Abstract
Background:
For almost a century it has been recognized that human possess a varied and dens microbial ecosystem called the human microbiota, yet we are still beginning to understand many of the roles that these microorganisms play in human health and development. It is thought that under certain circumstances such as dysbiosis, the microbiota can cause diseases, where the central nervous system (CNS) has an important relevance and where the “gut-brain axis” will play a major role.
Aims:
This review investigated the influence of the gut microbiota on brain function, trying to demonstrate whether dysbiosis influences CNS diseases or whether it is the disease that causes dysbiosis, highlighting the existing literature within this field.
Methods:
We performed a systematic literature search in EMBASE, PubMed, and Cochrane combining the terms “gut microbiota,” “dysbiosis,” and “CNS diseases” to identify those whom reported some influence or relation between dysbiosis of gut microbiota and CNS diseases. For the present systematic review, we only included systematic reviews or meta-analysis.
Results:
The EMBASE, PubMed, and Cochrane were systematically searched, considering only systematic reviews or meta-analysis. Nine studies comprising 705 articles were included in this review. Those 9 systematic reviews consist in 2 about autism spectrum disorder, 1 in dementia, 1 in depression, 2 in autoimmune diseases, 1 in schizophrenia, and 2 in some altered brain function. Available data characterizing several neural diseases demonstrate a significant correlation between dysbiosis and CNS diseases, strengthen the evidence that dysbiosis of gut microbiota may correlate with abnormalities in CNS patients.
Conclusions:
Although there is a clear need for more investigations to better understand the role of the gut microbiota in CNS diseases, the modulation of the nervous system by the microbiota is clear, continuing to be the subject of continuous research. We need to fully understand the mechanisms by which the microbiota interacts with the human brain, and therefore what's the connection between dysbiosis and pathologies such depression, dementia, autism, or schizophrenia.
Keywords: gut microbiota, dysbiosis, central nervous system, brain function
Introduction
The human body of an adult usually comprises 10 times more microbial cells than human cells, largely because of the high diversity of microorganisms in the gastrointestinal tract.1 The microorganisms that dominate this environment are mainly anaerobic bacteria, such as Bacteroidetes and Firmicutes, but others including virus, protozoa, archaea, and fungi are also involved in this environment.2,3 Together, these microorganisms and their genes constitute a dynamic microbial community that inhabits different areas of the body and therefore plays a vital role in the health, disease, growth, and development of its host. This is called the human microbiome.
Like the human genome, the microbiome is the genetic patrimony of the microorganisms that colonize diverse areas of the human body, being superficial or deep, like the genitourinary system, the respiratory system, and the gastrointestinal tract, among others. These microorganisms constituting the human microbiome may co-exist in commensal or mutual relations, and its composition is relatively stable in short time and host specific.
The microbiome participates in several functions, namely in the development of the immune and metabolic systems, influences absorption and distribution of nutrients, protects against pathogens or xenobiotics, and allows the maintenance of homeostasis and the maintenance of the intestinal barrier integrity, in addition to having an important role in the communication between the gut and the brain.3
The term “dysbiosis” refers to an imbalance of the microbiota and its functions, where its normal beneficial state may pass into a state detrimental to the host’ health.1 Thus, intestinal dysbiosis can negatively impact human health, leading to different diseases, ranging from inflammatory bowel disease to central nervous system (CNS) diseases, and where the “gut-brain axis” will play a major role.
A stable gut microbiota is critical for the maintenance of normal gut physiology and contributes to the correct transmission of signals along the gut-brain axis, which is a crosstalk between the brain and the gut involving multiple overlapping pathways, like enteroendocrine signaling, intestinal permeability, enteric reflexes, and immune activation, allowing the maintenance of the individual's health.4 On the contrary, intestinal dysbiosis can negatively influence the physiology of the gut, causing an inappropriate transmission of stimuli along the gut-brain axis and consequently causing the breakdown of the intestinal permeability and lead to an inflammatory condition where the cytokines could get into the bloodstream and reach the brain.4 Stress can also affect the microbiota by modifying its composition, since it can influence the functions of the gut.
Over the years, mental health has not been as prominent as other areas, particularly as regards oncology and cardiovascular diseases. Psychiatric disorders are, however, among the main causes of disability. Depression has a prominent place, accounting for approximately 40% of all disability cases, followed by anxiety disorders.5
Therefore, the aim of the present article was to focus on the influence of the gut microbiota on brain function, to summarize the current state-of-the-art literature on the association between dysbiosis and CNS diseases.
Materials and methods
A review of systematic reviews examining the role of the gut microbiota on nervous system was undertaken. In addition, we will discuss the results of studies examining the concept of dysbiosis in relation to brain function.
The objective of this work is to identify the impact that dysbiosis may have on the different pathologies of the CNS, not interested in this to deepening the effect of probiotics on the same diseases. Therefore, interventional studies were not included since they would add a lot of irrelevant information to this study, and they only provide information on possible interventions to overcome both the issue of dysbiosis and the CNS diseases.
The present review followed PRISMA guidelines for Systematic Review and Meta-analyses. The literature search was conducted to achieve the goal, and using different electronic databases such as PubMed, Embase, and Cochrane without any year restrictions, from inception through April 2019, allowed to identify pertinent publications using the keywords “Gut Microbiota” AND “Dysbiosis” AND “Nervous System.”
The inclusion criteria were systematic reviews or meta-analysis, human studies, and the language of published manuscripts (English, French, Portuguese, Spanish, and Italian). All studies that fulfilled the following inclusion criteria were considered.
To further restrict the research, exclusion criteria were applied. Articles were excluded based on some criteria. The exclusion criteria were not systematic reviews, not related to pathology, not getting the full text, not human studies, and if they were interventional studies. In addition, the group of retrieved articles and relevant systematic reviews were individually checked for relevance and examined for cross-references, and duplicates were discarded. Narrative reviews were not included in this article. The age of participants and the geographical location were not considered exclusion criteria.
Data were extracted by 1 reviewer (Cátia Almeida) using the predefined inclusion and exclusion criteria and checked for completeness and accuracy by 2 more reviewers (Rita Oliveira and Pedro Barata).
The extracted data included information about the diseases, the characteristics of the study sample like the aim of the review, the study population and its risk of bias, and adjusted estimates for the outcomes of interest. Furthermore, the correlation mechanisms and the bacteria involved were also extracted from the reviewed manuscripts.
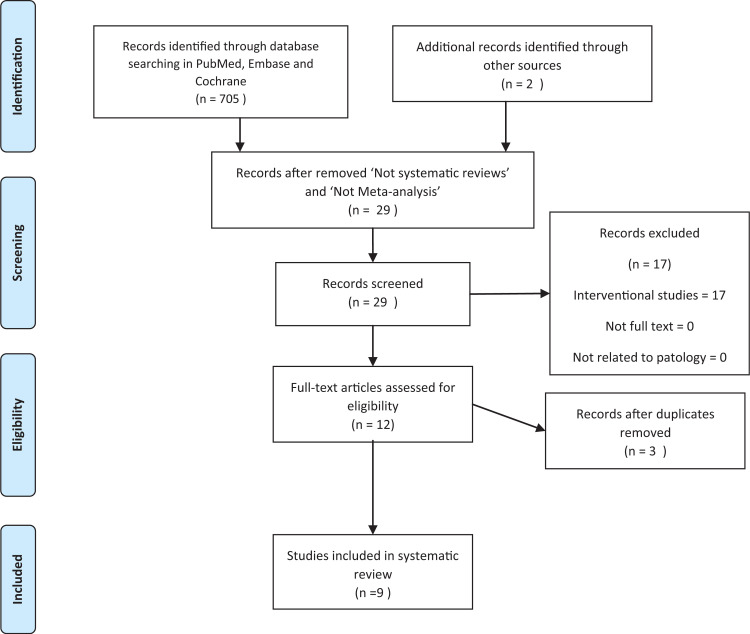
At the beginning of study selection, articles not found to be relevant were excluded via an assessment of the title, abstract, and keywords. The full-text of potentially relevant studies was then retrieved. The final group of articles reviewed included 9 systematic reviews and/or meta-analysis, comprising 705 studies.
Results and discussion
Literature research
Overall, the initial search yielded about 683 articles, and they were identified through the online research, of which only 9 fulfilled the search queries for being considered relevant on the study of the association between gut microbiota and nervous system diseases (Fig. 1).
Figure 1.
PRISMA flow diagram for selection of published systematic reviews and meta-analyses.
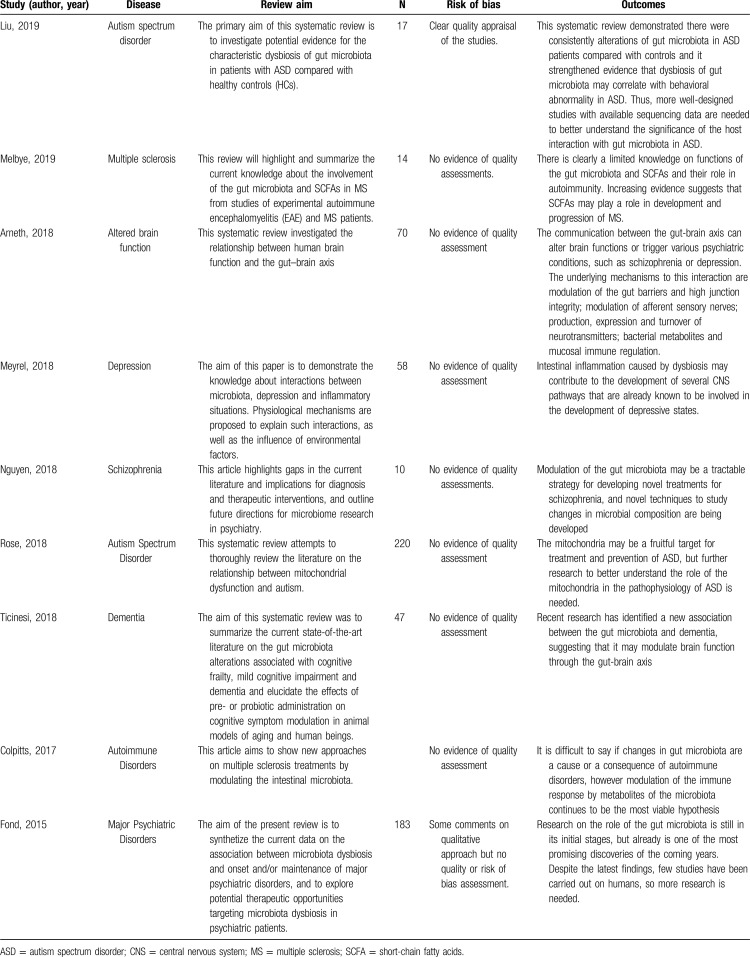
Thereby, the characteristics and main results of the systematic reviews and meta-analysis included are outlined in Table 1, which provides a brief summary.
Table 1.
Systematic reviews and/or meta-analysis characteristics
Table 1 lists the characteristics of the included studies. In terms of clinical populations examined, 2 investigations included individuals with autism spectrum, 2 studies with autoimmune diseases, and 1 for each other diseases such as depression, dementia, schizophrenia, and altered brain function. All included studies aimed to find a correlation between dysbiosis and disease, but of all them none could come to an accurate conclusion of this possible cause our consequence of disease. New paths have, however, been discovered in the evolution of science. Most of all indicate that the most likely target will be the modulation of the gut microbiota.
Gut-brain axis
It is known that anxiety, depression, and cognitive dysfunction can be side effects of an enteric infection, and this has already been proven with tests on animals.4
In recent years, researchers have discovered that the human microbiome affects the functioning and development of the CNS through interaction with the gut-brain axis, which is an interaction between the gastrointestinal tract and its flora, and the CNS, where a biochemical signaling occurs.
There are more and more studies that support the existence of a bidirectional communication along the gut-brain axis, which demonstrate that the gut microbiota influences the functioning of the CNS. This communication occurs through several mechanisms involving the endocrine, nervous, and immune system, both directly and indirectly.4
Although the mechanism by which the brain and gut communicate is not yet established and fully understood, evidence from animal and human studies has shown that the gut microbiota plays a very important role in brain behavior and cognitive development through the production of various hormones, immune factors, and metabolites, such as short-chain fatty acids (SCFAs). Furthermore, this connection is made through the vagus nerve and it can influence different processes, such as the production of metabolic precursors, neurotransmitters such as serotonin, melatonin and acetylcholine, and active metabolites. In addition, this axis has been shown to modulate brain functions, such as emotional behavior and stress-related responsiveness.4
Arneth have concluded that some microorganism such as pathogens or commensal bacteria found in the gastrointestinal tract may activate the signaling process of the CNS and the neural pathways and contribute to the development of mental illness such as depression and anxiety. Lactobacillus rhamnosus, Helicobacter pylori, L paracasei, Escherichia coli, and Pseudomonas are some of that microorganisms.4
It is also known that the gut microbiota regulates the development of lymphoid structures and modulates the differentiation of immune cells, thus maintaining the homeostatic interactions between host and microbiota. Some bacteria of the family Enterobacteriaceae appear to be more apt to survive in inhospitable conditions, like in an inflamed gut than the commensal anaerobic bacteria residing in the flora of a healthy individual. Given the anti-inflammatory effects of butyrate, it can be said that the depletion of butyrate bacteria will contribute to a situation of dysbiosis, caused by inflammation.1,4
Diseases such as bipolarity, depression, and schizophrenia are associated with dysregulation of the immune response, which leads to a high number of proinflammatory cytokines in the bloodstream due to modification of the intestinal permeability that allows those endotoxins into the circulation. Lipopolysaccharides (LPS) are a potent proinflammatory endotoxins of the cell walls of gram-negative bacteria, and they can activate microglia which will contribute to inflammation in the host nervous system.4
Thus, the underlying mechanisms to this interaction can be summarized as follows:
Modulation of the gut barriers and high junction integrity;
Modulation of afferent sensory nerves;
Production, expression, and turnover of neurotransmitters;
Bacterial metabolites and mucosal immune regulation.
Autism spectrum disorder
Autism is a developmental disorder of the CNS that usually begins in the first 3 years of life and is characterized by difficulties in communication and social interaction, and by repetitive and stereotyped behaviors, interests, or activities. There is no specific cause, but it is assumed that autism disorder results from abnormalities in the structure or function of the brain, and appears to involve a complicated interaction of genetic and environmental factors.6
There is increasing evidence that disorders in the pathway underlying the gut-brain axis, such as changes in the gut microbiota, may result in neurobehavioral and intestinal dysfunction in patients with autism. Among the comorbidities in autism, gastrointestinal symptoms are quite common, such as diarrhea and/or constipations, which are also correlated with the severity of the neurobehavioral disorder. Therefore, in recent years the gastrointestinal tract and its microbiota have gained increasing attention as a potential mediator of risk factors, especially concerning patients diagnosed with autism.
As already mentioned, the gut microbiota contributes to the maintenance of intestinal integrity, protection of the intestinal barrier, and preventing bacterial LPS and other toxins into the bloodstream. When we are faced with a state of dysbiosis these functions are, however, compromised, so it's common to find LPS and other toxins in the blood of the individual.6
Several teams have studied the gut microbiota of the autistic individuals and found a different composition of various microbial species in comparison to healthy controls. So far, a wide variety of mechanisms have been proposed to explain the association of the intestinal microbiota with autism, with emphasis on immune activation or dysfunction, toxins produced by the gut microbiota such as LPS, metabolites changes such as propionic acid and other SCFAs, and also the dysregulated metabolism of free amino acids.1
Fond et al have already showed increased levels of LPS in the blood of individuals with autism, a finding that goes with the theoretical assumption. Liu et al compared the gut microbiota of healthy children with the gut microbiota of children with autism and came to the conclusion that a greater number of Clostridium type germs were found 10 times more in autistic children. In addition, an increased number of Bacteroidetes and Lactobacillus, and a decreased number of in Prevotella, Firmicutes, and Bifidobacterium species were discovered. Intestinal permeability disorders have also been found in autism individuals.1,6
In fact, Bacteroides are a fairly abundant genus in all ages and this is the main producer of propionate (PPA) in the gut. The amount of propionate present in feces is directly related to the strong abundance of Bacteroides. This propionate will be used in the gluconeogenesis that occurs in the liver and then will serve as a source of glucose for the host. Studies have also shown that propionate leads to autism spectrum disorder (ASD)-like behaviors. Therefore, the characteristic abnormality in neurodevelopment in individuals with autism is accompanied by a poor metabolism of propionic acid, which may be related to a change in the propionate-producing gut microbiota. Clostridium has also been extensively studied in ASD due to its characteristic of producing toxins and propionate, which may aggravate the symptoms of the disease.1,6
Thus, some changes in the gut microbiota have been associated with autism, and the main mechanism by which microorganisms will influence host physiology is through the production of SCFAs. An example is Clostridium spp., which is one of the major producers of SCFAs and has been detected at very high levels in autistic individuals.
In the last years, several researchers have associated autism with some physiological disturbances such as abnormal mitochondrial metabolism. It is known that autism is associated with environmental factors such as metabolites from the gut microbiota, which may alter various physiological functions such as mitochondrial function. Incidentally, it has been described that autistic children have increasingly presented mitochondrial dysfunctions rather than mitochondrial diseases, and when compared with the general population it is known that they have a higher incidence of this dysfunction.7
Since the nervous system has a high metabolic rate, it will be expected than when abnormalities occur in mitochondrial function then the brain will suffer consequences, leading to dysfunctions that can translate into certain diseases.
Some organic compounds such as lactate, pyruvate, carnitine, and alanine aminotransferase were found at higher concentrations in the autistic group than in controls. Interestingly, it was through the high concentration of lactate that the connection between mitochondrial disturbances and autistic children was established. Indeed, when there are changes or mutations in metabolic genes required for mitochondrial function, it can lead to abnormalities in the CNS, leading to cognitive and behavioral abnormalities characteristic of ASD.6
From the studies to date it can be concluded that the majority of cases of ASD result from an interconnection between environmental and polygenetic factors. Thereby, mitochondrial dysfunction is one of the mechanisms that has aroused more curiosity in the scientific community regarding the interactions between genes and the environment, because the mitochondria is directly influenced by exogenous environmental agents and by intrinsic agents as inflammatory mediators and the gut microbiota. It seems to be closely associated with ASD individuals.7
Its etiology has not yet been fully established; however, preliminary studies suggest that mitochondria may prove to be a very significant target for the future treatment and prevention of ASD. Further studies are still needed.
Autoimmune disorders of the central nervous system
Autoimmune disorders are pathologies characterized by an inappropriate immune response against their own body, producing autoantibodies that mistakenly will attack healthy cells. This results in inflammation and destruction of tissue and organs.8
This CNS disorders have very complex etiologies that are not yet fully established and have debilitating consequences in afflicted patients, but environmental factors such as diet, drugs, infections, and life style, and certain genetic backgrounds have been proposed. Within this group of diseases multiple sclerosis (MS) stand out, involving both genetic and environmental factors.
Although the gut microbiota is resident in our body, it's composed for foreign microorganisms that evolved to live in symbiosis with its host, and therefore has the potential to influence the pathogenesis of several diseases.
Recently, some studies have focused in the important problematic that tries to answer the question about if there are significant differences between the gut microbiota of healthy and autoimmune individuals. In the last few years, researchers have profiled fecal gut microbiota form individuals with autoimmune disorders and these studies have shown that they exhibit gut microbiota dysbiosis, with both depletion and enrichment of certain microorganisms when compared to healthy controls.8
When analyzed it was shown that MS individuals have relatively low abundance of Bacteroidetes phylum such as Bacteroides, Parabacteroides, and Prevotella genera, and an increased abundance of certain Bifidobacterium and Pseudomonas. Among Firmicutes, some were found deplete, whereas others were enriched, such as Akkermansia and Dorea, which can metabolize mucin and induce proinflammatory cytokines. Based on a higher prevalence of Proteobacteria it's suggested that this microorganism can promote a proinflammatory response. Among the microorganisms showing lower abundance in MS individuals, those also have the ability to induce SCFAs production.8,9
In a healthy individual, the intestinal bacterial community is quite diverse and responsible for the homeostatic balance between pro- and anti-inflammatory immune responses. Multiple sclerosis, because it is an inflammatory disease, will not have this balance present, so it can be assumed that gut dysbiosis is responsible for the depletion of the number of microorganism with anti-inflammatory properties and the enrichment of those with the capacity to induce inflammation.9
Now the question of how the gut microbiota modulates autoimmune diseases of the CNS remains to be addressed; however, modulation of the immune response by metabolites of the microbiota continues to be the most viable hypothesis. In the end, there is depletion of bacteria with the ability to induce immune-regulatory cells and enrichment of bacteria that induce proinflammatory responses.
It is difficult to say if changes in gut microbiota are a cause or a consequence of autoimmune disorders, so further research is needed to determinate the microbiota role.
Cognitive frailty and dementia
In the past years there has been an aging of the population throughout the world, leading to the emergence of more and more age-related disabling diseases with serious personal, social, and economic consequences. Therefore, also the brain also suffers from aging leading the burden of cognitive frailty, a risk factor for dementia, to be one of the most daunting and costly consequences of longer life expectancies.
Cognitive frailty is a multidimensional geriatric condition characterized by increased vulnerability to several stressors, such as dietary variations, gastrointestinal infections, acute or chronic illness, constipation, and pharmacotherapy. All this factors will result as a reduced capacity of different physiological systems, and some adverse outcomes including falls, disability, and mortality.10
Dementia is a multifactorial incurable syndrome characterized by progressive deterioration in multiple cognitive domains that is severe enough to interfere with daily functioning with patients losing their ability to communicate and require continuous care. One of the principal neuropathological hallmarks of dementia is the deposition of β-amyloid particles that precedes cognitive deficits or clinical manifestations. Alzheimer disease is the most common cause of dementia in elderly people.10
In addition to all these changes, the gut microbiota also undergoes changes during aging, by beginning to lose its enormous microbial richness and to increase diversity among the elder ones. In fact, some studies have already confirmed that the gut microbiota of older people with physical frailty and mobility limitation experience lower biodiversity, specially in those microorganisms beneficial for the host physiology. In addition, these symptoms were also related to increased intestinal permeability, systemic, and local inflammation, and an enrichment of several bacteria with dire consequences.11
Some bacteria have a microbiological ability to produce amyloid proteins, which can be phagocytosed in enteric immune system cells and released into the CNS, but may also be directly related to a change in mucosal permeability induced by intestinal dysbiosis. It can thus promote systemic inflammation through the production of toxins such as LPS, and then the proinflammatory cytokines will activate the cerebral microglia, promoting neuroinflammation, neural apoptosis, and β-amyloid deposition.10
Recent research has identified a new association between the gut microbiota and dementia, suggesting that it may modulate brain function through the gut-brain axis. The existing of this axis has been widely spoken because changes in the microbiota have been associated as part of a mechanism that links high fat intake with a cognitive imbalance, more specifically a neuroinflammatory system breakdown can be caused by gut microbiota, which leads to deposition of β-amyloid in the brain. This seems to be the key to better understand the pathogenesis and progression of diseases such as dementia and Alzheimer.10
A low abundance of Bacteroides and a high number of other microorganisms is strongly associated with dementia, which turns to be stronger than the traditional biomarkers of the disease. Bacteroides are microorganisms known to regulate the function of endothelial cells and reduce inflammation, which is consistent with the inverse relationship between the amount of Bacteroides and the presence of dementia. Metabolites derived from tryptophan metabolism may also promote oxidative stress, cell death, tau phosphorylation, and tangle formation.10
Depression
Depression is probably the most common, life-disrupting and highly recurrent mental disorder worldwide. This disease is characterized by apathy, depressive mood, changes in appetite and weight, sleep disturbances, cognitive impairment, loss of productivity, and recurrent thoughts about suicide and death. Because of that, it represents an economic burden for public health. Although the causes of the disease are not yet clear, there are already several neurobiological mechanisms that seem to be kind of an explanation.1,5
Most of the studies on depression focus on the genetic, behavioral, and neurological aspects of the disease; however, in recent times the role of environmental factors and immunological dysregulation have gained significant attention. This illness is thought to begin when there is deregulation of neurofunctions and can largely be attributed to cytokines release secondary to an exaggerated systemic response to stressors.
It is known that physical and psychological stresses can induce internal dysbiosis, and this alteration of the microbiota composition may act directly or indirectly promoting the development of an inflammatory state in the central system leading to emotional disturbances.5
Several animal studies have come to support the hypothesis that the intestinal microbiota dysbiosis plays a very important role in the CNS, namely through inflammatory processes, the hypothalamic-pituitary-adrenal (HPA) axis and by affecting neurotransmission.9
The increase in intestinal permeability observed during depressive states seems to play a fundamental role in the constitution of the triangle dysbiosis-inflammation-depression, because it is through these breaches that the bacterial translocations occur, which is the origin of the inflammatory reaction, and so the source of depression.
The intestinal barrier is one of the greatest interfaces between the individual and their environment. Maintaining its integrity is important for the prevention of certain diseases, particularly those of the CNS, but when this integrity is not maintained, there is an increase in intestinal permeability, which results in a translocation of certain components of the bacterial wall, such as the LPS, into the bloodstream. These products will thus be involved in the activation of neuroinflammatory processes and in the increase of oxidative stress.1
Changes in gut microbiota were described in patients diagnosed with depression, consisting either of a predominance of certain potentially harmful bacteria or a reduction of potentially beneficial genera. Mostly these included enrichment in the relative abundance of Firmicutes, Actinobacteria, and Bacteroidetes.11
Several studies have shown that depression in humans is accompanied by an increase in the immune response against some metabolites such as LPS, produced by some gram-negative bacteria such as Pseudomonas and Klebsiella. Among the several studies within the theme, it was verified that LPS modulate the dose-dependent emotional memory. Also, there was a low gastric secretion reported by depressive patients, an increased in intestinal permeability, diarrhea, abdominal pain, constipation, and malabsorption syndrome.5,10
Another factor in favor of this hypothesis is that certain microorganisms such as Lactobacillus and Bifidobacterium secrete gamma aminobutyric acid; Escherichia, Bacillus, and Saccharomyces produce norepinephrine; Candida, Streptococcus, Escherichia, and Enterococcus produce serotonin; and Bacillus and Serratia can produce dopamine. This become important because all these neurotransmitters play a very important role both in depression and in antidepressive drugs, and all of these can be regulated by the microbiota.1
As already mentioned, activation of the HPA axis can lead to depressive complications. It is known that under conditions of stress the activation of the HPA axis can lead to increased cortisol and disturbance of intestinal permeability, altering the composition of the intestinal microbiota and also increasing the circulation of LPS which, as already mentioned, may promote local or systemic inflammations with important consequences for the CNS.11
Schizophrenia
Schizophrenia is a chronic and severe mental disorder that usually appears in late adolescence or early adulthood, and it's characterized by delusions, hallucinations, and other cognitive difficulties. Being a heterogeneous disease with a multiple pathophysiological contributors, many evidences point to the immune system as an important factor in the pathogenesis of this disease.
According to recent studies the existence of a mutual and dynamic relationship between the gut microbiota and schizophrenia has gained increasing importance; however, their relation is not yet fully understood. The involvement of the gut-brain axis has long been recognized, especially through the immune and inflammatory mechanisms.
Several mechanisms related to changes in the gut microbiota have been implicated in schizophrenia, highlighting chronic inflammation, oxidative stress, and other physiological dysfunctions, which might induce an immune response mediated by toll-like receptors and contribute to symptoms of the disorder.4,11
As seen in the pathologies presented previously, also in schizophrenia there is an increase in intestinal permeability through the process of microbial translocation, which leads us to the conclusion that this intestinal inflammation will be responsible for the stimulation of the immune system that will lead to the development of inflammatory states in the CNS, through the gut-brain axis. The presence of intestinal inflammation markers and increased serum levels of proinflammatory cytokines are also a good evidence of damage to the intestinal barrier.1,11
Patients diagnosed with schizophrenia have presented higher proportions of Firmicutes compared to healthy individuals, who also have high levels of Bacteroidetes and Actinobacteria. In these patients there was also a particular increase in Lactobacillus and Bifidobacterium, and studies have shown that the increase of these bacteria has result in regulation of inflammation induced by chronic stress and behavioral changes.11
In fact, these studies suggest that gut dysbiosis present in schizophrenia is different from the characteristic dysbiosis in depression disorders, since schizophrenic individuals present a high decrease in the abundance of Lachnospiraceae and Ruminococcaceae, which are very important in maintaining intestinal health. Increased levels of Megasphaera and Clostridium were also found in the gut microbiota of schizophrenic individuals when compared to controls, which is consistent with the fact that Megasphaera is associated with inflammations and cognitive delays, and Clostridium produces numerous toxins harmful to the brain, like phenylalanine derivative affecting CNS in a similar way as in autism.1,11
Recently there have been some studies that demonstrate the importance of the deregulation of tryptophan metabolism in the pathogenesis of the disease. Kynurenic acid resulting from this metabolism is an N-methyl-D-aspartate receptor antagonist, which means that an increase of this can lead to changes in cognitive activity, memory, and synaptic memory, leading to the development of certain pathologies. Although the role of kynurenic acid in schizophrenia is still not well understood, it is known that the intestinal microbiota may influence plasma levels of tryptophan. This has been found in schizophrenic individuals and its association has gained increasing importance in schizophrenia disease.11
There is an increase in the number of articles that describe a bidirectional interaction between the gut and the nervous system. It is still not clear whether changes in the gut microbiota composition are cause or consequence of disease, or even if simultaneous cause and consequence. We can expect further developments in the next years, as significant research is being done in this field. Nevertheless it's now clear that the gut microbiota composition will be a key player in the pathology therapeutic approach to the nervous system disease.
Limitations
The intestinal microbiota remains largely unexplored due to its complexity and difficulties in collecting and analyzing data. This is because microbiota is a new field of research and there is still little information on it. Therefore, the number and the methodologies of the articles included in the present systematic review should be interpreted with caution.
The presented keywords used in this systematic review (“Gut microbiota,” “dysbiosis,” and “nervous system”) may have conditioned the number of manuscripts identified, which is a limitation in our article. Other keywords could be considered, in a way to increase the number of identified manuscripts and therefore decreasing bias.
Conclusion
In recent years the study of the gut microbiota and its relationship with the CNS has revolutionized our understanding of what is thought about this large area. Thus the aim of this work was to try to reach a consensus about the impact of dysbiosis in different neurological disorders, trying to understand if it is the dysbiosis that influences the different pathologies or if it is the pathologies that lead to a situation of dysbiosis.
At this point there are, however, more questions than answers, and it becomes difficult to know precisely what causes what. Clear is the role of the gut microbiota in the modulation of the nervous system, continuing to be the subject of research and continuous innovations. The prevalence of mental disorders is expected to increase in the coming years, which makes our research increasingly necessary.
Therefore, more relevant clinical intervention studies should be carried out to elucidate the mechanisms by which the microbiota interacts with the human brain, and the complete study of the gut-brain axis will be crucial to understand its involvement in the different pathophysiology of disorders such as depression, dementia, autism, or schizophrenia.
Acknowledgments
None.
Conflicts of interest
None.
References
- [1].Fond G, Boukouaci W, Chevalier G, et al. The “psychomicrobiotic”: Targeting microbiota in major psychiatric disorders: A systematic review. Pathol Biol (Paris). 2015;63:35–42. [DOI] [PubMed] [Google Scholar]
- [2].Chong-Nguyen C, Duboc H, Sokol H. The gut microbiota, a new cardiovascular risk factor? Presse Med. 2017;46 (7–8 pt 1):708–713. [DOI] [PubMed] [Google Scholar]
- [3].Khanna S, Tosh PK. A clinician's primer on the role of the microbiome in human health and disease. Mayo Clin Proc. 2014;89:107–114. [DOI] [PubMed] [Google Scholar]
- [4].Arneth BM. Gut-brain axis biochemical signalling from the gastrointestinal tract to the central nervous system: gut dysbiosis and altered brain function. Postgraduate medical journal. 2018;94:446–452. [DOI] [PubMed] [Google Scholar]
- [5].Meyrel M, Varin L, Detaint B, et al. The intestinal microbiota: a new player in depression? [in French]. Encephale. 2018;44:67–74. [DOI] [PubMed] [Google Scholar]
- [6].Liu F, Li J, Wu F, et al. Altered composition and function of intestinal microbiota in autism spectrum disorders: a systematic review. Transl Psychiatry. 2019;9:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rose S, Niyazov DM, Rossignol DA, et al. Clinical and molecular characteristics of mitochondrial dysfunction in autism spectrum disorder. Mol Diagn Ther. 2018;22:571–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Colpitts SL, Kasper LH. Influence of the gut microbiome on autoimmunity in the central nervous system. J Immunol. 2017;198:596–604. [DOI] [PubMed] [Google Scholar]
- [9].Melbye P, Olsson A, Hansen TH, et al. Short-chain fatty acids and gut microbiota in multiple sclerosis. Acta Neurol Scand. 2019;139:208–219. [DOI] [PubMed] [Google Scholar]
- [10].Ticinesi A, Tana C, Nouvenne A, et al. Gut microbiota, cognitive frailty and dementia in older individuals: a systematic review. Clin Interv Aging. 2018;13:1497–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nguyen TT, Kosciolek T, Eyler LT, Knight R, Jeste DV. Overview and systematic review of studies of microbiome in schizophrenia and bipolar disorder. J Psychiatr Res. 2018;99:50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]