Abstract
As we know that, Oxadiazole or furadi azole ring containing derivatives are an important class of heterocyclic compounds. A heterocyclic five-membered ring that possesses two carbons, one oxygen atom, two nitrogen atoms, and two double bonds is known as oxadiazole. They are derived from furan by the replacement of two methylene groups (= CH) with two nitrogen (-N =) atoms. The aromaticity was reduced with the replacement of these groups in the furan ring to such an extent that it shows conjugated diene character. Four different known isomers of oxadiazole were existed such as 1,2,4-oxadiazole, 1,2,3-oxadiazole, 1,2,5-oxadiazole & 1,3,4-oxadiazole. Among them, 1,3,4-oxadiazoles & 1,2,4-oxadiazoles are better known and more widely studied by the researchers due to their broad range of chemical and biological properties. 1,3,4-oxadiazoles have become important synthons in the development of new drugs. The derivatives of the oxadiazole nucleus (1,3,4-oxadiazoles) show various biological activities such as antibacterial, anti-mycobacterial, antitumor, anti-viral and antioxidant activity, etc. as reported in the literature. There are different examples of commercially available drugs which consist of 1,3,4-oxadiazole ring such as nitrofuran derivative (Furamizole) which has strong antibacterial activity, Raltegravir as an antiviral drug and Nesapidil drug is used in anti-arrhythmic therapy. This present review summarized some pharmacological activities and various kinds of synthetic routes for 2, 5-disubstituted 1,3,4-oxadiazole, and their derived products.
Keywords: 1, 3, 4-oxadiazole, Heterocyclic compounds, Antiviral, Antitumor, Antitubercular
Background
Health problems were increasing day by day and become the most serious clinical problem. Recently, medicinal chemists have been looking for new drugs to be used safely to treat these serious clinical problems. There are a lot of heterocyclic compounds that are in clinical use to treat infectious disease [1].
The most common heterocyclic are those having five or six-member fused rings and possess nitrogen, oxygen, sulfur groups as heteroatoms. Some time boron, silicon, and phosphorus atoms can be used as hetero atoms [2].
Heterocyclic compounds containing nitrogen atom such as oxadiazole moieties are of interest to researchers in the fields of medicinal and pharmaceutical chemistry [3].
A heterocycles five-member ring that possesses one oxygen, two carbons, two nitrogen atoms, and two double bonds is known as oxadiazole [4]. This type of ring system is also known as azoximes, oxybiazole, biozole, diazoxole, furadiazole, and furoxans. Oxadiazole was first synthesized in 1965 by Ainsworth through the thermolysis of hydrazine. Its molecular formula is C2H2ON2 and having a molecular mass of 70.05 g/mol which is soluble in water [2].
Oxadiazoles are thermally stable compounds and their calculated resonance energy is equal to 167.4 kJ/mol. The thermal stability of oxadiazoles is increased with the substitution at the second position [5].
1,3,4-oxadiazole heterocyclic ring is one of the most important heterocyclic moieties due to its versatile biological actions [6]. These are the derivatives of furan in which two methylene groups were replaced with two nitrogen atoms. Replacement of these two methylene groups by two nitrogen atoms reduces the aromaticity of the ring & the resulting oxadiazole ring exhibits conjugated diene character [7]. Another heteroatom makes a weak base to the oxadiazole due to the inductive effect [6]. Hydrogen atoms were replaced by nucleophiles which are seen in nucleophilic substitution reaction [8].
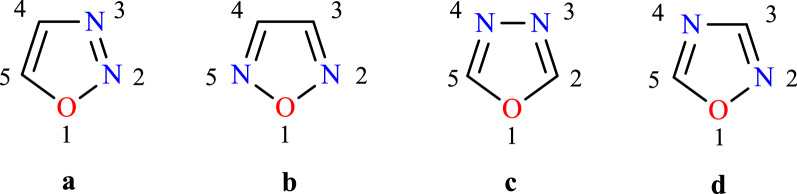
Nitrogen atoms are present in oxadiazole ring at different positions and based on the position there are four different possible isomers of oxadiazole such as 1,2,3-oxadiazole (a), 1,2,5-oxadiazole (b), 1,3,4-oxadiazole (c) and 1,2,4-Oxadiazole (d) were showed in Fig. 1 [6].
Fig. 1.
Oxadiazole
Among the different isomers, 1,3,4-oxadiazole isomer shows a lots of therapeutic activities like antibacterial [9, 10], anticonvulsant [11], antitumor [12–22], hypoglycemic, antipyretic [23], anti-tubercular [10, 24], anti-viral [25], immunosuppressive, spasmolytic, antioxidant [13, 26], anti-inflammatory [23, 27, 28], insecticidal [20], CNS stimulant, ant amoebic, antiemetic, antidepressant, anthelmintic activities, vasodilator activity, antimycotic activity [29], anti-allergic, anti-Alzheimer activity, ulcerogenic and antihypertensive activities etc. as reported in the literature [30]. Keeping the view of this, we have discussed different oxadiazole derivatives carrying urea, amide, and sulphonamide groups to investigate their anticancer, antiviral, antimicrobial, antitubercular, and antioxidant activities [31].
The presence of toxophoric –N = C–O– linkage in 1,3,4-oxadiazole ring might be responsible for their potent pharmacological activities. Among these, substituted 1,3,4-oxadiazoles are of considerable pharmaceutical interest. 2,5-disubstituted-1,3,4-oxadiazole derivatives are stable, especially 2,5-diaryl-1,3,4-oxadiazoles are more stable than the corresponding 2,5-dialkyl derivatives. Medicinal chemists have great perseverance in Research and development for the development of newer and safer antitumor agents. Tyrosine kinases (EGFR family) play a very important role in cancer proliferation. So those compounds which inhibit the activity of tyrosine kinases play a substantial role in cancer treatment. Therefore Tyrosine kinases (EGFR family) were selected and explore the binding mode of the novel compounds to EGFR tyrosine kinase active site [32].
There is various kind of synthetic route from which we can synthesize 1,3,4-oxadiazole, and their derived products. In general, 1,3,4-oxadiazole can be synthesized by the reaction of acid hydrazide or hydrazine along with carboxylic acids/acid chlorides and direct ring closure of diacyl hydrazines employing different kinds of the cyclizing agent such as phosphorus oxychloride, thionyl chloride, phosphorus pentaoxide, triflic anhydride, polyphosphoric acid, acetic anhydride and the direct reaction of an acid with (N-isocyananimino-) triphenylphosphorane [33]. In some reaction, carbon disulfide is also used for ring closure [34].
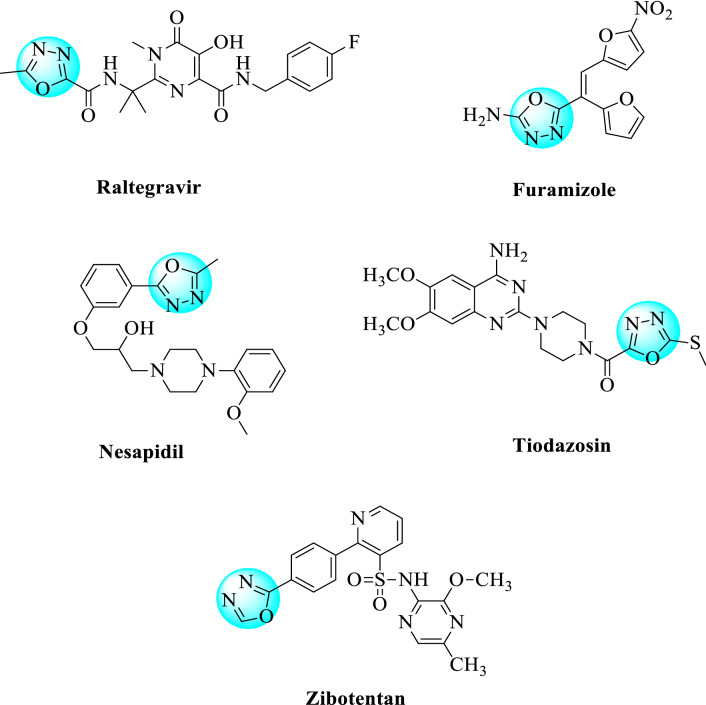
There are different examples of commercially available drugs containing 1,3,4-oxadiazole ring (Fig. 2) such as a nitrofuran derivative (Furamizole) which has strong antibacterial activity [35]. Raltegravir as an antiviral drug and Nesapidil drug is used in anti-arrhythmic therapy. The FDA approved anticancer agent Zibotentan is a 1,3,4-oxadiazole nucleus containing the most privileged derivatives available in the market [36]. Tiodazosin is used as an antihypertensive agent [37]. This present review summarized some pharmacological activities and various kinds of synthetic routes for 2,5-disubstituted 1,3,4-Oxadiazole, and their derived products during the last decade (2005–2020).
Fig. 2.
Commercially available drugs which contain 1,3,4-oxadiazole nucleus
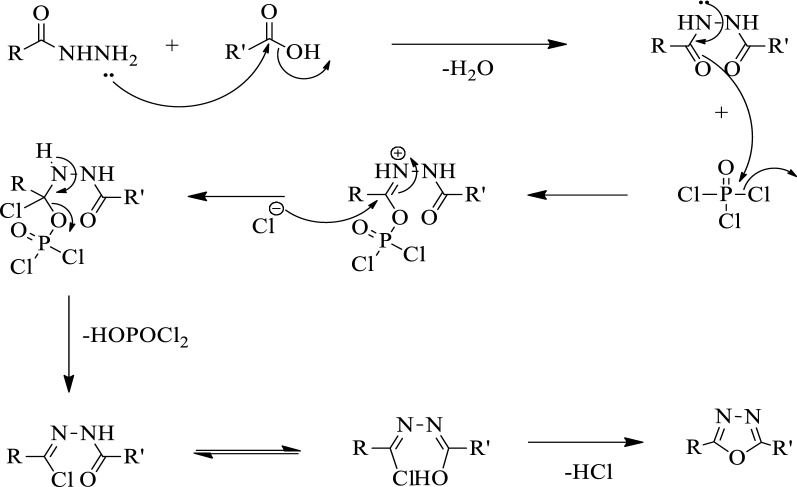
The mechanism for the formation of 2,5-disubstituted 1,3,4-oxadiazole
The probable mechanism for the formation of the 1,3,4-oxadiazole is given in (Fig. 3). The presence of lone pair of electron on the nitrogen atom of acid hydrazide attacks the carbonyl carbon atom of carboxylic acid eliminates a water molecule to form a hydrazide derivative which further reacts with phosphorus oxychloride, undergoes ring closure with the elimination of hydrogen chloride, and form 1,3,4-oxadiazole ring [38].
Fig. 3.
Mechanism for the formation of 2,5-disubstituted 1,3,4-oxadiazole using phosphorus oxychloride
Structure–activity relationship of 1,3,4-oxadiazole derivatives
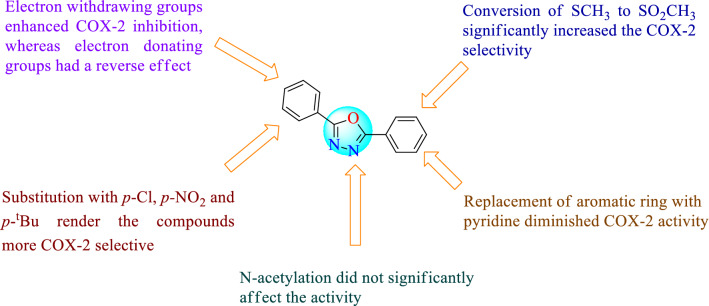
The structure–activity relationship of 1,3,4-oxadiazole is given in (Fig. 4). Substitution of phenyl ring with different substituents like p-Cl, p-NO2 & p-tBu further increases the activity. The conversion of the methylthio group into the methyl-sulfonyl group also increases the activity. The replacement of the phenyl ring along with the pyridine ring decreases the activity. If the acetyl group is present on the nitrogen atom of the oxadiazole ring did not significantly affect the activity [39]. Thus, based on the aforementioned results, we hypothesized that 2,5-disubstituted 1,3,4-oxadiazole scaffold may lead to novel potent agents with broad biological activity profile and improved pharmacokinetic properties.
Fig. 4.
Structure–activity relationship of 1,3,4-oxadiazole
Pharmacological profile of some oxadiazole derivatives
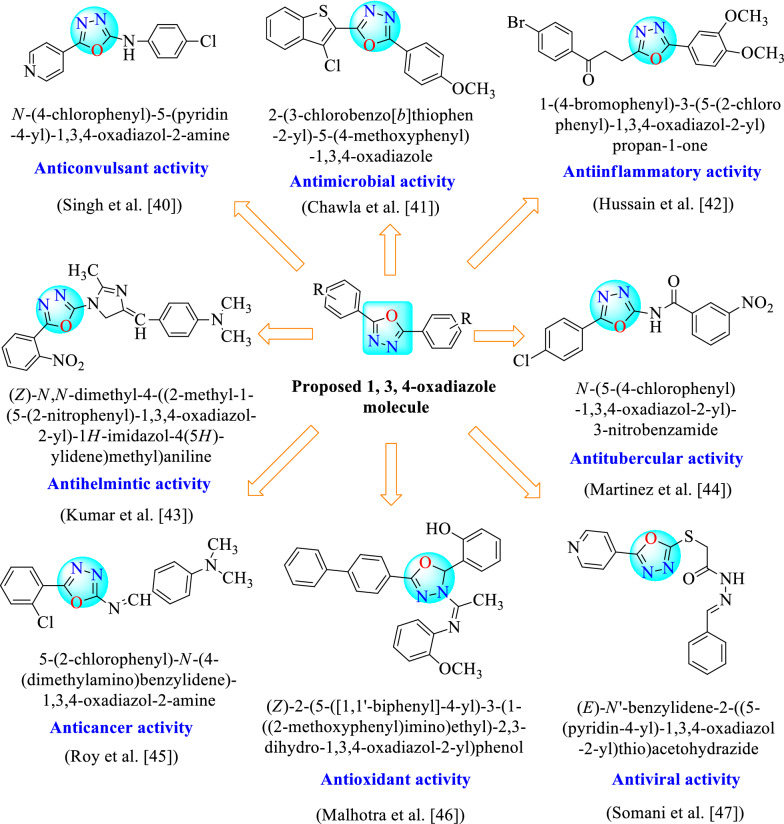
Compound N-(4 chlorophenyl) amino-5-(4-pyridyl)- 1,3,4-oxadiazole having electron-withdrawing group shows better anticonvulsant activity [40]. Compounds with p-methoxy group increase the antimicrobial potential [41] and 3, 4-dimethoxy containing compound increase anti-inflammatory activity as compared to reference drug [42]. 1,3,4-Oxadiazole nucleus containing compounds along with different substituents shows various kinds of activities (Fig. 5).
Fig. 5.
Therapeutic activity of 1,3,4-oxadiazole nucleus
Antimicrobial activity
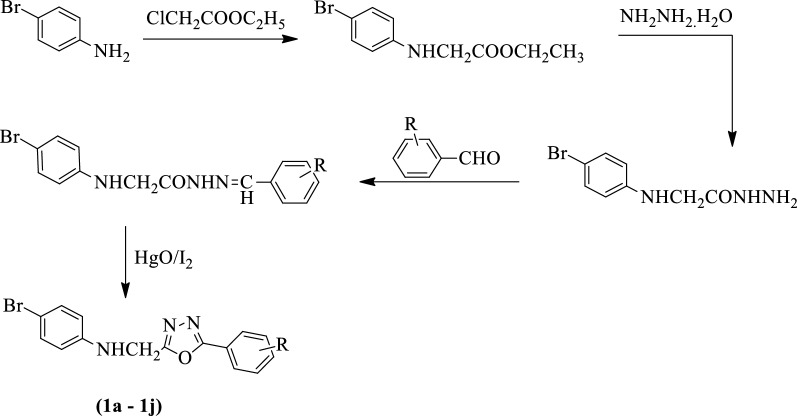
Bhat et al. [48] developed 4-bromo-N-[(5-(substituted phenyl)-1,3,4-oxadiazol-2yl)methyl]aniline (Scheme 1) and these derivatives were screened for antimicrobial activity against S. aureus, E. coli, B. Subtilis, and P. aeruginosa using amoxicillin as a positive control. The antimycotic activity was evaluated for these compounds against A. niger and C. albicans using ketoconazole as a reference standard. Derivatives with different groups like -OH, -NO2 [1b, 1c, 1d, 1g] shows good antimicrobial activity against fungal strains. Derivatives with groups like p-methoxy, p-chloro, p-methyl [1e, 1f, 1h] show better antimicrobial potential as compared to amoxicillin. The results of the antimicrobial activity of synthesized 1,3,4-oxadiazole derivatives were presented in (Table 1, Bhat et al. [48]).
Scheme 1.
Synthesis of substituted 1,3,4-oxadiazole (1a-j) with 4-bromoaniline starting material
Table 1.
Antimicrobial activity of titled compounds (1a-j) [48]
| Compound | Diameter of zone of inhibition (mm) | ||||
|---|---|---|---|---|---|
| S. aureus | B. subtilis | E. coli | P. aeruginosa | C. albicans | |
|
1a 1b 1c 1d 1e 1f 1 g 1 h 1i 1j Amoxicillin Ketoconazole |
13 14 14 15 18 19 14 18 16 15 21 – |
15 14 15 14 19 17 12 18 15 14 22 – |
14 13 14 13 18 18 15 19 14 15 21 – |
13 12 15 13 15 16 10 15 13 12 22 – |
08 15 14 15 08 09 15 09 10 11 – 23 |
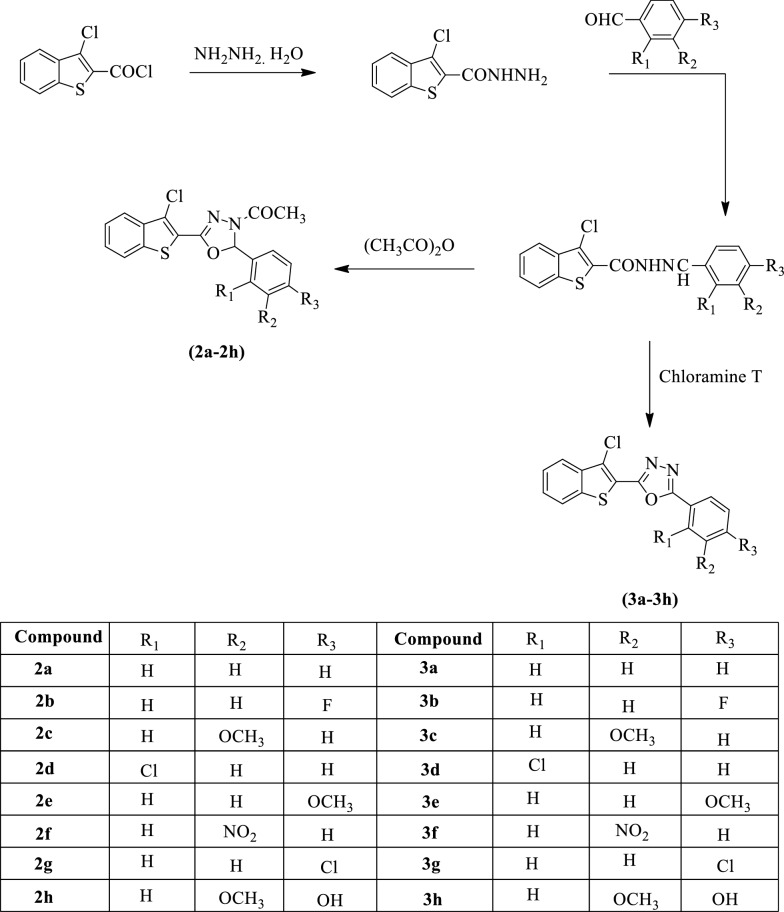
Chawla et al. [41] developed 1-(5-(3-chlorobenzo[b]thiophen-2-yl)-2-(2,3,4-trisubstituted phenyl)-1,3,4-oxadiazol-3(2H)-yl)ethanone and 2-(3-chlorobenzo[b]thiophen-2-yl)-5-(2,3,4-trisubstituted phenyl)-1,3,4-oxadiazole by using Scheme 2. The antibacterial activity of synthesized derivatives was evaluated against different bacterial strains such as (S. aureus, B. Subtilis, E. coli, and P. aeruginosa) using ciprofloxacin as standard drug. The antimycotic activity of these derivatives was evaluated against A. niger and C. albicans using fluconazole as a reference standard and the results were summarized in (Table 2, Chawla et al. [41]).
Scheme 2.
Synthesis of substituted 1,3,4-oxadiazole derivatives
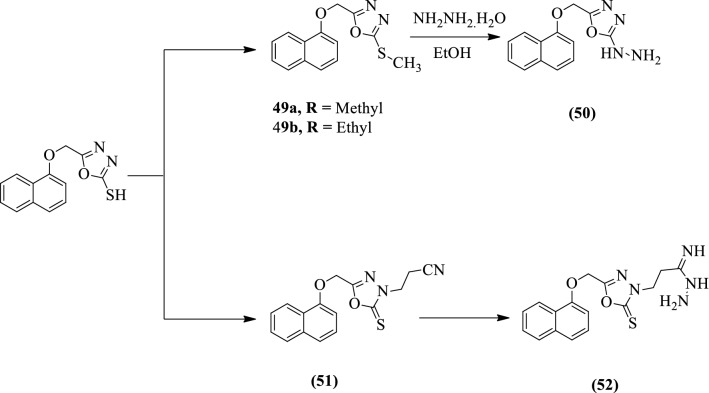
Table 2.
Antimicrobial activity of titled compounds (2a-h) and (3a-h) [41]
| Compound | Diameter of zone of inhibition (mm) | |||||
|---|---|---|---|---|---|---|
| Antibacterial activity | Antifungal activity | |||||
| S. aureus | B. subtilis | E. coli | P. aeruginosa | C. albicans | A. niger | |
| 2a | 14 | 21 | 10 | 17 | 09 | 10 |
| 2b | 18 | 19 | 12 | 15 | 10 | 11 |
| 2c | 30 | 27 | 14 | 18 | 09 | 11 |
| 2d | 19 | 22 | 11 | 18 | 10 | 11 |
| 2e | 28 | 28 | 14 | 14 | 10 | 09 |
| 2f | 14 | 19 | 10 | 15 | 10 | 10 |
| 2g | 21 | 23 | 13 | 19 | 11 | 09 |
| 2h | 14 | 20 | 10 | 16 | 09 | 10 |
| 3a | 11 | 12 | 10 | 09 | 11 | 11 |
| 3b | 10 | 12 | 09 | 11 | 12 | 12 |
| 3c | 20 | 21 | 12 | 13 | 11 | 11 |
| 3d | 20 | 22 | 16 | 18 | 10 | 11 |
| 3e | 18 | 19 | 11 | 13 | 11 | 10 |
| 3f | 11 | 13 | 10 | 11 | 10 | 11 |
| 3g | 12 | 14 | 09 | 12 | 10 | 10 |
| 3h | 10 | 13 | 09 | 11 | 10 | 11 |
| Ciprofloxacin | 26 | 26 | 28 | 25 | – | – |
| Fluconazole | – | – | – | – | 26 | 25 |
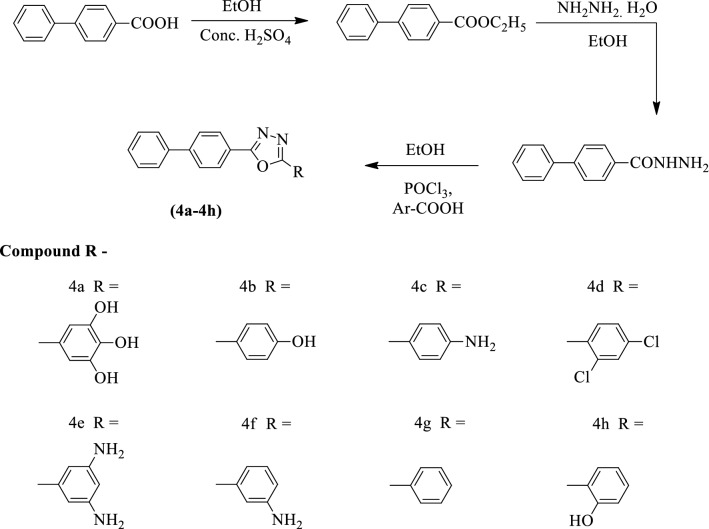
Kumar et al. [43] developed 2-((1, 1′-biphenyl)-4-yl)-5-(substituted phenyl)-1,3,4-oxadiazole by using Scheme 3. The antibacterial activity of these derivatives was evaluated against different Gram + ve (S. aureus) and Gram -ve (K. pneumonia, E. coli, and P. aeruginosa) strains using ofloxacin as a reference standard. The cup plate agar diffusion method was used for the determination of the zone of inhibition. The results of antibacterial activity were summarized in (Table 3, Kumar et al. [43]).
Scheme 3.
Synthesis of substituted 1,3,4-oxadiazole with 4-biphenyl carboxylic acid as starting material
Table 3.
In vitro antimicrobial activity of the titled compounds (4a-4 h) [43]
| Compound | Diameter of zone of inhibition (mm) | |||
|---|---|---|---|---|
| Antibacterial activity | ||||
| S. aureus | P. aeruginosa | K. pneumonia | E. coli | |
| 4a | 19 | 17 | 18 | 19 |
| 4b | 17 | 16 | 17 | 15 |
| 4c | 14 | 13 | 16 | 17 |
| 4d | 21 | 19 | 19 | 20 |
| 4e | 12 | 11 | 13 | 12 |
| 4f | 13 | 14 | 15 | 12 |
| 4g | 12 | 13 | 11 | 11 |
| 4h | 17 | 16 | 15 | 17 |
| Ofloxacin | 41 | 38 | 39 | 37 |
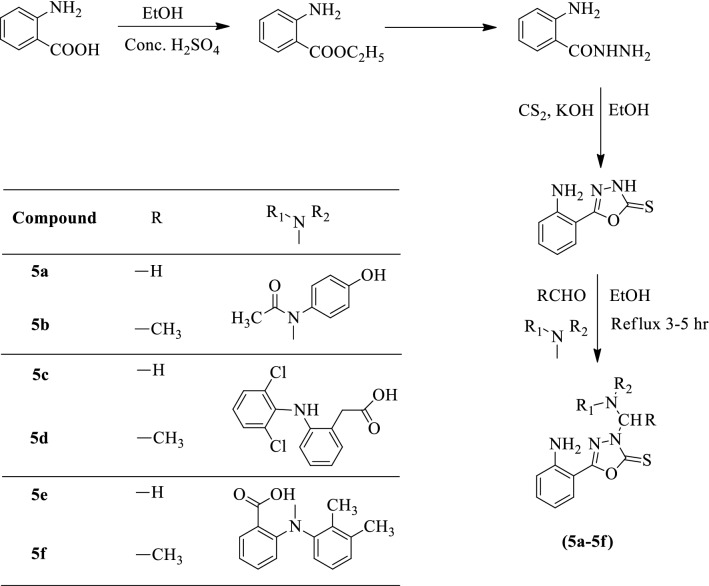
Kanthiah et al. [5] developed 5-(2-aminophenyl)-3-(substituted (disubstituted amino) methyl)-1,3,4-oxadiazole-2(3H)-thione by using Scheme 4. The antimicrobial activity of synthesized derivatives was evaluated against different two Gram + ve (S. aureus and S. pyogenes) and Gram -ve (E. coli and K. aerogenes) strains using amikacin as a reference standard. The antimycotic activity was also evaluated for these derivatives against C. albicans using ketoconazole as positive control and the results were summarized in (Table 4, Kanthiah et al. [5]).
Scheme 4.
Synthesis of substituted 1,3,4-oxadiazole with 2-aminobenzoic acid as starting material
Table 4.
Antimicrobial activity of the titled compounds (5a-5f) [5]
| Compound | Diameter of zone of inhibition (mm) | ||||
|---|---|---|---|---|---|
| Antibacterial activity | Antifungal activity | ||||
| S. aureus | S. pyrogenes | E. coli | P. aeruginosa | C. albicans | |
| 5a | 10 | 13 | 12 | 08 | 14 |
| 5b | 13 | 11 | 14 | 09 | 12 |
| 5c | 12 | 13 | 15 | 09 | 14 |
| 5d | 12 | 11 | 13 | 10 | 13 |
| 5e | 09 | 09 | 10 | 07 | 11 |
| 5f | 08 | 09 | 09 | 06 | 10 |
| Amikacin | 16 | 15 | 17 | 18 | – |
| Ketoconazole | – | – | – | – | 18 |
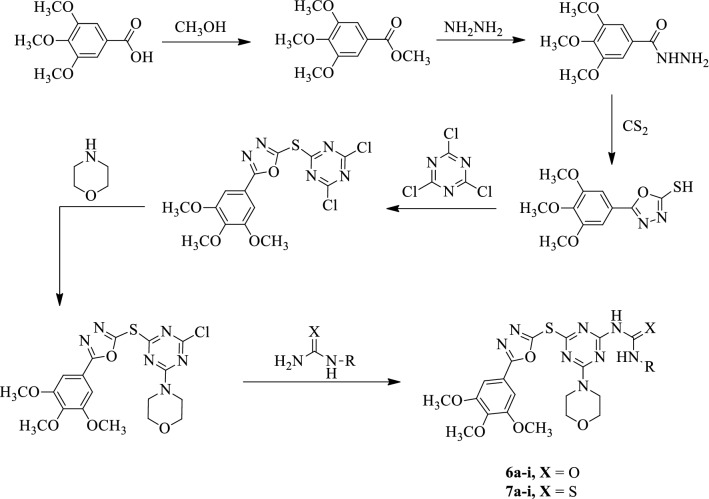
Chikhalia et al. [49] developed 1-substituted-3-(4-morpholino-6-((5-(3,4,5-trimethoxyphenyl)-1,3,4-oxadiazol-2-yl)thio)-1,3,5-triazin-2-yl)substituted urea (Scheme 5) and evaluated for antimicrobial activity against different strains such as (Staphylococcus aureus, Bacillus subtilis, Escherichia coli, and Pseudomonas aeruginosa) using ampicillin as a reference standard. The antifungal activity was also evaluated for these derivatives against C. albicans using fluconazole as a reference standard. Compound 6e shows better activity against E. coli and P. aeruginosa as compared to a positive control (ampicillin). Compound 6 g also shows better activity towards P. aeruginosa but lower than that of ampicillin. Compound 7c and 7g showed good activity against C. albicans but slightly lower than that of fluconazole. The results of antimicrobial activity were shown in (Table 5, Chikhalia et al. [49]).
Scheme 5.
Synthesis of substituted 1,3,4-oxadiazole with 3, 4, 5-trimethoxybenzoic acid as starting material
Table 5.
Minimum inhibitory concentration (MIC) of titled compounds [49]
| Compound | S. aureus | B. subtilis | P. aeruginosa | E. coli | C. albicans | ||
|---|---|---|---|---|---|---|---|
| R | X | ATCC 25923 | ATCC 6633 | ATCC 27853 | ATCC 27853 | ATCC 10231 | |
| 6a | C6H5 | O | 0.3 | 0.15 | 0.15 | 1.25 | 2.5 |
| 6b | 2-CH3 C6H5 | O | 0.31 | 0.07 | 1.25 | 0.625 | 5.0 |
| 6c | 3-CH3 C6H5 | O | 0.625 | 0.15 | 5.0 | 2.5 | 10 |
| 6d | 4-CH3 C6H5 | O | 2.5 | 2.5 | 0.03 | 5.0 | 1.25 |
| 6e | 2-Cl C6H5 | O | 0.15 | 1.25 | 0.019 | 0.019 | 5.0 |
| 6f | 3-Cl C6H5 | O | 0.15 | 0.625 | 1.25 | 1.25 | 2.5 |
| 6g | 4-Cl C6H5 | O | 0.15 | 0.3 | 0.019 | 0.07 | 0.15 |
| 6h | 3-NO2 C6H5 | O | - | 10 | 1.25 | – | – |
| 6i | 4-NO2 C6H5 | O | 2.5 | – | 0.625 | 5.0 | 10 |
| 7a | 2-CH3 C6H5 | S | 1.25 | – | 2.5 | 10 | – |
| 7b | 4-CH3 C6H5 | S | 1.25 | 5.0 | 2.5 | 1.25 | 5.0 |
| 7c | 3-OH C6H5 | S | 2.5 | 1.25 | 0.019 | 2.5 | 10 |
| 7d | 4-OH C6H5 | S | 0.15 | 0.625 | 2.5 | 0.625 | 1.25 |
| 7e | 4-Cl C6H5 | S | 0.625 | 0.07 | 5.0 | 0.03 | 0.31 |
| 7f | 3-NO2 C6H5 | S | 2.5 | 2.5 | 10 | 1.25 | 2.5 |
| 7g | 4-NO2 C6H5 | S | 2.5 | 5.0 | 5.0 | 0.1 | 0.15 |
| Ampicillin | 0.019 | 0.005 | 0.005 | 0.01 | – | ||
| Fluconazole | – | – | – | – | 0.01 | ||
Antitumor activity
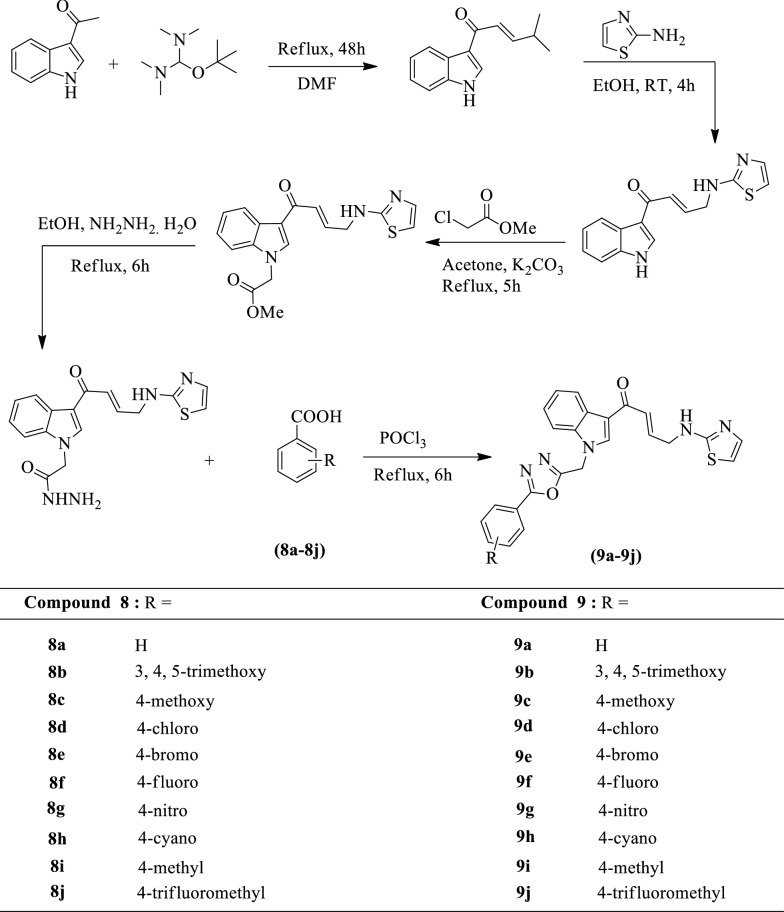
Srinivas et al. [30] developed (E)-1-(1-((5-substituted-1,3,4-oxadiazol-2-yl)methyl)-1H-indol-3-yl)-4-(thiazol-2-ylamino)but-2-en-1-one (Scheme 6) and evaluated for antitumor activity by MTT assay against four different cancer cell lines such as HT-29 (colon), A375 (melanoma), MCF-7 (breast) and A549 (lung) using combretastatin-A4 as reference standard. All derivatives of 1,3,4-oxadiazole fused indole ring was showed a variable degree of anticancer activity along with IC50 values ranging from 0.010 ± 0.004 and 18.50 ± 0.86 μM. Among the different derivatives 9a, 9b, 9f, 9g, 9h, and 9j were exhibited more potent than the positive control. The results of antitumor activity were presented in (Table 6, Srinivas et al. [30]).
Scheme 6.
Synthesis of substituted 1,3,4-oxadiazole derivatives
Table 6.
In vitro cytotoxicity (IC50Μ)a data of compounds (9a-j) [30]
| Compound | A549bc | MCF-7d | A375e | HT-29f |
|---|---|---|---|---|
| 9a | 1.20 ± 0.16 | 0.098 ± 0.004 | 2.56 ± 0.36 | 0.012 ± 0.001 |
| 9b | 0.023 ± 0.006 | 0.011 ± 0.001 | – | 1.90 ± 0.71 |
| 9c | 2.30 ± 0.21 | 2.19 ± 0.28 | – | 8.30 ± 1.60 |
| 9d | 3.56 ± 0.19 | 2.11 ± 0.23 | 6.13 ± 1.12 | 7.14 ± 0.86 |
| 9e | 5.02 ± 1.02 | 12.4 ± 0.96 | – | – |
| 9f | 0.27 ± 0.02 | 1.07 ± 0.59 | 2.81 ± 0.25 | 1.55 ± 0.65 |
| 9 g | 0.013 ± 0.001 | 0.80 ± 0.15 | 1.05 ± 0.53 | 1.24 ± 0.17 |
| 9 h | 1.02 ± 0.50 | 0.010 ± 0.004 | 1.99 ± 0.29 | 3.78 ± 0.16 |
| 9i | 13.9 ± 0.54 | 18.50 ± 0.86 | 8.23 ± 1.35 | – |
| 9j | 0.90 ± 0.09 | 0.12 ± 0.01 | 0.39 ± 0.012 | 1.10 ± 0.54 |
| Combretastatin-A4 | 0.11 ± 0.01 | 0.18 ± 0.01 | 0.21 ± 0.02 | 0.93 ± 0.03 |
aEach data represented as mean ± S.D values. From three different experiments performed in triplicates, bcA549: Human lung cancer cell line, dMCF-7: Human breast cancer cell line, eA375: Human melanoma cancer cell line, fHT-29: Human colon cancer cell line. –: Not active
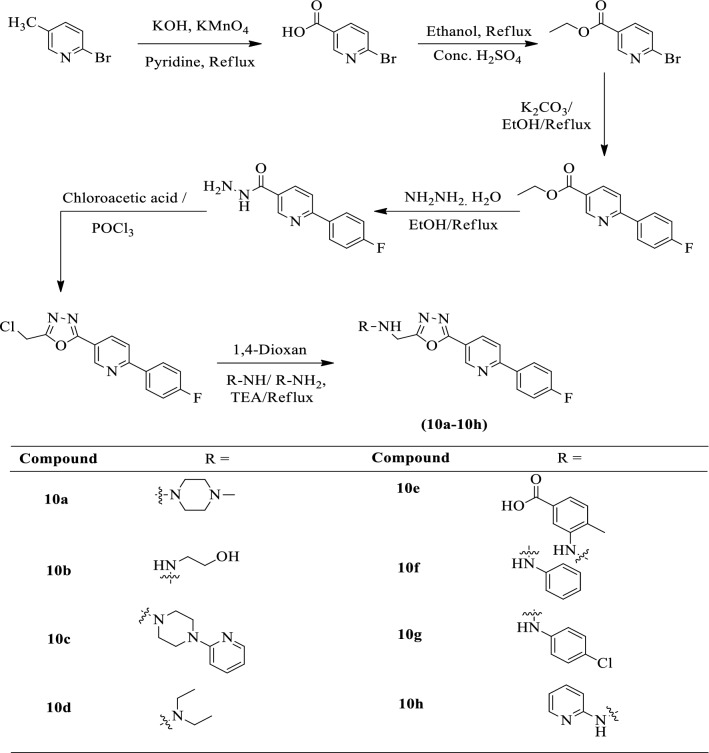
Vinayak et al. [50] developed N-[(5-(6-(4-fluorophenyl)pyridine-3-yl)1,3,4-oxadiazol-2-yl)methyl]-substituted-1-amine by using Scheme 7 and evaluated for antiproliferative activity against different cell lines such as HeLa, HepG2, and Caco by MTT assay using 5-Fluorouracil as a reference standard. The derivative 10a and 10d showed excellent activity against HepG2 cell lines. The compound 10f gives better results against the Caco-2 cancer cell line. The results of the anti-proliferative activity of synthesized derivatives were showed in (Table 7a, b, and c, Vinayak et al. [50]).
Scheme 7.
Synthesis of substituted 1,3,4-oxadiazole derivatives
Table 7.
(a) IC50 values of the synthesized novel amine derivatives. (b) CC50 values of the synthesized novel amine derivatives. (c) Selectivity index (SI) of the synthesized novel amine derivatives [50]
| Panel (a) | ||||
|---|---|---|---|---|
| Compound | IC50#values of 10(a-h) in (μM) | |||
| HeLa | Caco-2 | HepG2 | ||
| 10a | 212.4 ± 1.2 | 203.6 ± 2.3 | 2.6 ± 0.5 | |
| 10b | 85.6 ± 0.8 | 112.5 ± 1.2 | 45.6 ± 1.1 | |
| 10c | 34.8 ± 1.3 | 123.8 ± 1.4 | 128.9 ± 3.5 | |
| 10d | 112.9 ± 0.4 | 145.6 ± 0.4 | 5.8 ± 1.6 | |
| 10e | 118.4 ± 0.5 | 212.3 ± 0.4 | 32.2 ± 0.3 | |
| 10f | 78.3 ± 5.4 | 2.3 ± 0.5 | 23.5 ± 4.6 | |
| 10 g | 56.4 ± 3.4 | 56.8 ± 1.2 | 156.7 ± 2.3 | |
| 10 h | 88.6 ± 1.2 | 34.6 ± 0.9 | 176.4 ± 1.6 | |
| 5-FU | 7.6 ± 0.3 | 8.8 ± 0.6 | 7.6 ± 0.2 | |
| Panel (b) | ||||
|---|---|---|---|---|
| Compound | CC50* of the compound 10(a-h) in (μM) | |||
| HeLa | Caco-2 | HepG2 | ||
| 10a | 120 ± 1.2 | 112 ± 1.3 | 34 ± 0.5 | |
| 10b | 7.6 ± 0.6 | 145 ± 1.1 | 129 ± 0.3 | |
| 10c | 200 | 178 ± 2.3 | 102 ± 1.1 | |
| 10d | 450 | 100 ± 2.6 | 112 ± 1.4 | |
| 10e | 56 ± 2.4 | 62 ± 1.2 | 76 ± 3.4 | |
| 10f | 127 ± 3.4 | 87 ± 2.6 | 77 ± 0.4 | |
| 10 g | 200 | 23 ± 1.5 | 91 ± 4.3 | |
| 10 h | 123 ± 2.3 | 156 ± 0.4 | 73 ± 1.4 | |
| 5-FU | 57 ± 0.3 | 69 ± 2.3 | 52 ± 1.8 | |
| Panel (c) | ||||
|---|---|---|---|---|
| Compound | SI of the compound 10(a-h) | |||
| HeLa | Caco-2 | HepG2 | ||
| 10a | 0.566 | 0.551 | 13.06 | |
| 10b | 0.887 | 1.288 | 2.828 | |
| 10c | 5.747 | 1.437 | 0.791 | |
| 10d | 3.985 | 0.686 | 19.31 | |
| 10e | 0.472 | 0.292 | 0.236 | |
| 10f | 1.621 | 37.8 | 3.276 | |
| 10g | 3.546 | 0.404 | 0.580 | |
| 10h | 1.388 | 4.508 | 0.413 | |
| 5-FU | 7.5 | 7.84 | 6.84 | |
*Concentration of compound at 50% of the remaining viable cells
#Inhibitory concentration at 50% of the viable cells
± Average value of the two independent experiments
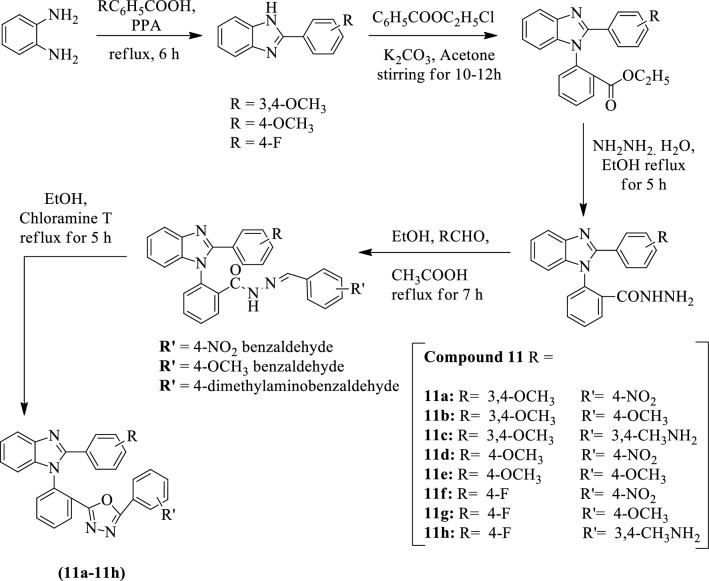
Kapoor et al. [51] developed 2-(substituted phenyl)-5-(2-(2-(substituted phenyl)-1H-benzo[d]imidazol-1-yl)phenyl)-1,3,4-oxadiazole by using Scheme 8 and evaluated for antitumor activity against MCF-7 (breast) cancer cell line by MTT assay. Compound 11e shows better cytotoxic activity as compare to 11a, 11b, and 11c. Compounds 11f, 11g, 11h also show the excellent cytotoxic activity as compared to the rest of the derivatives. Compounds 11e and 11h flourished potent cytotoxic activity with minimum percentage viability. Each compound was tested to calculate the percentage viability of cell line against the different concentrations which is presented in (Table 8, Kapoor et al. [51]).
Scheme 8.
Synthesis of substituted 1,3,4-oxadiazole with benzene 1, 2-diamine as starting material
Table 8.
In-vitro cytotoxicity of synthesized compounds against Breast cancer cell line (MCF-7) [51]
| Compound | % Viability | ||||
|---|---|---|---|---|---|
| 6.25 μg/ml | 12.5 μg/ml | 25 μg/ml | 50 μg/ml | 100 μg/ml | |
| 11a | 38.04 | 37.15 | 39.68 | 35.11 | 40.31 |
| 11b | 38.26 | 42.70 | 37.90 | 38.84 | 43.24 |
| 11c | 44.35 | 41.6 | 41.81 | 39.64 | 37.24 |
| 11d | 42.70 | 39.46 | 40.48 | 37.61 | 37.37 |
| 11e | 30.60 | 32.20 | 34.48 | 33.86 | 37.54 |
| 11f | 32.57 | 33.09 | 30.88 | 30.75 | 24.87 |
| 11 g | 34.39 | 33.58 | 28.80 | 32.40 | 30.96 |
| 11 h | 32.03 | 35.40 | 31.25 | 33.69 | 34.45 |
Control % viability = 100
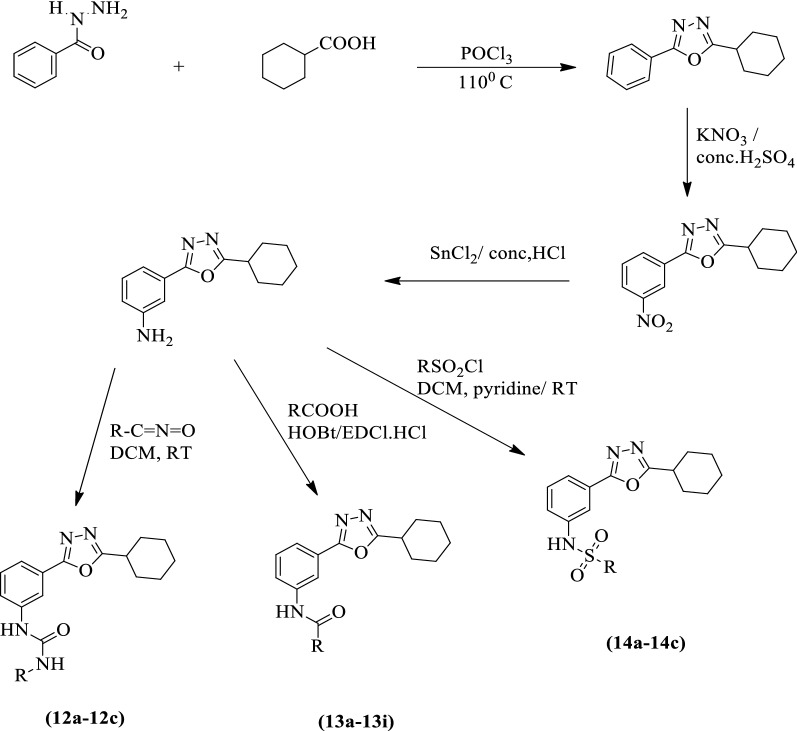
Kavitha et al. [31] developed N-substituted-(3-(5-cyclohexyl-1,3,4-oxadiazol-2-yl)phenyl)benzamide, urea, and substituted benzenesulfonamide derivatives by using Scheme 9. The anticancer activity of synthesized derivatives was evaluated against different cancer cell lines like HeLa and MCF-7 using cisplatin as a reference standard. Among the different derivatives, compounds 12a, 12b, 12c, 13c, 13d, and 14b showed significant activity after 48 h exposures. Further derivatives 12a, 13c, 13d, and 14b also showed excellent antitumor activity as compared to the positive control. Compound 12b showed excellent antitumor activity as compared to the rest of other compounds. The results of the antitumor activity of these derivatives were presented in (Table 9, Kavitha et al. [31]).
Scheme 9.
Synthesis of 1,3,4-oxadiazole derivatives
Table 9.
Preliminary cytotoxicity screening of synthesized 1,3,4-oxadiazole derivatives [31]
| Compound | IC50 μM | |
|---|---|---|
| HeLa | MCF-7 | |
| 12a | 79.7 | 81.6 |
| 12b | 30.4 | 23.5 |
| 12c | 45.6 | 28.6 |
| 13a | ≥ 100 | ≥ 100 |
| 13b | ≥ 100 | ≥ 100 |
| 13c | 80.1 | 78.3 |
| 13d | 58.8 | 62.4 |
| 13e | ≥ 100 | ≥ 100 |
| 13f | 100.3 | ≥ 100 |
| 13 g | ≥ 100 | ≥ 100 |
| 13 h | ≥ 100 | ≥ 100 |
| 13i | ≥ 100 | ≥ 100 |
| 14a | ≥ 100 | ≥ 100 |
| 14b | 62.9 | 60.9 |
| 14c | ≥ 100 | ≥ 100 |
| Standard | 3.5 | 3.5 |
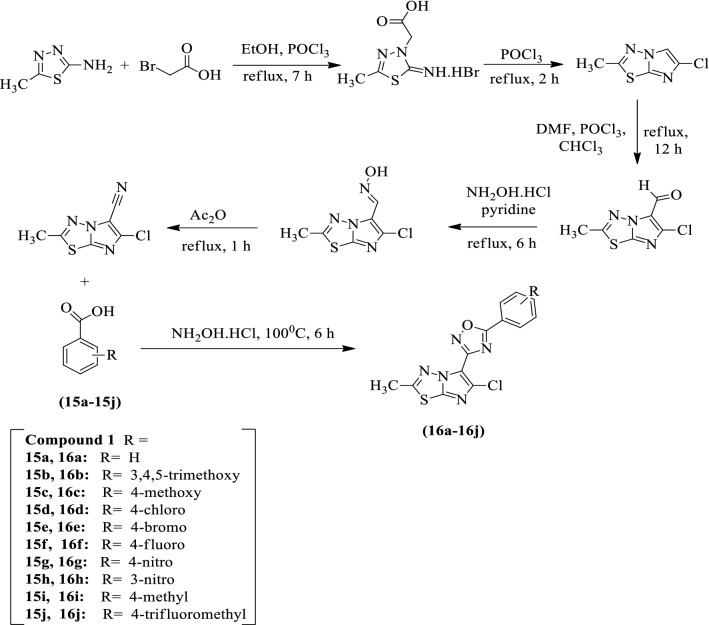
Chakrapani et al. [52] developed 3-(6-chloro-2-methylimidazo[2,1-b][1,3,4]thiadiazol-5-yl)-5-(substituted phenyl)-1,2,4-oxadiazole by using Scheme 10. The antitumor activity of the synthesized derivatives was evaluated by MTT assay against ACHN (renal), MCF-7 (breast), and A375 (melanoma) tumor cell line using doxorubicin as a reference standard. The compound 16b shows good cytotoxic activity in comparison to the reference drug. The compound 16j exhibits excellent activity towards melanoma cancer cell line (A375) and potent activities towards MCF-7 and ACHN cancer cell lines. The results of the antitumor activity of synthesized derivatives were presented in (Table 10, Chakrapani et al. [52]).
Scheme 10.
Synthesis of 1,2,4-oxadiazole derivatives
Table 10.
Cytotoxicity data for compound 16a-j [52]
| Compound | IC50 values, μM | ||
|---|---|---|---|
| A375 | MCF-7 | ACHN | |
| 16a | 11.4 | 10.2 | 18.5 |
| 16b | 1.22 | 0.23 | 0.11 |
| 16c | 2.98 | 0.70 | 1.89 |
| 16d | 14.6 | 19.1 | 6.47 |
| 16e | 8.20 | 11.2 | 7.7 |
| 16f | 2.70 | 8.41 | 17.6 |
| 16 g | 17.7 | 9.7 | 12.2 |
| 16 h | 2.20 | 5.98 | 10.6 |
| 16i | 9.56 | 13.7 | 2.44 |
| 16j | 0.37 | 1.47 | 0.33 |
| Doxorubicin | 5.51 | 2.02 | 0.79 |
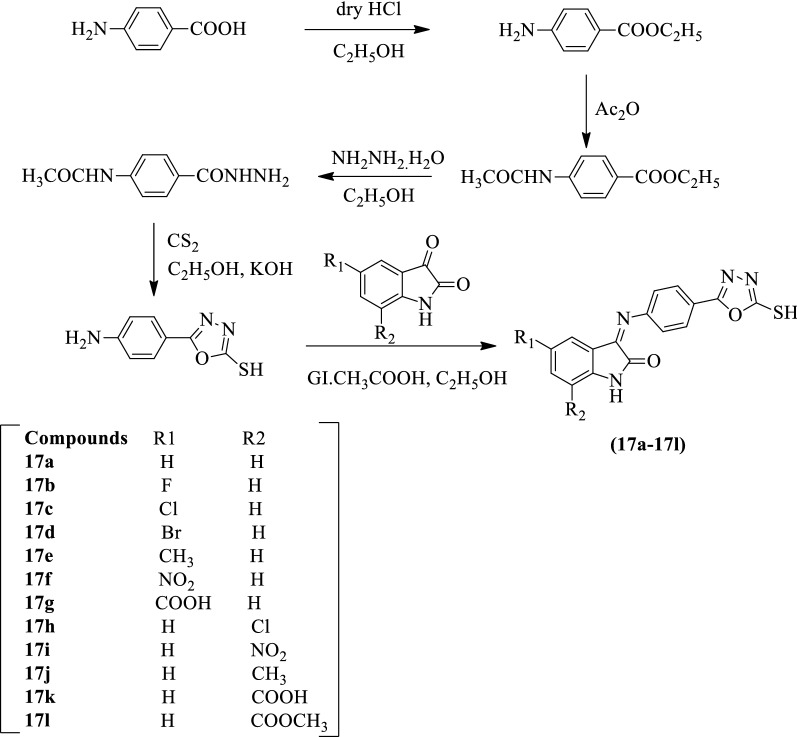
Gudipati et al. [53] developed (Z)-3-[(4-(5-mercapto-1,3,4-oxadiazol-2-yl)phenyl) imino]-5 or 7-substituted indolin-2-one (Scheme 11) and evaluated for antitumor activity by MTT assay against MCF-7, IMR-32, and HeLa tumor cell lines using cisplatin as a reference standard. The compounds 17b-17d showed the most potent antitumor activity than the rest of other derivatives. The results of antitumor activity were summarized in (Table 11, Gudipati et al. [53]).
Scheme 11.
Synthesis of substituted 1,3,4-oxadiazole with p-amino benzoic acid as starting material
Table 11.
Anticancer activity of synthesized compounds against HeLa, IMR-32 & MCF-7 cancer cells using MTT assay [53]
| Compound | R1 | R2 | IC50 (μM)*(HeLa) | IC50 (μM)*(IMR-32) | IC50 (μM)* (MCF-7) |
|---|---|---|---|---|---|
| Isatin | 521.9 | 352.74 | 410.95 | ||
| 17 | Intermediate | 309.59 | 176.85 | 206.95 | |
| 17a | H | H | 25.47 | 30.65 | 33.62 |
| 17b | F | H | 11.99 | 13.48 | 15.57 |
| 17c | Cl | H | 12.84 | 15.84 | 16.68 |
| 17d | Br | H | 10.64 | 12.68 | 16.06 |
| 17e | CH3 | H | 22.59 | 27.25 | 29.38 |
| 17f | NO2 | H | 18.60 | 22.51 | 24.48 |
| 17 g | COOH | H | 17.25 | 20.85 | 22.95 |
| 17 h | H | Cl | 18.69 | 22.51 | 24.92 |
| 17i | H | NO2 | 16.20 | 19.35 | 20.38 |
| 17j | H | CH3 | 15.12 | 18.32 | 20.95 |
| 17 k | H | COOH | 20.36 | 24.28 | 25.98 |
| 17 l | H | COOCH3 | 19.32 | 23.85 | 25.18 |
| Cisplatin | 14.08 | 13.64 | 13.54 |
Values are expressed as means (n = 4)
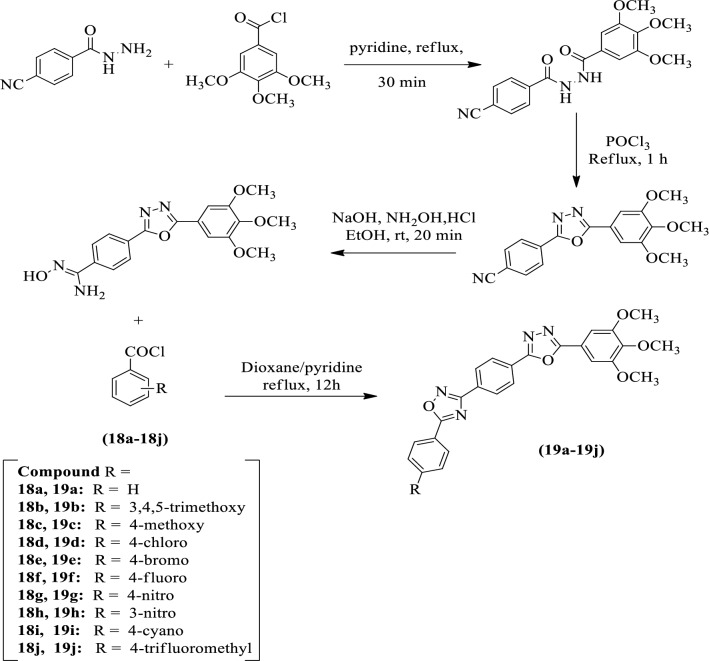
Polothi et al. [54] developed 5-(substituted phenyl)-3-(4-(5-(3,4,5-trimethoxyphenyl)-1,3,4-oxadiazol-2-yl)phenyl)-1,2,4-oxadiazole by using Scheme 12 and evaluated for antitumor activity by MTT assay against MDA MB-231, MCF-7 (breast cell line), A549 (lung cell line) cancer cell lines using doxorubicin as a reference standard. Among the different derivatives, compounds 19b, 19g, 19h, and 19i showed good cytotoxic activity as compared to the reference standard. The compound 19b with 3, 4, 5-trimethoxy group on phenyl ring shows excellent antitumor activity against human cancer cell lines such as A549 and MCF-7. The results of the antitumor activity of synthesized derivatives were showed in (Table 12, Polothi et al. [54]).
Scheme 12.
Synthesis of substituted 1,3,4-oxadiazole linked 1,2,4-oxadiazole
Table 12.
In vitro cytotoxic activity [IC50 (μM)a] of compounds (19a-j) [54]
| Compound | Lung cancerA549c | Breast cancer | |
|---|---|---|---|
| MCF-7b | MDA MB-231d | ||
| 19a | 9.78 ± 0.27 | 34.55 ± 2.34 | – |
| 19b | 0.45 ± 0.03 | 1.76 ± 0.34 | 2.11 ± 0.21 |
| 19c | 3.67 ± 0.18 | 2.89 ± 0.67 | 12.76 ± 0.81 |
| 19d | 4.56 ± 0.19 | 2.33 ± 0.56 | 7.34 ± 0.26 |
| 19e | 13.78 ± 1.78 | 12.4 ± 0.79 | 19.5 ± 2.11 |
| 19f | 34.9 ± 2.30 | 15.3 ± 1.72 | – |
| 19g | 1.03 ± 0.17 | 1.23 ± 0.30 | 1.89 ± 0.35 |
| 19h | 2.45 ± 0.23 | 0.34 ± 0.025 | 1.11 ± 0.18 |
| 19i | 1.89 ± 0.38 | 1.90 ± 0.41 | 3.78 ± 0.29 |
| 19j | 87.5 ± 4.67 | 6.30 ± 0.35 | 22.5 ± 1.28 |
| Doxorubicin | 2.10 ± 0.14 | 3.12 ± 0.17 | 3.41 ± 0.23 |
(–) not active, aEach data represents as mean ± S.D values. From three different experiments performed in triplicates. MCF-7: Human breast cancer cell line. cA549: Human lung cancer cell line. MDA MB-231d: Human breast cancer cell line
Antitubercular activity
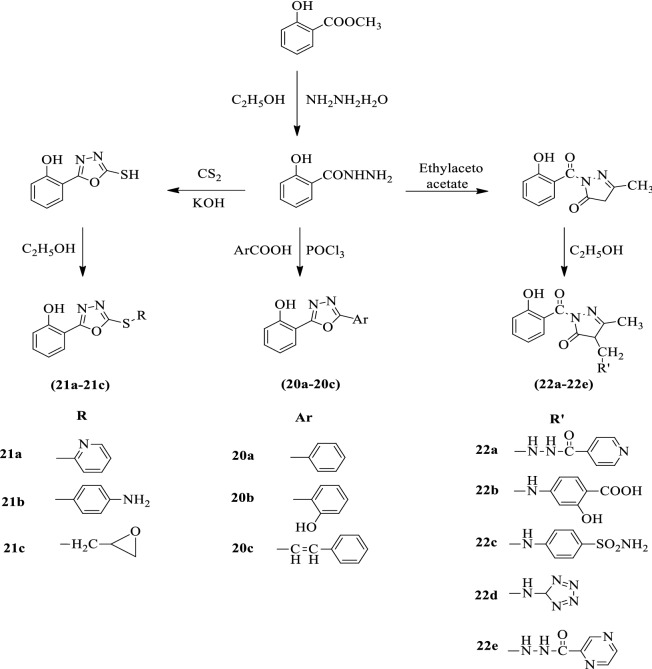
Pattan et al. [55] developed 2-(5-(substituted thio)-1,3,4-oxadiazol-2-yl) phenol and 4-(substituted-1-ylmethyl)-1-(2-hydroxy benzoyl)-3-methyl-1H-pyrazol-5(4H)-one by using Scheme 13. The antimycobacterial activity of the synthesized derivatives was evaluated against Mycobacterium tuberculosis (H37Rv) by MB 7H9 agar medium. Streptomycin was used as a reference standard. Compounds 20a, 21b, 22a, 22b, 22c, and 22e showed promising antitubercular activity. Compounds 20b, 20c, and 22d showed moderate activity and the results of activity were presented in (Table 13, Pattan et al. [55]).
Scheme 13.
Synthesis of 1,3,4-oxadiazole derivatives
Table 13.
Antitubercular activity data of the synthesized compounds [55]
| Compound | Antitubercular activity | |
|---|---|---|
| 50 μg/mL | 100 μg/mL | |
| 20a | S | S |
| 20b | R | R |
| 20c | R | R |
| 21a | R | R |
| 21b | S | S |
| 21c | R | R |
| 22a | S | S |
| 22b | S | S |
| 22c | S | S |
| 22d | R | R |
| 22e | S | S |
| Streptomycin | S | S |
R Resistant; S Sensitive
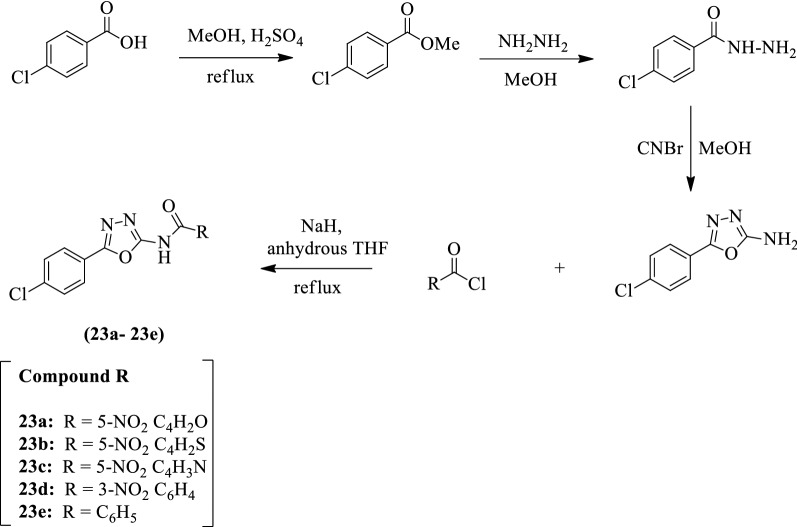
Martinez et al. [44] developed N-(5-(4-chlorophenyl)-1,3,4-oxadiazol-2-yl) substituted amide by using Scheme 14. The antimycobacterial activity of synthesized derivatives was evaluated against different Mycobacterium tuberculosis strains such as 209, H37Ra, and H37Rv using rifampin as a reference standard. Compound 23a shows more potent activity in comparison to the rest of other compounds. The results of the antitubercular activity of the synthesized derivatives were presented in (Table 14, Martinez et al. [44]).
Scheme 14.
Synthesis of substituted 1,3,4-oxadiazole derivatives
Table 14.
MIC100 values of 23a-e against virulent, non-virulent and RIF-resistant M. tuberculosis bacteria [44]
| Compound | R | MIC100 (μg/ml) in H37Rv ATCC 27294 | MIC100 (μg/ml) inH37Ra | MIC100 (μg/ml) in Mtb-209 (resistant) |
|---|---|---|---|---|
| 23a | 5-NO2C4H2O | 7.80 | 1–2.00 | 7.8 |
| 23b | 5-NO2C4H2S | 15.60 | 15.60 | 15.60 |
| 23c | 5-NO2C4H3O | 31.25 | 7.8 | 7.8 |
| 23d | 5-NO2C6H4 | 15.60 | 31.30 | 15.60 |
| 23e | 5-C6H5 | 15.60 | 62.50 | 31.25 |
| Rifampin | - | 0.06 | 0.008 | > 64 |
M. tuberculosis H37Rv ATCC 27294 reference strain; Mtb. M. tuberculosis H37Ra non-virulent strain; Mtb-209 RIF-resistant clinical isolate of M. tuberculosis
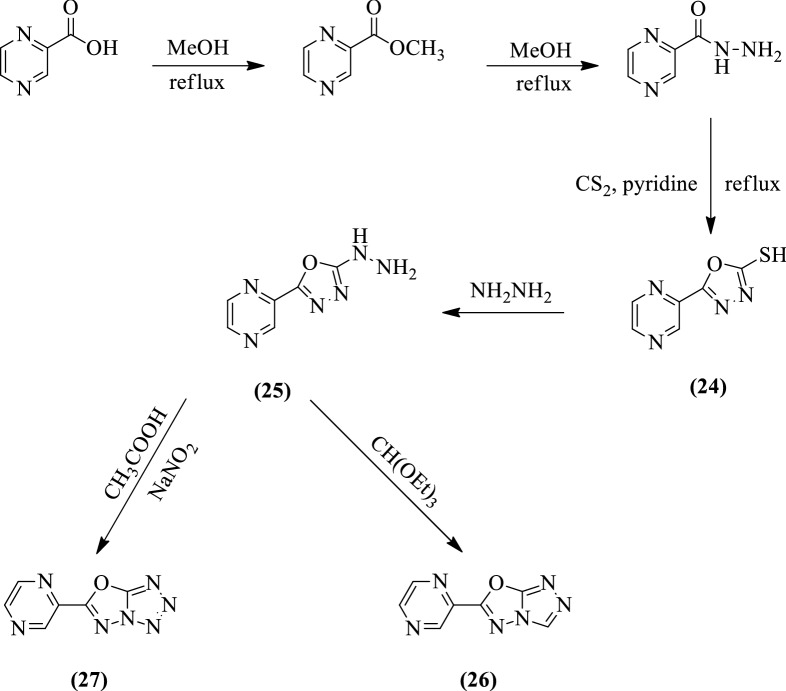
Das et al. [56] synthesized 6-(pyrazin-2-yl)-[1,3,4]oxadiazolo[3,2-d]tetrazole and 6-(pyrazin-2-yl)-[1,2,4]triazolo[3,4-b][1,3,4]oxadiazole (Scheme 15) and antimycobacterial activity of these derivatives were evaluated by (LJ) agar method against Mycobacterium tuberculosis H37Rv (MTCC200) using isoniazid and rifampicin as a reference standard. The compound 25 shows more potent antitubercular activity but still, it is lesser active than the reference standard. The results of antimycobacterial activity were showed in (Table 15, Das et al. [56]).
Scheme 15.
Synthesis of 1,3,4-oxadiazole linked triazole and tetrazole compounds
Table 15.
Anti Tuberculosis activity against Mycobacterium tuberculosis H37Rv (MTCC200) [56]
| Compound | MIC̽ (μg/ml) |
|---|---|
| 24 | > 100 |
| 25 | 6.25 |
| 26 | 50 |
| 27 | 50 |
| Rifampicin | 0.25 |
| Isoniazid | 0.20 |
MIC Minimum inhibitory concentration
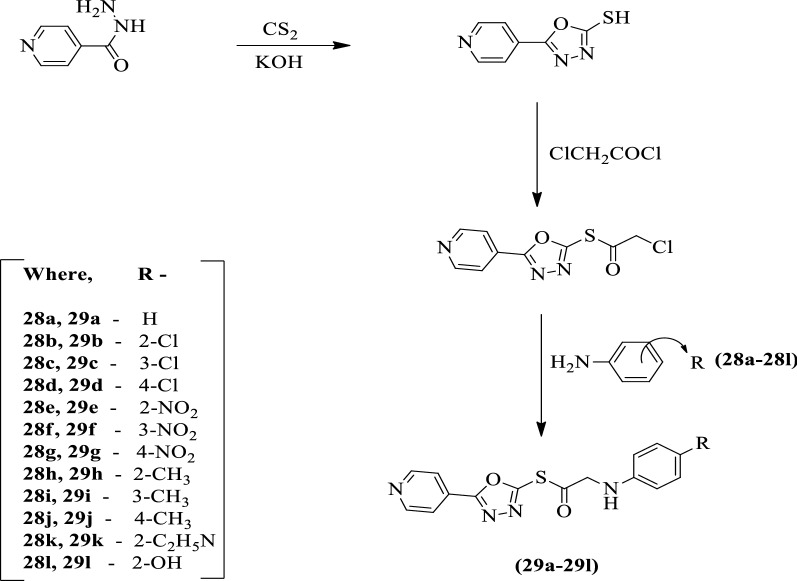
Raval et al. [57] developed S-(5-(pyridin-4-yl)-1, 3, 4-oxadiazol-2-yl)2-((substituted phenyl)amino)ethanethioate using Scheme 16. The antitubercular activity of synthesized derivatives was evaluated against Mycobacterium tuberculosis H37Rv (ATCC27294). Rifampin was used as a reference standard. Compounds 29e, 29g, and 29k show better activity and exhibited > 90% inhibition. The conclusion of antimycobacterial activity was presented in (Table 16, Raval et al. [57]).
Scheme 16.
Synthesis of substituted 1,3,4-oxadiazole
Table 16.
Antitubercular activity of the synthesized compounds (29a-l) against M. tuberculosis H37Rv [57]
| Compound | Primary screen (6.25 μg/ml) | % inhibition | Concentration (μM) | Actual MIC (μg/Ml) | Clog P̽ |
|---|---|---|---|---|---|
| 29a | > 6.25 | 64 | 0.0354 | – | 0.4996 |
| 29b | > 6.25 | 12 | 0.1640 | – | 1.5150 |
| 29c | > 6.25 | 32 | 0.1706 | – | 1.5150 |
| 29d | > 6.25 | 28 | 0.1735 | – | 1.5150 |
| 29e | > 6.25 | 92 | 0.0077 | 6.05 | 0.8964 |
| 29f | > 6.25 | 86 | 0.00132 | 5.92 | 0.8964 |
| 29g | > 6.25 | 96 | 0.0052 | 6.00 | 0.8964 |
| 29h | > 6.25 | 63 | 0.1130 | – | 0.9986 |
| 29i | 6.25 | 62 | 0.1138 | – | 0.9986 |
| 29j | > 6.25 | 64 | 0.1133 | – | 0.9986 |
| 29k | > 6.25 | 96 | 0.0089 | 5.77 | − 0.8943 |
| 29l | 6.25 | 69 | 0.1184 | – | − 9.1673 |
| Isoniazid | > 6.25 | 98 | 0.025 | 0.05 | − 0.6680 |
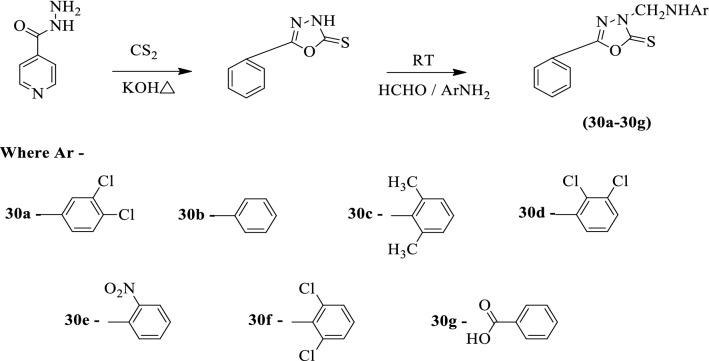
Somani et al. [58] developed 3-((substituted amino) methyl)-5-phenyl-1,3,4-oxadiazole-2(3H)-thione by using Scheme 17. The antimycobacterial activity of synthesized derivatives was evaluated against Mycobacterium tuberculosis H37Rv strain in MB 7H-9 agar medium using rifampicin as a reference standard. The conclusion of the antimycobacterial activity of synthesized derivatives was presented in (Table 17, Somani et al. [58]).
Scheme 17.
Synthesis of substituted 1,3,4-oxadiazole
Table 17.
Antitubercular activity of the synthesized compounds (30a-3g) against M. tuberculosis H37Rv [58]
| Compound | Antitubercular activity | ||
|---|---|---|---|
| 25 (µg/ml) | 50 (µg/ml) | 100 (µg/ml) | |
| 30a | R | R | S |
| 30b | R | S | S |
| 30c | S | S | S |
| 30d | S | S | S |
| 30e | S | S | S |
| 30f | R | R | S |
| 30g | R | R | S |
| Rifampicin | S | S | S |
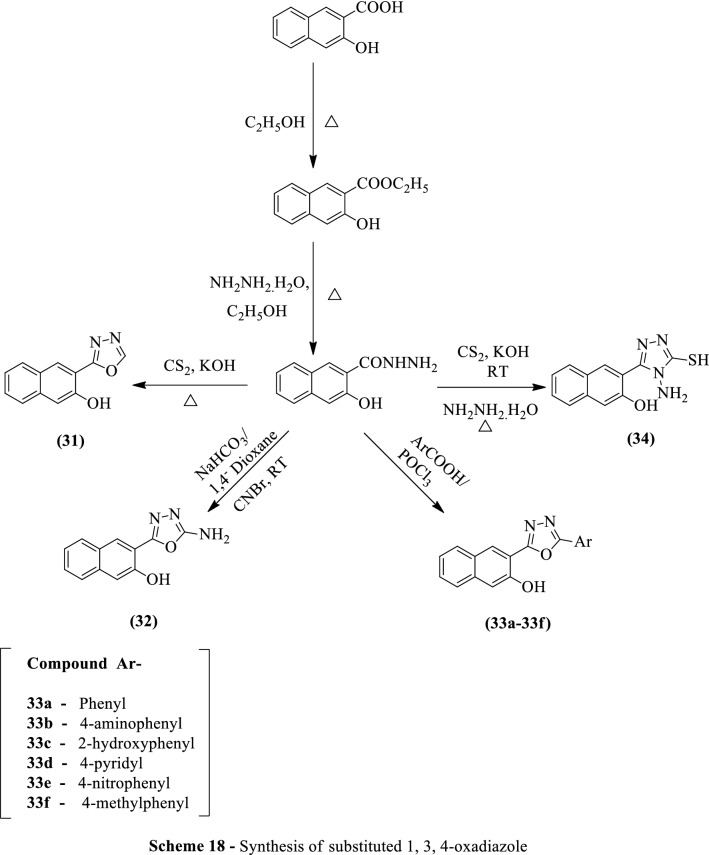
Gavarkar et al. [59] developed 3-(5-substituted-1,3,4-oxadiazol-2-yl) naphthalen-2-ol using Scheme 18. These derivatives were evaluated for antimycobacterial activity by tube dilution method against Mycobacterium tuberculosis H37Rv strain using MB 7H-9 agar broth. Streptomycin and Pyrazinamide were used as a reference standard. Compounds 31, 33c, and 33d exhibited good antitubercular activity as compare to reference standards and the results were summarized in (Table 18, Gavarkar et al. [59]).
Scheme 18.
Synthesis of substituted 1,3,4-oxadiazole
Table 18.
Antitubercular activity of the titled compounds against M. tuberculosis H37Rv [59]
| Compound | Antitubercular activity | ||
|---|---|---|---|
| 5 (µg/mL) | 10 (µg/mL) | 25 (µg/mL) | |
| 31 | R | S | S |
| 32 | R | R | R |
| 33a | R | R | R |
| 33b | R | R | R |
| 33c | R | S | S |
| 33d | S | S | S |
| 33e | R | R | R |
| 33f | R | R | R |
| 34 | R | S | R |
| Streptomycin | R | S | S |
| Pyrazinamide | R | S | S |
Antiviral activity
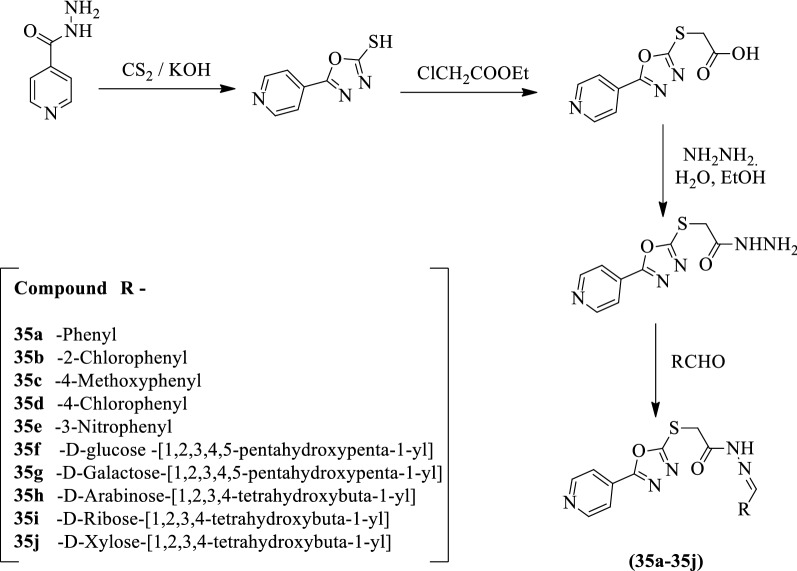
Somani et al. [47] developed N'-substituted-2-((5-(pyridin-4-yl)-1,3,4- oxadiazol-2-yl)thio)acetohydrazide (Scheme 19) and evaluated for antiviral activity against a different type of strains such as HIV-2 ROD and HIV-1 IIIB using MTT assay in MT-4 cells. Nevirapine was used as a reference standard. These derivatives were also evaluated for cytotoxic activity using MTT assay in uninfected MT-4 cells. The results of synthesized derivatives were expressed as CC50, IC50, and SI values which were summarized in Table 19a. The results of the antiviral activity of synthesized derivatives against other viruses in (HEL) and (Vero) culture were reported in (Table 19b, c, Somani et al. [47]).
Scheme 19.
Synthesis of substituted 1,3,4-oxadiazole
Table 19.
(a) Anti HIV activity of synthesized compounds. (b) Cytotoxicity and antiviral activity of titled compounds in Vero cell cultures. (c) Cytotoxicity and antiviral activity of titled compounds in HEL cell cultures [47]
| Panel (a) | ||||||
|---|---|---|---|---|---|---|
| Compound | HIV I (μg/ml) | SI | HIV II (μg/ml)) | SI | ||
| IC50 | CC50 | IC50 | CC50 | |||
| 35a | > 50 | = 50 | < 1 | > 57 | = 57 | < 1 |
| 35b | > 65 | = 65 | < 1 | > 60 | = 60 | < 1 |
| 35c | > 125 | > 125 | X1 | > 125 | > 125 | X1 |
| 35f | > 125 | > 125 | X1 | > 38 | > 125 | > 3 |
| 35 g | > 125 | > 125 | X1 | > 125 | > 125 | X1 |
| 35 h | > 125 | > 125 | X1 | > 125 | > 125 | X1 |
| 35i | > 125 | > 125 | X1 | > 125 | > 125 | X1 |
| 35j | > 125 | > 125 | X1 | > 125 | > 125 | X1 |
| Nevirapine(μM) | > 0.25 | > 200 | > 800 | – | – | – |
| DDI (μM) | > 5.37 | > 529 | > 98 | 2.71 | > 529 | > 195 |
| Panel (b) | ||||||
|---|---|---|---|---|---|---|
| Compound | Minimum cytotocic concentrationa (μg/mL) | EC50b (μg/mL) | ||||
| Para-influenza-3 virus | Retrovirus | Sindbis virus | Coxasacide B4 virus | Punta Toro virus | ||
| 35a | 20 | > 20 | > 20 | > 20 | > 20 | > 20 |
| 35b | 100 | > 20 | > 20 | > 20 | > 20 | > 20 |
| 35c | 100 | > 20 | > 20 | > 20 | > 20 | > 20 |
| 35f | > 100 | > 100 | > 100 | > 100 | > 100 | > 100 |
| 35 g | > 100 | > 100 | > 100 | > 100 | > 100 | > 100 |
| 35 h | > 100 | > 100 | > 100 | > 100 | > 100 | > 100 |
| 35i | > 100 | > 100 | > 100 | > 100 | > 100 | > 100 |
| 35j | > 100 | > 100 | > 100 | > 100 | > 100 | > 100 |
| Ribavirin (μM) | > 250 | 146 | 250 | > 250 | > 250 | 146 |
| Panel (c) | |||||
|---|---|---|---|---|---|
| Compound | Minimum cytotocic concentrationa (μg/mL) | EC50b (μg/mL) | |||
| Herpes simplex virus-1 | Herpes simplex virus-2 | Vaccinia virus | Vesicular stomatitis virus | ||
| 35a | > 100 | 50 | 100 | 45 | > 100 |
| 35b | 100 | > 20 | > 20 | > 20 | > 20 |
| 35c | > 100 | > 100 | > 100 | > 100 | > 100 |
| 35f | > 100 | > 100 | > 100 | > 100 | > 100 |
| 35g | > 100 | > 100 | > 100 | > 100 | > 100 |
| 35h | > 100 | > 100 | > 100 | > 100 | > 100 |
| 35i | > 100 | > 100 | > 100 | > 100 | > 100 |
| 35j | > 100 | > 100 | > 100 | > 100 | > 100 |
| Brivudin (μM) | > 250 | 0.04 | 50 | 2 | 250 |
| Cidofovir (μM) | > 250 | 1 | 1 | 2 | > 250 |
| Ganciclovir (μM) | > 100 | 0.02 | 0.07 | > 100 | > 100 |
aConcentration required to cause a microscopically detectable alteration of normal cell morphology, bConcentration required to reduce virus-induced cytopathogenicity by 50%
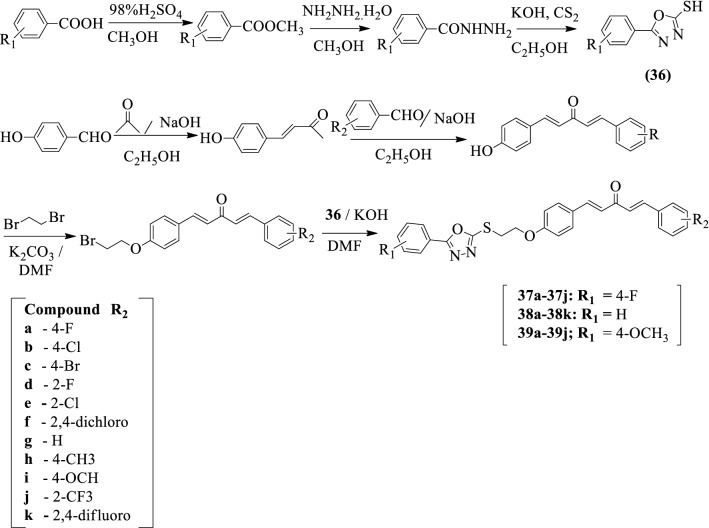
Gan et al. [25] developed (1E, 4E)-1-(substituted)-5-(4-(2-((5-substituted)-1,3,4-oxadiazol-2-yl)thio)ethoxy)phenyl)Penta-1,4-dien-3-one by using Scheme 20. The antiviral activity of synthesized compounds was evaluated against (TMV) using ribavirin as a reference standard. Among the synthesized derivatives, compounds 37a, 37c, 37f, 38a, 38b, 38c, 38d, 38e, 38f, 38g, 38h, 38i, 39e, and 39f exhibited potent curative activities as compared to a reference standard. Compounds 37a-37h and 38a-38g showed good protective activity against TMV as compared to the reference standard. Moreover, compounds 37a-37g, 38c, 38e, 38f, 38g, 38i, and 39a-39j showed better activities as compared to the positive control. Among them, compound 38f shows the best curative, inactivation, and protective activity as compare to the reference standard. The results of the antiviral activity of different derivatives were showed in (Table 20, Gan et al. [25]).
Scheme 20.
Synthesis of substituted 1,3,4-oxadiazole with benzoic acid as starting material
Table 20.
Antiviral activity of the titled compounds [25]
| Compound | R1 | R2 | Curative activity(%) | Protective activity(%) | Inactivation activity(%) |
|---|---|---|---|---|---|
| 37a | 4-F | 4-F | 43.2 ± 2.1 | 55.9 ± 1.7 | 84.4 ± 1.2 |
| 37b | 4-F | 4-Cl | 25.9 ± 1.8 | 52.5 ± 1.5 | 88.4 ± 0.8 |
| 37c | 4-F | 4-Br | 45.6 ± 1.9 | 67.9 ± 3.9 | 74.8 ± 1.3 |
| 37d | 4-F | 2-F | 31.1 ± 2.3 | 68.4 ± 3.2 | 83.4 ± 1.6 |
| 37e | 4-F | 2-Cl | 23.7 ± 3.1 | 56.8 ± 2.6 | 56.2 ± 1.9 |
| 37f | 4-F | 2,4-Di-Cl | 52.9 ± 4.5 | 65.1 ± 3.2 | 83.5 ± 2.7 |
| 37g | 4-F | H | 28.2 ± 1.1 | 52.9 ± 0.7 | 74.5 ± 0.9 |
| 37h | 4-F | 4-CH3 | 19.2 ± 0.9 | 60.5 ± 1.1 | 61.3 ± 0.8 |
| 37i | 4-F | 4-OCH3 | 27.5 ± 2.1 | 50.0 ± 1.5 | 61.4 ± 1.0 |
| 37j | 4-F | 2-CF3 | 28.3 ± 2.3 | 47.5 ± 2.4 | 60.2 ± 1.7 |
| 38a | H | 4-F | 45.8 ± 1.8 | 61.5 ± 2.9 | 69.1 ± 1.2 |
| 38b | H | 4-Cl | 44.1 ± 2.5 | 55.7 ± 1.6 | 59.4 ± 2.5 |
| 38c | H | 4-Br | 47.2 ± 3.6 | 53.8 ± 3.9 | 83.1 ± 2.4 |
| 38d | H | 2-F | 38.1 ± 2.6 | 66.3 ± 1.9 | 70.1 ± 2.0 |
| 38e | H | 2-Cl | 41.1 ± 4.2 | 61.5 ± 3.1 | 75.6 ± 2.1 |
| 38f | H | 2,4-Di-Cl | 49.8 ± 3.9 | 69.2 ± 2.1 | 90.4 ± 2.8 |
| 38g | H | H | 20.9 ± 2.1 | 66.7 ± 2.8 | 78.0 ± 2.5 |
| 38h | H | 4-CH3 | 48.1 ± 3.6 | 57.5 ± 2.7 | 72.7 ± 3.3 |
| 38i | H | 4-OCH3 | 40.6 ± 3.2 | 58.4 ± 3.8 | 79.3 ± 4.1 |
| 38j | H | 2-CF3 | 35.5 ± 1.7 | 50.5 ± 1.9 | 56.8 ± 2.1 |
| 39a | 4-OCH3 | 4-F | 20.8 ± 1.2 | 44.0 ± 0.9 | 83.0 ± 1.1 |
| 39b | 4- OCH3 | 4-Cl | 18.4 ± 0.9 | 34.4 ± 1.1 | 87.1 ± 1.8 |
| 39c | 4- OCH3 | 4-Br | 34.8 ± 2.1 | 41.1 ± 3.6 | 82.3 ± 5.1 |
| 39d | 4- OCH3 | 2-F | 25.4 ± 1.7 | 35.8 ± 1.4 | 81.3 ± 2.1 |
| 39e | 4- OCH3 | 2-Cl | 43.5 ± 2.2 | 46.1 ± 2.6 | 77.7 ± 2.0 |
| 39f | 4- OCH3 | 2,4-Di-Cl | 43.9 ± 2.4 | 49.6 ± 1.8 | 85.6 ± 1.9 |
| 39g | 4- OCH3 | H | 37.8 ± 1.6 | 42.5 ± 2.0 | 78.8 ± 2.1 |
| 39h | 4- OCH3 | 4-CH3 | 26.5 ± 1.2 | 42.1 ± 2.1 | 86.3 ± 5.4 |
| 39i | 4- OCH3 | 4-OCH3 | 35.1 ± 1.5 | 41.5 ± 1.8 | 81.5 ± 2.6 |
| 39j | 4- OCH3 | 2-CF3 | 30.5 ± 2.1 | 49.3 ± 2.3 | 77.9 ± 4.5 |
| 38k | H | 2,4-Di-F | 55.4 ± 2.8 | 71.3 ± 1.9 | 85.2 ± 4.0 |
| Ribavirin | 37.9 ± 1.9 | 51.8 ± 2.3 | 72.9 ± 2.4 |
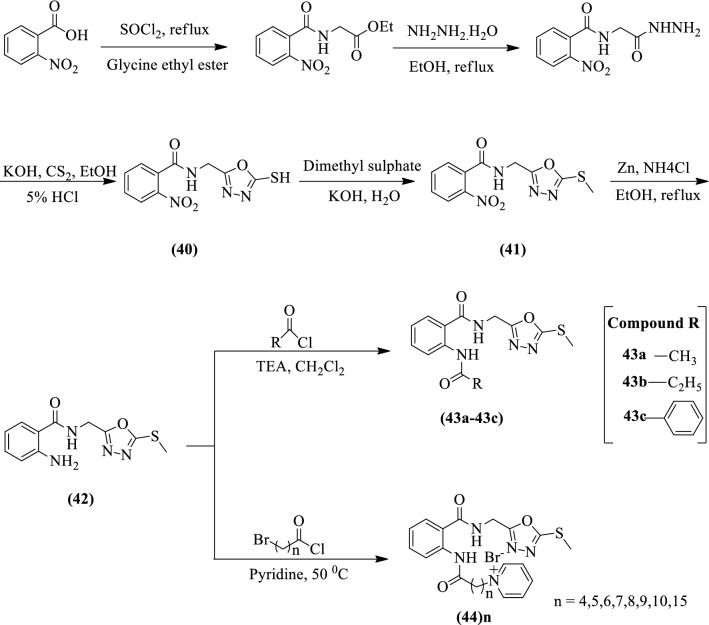
Wang et al. [1] developed N-((5-mercapto-1,3,4-oxadiazol-2-yl)methyl)-2-nitro benzamide, N-((5-(methylthio)-1,3,4-oxadiazol-2-yl)methyl)-2-nitro benzamide, 2-amino-N-((5-(methylthio)-1,3,4-oxadiazol-2-yl)methyl)benzamide and 2-(substituted)-N-((5-(methylthio)-1,3,4-oxadiazol-2-yl) methyl)benzamide (Scheme 21) and evaluated for antiviral activity. NNM was used as a reference standard. Among the synthesized derivatives, compounds 446, 448, and 4415 showed a more potent activity than the reference standard. The position of the substituent’s also affected the antiviral activity and the results of antiviral activity were represented in (Table 21, Wang et al. [1]).
Scheme 21.
Synthesis of 1,3,4-oxadiazole derivatives with 2-nitrobenzoic acid as starting material
Table 21.
Anti-TMV activities of titled compounds at 500 μg/mL in vivo [1]
| Compounds | Rate (%) | Compounds | Rate (%) | ||
|---|---|---|---|---|---|
| Curative activity | Protective activity | Curative activity | Protective activity | ||
| 40 | 38.5 ± 1.2 | 35.2 ± 3.1 | 448 | 60.0 ± 5.6 | 36.4 ± 1.0 |
| 41 | 36.9 ± 5.1 | 14.4 ± 2.9 | 449 | 26.9 ± 2.9 | 43.3 ± 3.0 |
| 42 | 26.8 ± 5.2 | 54.5 ± 2.9 | 4410 | 48.7 ± 5.1 | 25.2 ± 2.9 |
| 43a | 22.3 ± 6.4 | 54.6 ± 5.2 | 4415 | 51.9 ± 3.0 | 45.6 ± 4.2 |
| 43b | 47.2 ± 2.8 | 38.8 ± 4.5 | 40’ | 41.8 ± 1.0 | 41.7 ± 1.7 |
| 43c | 44.8 ± 9.5 | 36.8 ± 0.8 | 41’ | 17.5 ± 1.2 | 32.2 ± 1.6 |
| 444 | 7.1 ± 1.7 | 51.2 ± 7.6 | 42’ | 17.7 ± 1.2 | 42.6 ± 2.2 |
| 445 | 37.4 ± 3.5 | 27.8 ± 5.5 | 432’ | 49.3 ± 2.0 | 19.6 ± 2.4 |
| 446 | 50.6 ± 4.7 | 42.9 ± 2.5 | 4410’ | 33.9 ± 1.3 | 20.2 ± 1.0 |
| 447 | 37.1 ± 3.3 | 23.5 ± 1.1 | 4415’ | 35.3 ± 2.3 | 19.3 ± 0.8 |
| NNM | 54.2 ± 2.9 | 65.7 ± 2.2 | |||
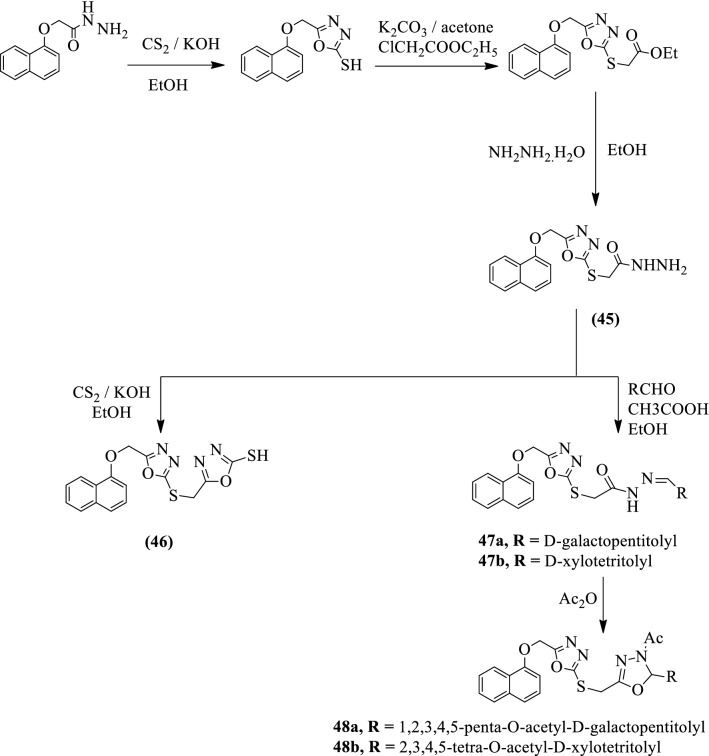
EI-Sayed et al. [60] developed 1,2,3,4,5-Penta-O-acetyl-D-galactopentitolyl and 2,3,4,5-tetra-O-acetyl-D-xylotetritolyl, hydrazide, and imidrazone of 1,3,4-oxadiazole by using Scheme 22a, b respectively. The antiviral activity of synthesized derivatives was evaluated as reverse transcriptase inhibitors with fresh human peripheral blood mononuclear cells. Compound 47b shows good antiviral activity followed by compounds 45 and 49a. Compounds 48b and 52 showed moderate activity while 47a and 48a showed the weakest activity among the series of tested compounds. The results of the antiviral activity of synthesized derivatives were presented in (Table 22, EI-Sayed et al. [60]).
Scheme 22.
a Synthesis of disubstituted 1,3,4-oxadiazoles.b Synthesis of hydrazide and imidrazone of 1,3,4-oxadiazoles
Table 22.
HIV inhibition activities (reverse transcriptase inhibitor) with therapeutic index [60]
| Compound | EC50 (μM) | IC50 (μM) | Therapeutic index |
|---|---|---|---|
| 45 | 3.24. 10–3 | 1.88 | 2.88. 10–7 |
| 47a | 1.1. 10–5 | 12.89 | 66.24. 10–8 |
| 47b | 5.26. 10–4 | 1.44 | 3.15. 10–7 |
| 48a | 5.23. 10–4 | 12.44 | 5.78. 10–6 |
| 48b | 1.56. 10–3 | 3.11 | 3.45. 10–6 |
| 49a | 3.81. 10–3 | 2.12 | 8.14. 10–6 |
| 52 | 2.72. 10–3 | 2.9 | 5.12. 10–6 |
Antioxidant activity
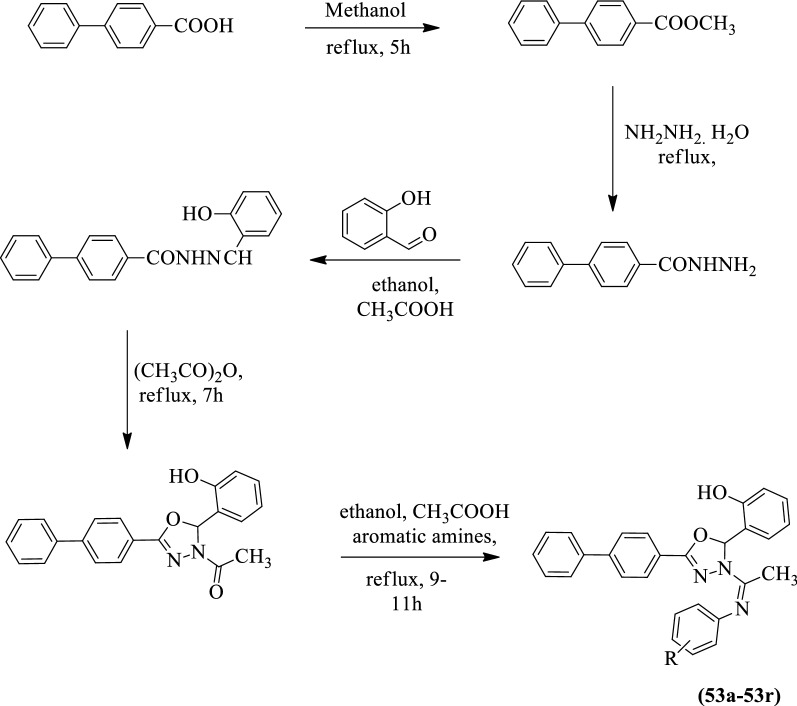
Malhotra et al. [46] developed (Z)-2-(5-[(1, 1-biphenyl)-4-yl]-3-(1-((substituted)imino) ethyl)-2,3-dihydro-1,3,4-oxadiazol-2yl)phenol (Scheme 23) and evaluated for antioxidant activity in terms of hydrogen peroxide scavenging activity. The results of the antioxidant activity of the synthesized derivatives were presented in (Table 23, Malhotra et al. [46]).
Scheme 23.
Synthesis of substituted 1,3,4-oxadiazole with 4-biphenyl carboxylic acid as starting material
Table 23.
Hydrogen peroxide scavenging activity of synthesized compounds [46]
| Compound | Scavenging of hydrogen peroxide at different concentration (%) | ||
|---|---|---|---|
| 100 (µg/ml) | 300 (µg/ml) | 500 (µg/ml) | |
| 53a | 41.55 | 39.84 | 41.22 |
| 53b | 46.34 | 44.55 | 45.77 |
| 53c | 51.11 | 48.12 | 44.59 |
| 53d | 41.92 | 42.33 | 41.72 |
| 53e | 45.65 | 46.19 | 45.91 |
| 53f | 51.21 | 43.12 | 39.57 |
| 53g | 39.58 | 42.61 | 43.18 |
| 53h | 43.45 | 41.37 | 45.27 |
| 53i | 41.88 | 45.19 | 48.11 |
| 53j | 47.52 | 54.15 | 53.18 |
| 53k | 45.35 | 50.27 | 52.15 |
| 53l | 51.15 | 52.27 | 58.18 |
| 53m | 45.87 | 41.37 | 41.93 |
| 53n | 42.98 | 39.72 | 39.57 |
| 53o | 41.03 | 43.06 | 44.14 |
| 53p | 51.62 | 52.18 | 52.91 |
| 53q | 54.18 | 53.76 | 57.36 |
| 53r | 49.87 | 51.35 | 48.74 |
| BHA | 63.27 | 66.19 | 68.25 |
| Ascorbic acid | 51.47 | 53.45 | 55.38 |
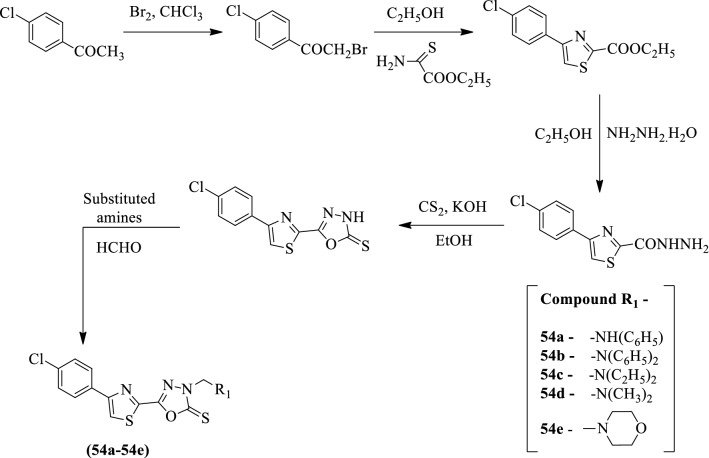
Rahul R. et al. [8] synthesized 5-(4-(4-chlorophenyl)thiazol-2-yl)-3-(substituted benzyl) -1,3,4-oxadiazole-2(3H)-thione by using Scheme 24 and evaluated for antioxidant activity by different methods such as Hydrogen peroxide scavenging, Nitric oxide scavenging, and DPPH assay. In DPPH assay compound 54c shows more significant activity in comparison to ascorbic acid. In other methods such as hydrogen peroxide and nitric oxide scavenging assay, compound 54c gives more potent activity than the rest of the other compounds but was not significant as compare to the results obtained in the DPPH assay. This shows that compound 54c gives more potent antioxidant activity as compared to the rest of the synthesized compounds. The results of the antioxidant activity of synthesized derivatives were presented in (Table 24, Rahul R. et al. [8]).
Scheme 24.
Synthesis of substituted 1,3,4-oxadiazole
Table 24.
(a) DPPH assay of synthesized compounds. (b) Nitric oxide scavenging of synthesized compounds. (c) Hydrogen peroxide scavenging of synthesized compounds
| Compound | % Scavenging activity at different concentrations | IC50 | ||||
|---|---|---|---|---|---|---|
| 20 (µg/ml) | 40 (µg/ml) | 60 (µg/ml) | 80 (µg/ml) | 100 (µg/ml) | ||
| Panel (a) | ||||||
| 54a | 39.94 ± 0.521 | 59.14 ± 0.652 | 61.38 ± 0.631 | 63.59 ± 0.245 | 65.34 ± 0.534 | 29.7 |
| 54b | 46.63 ± 0.342 | 49.7 ± 0.352 | 57.51 ± 0.421 | 60.51 ± 0.634 | 62.65 ± 0.453 | 43.3 |
| 54c | 44.86 ± 0.245 | 62.22 ± 0.214 | 64.66 ± 0.341 | 65.82 ± 0.372 | 67.76 ± 0.215 | 26.7 |
| 54d | 44.64 ± 0.234 | 53.89 ± 0.123 | 62.73 ± 0.223 | 64.02 ± 0.321 | 66.92 ± 0.431 | 27.1 |
| 54e | 47.34 ± 0.235 | 48.16 ± 0.516 | 49.54 ± 0.461 | 52.98 ± 0.371 | 55.75 ± 0.297 | 61.3 |
| Ascorbic acid | 49.38 ± 0.515 | 67.03 ± 0.541 | 75.78 ± 0.223 | 91.92 ± 0.561 | 95.34 ± 0.111 | 21.3 |
| Panel (b) | ||||||
| 54a | 34.83 ± 0.527 | 40.63 ± 0.654 | 43.87 ± 0.691 | 52.15 ± 0.215 | 53.11 ± 0.514 | 72.1 |
| 54b | 27.34 ± 0.372 | 29.81 ± 0.352 | 38.25 ± 0.421 | 42.55 ± 0.639 | 50.54 ± 0.450 | 98.3 |
| 54c | 33.57 ± 0.243 | 44.97 ± 0.211 | 48.69 ± 0.348 | 52.35 ± 0.442 | 53.15 ± 0.218 | 66.2 |
| 54d | 33.28 ± 0.232 | 44.40 ± 0.128 | 45.70 ± 0.224 | 52.01 ± 0.331 | 54.29 ± 0.481 | 69.8 |
| 54e | 26.67 ± 0.295 | 29.30 ± 0.506 | 44.95 ± 0.411 | 51.98 ± 0.381 | 52.07 ± 0.297 | 70.6 |
| Ascorbic acid | 47.53 ± 0.624 | 63.44 ± 0.521 | 84.28 ± 0.623 | 90.53 ± 0.411 | 93.56 ± 0.221 | 25.2 |
| Panel (c) | ||||||
| 54a | 35.75 ± 0.612 | 44.97 ± 0.237 | 55.19 ± 0.226 | 65.93 ± 0.662 | 67.14 ± 0.653 | 47.1 |
| 54b | 34.01 ± 0.563 | 43.51 ± 0.464 | 58.83 ± 0.152 | 60.48 ± 0.353 | 62.50 ± 0.452 | 49.1 |
| 54c | 34.24 ± 0.263 | 46.06 ± 0.533 | 58.82 ± 0.623 | 62.12 ± 0.621 | 63.63 ± 0.236 | 43.3 |
| 54d | 33.93 ± 0.235 | 46.81 ± 0.516 | 56.52 ± 0.532 | 59.89 ± 0.623 | 61.39 ± 0.425 | 45.6 |
| 54e | 34.48 ± 0.342 | 44.88 ± 0.345 | 55.57 ± 0.173 | 56.61 ± 0.535 | 58.63 ± 0.654 | 50.6 |
| Ascorbic acid | 44.53 ± 0.526 | 64.65 ± 0.653 | 71.74 ± 0.36 | 89.22 ± 0.621 | 96.19 ± 0.456 | 26.9 |
IC50 values in µg/ml for samples were determined using ED50 plus V 1.0 software. Data are the mean of three or more experiments and reported as mean ± standard error of the mean (SEM)
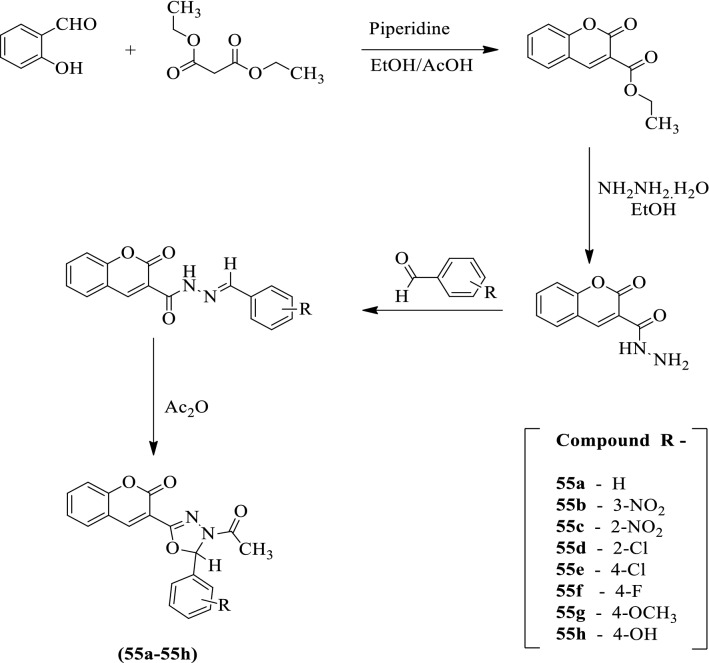
Dureja [61] developed 3-(4-acetyl-5-(substituted phenyl)-4, 5-dihydro-1,3,4-oxadiazol-2-yl)-2H-chromen-2-one (Scheme 25) and evaluated for antioxidant activity by using DPPH assay. Ascorbic acid was used as a reference standard and the results were summarized in (Table 25, Dureja [61]).
Scheme 25.
Synthesis of substituted 1,3,4-oxadiazole with 2-hydroxy benzaldehyde carboxylic acid as starting material
Table 25.
Antioxidant activity of synthesized compounds by DPPH method [61]
| Compound | % Scavenging activity | IC50 |
|---|---|---|
| 55a | 19.97–85.95 | 47.47 ± 2.473 |
| 55b | 3.07–64.92 | 197.96 ± 2.454 |
| 55c | 7.4–48.75 | > 500 |
| 55d | 13.87–77.45 | 60.93 ± 1.560 |
| 55e | 12.60–85.95 | > 500 |
| 55f | 14.70–69.70 | 130.8 ± 3.602 |
| 55g | 4.9–74.77 | 90.26 ± 2.442 |
| 55h | 6.85–69.42 | 91.70 ± 2.778 |
| Ascorbic acid | 44.95–95.5 | 12.7 ± 0.68 |
Conclusion
In this present review article, we have summarized different pharmacological activities of 1,3,4-oxadiazole containing compounds. From this study, we have found that 1,3,4-oxadiazole containing compounds can be synthesized by various kinds of synthetic routes, and these derivatives having a wide range of biological activities such as antitumor, antitubercular, antimicrobial, antiviral and antioxidant, etc. This review article established the fact that 1,3,4-oxadiazole as useful templates for further modification or derivatization to design more potent biologically active compounds.
Acknowledgements
Thanks to Head Prof. Sanju Nanda, Department of Pharmaceutical Sciences, M.D.U, Rohtak for providing library and internet facilities, etc.
Abbreviations
- CNS
Central Nervous System
- FDA
Food and Drug Administration
- MTT
3-(4, 5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- IC50
Half maximal inhibitory concentration
- L.J
Lowenstein-Jensen
- MB
Middle brook
- HeLa
Henrietta Lacks
- MIC
Minimum inhibitory concentration
- HIV
Human immunodeficiency virus
- CC50
Half maximal cytotoxic concentration
- SI
Selectivity index
- HEL
Human embryonic lung fibroblast
- VERO
Verda reno (means green kidney)
- TMV
Tobacco mosaic virus
- DPPH
2, 2-Diphenyl-1-picrylhydrazyl
- MTCC
Microbial type cell cultures
Authors’ contributions
PKV- endeavored and accomplished the scheme; AS-completed review work and wrote the manuscript. Both authors read and approved the final manuscript.
Funding
No funding was obtained for this study.
Availability of data and materials
All data are provided in the manuscript or cited in the references.
Ethics approval and consent to participate
Not applicable.
Competing interests
The author(s) have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ankit Siwach, Email: siwachaniket96@gmail.com.
Prabhakar Kumar Verma, Email: vermapk422@rediffmail.com.
References
- 1.Wang PY, Shao WB, Xue HT, Fang HS, Zhou J, Wu ZB, Song BA, Yang S. Synthesis of novel 1,3,4-oxadiazole derivatives containing diamides as promising antibacterial and antiviral agents. Res ChemIntermed. 2017;43(11):6115–6130. [Google Scholar]
- 2.Kaur M, Singh S, Kaur M. Synthesis, spectral study and biological activity of some 2,5-disubstituted 1,3,4-oxadiazole. Eur J Pharm Med Res. 2018;5(9):277–282. [Google Scholar]
- 3.Ahsan MJ. Synthesis and cytotoxicity evaluation of [(2, 4-dichloro phenoxy) methyl]-5-aryl-1,3,4-oxadiazole/4H-1,2,4-triazole analogues. Tur J Chem. 2018;42(5):1334–1343. doi: 10.3906/kim-1803-25. [DOI] [Google Scholar]
- 4.Alrazzak AN. Synthesis, characterization, and study some of physical properties of novel 1,3,4-oxadiazole derivatives. IOP ConfSer Mater SciEng. 2018 doi: 10.1088/1757-899X/454/1/012096. [DOI] [Google Scholar]
- 5.Kanthiah S, Kalusalingam A, Velayutham R, Vimala AT, Beyatricks J. 5-(2-aminophenyl)-1,3,4-oxadiazole-2 (3H)-thione derivatives: synthesis, characterization and antimicrobial evaluation. Int J Chem Tech Res. 2011;6(1):64–67. [Google Scholar]
- 6.Salahuddin MA, Yar MS, Mazumder R, Chakraborthy GS, Ahsan MJ, Rahman MU. Updates on synthesis and biological activities of 1,3,4-oxadiazole: a review. Synth Commun. 2017;47(20):1805–1847. doi: 10.1080/00397911.2017.1360911. [DOI] [Google Scholar]
- 7.Patel KD, Prajapati SM, Panchal SN, Patel HD. Review of synthesis of 1,3,4-oxadiazole derivatives. Synth Commun. 2014;44(13):1859–1875. doi: 10.1080/00397911.2013.879901. [DOI] [Google Scholar]
- 8.Rahul R, Jat RK, Saravanan J. Synthesis and in-vitro antioxidant activity of novel 1,3,4-oxadiazole-2-thione. J Innov Pharm Biol Sci. 2016;3:114–122. [Google Scholar]
- 9.Mishra P, Rajak H, Mehta A. Synthesis of Schiff bases of 2-amino-5-aryl-1,3,4-oxadiazoles and their evaluation for antimicrobial activities. J Gen Appl Microbiol. 2005;51(2):133–141. doi: 10.2323/jgam.51.133. [DOI] [PubMed] [Google Scholar]
- 10.Ahsan MJ, Samy JG, Khalilullah H, Nomani MS, Saraswat P, Gaur R, Singh A. Molecular properties prediction and synthesis of novel 1,3,4-oxadiazole analogues as potent antimicrobial and antitubercular agents. Bioorg Med Chem Lett. 2011;21(24):7246–7250. doi: 10.1016/j.bmcl.2011.10.057. [DOI] [PubMed] [Google Scholar]
- 11.Rajak H, Singour P, Kharya MD, Mishra P. A novel series of 2,5-disubstituted 1,3,4-oxadiazoles: synthesis and SAR study for their anticonvulsant activity. Chem Biol Drug Des. 2011;77(2):152–158. doi: 10.1111/j.1747-0285.2010.01066.x. [DOI] [PubMed] [Google Scholar]
- 12.Rajak H, Agarawal A, Parmar P, Thakur BS, Veerasamy R, Sharma PC, Kharya MD. 2, 5-Disubstituted-1,3,4-oxadiazoles/thiadiazole as surface recognition moiety: Design and synthesis of novel hydroxamic acid-based histone deacetylase inhibitors. Bioorg Med Chem Lett. 2011;21(19):5735–5738. doi: 10.1016/j.bmcl.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 13.Ahsan MJ, Bhandari L, Makkar S, Singh R, Hassan MZ, Geesi MH, Jadav BMA, SS, Balaraju T, Riadi Y, Rani S, Khalilullah H, Gorantla V, Hussain A, Synthesis, antiproliferative and antioxidant activities of substituted N-[(1,3,4-oxadiazol-2-yl) methyl] benzamines. Lett Drug Des Dis. 2020;17(2):145–154. doi: 10.2174/1570180816666181113110033. [DOI] [Google Scholar]
- 14.Ahsan MJ, Choupra A, Sharma RK, Jadav SS, Padmaja P, Hassan M, Al-Tamimi A, Geesi MH, Bakht MA. Rationale design, synthesis, cytotoxicity evaluation, and molecular docking studies of 1,3,4-oxadiazole analogues. Anti-Cancer Agents Med Chem. 2018;18(1):121–138. doi: 10.2174/1871520617666170419124702. [DOI] [PubMed] [Google Scholar]
- 15.Ahsan MJ, Meena R, Dubey S, Khan V, Manda S, Jadav SS, Sharma P, Geesi MH, Hassan MZ, Bakht MA, Riadi Y. Synthesis and biological potentials of some new 1,3,4-oxadiazole analogues. Med Chem Res. 2018;27(3):864–883. doi: 10.1007/s00044-017-2109-1. [DOI] [Google Scholar]
- 16.Ahsan MJ, Yadav RP, Saini S, Hassan M, Bakht MA, Jadav SS, Al-Tamimi S, Bin A, Geesi MH, Ansari MY, Khalilullah H. Synthesis, cytotoxic evaluation, and molecular docking studies of new oxadiazole analogues. Lett Org Chem. 2018;15(1):49–56. [Google Scholar]
- 17.Ahsan MJ. Rationale design, synthesis, and in vitro anticancer activity of new 2,5-disubstituted-1,3,4-oxadiazole analogues. ChemSelect. 2016;1(15):4713–4720. [Google Scholar]
- 18.Agarwal M, Singh V, Sharma SK, Sharma P, Ansari MY, Jadav SS, Yasmin S, Sreenivasulu R, Hassan MZ, Saini V, Ahsan MJ. Design and synthesis of new 2,5-disubstituted-1,3,4-oxadiazole analogues as anticancer agents. Med Chem Res. 2016;25(10):2289–2303. doi: 10.1007/s00044-016-1672-1. [DOI] [Google Scholar]
- 19.Ahsan MJ, Shastri S, Yadav R, Hassan M, Bakht MA, Jadav SS, Yasmin S. Synthesis and antiproliferative activity of some quinoline and oxadiazole derivatives. Org ChemInt. 2016 doi: 10.1155/2016/9589517. [DOI] [Google Scholar]
- 20.Ahsan MJ, Sharma J, Singh M, Jadav SS, Yasmin S. Synthesis and anticancer activity of N-aryl-5-substituted-1,3,4-oxadiazol-2-amine analogues. BioMed Res Int. 2014 doi: 10.1155/2014/814984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hatti I, Sreenivasulu R, Jadav SS, Ahsan MJ, Raju RR. Synthesis and biological evaluation of 1,3,4-oxadiazole-linked bisindole derivatives as anticancer agents. Monat Fur Chemie. 2015;146(10):1699–1705. doi: 10.1007/s00706-015-1448-1. [DOI] [Google Scholar]
- 22.Shaharyar M, Mazumder A, Ahsan MJ. Synthesis, characterization and anticancer evaluation of 2-(naphthalen-1-ylmethyl/naphthalen-2-yloxymethyl)-1-[5-(substituted phenyl) - [1,3,4] oxadiazol-2-ylmethyl]-1H-benzimidazole. Arab J Chem. 2014;7(4):418–424. doi: 10.1016/j.arabjc.2013.02.001. [DOI] [Google Scholar]
- 23.Biju CR, Ilango K, Prathap M, Rekha K. Design and microwave-assisted synthesis of 1,3,4-oxadiazole derivatives for analgesic and anti-inflammatory activity. J Young Pharm. 2012;4(1):33–37. doi: 10.4103/0975-1483.93576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahsan MJ, Samy JG, Jain CB, Dutt KR, Khalilullah H, Nomani MS. Discovery of novel antitubercular 1, 5-dimethyl-2-phenyl-4-([5-(arylamino)-1,3,4-oxadiazol-2-yl] methylamino)-1, 2-dihydro-3H-pyrazol-3-one analogues. Bioorg Med Chem Lett. 2012;22(2):969–972. doi: 10.1016/j.bmcl.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 25.Gan X, Hu D, Li P, Wu J, Chen X, Xue W, Song B. Design, synthesis, antiviral activity, and three-dimensional quantitative structure-activity relationship study of novel 1, 4-pentadien-3-one derivatives containing the 1,3,4-oxadiazole moiety. P Manag Sci. 2016;72(3):534–543. doi: 10.1002/ps.4018. [DOI] [PubMed] [Google Scholar]
- 26.Ahsan MJ, Hassan M, Jadav SS, Geesi MH, Bakht MA, Riadi Y, Akhtar M, Mallick MN, Akhter M. Synthesis and biological potentials of 5-aryl-N-[4-(trifluoromethyl) phenyl]-1,3,4-oxadiazol-2-amines. Lett Org Chem. 2020;17(2):133–140. doi: 10.2174/1570178616666190401193928. [DOI] [Google Scholar]
- 27.Yatam S, Jadav SS, Gundla R, Gundla KP, Reddy GM, Ahsan MJ, Chimakurthy J. Design, synthesis and biological evaluation of 2 (((5-aryl-1,2,4-oxadiazol-3-yl) methyl) thio) benzo [d] oxazoles: New anti-inflammatory and antioxidant agents. ChemSelect. 2018;3(37):10305–10310. [Google Scholar]
- 28.Rathore A, Sudhakar R, Ahsan MJ, Ali A, Subbarao N, Jadav SS, Umar S, Yar MS. In vivo anti-inflammatory activity and docking study of newly synthesized benzimidazole derivatives bearing oxadiazole and morpholine rings. Bioorg Chem. 2017;70:107–117. doi: 10.1016/j.bioorg.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 29.Ahsan MJ, Sharma J, Bhatia S, Kumar Goyal P, Shankhala K, Didel M. Synthesis of 2,5-disubstituted-1,3,4-oxadiazole analogs as novel anticancer and antimicrobial agents. Lett Drug Des Dis. 2014;11(4):413–419. doi: 10.2174/1570180810666131113211647. [DOI] [Google Scholar]
- 30.Srinivas M, Satyaveni S, Ram B. Design and synthesis of 1,3,4-oxadiazole incorporated indole derivatives as anticancer agents. J Pharm Res. 2018;12(5):758–763. [Google Scholar]
- 31.Kavitha S, Kannan K, Gnanavel S. Synthesis, characterization and biological evaluation of novel 2,5-substituted 1,3,4 oxadiazole derivatives. Saudi Pharm J. 2017;25(3):337–345. doi: 10.1016/j.jsps.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vismaya V. Insilico Design and Molecular Docking Studies of Novel 2-(4-chlorophenyl)-5-aryl-1,3,4-Oxadiazole Derivatives for Anti-cancer Activity. J Pharm Sci Res. 2019;11(7):2604–2609. [Google Scholar]
- 33.Yadav S, Vashist N, Gahlot V, Rajput A, Maratha S. Oxadiazole and their synthetic analogues: An updated review. Int J Pharm Biol Sci Arch. 2018;9(3):4–9. [Google Scholar]
- 34.Bala S, Kamboj S, Kajal A, Saini V, Prasad DN. 1,3,4-oxadiazole derivatives: synthesis, characterization, antimicrobial potential, and computational studies. Bio Med Res Int. 2014 doi: 10.1155/2014/172791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh R, Chauhan A. Biological importance of 1,3,4-Oxadiazole derivatives. Int J Adv Biol Res. 2013;3:140–149. [Google Scholar]
- 36.Sudeesh K, Gururaja R. Facile synthesis of some novel derivatives of 1,3,4-oxadiazole derivatives associated with quinolone moiety as cytotoxic and antibacterial agents. Org Chem Curr Res. 2017;6(2):2–5. [Google Scholar]
- 37.Nimavat B, Mohan S, Saravanan J, Deka S, Talukdar A, Sahariah BJ, Dey BK, Sharma RK. Synthesis and characterization of some novel oxadiazoles for in–vitro Anti-inflammatory activity. Int J Res Pharm Chem. 2012;2(3):594–602. [Google Scholar]
- 38.Prabhashankar J, Kariyappa AK (2013). Synthesis, characterization, and biological studies of five-membered nitrogen heterocycles. PhD Thesis, No 20912, ETH Zürich
- 39.Grover J, Bhatt N, Kumar V, Patel NK, Gondaliya BJ, Sobhia ME, Bhutani KK, Jachak SM. 2, 5-Diaryl-1,3,4-oxadiazoles as selective COX-2 inhibitors and anti-inflammatory agents. R Soc Chem Adv. 2015;5(56):45535–45544. [Google Scholar]
- 40.Singh P, Jangra PK. Oxadiazoles: a novel class of anti-convulsant agents. Der Chemica Sinica. 2010;1(3):118–123. [Google Scholar]
- 41.Arora RA, Parameswaran MK, Sharma PC, Michael S, Ravi TK. Synthesis of novel 1,3,4-oxadiazole derivatives as potential antimicrobial agents. Acta Pol Pharm. 2010;67:247–253. [PubMed] [Google Scholar]
- 42.Husain A, Ajmal M. Synthesis of novel 1,3,4-oxadiazole derivatives and their biological properties. Acta Pharm. 2009;59(2):223–233. doi: 10.2478/v10007-009-0011-1. [DOI] [PubMed] [Google Scholar]
- 43.Kumar B, Kumar A, Beheraand AK, Raj V. Latest update on pharmacological activities of 1,3,4-oxadiazole derivatives. J Cell Sci Ther. 2016;7(233):2. [Google Scholar]
- 44.Martínez R, Zamudio GJ, Pretelin-Castillo G, Torres-Ochoa RO, Medina-Franco JL, Pinzón CI, Miranda MS, Hernández E, Alanís-Garza B. Synthesis and antitubercular activity of new N-[5-(4-chlorophenyl)-1,3,4-oxadiazol-2-yl]-(nitroheteroaryl) carboxamides. Heterocycl. Commun. 2019;25(1):52–59. doi: 10.1515/hc-2019-0007. [DOI] [Google Scholar]
- 45.Roy PP, Bajaj S, Maity TK, Singh J. Synthesis and evaluation of anticancer activity of 1,3,4-oxadiazole derivatives against Ehrlich ascites carcinoma bearing mice and their correlation with histopathology of liver. Indian J Pharm Educ. 2017;15:16. [Google Scholar]
- 46.Malhotra M, Rawal RK, Malhotra D, Dhingra R, Deep A, Sharma PC. Synthesis, characterization and pharmacological evaluation of (Z)-2-(5-(biphenyl-4-yl)-3-(1-(imino) ethyl)-2, 3-dihydro-1,3,4-oxadiazol-2-yl) phenol derivatives as potent antimicrobial and antioxidant agents. Arab J Chem. 2017;1(10):S1022–1031. doi: 10.1016/j.arabjc.2013.01.005. [DOI] [Google Scholar]
- 47.Somani RR, Agrawal AG, Kalantri PP, Gavarkar PS, Clercq ED. Investigation of 1,3,4-oxadiazole scaffold as potentially active compounds. Int J Drug Des Dis. 2011;2:353–360. [Google Scholar]
- 48.Bhat KI, Sufeera K, Kumar PC. Synthesis, characterization and biological activity studies of 1,3,4-Oxadiazole analogs. J Young Pharm. 2011;3(4):310–314. doi: 10.4103/0975-1483.90243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chikhalia KH, Vashi DB, Patel MJ. Synthesis of a novel class of some 1,3,4-oxadiazole derivatives as antimicrobial agents. J. Enzyme Inhib Med Chem. 2009;24(3):617–622. doi: 10.1080/14756360802318936. [DOI] [PubMed] [Google Scholar]
- 50.Vinayak A, Sudha M, Lalita KS. Design, synthesis, and characterization of novel amine derivatives of 5-[5-(chloromethyl)-1,3,4-oxadiazol-2-yl]-2-(4-fluorophenyl)-pyridine as a new class of anticancer agents. Dhaka Univ J Pharm Sci. 2017;16(1):11–19. doi: 10.3329/dujps.v16i1.33377. [DOI] [Google Scholar]
- 51.Kapoor A, Dhiman N. Anticancer evaluation of 2-aryl substituted benzimidazole derivatives bearing 1,3,4-oxadiazole nucleus. Der Pharm Lett. 2016;8(12):149–156. [Google Scholar]
- 52.Chakrapani B, Ramesh V, Rao GP, Ramachandran D, Reddy TM, Chakravarthy AK, Sridhar G. Synthesis and anticancer evaluation of 1,2,4-oxadiazole linked imidazothiadiazole derivatives. Russ J Gen Chem. 2018;88(5):1020–1024. doi: 10.1134/S1070363218050304. [DOI] [Google Scholar]
- 53.Gudipati R, Anreddy RN, Manda S. Synthesis, characterization and anticancer activity of certain 3-{4-(5-mercapto-1,3,4-oxadiazole-2-yl) phenylimino} indolin-2-one derivatives. Saudi Pharm J. 2011;19(3):153–158. doi: 10.1016/j.jsps.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Polothi R, Raolji GS, Kuchibhotla VS, Sheelam K, Tuniki B, Thodupunuri P. Synthesis and biological evaluation of 1,2,4-oxadiazole linked 1,3,4-oxadiazole derivatives as tubulin-binding agents. Synth Commun. 2019;49(13):1603–1612. doi: 10.1080/00397911.2018.1535076. [DOI] [Google Scholar]
- 55.Pattan SR, Rabara PA, Pattan JS, Bukitagar AA, Wakale VS, Musmade DS. Synthesis and evaluation of some novel substituted 1,3,4-oxadiazole and pyrazole derivatives for antitubercular activity. Indian J Chem. 2009;48B:1453–1456. [Google Scholar]
- 56.Das R, Asthana GS, Suri KA, Mehta DK, Asthana A. Synthesis and assessment of antitubercular and antimicrobial activity of some novel triazolo and tetrazolo-fused 1,3,4-oxadiazole molecules containing pyrazine moiety. J Pharm Sci Res. 2015;7(10):806. [Google Scholar]
- 57.Raval JP, Akhaja TN, Jaspara DM, Myangar KN, Patel NH. Synthesis and in vitro antibacterial activity of new oxoethylthio-1,3,4-oxadiazole derivatives. J Saudi ChemSoc. 2014;18(2):101–106. doi: 10.1016/j.jscs.2011.05.019. [DOI] [Google Scholar]
- 58.Somani RR, Balkund VD, Nikam SR, Shirodkar PY, Zope DB. Synthesis, antibacterial, and anti-tubercular evaluation of some 1,3,4-oxadiazole based Mannich bases. Int J Chem Tech Res. 2013;5(5):2588–2592. [Google Scholar]
- 59.Gavarkar SP, Somani RR. Synthesis of novel azole heterocycles with their antitubercular and antifungal evaluation. Int J Chem Sci. 2015;13(1):432–440. [Google Scholar]
- 60.El-Sayed WA, El-Essawy FA, Ali OM, Nasr BS, Abdalla MM, Adel AH. Anti-HIV activity of new substituted 1,3,4-oxadiazole derivatives, and their acyclic nucleoside analogues. Z Naturforsch C. 2009;64(11–12):773–778. doi: 10.1515/znc-2009-11-1203. [DOI] [PubMed] [Google Scholar]
- 61.Dureja A (2011). Synthesis and biological evaluation of newer analogues of 2,5-disubstituted 1,3,4-oxadiazole as antioxidant, and anticonvulsant agents. PhD diss.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are provided in the manuscript or cited in the references.