE study at end of treatment (week 12) as percentage compared with baseline levels using flow cytometry. Box and whisker plots, the whiskers indicate the minimum and maximum range. Dotted lines represent the no change level in lymphocyte numbers compared with baseline (100%). (C) Heatmap representation of median per cent change of blood B and T lymphocyte subsets compared with baseline levels in patients with SLE treated with cenerimod or placebo from the phase 2 SLE study. Red represents high and white low percentage decrease. All p values shown were statistically significant (Mann–Whitney t-test for the non-interventional exploratory study and phase 2 SLE study part B; Kruskal–Wallis test with Dunn’s multiplicity-correction for the phase 2 SLE study part A). CM, central memory, EM, effector memory, EMRA, effector memory CD45RA positive; SLE, systemic lupus erythematosus.
In the phase 2 SLE study, cenerimod treatment normalises blood antibody-secreting cell numbers which correlate with IFN-associated biomarkers
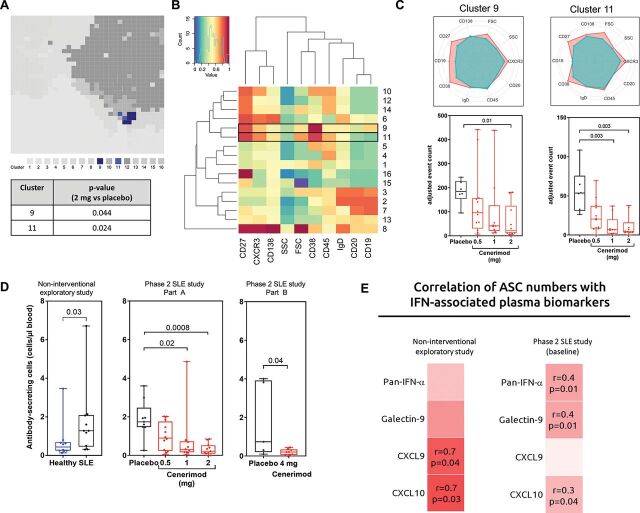
Based on the high expression of S1P1 receptor on blood B lymphocytes, the increase in B cell-associated biomarkers in SLE, and a cenerimod-driven blood B lymphocyte reduction, an unbiased analysis of B lymphocyte populations in the phase 2 SLE study part A was performed (figure 5A–C). The self-organising map algorithm used, identified 16 clusters based on surface marker expression. Of the B cell-specific clusters, clusters 9 and 11 were statistically significantly reduced following cenerimod treatment (figure 5A).
Figure 5.
In the phase 2 SLE study, cenerimod treatment normalises blood antibody-secreting cell numbers which correlate with IFN-associated biomarkers.
(A–C) Unbiased analysis and quantification of blood B lymphocytes from the phase 2 SLE study part A at week 12. (A) Total number of distinct clusters identified using a panel of B lymphocyte surface markers shown in a self-organising map. The relative size of each cluster is depicted in the map, with each individual cluster assigned a colour. B cell clusters with a statistically significant difference between 2 mg cenerimod treatment and placebo are shown in shades of blue (clusters 9 and 11). The p value for each statistically significant changed B cell cluster is given (Wilcoxon test). (B) Heatmap depicting relative expression of associated B lymphocyte cell surface expression markers with individual clusters ordered in hierarchical association horizontally, and hierarchical association of lymphocyte surface markers, SSC, and FSC ordered vertically. Red represents high, and blue low cell surface expression values. (C) Radar plots of clusters 9 and 11. Depicted are relative levels of cell surface marker expression for individual clusters. Red represents the individual cluster in comparison to the total population represented in turquoise. Enumeration of clusters shown as beads-adjusted event count of cells. (D) Enumeration of blood antibody-secreting cells based on the described phenotype5 from the non-interventional exploratory study, and phase 2 SLE study at week 12. All p values shown were statistically significant (Mann–Whitney t-test for the non-interventional exploratory study and phase 2 SLE study part B; Kruskal–Wallis test with Dunn’s multiplicity-correction for the phase 2 SLE study part A). (E) Heatmap of Spearman rank correlation (r) of blood antibody-secreting cells versus plasma IFN-associated biomarkers in patients with SLE from the non-interventional exploratory study, and phase 2 SLE study at baseline. Red represents positive and blue negative Spearman rank correlation. The r and p values are indicated for all correlations that are significant. FSC, forward scatter; SSC, side scatter; SLE, systemic lupus erythematosus. Results are displayed as box and whisker plots; the whiskers indicate the minimum and maximum range.
Cells in cluster 9 had higher expression of CD19, CD20, CD27, CD38 and CXCR3 when compared with the whole lymphocyte population; cells in cluster 11 had a similar expression pattern to cluster 9 except for lower expression of CD19 and higher expression of CD138 (figure 5B,C). Clusters 9 and 11 represented a subset of B lymphocytes resembling previously identified ASC which are known to be central to autoimmunity.5 Enumeration of these cenerimod-responsive cell clusters confirmed a dose-dependent reduction in absolute numbers in the cenerimod-treated groups compared with placebo control (figure 5C).
In the non-interventional exploratory study, patients with SLE showed a significant elevation in ASC numbers compared with healthy subjects (figure 5D). In the phase 2 SLE study, cenerimod treatment dose-dependently normalised ASC numbers to levels observed in healthy subjects (figure 5D and online supplemental figure 3A). The role of ASC in the production of pathogenic antibodies is well described5 and the increase in ASC was reflected in an increase of anti-dsDNA in patients from the non-interventional exploratory study (online supplemental figure 3B). Treatment with 4 mg cenerimod led to a significant decrease in systemic anti-dsDNA antibodies (online supplemental figure 3B) and total IgG (online supplemental figure 3C).
rmdopen-2020-001261supp004.pdf (144.6KB, pdf)
The relationship between ASC numbers and the inflammatory environment in patients with SLE was investigated. In the non-interventional exploratory study, ASC numbers correlated positively with the IFN-associated biomarkers CXCL9 (r=0.7, p=0.04) and CXCL10 (r=0.7, p=0.03); in the phase 2 SLE study prior to cenerimod treatment, ASC numbers correlated positively with pan-IFN-α (r=0.4, p=0.01), Galectin-9 (r=0.4, p=0.01) and CXCL10 (r=0.3, p=0.04) (figure 5E).
The phenotypically defined ASC from the non-interventional exploratory study were characterised by RNA sequencing analysis. ASC from patients with SLE had 601 differentially expressed genes (DEG) (false discovery rate <0.05 and fold change >1.5) compared with ASC from healthy subjects. Pathway analysis revealed IFN signalling to be the most significantly increased pathway (online supplemental figure 4A). Genes representing the previously reported 60-gene IFN signature identified in whole blood (WB) of patients with SLE30 were strongly upregulated, confirming the dominance of the IFN pathway in ASC (online supplemental figure 4B).
rmdopen-2020-001261supp005.pdf (164.2KB, pdf)
In summary, cenerimod treatment in patients with SLE normalised ASC numbers which correlated with the IFN-phenotype.
A whole blood gene expression signature of patients with SLE reflects an elevated IFN-associated phenotype
A WB gene expression analysis in patients with SLE versus healthy subjects was performed in the non-interventional exploratory study to investigate its relationship to the elevation of IFN-associated biomarkers and ASC numbers.
Gene expression analysis of WB revealed 830 DEG with 642 genes upregulated and 178 genes downregulated in patients with SLE versus healthy subjects. The most prominent canonical pathways perturbed in patients with SLE represented genes associated with IFN signalling, estrogen-mediated S-phase entry, and cyclins and cell cycle regulation/proliferation (figure 6A). Genes from the 60-gene IFN gene signature30 were strongly upregulated confirming the prominence of the IFN pathway, depicted as a heatmap (figure 6B), and captured in an overall score (figure 6C). In addition, increased plasma IFN-α protein levels correlated with the elevated IFN gene signature score (r=0.8, p<0.0001) (supplemental figure 4C). A correlation analysis demonstrated the WB-SLE gene signature to correlate stronger with the IFN-associated biomarkers than the IFN gene signature (figure 6D). Furthermore, the WB-SLE gene signature correlated with blood ASC numbers (r=0.6, p=0.006, online supplemental figure 4D).
Figure 6.

Whole blood gene expression signature of patients with SLE reflects an elevated IFN-associated phenotype which is decreased after cenerimod treatment.
(A) Analysis of the WB-SLE gene signature for the top three significantly enriched pathways. The dotted line represents the threshold defining the significance level of p=0.05. (B) Heatmap depicting the relative expression level of individual genes of the IFN gene signature identified by Li et al in WB of individuals from the non-interventional exploratory study. Individuals are shown vertically, genes are depicted horizontally. Red; high relative expression level. Blue; low relative expression level. (C) Overall IFN gene signature score from figure 6B for each individual from the non-interventional exploratory study. P value (Mann–Whitney test). (D) Heatmap of Spearman rank correlation (r) of plasma IFN-associated biomarkers and gene signatures in blood from individuals from the non-interventional exploratory study. Red represents positive Spearman rank correlation. The r and p values are indicated for all correlations between the WB-SLE signature or IFN signature and plasma IFN-associated biomarkers. (E) Quantification of plasma pan-IFN-α protein after 12 weeks of cenerimod treatment versus placebo in the phase 2 SLE study. Results are displayed as mean plus SEM (numerical reduction). (F) Heatmap of normalised z-score of IFN-associated protein biomarkers ranked according to the normalised z-score delta (numerical reduction) from the phase 2 SLE study part A (left panel) and part B (right panel) after 12 weeks of treatment. IFN, interferon; SEM: standard error of the mean; SLE, systemic lupus erythematosus; WB, whole blood.
Cenerimod treatment dose-dependently decreases IFN-associated biomarkers
Analysis of the IFN-associated biomarkers in the phase 2 SLE study following 12 weeks of cenerimod treatment demonstrated a numerical, dose-dependent reduction in absolute plasma pan-IFN-α levels (figure 6E) and in per cent to baseline (online supplemental figure 4E). Analysis of the IFN-associated biomarkers in the phase 2 SLE study after 12 weeks of cenerimod treatment also demonstrated a consistent reduction in CXCL9, CXCL10 and Galectin-9 (figure 6F, online supplemental figure 4F).
DISCUSSION
Despite significant improvements in disease understanding and management of patients with SLE, remission and preservation of organ function is still considered challenging due to overreliance on corticosteroids and restricted options of effective drugs.31 32 In this study, cenerimod, a novel, potent, selective and orally active S1P1 receptor modulator was characterised in the context of SLE.
The MRL/lpr mouse model presents with a systemic autoimmune phenotype, which possesses pathophysiological hallmarks of SLE, and is amenable to study histopathology in target organs typically associated with SLE.16 Cenerimod treatment decreased blood B and T lymphocyte numbers, consistent with its mechanism of action in sequestering lymphocytes in secondary lymphoid organs and is consistent with previous findings describing S1P receptor modulators in models of SLE.33–37 Importantly, the reduction of circulating lymphocytes translated to a reduction in organ-infiltrating lymphocytes in the kidney and brain. Consequently, organ-specific pathology and proteinuria were attenuated and culminated in improved survival. Cenerimod treatment also significantly reduced IFN-associated, inflammatory, and B cell-associated biomarkers. In summary, the preclinical data demonstrated that cenerimod treatment, by sequestering B and T lymphocytes, led to an efficient suppression of the inflammatory phenotype associated with SLE. Important for the translatability, the inflammatory phenotype in the blood and target organ of MRL/lpr mice, which could be reduced by cenerimod treatment, was also observed in the blood of patients with SLE.
Cenerimod was further investigated in patients with SLE following the promising results obtained in the MRL/lpr lupus mouse model. Cenerimod internalised the S1P1 receptor with equal potency on B and T lymphocytes isolated from healthy subjects and patients with SLE, thus rendering the lymphocytes non-responsive to S1P gradients. The results demonstrated that despite patients being treated with antimalarials and corticosteroids, cenerimod’s mechanism of action remained functional.
Patients with SLE present with a heterogeneous clinical and molecular phenotype.38 To capture this heterogeneous molecular phenotype, we characterised biomarkers associated with SLE immunopathogenesis. Patients showed a statistically significant increase of biomarkers related to B lymphocyte activity, the IFN pathway, and inflammation. B lymphocytes are an important source of IFN-α,39 40 which represents a relevant driver of SLE pathology, and has been employed for patient stratification in clinical studies.41 Whereas individual patients at time of analysis had no inflammation, others showed pronounced inflammation, reflected in elevation of all the evaluated biomarkers. These data highlight the interconnectivity of these biomarkers and indicate that the IFN pathway is only one component of the general inflammatory phenotype in SLE.
In the phase 2 SLE study, blood B and T lymphocytes were dose-dependently reduced, successfully translating cenerimod’s mechanism of action observed preclinically to patients with SLE. Cenerimod was equi-efficacious on CD19+ B and CD4+ T lymphocytes highlighting the essential role of the S1P–S1P1 receptor pathway not only in T cell, but also in B cell trafficking in SLE. The importance of B lymphocytes in patients with SLE is well documented42 and supported by the approval of an antibody targeting B cell activation.43 Although profound lymphopenia is considered a risk factor for infection, cenerimod treatment in the 12-week study did not affect rates of infection.29
An unbiased multivariate analysis of the B lymphocyte subsets revealed that cenerimod treatment significantly reduced a cell population resembling ASC. This cell population has been shown to be elevated in patients with SLE5 44 45 and correlates with increased anti-dsDNA levels.46 A focused phenotypical analysis of ASC confirmed that cenerimod treatment dose-dependently normalised their numbers to a level comparable to healthy subjects and reduced total IgG and anti-dsDNA levels. These data suggest that by normalising ASC numbers, cenerimod treatment may lead to a reduction of pathogenic autoantibodies. The strong effect of cenerimod treatment on reducing blood CD4+ T lymphocytes could provide an additional mechanism to limit B cell help and consequently ASC numbers and anti-dsDNA levels.
The IFN pathway was previously described as an important contributor to the loss of tolerance47 and in driving the increased frequency of ASC in patients.48 Furthermore, B lymphocytes were reported to produce significant amounts of IFN-α in patients with SLE,39 40 indicating that B lymphocytes (including ASC) might contribute to a systemic IFN-α increase. In line with these reported findings, both in the non-interventional exploratory and phase 2 SLE studies, ASC numbers correlated significantly with the IFN-associated biomarkers IFN-α, Galectin-9, CXCL9, and CXCL10. Next-generation sequencing of isolated ASC and WB confirmed a strong expression of IFN-associated genes in patients with SLE, as demonstrated by the striking upregulation of the published IFN gene signature.30 Elevation of IFN-associated gene expression was reflected in higher plasma IFN-α. The significance of blocking this pathway has been demonstrated in a phase 3 clinical study in patients with 2the non-interventional exploratory study, the WB gene expression signature identified in patients with SLE (WB-SLE) was superior to the IFN gene signature in reflecting the inflammatory phenotype. Besides IFN signalling, the WB-SLE signature also captured additionally activated pathways including estrogen-mediated S-phase entry, which is implicated in immune cell activation and cytokine production,50 and cyclins and cell cycle regulation which may be why the WB gene signature better reflected the phenotype. These data substantiate the observation that multiple pathways contribute to the overall activation of the adaptive and innate immune system in patients with SLE.38 51 In the phase 2 SLE study, concomitantly to the reduction in ASC number, cenerimod treatment reduced plasma IFN-α levels and had a small effect on other IFN-associated biomarkers. While cenerimod treatment directly reduced circulating lymphocytes, probably as a consequence it also indirectly dampened IFN-α levels. B cell-associated and inflammatory biomarkers were not impacted in this short 12-week study. It remains to be seen in a long-term clinical study and larger patient cohort if the above-mentioned effects can be confirmed and whether cenerimod treatment can decrease additional inflammatory biomarkers and associated pathologies in patients with SLE.
In summary, cenerimod treatment demonstrated preclinical efficacy in the MRL/lpr mouse model. In patients with SLE, cenerimod treatment reduced blood B and T lymphocytes, normalised ASC numbers, decreased autoantibodies and reduced IFN-associated biomarkers. Cenerimod is being investigated in a multiple-dose clinical study up to 12 months in patients with SLE (CARE: NCT-03742037).
Key messages.
What is already known about this subject?
Sphingosine-1-phosphate (S1P)-dependent chemotaxis is a key mechanism of regulating B and T lymphocyte egress from secondary lymphoid organs into the lymphatic and vascular circulation contributing to autoimmune disease pathogenesis.
S1P receptor modulators are approved for the treatment of relapsing-remitting and secondary progressive multiple sclerosis.
What does this study add?
Cenerimod, a novel potent and selective S1P1 receptor modulator, showed significant efficacy in the MRL/lpr mouse model of SLE; specifically, by reducing blood B and T lymphocytes, cenerimod treatment reduced proteinuria and tissue pathology, and controlled systemic and local inflammation resulting in increased survival.
In patients with SLE, cenerimod treatment reduced blood B and T lymphocytes, normalised blood antibody-secreting cell numbers, and reduced IFN-associated biomarkers.
How might this impact on clinical practice?
This study supports the further development of cenerimod in SLE where this treatment strategy may provide benefit to patients with SLE.
Acknowledgments
The authors would like to thank Baumlin N., Bourquin G., Farine H., Hartl D., Kulig P., Marrie J., Pouzol L., Raoult E., Runser C., Sassi A., Scherer J., Tunis M., von Münchow A., Wyss C., and Zurbach A., for their outstanding technical and scientific contributions.
Footnotes
Contributors: DSS, SF, MMM, MJM were responsible for the conception and design of the studies, data interpretation, and manuscript drafting. VS, EG, UG, GMP, EV, AKS, BR, JR, AH carried out experiments and analysed the data. MT and AKG were responsible for running the exploratory non-interventional study and manuscript review. UM, MPK and PMAG were involved in study concept and design, and manuscript review. All authors had access to and interpreted the data. All authors helped to critically revise the intellectual content of the manuscript and approved the final submission.
Funding: This research study was funded by Idorsia Pharmaceuticals Ltd.
Competing interests: DSS, SF, VS, EG, UG, GP, EV, AKS, BR, JR, AH, UM, PMAG, MPK, MMM, and MJM are employees of Idorsia Pharmaceuticals Ltd. MT, grant research support.
Patient consent for publication: Not required.
Ethics approval: All experimental procedures were conducted in accordance with the Swiss Animal Welfare Ordinance and Idorsia Animal Welfare Policy on the use of experimental animals. The study was approved by the Baselland Cantonal Veterinary Home Office. The clinical studies were run under the following ethics committee approvals: The non-interventional exploratory study (EK2017-02273, EK179/11 EK249/02, EKNZ, CH), the phase 2 SLE study.29
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
REFERENCES
- 1. Rahman A, Isenberg DA, Systemic lupus erythematosus. N Engl J Med 2008;358:929–39. 10.1056/NEJMra071297 [DOI] [PubMed] [Google Scholar]
- 2. Crispin JC, Kyttaris VC, Terhorst C, et al. , T cells as therapeutic targets in SLE. Nat Rev Rheumatol 2010;6:317–25. 10.1038/nrrheum.2010.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Simpson N, Gatenby PA, Wilson A, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum 2010;62:234–44. 10.1002/art.25032 [DOI] [PubMed] [Google Scholar]
- 4. Odendahl M, Jacobi A, Hansen A, et al. Disturbed peripheral B lymphocyte homeostasis in systemic lupus erythematosus. J Immunol 2000;165:5970–9. 10.4049/jimmunol.165.10.5970 [DOI] [PubMed] [Google Scholar]
- 5. Tipton CM, Fucile CF, Darce J, et al. Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat Immunol 2015;16:755–65. 10.1038/ni.3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Isenberg DA, McClure C, Farewell V, et al. Correlation of 9G4 idiotope with disease activity in patients with systemic lupus erythematosus. Ann Rheum Dis 1998;57:566–70. 10.1136/ard.57.9.566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bennett L, Palucka AK, Arce E, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med 2003;197:711–23. 10.1084/jem.20021553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lovgren T, Eloranta ML, Bave U, et al. , Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum 2004;50:1861–72. 10.1002/art.20254 [DOI] [PubMed] [Google Scholar]
- 9. Crow MK, Kirou KA, Wohlgemuth J. Microarray analysis of interferon-regulated genes in SLE. Autoimmunity 2003;36:481–90. 10.1080/08916930310001625952 [DOI] [PubMed] [Google Scholar]
- 10. Kaul A, Gordon C, Crow MK, et al. Systemic lupus erythematosus. Nat Rev Dis Primers 2016;16:16039 10.1038/nrdp.2016.39 [DOI] [PubMed] [Google Scholar]
- 11. Chiba K, Matsuyuki H, Maeda Y, et al. Role of sphingosine 1-phosphate receptor type 1 in lymphocyte egress from secondary lymphoid tissues and thymus. Cell Mol Immunol 2006;3:11–19. 10.1016/j.pharmthera.2005.05.002 [DOI] [PubMed] [Google Scholar]
- 12. Sanford M. Fingolimod: a review of its use in relapsing-remitting multiple sclerosis. Drugs 2014;74:1411–33. 10.1007/s40265-014-0264-y [DOI] [PubMed] [Google Scholar]
- 13. Goodman AD, Anadani N, Gerwitz L. Siponimod in the treatment of multiple sclerosis. Expert Opin Investig Drugs 2019;3:1–7. 10.1080/13543784.2019.1676725 [DOI] [PubMed] [Google Scholar]
- 14. Stepanovska B, Huwiler A. Targeting the S1P receptor signaling pathways as a promising approach for treatment of autoimmune and inflammatory diseases. Pharmacol Res 2020;154:104170 10.1016/j.phrs.2019.02.009 [DOI] [PubMed] [Google Scholar]
- 15. Piali L, Birker-Robaczewska M, Lescop C, et al. Cenerimod, a novel selective S1P1 receptor modulator with unique signaling properties. Pharmacol Res Perspect 2017;5:6 10.1002/prp2.370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Celhar T, Fairhurst AM. Modelling clinical systemic lupus erythematosus: similarities, differences and success stories. Rheumatology (Oxford) 2017;56:i88–i99. 10.1093/rheumatology/kew400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu J, Karypis G, Hippen KL, et al. Genomic view of systemic autoimmunity in MRLlpr mice. Genes Immun 2006;7:156–68. 10.1038/sj.gene.6364286 [DOI] [PubMed] [Google Scholar]
- 18. Mathian A, Mouries-Martin S, Dorgham K, et al. Monitoring disease activity in systemic lupus erythematosus with single-molecule array digital enzyme-linked immunosorbent assay quantification of serum interferon-alpha. Arthritis Rheumatol 2019;71:756–65. 10.1002/art.40792 [DOI] [PubMed] [Google Scholar]
- 19. van den Hoogen LL, JAG VR, Mertens JS, et al. Galectin-9 is an easy to measure biomarker for the interferon signature in systemic lupus erythematosus and antiphospholipid syndrome. Ann Rheum Dis 2018;77:1810–14. 10.1136/annrheumdis-2018-213497 [DOI] [PubMed] [Google Scholar]
- 20. Bauer JW, Baechler EC, Petri M, et al. Elevated serum levels of interferon-regulated chemokines are biomarkers for active human systemic lupus erythematosus. PLoS Med 2006;3:e491 10.1371/journal.pmed.0030491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bauer JW, Petri M, Batliwalla FM, et al. Interferon-regulated chemokines as biomarkers of systemic lupus erythematosus disease activity: a validation study. Arthritis Rheum 2009;60:3098–107. 10.1002/art.24803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weckerle CE, Mangale D, Franek BS, et al. Large-scale analysis of tumor necrosis factor alpha levels in systemic lupus erythematosus. Arthritis Rheum 2012;64:2947–52. 10.1002/art.34483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Illei GG, Shirota Y, Yarboro CH, et al. Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheum 2010;62:542–52. 10.1002/art.27221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ripley BJ, Goncalves B, Isenberg DA, et al. Raised levels of interleukin 6 in systemic lupus erythematosus correlate with anaemia. Ann Rheum Dis 2005;64:849–53. 10.1136/ard.2004.022681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Litinskiy MB, Nardelli B, Hilbert DM, et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol 2002;3:822–9. 10.1038/ni829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Suurmond J, Calise J, Malkiel S, et al. DNA-reactive B cells in lupus. Curr Opin Immunol 2016;43:1–7. 10.1016/j.coi.2016.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tackey E, Lipsky PE, Illei GG. Rationale for interleukin-6 blockade in systemic lupus erythematosus. Lupus 2004;13:339–43. 10.1191/0961203304lu1023oa [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Geginat J, Larghi P, Paroni M, et al. The light and the dark sides of interleukin-10 in immune-mediated diseases and cancer. Cytokine Growth Factor Rev 2016;30:87–93. 10.1016/j.cytogfr.2016.02.003 [DOI] [PubMed] [Google Scholar]
- 29. Hermann V, Batalov A, Smakotina S, et al. First use of cenerimod, a selective S1P1 receptor modulator, for the treatment of SLE: a double-blind, randomised, placebo-controlled, proof-of-concept study. Lupus Sci Med 2019;6:e000354 10.1136/lupus-2019-000354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li QZ, Zhou J, Lian Y, et al. Interferon signature gene expression is correlated with autoantibody profiles in patients with incomplete lupus syndromes. Clin Exp Immunol 2010;159:281–91. 10.1111/j.1365-2249.2009.04057.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Murphy G, Isenberg DA, New therapies for systemic lupus erythematosus – past imperfect, future tense. Nat Rev Rheumatol 2019;15:403–12. 10.1038/s41584-019-0235-5 [DOI] [PubMed] [Google Scholar]
- 32. Durcan L, O’Dwyer T, Petri M. Management strategies and future directions for systemic lupus erythematosus in adults. Lancet 2019;393:2332–43. 10.1016/S0140-6736(19)30237-5 [DOI] [PubMed] [Google Scholar]
- 33. Ando S, Amano H, Amano E, et al. FTY720 exerts a survival advantage through the prevention of end-stage glomerular inflammation in lupus-prone BXSB mice. Biochem Biophys Res Commun 2010;394:804–10. 10.1016/j.bbrc.2010.03.078 [DOI] [PubMed] [Google Scholar]
- 34. Okazaki H, Hirata D, Kamimura T, et al. Effects of FTY720 in MRL-lpr/lpr mice: therapeutic potential in systemic lupus erythematosus. J Rheumatol 2002;29:707–16. [PubMed] [Google Scholar]
- 35. Wenderfer SE, Stepkowski SM, Braun MC. Increased survival and reduced renal injury in MRL/lpr mice treated with a novel sphingosine-1-phosphate receptor agonist. Kidney Int 2008;74:1319–26. 10.1038/ki.2008.396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sugahara K, Maeda Y, Shimano K, et al. Amiselimod (MT-1303), a novel sphingosine 1-phosphate receptor-1 modulator, potently inhibits the progression of lupus nephritis in two murine SLE models. J Immunol Res 2019;2019:5821589 10.1155/2019/5821589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Taylor Meadows KR, Steinberg MW, Clemons B, et al. Ozanimod (RPC1063), a selective S1PR1 and S1PR5 modulator, reduces chronic inflammation and alleviates kidney pathology in murine systemic lupus erythematosus. PLoS One 2018;13:e0193236 10.1371/journal.pone.0193236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Banchereau R, Hong S, Cantarel B, et al. Personalized immunomonitoring uncovers molecular networks that stratify lupus patients. Cell 2016;165:551–65. 10.1016/j.cell.2016.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ward JM, Ratliff ML, Dozmorov MG, et al. Human effector B lymphocytes express ARID3a and secrete interferon alpha. J Autoimmun 2016;75:130–40. 10.1016/j.jaut.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hamilton JA, Hsu HC, Mountz JD. Role of production of type I interferons by B cells in the mechanisms and pathogenesis of systemic lupus erythematosus. Discov Med 2018;25:21–9. [PubMed] [Google Scholar]
- 41. Furie R, Khamashta M, Merrill JT, et al. Anifrolumab, an anti-interferon-alpha receptor monoclonal antibody, in moderate-to-severe systemic lupus erythematosus. Arthritis Rheumatol 2017;69:376–86. 10.1002/art.39962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dorner T, Giesecke C, Lipsky PE. Mechanisms of B cell autoimmunity in SLE. Arthritis Res Ther 2011;13:243 10.1186/ar3433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Furie R, Petri M, Zamani O, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum 2011;63:3918–30. 10.1002/art.30613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nutt SL, Hodgkin PD, Tarlinton DM, et al. The generation of antibody-secreting plasma cells. Nat Rev Immunol 2015;15:160–71. 10.1038/nri3795 [DOI] [PubMed] [Google Scholar]
- 45. Sakakibara S, Arimori T, Yamashita K, et al. Clonal evolution and antigen recognition of anti-nuclear antibodies in acute systemic lupus erythematosus. Sci Rep 2017;7:16428 10.1038/s41598-017-16681-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jacobi AM, Odendahl M, Reiter K, et al. Correlation between circulating CD27 high plasma cells and disease activity in patients with systemic lupus erythematosus. Arthritis Rheum 2003;48:1332–42. 10.1002/art.10949 [DOI] [PubMed] [Google Scholar]
- 47. Domeier PP, Chodisetti SB, Schell SL, et al. B-cell-intrinsic type 1 interferon signaling is crucial for loss of tolerance and the development of autoreactive B cells. Cell Rep 2018;24:406–18. 10.1016/j.celrep.2018.06.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Eloranta ML, Ronnblom L. Cause and consequences of the activated type I interferon system in SLE. J Mol Med (Berl) 2016;94:1103–10. 10.1007/s00109-016-1421-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Furie R, Tummala R. A phase 3 randomized controlled trial of anifrolumab in patients with moderate to severe systemic lupus erythematosus. Arthritis Rheumatol 2019;71:1–5362. 10.1002/art.41108 [DOI] [Google Scholar]
- 50. Khan D, Ansar Ahmed S. The immune system is a natural target for estrogen action: opposing effects of estrogen in two prototypical autoimmune diseases. Front Immunol 2015;6:635 10.3389/fimmu.2015.00635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Haynes WA, Haddon DJ, Diep V, et al. Integrated, multi-cohort analysis reveals unified signature of systemic lupus erythematosus. JCI Insight 2020. Jan 23 10.1172/jci.insight.122312 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2020-001261supp001.pdf (151.1KB, pdf)
rmdopen-2020-001261supp002.pdf (121.9KB, pdf)
rmdopen-2020-001261supp003.pdf (369.5KB, pdf)
rmdopen-2020-001261supp004.pdf (144.6KB, pdf)
rmdopen-2020-001261supp005.pdf (164.2KB, pdf)