Abstract
Cardiovascular diseases are patterned by race and socioeconomic status, and chronic low-grade inflammation is proposed as a key underlying mechanism. Theories for how racial and socioeconomic disadvantages foster inflammation emphasize a lifecourse approach: social disadvantages enable chronic or repeated exposure to stressors, unhealthy behaviors, and environmental risks that accumulate across the lifecourse to increase low-grade inflammation. However, single samples rarely include multiple racial and socioeconomic groups that each span a wide age range, precluding examination of this proposition. To address this issue, the current study combined seven studies that measured C-reactive protein and interleukin-6, producing a pooled sample of 1,650 individuals aged 11 to 60 years. We examined (a) whether race and socioeconomic disparities in inflammatory biomarkers vary across the lifecourse, (b) whether adiposity operates as a pathway leading to these disparities, and (c) whether any indirect pathways through adiposity also vary across the lifecourse. Relative to White individuals, Black individuals exhibited higher, whereas Asian individuals exhibited lower, levels of inflammatory biomarkers and adiposity accounted for these racial differences. Similarly, lower socioeconomic status was associated with higher inflammatory biomarkers via elevated adiposity. Importantly, both racial and socioeconomic disparities, as well as their pathways via adiposity, widened across the lifecourse. This pattern suggests that the impact of social disadvantages compound with age, leading to progressively larger disparities in low-grade inflammation. More broadly, these findings highlight the importance of considering age when examining health disparities and formulating conceptual models that specify how and why disparities may vary across the lifecourse.
Keywords: health disparities, socioeconomic status, race, adiposity, low-grade inflammation, lifecourse
Cardiovascular diseases are among the most prevalent, disabling, and costly health problems in the United States today (Benjamin et al., 2018; NCHS, 2017), and their rates are patterned by socioeconomic status. For example, individuals with low socioeconomic status have higher incidence of and mortality from heart attack, stroke, and heart failure (Addo et al., 2012; Alter et al., 2006; Hawkins et al., 2012). Over the past decade, research has increasingly highlighted chronic low-grade inflammation as a pathway contributing to this phenomenon (Danese and McEwen, 2012; Matthews et al., 2010; Miller et al., 2011; Nusslock and Miller, 2016). Indeed, studies have shown that relative to their more affluent peers, individuals with low socioeconomic status exhibit higher levels of circulating inflammatory biomarkers, including C-Reactive Protein (CRP) and Interleukin-6 (IL-6) (Muscatell et al., 2018). In turn, preclinical studies in animals have shown that inflammation is pivotal in the pathogenesis of atherosclerotic cardiovascular disease (Frenois et al., 2007; Pan et al., 2013). Paralleling these findings, prospective studies have shown relevance in human diseases, illustrating that higher levels of circulating inflammatory biomarkers, in particular CRP and IL-6, forecasted the development of heart attacks in otherwise healthy individuals (e.g., Danesh et al., 2000; Ridker, 2007).
As inflammation can be reliably assessed before clinical manifestation of cardiovascular diseases, recognizing its role in linking disadvantage to cardiovascular diseases is among the first steps to prevention and intervention efforts. However, changing an individual’s socioeconomic status is often not feasible. Therefore, it is crucial to identify the behavioral and biological pathways through which disadvantage operates to promote inflammation, as they can provide more pragmatic target points for prevention and intervention purposes. Mounting evidence highlights adiposity as one such mechanism through which socioeconomic disadvantage becomes associated with inflammation. Indeed, conceptual models suggest that socioeconomic disadvantage can promote adiposity through individual, family, and neighborhood pathways (Braveman and Gottlieb, 2014; Chen and Miller, 2013; Cohen et al., 2010; Matthews and Gallo, 2011; Miller et al., 2011; Schreier and Chen, 2013).
At the individual level, chronic psychological stressors associated with disadvantage, and efforts to cope with them, can promote behavioral patterns that contribute to adiposity. For example, stress can increase appetite, food cravings, and intake of foods high in caloric, fat, or sugar content (Chao et al., 2015; Kandiah et al., 2008; Morris et al., 2015), as well as decrease physical activity (Stults-Kolehmainen and Sinha, 2014). At the family level, socioeconomically disadvantaged families are less likely to eat meals together (Neumark-Sztainer et al., 2003; Tubbs et al., 2005) and are more likely to watch television during mealtimes (Coon et al., 2001; Eloise-kate et al., 2017). These mealtime practices have relevance for adiposity (Fiese and Schwartz, 2008)— common family mealtimes has been linked to higher consumption of fruits and vegetables and lower risk for obesity (Berge et al., 2015; Larson et al., 2013; Rollins et al., 2010), whereas television-watching during mealtimes has been linked to lower fruit and vegetable consumptions and greater adiposity (Ford et al., 2012; Gable et al., 2007). Finally, structural inequalities such as residential segregation and economic disinvestment result in disproportionate access to health-promoting resources across residential neighborhoods (Braveman et al., 2011). For example, socioeconomically disadvantaged groups have less access to green spaces, athletic facilities, and produce markets, but more access to fast food outlets in their residential neighborhoods (Abbott et al., 2014; Ball et al., 2009; Fraser et al., 2010). In sum, socioeconomic disadvantage can promote adiposity through individual, family, and neighborhood factors (Abbott et al., 2014; Wardle et al., 2011).
In turn, adiposity plays a central role in inflammation. Adipocytes secrete a number of proinflammatory cytokines, with about a quarter to a third of circulating IL-6 released from adipose tissue (Black, 2003; Mohamed-Ali et al., 1998). Increased lipid and fat accumulation also activate proinflammatory pathways, which facilitates recruitment of extant macrophages to adipose tissues, further perpetuating a proinflammatory state (Elks and Francis, 2010; Henegar et al., 2008; Weisberg et al., 2003). Indeed, greater adiposity, as measured by waist circumference and Body Mass Index, has been consistently and strongly linked to higher levels of inflammatory biomarkers (Choi et al., 2013). Collectively, these observations suggest that adiposity could be an important mechanism connecting disadvantage to inflammation.
Importantly, the relationship between social disadvantages and inflammatory activity is theorized to strengthen across the lifecourse. The sequelae of social disadvantages involve the wearing and tearing down of systems, a process that takes time to arise, accumulate, and become established. It is unlikely that a single incident of discrimination or a single high-fat meal produces a chronic state of low-grade inflammation. It is much more likely that social disadvantage entails sustained exposure to psychological stressors, unhealthy behaviors, and environmental risks, which accumulate across the lifecourse to foster a chronic state of inflammation (e.g., Braveman and Barclay, 2009; Chen et al., 2002; Danese and McEwen, 2012; Seeman et al., 2010). However, it is methodologically challenging to test this proposition, as few studies that assessed inflammatory biomarkers have samples with multiple racial and SES groups that each spans a sufficiently wide age range. This results in inadequate cell sizes for each age by race or SES combination, precluding formal test of age by disadvantage interactions.
To address this issue, we integrated data from all seven of our lab’s studies with relevant measures, conducted over 15 years, to carry out a pooled data analysis. Specifically, we extracted and harmonized measures of age, race (Black, Asian, White), SES (income, savings, education), adiposity (waist circumference), and biomarkers of low-grade inflammation (CRP, IL-6) to create a pooled sample of over 1,600 individuals aged from 11 to 60 years, allowing examination of whether the magnitude of the associations from race and SES to low-grade inflammation varied across the lifespan. We expected to replicate previous findings on race and SES disparities in low-grade inflammation, with Black or low-SES individuals exhibiting the highest levels of biomarkers. We also hypothesized that Asian individuals would exhibit lower inflammatory biomarkers relative to White individuals, as the prevalence of cardiovascular diseases in Asian individuals are estimated to be lower than the general population (Leigh et al., 2016). Because putative mechanisms linking social disadvantage to inflammation are theorized to accumulate across time, we further hypothesized that race and SES disparities would widen across the lifecourse, as evidenced by significant race by age and SES by age interactions. Finally, we examined whether racial and SES disparities in inflammation would emerge through adiposity pathways, hypothesizing that adiposity would emerge as a mediator of these disparities and account for increasing proportions of the disparities in inflammation across the lifecourse.
1. Method
This pooled data analysis combined all seven of our lab’s studies (A-G) that included relevant measures of demographics (race, SES, and sex at birth), adiposity (waist circumference), and low-grade inflammation (IL-6 and CRP). Studies were selected prior to data analyses based on the single criterion that it included all relevant measures. Table 1 provides the year and location of data collection (US or Canada). Studies were approved by the institutional review board of the university where they were conducted. Adults gave written consent, and youth gave assent and a guardian gave consent. Study A (Miller et al., 2018) recruited 277 eighth-graders to examine psychosocial contributors to early cardiovascular disparities. Study B (Chen et al., 2013) recruited 261 youth-parent dyads to examine how life experiences of family members may influence the health of other family members. Study C (Miller and Cole, 2012) recruited 147 female adolescents to understand the association between depression and inflammation. Thus, participants had to be at high risk for a first episode of depression as indicated by family history or cognitive vulnerability. Study D (Forlenza and Miller, 2006) is comprised of two studies, which together recruited 171 adults to examine the pathophysiological effects of depression. Half of the participants met diagnostic criteria for clinical depression and the remaining half had no lifetime history of psychiatric illness. Study E (Hostinar et al., 2017) recruited 360 youth and adults to examine how early life and current SES contribute to health disparities. Thus, participants had to fit into one of four groups defined by childhood (low vs. high) and adulthood (low vs. high) SES. Study F is comprised of two studies (Miller et al., 2014b; Rohleder et al., 2009) that examined the health effects of caregiving. The sample included 53 adults who were caring for a family member with brain cancer (glioblastoma) and 67 age- and sex-matched controls who did not have any major stressors in the past year. Study G (Chen et al., 2017) included 311 adults, who were parents of youth with asthma, recruited for a study on social disparities and childhood asthma.
Table 1.
Descriptive statistics of sample characteristics by study (N = 1,650).
| Study A | Study B: Children | Study B: Parents | Study C | Study D | Study E | Study F | Study G | |
|---|---|---|---|---|---|---|---|---|
| Analysis n | 193 | 231 | 242 | 141 | 171 | 330 | 81 | 261 |
| Location | Chicago | Vancouver | Vancouver | Vancouver | St. Louis | Vancouver | Vancouver | Chicago |
| Year | 2015–2017 | 2010–2012 | 2010–2012 | 2005–2007 | 2001–2003 | 2009–2012 | 2005–2012 | 2012–2017 |
| Age: range; Mean (SD) | 11–15; 13.45 (.64) |
13–16; 14.55 (1.07) |
34–60; 45.87 (5.23) |
14–19; 17.04 (1.35) |
18–58; 28.7 (8.95) |
15–55; 36.64 (10.76) |
19–60; 44.95 (11.45) |
26–60; 45.21 (6.43) |
| Race: n (%) | ||||||||
| White | 75 (39%) | 128 (55%) | 155 (64%) | 73 (52%) | 80 (47%) | 260 (79%) | 64 (79%) | 170 (65%) |
| Black | 104 (54%) | 12 (5%) | 4 (2%) | 2 (1%) | 80 (47%) | 2 (1%) | 0 (0%) | 65 (25%) |
| Asian | 14 (7%) | 91 (39%) | 83 (34%) | 66 (47%) | 11 (6%) | 68 (21%) | 17 (21%) | 26 (10%) |
| Female: n (%) | 122 (63%) | 111 (48%) | 188 (78%) | 141 (100%) | 140 (82%) | 177 (54%) | 55 (68%) | 228 (87%) |
| SES: Mean (SD) | ||||||||
| Family income | 5.47 (2.31) | 5.44 (1.77) | 5.41 (1.78) | n/a | 2.54 (1.35) | 4.73 (1.78) | 4.99 (1.93) | 6.43 (2.07) |
| Family savings | 4.18 (2.69) | 4.70 (2.43) | 4.64 (2.46) | n/a | n/a | 3.07 (1.84) | n/a | 5.02 (2.75) |
| Education | 3.76 (1.21) | 3.79 (0.90) | 3.79 (0.89) | 3.72 (1.04) | 3.36 (1.04) | 3.27 (1.18) | 3.65 (1.20) | 4.00 (1.06) |
| Adiposity | 75.23 (12.1) | 76.34 (10.37) | 86.41 (12.99) | 71.88 (7.26) | 89.71 (16.03) | 85.74 (13.74) | 86.12 (13.5) | 92.29 (15.41) |
| CRP: Mean (SD) | 2.1 (2.46) | 1.51 (2.38) | 3.33 (2.72) | 1.88 (2.21) | 3.59 (2.35) | 3.59 (2.71) | 4.33 (3.01) | 4.66 (2.72) |
| CV (%) | 2.5 | 2.2 | 2.2 | 2.2 | < 3 | 2.2 | 2.2 | 2.5 |
| IL-6: Mean (SD) | 3.78 (1.87) | 3.96 (1.88) | 4.27 (1.89) | 4.25 (1.93) | 5.37 (1.64) | 4.54 (1.72) | 3.81 (2.28) | 4.51 (1.89) |
| CV (%) | 3.7 | < 10 | < 10 | < 10 | 4.6 | < 10 | < 10 | 3.4 |
Note. Location refers to the city in which the study was conducted. Year refers to the years during which data was collected. Family income was coded in a 9-point scale: 1 = less than 5,000; 2 = 5,000 to 19,999; 3 = 20,000 to 34,999; 4 = 35,000 to 49,999; 5 = 50,000 to 74,999; 6 = 75,000 to 99,999; 7 = 100,000 to 149,999; 8 = 150,000 to 199,999; 9 = 200,000 and higher. Family savings was coded in a 9-point scale: 1 = less than 500; 2 = 500 to 4,999; 3 = 5,000 to 9,999; 4 = 10,000 to 19,999; 5 = 20,000 to 49,999; 6 = 50,000 to 99,999; 7 = 100,000 to 199,999; 8 = 200,000 to 499,999; 9 = 500,000 and higher. Education was coded in a 5-point scale: 1 = less than high school, 2 = high school diploma, 3 = some college, 4 = bachelor’s degree, and 5 = graduate degree. Adiposity was assessed with waist circumference expressed in centimeters. C-Reactive Protein (CRP) and Interleukin-6 (IL-6) are expressed in POMP scores that ranged from 0 to 10.
1.1. Measures
Table 1 presents descriptive statistics by study.
1.1.1. Age.
Participants self-reported age, which ranged from 11 to 80 years (M = 28.87, SD = 15.84). Upon inspection of the distribution, we excluded participants ages 61 to 80 because there were too few participants (n=26, 1.4%) and insufficient variation in race (86 % White) in that range. As such, the analytical sample age ranged from 11 to 60 years (M = 31.45, SD = 14.83).
1.1.2. Sex.
Participants self-reported sex at birth as female (n= 1162, 70%) or male (n= 479, 29%), which was effect-coded with male being the reference group.
1.1.3. Race.
Adult participants self-reported race, while parents of youth reported race on youth’s behalf. Race was coded as White, Black, and Asian. Given our interest in racial differences in inflammation across the lifespan, we restricted analyses to groups with at least 246 participants1 and well-represented across the age spectrum. As a result, Latino/Hispanic (n = 192 with 71% ≤ age 16) and Native Hawaiian/Pacific Islander (n = 60) participants were excluded from analyses. Multi-racial participants who identified as Black and of any other race categories were coded as Black. Participants who identified as Asian and any other race except Black was coded as Asian. White participants were coded as such only if they did not identify as any other race. Two effect-coded variables were created with White being the reference group.
1.1.4. Socioeconomic Status (SES).
Family gross income, family savings, and education were used to measure SES. Adult participants self-reported measures of SES and parents of youth completed measures of SES on youth’s behalf.
1.1.4.1. Family Income.
Participants from Studies A, B, and G reported the dollar amount of their family’s gross income in the past twelve months, while participants from Studies D, E, and F reported family’s gross income in the past twelve months using scales of income brackets. Specifically, Study D used a 12-point scale (1 = less than 5,000; 12 = 125,000 and higher), Study E used a 10-point scale (1 = less than 5,000; 10 = 200,000 and higher), and Study F used a 8-point scale (1 = less than 5,000; 8 = 200,000 and higher). Sample C did not assess family income. To harmonize measures of family income across studies, we first converted income that was reported in Canadian Dollars (CAD; Studies B, C, E, and F) to United States Dollars (USD) using conversion rates at the time of data collection. Next, we recoded income into a 9-point scale with 1 being less than 5,000; 2 being 5,000 to 19,999; 3 being 20,000 to 34,999; 4 being 35,000 to 49,999; 5 being 50,000 to 74,999; 6 being 75,000 to 99,999; 7 being 100,000 to 149,999; 8 being 150,000 to 199,999; 9 being 200,000 and higher (M = 5.15, SD = 2.15).
1.1.4.2. Family savings.
Participants from Studies A, B, and G reported the dollar amount of their family savings, including stocks and bonds, while participants from Study E reported their family savings using a 9-point scale (1= less than 500; 9 = 500,000 and higher). Studies C, D, and F did not have measures of family savings. Similar to family income, we harmonized these measures by first converting income that was reported in CAD to USD, and then recoded savings into the same 9-point scale used in Study E: 1 being less than 500; 2 being 500 to 4,999; 3 being 5,000 to 9,999; 4 being 10,000 to 19,999; 5 being 20,000 to 49,999; 6 being 50,000 to 99,999; 7 being 100,000 to 199,999; 8 being 200,000 to 499,999; 9 being 500,000 and higher (M = 4.27, SD = 2.53).
1.1.4.3. Education.
Adult participants reported the highest education degree attained. For youth, the higher of the two parental education degrees was used to gauge youth’s SES. Highest education degree attained was reported on a 5-point scale (1 = less than high school; 5 = graduate degree) in Studies A, B, and G, on a 8-point scale (1 = less than high school; 8 = doctoral degree) in Study C, and on a 7-point scale (1 = less than high school; 7 = professional degree) in Studies E and F. Participants from Study D reported the total number of years of education. To harmonize this measure across studies, we first recoded the number of education years to education degree based on conventions (e.g., 12 years coded high school graduate, 16 years coded as bachelor’s degree) and then recoded all scales in to the same 5-point scale with 1 being less than high school, 2 being high school diploma, 3 being some college, 4 being bachelor’s degree, and 5 being graduate degree (M = 3.66, SD = 1.09).
Because three studies did not have all three SES indicators (see Table 1), we created a composite by averaging the standardized measures of family income (r = .60 with savings and r = .40 with education), family savings (r = .39 with education), and education (Cronbach’s α = .71).
1.1.5. Central Adiposity.
Waist circumference was used to measure abdominal adiposity, because evidence suggests it better predicts disease risks than body mass index, which captures both general fat and muscle mass. Waist circumference was assessed at the middle point between iliac crest and lower rib using measuring tape, expressed in centimeters (M = 83.52, SD = 14.75).
1.1.6. Low-Grade Inflammation.
In all studies, antecubital blood was drawn into serum separator tubes. Serum was harvested by centrifugation and kept frozen at −30 or −80 degrees Celsius until assays for C-Reactive Protein (CRP) and Interleukin-6 (IL-6) were performed (Table 1 presents the coefficients of variation).
To harmonize inflammation across studies, we first excluded outliers (defined as ± 3SDs from mean) and participants with CRP of 10 mg/L or higher, following conventions in the literature that values in this range indicate acute infection or chronic inflammatory disease. Both the American Heart Association and the Centers for Disease Control and Prevention recommended excluding values > 10 in studies of cardiovascular risk (Best et al., 2005; Pearson et al., 2003). We then natural-log transformed values to correct skew within studies. Next, to harmonize the methodological differences in assay procedures across studies, and calibrate values into comparable units, we adapted the proportion of maximum possible score (POMP scores), a technique used in previous coordinated analyses (Stawski et al., 2019). Specifically, within each study, we subtracted the minimum value from each observed value. We then divided the difference by the sample range and multiplied the quotient by ten. Thus, despite differences across studies, inflammation was scaled to range from 0 to 10. Finally, we created an inflammation composite by averaging the standardized CRP and IL-6 POMP scores (r = .43 between CRP and IL-6).
2. Statistical Approach
2.1. Primary Analyses
First, we examined the independent effects of age, race, and SES on inflammation in a regression model. For age, we first modeled a linear prediction and then a curvilinear prediction to examine if the magnitude of the age-inflammation link varied across the lifecourse. We then examined the race by age and SES by age interaction effects on inflammation in separate models. Specifically, for both race and SES, we first tested linear age moderations and then conducted exploratory analyses to examine curvilinear age moderations. All models included age, race, SES, and sex at birth as predictors. Significant moderations were probed by computing simple effects at mean age of 31 and at −1 and +1 SD from mean (ages 16 and 46, respectively), and then followed-up with regions of significance tests.
Next, we conducted path analyses to examine whether the links from race and SES to inflammation were in part through associations with adiposity and whether the magnitude of these indirect effects differed by age. Specifically, we conducted indirect effect models specifying paths from race/SES to adiposity, adiposity to inflammation, and race/SES to inflammation, and then conducted moderated indirect effect models with age moderating the paths from race/SES to inflammation as well as the paths from race/SES to adiposity. Indirect and moderated indirect effects as well as their 95% confidence intervals were estimated using 10,000 bootstrapped samples. Significant moderated indirect effects were probed at −1 and +1 SD from mean (ages 16 and 46, respectively). All models included age, sex at birth, race, and SES as predictors. Continuous variables were mean-centered and categorical variables were effect-coded, except for the omnibus groups regions of significance test (Hayes and Montoya, 2017).
2.2. Sensitivity Analyses
A series of sensitivity analyses were conducted to examine whether results were robust to sample characteristics and independent of each other. First, we accounted for country in which the study was conducted, US (n=625) vs. Canada (n=1025), with an effect-coded variable entered as a covariate in the main effects model and as interaction variables (country by race and country by SES) for the interaction models (Yzerbyt et al., 2004).
Second, as depression and chronic caregiver stress have known associations with low-grade inflammation (Miller and Cole, 2012; Wright et al., 2004), we accounted for whether participants had clinical depression, were at-risk for depression, or were caregivers to a patient. Specifically, we created an effect-coded variable that categorized Study C’s participants who were at high-risk for depression, Study D’s participants who had clinical depression, and Study E’s participants who were caregivers to a family member with brain cancer as caregiver/clinical participants (n=262). All remaining participants were categorized as non-clinical/caregiver participants (n=1388). Similar to country, this variable was entered as a covariate in main effects model and as interaction variables in interaction models. In addition, as smoking also has known associations with low-grade inflammation (Danesh et al., 2000; Pirkola et al., 2010), we accounted for whether participants smoked at least one cigarette daily. Specifically, smoking status was effect-coded (non-smoker as reference group) and the entered as a covariate in main effect models and as a moderator in interaction models.
Third, to account for sex differences in body composition (Stevens et al., 2010), we standardized waist circumference scores separately for male and female participants. Sex-adjusted values were used in indirect, and moderated indirect, effect models as a mediator.
Fourth, Study B includes youth-parent dyad, which can create dependencies in data. To ensure results were not driven by such dependencies, sensitivity analyses re-conducted models with Study B’s youth removed. We opted to exclude youth, rather than parents, because parents’ age spanned a wider range (age 34 to 60) comparing to youth (age 13 to 16), and because removing parents would substantially decrease the number of Asian participants in that wide age range by 60%, compromising our position to examine Asian race by age effects.
Finally, to examine whether linear or curvilinear age moderation of race and SES on inflammation were independent of each other, we entered all the main, quadratic, interaction, and quadratic interaction effects into a single model.
2.3. Power Analyses
The analytical sample size was 1,650. Statistical significance was determined at alpha of .05. We expected small effect sizes for interactions, and power analyses suggested that to detect correlations of .08, .10, and .12 (i.e., delta R2 of .006, .01, and .014, respectively) for the specific interaction term when seven other predictors are included with statistical power of .80, sample sizes of 1311, 787, and 548 are necessary, respectively. Furthermore, for the single model wherein all terms were included to examine independent interaction effects, we expected to detect three significant interaction effects (age by SES, age by Black race, and age by Asian race) when nine other terms are included (i.e., the main effect and quadratic effect terms). Power analyses suggested that to detect correlations of .08, .10, and .12 for the three interaction terms with statistical power of .80, sample sizes of 1822, 1095, and 783 are necessary, respectively.
3. Results
As shown in Table 2, Black individuals (vs. White and Asian individuals) were younger, more likely to be female, lower in SES, higher in adiposity, and higher in inflammation. Asian individuals (vs. White individuals) were younger, and lower in adiposity and inflammatory biomarkers. Across participants, increasing age was associated with higher SES, adiposity, and inflammatory biomarkers. Further, higher SES was associated with lower adiposity and inflammatory biomarkers. Of note, the association between race and SES was modest (rs ≤ .23), allowing testing of independent effects of race and SES with little concern over collinearity.
Table 2.
Descriptive statistics by race and simple correlations among study variables (N = 1607 to 1650).
| Descriptive Statistics | Bivariate Correlations | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Black participants (n = 269) | Asian participants (n = 376) | White participants (n = 1005) | Statistical tests | Age | SES | Female | Adiposity | Inflammation | |
| Age | 25.62 (12.86) | 28.35 (14.11) | 34.18 (14.92) | F (2,1647) = 48.6* Black < Asian < White | 1 | ||||
| SES | −0.53 (0.77) | 0.06 (0.84) | 0.07 (0.80) | F (2,1642) = 60.8* Black < Asian = White | .10* | 1 | |||
| Female | 209 (78%) | 258 (69%) | 695 (69%) | χ2 (2,1647) = 7.49* Black > Asian = White | .05 | .00 | 1 | ||
| Adiposity | 88.50 (18.50) | 79.23 (12.25) | 83.77 (13.99) | F (2,1613) = 32.1* Black > White > Asian | .42* | −.16* | −.14* | 1 | |
| Inflammation | 0.25 (0.96) | −0.16 (0.85) | 0.01 (0.83) | F (2,1647) = 18.04* Black > White > Asian | .24* | −.19* | .08* | .56* | 1 |
| Black (vs. White) | - | - | - | - | −.23* | −.23* | .05* | .06* | .06* |
| Asian (vs. White) | - | - | - | - | −.19* | −.06* | .01 | −.09* | −.05* |
Note.
p < .05 for simple correlations. Means, and standard deviations in parentheses, are presented for continuous variables. Frequencies, and percentages in parentheses, are presented for sex. SES refers to socioeconomic status and was assessed with a standardized composite of family income, family savings, and education degree (parental education for youth). Inflammation was assessed with a standardized composite of C-Reactive Protein and Interleukin-6 levels. Both standardized composites were expressed in standard deviation units. Adiposity was assessed with waist circumference expressed in centimeters. Racial group differences in continuous study variables were tested with analyses of variance (ANOVAs) and followed-up with Tukey’s HSD post-hoc tests. Racial group differences in sex was assessed with a Chi-Square Test.
3.1. Main effects of age, race, and SES
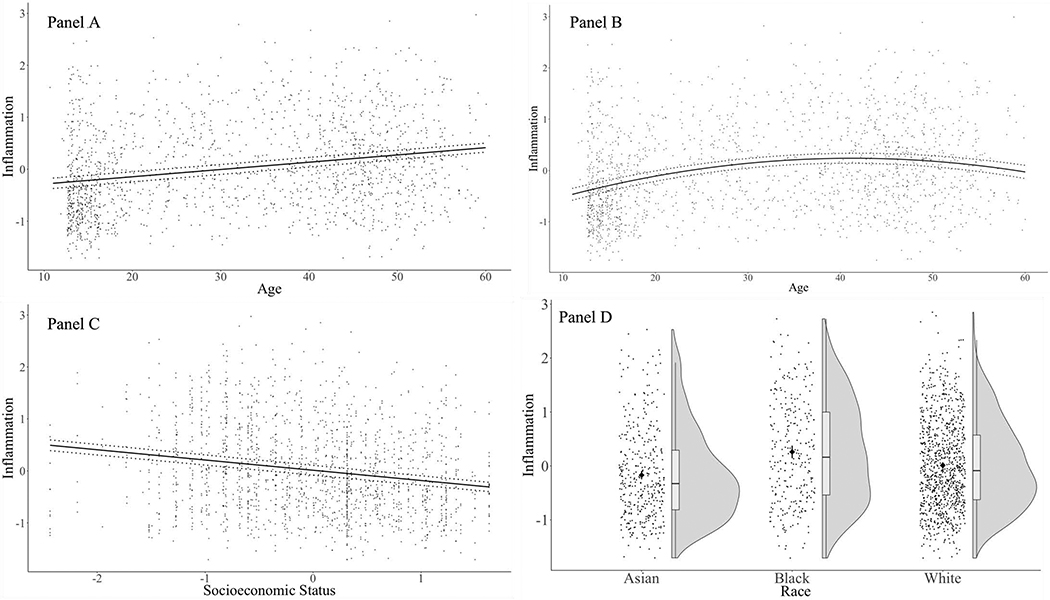
First, we examined the independent associations of age, race, and SES with inflammatory biomarkers, as shown in Table 3 and Figure 1. Replicating previous findings, older age, Black (vs. White) race, and low SES were independently associated with higher biomarker levels. By contrast, Asian (vs. White) race was independently associated with lower biomarker levels. As shown in Table 3 and Figure 1B, there was also a significant curvilinear effect of age, such that inflammatory biomarkers increased across the lifespan, but this slope gradually flattened. Specifically, regions of significance test showed that inflammation increased up to age 42, at which point the gradient was no longer statistically significant (b = .005, SE = .003, p = .13), and remained non-significant through age 60, the sample’s maximum age.
Table 3.
Main effects of age, race, and SES, quadratic effect of age, and interaction effects between race/SES and age (listwise deletion N = 1636).
| Unstandardized b (Standard Error), p-value | 95% Confidence Interval | Effect Size (r) | |
|---|---|---|---|
| Main effects | |||
| Age | .02 (.001), p < .001 | [.013, .018] | .26 |
| Black (vs. White) | .39 (.077), p < .001 | [.238, .542] | .12 |
| Asian (vs. White) | −.29 (.070), p < .001 | [−.420, −.152] | .10 |
| SES | −.19 (.025), p < .001 | [−.243, −.145] | .18 |
| Female (vs. Male) | .11 (.044), p = .011 | [.026, .200] | .06 |
|
Quadratic and Interaction effects | |||
| Age x Age | −.0005 (.0001), p < .001 | [−.0007, −.0002] | .08 |
| Black (vs. White) x Age | .02 (.006), p < .001 | [.011, .033] | .09 |
| Asian (vs. White) x Age | −.01 (.005), p = .006 | [−.023, −.004] | .06 |
| Black (vs. White) x Age x Age | .0004 (.0006), p = .530 | [−.001, .002] | .01 |
| Asian (vs. White) x Age x Age | −.001 (.0005), p = .011 | [−.0023, −.0003] | .06 |
| SES x Age | −.006 (.002), p < .001 | [−.009, −.003] | .09 |
| SES x Age x Age | −.0002 (.0001), p = .139 | [−.0006, .0000] | .03 |
|
Single model | |||
| Age | .02 (.002), p < .001 | [.015, .022] | .22 |
| Black (vs. White) | .46 (.137), p < .001 | [.194, .733] | .08 |
| Asian (vs. White) | −.11 (.126), p = .372 | [−.360, .135] | .02 |
| SES | −.12 (.046), p = .010 | [−.206, −.025] | .06 |
| Female (vs. Male) | .10 (.045), p = .028 | [.010, .186] | .05 |
| Age x Age | −.0006 (.0002), p < .001 | [−.001, −.0003] | .08 |
| Black (vs. White) x Age | .01 (.006), p = .024 | [.002, .027] | .05 |
| Asian (vs. White) x Age | −.01 (.005), p = .072 | [−.019, .001] | .04 |
| Black (vs. White) x Age x Age | .000 (.0006), p = .896 | [−.001, .001] | .00 |
| Asian (vs. White) x Age x Age | −.001 (.0005), p = .034 | [−.002, −.000] | .05 |
| SES x Age | −.004 (.002), p = .018 | [−.008, −.001] | .06 |
| SES x Age x Age | −.0003 (.0002), p = .080 | [−.001, .000] | .04 |
Note. Continuous predictors were mean-centered, and binary predictors were effect-coded. SES refers to socioeconomic status. Effect sizes are expressed in semi-partial correlation coefficients (i.e., the square root of delta R2) of the specific terms. Main effects of age, race, SES, and sex were always entered simultaneously as predictors of inflammation in the same block. For the quadratic and interaction effects section, each quadratic effect, linear interaction effect, or quadratic interaction effect was entered as a separate model (but both race codes entered when testing and race interaction effects). For the single model section, all main, quadratic, and interaction effects were entered simultaneously as predictors of inflammation.
Figure 1.
Main effects of linear age (Panel A), curvilinear age (Panel B), SES (Panel C), and race (Panel D) on inflammation. Solid circles represent unadjusted inflammation data. Panels A, B, and C present the adjusted regression slopes in solid lines and the associated 95% CIs in dotted lines. Panel D presents raincloud plots that combine rhombuses displaying the means for each level of race (as well as bars displaying 95% CIs), boxplots displaying the median and quartiles, as well as violin plots shaded in grey displaying the distribution. Main effects were entered simultaneously as predictors along with sex at birth. In Panel C, the high frequency of SES value at 0.31 is due to Study C only having one of the three indicators, education, reflecting individuals who rated a 4 on the 5-point scale (see Table 1).
3.2. Age-moderated effects of race
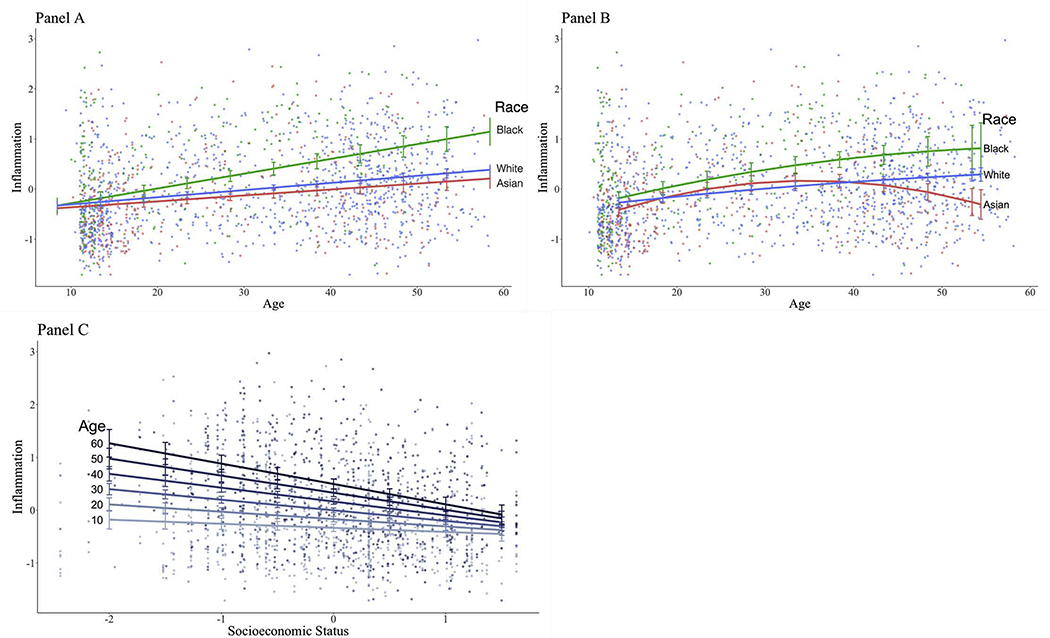
We then examined whether the magnitude of the relationships between race and inflammatory biomarkers varied by age. As shown in Table 3 and Figure 2A, there was a significant interaction between race and age. Specifically, racial differences in inflammatory biomarkers widened with age, such that Black-White difference was smallest among younger participants at age 16 (b =.09, SE = .047, p = .051), but this difference was more evident at mean age of 31 (b = .25, SE = .041, p < .001), and widened among older participants at age 45 (b = .42, SE = .069, p < .001). Similarly, Asian-White difference was smallest among younger participants (b = −.08, SE = .044, p = .064), but this difference was again more evident at mean age (b = −.18, SE = .036, p = < .001), and widened older participants (b = −.28, SE = .057, p < .001). Omnibus groups regions of significance test showed that racial differences emerged starting at about age 18 and remained significant through age 602.
Figure 2.
Interaction effect between linear age and race (Panel A), curvilinear age and race (Panel B), and linear age and SES (Panel C) on inflammation. Points represent unadjusted inflammation data and differences in color represent different levels of race and age as indicated by the corresponding legends. Panels A and B presents the linear and curvilinear adjusted slopes between age and inflammation at each level of race. Panel C presents the adjusted slopes between SES and inflammation at each decade of age. All models were adjusted for main effects of age, race, SES, and sex at birth.
As we observed a significant curvilinear effect of age (described above), exploratory analyses were conducted to examine whether there was a curvilinear age moderation of the association between race and inflammation. As shown in Table 3, there was no significant curvilinear age moderation for the association between Black race and inflammation, but there was a significant curvilinear age moderation for the association between Asian race and inflammatory biomarkers. Specifically, as depicted in Figure 2B, a significant curvilinear age effect was found among Asian participants (b = −.001, SE = .0003, p < .001), but not among White participants (b = −.0004, SE = .0004, p = .320).
3.3. Age-moderated effects of SES
As shown in Table 3 and Figure 2C, there was also a significant interaction between SES and age. Specifically, although lower SES was associated with higher biomarkers in younger participants (at age 16; b = −.11, SE = .03, p = .001), the magnitude of this relationship was larger among participants at mean age of 31 (b = −.20, SE = .03, p < .001), and even larger among older participants at age 45 (b = −.29, SE = .04, p < .001). Regions of significance test showed that SES became significantly associated inflammatory biomarkers starting at about age 12 and remained significant through age 60. Finally, we examined whether this age moderation of the SES-inflammation link followed a curvilinear pattern. As shown in Table 3, we found no evidence of a SES by curvilinear age moderation.
3.4. Sensitivity analyses for main, quadratic, interaction, and quadratic interaction effects
All main, quadratic and interaction effects remained the same when accounting for country in which study was conducted, whether participants were chronic caregivers/clinical vs. non-clinical participants, and whether participants were smokers. In addition, when Study B’s youth participants were excluded to eliminate potential youth-dyad dependencies in data, the main, quadratic, and interaction effects remained the same. Supplementary Material present statistics for these models. Finally, as shown in Table 3, when all main, quadratic, interaction, and quadratic interaction terms were entered simultaneously into a single model, all results remained the same except for the linear age moderation of the link between Asian race and inflammatory biomarkers, which was no longer significant (p = .07). However, the curvilinear age moderation of the same link remained significant, suggesting that the curvilinear age moderation may be more robust than the linear moderation for Asian vs. White comparisons. These results suggest that the race by age and SES by age interaction effects were independent of each other.
3.5. Indirect effects of race and SES through adiposity
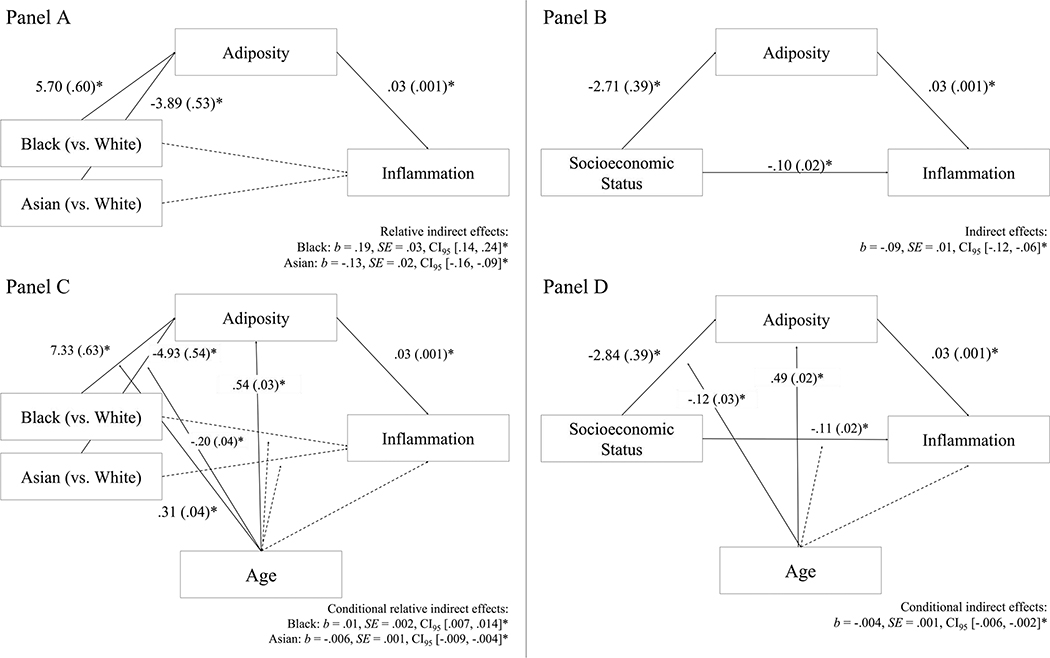
Next, we tested whether the race and SES disparities in inflammatory biomarkers were partly via associations with adiposity. As shown in Figures 3A and 3B, independent of SES, there was a significant indirect effect such that Black (vs. White) participants had greater adiposity, which in turn was associated with higher inflammatory biomarkers (b = .19, SE = .03, p < .001). By contrast, Asian (vs. White) participants had lower adiposity, which in turn was associated with lower inflammatory biomarkers (b = −.13, SE = .02, p < .001). There was no direct effect of race when accounting for adiposity. Independent of race, there was also a significant indirect effect such that lower SES was associated with greater adiposity, which in turn was associated with higher inflammatory biomarkers (b = −.09, SE = .01, p < .001). There was significant residual covariance in the SES-inflammation relationship, above and beyond adiposity.
Figure 3.
*p < .05. Panels A and B present indirect effect models specifying that race and SES were associated with adiposity, which in turn was associated with inflammation. Panels C and D present the conditional indirect effect model wherein the indirect effect from race and SES to adiposity to inflammation strengthened with age. Solid lines indicate significant paths or interactions and dotted lines indicate non-significant paths or interactions. Coefficients in race models were adjusted for sex at birth and SES and coefficients in SES models were adjusted for race and SES.
3.6. Age-moderated indirect effects of race and SES through adiposity
We next tested conditional indirect effect models to determine if there were age-related variations in the observed indirect effects via adiposity. As shown in Figure 3C, there was a significant conditional indirect effect for Black race (b = .01, SE = .002, CI95 [.007, .014], p < .001), such that the pathway linking Black race, high adiposity, and high inflammation was stronger for older participants (b = .39, SE = .05, CI95 [.30, .49]) than for younger participants (b = .09, SE = .03, CI95 [.04, .14]). There was also a significant conditional indirect effect for Asian race (b = −.006, SE = .001, CI95[−.009, −.004], p < .001), such that the indirect effect linking Asian race, low adiposity, and low inflammation was stronger for older participants (b = −.26, SE = .03, CI95 [−.32, −.20]) than younger participants (b = −.07, SE = .02, CI95 [−.11, −.03]). Similarly, as shown in Figure 3D, there was also a significant conditional indirect effect for SES, adiposity, and inflammation linkage (b = −.004, SE = .001, p < .001), such that the indirect effect linking low SES, high adiposity, high inflammatory biomarkers was stronger for older participants (b = −.15, SE = .02, CI95 [−.19, −.11]) than for younger participants (b = −.03, SE = .02, CI95 [−.06, −.004]).
3.7. Sensitivity analyses for indirect and age-moderated indirect effect
All indirect effects and age-moderated indirect effects remained significant when accounting for country in which study was conducted, whether participants were chronic caregivers/clinical vs. non-clinical participants, whether participants were smokers, and whether waist circumference was adjusted for sex differences. Supplementary Material present statistics for these models.
4. Discussion
Cardiovascular diseases disproportionately affect certain racial and SES groups, and low-grade inflammation has been proposed as an underlying mechanism. Although studies have documented race and SES differences in inflammatory activity, methodological challenges precluded previous studies from discerning how these differences, and the underlying contributors, change from childhood, to adolescence, to adulthood. To address these questions, we integrated data from seven studies to produce a sample with multiple racial and SES groups that spanned a wide age range. First, we found that, even at relatively younger ages, Black individuals exhibited higher, and Asian individuals exhibited lower, levels of inflammatory biomarkers relative to White individuals. We also found that individuals with low SES had higher levels of inflammatory biomarkers. Second, extending previous research on health disparities, the magnitude of inflammation disparities increased with age for both race and SES. Third, adiposity was a pathway that not only accounted for race and SES differences, but also accounted for how these differences widened across developmental stages.
These results converge with nascent findings on age variation in health disparities. For example, one prospective study of adults found that, relative to White individuals, Black individuals had larger increases in low-grade inflammation over a 15-year span (Fuller-Rowell et al., 2015). In addition, a recent study examined SES disparities in CRP using four datasets that differed in sample age ranges (Yang et al., 2017). Although age interactions were not conducted as the datasets were not integrated into a single pooled sample, the magnitude of the SES-CRP link appeared to increase from a sample of younger adults (age 24–32; r = −.03) to a sample of older adults (age 50–74; r = −.14).3 Another study reported widening of SES gaps in prevalence of high CRP from age 20 to 69 among men (Martinson et al., 2016). Using data from the National Health and Nutrition Examination Survey (NHANES), one study reported increased prevalence of very high CRP levels (CRP > 10 mg/L) among those in poverty (vs. those above poverty) at every decade age group from age 20 to age 80. Although no formal age group by poverty status interaction was conducted, the greatest numeric difference by poverty status occurred in the older age group of 70–79 (Alley et al., 2006). As these studies lack statistical interaction tests and only utilized adult samples, our findings extend knowledge by first demonstrating that disparities in low-grade inflammation were present by childhood and adolescence, with SES gaps statistically emerging by age 12 and race differences statistically emerging by age 18, and then further demonstrating that these disparities widen across the lifecourse, from childhood to adolescence to adulthood.
We also advance knowledge by documenting a parallel age-graded pattern in disparities related to central adiposity, a known contributor to inflammation (Choi et al., 2013; Ferrante Jr, 2007). These results converged with findings from a longitudinal study that examined race and SES disparities in trajectories of body mass index (BMI) across adulthood (age 18 to 45). Specifically, BMI increased with age, but this increase was accelerated among Black (vs. White) individuals and among those with lower SES (Clarke et al., 2009). Our findings further knowledge by demonstrating that the age-graded patterns in adiposity may account for the age-graded patterns in inflammation disparities. These findings are consistent with previous literature that socioeconomic and racial disadvantages are associated with adiposity-promoting behaviors, including lower consumption of vegetables and fruits, increased consumption of fried foods, and more sedentary lifestyle among youth and adults (Delva et al., 2007, 2006; Fahlman et al., 2010; Singh et al., 2008). While the role of individual health behaviors should be acknowledged, group differences in adiposity should not be interpreted simply as reflecting variation in choices of lifestyle behaviors, which may lead to victim-blaming conclusions (Adler and Stewart, 2009; Braveman et al., 2011). Rather, a plethora of theoretical and empirical accounts suggest that these variations may be reflective of structural inequalities, such as residential segregation and economic disinvestment, that limit access to health-promoting resources, including full-service supermarkets, safe recreational facilities for exercise, and health care facilities (Adler and Stewart, 2009; Bader et al., 2010; Fleischhacker et al., 2011) (Abbott et al., 2014). These environmental factors can increase the probability of engaging in the same adiposity-promoting behaviors described above. Thus, together with contextual stressors, such as discrimination, unemployment, and exposure to violence, which have known associations with adiposity (Kwarteng et al., 2016; Wardle et al., 2011), these disparate environmental and psychosocial experiences may contribute to the observed race and SES differences in adiposity, and the widening of such differences across the lifecourse, eventuating in chronic low-grade inflammation.
Exploratory analyses suggested a curvilinear relationship between age and low-grade inflammation among Asian, but not Black or White, participants (Figure 2B). We are reluctant to read too much into these patterns for two reasons. First, the number of Asian individuals at the upper end of the age spectrum - where the curvilinear pattern is apparent - was relatively small in this sample (69 participants, 18% of Asian participants, were older than age 45). Hence, this pattern could be imprecisely estimated. Second, we cannot locate other studies with similar results. There are very few empirical studies of aging-related inflammation disparities and that include Asian individuals, and the ones that do have not tested for curvilinear effects (e.g., Costello-White et al., 2015; Karim et al., 2020). As such, we believe these exploratory results should be considered tentative until replicated.
4.1. Implications
The present findings highlight the importance of considering age when examining health disparities and formulating conceptual models that specify how and why disparities may vary across the lifecourse. Our findings begin to shed light on lifecourse questions, but also invite additional mechanistic questions about why the inflammation disparities would strengthen with age. Specifically, results suggest that social disadvantages can have cumulative effects that eventuate a chronic state of inflammation, but how this accumulation occurs is unclear. Do cumulative effects occur because of continuity or worsening of disadvantages and environmental circumstances (e.g., persistent or increasing exposure to inflammation-stimulating stressors or pollutants)? Or, do cumulative effects occur through embedding mechanisms, whereby early disadvantages set into motion biological and psychosocial trajectories that accumulate and solidify with development? Or, do both early embedding and persistent disadvantages accumulate in synergistic ways that confer additive and interactive risks across the lifecourse? In addition, the duration of such cumulative effects remains unclear: do these cumulative effects of social disadvantages begin and end within an individual’s lifecourse, or do they extend beyond and have intergenerational effects? These open questions call for prospective methodologies like accelerated longitudinal designs (ALD) that follow multiple cohorts of individuals with retrospective measures of parental or grandparental disadvantages as well as repeated concurrent measures of disadvantages, mechanisms, and inflammation. As ALDs that cover the lifecourse are typically difficult to carry out, an alternative is to “build” them with existing data using pooled or integrative data-analytic techniques that combine multiple separate longitudinal studies that utilized samples of different developmental stages. Using these methodologies, trajectories of disadvantages can be estimated along with trajectories of mechanisms, which can be modeled as additive or interactive antecedents to trajectories of low-grade inflammation across time and cohorts.
Because inflammation is key to the pathogenesis of cardiovascular diseases, the present findings can have practical implications for efforts to prevent or mitigate health disparities. Although disparities in low-grade inflammation widened with age, as suggested by regions of significance analyses, they were already present by the first two decades of life (by age 12 for socioeconomic disparities and by age 18 for racial disparities). If replicated, these patterns suggest that prevention efforts might best be implemented during childhood, a period of life characterized by high levels of behavioral and biological plasticity, and where intervention-related benefits have multiple decades to compound. Indeed, a family-oriented intervention that targeted Black children from low SES backgrounds showed promising effects on low-grade inflammation as young adults (Miller et al., 2014a). Another intervention that targeted disadvantaged children produced evidence of better cardiometabolic health by the time participants reached their mid-30s (Campbell et al., 2014). These preliminary studies suggest that early interventions may confer lasting health benefits, though larger and longer trials are needed to confirm this interpretation.
4.2. Limitations
Findings from the present study should be interpreted in light of its limitations. First, results were drawn from cross-sectional data, precluding inferences about causality for both the observed main and indirect effects. As mentioned above, future studies may benefit from combining multiple longitudinal studies to establish temporal precedence in assessing indirect effects. Second, the analytical sample age range (age 11 to 60) did not span the entire lifecourse, and thus it is unknown whether the age-gradient effects in inflammation disparities would extend to early childhood or late adulthood. Third, most of the Black participants were Americans (92%) and most of the Asian participants were Canadians (88%). Consequently, race may have confounded with country such that the observed racial differences may be attributable to geographical differences between the US and Canada. However, analyses were conducted such that Black and Asian individuals were not compared against each other, but against White individuals, a group that consisted of both Americans and Canadians. In addition, studies have shown that the US and Canada have similar rates of cardiovascular risks (Lasser et al., 2006), and that these health problems are also patterned by race in Canada (Lebrun and LaVeist, 2011). Thus, it is unlikely that racial differences observed in this study were solely driven by geographical differences. Fourth, racial minority status is used as a proxy for relative social disadvantage compared to White individuals in this study. However, the extent to which membership in racial minority status is an accurate proxy for relative social disadvantage may vary across racial groups. Race-based social disadvantages should be most optimally assessed with direct measures of such (e.g., racial discrimination). Furthermore, emerging evidence suggests heterogeneity within broad categories can have relevance for health. As we do not have adequate information to examine subgroups, heterogeneity of inflammation among diverse subgroups may be masked. Fifth, interpretation of race and SES by age interactions as cumulative effects across the lifecourse assume that these social disadvantages are stable across development, which may not be true as there can be mobility in SES. However, as mobility can happen in upward or downward directions, mobility alone likely does not serve as an alternative explanation for the observed strengthening of the SES-inflammation link with age. Nonetheless, future research should replicate these findings using longitudinal methods. Sixth, as not all studies included measures of diet, physical activities, or neighborhood factors associated with adiposity, we were unable to test mechanisms linking disadvantage to adiposity. In addition, there are multiple pathways through which disadvantages may confer health risks, this study only examined adiposity. Future research should consider other psychosocial, biological, and environmental pathways. Seventh, the conditional indirect effects only modeled age moderation of the paths from race/SES to adiposity and race/SES to inflammation, but not adiposity to inflammation. Although it is possible to model age moderations of all three paths, there is currently no method of computing a moderated mediation coefficient for significance testing under such a scenario due to nonlinearity.
4.3. Conclusions
Low-grade inflammation contributes to multiple health problems across the lifespan, including diseases that are disproportionately common among disadvantaged groups. This pooled data analysis found that race and SES health disparities in inflammation, and their pathways via adiposity, strengthen across the lifecourse, suggesting cumulative effects of disadvantages across the lifecourse to confer health problems.
Supplementary Material
Highlights.
Socioeconomic and racial disparities in low-grade inflammation emerged in the first two decades of life.
Socioeconomic and racial disparities in low-grade inflammation widened across the lifecourse.
Central adiposity was a pathway through which socioeconomic and racial disadvantages related with low-grade inflammation.
Central adiposity also accounted for how socioeconomic and racial differences widened across the lifecourse.
Sources of Funding
This research was supported by grants from the National Institutes of Health R01 HD058502 (GEM), R01 HL122328 (GEM), R01 HL108723 (EC), F31 HL147509 (PHL), grants from the Canadian Institutes of Health Research 67191 (GEM) and 97872 (EC), and a Grant-In-Aid from the American Heart Association (GEM).
Footnotes
Declarations of interest
We, the authors, declare no conflict of interests.
This criterion is based on a power analysis showing that given the cell size of the reference group (White; n=1005), a cell size of 246 is necessary to detect a small effect size (d=.20, r=.10) with .80 statistical power.
For omnibus groups regions of significance test, race was indicator-coded rather than effect-coded (Hayes and Montoya, 2017).
Correlation coefficients were transformed from the reported exponentiated logit regression coefficients and their confidence intervals for ease of interpretation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott G, Backholer K, Peeters A, Thornton L, Crawford D, Ball K, 2014. Explaining educational disparities in adiposity: the role of neighborhood environments. Obesity 22, 2413–2419. [DOI] [PubMed] [Google Scholar]
- Addo J, Ayerbe L, Mohan KM, Crichton S, Sheldenkar A, Chen R, Wolfe CD, McKevitt C, 2012. Socioeconomic status and stroke: an updated review. Stroke 43, 1186–1191. [DOI] [PubMed] [Google Scholar]
- Adler NE, Stewart J, 2009. Reducing obesity: motivating action while not blaming the victim. Milbank Q. 87, 49–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alley DE, Seeman TE, Kim JK, Karlamangla A, Hu P, Crimmins EM, 2006. Socioeconomic status and C-reactive protein levels in the US population: NHANES IV. Brain. Behav. Immun. 20, 498–504. [DOI] [PubMed] [Google Scholar]
- Alter DA, Chong A, Austin PC, Mustard C, Iron K, Williams JI, Morgan CD, Tu JV, Irvine J, Naylor CD, for the SESAMI Study Group*, 2006. Socioeconomic Status and Mortality after Acute Myocardial Infarction. Ann. Intern. Med. 144, 82 10.7326/0003-4819-144-2-200601170-00005 [DOI] [PubMed] [Google Scholar]
- Bader MD, Purciel M, Yousefzadeh P, Neckerman KM, 2010. Disparities in neighborhood food environments: Implications of measurement strategies. Econ. Geogr. 86, 409–430. [DOI] [PubMed] [Google Scholar]
- Ball K, Timperio A, Crawford D, 2009. Neighbourhood socioeconomic inequalities in food access and affordability. Health Place 15, 578–585. [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, 2018. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation 137, e67. [DOI] [PubMed] [Google Scholar]
- Berge JM, Wall M, Hsueh T-F, Fulkerson JA, Larson N, Neumark-Sztainer D, 2015. The protective role of family meals for youth obesity: 10-year longitudinal associations. J. Pediatr. 166, 296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best LG, Zhang Y, Lee ET, Yeh J-L, Cowan L, Palmieri V, Roman M, Devereux RB, Fabsitz RR, Tracy RP, 2005. C-reactive protein as a predictor of cardiovascular risk in a population with a high prevalence of diabetes: the Strong Heart Study. Circulation 112, 1289–1295. [DOI] [PubMed] [Google Scholar]
- Black PH, 2003. The inflammatory response is an integral part of the stress response: Implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain. Behav. Immun. 17, 350–364. 10.1016/S0889-1591(03)00048-5 [DOI] [PubMed] [Google Scholar]
- Braveman P, Barclay C, 2009. Health disparities beginning in childhood: a life-course perspective. Pediatrics 124 Suppl 3, S163–75. 10.1542/peds.2009-1100D [DOI] [PubMed] [Google Scholar]
- Braveman P, Gottlieb L, 2014. The social determinants of health: it’s time to consider the causes of the causes. Public Health Rep. 129, 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braveman P, Kumanyika S, Fielding J, LaVeist T, Borrell LN, Manderscheid R, Troutman A, 2011. Health disparities and health equity: the issue is justice. Am. J. Public Health 101, S149–S155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F, Conti G, Heckman JJ, Moon SH, Pinto R, Pungello E, Pan Y, 2014. Early childhood investments substantially boost adult health. Science 343, 1478–1485. 10.1126/science.1248429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao A, Grilo CM, White MA, Sinha R, 2015. Food cravings mediate the relationship between chronic stress and body mass index. J. Health Psychol. 20, 721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Lee WK, Cavey L, Ho A, 2013. Role models and the psychological characteristics that buffer low- socioeconomic- status youth from cardiovascular risk. Child Dev. 84, 1241–1252. [DOI] [PubMed] [Google Scholar]
- Chen E, Matthews KA, Boyce WT, 2002. Socioeconomic differences in children’s health: how and why do these relationships change with age? Psychol. Bull. 128, 295. [DOI] [PubMed] [Google Scholar]
- Chen E, Miller GE, 2013. Socioeconomic status and health: mediating and moderating factors. Annu Rev Clin Psychol 9, 723–49. 10.1146/annurev-clinpsy-050212-185634 [DOI] [PubMed] [Google Scholar]
- Chen E, Miller GE, Shalowitz MU, Story RE, Levine CS, Hayen R, Sbihi H, Brauer M, 2017. Difficult Family Relationships, Residential Greenspace, and Childhood Asthma. Pediatrics 139, e20163056 10.1542/peds.2016-3056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Joseph L, Pilote L, 2013. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes. Rev. 14, 232–244. 10.1111/obr.12003 [DOI] [PubMed] [Google Scholar]
- Clarke P, O’Malley PM, Johnston LD, Schulenberg JE, 2009. Social disparities in BMI trajectories across adulthood by gender, race/ethnicity and lifetime socio-economic position: 1986–2004. Int. J. Epidemiol. 38, 499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Janicki- Deverts D, Chen E, Matthews KA, 2010. Childhood socioeconomic status and adult health. Ann. N. Y. Acad. Sci. 1186, 37–55. 10.1111/j.1749-6632.2009.05334.x [DOI] [PubMed] [Google Scholar]
- Coon KA, Goldberg J, Rogers BL, Tucker KL, 2001. Relationships between use of television during meals and children’s food consumption patterns. Pediatrics 107, e7–e7. [DOI] [PubMed] [Google Scholar]
- Costello-White R, Ryff CD, Coe CL, 2015. Aging and low-grade inflammation reduce renal function in middle-aged and older adults in Japan and the USA. Age 37, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, McEwen BS, 2012. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol. Behav., Allostasis and Allostatic Load 106, 29–39. 10.1016/j.physbeh.2011.08.019 [DOI] [PubMed] [Google Scholar]
- Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, Gallimore JR, Pepys MB, 2000. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. Bmj 321, 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delva J, Johnston LD, O’Malley PM, 2007. The epidemiology of overweight and related lifestyle behaviors: racial/ethnic and socioeconomic status differences among American youth. Am. J. Prev. Med. 33, S178–S186. [DOI] [PubMed] [Google Scholar]
- Delva J, O’Malley PM, Johnston LD, 2006. Racial/ethnic and socioeconomic status differences in overweight and health-related behaviors among American students: national trends 1986–2003. J. Adolesc. Health 39, 536–545. [DOI] [PubMed] [Google Scholar]
- Elks CM, Francis J, 2010. Central adiposity, systemic inflammation, and the metabolic syndrome. Curr. Hypertens. Rep. 12, 99–104. [DOI] [PubMed] [Google Scholar]
- Eloise-kate VL, Campbell KJ, Spence AC, 2017. Family meals with young children: an online study of family mealtime characteristics, among Australian families with children aged six months to six years. BMC Public Health 17, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlman MM, McCaughtry N, Martin J, Shen B, 2010. Racial and socioeconomic disparities in nutrition behaviors: targeted interventions needed. J. Nutr. Educ. Behav. 42, 10–16. [DOI] [PubMed] [Google Scholar]
- Ferrante A Jr, 2007. Obesity- induced inflammation: a metabolic dialogue in the language of inflammation. J. Intern. Med. 262, 408–414. [DOI] [PubMed] [Google Scholar]
- Fiese BH, Schwartz M, 2008. Reclaiming the family table: Mealtimes and child health and wellbeing. Soc. Policy Rep. 22, 1–20. [Google Scholar]
- Fleischhacker SE, Evenson KR, Rodriguez DA, Ammerman AS, 2011. A systematic review of fast food access studies. Obes. Rev. 12, e460–e471. [DOI] [PubMed] [Google Scholar]
- Ford C, Ward D, White M, 2012. Television viewing associated with adverse dietary outcomes in children ages 2–6. Obes. Rev. 13, 1139–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlenza MJ, Miller GE, 2006. Increased serum levels of 8-hydroxy-2′-deoxyguanosine in clinical depression. Psychosom. Med. 68, 1–7. [DOI] [PubMed] [Google Scholar]
- Fraser LK, Edwards KL, Cade J, Clarke GP, 2010. The geography of fast food outlets: a review. Int. J. Environ. Res. Public. Health 7, 2290–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenois F, Moreau M, O’Connor J, Lawson M, Micon C, Lestage J, Kelley KW, Dantzer R, Castanon N, 2007. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology 32, 516–531. 10.1016/j.psyneuen.2007.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller-Rowell TE, Curtis DS, Doan SN, Coe CL, 2015. Racial Disparities in the Health Benefits of Educational Attainment: A Study of Inflammatory Trajectories Among African American and White Adults. Psychosom. Med. 77, 33 10.1097/PSY.0000000000000128 [DOI] [PubMed] [Google Scholar]
- Gable S, Chang Y, Krull JL, 2007. Television watching and frequency of family meals are predictive of overweight onset and persistence in a national sample of school-aged children. J. Am. Diet. Assoc. 107, 53–61. [DOI] [PubMed] [Google Scholar]
- Hawkins NM, Jhund PS, McMurray JJV, Capewell S, 2012. Heart failure and socioeconomic status: accumulating evidence of inequality. Eur. J. Heart Fail. 14, 138–146. 10.1093/eurjhf/hfr168 [DOI] [PubMed] [Google Scholar]
- Hayes AF, Montoya AK, 2017. A Tutorial on Testing, Visualizing, and Probing an Interaction Involving a Multicategorical Variable in Linear Regression Analysis. Commun. Methods Meas. 11, 1–30. 10.1080/19312458.2016.1271116 [DOI] [Google Scholar]
- Henegar C, Tordjman J, Achard V, Lacasa D, Cremer I, Guerre-Millo M, Poitou C, Basdevant A, Stich V, Viguerie N, 2008. Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity. Genome Biol. 9, R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Ross KM, Chen E, Miller GE, 2017. Early-life socioeconomic disadvantage and metabolic health disparities. Psychosom. Med. 79, 514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandiah J, Yake M, Willett H, 2008. Effects of stress on eating practices among adults. Fam. Consum. Sci. Res. J. 37, 27–38. [Google Scholar]
- Karim MA, Kartsonaki C, Bennett DA, Millwood IY, Hill MR, Avery D, Bian Z, Du H, Guo Y, Qian Y, 2020. Systemic inflammation is associated with incident stroke and heart disease in East Asians. Sci. Rep. 10, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwarteng JL, Schulz AJ, Mentz GB, Israel BA, Shanks TR, Perkins DW, 2016. Neighbourhood poverty, perceived discrimination and central adiposity in the USA: independent associations in a repeated measures analysis. J. Biosoc. Sci. 48, 709–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson N, MacLehose R, Fulkerson JA, Berge JM, Story M, Neumark-Sztainer D, 2013. Eating breakfast and dinner together as a family: associations with sociodemographic characteristics and implications for diet quality and weight status. J. Acad. Nutr. Diet. 113, 1601–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasser KE, Himmelstein DU, Woolhandler S, 2006. Access to care, health status, and health disparities in the United States and Canada: results of a cross-national population-based survey. Am J Public Health 96, 1300–7. 10.2105/AJPH.2004.059402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun LA, LaVeist TA, 2011. Black/White racial disparities in health: a cross-country comparison of Canada and the United States. Arch. Intern. Med. 171, 1591–1593. [DOI] [PubMed] [Google Scholar]
- Leigh JA, Alvarez M, Rodriguez CJ, 2016. Ethnic Minorities and Coronary Heart Disease: an Update and Future Directions. Curr. Atheroscler. Rep. 18, 9 10.1007/s11883-016-0559-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinson ML, Teitler JO, Plaza R, Reichman NE, 2016. Income disparities in cardiovascular health across the lifespan. SSM-Popul. Health 2, 904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA, Gallo LC, 2011. Psychological perspectives on pathways linking socioeconomic status and physical health. Annu. Rev. Psychol. 62, 501–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA, Gallo LC, Taylor SE, 2010. Are psychosocial factors mediators of socioeconomic status and health connections? A progress report and blueprint for the future. Ann N Acad Sci 1186, 146–73. 10.1111/j.1749-6632.2009.05332.x [DOI] [PubMed] [Google Scholar]
- Miller GE, Brody GH, Yu T, Chen E, 2014a. A family-oriented psychosocial intervention reduces inflammation in low-SES African American youth. Proc. Natl. Acad. Sci. 111, 11287–11292. 10.1073/pnas.1406578111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Armstrong CC, Carroll AL, Ozturk S, Rydland KJ, Brody GH, Parrish TB, Nusslock R, 2018. Functional connectivity in central executive network protects youth against cardiometabolic risks linked with neighborhood violence. Proc. Natl. Acad. Sci. 115, 12063–12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ, 2011. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol. Bull. 137, 959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Cole SW, 2012. Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biol. Psychiatry 72, 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Murphy ML, Cashman R, Ma R, Ma J, Arevalo JM, Kobor MS, Cole SW, 2014b. Greater inflammatory activity and blunted glucocorticoid signaling in monocytes of chronically stressed caregivers. Brain. Behav. Immun. 41, 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed-Ali V, Pinkney JH, Coppack SW, 1998. Adipose tissue as an endocrine and paracrine organ. Int. J. Obes. 22, 1145–1158. [DOI] [PubMed] [Google Scholar]
- Morris MJ, Beilharz JE, Maniam J, Reichelt AC, Westbrook RF, 2015. Why is obesity such a problem in the 21st century? The intersection of palatable food, cues and reward pathways, stress, and cognition. Neurosci. Biobehav. Rev. 58, 36–45. [DOI] [PubMed] [Google Scholar]
- Muscatell KA, Brosso SN, Humphreys KL, 2018. Socioeconomic status and inflammation: a meta-analysis. Mol. Psychiatry 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCHS, 2017. National Ambulatory Medical Care Survey: 2015 state and national summary tables. Hyattsville MD NCHS. [Google Scholar]
- Neumark-Sztainer D, Hannan PJ, Story M, Croll J, Perry C, 2003. Family meal patterns: associations with sociodemographic characteristics and improved dietary intake among adolescents. J. Am. Diet. Assoc. 103, 317–322. [DOI] [PubMed] [Google Scholar]
- Nusslock R, Miller GE, 2016. Early-life adversity and physical and emotional health across the lifespan: A neuroimmune network hypothesis. Biol. Psychiatry 80, 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Lin W, Wang W, Qi X, Wang D, Tang M, 2013. The effects of central pro-and anti-inflammatory immune challenges on depressive-like behavior induced by chronic forced swim stress in rats. Behav. Brain Res. 247, 232–240. 10.1016/j.bbr.2013.03.031 [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon III RO, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, 2003. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 107, 499–511. [DOI] [PubMed] [Google Scholar]
- Pirkola J, Vääräsmäki M, Ala-Korpela M, Bloigu A, Canoy D, Hartikainen A-L, Leinonen M, Miettola S, Paldanius M, Tammelin TH, 2010. Low-grade, systemic inflammation in adolescents: association with early-life factors, gender, and lifestyle. Am. J. Epidemiol. 171, 72–82. [DOI] [PubMed] [Google Scholar]
- Ridker PM, 2007. Inflammatory biomarkers and risks of myocardial infarction, stroke, diabetes, and total mortality: implications for longevity. Nutr. Rev. 65, S253–S259. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Marin TJ, Ma R, Miller GE, 2009. Biologic cost of caring for a cancer patient: dysregulation of pro-and anti-inflammatory signaling pathways. J Clin Oncol 27, 2909–2915. [DOI] [PubMed] [Google Scholar]
- Rollins BY, Belue RZ, Francis LA, 2010. The beneficial effect of family meals on obesity differs by race, sex, and household education: the national survey of children’s health, 2003–2004. J. Am. Diet. Assoc. 110, 1335–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier H, Chen E, 2013. Socioeconomic status and the health of youth: a multilevel, multidomain approach to conceptualizing pathways. Psychol. Bull. 139, 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman T, Epel E, Gruenewald T, Karlamangla A, McEwen BS, 2010. Socio-economic differentials in peripheral biology: cumulative allostatic load. Ann N Acad Sci 1186, 223–39. 10.1111/j.1749-6632.2009.05341.x [DOI] [PubMed] [Google Scholar]
- Singh GK, Kogan MD, Van Dyck PC, Siahpush M, 2008. Racial/ethnic, socioeconomic, and behavioral determinants of childhood and adolescent obesity in the United States: analyzing independent and joint associations. Ann Epidemiol 18, 682–95. 10.1016/j.annepidem.2008.05.001 [DOI] [PubMed] [Google Scholar]
- Stawski RS, Scott SB, Zawadzki MJ, Sliwinski MJ, Marcusson-Clavertz D, Kim J, Lanza ST, Green PA, Almeida DM, Smyth JM, 2019. Age differences in everyday stressor-related negative affect: A coordinated analysis. Psychol. Aging 34, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J, Katz EG, Huxley RR, 2010. Associations between gender, age and waist circumference. Eur. J. Clin. Nutr. 64, 6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stults-Kolehmainen MA, Sinha R, 2014. The effects of stress on physical activity and exercise. Sports Med. 44, 81–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubbs CY, Roy KM, Burton LM, 2005. Family ties: Constructing family time in low- income families. Fam. Process 44, 77–91. [DOI] [PubMed] [Google Scholar]
- Wardle J, Chida Y, Gibson EL, Whitaker KL, Steptoe A, 2011. Stress and Adiposity: A Meta-Analysis of Longitudinal Studies. Obesity 19, 771–778. 10.1038/oby.2010.241 [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann DC, Desai M, Rosenbaum M, Leibel RL, Ferrante AW, 2003. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RJ, Finn P, Contreras JP, Cohen S, Wright RO, Staudenmayer J, Wand M, Perkins D, Weiss ST, Gold DR, 2004. Chronic caregiver stress and IgE expression, allergen-induced proliferation, and cytokine profiles in a birth cohort predisposed to atopy. J. Allergy Clin. Immunol. 113, 1051–1057. 10.1016/j.jaci.2004.03.032 [DOI] [PubMed] [Google Scholar]
- Yang YC, Gerken K, Schorpp K, Boen C, Harris KM, 2017. Early-Life Socioeconomic Status and Adult Physiological Functioning: A Life Course Examination of Biosocial Mechanisms. Biodemography Soc. Biol. 63, 87–103. 10.1080/19485565.2017.1279536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yzerbyt VY, Muller D, Judd CM, 2004. Adjusting researchers’ approach to adjustment: On the use of covariates when testing interactions. J. Exp. Soc. Psychol. 40, 424–431. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.