Abstract
Background
Accumulating evidence has revealed that circulating microRNAs (miRNAs) can serve as non-invasive biomarkers for cancer diagnosis. This study aimed to identify differentially expressed miRNAs in serum which might become potential biomarkers for non-invasive diagnosis of papillary thyroid carcinoma (PTC).
Methods
The experiment was carried out between 2015 and 2017. In the screening stage, the Exiqon miRNA quantitative real-time polymerase chain reaction (qPCR) panel was applied to select candidate miRNAs. In the following training, testing, and external validation stages, the serum samples of 100 patients and 96 healthy controls (HCs) were analyzed to compare the expression levels of the identified miRNAs. The areas under the receiver operating characteristic curves (AUCs) were calculated to assess the diagnostic value of the identified signature.
Results
Three miRNAs (miR-25-3p, miR-296-5p, and miR-92a-3p) in serum were consistently up-regulated in PTC patients compared with HCs. A three-miRNA panel was constructed by logistic regression analysis and showed better diagnostic performance than a single miRNA for PTC detection. The AUCs of the panel were 0.727, 0.771, and 0.862 for the training, testing, and external validation stage, respectively. Meanwhile, the panel showed stable capability in differentiating PTC patients from patients with benign goiters, with an AUC as high as 0.969. For further exploration, the three identified miRNAs were analyzed in tissue samples (23 PTC vs. 23 HCs) and serum-derived exosomes samples (24 PTC vs. 24 HCs), and the altered expression in the tumor also indicated their close relationship with PTC disease.
Conclusion
We identify a three-miRNA panel in serum which might serve as a promising biomarker for PTC diagnosis.
Keywords: MicroRNA, Serum, Papillary thyroid carcinoma, Diagnosis, Biomarkers
Introduction
Thyroid cancer is one of the most prevalent malignant tumors in the endocrine system.[1,2] It can be classified into three main types based on histopathological characteristics: papillary thyroid carcinoma (PTC), follicular thyroid carcinoma (FTC), and anaplastic thyroid carcinoma (ATC). PTC is the most common type, accounting for about 85% to 90% of all thyroid cancer cases.[3] Surgery remains the major treatment option for most cases, especially those with non-invasive and small tumors.[4] The accurate diagnosis of PTC plays a very important role in reducing disease risk and obtaining a better prognosis.[5] Most PTC cases are discovered in a routine health examination. Fine-needle aspiration biopsy (FNA) is widely conducted to determine the properties of the masses.[6] Ultrasound imaging, positron emission tomography–computed tomography (PET-CT), and other image evaluation methods also help a lot in diagnosis.[7] However, these strategies still have their limitations of being expensive, invasive, time-consuming, or overly dependent on precise instrument and technical level of medical staff.[8,9] Therefore, novel non-invasive detecting methods are in great demand for PTC diagnosis.
MicroRNAs (miRNAs) are classes of endogenous small non-coding RNAs with the length of about 22 nucleotides. They are important regulatory molecules, functioning in post-transcriptional regulation of gene expression by targeting mRNA or mediating mRNA degradation.[10] The stable existence of miRNAs in body fluids have been detected, and the relationship between circulating miRNAs and cancers has also been continuously discovered, which indicates their potential roles as non-invasive diagnostic biomarkers for various cancers, including PTC.[11–16] However, these results are still inconsistent due to the varied study cohorts, sample sizes, or testing procedures. The present study conducted four-stage experiments to explore the patterns of serum miRNA expression among 100 PTC patients and 96 healthy controls (HCs) by quantitative real-time polymerase chain reaction (qRT-PCR). The expression levels of the identified miRNAs in serum were also compared between PTC and nodular goiter (NG) patients. Moreover, the miRNA expression levels were further analyzed in tissue specimens and serum-derived exosomes to improve our understanding. We expect to discover novel circulating miRNA signatures that may be more reliable and sensitive for PTC diagnosis.
Methods
Ethical approval
The study was conducted with the approval of the Institutional Review Board and the Ethics Committee of Nanjing Medical University (No. 2016-SRFA-148).
Patients and samples
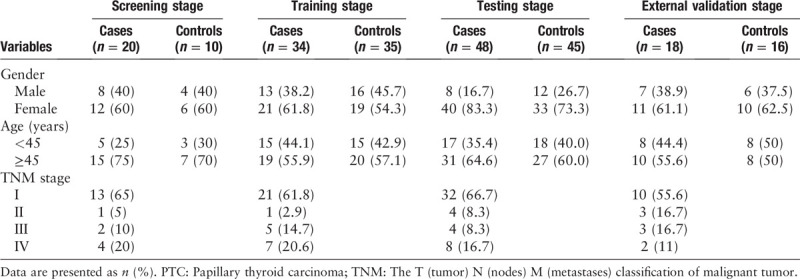
A total of 100 PTC patients, 30 NG patients, and 96 healthy donors were recruited from the First Affiliated Hospital of Nanjing Medical University during 2015 and 2017. All the patients were pathologically confirmed as PTC. The blood samples were taken without any therapeutic intervention such as surgery, radiotherapy, or drug treatment. The clinical characteristics of the participants were listed in Table 1. There was no significant difference in the distribution of age or gender between patients and HCs in any stage (P > 0.05). The written informed consent was obtained from each participant before samples were taken.
Table 1.
Clinical characteristics of the 100 PTC patients and 96 healthy controls.
All the blood samples were stored in serum separation tubes (SST) advance tubes (Becton, Dickinson and Company, NJ, USA) after collected. Then a centrifugal process (1600 × g for 10 min) was followed to separate serum from whole blood within 6 h. The serum samples were kept at −80°C for future processing. The tissue specimens were stored in liquid nitrogen.
Study design
The study was carried out in four stages: the screening, training, testing, and external validation stage [Figure 1]. In the initial screening stage, we randomly selected 20 serum samples of PTC patients, 20 of patients with NG, and 10 of HCs, and then gathered them together into 2 PTC, 2 NG, and 1 HC pools (per 10 individual samples into one pool sample). According to the manufacturer's protocol, approximately 25 ng total RNA was extracted from each serum pool sample and then reverse transcribed to cDNA using the miRCURY Locked Nucleic Acid (LNATM) Universal Reverse Transcription (RT) miRNA PCR, Polyadenylation and cDNA synthesis kit (Exiqon, Vedbaek, Denmark). The Exiqon-miRCURY-Ready-to-Use-PCR-Human-panel-I+II-V1.M (Exiqon, Vedbaek, Denmark) which could detect 179 miRNAs in serum/plasma was then applied for the preliminary screening of differentially expressed miRNAs. The platform was applied to compare the miRNA expression levels of each PTC or NG pool with the HC pool. In this step, miRNAs that meet all the three criteria could be included in the candidate list and further evaluated in the following stages: (i) the Ct-value was less than 37; (ii) the Ct-value was at least five lower than that of the negative control; (iii) the expression level was significantly altered in both PTC samples compared to the HC pool sample with the fold change (FC) being more than 1.5 or less than 0.67. Meanwhile, the four miRNAs (miR-95-5p, miR-190a-5p, miR-151a-5p, and miR-222-3p) reported in literature were also included in the candidate list.[12,17,18] For the precise verification of the dysregulated miRNAs, 69 serum samples (34 PTC vs. 35 HCs) in the training stage and 93 serum samples (48 PTC vs. 45 HCs) in the testing stage were analyzed by quantitative real-time polymerase chain reaction (qRT-PCR). In the external validation stage, a cohort containing the serum samples of 18 PTC cases and 16 HCs was detected to verify the diagnostic value of the identified miRNA signatures. Meanwhile, the serum samples of 30 PTC and 30 NG cases were further analyzed to evaluate the differential diagnostic ability of the identified miRNAs.
Figure 1.
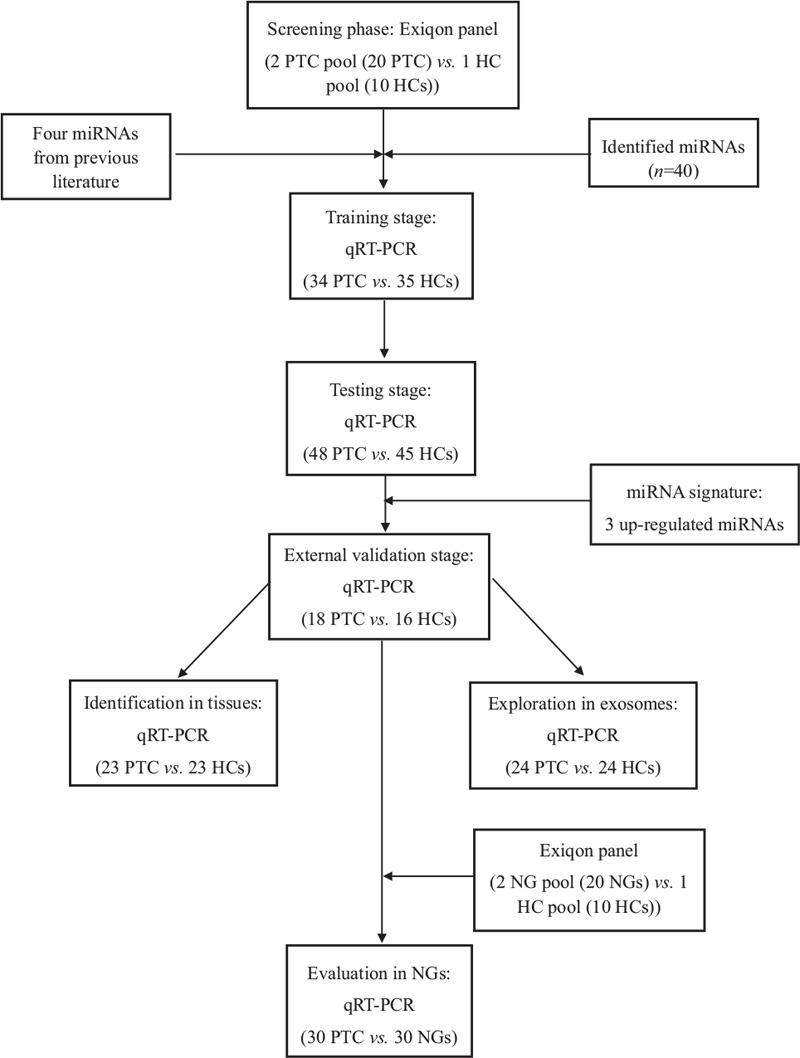
The flow chart of the experiment design. PTC: Papillary thyroid carcinoma; HC: Healthy control; NG: Nodular goiter; qRT-PCR: Quantitative real-time polymerase chain reaction.
In addition, the expression levels of the identified miRNAs were further explored in 23 pairs of PTC tissues and the matched adjacent normal tissues, and 59 pairs of PTC tissue samples from the TCGA database (http://cancergenome.nih.gov/). Serum-derived exosomes were isolated from 24 PTC patients and 24 HCs to analyze miRNAs expression to better understand the existing form of the identified signatures.
Isolation of exosomes
Exosomes were isolated from serum using the ExoQuick Exosome Precipitation Solution (System Biosciences, Mountain View, CA, USA). According to the manufacturer's protocol, 200 μL serum and 50 μL ExoQuick exosomes precipitation solution were mixed and then kept at 4°C for 30 min before centrifuged by 12,000 × g for 2 min. The supernatants were removed and the exosomes pellets were lysed in 200 μL RNase-free water for future analysis.
RNA extraction
Total RNA was extracted from 200 μL serum or exosomes using the mirVana PARIS Kit (Ambion, Austin, TX, USA) following the given protocol. 5 μL of synthetic C.elegans miR-39 (5 mmol/L, RiboBio, Guangzhou, China) was added for sample-to-sample normalization after the addition of denaturing solution (Ambion, Austin, TX, USA). Total RNA of tissue samples was obtained using Trizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. After being extracted, total RNA was dissolved in 100 μL RNase-free water and stored at −80°C until analysis. The concentration and purification of the RNA sample were assessed by the ultraviolet spectrophotometer.
Quantitative real-time polymerase chain reaction (qRT-PCR)
MiRNAs were amplified using the specific primers of reverse transcription (RT) and polymerase chain reaction (PCR) from Bulge-LoopTM miRNA qRT-PCR Primer Set (RiboBio, Guangzhou, China). The RT reaction was performed in the condition of 42°C for 60 min, and then 70°C for 10 min. The qRT-PCR reaction was carried out under the process of 95°C for 20 sec, 40 cycles of 95°C for 10 s followed by 60°C for 20 s, and then 70°C for 10 s. The reaction was performed triply on 384-well plates on the LightCycler® 480 Real-Time PCR System (Roche Diagnostics, Mannheim, Germany). The amount of PCR product was evaluated by the level of fluorescence emitted by SYBR Green (SYBR® Premix Ex TaqTM II, TaKaRa, Dalian, China). Melting analysis was finally added to evaluate the specificity of the PCR products. The expression levels of miRNAs were quantified by the 2−△△Ct method (ΔCt = CtmiRNA− Ctcel-miR-39). For tissue specimens, RNU6B (U6) was regarded as the endogenous reference for sample normalization.
Statistical analysis
The difference of miRNAs expression between PTC patients and HCs was evaluated using Mann-Whitney U test. χ2 test was used to compare the demographic and clinical characteristics of participants. A logistic regression model was built for the construction of the diagnostic panel. The diagnostic value of the identified signature was evaluated by receiver operating characteristic (ROC) curve analysis and the area under the ROC curve (AUC). All the statistical analyses were processed using SPSS software (version 25.0, IBM, North Castle, NY, USA) and GraphPad Prism 7.0 (GraphPad Software, CA, USA). A two-sided P < 0.05 was considered to be statistically significant.
Results
miRNA profiling in the screening stage
In this stage, we identified 40 differentially expressed miRNAs (36 up-regulated and four down-regulated) using the Exiqon miRNA panel [Supplementary Table 1]. These miRNAs were selected with more than 1.5-fold or less than 0.67-fold altered expression in both 2 PTC pools compared with the HC pool sample. In addition, four miRNAs (miR-95-5p, miR-190a-5p, miR-151a-5p, and miR-222-3p) once reported in previous literature were added and formed a candidate list containing 44 candidate miRNAs.[12,17,18]
Confirmation of the candidate miRNAs by qRT-PCR
For the further validation of the 44 candidate miRNAs, we detected miRNA expression in the serum samples of 34 PTC patients and 35 HCs by qRT-PCR, and screened out nine consistently up-regulated miRNAs (let-7b-5p, miR-150-5p, miR-204-5p, miR-485-3p, miR-139-5p, miR-200a-3p, miR-20a-5p, miR-92b-3p, and miR-93-5p) in the training stage. Then, we analyzed the serum samples of 48 PTC patients and 45 HCs in the testing stage and finally confirmed three differentially expressed miRNAs (miR-25-3p, miR-296-5p, and miR-92a-3p) with mean fold change (FC) >1.5 and P < 0.05 [Table 2]. The expression levels of the miRNAs that failed to pass through the following two-stage screening were shown in Supplementary Table 2. In the external validation stage, we verified the expression levels of miR-25-3p, miR-296-5p, and miR-92a-3p in 18 PTC patients and 16 HCs. The expression difference turned out to be consistent [Supplementary Table 3]. We combined the data of the three stages and found that all the three miRNAs were significantly up-regulated in PTC compared to HCs (miR-25-3p: P < 0.001, Z = −4.086; miR-296-5p: P < 0.001, Z = −3.577; miR-92a-3p: P < 0.001, Z = −5.295) [Figure 2].
Table 2.
The expression levels of the three serum miRNAs in the training and testing stages.
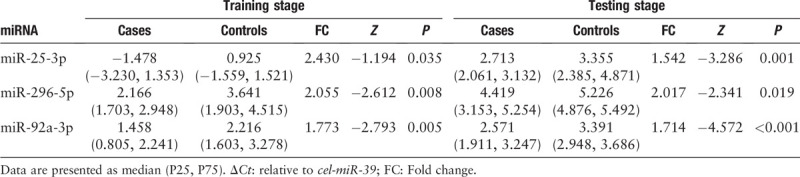
Figure 2.
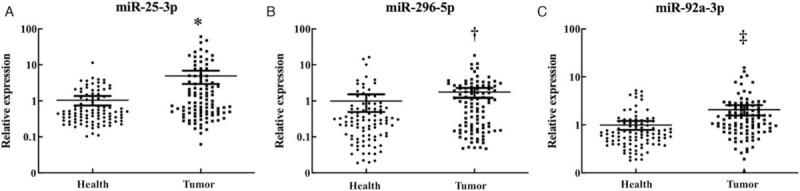
Expression levels of the three miRNAs in the serum of 100 papillary thyroid carcinoma (PTC) patients and 96 healthy controls (HCs). Horizontal line: Mean with 95% confidence interval; ∗P < 0.001, Z = −4.086; †P < 0.001, Z = −3.577; ‡P < 0.001, Z = −5.295.
Diagnostic value of the identified miRNAs in serum
The diagnostic value of each identified miRNA was evaluated by ROC curve analysis. As shown in Supplementary Figure 1, when the data of the three stages were combined, the AUCs of miR-25-3p, miR-296-5p, and miR-92a-3p were 0.623 (95% confidence interval[CI]: 0.542–0.704, sensitivity: 64.1%, specificity: 60.4%), 0.621(95% CI: 0.540–0.703, sensitivity: 75%, specificity: 50.5%), and 0.702(95% CI: 0.626–0.778, sensitivity: 67.4%, specificity: 69.2%), respectively. Since combined biomarkers might perform better than an individual one in diagnosis, a diagnostic panel consisting of the three identified miRNAs was constructed using a multiple logistic regression model. The formula to calculate the predicted probability of PTC was ‘Logit(P)=1.768 – 0.009 × miR-25-3p – 0.187 × miR-296-5p – 0.797 × miR-92a-3p’. The values of probability were used to construct ROC curves, and the AUCs for the three-miRNA panel in each stage were calculated separately: 0.727 (95% CI: 0.600–0.855, sensitivity: 65.7%, specificity: 73.3%, Figure 3B) for the training stage, 0.771(95% CI: 0.669–0.874, sensitivity: 88.9%, specificity: 68.9%, Figure 3C) for the testing stage, and 0.862(95% CI: 0.734–0.990, sensitivity: 93.3%, specificity: 66.7%, Figure 3D) for the external validation stage. When data of the three stages were combined, the AUC of the panel was 0.775 (95% CI: 0.707–0.843, sensitivity: 84.8%, specificity: 62.2%, Figure 3A), representing a better diagnostic ability than any individual miRNA.
Figure 3.
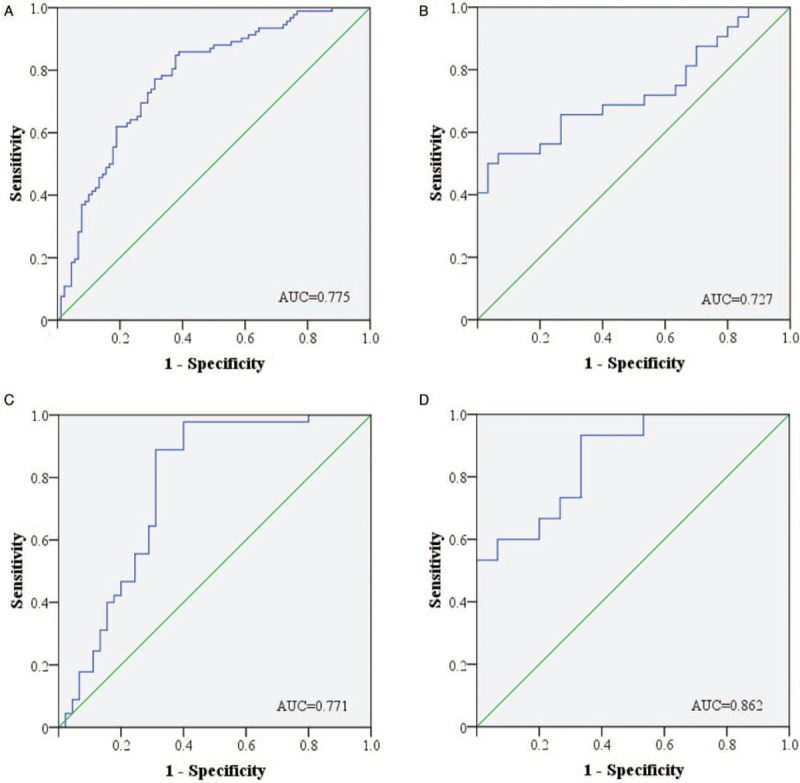
Receiver-operating characteristic (ROC) curves for the three-miRNA panel to discriminate papillary thyroid carcinoma (PTC) patients from healthy controls (HCs). (A) the combined training, testing and external validation stages (100 PTC vs. 114 HCs), 95% CI: 0.707–0.843, P < 0.001; (B) the training stage (34 PTC vs. 35 HCs), 95% CI: 0.600–0.855, P = 0.002; (C) the testing stage (48 PTC vs. 45 HCs), 95% CI: 0.669–0.874, P < 0.001; (D) the external validation stage (18 PTC vs.16 HCs), 95% CI: 0.734–0.990, P = 0.001. AUC: Areas under the curve; CI: Confidence interval.
Differential diagnosis between PTC and NG
To further assess the differential diagnostic capability of the miRNA panel, patients with NG were also analyzed in the study. None of the three identified miRNAs was found to be differentially expressed in the pool profile conducted between NG and HCs (2 NG pools vs. 1 HC pool) [Supplementary Table 4]. Then the diagnostic value of the three-miRNA panel was evaluated in the serum samples of 30 PTC and 30 NG patients. It was confirmed that all the three serum miRNAs were significantly up-regulated in PTC patients compared to patients with NG [Supplementary Figure 2], while no significant difference was found between NG and HCs. Finally, the three-miRNA panel could also perform well in discriminating PTC patients from NG patients with the AUC being as high as 0.969 (95% CI: 0.927–1.000, Supplementary Figure 3).
Evaluation of miRNA expression in tissue samples
We further detected the expression levels of the three miRNAs in 23 pairs of tissue samples (23 PTC vs. 23 HCs) to explore the relationship between tissue and serum miRNA expression patterns. As showed in Figure 4, the expression levels of miR-25-3p (P = 0.025, t = −2.405) and miR-92a-3p (P = 0.043, t = −2.152) were conversely decreased in tumor tissue samples, while miR-296-5p (P = 0.225, t = −1.246) showed no statistical significance. The TCGA miRNA sequencing data of 59 pairs of PTC tumor tissues and the matched normal tissues were further analyzed for validation. In this cohort, the expression of miR-25 and miR-296 was much lowered in PTC tumor tissues than those in normal tissues [Supplementary Figure 4].
Figure 4.
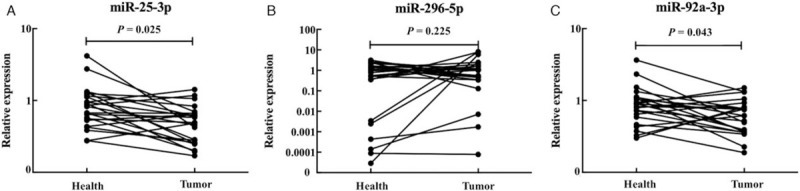
The expression levels of the three miRNAs in 23 pairs of tumor tissues. Horizontal line: Mean with SEM.
Evaluation of miRNA expression in serum exosomes
To explore the potential form of the identified miRNAs, we further analyzed miRNA expression in the serum-based exosomes samples (24 PTC vs. 24 HCs) by qRT-PCR. As shown in Figure 5, miR-296-5p was still significantly up-regulated (P = 0.019, Z = −2.340), while miR-25-3p (P < 0.001, Z = −3.980) and miR-92a-3p (P = 0.006, Z = −2.722) were down-regulated in serum-derived exosomes.
Figure 5.
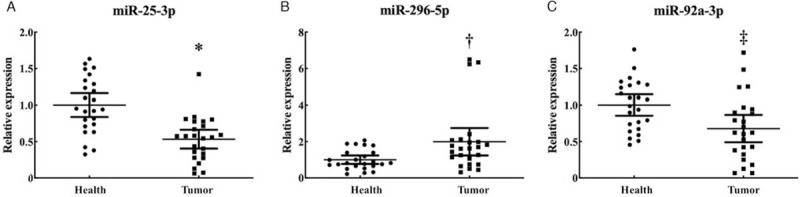
The expression levels of the three miRNAs in the serum exosomes of 24 papillary thyroid carcinoma (PTC) patients and 25 healthy controls (HCs). Horizontal line: Mean with SEM. ∗P < 0.001, Z = −3.980; †P = 0.019, Z = −2.340; ‡P = 0.006, Z = −2.722.
Bioinformatics analysis of candidate miRNAs
We applied DIANA-TarBasev7.0 to explore the potential target genes of each miRNA. Then the DIANA-miRPath v3.0 (a pathway analysis web-server) was used to investigate the miRNA-involved pathways within the Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) datasets. The KEGG analysis revealed the roles of these miRNAs in several tumor-related biological processes, such as viral carcinogenesis, lysine degradation, and cell cycle. The results of GO analysis also showed several cancer-related biological processes involved by the three miRNAs, including cell death, cellular nitrogen compound metabolic process, and protein binding transcription factor activity. The heatmaps of the targeted pathways were shown in Figure 6.
Figure 6.
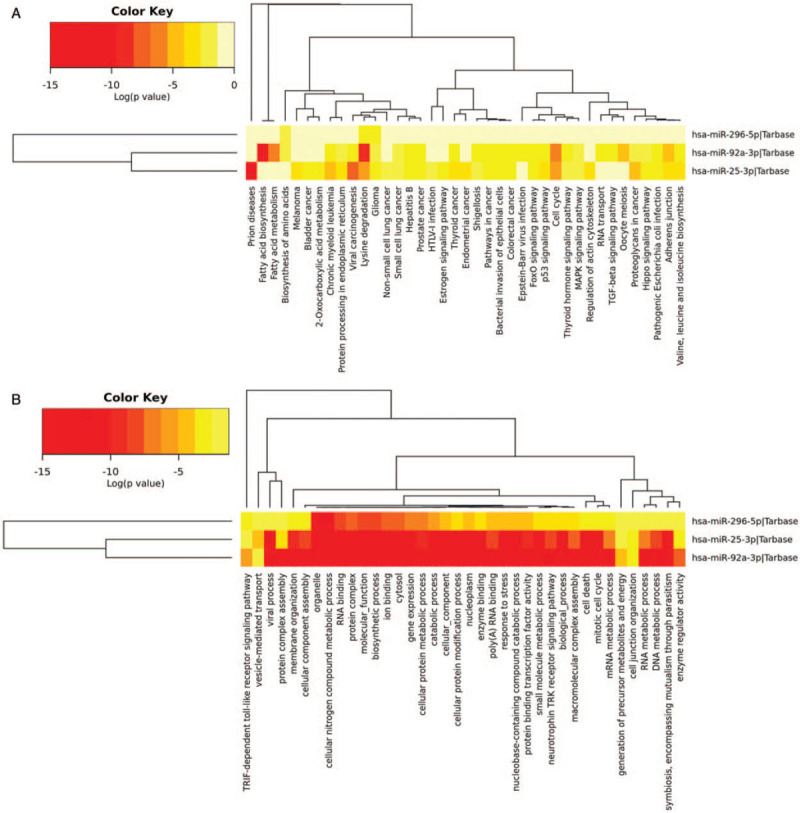
Heatmap of KEGG (A) and GO (B) analysis with the experimentally validated target genes of the three miRNAs. KEGG: Kyoto Encyclopedia of Genes and Genomes; GO: Gene Ontology.
Discussion
PTC is the most common malignant tumor of the thyroid.[3] With a growing incidence rate in recent years, it has posed great health danger for more and more people worldwide, especially for females.[2] Although most cases have a favorable prognosis after timely treatment, there is still a considerable proportion of patients with high-grade malignancy and poor overall survival.[19] A previous study has validated the stable existence of miRNAs in blood circulation.[20] Circulating miRNAs have a great advantage of being stable and non-invasive as diagnostic biomarkers for PTC. Accumulating studies have also focused on the roles of circulating miRNAs in PTC development. For example, Lee et al[21] reported that circulating miR-222 and miR-146b might be correlated with PTC tumorigenesis and recurrence. Shuang et al[12] found that serum let-7e, miR-151-5p, and miR-222 were significantly up-regulated in PTC patients compared with HCs. However, these studies are still incomplete and usually have poor consistency. For further exploration, we designed a four-stage study and established a serum three-miRNA panel which might contribute to the non-invasive diagnosis of PTC.
Briefly, 44 candidate miRNAs (40 identified by the Exiqon miRNA qPCR panel and four identified by the previous kinds of literature) were screened out in the screening stage. According to the manufacturer's introduction, the Exiqon platform is designed for the profiling analysis of 179 human miRNAs in serum/plasma samples. Over 1 million in-house and collaborative data points from serum/plasma samples of healthy and diseased individuals have been used in the selection of the 179 miRNAs list for the panel, which enhances its reference value for preliminary screening. Several previous studies have also confirmed its advantage for the detection of clinical samples with low miRNA abundance, such as serum samples.[22,23] The expression levels of the candidate miRNAs were further evaluated by qRT-PCR in the following training, testing, and external validation stages. Finally, we identified three miRNAs in serum (miR-25-3p, miR-296-5p, and miR-92a-3p) which were consistently up-regulated in PTC patients compared to HCs. A three-miRNA panel was thus constructed and showed impressive specificity and sensitivity in PTC discrimination. Meanwhile, the identified biomarker also had a good differential diagnostic capability in identifying PTC patients from patients with NG.
Bioinformatics analysis using the DIANA-miRPath v3.0 was conducted for the preliminary exploration of mechanisms. The role of miR-25-3p in tumorigenesis and metastasis of thyroid cancer has been explored by many researchers. Min et al[14] found that for PTC patients, the expressing levels of miR-25-3p in plasma and tumor tissues were much higher than those with benign nodules or HCs, and could decrease significantly after tumor excision. However, with a limited sample size, the results were short of conviction to some extent. Mei et al[24] reported that miR-25 could promote the migration and proliferation of thyroid cancer by mediating the inhibition of SOCS4. Aherne et al[25] discovered that the dysregulation of miR-25 might affect the gene expression of TRAIL and MEK4 in thyroid cells. According to Esposito et al,[26] the down-regulation of miR-25 could facilitate the development of thyroid carcinoma by influencing the EZH2 protein level. For miR-296-5p, Chi et al[27] found its down-regulation in FTC tissues compared with follicular thyroid adenoma (FA) tissues. It also functioned as an important regulator in the promotion or suppression of various cancers. For example, by interacting with the 3’-UTR of PIN1 mRNA, miR-296-5p could suppress the development of prostate cancer; while in non-small cell lung cancer, miR-296-5p inhibited cancer progression by regulating PLK1 expression.[28,29] On the contrary, Li et al[30] suggested that overexpressed miR-296-5p could reduce the anti-growth effect of CDX1 and promoted cell growth in gastric cancer. The conflicting results in different studies indicate that miR-296-5p may have complex functions in cancer development, but evidence in PTC is still quite limited up to now. The potential of circulating miR-92a as a biomarker was reported in several cancers, like colorectal cancer, breast cancer, and hepatocellular carcinoma.[31–33] According to Todorović et al,[34] the dysregulation of miR-92a in PTC tissues was correlated with the change of VHL (a tumor suppressor gene) expression. However, it is worth noting that the molecular mechanisms of the three identified miRNAs in PTC are still unclear and requires more research in the future.
Furthermore, to compare the expression levels of the identified miRNAs in tissues, we further analyzed 23 pairs of PTC tumor tissues and the matched adjacent normal tissues. Interestingly, the expression of miR-25-3p and miR-92a-3p were down-regulated in PTC tumor tissues, contrary to the results found in serum. miRNA expression alterations between tissue and blood samples have been frequently observed in previous studies.[35–37] In fact, the expression patterns of circulating miRNAs may greatly differ from their respective parental cells regardless of their origins, and not all circulating miRNAs can be found in the originating cells.[38,39] We suspected that the phenomenon could be partly due to the communication of miRNAs between tumor, tumor microenvironment, and peripheral blood circulation. Different biological states of tumors, the influence of other non-tumor cells, or change of immunological states might also alter the expression of miRNAs. Moreover, the profiles of circulating miRNAs might reflect the general change in the body fluids of patients, while the profiles of intracellular miRNAs might just reflect the disorder in local organs. In addition, according to the results of TCGA analysis, the expression levels of miR-25 and miR-296 were significantly lowered in tumor tissues. The result was only partly overlapped with our findings. We suspected that such discrepancy might be caused by different racial types, specimen origins, and limited sample sizes. Thus, still a larger number of tissue samples especially serum-tissue matched samples were in demand for further validation.
Exosomes, released from many cell types, are small membrane-bound vesicles present in nearly all kinds of body fluids.[40] The stable existence of miRNAs has been identified in exosomes.[41] Exosomes-based miRNAs may serve as signaling molecules that can reveal the physiological state of cells of origin, or serve as potential biomarkers for various diseases including cancers.[42] Therefore, to explore the potential form of miRNAs in the blood circulation and further detect their capability to be PTC biomarkers, we isolated exosomes from serum samples (24 PTC vs. 24 HCs) and detected miRNA expression levels by qRT-PCR. As a result, only miR-296-5p was consistently up-regulated in serum-derived exosomes, while miR-25-3p and miR-92a-3p were significantly down-regulated. The inconsistency of miRNA expression between serum samples and serum exosomes samples might be due to the varied existing form of circulating miRNAs. Recently, a study by Arroyo et al.[43] revealed that some specific miRNAs in serum (ie, miR-25-3p, miR-92a-3p) were mainly carried by the Argonaute2 complexes rather than exosomes, which could partly explain the discrepancy of miRNA expression levels between serum and exosomes samples. Our findings also suggested that the existing form of miRNAs in blood circulation is really complicated and requires further exploration.
In this study, we identified a three-miRNA signature in serum for PTC diagnosis. However, there are still some limitations to this research. First, the sample size was not large enough especially for tissues and exosomes samples, and study on larger cohorts was still essential for further validation. Second, the roles of these miRNAs in the biological process of PTC were still unclear, and deeper research was needed to explore the exact inner mechanisms. In addition, miRNA expression in other pathological types including FTC and ATC is worthy to explore for the confirmation of the specificity and sensitivity of the identified signature. Last but not least, our study was preclinical research, and there will be a long way to go for future clinical applications.
In conclusion, we identified a three-miRNA signature (miR-25-3p, miR-296-5p, and miR-92a-3p) in serum for PTC diagnosis. This panel could probably serve as a stable, sensitive, and non-invasive biomarker for PTC detection. Further studies with larger cohorts are needed and should focus on the exploration of the exact mechanisms of these miRNAs in PTC in the future.
Funding
The work was supported by grants from the National Natural Science Foundation of China (No. 81672400, and No. 81702364) and the Natural Science Foundation of Jiangsu Provincial Department of Education (No. BK20171085).
Conflicts of Interest
None.
Supplementary Material
Supplementary Material
Footnotes
How to cite this article: Zou X, Gao F, Wang ZY, Zhang H, Liu QX, Jiang L, Zhou X, Zhu W. A three-microRNA panel in serum as novel biomarker for papillary thyroid carcinoma diagnosis. Chin Med J 2020;133:2543–2551. doi: 10.1097/CM9.0000000000001107
Xuan Zou and Feng Gao contributed equally to this work.
Supplemental digital content is available for this article.
References
- 1.National Cancer Institute. Thyroid Cancer Treatment (Adult) (PDQ®)–Patient Version. Available at: https://www.cancer.gov/types/thyroid/patient/thyroid-treatment-pdq (Accessed July 7, 2019). [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017; 67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Tuttle RM, Ball DW, Byrd D, Dilawari RA, Doherty GM, Duh QY, et al. Thyroid carcinoma. J Natl Compr Canc Netw 2010; 8:1228–1274. doi: 10.6004/jnccn.2010.0093. [DOI] [PubMed] [Google Scholar]
- 4.Meng X, Wen S, Wang B, Feng Y, Yang L, Kong L. Surgical treatment for primary papillary thyroid cancer: a Meta-analysis (In Chinese). J Clin Otolaryngol Head Neck Surg 2015; 29:835–840. doi: 10.13201/j.issn.1001-1781.2015.09.016. [PubMed] [Google Scholar]
- 5.Clement SC, Kremer LC, Links TP, Mulder RL, Ronckers CM, van Eck-Smit BL, et al. Is outcome of differentiated thyroid carcinoma influenced by tumor stage at diagnosis? Cancer Treat Rev 2015; 41:9–16. doi: 10.1016/j.ctrv.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Yoon RG, Baek JH, Lee JH, Choi YJ, Hong MJ, Song DE, et al. Diagnosis of thyroid follicular neoplasm: fine-needle aspiration versus core-needle biopsy. Thyroid 2014; 24:1612–1617. doi: 10.1089/thy.2014.0140. [DOI] [PubMed] [Google Scholar]
- 7.Guille JT, Opoku-Boateng A, Thibeault SL, Chen H. Evaluation and management of the pediatric thyroid nodule. Oncologist 2015; 20:19–27. doi: 10.1634/theoncologist.2014-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papini E, Guglielmi R, Bianchini A, Crescenzi A, Taccogna S, Nardi F, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab 2002; 87:1941–1946. doi: 10.1210/jcem.87.5.8504. [DOI] [PubMed] [Google Scholar]
- 9.Caraway NP, Sneige N, Samaan NA. Diagnostic pitfalls in thyroid fine-needle aspiration: a review of 394 cases. Diagn Cytopathol 1993; 9:345–350. doi: 10.1002/dc.2840090320. [DOI] [PubMed] [Google Scholar]
- 10.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 11.Turchinovich A, Weiz L, Burwinkel B. Extracellular miRNAs: the mystery of their origin and function. Trends Biochem Sci 2012; 37:460–465. doi: 10.1016/j.tibs.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Yu S, Liu Y, Wang J, Guo Z, Zhang Q, Yu F, et al. Circulating microRNA profiles as potential biomarkers for diagnosis of papillary thyroid carcinoma. J Clin Endocrinol Metab 2012; 97:2084–2092. doi: 10.1210/jc.2011-3059. [DOI] [PubMed] [Google Scholar]
- 13.Lee JC, Gundara JS, Glover A, Serpell J, Sidhu SB. MicroRNA expression profiles in the management of papillary thyroid cancer. Oncologist 2014; 19:1141–1147. doi: 10.1634/theoncologist.2014-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li M, Song Q, Li H, Lou Y, Wang L. Circulating miR-25-3p and miR-451a May Be Potential Biomarkers for the Diagnosis of Papillary Thyroid Carcinoma. Plos One 2015; 10:e0132403.doi: 10.1371/journal.pone.0132403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu SR, Wu Q, Shi YQ. Recent advances of miRNAs in the development and clinical application of gastric cancer. Chin Med J 2020; 133:1856–1867. doi: 10.1097/CM9.0000000000000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li CY, Zhang WW, Xiang JL, Wang XH, Li J, Wang JL. Identification of microRNAs as novel biomarkers for esophageal squamous cell carcinoma: a study based on The Cancer Genome Atlas (TCGA) and bioinformatics. Chin Med J 2019; 132:2213–2222. doi: 10.1097/CM9.0000000000000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cantara S, Pilli T, Sebastiani G, Cevenini G, Busonero G, Cardinale S, et al. Circulating miRNA95 and miRNA190 are sensitive markers for the differential diagnosis of thyroid nodules in a Caucasian population. J Clin Endocrinol Metab 2014; 99:4190–4198. doi: 10.1210/jc.2014-1923. [DOI] [PubMed] [Google Scholar]
- 18.Stokowy T, Eszlinger M, Swierniak M, Fujarewicz K, Jarzab B, Paschke R, et al. Analysis options for high-throughput sequencing in miRNA expression profiling. BMC Res Notes 2014; 7:144.doi: 10.1186/1756-0500-7-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baudin E, Schlumberger M. New therapeutic approaches for metastatic thyroid carcinoma. Lancet Oncol 2007; 8:148–156. doi: 10.1016/S1470-2045(07)70034-7. [DOI] [PubMed] [Google Scholar]
- 20.Larrea E, Sole C, Manterola L, Goicoechea I, Armesto M, Arestin M, et al. New concepts in cancer biomarkers: circulating miRNAs in liquid biopsies. Int J Mol Sci 2016; 17: doi: 10.3390/ijms17050627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JC, Zhao JT, Clifton-Bligh RJ, Gill A, Gundara JS, Ip JC, et al. MicroRNA-222 and microRNA-146b are tissue and circulating biomarkers of recurrent papillary thyroid cancer. Cancer-Am Cancer Soc 2013; 119:4358–4365. doi: 10.1002/cncr.28254. [DOI] [PubMed] [Google Scholar]
- 22.Jensen SG, Lamy P, Rasmussen MH, Ostenfeld MS, Dyrskjot L, Orntoft TF, et al. Evaluation of two commercial global miRNA expression profiling platforms for detection of less abundant miRNAs. Bmc Genomics 2011; 12:435.doi: 10.1186/1471-2164-12-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, Gelfond JA, McManus LM, Shireman PK. Reproducibility of quantitative RT-PCR array in miRNA expression profiling and comparison with microarray analysis. Bmc Genomics 2009; 10:407.doi: 10.1186/1471-2164-10-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mei Z, Chen S, Chen C, Xiao B, Li F, Wang Y, et al. Interleukin-23 facilitates thyroid cancer cell migration and invasion by inhibiting SOCS4 expression via MicroRNA-25. Plos One 2015; 10:e0139456.doi: 10.1371/journal.pone.0139456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aherne ST, Smyth P, Freeley M, Smith L, Spillane C, O’Leary J, et al. Altered expression of mir-222 and mir-25 influences diverse gene expression changes in transformed normal and anaplastic thyroid cells, and impacts on MEK and TRAIL protein expression. Int J Mol Med 2016; 38:433–445. doi: 10.3892/ijmm.2016.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esposito F, Tornincasa M, Pallante P, Federico A, Borbone E, Pierantoni GM, et al. Down-regulation of the miR-25 and miR-30d contributes to the development of anaplastic thyroid carcinoma targeting the polycomb protein EZH2. J Clin Endocrinol Metab 2012; 97:E710–718. doi: 10.1210/jc.2011-3068. [DOI] [PubMed] [Google Scholar]
- 27.Chi J, Zheng X, Gao M, Zhao J, Li D, Li J, et al. Integrated microRNA-mRNA analyses of distinct expression profiles in follicular thyroid tumors. Oncol Lett 2017; 14:7153–7160. doi: 10.3892/ol.2017.7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee KH, Lin FC, Hsu TI, Lin JT, Guo JH, Tsai CH, et al. MicroRNA-296-5p (miR-296-5p) functions as a tumor suppressor in prostate cancer by directly targeting Pin1. Biochim Biophys Acta 2014; 1843:2055–2066. doi: 10.1016/j.bbamcr.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Xu C, Li S, Chen T, Hu H, Ding C, Xu Z, et al. miR-296-5p suppresses cell viability by directly targeting PLK1 in non-small cell lung cancer. Oncol Rep 2016; 35:497–503. doi: 10.3892/or.2015.4392. [DOI] [PubMed] [Google Scholar]
- 30.Li T, Lu YY, Zhao XD, Guo HQ, Liu CH, Li H, et al. MicroRNA-296-5p increases proliferation in gastric cancer through repression of Caudal-related homeobox 1. Oncogene 2014; 33:783–793. doi: 10.1038/onc.2012.637. [DOI] [PubMed] [Google Scholar]
- 31.Liu GH, Zhou ZG, Chen R, Wang MJ, Zhou B, Li Y, et al. Serum miR-21 and miR-92a as biomarkers in the diagnosis and prognosis of colorectal cancer. Tumour Biol 2013; 34:2175–2181. doi: 10.1007/s13277-013-0753-8. [DOI] [PubMed] [Google Scholar]
- 32.Si H, Sun X, Chen Y, Cao Y, Chen S, Wang H, et al. Circulating microRNA-92a and microRNA-21 as novel minimally invasive biomarkers for primary breast cancer. J Cancer Res Clin Oncol 2013; 139:223–229. doi: 10.1007/s00432-012-1315-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wen Y, Han J, Chen J, Dong J, Xia Y, Liu J, et al. Plasma miRNAs as early biomarkers for detecting hepatocellular carcinoma. Int J Cancer 2015; 137:1679–1690. doi: 10.1002/ijc.29544. [DOI] [PubMed] [Google Scholar]
- 34.Todorovic L, Stanojevic B, Mandusic V, Petrovic N, Zivaljevic V, Paunovic I, et al. Expression of VHL tumor suppressor mRNA and miR-92a in papillary thyroid carcinoma and their correlation with clinical and pathological parameters. Med Oncol 2018; 35:17.doi: 10.1007/s12032-017-1066-3. [DOI] [PubMed] [Google Scholar]
- 35.Huang Z, Zhu D, Wu L, He M, Zhou X, Zhang L, et al. Six serum-based miRNAs as potential diagnostic biomarkers for gastric cancer. Cancer Epidemiol Biomarkers Prev 2017; 26:188–196. doi: 10.1158/1055-9965.EPI-16-0607. [DOI] [PubMed] [Google Scholar]
- 36.Huang Z, Zhang L, Zhu D, Shan X, Zhou X, Qi LW, et al. A novel serum microRNA signature to screen esophageal squamous cell carcinoma. Cancer Med 2017; 6:109–119. doi: 10.1002/cam4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagy ZB, Bartak BK, Kalmar A, Galamb O, Wichmann B, Dank M, et al. Comparison of circulating miRNAs expression alterations in matched tissue and plasma samples during colorectal cancer progression. Pathol Oncol Res 2019; 25:97–105. doi: 10.1007/s12253-017-0308-1. [DOI] [PubMed] [Google Scholar]
- 38.Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, Gonzalez S, Sanchez-Cabo F, Gonzalez MA, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun 2011; 2:282.doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007; 9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 40.Simons M, Raposo G. Exosomes--vesicular carriers for intercellular communication. Curr Opin Cell Biol 2009; 21:575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 41.Gallo A, Tandon M, Alevizos I, Illei GG. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. Plos One 2012; 7:e30679.doi: 10.1371/journal.pone.0030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J, Li S, Li L, Li M, Guo C, Yao J, et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics 2015; 13:17–24. doi: 10.1016/j.gpb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A 2011; 108:5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


