Abstract
The population prevalence of insect venom allergy ranges between 3–5%, and it can lead to potentially life-threatening allergic reactions. Patients who have experienced a systemic allergic reaction following an insect sting should be referred to an allergy specialist for diagnosis and treatment. Due to the widespread reduction in outpatient and inpatient care capacities in recent months as a result of the COVID-19 pandemic, the various allergy specialized centers in Germany, Austria, and Switzerland have taken different measures to ensure that patients with insect venom allergy will continue to receive optimal allergy care. A recent data analysis from the various centers revealed that there has been a major reduction in newly initiated insect venom immunotherapy (a 48.5% decline from March–June 2019 compared to March–June 2020: data from various centers in Germany, Austria, and Switzerland). The present article proposes defined organizational measures (e.g., telephone and video appointments, rearranging waiting areas and implementing hygiene measures and social distancing rules at stable patient numbers) and medical measures (collaboration with practice-based physicians with regard to primary diagnostics, rapid COVID-19 testing, continuing already-initiated insect venom immunotherapy in the outpatient setting by making use of the maximal permitted injection intervals, prompt initiation of insect venom immunotherapy during the summer season, and, where necessary, using outpatient regimens particularly out of season) for the care of insect venom allergy patients during the COVID-19 pandemic.
Keywords: Venom, Allergy, Immunotherapy, SARS-Co-2, Lockdown
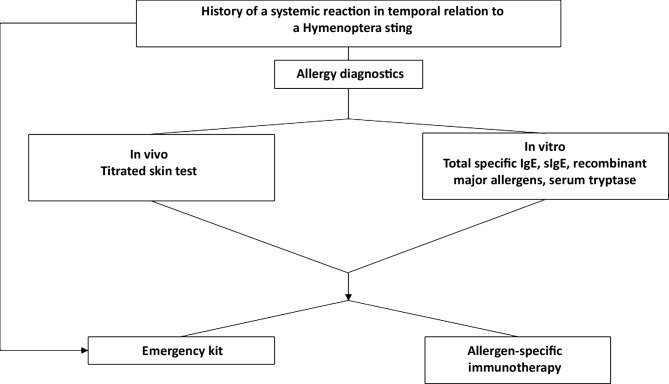
At a population prevalence of 3–5%, insect venom allergy is common and can potentially trigger life-threatening allergic reactions [1]. Therefore, patients who have experienced a systemic allergic reaction to an insect sting should be referred to an allergy specialist for diagnosis and treatment. In addition to patient history taking, where the symptoms and concomitant circumstances of the reaction are recorded, the standard procedure includes titrated skin prick testing and, if necessary, intracutaneous testing and/or determination of specific immunoglobulin (Ig)-E antibodies to insect venom and, where appropriate, their components to identify immediate-type allergy (Fig. 1). For a better risk assessment, especially after the onset of severe reactions, the determination of basal serum tryptase is also recommended. If the above-mentioned findings are positive and the patient has a clear history of a systemic allergic reaction in the context of a venom sting, the initiation of allergen-specific immunotherapy with the relevant insect venom is recommended [2].
Fig. 1.
Diagnostic algorithm for insect venom allergy (from [2]). IgE immunoglobulin E, sIgE specific immunoglobulin E
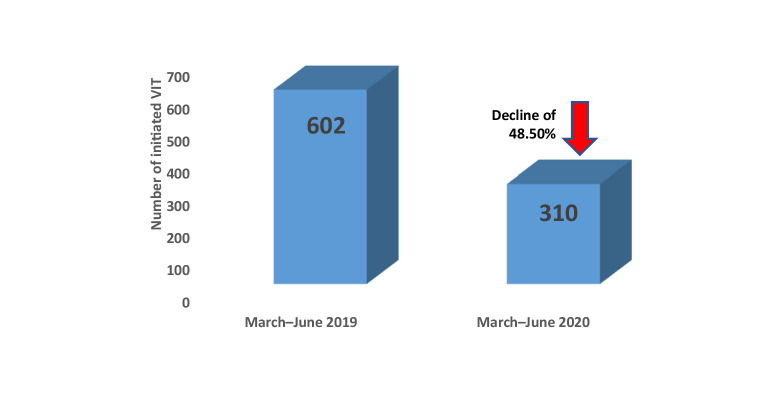
The failure to initiate specific immunotherapy in at-risk patients in a timely manner, leads to an increase of their health risks and may result in an increased need of emergency care for insect sting reactions. Such situations should be avoided during possible healthcare shortage. The significance of the COVID-19 pandemic for allergology has recently been discussed in a number of position papers [3, 4]. Due to the widespread reduction in outpatient and inpatient care capacities in recent months as a result of the COVID-19 pandemic, the various allergy specialists from Germany, Austria, and Switzerland have taken different measures to ensure that patients with insect venom allergy continue to receive optimal allergy care. However, overall, there has been a large reduction in newly initiated insect venom immunotherapy (Table 1) during the lock down. A survey among large allergy centers with regard to newly initiated venom immunotherapy (VIT) revealed an almost 50% reduction for the months March–June 2020 compared to the similar period in 2019 (Fig. 2). This decline was related to reduced hospital capacities, but also the fact that patients considered to visit a physician or a hospital as a high-risk due to the COVID-19 pandemic.
Table 1.
Overview of the number of VIT initiated in the period March–June 2019 and 2020 at a number of different centers
| Centers | Initiated VIT March–June 2019 |
Initiated VIT March–June 2020 |
|---|---|---|
| Allergology and Dermatology, University Hospital Basel, Switzerland | Not specified | Not specified |
| Department of Dermatology, Venereology and Allergology, Charité—University Hospital Berlin, Germany | 28 | 9 |
| Outpatient Department, University Department of Rheumatology, Immunology and Allergology, University Hospital, Switzerland | ~30 | 12 |
| Department and Outpatient Clinic for Dermatology and Allergology, University Hospital Bonn, Germany | 28 | 21 |
| ENT Department and Outpatient Clinic, Carl Gustav Carus University Hospital Dresden, Germany | 23 | 25 |
| Department of Dermatology, University Hospital Düsseldorf, Germany | Not specified | Not specified |
| Department of Dermatology and Allergology, University Hospital Gießen and Marburg, Germany | 76 (36 G, 40 M) | 42 (33 G, 9 M) |
| University Department of Dermatology and Venereology, Medical University of Graz, Austria | 50 | 2 |
| Department of Dermatology, Allergology and Venereology, Hannover Medical University, Hannover, Germany | 89 | 18 |
| Department of Dermatology, University Hospital Heidelberg, Germany | 15 | 17 |
| Department and Outpatient Clinic for Dermatology, Venereology and Allergology, University Hospital Leipzig, Germany | 35 | 33 |
| Department of Dermatology and Venereology, Kepler University Hospital, Linz, Austria | 31 | 16 |
| Department and Outpatient Clinic for Dermatology, Johannes Gutenberg University Mainz, Germany | Not specified | Not specified |
| Department and Outpatient Clinic for Dermatology and Allergology, University of Munich, Germany | 87 | 53 |
| Department of Dermatology, University Hospital Münster, Germany | 61 | 40 |
| University Department for Pediatric and Adolescent Medicine, Paracelsus Private Medical University, Salzburg, Austria | 29 | 17 |
| Department of Dermatology, Venereology and Allergology, Cantonal Hospital St. Gallen, Switzerland | 20 | 5 |
| Department and Outpatient Clinic for Dermatology, Venereology and Allergology, University Hospital Würzburg, Germany | Not specified | Not specified |
VIT venom immuntherapy, G Gießen, M Marburg
Fig. 2.
Number of initiated VIT (total from 14 centers in Germany, Austria, and Switzerland) between March and June in 2019 compared to 2020. VIT venom immunotherapy
Thus, the authors propose measures to ensure allergy care for insect venom-allergic individuals during times of emergency regulations in the healthcare system, such as during the COVID-19 pandemic (Table 2).
Table 2.
Recommended measures for the care of insect venom allergy sufferers during the COVID-19 pandemic
| Increased use of telephone and video appointments |
| Rearranging waiting areas and implementing hygiene measures and social distancing rules at stable patient numbers |
| Collaboration with practice-based physicians with regard to primary diagnostics (determination of bee/wasp sIgE) |
| Rapid COVID-19 testing (preadmission or on admission) |
| Unrestricted outpatient continuation of already-initiated insect venom immunotherapy (except in patients suffering from COVID-19 themselves) by making use of the permitted injection intervals |
| Prompt new initiation of insect venom immunotherapy during the season; if necessary, use of outpatient protocols, especially out of season |
| Explicitly addressing the COVID-19 situation with patients (either personally or in the scheduling letter) |
| In the case of shortages, triage according to the severity of the sting reaction |
| Adapting departmental organization, e.g., collaboration with other departments, extended outpatient clinic times, up-titration at weekends |
sIgE specific Immunoglobin E
Continuation of already-initiated insect venom immunotherapy
Allergen-specific immunotherapy with insect venom that has already been initiated should be continued as consistently as possible, despite eventual limitations in medical resources, by making use of the permissible length of intervals (see also [3]). Interrupting specific immunotherapy can cause a loss of protection and leads to unnecessary expense at a later point as a result of having to re-start therapy if the treatment interval has been exceeded. If the patient has COVID-19 themselves, a pause in treatment is recommended until recovery. Following recovery, the dose should be re-up-titrated (if still within the permitted interval) or allergen-specific immunotherapy newly initiated if necessary. In some cases, it may be beneficial to contact the patient by telephone or telehealth appointment prior to their personal visit for the immunotherapy injection in order to rule out current contraindications to the injection, thereby potentially saving the patient an unnecessary visit.
New initiation of insect venom immunotherapy
It is possible to postpone the new initiation of insect venom immunotherapy out of season, assuming the time window is taken into account (see also [3]). Postponing initiation therapy during the summer season should be avoided, in order that the patient is not exposed to the risk of a repeat severe reaction to an accidental sting. Treatment initiation should preferably be performed as ultra-rush therapy under medical supervision. One- to five-day protocols have proven successful to this end [5, 6]. They have the advantage that the maximum dose is achieved after a short initiation treatment phase. Shortened outpatient up-titration protocols have also been investigated for vespid venom allergy patients and show good results in terms of safety [7]. However, they require a longer initiation phase (7 weeks), implying that such treatment protocols should be preferred out of the season.
In summary, the diagnostic work-up of insect venom allergy, including the patient history and skin testing, should be adapted to the prevailing conditions. Initiation of immunotherapy should continue to be started with ultra-rush protocols and, above all, not postponed during the summer season. During the out-of-season period and in case of shortages of inpatient resources, or in case of certain regional requirements, an up-titration can be performed in an outpatient setting. A shortened, 7‑week protocol for vespid venom allergy patients has been recently published [7]. Whenever possible, outpatient up-titration should be performed at a center experienced with this therapy and is able to provide emergency medical care.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
M. Worm declares that she has received lecture fees and honoraria for participation on the advisory boards of Allergopharma, ALK-Abelló, Mylan, Leo Pharma, Sanofi-Aventis Deutschland, Regeneron Pharmaceuticals, DBV Technologies, Stallergenes, HAL Allergie, Bencard Allergie, Aimmune Therapeutics UK Limited, Actelion Pharmaceuticals Deutschland, Novartis, Biotest, AbbVie Deutschland, and Lilly Deutschland, outside the present work. B. Ballmer-Weber declares that she received consultancy fees from ALK during the course of the present work. Outside the present work, Ballmer-Weber declares that she has received lecture fees from ThermoFisher, Novartis, and Menarini, as well as consultancy fees from Allergopharma. R. Brehler reports lecture fees from ALK, Allergopharma, Almirall, Astra Zeneca, Bencard, Behring, Gesellschaft zur Förderung der Dermatologischen Forschung und Fortbildung e. V., Gesellschaft für Information und Organisation mbH, GSK, Dr. Pfleger HAL, Leti, med update, Merck, Novartis, Oto-Rhino-Laryngologischer Verein, Pierre Fabre, Shire, Stallergenes, Takeda, and Thermo-Fischer; consultant activity for Allergopharma, Bencard, HAL, Leti, Novartis, and Takeda; fees (institution) for clinical trials from Bencard, Biotech Tools, Genentech, Leti, Novartis, Circassia, and Shire. M. Cuevas and K. Hartmann declare that they have no conflict of interests. A. Gschwend declares that she received honoraria from ALK-Abelló (participation in the scientific advisory board) during the course of the present work. T. Hawranek declares that he has received honoraria and non-financial support from ALK, outside the present work. W. Hötzenecker declares that he received honoraria from ALK-Abelló during the course of and outside the present work. B. Homey reports lecturing, consulting, and research support: Novartis, Galderma, Regeneron/Sanofi, ALK Abello, Celgene, AbbVie, Janssen-Cilag, Lilly and Pfizer. T. Jakob reports grants and/or personal fees and/or non-financial support from Novartis, ALK Abello, Bencard, Allergopharma, Thermo Fisher, and Celgene, outside the submitted work. N. Novak declares that she received grants and honoraria from ALK-Abelló, as well as honoraria from HAL Allergy and Bencard Allergy Therapeutics, during the course of the present work. J. Pickert declares that she has received honoraria from ALK, Novartis, and Sanofi, outside the present work. J. Saloga reports personal fees from ALK-Abelló, Novartis, and Educational Institutions, outside the submitted work. In addition, Dr. Saloga has a patent Encapsulation of allergens issued. K. Schäkel declares that he received honoraria from ALK-Abelló during the course of and outside the present work. A. Trautmann declares that he received honoraria from ALK-Abelló during the course of the present work. R. Treudler declares that she received honoraria from ALK-Abelló during the course of the present work. Outside the present work, Treudler declares that she has received honoraria from ALK-Abelló, Novartis, Takeda, Gesundheitsnetz Leipzig, GEKA mbH, Sanofi, and AbbVie, as well as grants from Hautnetz Leipzig e. V.; she also declares a scientific collaboration with the Fraunhofer Institute. B. Wedi declares that she has received grants, lecture fees, and honoraria for advisory board participation, travel cost reimbursement for congresses, research grants, as well as financial support from Novartis. Wedi also declares that she has received research grants, lecture fees, honoraria for advisory board participation, travel cost reimbursement for congresses, and non-financial support from Shire, as well as lecture fees and honoraria for advisory board participation from ALK-Abéllo. Wedi has also received lecture fees from HAL-Allergy and Bencard, as well as honoraria from Sobi, all outside the present work. G. Sturm declares that he has received grants from ALK-Abelló, as well as honoraria from Novartis, Bencard, Stallergens, HAL, Allergopharma, and Mylan, outside the present work. F. Rueff declares that she has received grants from Novartis, consultancy fees from Bencard, LEO-Pharma, Novartis, and UCB, as well as lecture fees from Abbvie, ALK, Allergopharma, Bencard, HAL, MEDA Pharma, Mylan, Novartis, and UCB, outside the present work.
References
- 1.Worm M, Moneret-Vautrin A, Scherer K, Lang R, Fernandez-Rivas M, Cardona V, et al. First European data from the network of severe allergic reactions (NORA) Allergy. 2014;69:1397–1404. doi: 10.1111/all.12475. [DOI] [PubMed] [Google Scholar]
- 2.Przybilla B, Ruëff F, Walker A, Räwer HC, Aberer W, Bauer CP, et al. Diagnose und Therapie der Bienen- und Wespengiftallergie. Allergo J. 2011;20:318–339. doi: 10.1007/BF03362543. [DOI] [Google Scholar]
- 3.Klimek L, Pfaar O, Worm M, Bergmann KC, Bieber T, Buhl R, et al. Allergen-Immuntherapie in der Aktuellen Covid-19-Pandemie. Allergo J. 2020;29(3):17–25. doi: 10.1007/s15007-020-2539-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaker MS, Wallace DV, Golden DBK, Oppenheimer J, Bernstein JA, Campbell RL, et al. Anaphylaxis-a 2020 practice parameter update, systematic review, and grading of recommendations, assessment, development and evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020;145:1082–1123. doi: 10.1016/j.jaci.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 5.Brehler R, Wolf H, Kütting B, Schnitker J, Luger T. Safety of a two-day ultrarush insect venom immunotherapy protocol in comparison with protocols of longer duration and involving a larger number of injections. J Allergy Clin Immunol. 2000;105:1231–1235. doi: 10.1067/mai.2000.105708. [DOI] [PubMed] [Google Scholar]
- 6.Lee H, Roediger C, Bauer A, Zuberbier T, Worm M. Prospective safety analysis of an ultrarush specific immunotherapy in adults with wasp venom allergy. Allergy. 2006;61:1237–1238. doi: 10.1111/j.1398-9995.2006.01178.x. [DOI] [PubMed] [Google Scholar]
- 7.Schrautzer C, Arzt-Gradwohl L, Bokanovic D, Schwarz I, Čerpes U, Koch L, et al. A safe and efficient 7-week immunotherapy protocol with aluminum hydroxide adsorbed vespid venom. Allergy. 2020;75:678–680. doi: 10.1111/all.14012. [DOI] [PMC free article] [PubMed] [Google Scholar]