Abstract
Wastewater based epidemiology (WBE) has emerged as a reliable strategy to assess the coronavirus disease 2019 (COVID-19) pandemic. Recent publications suggest that SARS-CoV-2 detection in wastewater is technically feasible; however, many different protocols are available and most of the methods applied have not been properly validated. To this end, different procedures to concentrate and extract inactivated SARS-CoV-2 and surrogates were initially evaluated. Urban wastewater seeded with gamma-irradiated SARS-CoV-2, porcine epidemic diarrhea virus (PEDV), and mengovirus (MgV) was used to test the concentration efficiency of an aluminum-based adsorption-precipitation method and a polyethylene glycol (PEG) precipitation protocol. Moreover, two different RNA extraction methods were compared in this study: a commercial manual spin column centrifugation kit and an automated protocol based on magnetic silica beads. Overall, the evaluated concentration methods did not impact the recovery of gamma-irradiated SARS-CoV-2 nor MgV, while extraction methods showed significant differences for PEDV. Mean recovery rates of 42.9 ± 9.5%, 27.5 ± 14.3% and 9.0 ± 2.2% were obtained for gamma-irradiated SARS-CoV-2, PEDV and MgV, respectively. Limits of detection (LoD95%) for five genomic SARS-CoV-2 targets (N1, N2, gene E, IP2 and IP4) ranged from 1.56 log genome equivalents (ge)/mL (N1) to 2.22 log ge/mL (IP4) when automated system was used; while values ranging between 2.08 (N1) and 2.34 (E) log ge/mL were observed when using column-based extraction method. Different targets were also evaluated in naturally contaminated wastewater samples with 91.2%, 85.3%, 70.6%, 79.4% and 73.5% positivity, for N1, N2, E, IP2 and IP4, respectively. Our benchmarked comparison study suggests that the aluminum precipitation method coupled with the automated nucleic extraction represents a method of acceptable sensitivity to provide readily results of interest for SARS-CoV-2 WBE surveillance.
Keywords: SARS-CoV-2, Porcine epidemic diarrhea virus, Polyethylene glycol precipitation, Aluminum-based adsorption-precipitation, Wastewater based epidemiology, RT-qPCR
Abbreviations: COVID-19, Coronavirus disease 2019; EC, European Commission; MgV, Mengovirus; PEDV, Porcine Epidemic Diarrhea virus; PEG, polyethylene glycol; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; WBE, wastewater-based epidemiology; WHO, World Health Organization
Graphical abstract
1. Introduction
The use of wastewater as a tool for epidemiology tracking, known as wastewater-based epidemiology (WBE), has a long history of use in public health, particularly for human enteric viruses (Asghar et al., 2014; Cuevas-Ferrando et al., 2020; Hellmér et al., 2014; Miura et al., 2016; Prevost et al., 2015; Santiso-Bellón et al., 2020). In the midst of the ongoing COVID-19 pandemic, WBE is being implemented globally for the detection of SARS-CoV-2 RNA shed into wastewater, sewers, and sludge (Ahmed et al., 2020a; Bivins et al., 2020; Guerrero-Latorre et al., 2020; Haramoto et al., 2020; Kumar et al., 2020; La Rosa et al., 2020; Lodder and de Roda Husman, 2020; Medema et al., 2020; Prado et al., 2020; Randazzo et al., 2020a, Randazzo et al., 2020b; Rimoldi et al., 2020; Sherchan et al., 2020; Wang et al., 2020; Wu et al., 2020). All these studies have been implemented in research contexts; nevertheless, different countries are currently implementing wastewater surveillance into their national or regional COVID-19 monitoring programs for early warning of SARS-CoV-2 community spread and disease outbreaks (WHO, 2020). Additionally, WBE has the potential to be applied in high-risk settings such as nursing homes and hospital or in low-resource settings (WHO, 2020). As recently stated by WHO, WBE research should be seen as an important public health objective to advance knowledge about COVID-19, however, many technical issues still need to be addressed (Ahmed et al., 2020b; Polo et al., 2020; Rusiñol et al., 2020; WHO, 2020). In an attempt to coordinate current knowledge and data gaps, the European Commission (EC) created a Pan-European Umbrella Study to better understand the limitations and challenges of this approach including the development of a roadmap for a systemic rollout of complementing ongoing national and regional surveillances in a unique approach (EC, 2020). One of the problems highlighted by these collaborative studies is the need of standardized procedures, spanning from sampling to data analysis. In this sense, viral concentration and nucleic acid extraction methods are two critical steps for the analysis of viruses in wastewater and quality controls must be accurately defined. To our knowledge, three studies have compared different concentration methods using SARS-CoV-2 surrogates (Ahmed et al., 2020c; Jafferali et al., 2020; Rusiñol et al., 2020). However, the analytical performances of SARS-CoV-2 concentration, extraction, and detection procedures tested alongside are not yet characterized for wastewater samples. Thus, the aim of this work was to evaluate different concentration methods, nucleic acid extraction procedures, and quantitative RT-qPCR assays to efficiently detect SARS-CoV-2 in wastewater using gamma-irradiated SARS-CoV-2, porcine epidemic diarrhea virus (PEDV) as coronavirus model, and mengovirus as non-enveloped counterpart. Importantly, limits of detection were established using gamma-irradiated SARS-CoV-2 particles and five different RT-qPCR assays targeting various genetic fragments. We finally validate the selected RT-qPCR assays in wastewater samples collected during the COVID-19 pandemic in different regions of Spain.
2. Materials and methods
2.1. Concentration methods
An aluminum-based adsorption-precipitation and a polyethylene-glycol (PEG) precipitation methods were compared to assess their analytical performance and thus their suitability in concentrating SARS-CoV-2 from wastewater. To this end, 200 mL of grab wastewater samples (n = 8) that previously tested negative for SARS-CoV-2 (Randazzo et al., 2020a) were inoculated with 105 genome equivalents (ge) gamma-irradiated (5 × 106 RADs) SARS-CoV-2 (Bei Resources; NR-52287), 106 PCR units (PCRU) PEDV strain CV777, an enveloped virus member of the Coronaviridae family and surrogate for SARS-CoV-2; and, 106 PCRU mengovirus (MgV) vMC0 (CECT 100000), a non-enveloped member of the Picornaviridae designated in the ISO 15216-1:2017 standard method as process control. The PEDV cytopathogenic CV777 strain (Friedrich-Loeffler-Insitut, Greifswald, Germany) and MgV vMC0 were propagated in Vero and HeLa cell monolayers, respectively (Puente et al., 2020). Two hundred milliliters of seeded wastewater samples (n = 4) were concentrated through aluminum-based adsorption-precipitation (Randazzo et al., 2019, Randazzo et al., 2020a, Randazzo et al., 2020b). A final concentrate was then formed by centrifugation at 1900 ×g for 30 min and the resulting pellet was resuspended in 1 mL of PBS, pH 7.4. Alternatively, 200 mL of seeded wastewater samples (n = 4) were concentrated through precipitation with 20% polyethylene glycol (PEG) 8000 (PanReac, Spain) and resuspended in 1 mL of PBS, pH 7.4. Briefly, 25 mL of Tris Glycine-Beef Extract buffer (TGEB) pH 9.5 were added to each sample and incubated in agitation at 300 rpm for 2 h at 4 °C. After incubation, samples were centrifuged at 2500 ×g for 10 min at 4 °C. Supernatant was adjusted to pH 7.0–7.2. PEG and NaCl were added to a final concentration of 20% and 0.3 M, respectively, and mixed gently. Sample was incubated in agitation overnight at 4 °C and then centrifuged at 3500 ×g for 30 min at 4 °C. Pellet was resuspended in 1 mL of PBS and concentrated samples stored at −80 °C for further analysis.
For both concentration methods, recovery controls were prepared by spiking each virus at the concentration detailed above in 1 mL of PBS. For each sample, the percentage recovery was calculated dividing the viral titer of concentrated sample by the titer of the recovery control.
2.2. Viral extraction, detection and quantification
2.2.1. Nucleic acid extraction
Viral extraction from wastewater concentrates was carried out comparing a manual column-based commercial kit and an automated instrument relying on magnetic beads for nucleic acid purification.
Manual nucleic acid extraction was performed from 150 μL of concentrated sample using the Nucleospin RNA virus Kit (referred as MN) (Macherey-Nagel GmbH & Co., Germany) following the manufacturer's protocol together with an initial pre-treatment step with Plant RNA Isolation Aid (Ambion, USA) (Cuevas-Ferrando et al., 2020; Randazzo et al., 2020a, Randazzo et al., 2020b). In parallel, Maxwell® RSC Instrument (Promega, Spain) was used for automated nucleic acid isolation using the Maxwell RSC Pure Food GMO and authentication kit (Promega) (referred as Max). Some modifications of the original provider's protocol were established based on preliminary laboratory results during a method optimization step (data not shown). Finally, 400 μL of cetyltrimethyl ammonium bromide (CTAB) and 40 μL of proteinase K solution (both provided with the kit) were added to 300 μL of concentrated water samples, the mix was then incubated at 60 °C for 10 min and centrifuged for 10 min at 16,000 ×g. Then, the supernatant was transferred to the loading cartridge along with 300 μL of lysis buffer. The cartridge was loaded in the Maxwell® RSC Instrument and the extraction performed by selecting the “Maxwell RSC Viral total Nucleic Acid” running program in the instrument software. For both manual and automated extractions, RNA was finally eluted in 100 μL nuclease-free water. Negative controls constituted by nuclease-free water instead of concentrated sample were included in both extraction methods.
2.2.2. Viral detection and quantification
Viral detection of SARS-CoV-2, PEDV, and MgV was performed by RT-qPCR using One Step PrimeScript™ RT-PCR Kit (Perfect Real Time) (Takara Bio, USA). SARS-CoV-2 detection was performed targeting N1 region of the nucleocapsid gene (CDC, 2020) while membrane gene (M) specific primers were used for PEDV detection as described by Puente et al., 2020. For mengovirus, detection was carried out using primers and probe described in ISO 15216-1:2017. Reaction mixes, thermal cycling conditions and sequences for primers and probes are shown in Tables S1, S2 and S3, respectively. All RT-qPCR assays were performed in duplicate on a LightCycler 480 instrument (Roche Diagnostics, Germany). Positive (genomic RNA) and negative (nuclease-free water) controls were always included. Standard curves for PEDV, MgV and SARS-CoV-2 quantifications were performed using the genomic RNA of each virus with serial 10-fold dilutions in triplicate. Differences between methods were statistically analyzed using Saphiro test for normal distribution and T-student for mean comparison (p < 0.05). Influence of concentration and extraction methods was analyzed using multifactorial ANOVA for each virus (p < 0.05).
2.3. SARS-CoV-2 detection limit in wastewater
The limits of detection at 95% and 50% confidence intervals (LoD95% and LoD50%, respectively) were obtained by detecting gamma-irradiated SARS-CoV-2 (Bei Resources, NR-52287) ten-fold serially diluted from 1.7 × 103 to 1.7 ge/mL and seeded in 200 mL of wastewater samples tested negative for SARS-CoV-2. Samples were also spiked with PEDV (107 gc/mL) as process control. Viral particles were concentrated by the aluminum-based adsorption-precipitation method and RNA extracted using both RNA extraction protocols as described above. Experiments were performed in triplicate by concentrating three independent samples for each inoculation level. LoD95% and LoD50% were calculated according to Wilrich and Wilrich (2009).
To determine SARS-CoV-2 detection limits, five different targets were used: N1 and N2 regions of the nucleocapsid gene, the envelope gene (E), and regions IP2 and IP4 of the RNA-dependent RNA polymerase gene (RdRp). The amplification of the N1 region was conducted as previously described. Region N2 detection was fulfilled using primers and probes available at the diagnostic panel assays 2019-nCoV RUO Kit from the US CDC (CDC, 2020). Detection of gene E was performed using primers and probes described by Corman et al. (2020) (Table S1 and S2). To amplify and quantify IP2 target, One Step PrimeScript™ III RT-PCR kit (Takara Bio) was used.
2.4. RT-qPCR comparison in naturally contaminated wastewater samples
A total of 34 influent wastewater samples collected in different regions of Spain were analyzed for the detection and quantification of SARS-CoV-2. Samples were concentrated using the aluminum-based adsorption-precipitation method as described before. Detection of SARS-CoV-2 was conducted through the analysis of five aforementioned SARS-CoV-2 genome targets (Tables S1, S2, and S3). Each reaction was performed in duplicate. Genomic RNA of SARS-CoV-2 (ATCC VR-1986D) and nuclease free water were used as positive and negative controls, respectively. Viral quantifications were calculated by using two different standard curves for N1, N2 and E genes. The standard curves were built by using N1, N2 (2019-nCoV_N_Positive Control from CDC, IDT Catalog No. 10006625) and E gene (2019-nCoV_E Positive Control from Charité/Berlin, IDT Catalog No. 10006896) plasmids and a complete genomic RNA of SARS-CoV-2 (ATCC VR-1986D).
3. Results and discussion
3.1. Concentration and extraction method comparison
Fig. 1 shows the viral recovery rates of eight wastewater samples tested negative for SARS-CoV-2 spiked with gamma-irradiated SARS-CoV-2, PEDV and MgV, subjected to two different concentration and two nucleic acid extraction methods (Fig. 1). To determine the presence of potential inhibitors, a 10-fold dilution of each sample was also analyzed by RT-qPCR for the three targeted viruses. RT-qPCR results showed that no significant inhibitions occurred (data not shown).
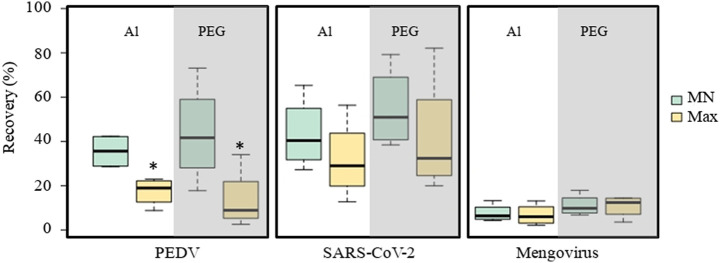
Fig. 1.
Virus recovery (%) in wastewater samples using the aluminum-based adsorption-precipitation (Al) and polyethylene glycol (PEG) precipitation methods and two RNA extraction assays (MN and Max). SARS-CoV-2 detection was performed using N1 target. Abbreviations: MN, NucleoSpin RNA virus kit (Macherey-Nagel GmbH & Co.); Max, Maxwell RSC (Promega). * p < 0.05 in comparison with the Al-MN protocol.
SARS-CoV-2 mean recoveries ranged from 30.2 ± 17.7% (Al-Max) to 52.8 ± 18.2% (PEG-MN). In the case of MgV, mean recoveries were lower and showed less variability than those obtained for SARS-CoV-2, ranging from 6.8 ± 4.8% (Al-Max) to 11.1 ± 4.9% (PEG-MN). Despite the observed differences, mean recoveries of SARS-CoV-2 and MgV were not significantly different among the tested concentration and extraction methods. In light of those results, recoveries of SARS-CoV-2 and MgV would not be significantly affected by any combination of concentration and extraction methods tested in this study (Fig. 1).
PEDV showed a global mean recovery of 27.5 ± 14.3%, with values ranging from 2.6% (PEG-Max) to 73% (PEG-MN). Results obtained with PEG concentration showed high variability (coefficient of variation (CV) of 82.99%) in comparison with the aluminum method (CV of 44.16%). Significant differences (p < 0.05) were observed for Al-MN with Al-Max (p-value = 0.012) and PEG-Max (p-value = 0.043) (Fig. 1). These results highlight the suitability of tested methods for the analysis of enveloped viruses in wastewater.
Ahmed et al. (2020c) recently reported similar mean recoveries ranging from 26.7 to 65.7% using murine hepatitis virus as surrogate for SARS-CoV-2 concentrated from wastewater by ultracentrifugation, filtration and flocculation methods. Interestingly, the authors report mean recovery of 44.0 ± 27.7% for PEG flocculation that is similar to the recovery 43.5 ± 22.8% obtained for PEDV using PEG and MN in this study. In the study of Gonzalez et al. (2020), recovery percentages of bovine coronavirus were 5.5% and 4.8% when using InnovaPrep and electronegative filtration methods for viral concentration. These recovery values were more in concordance with the ones obtained in our study for the non-enveloped MgV. From what we know, this is the first study that used gamma-irradiated SARS-CoV-2 for methods assessment and comparison. However, taking into account that these protocols are intended to be used for early SARS-CoV-2 monitoring, in which the readily availability of results is crucial to set a timely public health response up, the choice of a suitable analytical method should be based on the bench work time needed for each procedure, alongside its sensitivity. Since the PEG protocol includes an overnight incubation step, unlike the aluminum-based adsorption-precipitation method (total time less than 2 h), we selected the aluminum protocol for further comparisons.
3.2. SARS-CoV-2 detection limit in wastewater
Detection limits of five SARS-CoV-2 genome targets in wastewater were evaluated through the analysis of serial diluted spiked samples. For the detection of IP2 target, no amplification was obtained when using the One Step PrimeScript™ RT-PCR Kit (Perfect Real Time) (RR064) due to the presence of inhibitors. Therefore, the One Step PrimeScript™ III RT-PCR kit (RR600) was used since is claimed to be highly resistant to a wide variety of inhibitory substances. Limit of detection values (LoD95% and LoD50%) obtained for each gene target processed with the two extraction protocols analyzed in this study are shown in Table 1 . LoD95% values for MN protocol ranged from 2.08 to 2.34 log ge/mL; while Max protocol showed values between 1.56 and 2.22 log ge/mL. These results suggest that Maxwell RSC instrument coupled with Maxwell RSC Pure Food GMO and authentication kit are slight more sensitive than the MN protocol. However, given the large demand for commercial RNA extraction kits and the shortages of provisions, different suitable alternatives are worthy to further evaluate.
Table 1.
Detection ratio and limit of detection (LoD95% and LoD50%) of gamma-irradiated SARS-CoV-2 viral particles in wastewater using the aluminum-precipitation protocol. Viral RNA was extracted using two different nucleic acid extraction protocols and detected by targeting N1, N2, E, IP2 and IP4 genomic fragments. Abbreviations: MN, NucleoSpin RNA virus kit (Macherey-Nagel GmbH & Co.); Max, Maxwell RSC (Promega).
| Method | Target | Levels of inoculated gamma-irradiated SARS-CoV-2 (ge/mL) |
LoD95% (ge/mL)a | LoD50% (ge/mL)a | |||
|---|---|---|---|---|---|---|---|
| 1700 | 170 | 17 | 1.7 | ||||
| MN | N1 | 6/6 | 6/6 | 2/6 | 0/6 | 2.08 | 1.44 |
| N2 | 6/6 | 6/6 | 2/6 | 0/6 | 2.08 | 1.44 | |
| E | 6/6 | 6/6 | 0/6 | 0/6 | 2.34 | 1.71 | |
| IP2 | 6/6 | 6/6 | 1/6 | 0/6 | 2.22 | 1.58 | |
| IP4 | 6/6 | 6/6 | 1/6 | 0/6 | 2.22 | 1.58 | |
| Max | N1 | 6/6 | 6/6 | 5/6 | 0/6 | 1.56 | 0.92 |
| N2 | 6/6 | 6/6 | 4/6 | 0/6 | 1.74 | 1.10 | |
| E | 6/6 | 6/6 | 3/6 | 0/6 | 1.91 | 1.28 | |
| IP2 | 6/6 | 6/6 | 4/6 | 0/6 | 1.74 | 1.10 | |
| IP4 | 6/6 | 6/6 | 1/6 | 0/6 | 2.22 | 1.58 | |
Calculated according to Wilrich and Wilrich (2009).
Validation of the two extractions methods was performed in wastewater samples naturally contaminated with SARS-CoV-2 using N1 target. As occurred with spiked samples, slightly better results were obtained when automated system was used (Supplementary Table S4). Regarding the LoD95% values, E gene with MN (2.34 log ge/mL) and IP4 gene with Max (2.22 log ge/mL) showed the highest detection values. Randazzo et al. (2020a) established the theoretical LoD95% as 1.45 and 1.91 log gc/mL for N1 and N2, respectively. By spiking gamma-irradiated SARS-CoV-2 we are now able to establish rigorous LoD95% values for N1 and N2, being of 2.08 log ge/mL with MN for both genes, and 1.56 for N1 and 1.74 log ge/mL for N2 with Max. In line, Cuevas-Ferrando et al. (2020), applying the aluminum concentration method combined with the MN extraction, reported LoD95% of 2.46 log gc/mL for HEV in wastewater.
3.3. Bias of RT-qPCR assays for SARS-CoV-2 detection in wastewater samples
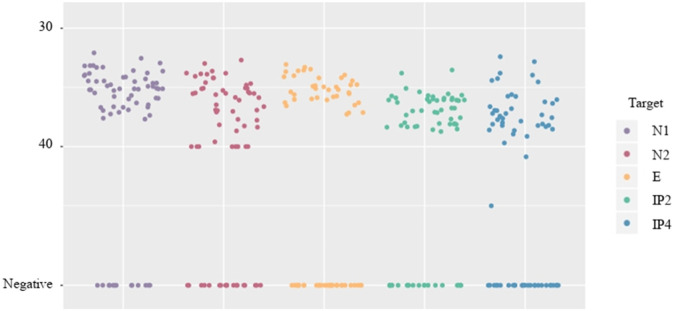
Naturally contaminated wastewater samples were analyzed by targeting five different SARS-CoV-2 genomic fragments (N1, N2, E, IP2 and IP4) to evaluate the sensitivity of each RT-qPCR assay. As shown in Fig. 2 , several differences were found in the detection of SARS-CoV-2 depending on the target used. The percentage of positive samples for each target resulted as 91.2%, 85.3%, 70.6%, 79.4% and 73.5%, for N1, N2, E, IP2 and IP4, respectively. These results evidenced the variability that can be obtained in positive samples depending on the primer set used. Additionally, reproducibility of SARS-CoV-2 detection varied within each genomic target. For example, for 10 out of 24 samples that tested positive for gene E, no fluorescence was detected in any of its technical replicates. In this sense, N1 was the target with the highest percentage of positive replicates (77%) in line of results previously reported (Muenchhoff et al., 2020). The lower sensitivity to detect gamma-irradiated SARS-CoV-2 by targeting gene E could be due to the presence of mutations in the primer binding site that would hamper amplification and, therefore, its detection (Artesi et al., 2020).
Fig. 2.
Distribution of cycle threshold values for N1, N2, E, IP2 and IP4 genomic targets of SARS-CoV-2 detected in wastewater samples (n = 34 samples).
For viral quantification of N1, N2 and gene E, standard curves were built using complete genomic RNA of SARS-CoV-2 (ATCC VR-1986D) and synthetic plasmids developed for genes N and E (Supp. Fig. S1). Fig. 3 shows the difference in the logarithm of gc/L obtained with each standard curve for each gene. Mean values of these differences were 1.27 ± 0.01, 0.27 ± 0.03, and 1.61 ± 0.01 log (gc/L) for N1, N2, and E, respectively. This overestimation was produced by the plasmid quantification as described previously (Lin et al., 2011). Quantification bias was observed depending on the reference material used, which is very important when comparing quantification data from different studies. Thus, the use of genomic RNA of SARS-CoV-2 as standard material should be recommended. Moreover, given the final WBE aim, that is the estimation of the number of infected people in a given community, this bias needs to be accurately assessed before the introduction of the quantification values into predictive epidemiological models.
Fig. 3.
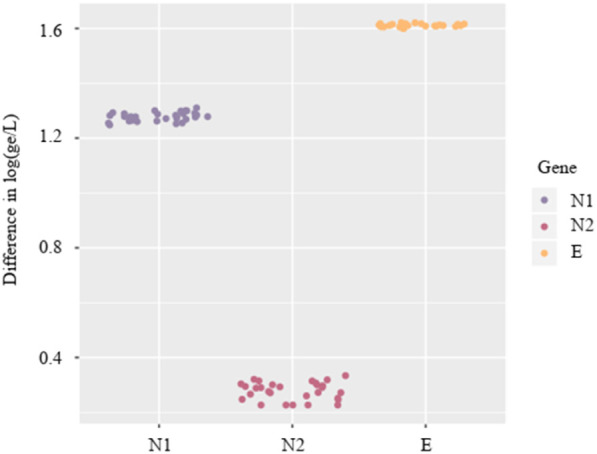
Differences in SARS-CoV-2 RNA concentrations (log10 gc/L) in wastewaters (n = 34) calculated according to standard curves generated by using genomic RNA (ATCC VR-1986D) and synthetic plasmids (IDT 10006625 for N1 and N2; IDT 10006896 for E gene).
4. Conclusions
The introduction of SARS-CoV-2 and its spread to the pandemic status have put all countries on alert and WBE has been readily implemented as an early warning tool for outbreaks. In most of the countries, wastewater surveillance for monitoring COVID-19 started very hastily, even before the scientific community could have robust data on the optimal methodologies. In fact, procedures used for viral detection have been little studied and standardization is still needed. This study benchmarked two concentration methods and two nucleic acid extraction methods widely used in environmental virology. Results obtained in this study reveal the variability obtained depending on the surrogate used as process control to validate the analyses, the extraction method, and the molecular target used for SARS-CoV-2 detection. These are critical decision which will affect the sensitivity of the analyses. On the other hand, despite the difference on sample processing time, both aluminum and PEG concentration methods can be indiscriminately used, as they did not show significant differences. However, the reduced time needed for the concentration of the samples using the aluminum-based adsorption-precipitation method, makes it the preferred method for this step. In contrast, a different sensitivity of the RT-qPCR assay has been observed suggesting that the selection of the molecular target for detection is crucial. The findings of this study expand the knowledge on the analytical procedures and its efficiencies for SARS-CoV-2 detection in wastewater constituting a step forward for the global implementation of COVID-19 WBE.
CRediT authorship contribution statement
Alba Pérez-Cataluña: Investigation, formal analysis, writing, and reviewing. Enric Cuevas-Ferrando: Investigation, formal analysis, visualization, writing, and reviewing. Walter Randazzo: writing and reviewing. Irene Falcó: Resources, writing, and reviewing. Ana Allende: writing and reviewing. Gloria Sánchez: Conceptualization, supervision, funding acquisition, writing, and reviewing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors thank Agustin Garrido Fernández, Azahara Reolid, and Andrea Lopez de Mota for their technical support.
We acknowledge Global Omnium S.L., NILSA, Aguas de Malaga, ESAMUR and FACSA for managing water sampling.
Funding
The study was funded by grants from CSIC (202070E101), Generalitat Valenciana (Covid_19-SCI), MICINN co-founded by AEI/FEDER, UE (AGL2017-82909), and MICINN/AEI (PID2019-105509RJ-I00). EC-F is recipient of a predoctoral contract from the MICINN, Call 2018. WR is holder of the APOSTD/2018/150 postdoctoral contract from Generalitat Valenciana.
Editor: Damia Barcelo
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2020.143870.
Appendix A. Supplementary data
Supplementary material
References
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728:138764. doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bivins A., Bertsch P.M., Bibby K., Choi P.M., Farkas K., Gyawali P., Hamilton K.A., Haramoto E., Kitajima M., Simpson S.L., Tandukar S., Thomas K., Mueller J.F. Surveillance of SARS-CoV-2 RNA in wastewater: Methods optimization and quality control are crucial for generating reliable public health information. Curr. Opin. Environ. Sci. Heal. 2020;17:82–93. doi: 10.1016/j.coesh.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Bivins A., Bibby K., Farkas K., Gathercole A., Haramoto E., Gyawali P., Korajkic A., McMinn B.R., Mueller J.F., Simpson S.L., Smith W.J.M., Symonds E.M., Thomas K.V., Verhagen R., Kitajima M. Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater. Sci. Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.139960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artesi M., Bontems S., Göbbels P., Franckh M., Maes P., Boreux R., Meex C., Melin P., Hayette M.-P., Bours V., Durkin K. A recurrent mutation at position 26,340 of SARS-CoV-2 is associated with failure of the E-gene qRT-PCR utilized in a commercial dual-target diagnostic assay. J. Clin. Microbiol. 2020 doi: 10.1128/jcm.01598-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghar H., Diop O.M., Weldegebriel G., Malik F., Shetty S., El Bassioni L., Akande A.O., Al Maamoun E., Zaidi S., Adeniji A.J., Burns C.C., Deshpande J., Oberste M.S., Lowther S.A. Environmental surveillance for polioviruses in the global polio eradication initiative. J. Infect. Dis. 2014;210:S294–S303. doi: 10.1093/infdis/jiu384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivins A., North D., Ahmad A., Ahmed W., Alm E., Been F., Bhattacharya P., Bijlsma L., Boehm A.B., Brown J., Buttiglieri G., Calabro V., Carducci A., Castiglioni S., Cetecioglu Gurol Z., Chakraborty S., Costa F., Curcio S., De Los Reyes F.L., Delgado Vela J., Farkas K., Fernandez-Casi X., Gerba C., Gerrity D., Girones R., Gonzalez R., Haramoto E., Harris A., Holden P.A., Islam M.T., Jones D.L., Kasprzyk-Hordern B., Kitajima M., Kotlarz N., Kumar M., Kuroda K., La Rosa G., Malpei F., Mautus M., McLellan S.L., Medema G., Meschke J.S., Mueller J., Newton R.J., Nilsson D., Noble R.T., Van Nuijs A., Peccia J., Perkins T.A., Pickering A.J., Rose J., Sanchez G., Smith A., Stadler L., Stauber C., Thomas K., Van Der Voorn T., Wigginton K., Zhu K., Bibby K. Wastewater-based epidemiology: global collaborative to maximize contributions in the fight against COVID-19. Environ. Sci. Technol. 2020 doi: 10.1021/acs.est.0c02388. [DOI] [PubMed] [Google Scholar]
- CDC. CDC 2019-novel coronavirus (2019-nCoV) real-time RT-PCR diagnostic panel. https://www.fda.gov/media/134922/download. Accessed October 2020. [DOI] [PMC free article] [PubMed]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brünink S., Schneider J., Schmidt M.L., Mulders D.G., Haagmans B.L., van der Veer B., van den Brink S., Wijsman L., Goderski G., Romette J.-L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopmans M.P., Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas-Ferrando E., Randazzo W., Pérez-Cataluña A., Sánchez G. HEV occurrence in waste and drinking water treatment plants. Front. Microbiol. 2020;10 doi: 10.3389/fmicb.2019.02937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission (EC) Feasibility assessment for an EU-wide Wastewater Monitoring System for SARS-CoV-2 Surveillance. 2020. https://ec.europa.eu/jrc/en/science-update/call-notice-feasibility-assessment-eu-wide-wastewater-monitoring-system-sars-cov-2-surveillance (accessed 10.6.20)
- Gonzalez R., Curtis K., Bivins A., Bibby K., Weir M.H., Yetka K., Thompson H., Keeling D., Mitchell J., Gonzalez D. COVID-19 surveillance in Southeastern Virginia using wastewater-based epidemiology. Water Res. 2020;186:116296. doi: 10.1016/j.watres.2020.116296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Latorre L., Ballesteros I., Villacrés-Granda I., Granda M.G., Freire-Paspuel B., Ríos-Touma B. SARS-CoV-2 in river water: implications in low sanitation countries. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020;737:140405. doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmér M., Paxéus N., Magnius L., Enache L., Arnholm B., Johansson A., Bergström T., Norder H. Detection of pathogenic viruses in sewage provided early warnings of hepatitis A virus and norovirus outbreaks. Appl. Environ. Microbiol. 2014;80:6771–6781. doi: 10.1128/AEM.01981-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISO 15216-1:2017 . 2017. Microbiology of the Food Chain—Horizontal Method for Determination of Hepatitis A Virus and Norovirus Using Real-Time RT-PCR—Part 1: Method for Quantification; ISO:Geneva, Switzerland. [Google Scholar]
- Jafferali M.H., Khatami K., Atasoy M., Birgersson M., Williams C., Cetecioglu Z. Benchmarking virus concentration methods for quantification of SARS-CoV-2 in raw wastewater. Sci. Total Environ. 2020;142939 doi: 10.1016/j.scitotenv.2020.142939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Patel A.K., Shah A.V., Raval J., Rajpara N., Joshi M., Joshi C.G. First proof of the capability of wastewater surveillance for COVID-19 in India through detection of genetic material of SARS-CoV-2. Sci. Total Environ. 2020;746:141326. doi: 10.1016/j.scitotenv.2020.141326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P., Bonanno Ferraro G., Veneri C., Bonadonna L., Lucentini L., Suffredini E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020;736:139652. doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.-H., Chen Y.-C., Pan T.-M. Quantification bias caused by plasmid DNA conformation in quantitative real-time PCR assay. PLoS One. 2011;6 doi: 10.1371/journal.pone.0029101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodder W., de Roda Husman A.M. SARS-CoV-2 in wastewater: potential health risk, but also data source. Lancet Gastroenterol. Hepatol. 2020 doi: 10.1016/S2468-1253(20)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environ. Sci. Technol. Lett. 2020;7:511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Miura T., Lhomme S., Le Saux J.C., Le Mehaute P., Guillois Y., Couturier E., Izopet J., Abranavel F., Le Guyader F.S. Detection of hepatitis E virus in sewage after an outbreak on a French Island. Food Environ. Virol. 2016;8:194–199. doi: 10.1007/s12560-016-9241-9. [DOI] [PubMed] [Google Scholar]
- Muenchhoff M., Mairhofer H., Nitschko H., Grzimek-Koschewa N., Hoffmann D., Berger A., Rabenau H., Widera M., Ackermann N., Konrad R., Zange S., Graf A., Krebs S., Blum H., Sing A., Liebl B., Wölfel R., Ciesek S., Drosten C., Protzer U., Boehm S., Keppler O.T. Multicentre comparison of quantitative PCR-based assays to detect SARS-CoV-2, Germany, March 2020. Eurosurveillance. 2020:25. doi: 10.2807/1560-7917.ES.2020.25.24.2001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo D.D., Quintela-Baluja M., Corbishley A., Jones D.L., Singer A.C., Graham D.W., Romalde P.J.L. Making waves: wastewater-based epidemiology for SARS-CoV-2 – developing robust approaches for surveillance and prediction is harder than it looks. Water Res. 2020;186:116404. doi: 10.1016/j.watres.2020.116404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado T., Fumian T.M., Mannarino C.F., Maranhão A.G., Siqueira M.M., Miagostovich M.P. Preliminary results of SARS-CoV-2 detection in sewerage system in Niterói municipality, Rio de Janeiro, Brazil. Mem. Inst. Oswaldo Cruz. 2020;115 doi: 10.1590/0074-02760200196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevost B., Lucas F.S., Ambert-Balay K., Pothier P., Moulin L., Wurtzer S. Deciphering the diversities of astroviruses and noroviruses in wastewater treatment plant effluents by a high-throughput sequencing method. Appl. Environ. Microbiol. 2015;81:7215–7222. doi: 10.1128/AEM.02076-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puente H., Randazzo W., Falcó I., Carvajal A., Sánchez G. Rapid selective detection of potentially infectious porcine epidemic diarrhea coronavirus exposed to heat treatments using viability RT-qPCR. Front. Microbiol. 2020;11:1911. doi: 10.3389/fmicb.2020.01911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Piqueras J., Evtoski Z., Sastre G., Sancho R., Gonzalez C., Sánchez G. Interlaboratory comparative study to detect potentially infectious human enteric viruses in influent and effluent waters. Food Environ. Virol. 2019 doi: 10.1007/s12560-019-09392-2. [DOI] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181:115942. doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Cuevas-Ferrando E., Sanjuán R., Domingo-Calap P., Sánchez G. Metropolitan wastewater analysis for COVID-19 epidemiological surveillance. Int. J. Hyg. Environ. Health. 2020;230:1438–4639. doi: 10.1016/j.ijheh.2020.113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi S.G., Stefani F., Gigantiello A., Polesello S., Comandatore F., Mileto D., Maresca M., Longobardi C., Mancon A., Romeri F., Pagani C., Cappelli F., Roscioli C., Moja L., Gismondo M.R., Salerno F. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Sci. Total Environ. 2020;744:140911. doi: 10.1016/j.scitotenv.2020.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusiñol M., Martínez-Puchol S., Forés E., Itarte M., Girones R., Bofill-Mas S. Concentration methods for the quantification of coronavirus and other potentially pandemic enveloped virus from wastewater. Curr. Opin. Environ. Sci. Heal. 2020 doi: 10.1016/j.coesh.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiso-Bellón C., Randazzo W., Pérez-Cataluña A., Vila-Vicent S., Gozalbo-Rovira R., Muñoz C., Buesa J., Sanchez G., Rodríguez Díaz J. Epidemiological surveillance of norovirus and rotavirus in sewage (2016–2017) in Valencia (Spain) Microorganisms. 2020;8:458. doi: 10.3390/microorganisms8030458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherchan S.P., Shahin S., Ward L.M., Tandukar S., Aw T.G., Schmitz B., Ahmed W., Kitajima M. First detection of SARS-CoV-2 RNA in wastewater in North America: a study in Louisiana, USA. Sci. Total Environ. 2020;743:140621. doi: 10.1016/j.scitotenv.2020.140621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Feng H., Zhang S., Ni Z., Ni L., Chen Y., Zhuo L., Zhong Z., Qu T. SARS-CoV-2 RNA detection of hospital isolation wards hygiene monitoring during the Coronavirus Disease 2019 outbreak in a Chinese hospital. Int. J. Infect. Dis. 2020;94:103–106. doi: 10.1016/j.ijid.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Status of environmental surveillance for SARS-CoV-2 virus. 2020. https://covid19.who.int/
- Wilrich C., Wilrich P.T. Estimation of the pod function and the LOD of a qualitative microbiological measurement method. J. AOAC Int. 2009;92:1763–1772. doi: 10.1093/jaoac/92.6.1763. [DOI] [PubMed] [Google Scholar]
- Wu F., Zhang J., Xiao A., Gu X., Lee W.L., Armas F., Kauffman K., Hanage W., Matus M., Ghaeli N., Endo N., Duvallet C., Poyet M., Moniz K., Washburne A.D., Erickson T.B., Chai P.R., Thompson J., Alm E.J. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. mSystems. 2020;5 doi: 10.1128/msystems.00614-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material