Abstract
Background:
Three-dimensional (3D) camera systems are increasingly used for computerized volume calculations. In this study we investigate whether the Vectra XT 3D imaging system is a reliable tool for determination of breast volume in clinical practice. It is compared with the current gold standard in literature, magnetic resonance imaging (MRI), and current clinical practice (plastic surgeon’s clinical estimation).
Methods:
Breast volumes of 29 patients (53 breasts) were evaluated. 3D images were acquired by Vectra XT 3D imaging system. Pre-existing breast MRI images were collected. Both imaging techniques were used for volume analyses, calculated by two independent investigators. Breast volume estimations were done by plastic surgeons during outpatient consultations. All volume measurements were compared using paired samples t-test, intra-class correlation coefficient, Pearson’s correlation, and Bland–Altman analysis.
Results:
Two 3D breast volume measurements showed an excellent reliability (intra-class correlation coefficient: 0.991), which was comparable to the reliability of MRI measurements (intra-class correlation coefficient: 0.990). Mean (SD) breast volume measured with 3D breast volume was 454 cm3 (157) and with MRI was 687 cm3 (312). These volumes were significantly different, but a linear association could be found: y(MRI) = 1.58 × (3D) – 40. Three-dimensional breast volume was not significantly different from volume estimation made by plastic surgeons (472 cm3 (69), P = 0.323).
Conclusions:
The 3D imaging system measures lower volumes for breasts than MRI. However, 3D measurements show a linear association with MRI and have excellent reliability, making them an objective and reproducible measuring method suitable for clinical practice.
Introduction
Breast volumetry is an important tool in breast reconstructive surgery. Accurate breast volume assessment is needed for pre-operative planning and follow-up results. It influences the degree of breast reduction, the choice of breast implants, or the amount of tissue needed for autologous breast reconstruction, and is used for progression control in autologous fat transplantation.1,2
There is no widely accepted technique for breast volume measurement due to a lack of information regarding the accuracy and comparability of each method. Many have not met the requirements of reproducibility, patient compliance, and cost efficiency. This has limited the use of breast volume measurement methods in routine clinical practice.3
Many measurement techniques have been proposed to determine breast volume. Available techniques include medical imaging modalities (eg, mammography, magnetic resonance imaging (MRI), and computed tomography), casting, anthropometric measurements, and three-dimensional (3D) imaging.2,4–7 Despite the fact that the community accepts water displacement of excided breast tissue as the gold standard,7 a review of different measurement techniques for breast tissue has led to a different conclusion.3 Most available methods to measure breast volume are associated with a large (>200 ml) uncertainty in breast volume.5,6,8,9 MRI scanning consistently demonstrated the highest accuracy, reporting errors lower than 10%, and highest reproducibility.1 It was regarded as the best method available to determine the breast volume. However, this technique is relatively expensive and time-consuming.
3D imaging is regaining popularity worldwide. Volumetric analysis can be used to document changes in breast morphology and is described in several studies.10–13 However, there are a few studies available directly comparing 3D breast volume with MRI measured volume of the breast, which is the best objective standard for breast volume. Kovacs et al. have a limited study population of 12 breasts in 6 patients.6 Koch et al. described a correlation between 3D imaging and MRI in only 22 women.14 Others lack an objective gold standard or a sufficient study population.
As a result, objective breast volume measurement is not often performed in clinical practice. 3D imaging is however well-tolerated, non-invasive, and performed within a short time and easy to handle. Plastic surgeons now prefer to use their clinical judgment to estimate breast volume instead. This might lead to subjective results of breast volume, which makes operative planning more difficult and could eventually even lead to post-operative asymmetry.
In this study we want to investigate whether the Vectra XT 3D imaging is a reliable tool for objective breast volume determination in clinical practice. First, 3D breast volumes will be compared with MRI measurements because the authors regard this as the current gold standard in literature. Second, 3D measurements will be compared with the surgeon’s estimation of breast volume, which reflects the current standard in clinical practice.
Materials and Methods
Study Design
Inclusion criteria for this study were: >18 years of age, patients undergoing prophylactic, oncologic, unilateral or bilateral mastectomy or lumpectomy followed by breast reconstruction, and availability of a recent breast MRI scan. Exclusion criteria were breast implants, tissue expanders, or bilateral mastectomy in the past.
No breast surgery had occurred between MR image acquisition and breast volume assessment by the plastic surgeon and 3D breast measurements. Estimation of both breast volume and 3D-images are part of standard care at our institute. The study was approved by the institute’s medical ethical committee. After providing written informed consent, surgical estimation values, 3D-images, and MRI-scans were acquired and collected in a central database.
3D Imaging
A Vectra XT 3D imaging system (Canfield Imaging Systems, Fairfield, N.J.) is used as part of standard care at our hospital. 3D images are taken after every pre-operative consultation for breast reconstruction. The breasts were photographed in standing position with the arms positioned akimbo. The camera system is adjustable to the body height. It contains six color cameras positioned in a triangulated configuration. This camera system can capture images in 180 degrees. Vectra software (Vectra Breast Sculptor, v 5.5.7, Canfield Scientific Inc) automatically processes the images into a high-resolution 3D image model. These images were used for calculation of breast volumes.
To determine breast boundaries, the Vectra XT software automatically identifies landmarks on the following locations: sternal notch (SN), midclavicular, nipple, areola, medial mammary fold (MMF), and lateral mammary fold (LMF). If automatic landmark detection was unsuccessful or unsatisfactory, the landmarks were manually placed or adjusted. From these landmarks, a region of interest (ROI) is formed (see Fig. 1A). The ROI was formed by a medial border, cranial border, lateral border, and caudal border. The medial border comes from a fractional line (0.7) from the midpoint of the MMF landmarks to SN. The caudal border is derived from a circle formed by the MMF, IMF, and LMF landmarks, with an extra radius of factor 1.22 for a good measure. The lateral end goes little past the LMF point—the MMF to LMF circle is divided into 12 segments and then there is 1 additional segment past the LMF. The cranial border comes from the SN to midclavicular, the length of the upper margin is a fractional line (0.8) from MMF to LMF. The lateral border is formed by the shortest path from the lateral end of the cranial border to the lateral end of the caudal border. The main challenge with trunk surface 3D scans is the delimitation of the breast’s dorsal boundary called the “chest wall.” The chest wall plane is a curved plane that matches the patient’s torso. The internal boundary is set at the skin level and guided by the shape of the skin surface around the breast.
Fig. 1.
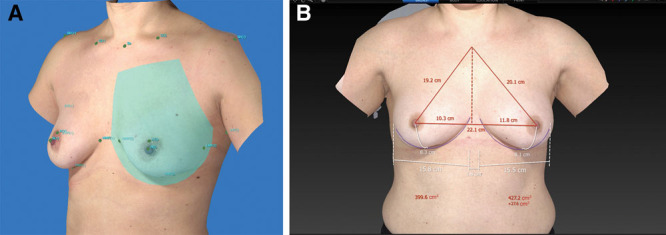
Three-dimensional image of the breast. A, ROI is defined by the landmarks of the breast. B, Automatic calculation of breast volume with landmarks, using Vectra Breast Sculptor.
Now, the volume is calculated from the ROI as closed object with the defined chest wall. The Vectra XT 3D imaging system has the algorithm for calculation of breast volume (a closed object of ROI and defined chest wall) using the previous described method integrated in its system. It is processed automatically and takes only a few seconds (Fig. 1B).
Assessments of breast volume for 3D images were done independently by two investigators (RK and MP) of this study. Both investigators were blinded for breast MRI measurements, the surgeon’s estimation of breast volume, and for the 3D analysis from the other investigator.
Breast MRI
Breast MRI examinations were performed for assessing disease extent in invasive lobular carcinoma, response monitoring during neoadjuvant chemotherapy, or breast cancer screening in high risk patients (eg, BRCA gene mutation carriers). At our institute, a Philips Ingenia 1.5T MRI system is used. Also existing MRI-scans from other hospitals, when available, were used in case they were the most recent scans. Volumes were calculated on T2-weighted sequences, as these were considered to best represent the anatomic landmarks needed for this measurement. There were no relevant differences in the sequence protocol settings of the different systems (Table 1). One MRI image (one breast image) was excluded because there was only a T1-weighted sequence available and parameters were different from other scans. First, multiplanar reformatted images of the breast with a thickness of 3 mm was created. Then, the cranial and caudal boundaries of the breast were defined by drawing an ROI on the sagittal plane at the level of the nipple. For this ROI, the breast contour was followed using the borders of the convexity of the breast to define the lower and upper borders (Fig. 2A). Next, a switch was made to the axial plane and, using the previously defined borders, new ROIs were drawn at the most cranial and caudal image. Using a similar method as in the sagittal plane, the breast contours were followed and the medial and lateral boundaries were defined by drawing the line perpendicular to the major pectoral muscle. Between these upper and lower boundaries, additional ROIs were drawn on the axial images (Fig. 2B). As a rule of thumb every fourth slice was used, unless there were significant alterations in the breast contour. The software Syngo.via (Siemens Healthcare GmbH, Erlangen, Germany) could interpolate the predefined ROIs into a continuous volume of interest, computed as cubic centimeters (cm3). These results were subsequently verified and if necessary “the nudge tool” was used for small corrections. All measurements were conducted by two independent and blinded radiology residents (N. dV, C. vB), who are specialized in breast imaging.
Table 1.
Sequence Parameters of T2w MR Images Used for Measuring Breast Volume
| Institute 1 | Institute 2 | Institute 3 | Institute 4 | |
|---|---|---|---|---|
| No. scans | 25 | 1 | 1 | 1 |
| Vendor | Philips Ingenia | Siemens Spectra | Philips Achieva | Philips Ingenia |
| Sequence | T2w TSE (VISTA) 3D scanmode | T2w TSE 2D scanmode | T2w TSE 2D scanmode | T2 TSE 2D scanmode |
| Field strength | 1.5 T | 3.0 T | 1.5 T | 1.5 T |
| Coil | 16-channel breast coil | 16-channel breast coil | 7-channel breast coil | 16-channel breast coil |
| Echo time (TE) | 225 ms | 75 ms | 120 ms | 120 ms |
| Repetition time (TR) | 2000 ms | 5000 ms | 5827 ms | 3789 ms |
| Flip angle (FA) | 90 degrees | 80 degrees | 90 degrees | 90 degrees |
| In-plane resolution | 1.0 × 1.0 × 2.00 Mm | 0.75 × 0.75 × 4.0 mm | 0.68 × 0.68 × 3.0 mm | 0.66 × 0.66 × 3.0 mm |
| No. signal acquisitions (NSA) | 1 | 2 | 1.5 | 2 |
Fig. 2.
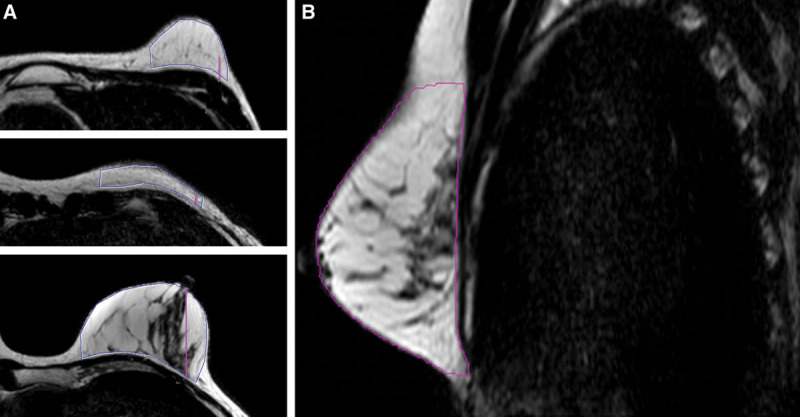
MRI image of the breast. A, For this ROI, the breast contour was followed using the borders of the convexity of the breast to define the lower and upper borders. B, Several extra ROIs were drawn on the axial images in between the upper and lower boundary.
Surgeons’ Estimation
A total of seven plastic surgeons took part in this study. They assessed the distance from sternal notch to nipple, breast width at its widest point, and projection of the breast from the thorax by measuring tape to assist in the estimation of breast volume. Assessment was done with the patient in standing position. If possible, measurements were performed on both breasts. At last, plastic surgeons were asked for an estimation of breast volume (in cm3). They were blinded for results of breast volume measured by 3D imaging or breast MRI.
Statistical Analysis
Statistical analysis was performed using SPSS (IBM SPSS Statistics, v 23.0.0, IBM Corporation). Descriptive statistics were used to describe patient characteristics. Mean (SD) values of 3D images and MRI and plastic surgeon’s estimation were used. Absolute and relative differences between 3D and MRI were given.
The level of reliability of breast volume assessment by 3D and MRI was analyzed using the intracIass correlation coefficient (ICC). Inter-rater reliability was calculated for breast volume assessment by 3D and MRI, also using ICC. ICC values of 0.00–0.20, 0.21–0.40, 0.41–0.60, 0.61–0.80, and 0.81–1.00 were used to indicate poor, fair, moderate, substantial, and excellent to perfect reliability, respectively.15
However, the ICC value can be high if the methods show a similar variation pattern, even if the measurement results do not indicate a high agreement between the methods. Thus, Bland–Altman plots were used to analyze the agreement between single measurements, as well as for the measurements from the separate techniques. Limits of agreement were determined using the mean difference in volumes ±1.96 SD of the volume difference. If necessary, proportional and systematic differences were determined using a linear regression analysis of the difference and the mean of the two variables. Pearson’s correlation was used to evaluate the correlation between variables. Paired samples t-test was used to test for differences between the three methods (plastic surgeons’ estimation, 3D, MRI). A value of P < 0.05 was considered to be statistically significant.
Results
The study was conducted at our outpatient clinic between December 2016 and June 2017. A total of 34 women fulfilled the inclusion criteria and were asked to participate in the study. In total, 29 women agreed to participate in the study. Fifty-three breast volumes were assessed. Mean age (SD) of participants was 5011 years. Mean (SD) body mass index was 25.5 kg/m2 (4.46). Plastic surgeons who performed the clinical assessment were specialized in breast surgery, with a mean experience of 7.5 years (range, 3–16 years).
3D Breast Volume Compared with MRI (Gold Standard in Literature)
The ICC between the two 3D breast measurements was 0.991 (95% confidence interval (CI): 0.984–0.995). The Bland–Altman plot comparing one observer’s 3D measurement with the other’s demonstrated a mean difference between the two observers of 1.73 and limits of agreement ranging from –56.9 to 60.3. (Fig. 3A). The ICC between the two MRI measurements was 0.990 (95% CI: 0.982–0.994). The Bland–Altman plot comparing one observer’s MRI measurement with the other’s demonstrated a mean difference between the two observers of 38.4 and limits of agreement ranging from –84.5 to 161.3. (Fig. 3B)
Fig. 3.
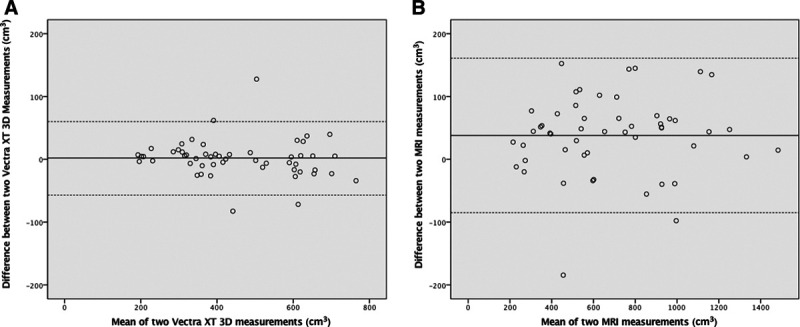
Comparison of reliability 3D and MRI breast measurements. A, The Bland–Altman plot comparing the 3D measurements demonstrates a mean difference of 1.73 (limits of agreement ranging from –56.9 to 60.3) between the 2 observers. B, The Bland–Altman analysis comparing 2 MRI measurements demonstrates a mean difference of 38.4 (limits of agreement ranging from –84.5 to 161.3) between the 2 observers.
Mean (SD) breast volume measured by 3D imaging was 454.0 cm3 (157.9), ranging from 192 to 764 cm3. Mean (SD) breast volume measured by MRI was 686.6 (312.5) ranging from 215 cm3 to 1483 cm3. There was a significant difference between 3D measurement and MRI (P < 0.001). ICC between the 3D and MRI measurements was 0.810 (95% CI: 0.670–0.891). The Bland–Altman analysis yielded a mean difference of 229.7, with limits of agreement ranging from –158.0 to 617.4 (Fig. 4A). A proportional difference of 0.70 (95% CI: 0.56–0.85) was seen, indicating that the volume difference between the measurement methods increases with higher breast volumes. The systematic difference between the two methods was –173.2 cm3 (95% CI: –262.5 to –83.9), indicating that breast volumes obtained by MRI were overall larger than obtained by 3D.
Fig. 4.
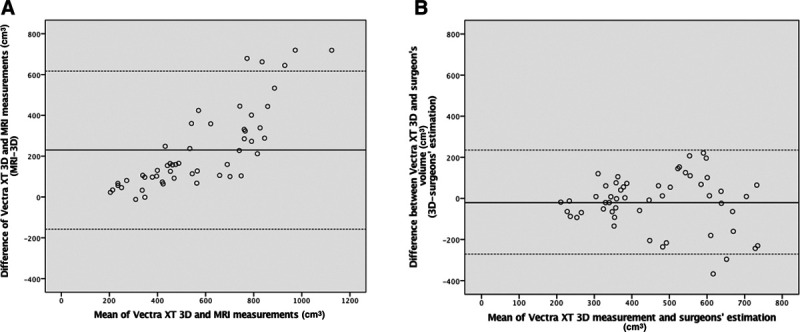
Comparison of measured volume in 3D, MRI and surgeon’s estimation. A, The Bland–Altman analysis between 3D and MRI demonstrates a mean difference of 229.7 (limits of agreement ranging from –158.0 to 617.4). B, The Bland–Altman analysis between 3D and plastic surgeon’s estimation demonstrates a mean difference of –17.7 (limits of agreement ranging from –270.7 to 235.3).
3D breast measurements were however significantly correlated (R = 0.84, P < 0.001). The relationship between 3D measurements and MRI was demonstrated in Figure 5. A linear regression was demonstrated between MRI and 3D, indicating y (MRI) = 1.58 × (3D) – 40.
Fig. 5.
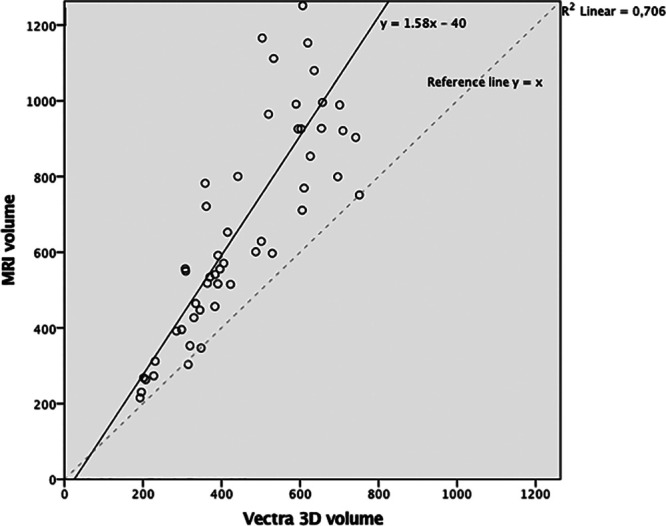
A scatter plot of MRI and 3D measurements. A simple linear regression could be performed to predict MRI from 3D measurements (F = 122.6; P < 0.001; R2 = 0.71). Y (predicted MRI) = 1.58 × (3D) – 40.
3D Breast Volume Compared with Clinical Standard (Plastic Surgeon’s Estimation)
Mean (SD) volume estimated by plastic surgeons was 471.7 cm3 (168.9) for 1 breast. The discrepancy between the volume estimated by plastic surgeons and measured by 3D was only 17.7 cm3 (95% CI: –53.3 to 17.9). ICC between plastic surgeon’s estimation and 3D volume was 0.815 (95% CI: 0.68–0.893).
Paired samples t-test showed no significant difference between 3D measurement and plastic surgeons’ estimation (P = 0.323). The Bland–Altman plot yielded a mean difference of –17.7, with limits of agreement ranging from –270.7 to 235.3 (Fig. 4B).
Discussion
This study investigated the Vectra XT 3D imaging system for determination of breast volume in comparison with the gold standard in literature (MRI) and the clinical standard (assessment by the plastic surgeon). Our study suggests that the use of 3D measured breast volumes is reliable. Both 3D breast volume and MRI breast volume showed excellent reliability. In Bland–Altman analysis, 3D measurements had even smaller limits of agreement compared with MRI, emphasizing a good reliability for 3D for breast volume measurement. However, the exactness of breast volume measured by the two methods differed significantly.
Three-dimensional breast measurements were lower than MRI breast measurements. Volume outcomes and differences depended on breast size. This was further analyzed using Bland–Altman analysis (Fig. 3A). A fixed and proportional difference from the Bland–Altman plot could be seen, meaning that breast volume differences measured by MRI and 3D imaging are dependent on size, showing smaller differences for small breast volumes and larger differences for high breast volumes.
This was earlier demonstrated in a study of Yang et al, where proportional errors were found with respect to breast volume.16 An increase of measurement error is seen with an increasing breast size, which is regarded as proportional difference. Secondly, the increasing slope of the plot suggests that the two can be related using a linear regression model. This was also demonstrated in Figure 4, in which a linear regression could be found between MRI and 3D. A similar linear association between 3D and MRI was elaborated earlier in a study by Yang et al. As results change in parallel, 3D measured volume is capable of predicting the MRI breast volume.16 In 2011, Koch et al. also found significantly smaller volumes in 3D compared with MRI, but found 3D volume applicable for predicting MRI-measured volume using a linear regression.14
An possible explanation for the difference in measurement methods was suggested in the definition of dorsal boundary of the breast. The internal boundary can only be guided by the shape of the skin surface around the breast. This defines the closed object of a breast and can influence the dorsal limit used for volume calculation. The amount of subcutaneous fatty tissue or even bad posture can have a relevant influence on the interpolation of the breast.
This also means that this tool is not suitable for patients who have pectus excavatum deformities or scoliosis. A prone breast MRI, where the chest wall can exactly be defined (as the anterior border of the pectoralis muscle), is well suited for diagnosis, but it is less suitable for use in clinical applications of defining breast parameters.
The disadvantage of the clinical application of MRI is its measuring position. During the MRI examination, a patient is in a prone position so that the axillary tissue is shifted to the front and potentially added to the breast. In 3D imaging, the patient is in a standing position. This might minimalize the amount of lateral subcutaneous tissue that is included in the breast volume measurement,14 and might also be influenced by the definition of chest wall, which is further to the front compared with posterior delineation of the manually segmented breasts on MRI.17
Some have tried to introduce a special MRI apparatus for unilateral breast MRI in a supine position, showing promising results with breast images comparable to prone position.9,18,19 These have been suggesting a better clinical breast representation. However, supine positioning in MRI is not part of standard care and needs further development and use in clinical practice. Moreover, Khatam et al. demonstrated that a change in the subject’s position from supine to upright can result in significant stretches in some parts of the breast skin, especially above the nipple, resulting in different volumes for a different patient positioning.20
All this implies that measuring position might have consequences for the breast volume that is measured. When a patient looks at herself in the mirror, she does not see the mammary gland but the external shape of the breast. As we are interested in quantifying the breast size in the way the patient perceives it in the mirror, the most adequate measuring position for measuring breast volume seems to be in standing position, instead of supine or prone.
Since 3D measurements are captured in standing position, it makes the breast measurement outcome the most clinically applicable. The Vectra XT 3D software uses automated calculation of volume, which is programmed by the software. This automated process is time-reducing and efficient. Unlike some estimation errors, especially in ptotic breasts, where the submammarian fold is difficult to determine and hence the caudal limit of the breast,14,21 it is an objective way of determining breast parameters. In this study, no detailed information was reported on ptosis grading of the breasts. However, a large variety of breast volumes were included in the study, representing a varied patient population. No errors were present in volume analysis with 3D imaging, and the extend of incorrect measurement in ptotic breast did not appear to be a relevant problem in this study.
Additionally, reliability of MRI and 3D imaging was compared. The results on reproducibility of both techniques showed excellent results. ICC of 3D breast measurements was 0.991, whereas the ICC of MRI measurements was 0.990.
Bland–Altman analysis showed only a good agreement between 2 measurements, which was even better than agreement between 2 MRI measurements. No proportional difference nor fixed difference was seen from this analysis. This means that reliability of 3D breast volume was high for small and for large breast volumes, and not depending on breast size. Earlier measurements with 3D imaging for other clinical applications than breast volumes have also shown high reproducibility.21–25
When looking at clinical aspects, 3D has several advantages over MRI for breast assessment. Three-dimensional measurements have the advantage over MRI to be non-invasive, and capturing images takes minimal time. No extra appointment needs to be planned for a breast image. Patients with claustrophobia, metal implants or cardiac pacemakers can benefit from a 3D image compared with MRI. This makes the camera suitable for the use in daily practice in clinic hours with patients. MRI avoids x-ray dosage, but the process is expensive and performed only for a small number of women in routine practice.26 The costs for an MRI examination are estimated to be around 280–1400 USD per scan. Moreover, segmentation on imaging software is still mostly conducted manually, which is labor-intensive, and evidence supporting automatic segmentation tools are scarce.27 Koch et al. have found significantly shorter time for 3D recording and volume assessment compared with MRI recording and volume assessment. For the Vectra XT 3D imaging system, the major limitation is its relatively high acquisition costs of 40,000 USD and its lack of portability. Many large academic centers as well as aesthetic plastic surgeons have already a 3D imaging system available. Until now, it does not seem to be cost-effective for smaller peripheral centers.27
Still, many plastic surgeons use only some standardized measurements to estimate volume. The difference between 3D imaging and the plastic surgeon’s volume assessment seemed comparable: 17.7 cm3 (95% CI: –53.3 to 17.9; P = 0.323). However, in Bland–Altman analysis, limits of agreement ranged from –270.7 to 235.3 (Fig. 5). Considering that the minimum volume difference detectable by the human eye is 50 cm3 by subjective judgment,28 we suggest a low agreement between the plastic surgeon’s estimation and the 3D measurement. From analyzing the plot, it seems that the scatter around the bias line gets larger as the average gets higher. This might suggest that for larger breasts, a larger variability of estimated breast volume is found.
This study did not aim to investigate the reproducibility of plastic surgeon’s estimation. The answers to this question are limited by our current study design. For future research, the next step would be to compare two surgeon’s estimations of breast.
We consider our current data useful for clinical practice since this study was, to our knowledge, the first which has compared 3D breast volume to a clinical assessment of breast volume with the patient in standing position. Secondly, we showed that volume measurement performed by MRI or 3D imaging is an objective parameter which provide reliable results for breast volume. We think that the breast volume can be different for both methods because of different measuring position (prone position versus standing position).
Future research should focus on reproducibility of plastic surgeon’s estimation of breast parameters to see if 3D breast volumes are superior in the clinical assessment of breasts. This could increase the clinical utility of 3D imaging for breast assessment and could represent an important step toward a more standardized approach to breast surgery.
Conclusions
The 3D imaging system used in this study shows excellent reliability for measurements of breast volume, which is even higher than reproducibility of measurements made with MRI, the current gold standard. However, 3D breast volumes are significantly lower, but show a strong correlation to MRI volumes from which a linear regression line can be derived. This makes 3D imaging an objective and reproducible measuring method suitable for clinical practice. Breast volume measured by 3D imaging seems comparable to the assessment of the breast by a plastic surgeon, but further research is needed to compare reliability of these two methods. Since 3D imaging is not variable to individual experience, this could represent an important step towards a standardized approach of breast evaluation and surgery.
ACKNOWLEDGMENT
All procedures performed involving human participants were in accordance with the ethical standards of the institutional research committee (METC 17-4-009) and with the ethical standards as laid in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Supplementary Material
Footnotes
Published online 30 November 2020.
Disclosure: None of the authors has a financial interest in any of the products or devices mentioned in this article.
References
- 1.Herold C, Ueberreiter K, Busche MN, et al. Autologous fat transplantation: Volumetric tools for estimation of volume survival. A systematic review. Aesthetic Plast Surg. 2013;37:380–387. [DOI] [PubMed] [Google Scholar]
- 2.Yip JM, Mouratova N, Jeffery RM, et al. Accurate assessment of breast volume: A study comparing the volumetric gold standard (direct water displacement measurement of mastectomy specimen) with a 3D laser scanning technique. Ann Plast Surg. 2012;68:135–141. [DOI] [PubMed] [Google Scholar]
- 3.Choppin SB, Wheat JS, Gee M, et al. The accuracy of breast volume measurement methods: A systematic review. Breast. 2016;28:121–129. [DOI] [PubMed] [Google Scholar]
- 4.Yoo A, Minn KW, Jin US. Magnetic resonance imaging-based volumetric analysis and its relationship to actual breast weight. Arch Plast Surg. 2013;40:203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee WY, Kim MJ, Lew DH, et al. Three-dimensional surface imaging is an effective tool for measuring breast volume: A validation study. Arch Plast Surg. 2016;43:430–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kovacs L, Eder M, Hollweck R, et al. Comparison between breast volume measurement using 3D surface imaging and classical techniques. Breast. 2007;16:137–145. [DOI] [PubMed] [Google Scholar]
- 7.Bulstrode N, Bellamy E, Shrotria S. Breast volume assessment: Comparing five different techniques. Breast. 2001;10:117–123. [DOI] [PubMed] [Google Scholar]
- 8.Parmar C, West M, Pathak S, et al. Weight versus volume in breast surgery: An observational study. JRSM Short Rep. 2011;2:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Losken A, Seify H, Denson DD, et al. Validating three-dimensional imaging of the breast. Ann Plast Surg. 2005;54:471–6; discussion 477–478. [DOI] [PubMed] [Google Scholar]
- 10.Koban KC, Etzel L, Li Z, et al. Three-dimensional surface imaging in breast cancer: A new tool for clinical studies? Radiat Oncol. 2020;15:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nahabedian MY, Galdino G. Symmetrical breast reconstruction: is there a role for three-dimensional digital photography? Plast Reconstr Surg. 2003;112:1582–1590. [DOI] [PubMed] [Google Scholar]
- 12.Tepper OM, Choi M, Small K, et al. An innovative three-dimensional approach to defining the anatomical changes occurring after short scar-medial pedicle reduction mammaplasty. Plast Reconstr Surg. 2008;121:1875–1885. [DOI] [PubMed] [Google Scholar]
- 13.Tepper OM, Small KH, Unger JG, et al. 3D analysis of breast augmentation defines operative changes and their relationship to implant dimensions. Ann Plast Surg. 2009;62:570–575. [DOI] [PubMed] [Google Scholar]
- 14.Koch MC, Adamietz B, Jud SM, et al. Breast volumetry using a three-dimensional surface assessment technique. Aesthetic Plast Surg. 2011;35:847–855. [DOI] [PubMed] [Google Scholar]
- 15.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 16.Yang J, Zhang R, Shen J, et al. The three-dimensional techniques in the objective measurement of breast aesthetics. Aesthetic Plast Surg. 2015;39:910–915. [DOI] [PubMed] [Google Scholar]
- 17.Seoud L, Ramsay J, Parent S, et al. A novel fully automatic measurement of apparent breast volume from trunk surface mesh. Med Eng Phys. 2017;41:46–54. [DOI] [PubMed] [Google Scholar]
- 18.Siegler P, Holloway CM, Causer P, et al. Supine breast MRI. J Magn Reson Imaging. 2011;34:1212–1217. [DOI] [PubMed] [Google Scholar]
- 19.Wang CB, Lee S, Kim T, et al. Breast tumor movements analysis using MRI scans in prone and supine positions. Sci Rep. 2020;10:4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khatam H, Reece GP, Fingeret MC, et al. In-vivo quantification of human breast deformation associated with the position change from supine to upright. Med Eng Phys. 2015;37:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Menezes M, Rosati R, Ferrario VF, et al. Accuracy and reproducibility of a 3-dimensional stereophotogrammetric imaging system. J Oral Maxillofac Surg. 2010;68:2129–2135. [DOI] [PubMed] [Google Scholar]
- 22.Roostaeian J, Adams WP., Jr. Three-dimensional imaging for breast augmentation: Is this technology providing accurate simulations? Aesthet Surg J. 2014;34:857–875. [DOI] [PubMed] [Google Scholar]
- 23.Rosati R, De Menezes M, Rossetti A, et al. Digital dental cast placement in 3-dimensional, full-face reconstruction: A technical evaluation. Am J Orthod Dentofacial Orthop. 2010;138:84–88. [DOI] [PubMed] [Google Scholar]
- 24.Preuß M, Killaars R, Piatkowski de Grzymala A, et al. Validity and reliability of three-dimensional imaging for measuring breast cancer-related lymphedema in the upper limb: A cross-sectional study. Lymphat Res Biol. 2018;16:525–532. [DOI] [PubMed] [Google Scholar]
- 25.Casale T, Caciari T, Rosati MV, et al. Anesthetic gases and occupationally exposed workers. Environ Toxicol Pharmacol. 2014;37:267–274. [DOI] [PubMed] [Google Scholar]
- 26.Caruso MK, Guillot TS, Nguyen T, et al. The cost effectiveness of three different measures of breast volume. Aesthetic Plast Surg. 2006;30:16–20. [DOI] [PubMed] [Google Scholar]
- 27.Chae MP, Rozen WM, Spychal RT, et al. Breast volumetric analysis for aesthetic planning in breast reconstruction: A literature review of techniques. Gland Surg. 2016;5:212–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sigurdson LJ, Kirkland SA. Breast volume determination in breast hypertrophy: An accurate method using two anthropomorphic measurements. Plast Reconstr Surg. 2006;118:313–320. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


