Abstract
Aim
Neurofibromatosis type 1 (NF1) is a multifaceted disease, and frequently comorbid with neurodevelopmental disorders such as autism spectrum disorder (ASD) and learning disorder. Dysfunction of adenylyl cyclase (AC) is one of the candidate pathways in abnormal development of neuronal cells in the brain of NF1 patients, while its dynamic abnormalities have not been observed. Direct conversion technology can generate induced‐neuronal (iN) cells directly from human fibroblasts within 2 weeks. Just recently, we have revealed that forskolin, an AC activator, rescues the gene expression pattern of iN cells derived from NF1 patients (NF1‐iN cells). In this microreport, we show the dynamic effect of forskolin on NF1‐iN cells.
Methods
iN cells derived from healthy control (HC‐iN cells) and NF1‐iN cells were treated with forskolin (final concentration 10 μM), respectively. Morphological changes of iN cells were captured by inverted microscope with CCD camera every 2 minutes for 90 minutes.
Results
Prior to forskolin treatment, neuron‐like spherical‐form cells were observed in HC‐iN cells, but most NF1‐iN cells were not spherical‐form but flatform. Only 20 minutes after forskolin treatment, the morphology of the iN cells were dramatically changed from flatform to spherical form, especially in NF1‐iN cells.
Conclusion
The present pilot data indicate that forskolin or AC activators may have therapeutic effects on the growth of neuronal cells in NF1 patients. Further translational research should be conducted to validate our pilot findings for future drug development of ASD.
Keywords: adenylyl cyclase, autism spectrum disorder, cyclic adenosine monophosphate, forskolin, induced pluripotent stem cells, induced‐neuronal cells, learning disorder, neurofibromatosis type 1
Neurofibromatosis type 1 (NF1) is highly comorbid with neurodevelopmental disorders such as autism spectrum disorder (ASD) and learning disorder, and underlying mechanisms have not been well clarified. We herein showed that forskolin, an AC activator, rapidly enhances neuron‐like morphological change of directly induced‐neuronal (iN) cells from NF1 patients. The present pilot data using the direct conversion technology indicate that forskolin or AC activators may have therapeutic effects on the growth of neuronal cells in NF1 patients.
![]()
1. INTRODUCTION
Neurofibromatosis type 1 (NF1: also known as von Recklinghausen disease) is a multifaceted disease, which shows a variety of physical symptoms including multiple café‐au‐lait spots, Lisch nodules, neurofibromas, scoliosis, and vision disorder, 1 , 2 , 3 and also shows a variety of mental symptoms including mental retardation, epilepsy, and cognitive impairment/ learning disorder. 4 , 5 About 30% of NF1 patients are comorbid with autism spectrum disorder (ASD). 6 , 7 , 8 , 9 , 10 These clinical reports have suggested some neurodevelopmental pathophysiology in the brains of NF1 patients.
Neurofibromatosis type 1 is a monogenic disease, and its causative gene is the NF1 gene coding “neurofibromin 1”. A strong association between neurofibromin 1 and Ras‐GTPase has widely been known. 11 Neurofibromin 1 regulates not only Ras‐GTPase but also adenylyl cyclases (ACs) in various cell types. 12 Detailed molecular basis of ACs‐mediated neurofibromin 1 has not been well clarified. Interestingly, a recent study using a zebrafish model of NF1 has shown that the AC signaling pathway is involved in learning and that the Ras‐GTPase pathway is associated with memory. 13
Regenerative medicine technologies using induced pluripotent stem (iPS) cells from human tissues have been highlighted to clarify the cellular‐level pathophysiology of brain disorders including psychiatric disorders. 14 , 15 Direct conversion technologies, not using iPS cells, have also been attracting attention as a useful translational research tool. 16 , 17 Directly converted neuronal cells, called “induced‐neuronal (iN) cells,” were developed from mouse fibroblasts transfected with three transcriptional factors: Brn2, Ascl1, and Myt1l (BAM factors), without via iPS cells. 18 Human iN cells have been utilized for neuropsychiatric research, 19 , 20 and some previous reports have shown that iN cells have the advantage of retaining some of the physiological conditions that are lost in iPS cells. 21 , 22 We have successfully induced iN cells from adult human fibroblasts using human BAM factors in 2 weeks. 23 , 24 , 25 , 26 , 27 Recently, we have reported on gene expression analysis of iN cells derived from NF1 patients (NF1‐iN cells). 26 Interestingly, forskolin, an activator of AC pathway, rescued the abnormal gene expression in NF1‐iN cells to the levels of gene expression in iN cells derived from healthy controls (HC‐iN cells).
Therefore, we hypothesize that forskolin could compensate for some of the dynamic neuronal abnormalities in NF1‐iN cells. To clarify our hypothesis, we herein performed cytomorphological observations of iN cells in the presence or absence of forskolin.
2. METHODS
All methods of this study were performed in accordance with the Declaration of Helsinki and were approved by the ethics committees of Kyushu University (Fukuoka, Japan).
Human iN cells were generated as reported previously. 26 We used two fibroblasts cell lines from a NF1 patient and a healthy volunteer in the present study, which were already used in our previous report. 26 Briefly, on Day −3, fibroblasts were seeded in 35‐mm glass‐bottom dishes (Matsunami Glass) at a density of 1 × 105 cells/dish. On Day 0, lentiviruses infected to fibroblasts to each of the human BAM factors (human BRN2, ASCL1, MYT1L, MOI = 10 each) in Fibroblast Growth Medium (FGM) that contained 15% fetal bovine serum (Japan Bioserum), 0.1 mmol/L MEM Non‐Essential Amino Acids (Thermo Fisher Scientific), and 1% Pen Strep (Thermo Fisher Scientific) in Minimal Essential Medium Eagle (Sigma Chemical) which contained 8 µg/mL of polybrene (Sigma Chemical) for 24 hours. On Day 1, the medium was changed with fresh FGM. After Day 2, the medium was changed every 3 days with iN Medium (10 ng/mL FGF2 [Peprotech], 1 mmol/L valproic acid [Sigma Chemical], 0.8% N2 supplement [Thermo Fisher Scientific], 0.4% B27 supplement [Thermo Fisher Scientific], 1% Pen Strep, 10 µg/mL blasticidin [Thermo Fisher Scientific] in Dulbecco's Modified Eagle's Medium/Nutrient Mixture F12 Ham [Sigma Chemical]: Neurobasal medium [Thermo Fisher Scientific] = 4:1).
On Day 14, Forskolin (Nacalai Tesque) was added to the cell culture medium (final concentration 10 µmol/L), and images of cell contour were captured every 2 minutes for 90 minutes using an Olympus IX70‐22FL inverted microscope and ×20 objective (Olympus).
For cell counting, ImageJ was used to measure the number of cells with an apparently spherical‐form in all cells. To preclude bias, the cell measurements were performed blind to cell type and condition. To determine the differences between the groups, two‐way ANOVA followed by Tukey's correction was used by GraphPad Prism 7.04 (GraphPad Software).
3. RESULTS
In iN cells from healthy control (HC‐iN cells), many cells were neuron‐like spherical‐form (Figure A, magenta arrow head). On the other hand, most cells of iN cells derived from NF1 patient were thin and flat (NF1‐iN cells: Figure C, cyan arrow head). This result suggests that most NF1‐iN cells could not form neuron‐like spherical‐form cell morphology because of deficit of AC ability. Interestingly, only 20 minutes after AC activation by forskolin treatment, most NF1‐iN cells began to have a dense cell contour and the cell morphology dramatically changed to neuron‐like spherical‐form (Figure D, cyan arrow head, Video S1). Furthermore, since forskolin appeared to promote neurite outgrowth of iN cells (Figure E‐H, yellow arrow head, Video S2), quantitative experiments and analysis should be performed on more samples in the near future.
FIGURE 1.
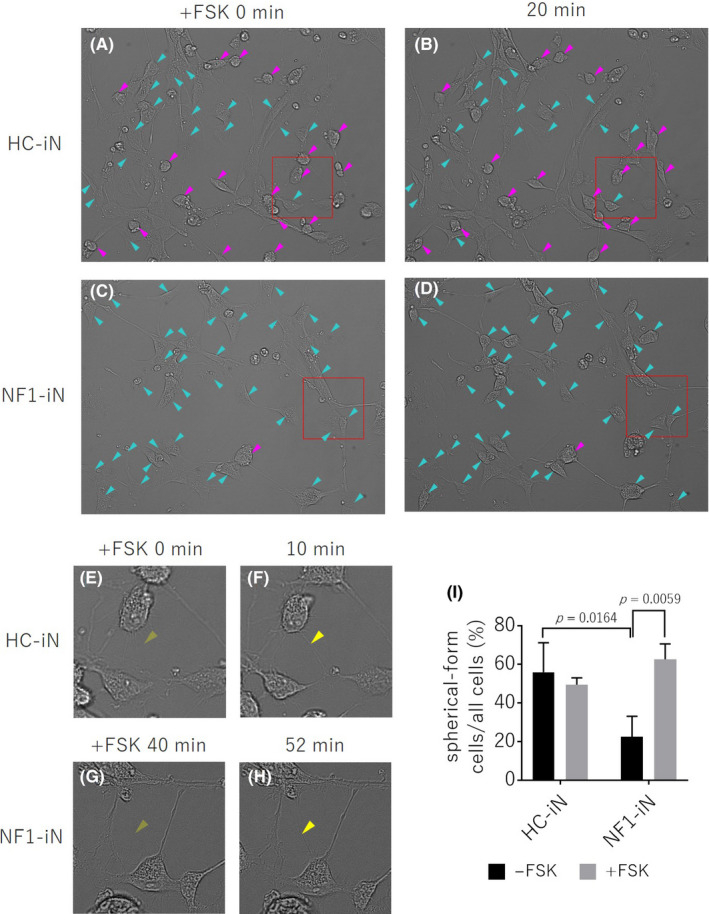
FIGURE (A‐D) Phase difference images of iN cells derived from healthy control (HC‐iN, A, B) and NF1 patient (NF1‐iN, C, D) after forskolin (FSK) treatment. Neuron‐like spherical‐form cells mainly appeared in HC‐iN cells (magenta arrow head). Flat cells with a thin cell contour obtain a dense cell contour only 20 minutes after forskolin treatment (cyan arrow head). (E‐H) Enlarged images of the part surrounded by the red frame on FIGURE A‐D. Forskolin appeared to enhanced neurite outgrowth in the iN cells (yellow arrow head). (I) The ratio of the number of neuronal‐like spherical‐form cells to the total number of cells. NF1‐iN cells in the absence of forskolin had a significantly lower percentage of the spherical‐form cells compared to HC‐iN cells (p = 0.0164, two‐way ANOVA / Tukey’s test, n = 3 each group). In the presence of forskolin, the spherical‐form cell morphology of NF1‐iN cells was significantly higher (p = 0.0059, two‐way ANOVA / Tukey’s test, n = 3 each group)
Cell counts showed that NF1‐iN cells were significantly flattened compared to HC‐iN cells (Figure 1, P = .0164), and their cell morphology was significantly recovered by forskolin treatment (Figure 1, P = .0059).
4. DISCUSSION
We have previously reported that forskolin improves gene expression abnormalities in NF1‐iN cells. 26 Here, we showed that forskolin dramatically changed the cell morphology from flatform to spherical form, especially in NF1‐iN cells, and the possibility of enhancing neurite outgrowth of NF1‐iN cells to the similar level of HC‐iN cells. These results only suggest a possible effect of forskolin, and further experiments will be needed to confirm these results.
How does forskolin change the cell morphology? Forskolin is known to activate intracellular ACs and increase intracellular cyclic adenosine monophosphate (cAMP) levels, and a previous report has shown that forskolin regulates cytoskeletal formation in mouse adrenal cortex tumor‐derived cell line Y1 cells. 28 Elevated intracellular cAMP levels cause dephosphorylation of paxillin at the edge of the cells, and paxillin moves from focal adhesion to cytoplasm. 28 NF1 patients have aberrant gene expression of neurofibromin 1 which is known to regulate AC activity and the intracellular cAMP levels. 12 We have recently shown that gene expression of neurofibromin 1 was also low in NF1‐iN cells, 26 suggesting low intracellular cAMP levels in NF1‐iN cells. We herein showed that NF1‐iN cells tend to have flatform cell morphology compared to HC‐iN cells and that these cell morphologies were rescued by the application of forskolin. Thus, such morphological abnormalities may be caused by abnormal cytoskeleton development due to lower levels of paxillin dephosphorylation from lower ACs activation and lower intracellular cAMP levels in NF1‐iN cells.
Paxillin is known to be involved in neurite outgrowth in experiments using rat adrenal medulla pheochromocytoma‐derived cell line PC12 cells. 29 Similarly, the present data have suggested that forskolin alters the phosphorylation state of paxillin and activated neurite outgrowth.
The present pilot experiment has indicated that AC and cAMP activation can normalize neuronal development in the brain of NF1 patients. We propose that the administration of forskolin or forskolin‐like AC activators into the brain of NF1 patients during neurodevelopmental periods may contribute to prevent neurodevelopmental disorders including ASD and neuropsychiatric disorders in later life.
4.1. Limitations
The present data should be validated with multiple further experiments. Further verification is necessary using neurofibromin 1 and/ or paxillin gene knockdown/ knockout iN cells or human iPS cell‐derived neurons, or in vivo NF1 model animals. Mechanism studies using the other AC activators/ inhibitors, regarding the association between NF1 and ACs, are also needed to validate the present pilot data. Moreover, the present findings based on morphological observation should be validated by additional analysis such as protein level analysis to determine the expression/ localization/ phosphorylation status of paxillin in NF1‐iN cells.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
TAK was the principal investigator of the present research. NS was the first author created the conception and design of the project and wrote the protocol. TAK, TN, and M.N‐K performed clinical recruitment and sampling collection. TAK, NS, S‐IK, MO, SI, YS, HK, and KM involved in the performance of experiments and data analyses/interpretation. NS wrote the first draft of the manuscript. TAK, SO, MF, AS, and SK made critical revisions of the manuscript. All authors contributed substantially to the scientific process leading up to the writing of the present manuscript and approved this submission in its current form.
ETHICAL APPROVAL OF THE RESEARCH PROTOCOL BY AN INSTITUTIONAL REVIEWER BOARD
All methods of this study were performed in accordance with the Declaration of Helsinki and were approved by the ethics committees of Kyushu University (Fukuoka, Japan).
INFORMED CONSENT
Informed consent was obtained from all the healthy volunteers and patients before donating skin fibroblasts.
REGISTRY AND THE REGISTRATION NO. OF THE STUDY/TRIAL
28‐89/2019‐541 (the ethics committees of Kyushu University).
ANIMAL STUDIES
This study did not include animal experiments (all data are derived from human cell experiments.)
Supporting information
Video S1
Video S2
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Ms Yuka Matsushita and Ms Aya Yamada for their technical assistance. This work was supported by Grant‐in‐Aid for Scientific Research on (1) Innovative Areas “Will‐Dynamics” of The Ministry of Education, Culture, Sports, Science, and Technology, Japan (JP16H06403 to TAK), (2) The Japan Agency for Medical Research and Development (AMED) (“Syogaisya‐Taisaku‐Sogo‐Kenkyu‐Kaihatsu‐Jigyo” JP19dk0307047 & JP19dk0307075, and “Yugo‐no” JP19dm0107095 to TAK), (3) KAKENHI—the Japan Society for the Promotion of Science (“Wakate A” JP26713039 and “Kiban A” JP18H04042 to TAK, and “Wakate B” JP26860932 & JP17K16386 to NS), (4) SENSHIN Medical Research Foundation (to TAK and SK), (5) Mochida Memorial Foundation for Medical and Pharmaceutical Research (to TAK), (6) NIH R00 MH093458 (S‐IK), (7) NIH RO1 MH‐105660 (AS), (8) the National Institute of Mental Health MH‐084018 (AS), (9) MH‐094268 Silvio O. Conte center (AS), (10) MH‐092443 (AS), (11) Stanley (AS), (12) S‐R/RUSK (AS), (13) NARSAD (AS), and (14) Maryland Stem Cell Research Fund (AS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Sagata N, Kano S‐I, Ohgidani M, et al. Forskolin rapidly enhances neuron‐like morphological change of directly induced‐neuronal cells from neurofibromatosis type 1 patients. Neuropsychopharmacol. Rep. 2020;40:396–400. 10.1002/npr2.12144
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the supplementary material of this article.
REFERENCES
- 1. Neurofibromatosis. Conference statement. National Institutes of Health Consensus Development Conference. Arch Neurol. 1988;45, 575–8. [PubMed] [Google Scholar]
- 2. Ferner RE. Neurofibromatosis 1 and neurofibromatosis 2: a twenty first century perspective. Lancet Neurol. 2007;6:340–51. [DOI] [PubMed] [Google Scholar]
- 3. Jouhilahti EM, Peltonen S, Heape AM, Peltonen J. The pathoetiology of neurofibromatosis 1. Am J Pathol. 2011;178:1932–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johnson NS, Saal HM, Lovell AM, Schorry EK. Social and emotional problems in children with neurofibromatosis type 1: evidence and proposed interventions. J Pediatr. 1999;134:767–72. [DOI] [PubMed] [Google Scholar]
- 5. Barton B, North K. Social skills of children with neurofibromatosis type 1. Dev Med Child Neurol. 2004;46:553–63. [DOI] [PubMed] [Google Scholar]
- 6. Morris SM, Acosta MT, Garg S, Green J, Huson S, Legius E, et al. Disease burden and symptom structure of autism in neurofibromatosis type 1: a study of the international NF1‐ASD Consortium Team (INFACT). JAMA Psychiatry. 2016;73:1276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Plasschaert E, Descheemaeker M‐J, Van Eylen L, Noens I, Steyaert J, Legius E, et al. Prevalence of autism spectrum disorder symptoms in children with neurofibromatosis type 1. Am J Med Genet B Neuropsychiatr Genet. 2015;168B:72–80. [DOI] [PubMed] [Google Scholar]
- 8. Garg S, Lehtonen A, Huson SM, Emsley R, Trump D, Evans DG, et al. Autism and other psychiatric comorbidity in neurofibromatosis type 1: evidence from a population‐based study. Dev Med Child Neurol. 2013;55:139–45. [DOI] [PubMed] [Google Scholar]
- 9. Garg S, Green J, Leadbitter K, Emsley R, Lehtonen A, Evans DG, et al. Neurofibromatosis type 1 and autism spectrum disorder. Pediatrics. 2013;132:e1642–e1648. [DOI] [PubMed] [Google Scholar]
- 10. Walsh KS, Vélez JI, Kardel PG, Imas DM, Muenke M, Packer RJ, et al. Symptomatology of autism spectrum disorder in a population with neurofibromatosis type 1. Dev Med Child Neurol. 2013;55:131–8. [DOI] [PubMed] [Google Scholar]
- 11. Weiss B, Bollag G, Shannon K. Hyperactive Ras as a therapeutic target in neurofibromatosis type 1. Am J Med Genet 1999;89:14–22. [PubMed] [Google Scholar]
- 12. Tong J, Hannan F, Zhu Y, Bernards A, Zhong Y. Neurofibromin regulates G protein‐stimulated adenylyl cyclase activity. Nat Neurosci. 2002;5:95–6. [DOI] [PubMed] [Google Scholar]
- 13. Wolman MA, de Groh ED, McBride SM, Jongens TA, Granato M, Epstein JA, et al. Modulation of cAMP and ras signaling pathways improves distinct behavioral deficits in a zebrafish model of neurofibromatosis type 1. Cell Rep. 2014;8:1265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ishii T, Ishikawa M, Fujimori K, Maeda T, Kushima I, Arioka Y, et al. In vitro modeling of the bipolar disorder and schizophrenia using patient‐derived induced pluripotent stem cells with copy number variations of PCDH15 and RELN. eNeuro. 2019;6: 0403–18. 10.1523/eneuro.0403-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang M, Zhang L, Gage FH. Modeling neuropsychiatric disorders using human induced pluripotent stem cells. Protein Cell. 2020;11:45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gamo NJ, Sawa A. Human stem cells and surrogate tissues for basic and translational study of mental disorders. Biol Psychiatry. 2014;75:918–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu YN, Lu SY, Yao J. Application of induced pluripotent stem cells to understand neurobiological basis of bipolar disorder and schizophrenia. Psychiatry Clin Neurosci. 2017;71(9):579–99. [DOI] [PubMed] [Google Scholar]
- 18. Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M, et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Srivastava R, Faust T, Ramos A, Ishizuka K, Sawa A. Dynamic changes of the mitochondria in psychiatric illnesses: new mechanistic insights from human neuronal models. Biol Psychiatry. 2018;83:751–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yoshimizu T, Pan JQ, Mungenast AE, Madison JM, Su S, Ketterman J, et al. Functional implications of a psychiatric risk variant within CACNA1C in induced human neurons. Mol Psychiatry. 2015;20:162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Victor MB, Richner M, Olsen HE, Lee SW, Monteys AM, Ma C, et al. Striatal neurons directly converted from Huntington's disease patient fibroblasts recapitulate age‐associated disease phenotypes. Nat Neurosci. 2018;21:341–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tang BL. Patient‐derived iPSCs and iNs‐shedding new light on the cellular etiology of neurodegenerative diseases. Cells. 2018;7: E38 10.3390/cells7050038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kano S, Yuan M, Cardarelli RA, Maegawa G, Higurashi N, Gaval‐Cruz M, et al. Clinical utility of neuronal cells directly converted from fibroblasts of patients for neuropsychiatric disorders: studies of lysosomal storage diseases and channelopathy. Curr Mol Med. 2015;15:138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Passeri E, Wilson AM, Primerano A, Kondo MA, Sengupta S, Srivastava R, et al. Enhanced conversion of induced neuronal cells (iN cells) from human fibroblasts: utility in uncovering cellular deficits in mental illness‐associated chromosomal abnormalities. Neurosci Res. 2015;101:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Passeri E, Jones‐Brando L, Bordón C, Sengupta S, Wilson AM, Primerano A, et al. Infection and characterization of Toxoplasma gondii in human induced neurons from patients with brain disorders and healthy controls. Microbes Infect. 2016;18:153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sagata N, Kato TA, Kano S‐I, Ohgidani M, Shimokawa N, Sato‐Kasai M, et al. Dysregulated gene expressions of MEX3D, FOS and BCL2 in human induced‐neuronal (iN) cells from NF1 patients: a pilot study. Sci Rep. 2017;7:13905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Akamine S, Sagata N, Sakai Y, Kato TA, Nakahara T, Matsushita Y, et al. Early‐onset epileptic encephalopathy and severe developmental delay in an association with de novo double mutations in NF1 and MAGEL2. Epilepsia Open. 2018;3:81–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Han JD, Rubin CS. Regulation of cytoskeleton organization and paxillin dephosphorylation by cAMP. Studies on murine Y1 adrenal cells. J Biol Chem. 1996;271:29211–5. [DOI] [PubMed] [Google Scholar]
- 29. Ishitani T, Ishitani S, Matsumoto K, Itoh M. Nemo‐like kinase is involved in NGF‐induced neurite outgrowth via phosphorylating MAP1B and paxillin. J Neurochem. 2009;111:1104–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1
Video S2
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.


