Highlights
-
•
This study describes for the first time the clinical course of SARS-CoV-2 infection in a male patient with Good's syndrome.
-
•
Absence of peripheral B cells may not always correlate with mild clinical course of COVID-19.
-
•
Immunological findings in COVID-19 may not apply to all clnical settings in the same manner.
Keywords: SARS-CoV-2, Good's sydrome (GS), COVID-19
To the Editor
Good's syndrome (GS) is a rare disorder that typically affects both sexes after the 5th decade of life. GS is characterized by the presence of thymoma associated with hypogammaglobulinemia and low to absent peripheral B cells in most cases [1,2]. In addition, altered T cell function in the presence of normal or elevated T cell counts has been reported [1,2]. Opportunistic and viral infections may complicate the clinical course of affected patients and frequently lead to exitus [1,2]. Recently, a novel virus, namely SARS-CoV-2, has been identified as responsible for a pandemic causing millions of affected patients and more than 1 million of deaths worldwide [3]. To date, no cases of patients with GS and COVID-19 have been reported. We report on the first GS patient that was infected with SARS-CoV-2 and albeit appropriate treatment was initiated, he succumbed to the infection.
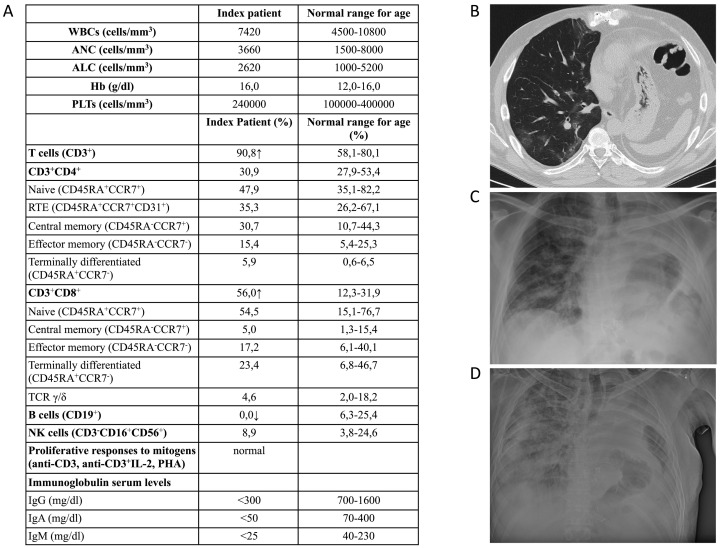
The index patient was a 51 years old man with a positive maternal family history for thymoma and myasthenia gravis. At the age of 48, he was diagnosed with thymoma and underwent surgical excision and left pneumonectomy. Upon dismissal, he presented recurrent respiratory and gastrointestinal infections. The first immunological evaluation showed a reduction of the gamma-globulin peak in serum electrophoresis with reduced immunoglobulin serum levels: IgG = 351 mg/dl, IgA <8 mg/dl, IgM = 6 mg/dl. He came to our attention at the age of 49. His immunological work-up is summarized in Fig. 1A. Briefly, while his differential white blood cell count was normal, evaluation of peripheral lymphocyte subsets showed inversion of the CD4/CD8 ratio with complete lack of peripheral B cells. Proliferative responses to mitogens were normal. Immunoglobulin serum levels were extremely low for all classes (Fig. 1A). The patient was diagnosed with Good's syndrome (GS) and was put on facilitated subcutaneous immunoglobulin replacement treatment, with good control of the respiratory infections.
Fig. 1.
Immunological and imaging evaluation of the GS index patient with COVID-19. A. Patient's immunological evaluation at diagnosis of GS. B. High resolution computed tomography performed at admission shows multiple small ground-glass opacities located mainly at the right lower lobe. C. Scattered pulmonary opacities can be appreciated at day 4. D. Chest X-ray at day 12 showing progressive extension and consolidation of lung opacities.
The patient encountered SARS-CoV-2 during the second wave in Italy in November 2020. His initial symptoms were low grade fever, myalgias and asthenia. Nasopharyngeal swab resulted positive for SARS-CoV-2 and the patient was put on combined home treatment with enoxaparin, azythromycin and prednisolone. His clinical conditions deteriorated after two days with development of breathing difficulty and was thus admitted to the hospital. While the chest X-ray at admission did not show any opacities in the right lung, lung HRTC revealed the presence of multiple small ground-glass opacities both peripheral and central, mainly at the lower lobe (Fig. 1B). Mild hypoxemia (PaO2 77 mmHg) with hypocapnia (PaCO2 24.4 mmHg) and respiratory alkalosis (pH 7.51) were present. While a moderate increase in CRP (8.38 mg/dl; normal values <3 mg/dl) was noted, D-dimer levels (231 ng/ml; normal values <250), absolute lymphocyte counts (1200 cells/ul; normal values: 900–4000) renal and liver function, LDH and CK were within normal range. Low flow O2 with nasal cannula (FiO2 28%) was started with normalization of hypoxemia and hypocapnia. The patient was put on intravenous dexametasone (6 mg), enoxaparin 6000 UI/daily was maintained, while azythromycin 500 mg/daily was stopped after six days. According to local COVID-19 treatment guidelines, Remdesivir was not administered due to the interval from symptoms' onset.
The patient responded positively for the following four days, remained afebrile, with normalization of CRP (<3 mg/dl). Ferritin was 659 ng/ml, while IgG were 1022 mg/dl. Oxygen saturation was 96%. Nonetheless, control chest x-ray at day 4 showed some scattered pulmonary opacities on the right lung (Fig. 1C). Starting on day 6 after admission, the index patient presented breathing difficulty, with an increase of respiratory rate (32/min) that required high flow O2 administration (fiO2 50 to 90%). CRP rapidly increased to 19 mg/dl, together with D-Dimer (779 ng/ml) and LDH (554 U/L). Lymphocytes showed transient and mild decrease. Procalcitonin was negative and so were NtproBNP and t-troponin. Blood cultures, Legionella/Pneumococcus urinary antigens and blood galactomann antigen were negative. IgG plasma levels decreased to 790 mg/dl. Broad-spectrum antibiotic therapy with Piperacillin-Tazobactam, Linezolid and Levofloxacin was initiated empirically, and intravenous immunoglobulins (30 g) were administered.
Invasive ventilation with helmet CPAP was started due to severe hypoxemia in O2 reservoir bag 15 l/min (PaO2 64 mmHg P/F 71) but was not tolerated well from the patient. Pronation protocol was also attempted, even though not well tolerated by the patient. Oro-tracheal intubation was excluded by ICU specialist because of underlying oncological disease and anatomical condition. Subsequent chest X-ray showed progressive extension and consolidation of lung opacities, leading to a “white lung” (Fig. 1D). Palliative therapy to relieve severe respiratory distress was started 24 h before the death that occurred at day 13.
Recent evidence from patients affected with primary immunodeficiencies that developed COVID-19 suggests that the lack of B cells, as observed in agammaglobulinemic patients (X-linked agammaglobulinemia, XLA; autosomal recessive agammaglobulinemia, ARA), may play a protective role on the evolution of the SARS-CoV-2 infection [4,5]. Nonetheless, this was not the case for the index patient that completely lacked peripheral B cells but presented a severe clinical course. Considering the diversity of these conditions, GS vs XLA/ARA, this different clinical outcome may be due to T cell, NK cell or other immune cell dysfunction in the setting of GS but may also be related to anatomical alterations due to thymoma and/or lung surgery.
In conclusion, we report on the first patient with GS that developed COVID-19 and despite medical treatment, the viral infection resulted fatal. This should underline the fact that the immunological findings so far reported in COVID-19 patients may not be applicable in all conditions and therefore cautiousness should always be used when SARS-CoV-2 infection affects patients with rare diseases such as GS.
Funding
The research leading to these results has received funding from the European Community's Seventh Framework Programme FP7/2007–2013 under grant agreement no 201549 (EURO-PADnet HEALTH-F2-2008-201549) and from the Italian Ministerial Grant GR-2010-2315762. The research leading to these results also received funding from the “Fondazione C. Golgi”, Brescia, Italy and the Jeffrey Modell Foundation.
Capsule summary
This study describes for the first time the clinical course of SARS-CoV-2 infection in a male patient affected with Good's syndrome.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
We would like to thank the patient, the patient's family and the nurses for all their efforts. Several of us are members of the European Reference Network for Rare Immunodeficiency, Autoinflammatory and Autoimmune Diseases (project identification No. 739543).
References
- 1.Kelesidis T., Yang O. Good’s syndrome remains a mystery after 55 years: a systematic review of the scientific evidence. Clin. Immunol. 2010 Jun;135(3):347–363. doi: 10.1016/j.clim.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malphettes M., Gérard L., Galicier L., Boutboul D., Asli B., Szalat R., Perlat A., Masseau A., Schleinitz N., Le Guenno G., Viallard J.F., Bonnotte B., Thiercelin-Legrand M.F., Sanhes L., Borie R., Georgin-Lavialle S., Fieschi C., Oksenhendler E. DEFicit Immunitaire de l’adulte study group. Good syndrome: an adult-onset immunodeficiency remarkable for its high incidence of invasive infections and autoimmune complications. Clin. Infect. Dis. 2015 Jul 15;61(2):e13–e19. doi: 10.1093/cid/civ269. [DOI] [PubMed] [Google Scholar]
- 3.Yuki K., Fujiogi M., Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin. Immunol. 2020 Jun;215 doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quinti I., Lougaris V., Milito C., Cinetto F., Pecoraro A., Mezzaroma I., Mastroianni C.M., Turriziani O., Bondioni M.P., Filippini M., Soresina A., Spadaro G., Agostini C., Carsetti R., Plebani A. A possible role for B cells in COVID-19? Lesson from patients with agammaglobulinemia. J. Allergy Clin. Immunol. 2020 Jul;146(1):211–213.e4. doi: 10.1016/j.jaci.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyts I., Bucciol G., Quinti I., Neven B., Fischer A., Seoane E., Lopez-Granados E., Gianelli C., Robles-Marhuenda A., Jeandel P.Y., Paillard C., Sankaran V.G., Demirdag Y.Y., Lougaris V., Aiuti A., Plebani A., Milito C., Dalm V.A., Guevara-Hoyer K., Sánchez-Ramón S., Bezrodnik L., Barzaghi F., Gonzalez-Granado L.I., Hayman G.R., Uzel G., Mendonça L.O., Agostini C., Spadaro G., Badolato R., Soresina A., Vermeulen F., Bosteels C., Lambrecht B.N., Keller M., Mustillo P.J., Abraham R.S., Gupta S., Ozen A., Karakoc-Aydiner E., Baris S., Freeman A., Yamazaki-Nakashimada M., Scheffler-Mendoza S., Espinosa-Padilla S., Gennery A.R., Jolles S., Espinoza Y., Poli M.C., Fieschi C., Hauck F., Cunningham-Rundles C., Mahlaoui N., IUIS Committee of Inborn Errors of Immunity, Warnatz K., Sullivan K.E., Tangye S.G. Coronavirus Disease 2019 in patients with inborn errors of immunity: an international study. J. Allergy Clin. Immunol. 2020 Sep 24 doi: 10.1016/j.jaci.2020.09.010. S0091-6749(20)31320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]