Summary
Perforator selection is of paramount importance when performing a Deep Inferior Epigastric Perforator flap. Technological advancements within imaging modalities have proved invaluable in preoperative planning and intraoperative assessment. Computed tomographic angiography remains the gold standard for preoperative perforator mapping, while color ultrasound Doppler is considered more reliable for determining vessel caliber. Intraoperatively, an imaging modality that provides sequential, real-time assessment of various perforators’ supply to the flap would provide helpful insight to determine which perforator will optimize flap viability, especially of the most distal, lateral margins. Multispectral imaging, a variant of near infrared imaging, has emerged as an alternative method to assess tissue viability in the operating room as well as postoperatively. Unlike Spy technology, which is invasive and cost ineffective, the SnapshotNIR (KD203) is a handheld multispectral imaging device utilizing NIR to measure the oxygenation of the hemoglobin in the area to calculate the tissue oxygen content (StO2) displayed in a color image. The following case of a 46-year-old woman undergoing tertiary breast reconstruction for treatment of progressive grade 2 capsular contracture illustrates the utility and ease of KD203 application to intra-operative perforator determination in deep inferior epigastric perforator flap assessment.
Introduction
In 1994, Allen and Treece reported the first Deep Inferior Epigastric Perforator (DIEP) flap in autologous breast reconstruction.1 Compared with the transverse rectus abdominis myocutaneous flap, this technique relies on meticulous dissection to isolate the desired perforator(s) to supply the flap while sparing the rectus muscle and fascia. In addition to the added benefit of an abdominoplasty, the DIEP flap has been found to decrease donor site morbidity.2 The challenge remains to identify the ideal perforator that will optimize flap viability, especially of the most distal, lateral margins. Technological advancements within imaging modalities have proved invaluable in preoperative planning and intraoperative assessment. Computed tomographic angiography remains the gold standard for preoperative perforator localization and mapping while color ultrasound Doppler, although operator dependent, has been found to be superior for assessing vessel caliber.3,4 Indocyanine Green Fluorescence (ICG) imaging, such as Spy technology, is commonly utilized to visualize tissue perfusion for mastectomy flap assessment in breast reconstruction and more recently, it is providing utility in free flap assessment. Intraoperative indocyanine green fluorescence perfusion assessment decreases rates of postoperative necrosis and flap loss,5 but not without its own disadvantages. Indocyanine green fluorescence is invasive, contraindicated in patients with liver disease and uremia, limited in its sequential monitoring capabilities, introduces risk of anaphylaxis, and is expensive to upkeep.6
Multispectral imaging (MSRI) and hyperspectral technologies have emerged as alternative methods to assess tissue viability in the operating room as well as postoperatively. MSRI technology emits specific near infrared light (NIR) wavelengths to the area of interest and measures the amount reflected back. Oxygenated and deoxygenated hemoglobin absorb NIR differently, thus enabling MSRI to provide a measure of tissue oxygen content (StO2) for the imaged area. Hyperspectral technologies are similar to the aforementioned MSRI, except in their utilization of visible light wavelength spectrums.7 NIR penetrates tissues more deeply than visible light and is less affected by high epidermal melanin content, providing a more accurate measurement of darker skin types.
The SnapshotNIR (KD203) received FDA clearance in 2017 for non-invasive tissue oxygenation assessment. This lightweight, hand-held MSRI device captures color pictures with their associated StO2, providing sequential, real-time assessment of the tissue to aid with viability determination, without the need for intravenous injection. In a head-to-head comparison, MSRI was found to be as effective as SPY at predicting skin necrosis while also being more cost effective.8
The following case illustrates the utility and ease of KD203 application to intra-operative perforator determination in DIEP flap assessment.
Patient
A 46-year-old woman, having a body mass index of 39.5 kg/m2 with no other significant past medical history, underwent unilateral mastectomy with 2-stage expander to implant reconstruction for stage II invasive ductal carcinoma, ER+, PR+, and Her2+. Following radiation treatment, she presented with progressive grade 2 capsular contracture with deformity and worsening pain, prompting tertiary reconstruction with a DIEP free flap.
Technique
An MRA of the pelvis with and without contrast was obtained before surgery for perforator visualization. The bilateral deep inferior epigastric arteries were successfully visualized with no occlusion present on either side. The MRA revealed 3 perforator branches on the right and 2 on the left. An image of the flap was taken before incision to visualize the oxygenation status of the undisturbed tissue (Fig. 1). When using the device, specific locations on the image can be chosen to show their associated StO2. Synchronous dissection of the internal mammary vessels and harvesting of the abdominal flap commenced by a 2 surgeon team. The flap was raised from lateral to medial until the most lateral row of perforators were visualized on either side. The deep inferior epigastric perforators were dissected out using careful scissor dissection augmented with monopolar cautery according to the technique described by Buchel, who personally taught the author this technique. Various perforators identified by MRA were isolated. An image was taken after all identified perforators were clamped for 5 minutes (Fig. 2A). A subsequent image was taken 5 minutes after the selected perforator was unclamped, revealing the extent of its oxygenation of the flap (Fig. 2B). The other perforators remained clamped during imaging. The perforator providing the most thorough oxygenation of the flap was selected on the patient’s left side, and the flap was harvested based on this single perforator.
Fig. 1.
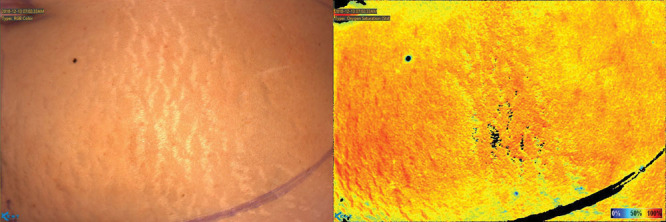
Preoperative image of the hemi-abdomen. The color scale corresponds to StO2 percentages, with black being 0%, deep blue ≤ 25%, and deep red 100%.
Fig. 2.
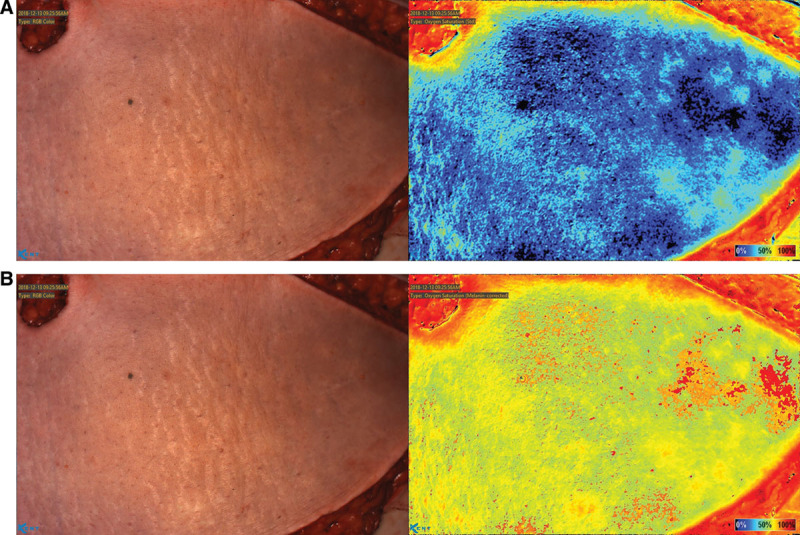
Imaging of flap while perforators are clamped. A, Effect of clamping all identified perforators for 5 minutes, on flap StO2. B, Flap StO2 5 minutes after the selected perforator was unclamped.
Direct end-to-end anastomosis of the internal mammary artery with the free flap arterial perforator was performed with 9-0 nylon. The respective veins were coupled using a 2.5 mm coupler. The flap rapidly regained perfusion, was warm and healthy. Zones 1, 2, and part of 3 were utilized based upon the needed volume. The donor site and breast were closed in layers. A ViOptix monitor was applied to the area identified to have the strongest perforator signal, through the skin by KD203 imaging. The site was found to have 60% saturation with 95% signal strength. A final image of the breast was taken before the patient left the operating room (Fig. 3). The patient experienced no complications during her hospital admission, maintaining a ViOptix reading >70% and drain output decreasing steadily throughout. She was discharged home on postoperative day 4.
Fig. 3.
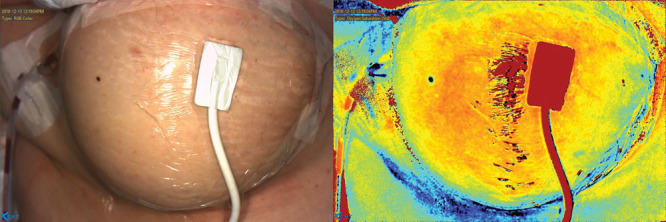
Final image of the breast with a ViOptix monitor in place, before patient leaving the operating room.
Discussion
Over 20 years since their introduction for use in breast reconstruction, DIEP flaps are considered to be a desired option in autologous breast reconstruction, but the ever-present concern is perforator selection. Patel and Keller concluded that the largest caliber vessel is best suited to perfuse the flap, consistent with the current general consensus of 1.5-mm diameter.9 With multiple perforators meeting such criteria, the next step is determining which will supply the flap best. In some institutions, the Spy is utilized to view perfusion of the flap. No cases have been reported in which various perforators were clamped and then specifically assessed in their ability to perfuse the flap. A unique intra-operative modality reported by de Weerd et al is the use of dynamic infrared thermography. He describes subjecting the flap to a thermal cold challenge and subsequently taking images with an infrared camera to capture the rate and pattern of skin rewarming to localize the most robust perforators.10 The ViOptix, another device utilizing NIR, is often utilized during the hospital admission following vascular anastomosis of a free flap for continuous monitoring of tissue oxygen saturation, as this is a critical time period for flap viability. This is the first reported case to utilize the KD203 for intra-operative perforator assessment, providing real-time oxygenation values of the tissue to assist with perforator selection and flap margins. Additional studies with long-term follow-up of flap survival are needed to assess the efficacy of this perforator selection technique.
Conclusion
The KD203 effectively provides intra-operative visualization of flap oxygenation supplied by various perforators, thus assisting with the determination of the vessel that will supply the flap efficiently in its entirety.
ACKNOWLEDGMENT
The authors acknowledge the Research Open Access Publishing (ROAAP) Fund of the University of Illinois at Chicago for financial support towards the open access publishing fee for this article.
Footnotes
Published online 25 November 2020.
Disclosure: Dr. Jones is a speaker for LifeCell and Allergan. The remaining authors have no financial interest to declare.
Statement of Conformity:
We affirm that this case report is in accordance with the Helsinki declaration. As it is a report of one patient’s procedure, IRB approval was not warranted.
References
- 1.Allen RJ, Treece P. Deep inferior epigastric perforator flap for breast reconstruction. Ann Plast Surg. 1994;32:32–38. [DOI] [PubMed] [Google Scholar]
- 2.Knox AD, Ho AL, Leung L, et al. Comparison of outcomes following autologous breast reconstruction using the DIEP and pedicled TRAM flaps: a 12-year clinical retrospective study and literature review. Plast Reconstr Surg. 2016;138:16–28. [DOI] [PubMed] [Google Scholar]
- 3.Aubry S, Pauchot J, Kastler A, et al. Preoperative imaging in the planning of deep inferior epigastric artery perforator flap surgery. Skeletal Radiol. 2013;42:319–327. [DOI] [PubMed] [Google Scholar]
- 4.Cina A, Salgarello M, Barone-Adesi L, et al. Planning breast reconstruction with deep inferior epigastric artery perforating vessels: multidetector CT angiography versus color Doppler US. Radiology. 2010;255:979–987. [DOI] [PubMed] [Google Scholar]
- 5.Phillips BT, Lanier ST, Conkling N, et al. Intraoperative perfusion techniques can accurately predict mastectomy skin flap necrosis in breast reconstruction: results of a prospective trial. Plast Reconstr Surg. 2012;129:778e–88e. [DOI] [PubMed] [Google Scholar]
- 6.Smeeth L, Thomas SL, Hall AJ, et al. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–2618. [DOI] [PubMed] [Google Scholar]
- 7.Chin MS, Chappell AG, Giatsidis G, et al. Hyperspectral imaging provides early prediction of random axial flap necrosis in a preclinical model. Plast Reconstr Surg. 2017;139:1285e–1290e. [DOI] [PubMed] [Google Scholar]
- 8.Jones GE, Yoo A, King VA, et al. Snapshot multispectral imaging is not inferior to SPY laser fluorescence imaging when predicting murine flap necrosis. Plast Reconstr Surg. 2020;145:85e–93e. [DOI] [PubMed] [Google Scholar]
- 9.Patel SA, Keller A. Theoretical model describing arterial flow in the DIEP flap related to number and size of perforator vessels. J Plast Reconstr Aesthet Surg. 2008;61:1316–1320. [DOI] [PubMed] [Google Scholar]
- 10.de Weerd L, Weum S, Mercer JB. The value of dynamic infrared thermography (DIRT) in perforator selection and planning of free DIEP flaps. Ann Plast Surg. 2009;63:247–249. [DOI] [PubMed] [Google Scholar]


