Abstract
Pathological angiogenesis is an important component of hepatic fibrosis along with fibrous deposition, but its role is not well understood. Here, we demonstrated that fibronectin containing extra domain A(FN-EDA), a fibronectin splice variant highly expressed in hepatic fibrosis, mediated angiogenesis in disease progression. FN-EDA was positively correlated with pathological angiogenesis in hepatic fibrosis, and a reduction in FN-EDA expression was associated with diminished intrahepatic angiogenesis and fibrosis. FN-EDA mostly colocalized with hepatic stellate cells (HSCs) and interference or blockage of FN-EDA attenuated migration and tube formation in co-cultured endothelial cells. Mechanistic studies indicated that FN-EDA was secreted to promote phosphorylation of VEGFR2 with the assistance of integrin and CD63. Targeting FN-EDA-integrin combination postponed the progression of hepatic angiogenesis and fibrosis in vivo. These results indicated that FN-EDA plays an emerging role in angiogenesis in hepatic fibrosis and could be a potential therapeutic intervention for the disease.
Subject terms: Liver fibrosis, Liver cirrhosis
Introduction
Hepatic fibrosis is a successive process that is accompanied by excessive deposition of extracellular matrixes (ECM) and pathological angiogenesis, which is frequently observed in patients with chronic liver diseases1. Without proper and timely intervention, hepatic fibrosis gradually tends to become hepatic cirrhosis, one of the most common lethal diseases worldwide2. Recently, increasing evidence have indicated that intrahepatic pathological angiogenesis with an aberrant angioarchitecture is an indispensable part of hepatic fibrogenesis3,4. Pathological angiogenesis triggered by vascular endothelial growth factor (VEGF) overproduction is believed to be central to liver fibrosis progress and the development of portal hypertension5–7. VEGF/VEGFR2 signaling is essential in angiogenesis and the crosstalk between hepatocytes and hepatic sinusoidal endothelial cells (HSECs), some studies even suggest VEGFR2 inhibitor Bevacizumab could attenuate hepatic fibrosis8–12.
Fibronectin (FN) is a high-molecular-weight multifunctional glycoprotein whose pre-mRNA has three alternative splicing sites (extra domain A (EDA), extra domain B (EDB), and type III homology connecting segment (IIICS)) which generates twenty different isoforms of the FN protomer. Traditionally, circulating soluble plasma FN (pFN) lacks both the EDA and EDB segments secreted by hepatocytes, while cellular FN (cFN) contains variable proportions of EDA or EDB or both which are enriched in the extracellular matrix13–15. FN-EDA and FN-EDB are expressed nearly ubiquitously in embryonic tissues and are associated with cardiovascular development16–18 while their expressions are strictly limited in normal adult tissues but is increased in various pathological states. Accumulated evidence has demonstrated that FN-EDA participates in some fibrotic diseases of many organs, including the dermis, lung and bone marrow19–21, and several physiopathologic processes such as intimal proliferation, wound healing and ischemia reperfusion injury22–25.
The explicit role of FN-EDA in hepatic fibrosis is still controversial. Previous studies reported that FN-EDA is upregulated by TGF-β in hepatic fibrosis model26, and male FN-EDA KO mice are protected from CCl4-induced hepatic fibrosis27. However, an in vivo study indicated that total fibronectin is dispensable for hepatic fibrogenesis28. In addition, although some reports suggest that FN-EDA promotes HSC activation29, others found that FN-EDA could promote only HSC motility but not differentiation27. FN-EDA participates in hepatic fibrosis but has a limited effect on fibrogenesis. Our previous works have demonstrated that the expression of FN is elevated in HSCs via the LPS/TLR4 pathway in a mouse liver fibrosis model, and we preliminary verified its association with vascular changes30. Therefore, considering our previous works and that FN-EDA participates in embryonic vascular morphogenesis and retinal neovascularization18,31, we propose that FN-EDA may mediate pathological angiogenesis in hepatic fibrosis.
Herein, we investigated the expression of FN-EDA and analyzed its relationship with intrahepatic angiogenesis in hepatic fibrosis patients and a CCl4-induced mouse hepatic fibrosis model. Mechanistically, we demonstrated that FN-EDA secreted from HSCs promoted pathological angiogenesis by activating the VEGFR2 pathway in endothelial cells with the assistance of integrin and CD63 in a paracrine manner. Meanwhile, we preliminarily evaluated the potential therapeutic effect of blocking FN-EDA in vivo. Our studies identified a new molecular mechanism of FN-EDA on pathological angiogenesis and provided a potential target for therapeutic interventions in hepatic fibrosis.
Results
FN-EDA expression was elevated in hepatic fibrosis and positively correlated with angiogenesis
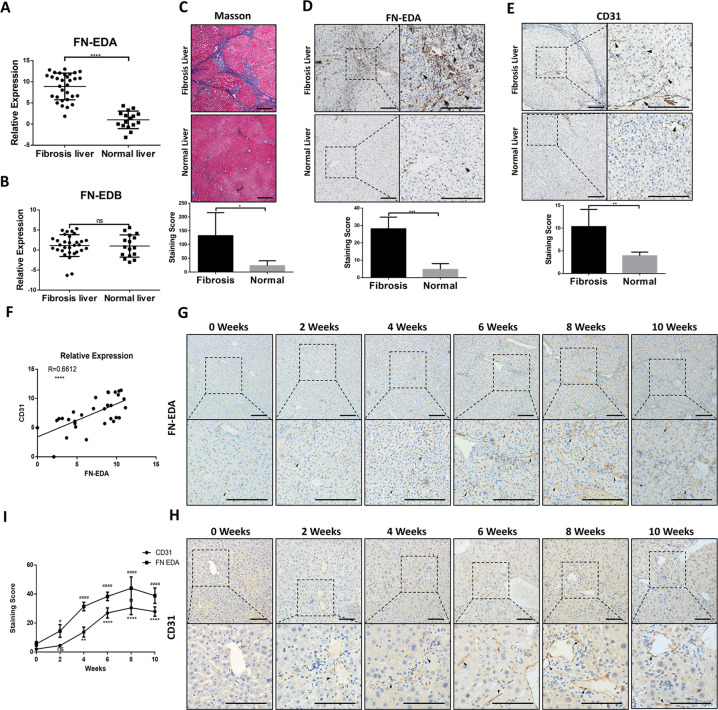
Our previous study demonstrated that FN is overexpressed in HSCs in mouse hepatic fibrosis. As EDA and EDB are the segments of FN unique to pathological states and are independently expressed due to alternative splicing32,33, we first detected their expression in normal and fibrotic livers. qRT-PCR analysis showed that EDA expression was significantly increased in the fibrotic group versus the healthy group (Fig. 1A) while the expression of EDB was not significantly different between the two groups (Fig. 1B). Thus, we obtained human hepatic sections and detected the expression of FN-EDA. Masson staining showed the differences in collagen deposition between patients and healthy individuals (Fig. 1C), and immunohistochemical analysis suggested that FN-EDA expression was significantly stronger in human fibrotic hepatic tissues than in normal tissues (Fig. 1D). We observed FN-EDA expression mainly in the Disse space, indicating that FN-EDA may be mechanistically involved in the biological functions of endothelial cells.
Fig. 1. Expression of FN-EDA is associated with CD31 in hepatic fibrosis.
A, B qRT-PCR analysis of FN-EDA and FN-EDB mRNA levels between liver tissue of CHD patients (n = 30) and healthy control (n = 15). (GAPDH was used as an internal control. Data are shown as the mean ± SEM. FN-EDA: ****P < 0.0001; FN-EDB: P: ns) C Masson staining in human fibrosis hepatic tissues (n = 5) and normal tissues (n = 5). (quantification relative area of Masson staining. Data are shown as mean ± SEM. *P = 0.0217. Scale bar = 200 μm. D Immunohistochemical staining of FN-EDA in human fibrosis hepatic tissues (n = 5) and normal tissues (n = 5). (Data are shown as mean ± SEM. ***P = 0.0001. Scale bar = 200 μm). E Immunohistochemical staining of CD31 in human fibrosis hepatic tissues (n = 5) and normal tissues (n = 5). (Data are shown as mean ± SEM. **P = 0.0060. Scale bar = 200 μm). F qRT-PCR analysis of correlation between FN-EDA and CD31 in human fibrosis hepatic tissues (n = 30). (R = 0.6612 ****P < 0.0001) G, H FN-EDA and CD31 staining in livers of CCl4-treated mice biweekly until tenth weeks (n = 18) (Scale bar = 200 μm; arrows: staining) I Analysis of FN-EDA and CD31 expression in IHC (n = 3).
Therefore, we further explored the relationship between FN-EDA and pathological angiogenesis in liver fibrosis. First, we detected CD31 (a reliable marker for angiogenesis) expression in the above hepatic tissues and found that CD31 was highly expressed in fibrotic livers (Fig. 1E). Meanwhile, FN-EDA expression was positively correlated with CD31 in human fibrosis liver samples by qRT-PCR analysis (Fig. 1F). Furthermore, we produced CCl4-treated mice, harvested hepatic tissue and evaluated the expression of FN-EDA and CD31 in these tissues at the histological level biweekly until ten weeks. We found that both FN-EDA and CD31 expression were elevated over time with CCl4 treatment especially in the first 8 weeks and were significantly higher than at the beginning (Fig. 1G–I). The above results suggested that FN-EDA was positively correlated with angiogenesis in hepatic fibrosis.
FN-EDA derived from HSCs promoted pathological angiogenesis in hepatic fibrosis
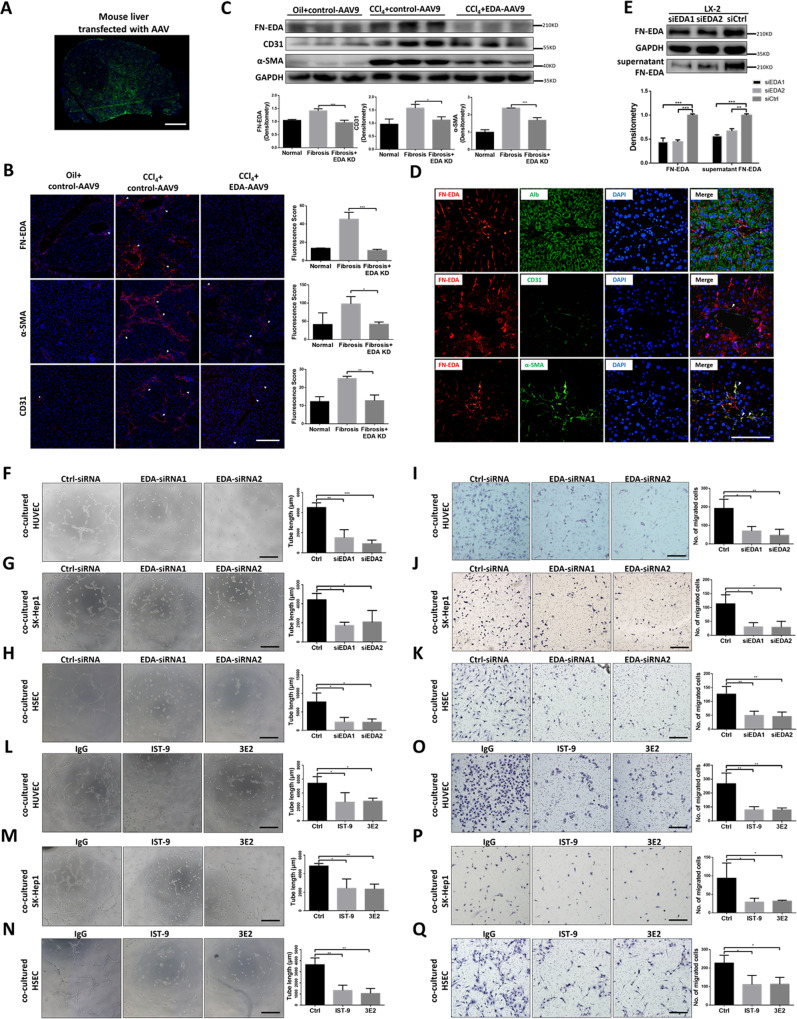
To determine whether FN-EDA promoted angiogenesis in hepatic fibrosis, we produced FN-EDA knockdown mice through tail vein injection using a recombinant EDA-AAV9 vector and then treated the mice with CCl4 for 8 weeks. EGFP fluorescence showed the transfection efficiency in mouse livers (Fig. 2A), and the expression of FN-EDA was significantly decreased (Fig. 2B, C). FN-EDA KD mice had diminished neovessel density, CD31 expression and fibrosis level compared with the control group (Fig. 2B, C). The above results indicated that FN-EDA mediated pathologic angiogenesis in hepatic fibrosis to some extent. Next, we defined the cellular sources of FN-EDA during chronic hepatic fibrosis. We examined the colocalization of FN-EDA with albumin (hepatocyte marker), CD31 (endothelial marker), and α-SMA (activated HSC marker) in mouse fibrosis hepatic tissues. The expression of FN-EDA largely overlapped with that of α-SMA, slightly overlapped with that of CD31 and did not overlap with that of albumin (Fig. 2D), indicating that FN-EDA was mainly expressed in HSCs during chronic hepatic fibrosis.
Fig. 2. FN-EDA promoted angiogenesis in vivo and vitro.
A Overall view of mouse liver infected with AAV9 (Green: EGFP). Six-week-old male wild-type C57BL/6 mice were intraperitoneally injected with 20% CCl4 dissolved in olive oil, or olive oil alone twice a week (2 ml/kg) for 8 weeks. AAV9 control vector (AAV9-Ctrl) or AAV9-FN-EDA-shRNA were injected into mice through tail vein at the beginning of week 1 and week 5. Mouse livers were harvest at the end of week 8. (Scale bar = 2.5 mm.) B Immunofluorescence staining of FN-EDA, α-SMA and CD31 in AAV9-FN-EDA-shRNA or AAV9-Ctrl infected mice with experimental hepatic fibrosis (n = 3). (Arrows: staining. Data are shown as mean ± SEM. FN-EDA: ***P < 0.0001; α-SMA: *P < 0.01; CD31: **P < 0.001. Scale bar = 200 μm.) C Immunoblot analysis of FN-EDA, α-SMA and CD31 in AAV9-FN-EDA-shRNA or AAV9-Ctrl infected mice with experimental hepatic fibrosis (n = 3). (Data are shown as mean ± SEM. α-SMA: ***P < 0.0001; FN-EDA: ***P < 0.0001; CD31: **P < 0.001.) D Mouse experimental hepatic fibrosis samples were processed for immunofluorescence co-staining for FN-EDA (red) with α-SMA (activated HSC marker), CD31 (endothelial marker), and Alb (hepatocyte marker) (green). Nucleus were counterstained with DAPI. (Arrows: co-staining. Scale bar = 100 μm.) E Immunoblot result of FN-EDA in LX-2 cells and its supernatant. LX-2 cells were transfected with two FN-EDA-siRNAs or Ctrl-siRNA following the general procedure. Serum-free DMEM was replaced for 24 h culturing and then collected supernatant and add loading buffer for immunoblot. (Data are shown as mean ± SEM; FN-EDA: EDA-siRNA1 versus Ctrl, ***P < 0.0001; EDA-siRNA2 versus Ctrl, ***P < 0.0001; supernatant FN-EDA: EDA-siRNA1 versus Ctrl, ***P < 0.0001; EDA-siRNA2 versus Ctrl, **P < 0.001.) F–H Tube formation assay of HUVECs, SK-hep1 cells and HSECs co-cultured with LX-2 cells for 6 h. LX-2 cells were transfected with two FN-EDA-siRNAs or Ctrl-siRNA (n = 3). (Data are shown as mean ± SEM; HUVEC: EDA-siRNA1 versus Ctrl, **P < 0.001; EDA-siRNA2 versus Ctrl, ***P < 0.0001; SK-hep1: EDA-siRNA1 versus Ctrl, *P < 0.01; EDA-siRNA2 versus Ctrl, *P < 0.01. HSEC: EDA-siRNA1 versus Ctrl, *P < 0.01; EDA-siRNA2 versus Ctrl, *P < 0.01. Scale bar = 100 μm.) I–K Migration assay of HUVECs, SK-hep1 cells and HSECs co-cultured with LX-2 for 24 h (n = 3). (Data are shown as mean ± SEM; HUVECs: EDA-siRNA1 versus Ctrl, *P < 0.01; EDA-siRNA2 versus Ctrl, ***P < 0.0001; SK-hep1 cells: EDA-siRNA1 versus Ctrl, *P < 0.01; EDA-siRNA2 versus Ctrl, *P < 0.01; HSECs: EDA-siRNA1 versus Ctrl, **P < 0.001; EDA-siRNA2 versus Ctrl, **P < 0.001; scale bar = 100 μm.) L–N Tube formation assay of HUVECs, SK-hep1 cells and HSECs co-cultured with LX-2 for 6 h. FN-EDA specific neutralizing antibodies IST-9 or 3E2 or mouse IgG (40 μg/ml) were pretreated for 0.5 h (n = 3). (Data are shown as mean ± SEM; HUVEC: IST-9 versus Ctrl, *P < 0.01; 3E2 versus Ctrl, *P < 0.01; SK-hep1: IST-9 versus Ctrl, *P < 0.01; 3E2 versus Ctrl, **P < 0.001. HSEC: IST-9 versus Ctrl, **P < 0.001; 3E2 versus Ctrl, **P < 0.001, scale bar = 100 μm.) O–Q Migration assay of HUVECs, SK-hep1 cells and HSECs co-cultured with LX-2 for 24 h. FN-EDA specific neutralizing antibodies IST-9 or 3E2 or mouse IgG (40 μg/ml) was pretreated for 0.5 h (n = 3). (Data are shown as mean ± SEM; HUVEC: IST-9 versus Ctrl, **P < 0.001; 3E2 versus Ctrl, **P < 0.001; SK-hep1: IST-9 versus Ctrl, *P < 0.01; 3E2 versus Ctrl, **P < 0.001; HSEC: IST-9 versus Ctrl, *P < 0.01; 3E2 versus Ctrl, *P < 0.01, scale bar = 100 μm).
To further determine how FN-EDA participates in pathological angiogenesis, we first detected the expression of FN-EDA in LX-2 cells in vitro. Consistent with expectations, FN-EDA was highly detected in LX-2 and its supernatant and was decreased after knockdown by two different FN-EDA-siRNAs (Fig. 2E). Then, we used a transwell plate to coculture LX-2 cells with several endothelial cells, including HUVECs (a standard endothelial cell line), SK-hep1 cells (a liver derived endothelial cell line) and primary HSECs. Tube formation and migration of endothelial cells were significantly decreased after knocking down FN-EDA in LX-2 cells (Fig. 2F–K). To further determine whether FN-EDA itself could activate endothelial cells in a direct manner, we used two different FN-EDA specific neutralizing antibodies, IST-9 and 3E2, to block FN-EDA in the above coculture environment. Tube formation and migration of endothelial cells promoted by LX-2 were also significantly attenuated (Fig. 2L–Q). In summary, all the results indicated that HSC derived FN-EDA promoted pathological angiogenesis in hepatic fibrosis in a paracrine manner.
FN-EDA activated VEGFR2 phosphorylation not completely dependent on VEGFA
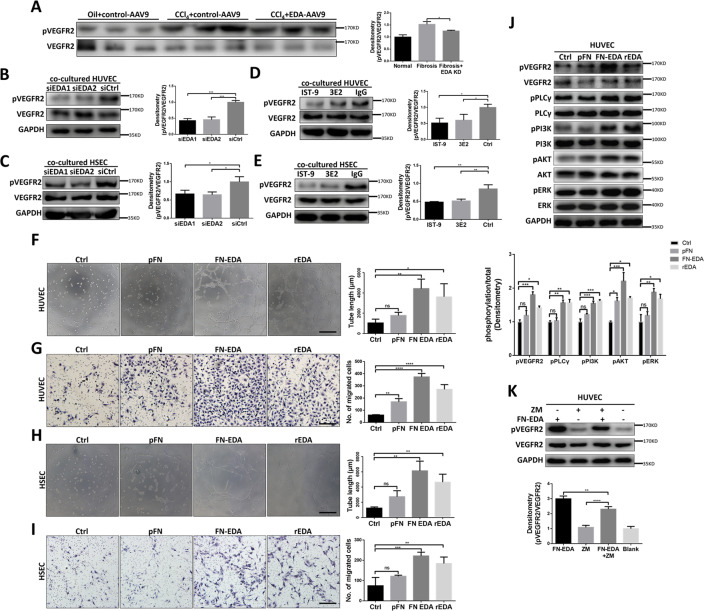
Continuous hyperactivation of the VEGFR2 related pathway is considered the most critical aspect of pathological angiogenesis during chronic hepatic fibrosis34, so we examined the phosphorylation of VEGFR2 after knocking down and blocking FN-EDA. Decreasing phosphorylation levels of VEGFR2 were observed in FN-EDA KD mice comparing with fibrotic control (Fig. 3A), and knocking down of FN-EDA in LX-2 and blocking FN-EDA by neutralizing antibodies in vitro, decreased level phosphorylation levels of VEGFR2 was observed in co-cultured HUVEC and HSEC (Fig. 3B–E). To further determine whether the EDA segment itself plays a decisive role, we used recombinant EDA (rEDA), FN-EDA and pFN (EDA lacking FN) to treat HUVECs and HSECs, respectively. FN-EDA and rEDA but not pFN significantly enhanced the motility and tube formation of HUVECs and HSECs (Fig. 3F–I). Meanwhile, the phosphorylation of VEGFR2 and its downstream pathways including PI3K, AKT, PLCγ, and ERK were dramatically increased after stimulation by FN-EDA and rEDA compared with the control (Fig. 3J). A previous study suggested that FN-EDA increases VEGF-C expression in colorectal carcinoma35, so we considered whether this FN-EDA influenced VEGFR2 phosphorylation was VEGF dependent. We pretreated FN-EDA-stimulated HUVECs with the inhibitor ZM323881, which selectively inhibited VEGF stimulated VEGFR2 phosphorylation. Immunoblot results suggested ZM323881 only partly weakened the phosphorylation of VEGFR2 after treatment with FN-EDA (Fig. 3K), indicating that in addition to VEGF, there was another potential pathway through which FN-EDA could stimulate VEGFR2 phosphorylation. In conclusion, the above results provide evidence that FN-EDA promotes the phosphorylation of VEGFR2.
Fig. 3. FN-EDA promoted phosphorylation of VEGFR2 in vivo and vitro.
A Immunoblot result of pVEGFR2 and total VEGFR2 in AAV9-FN-EDA-shRNA or AAV9-Ctrl infected mice with experimental hepatic fibrosis (n = 3). (Data are shown as mean ± SEM; *P < 0.01.) B, C Immunoblot result of pVEGFR2 in HUVECs and HSECs co-cultured with FN-EDA-siRNAs or Ctrl-siRNA transfected LX-2 cells for 8 h (n = 3). (Data are shown as mean ± SEM; HUVEC: EDA-siRNA1 versus Ctrl, ***P < 0.0001; EDA-siRNA2 versus Ctrl, **P < 0.0001; HSEC: EDA-siRNA1 versus Ctrl, *P < 0.01; EDA-siRNA2 versus Ctrl, *P < 0.01.) D, E Immunoblot result of pVEGFR2 in HUVECs and HSECs co-cultured with LX-2 for 8 h. LX-2 were pretreated with neutralizing antibodies IST-9 or 3E2 or mouse IgG (40 μg/ml) for 0.5 h (n = 3). (Data are shown as mean ± SEM; HUVEC: IST-9 versus Ctrl, *P < 0.01; 3E2 versus Ctrl, *P < 0.01; HSEC: IST-9 versus Ctrl, **P < 0.001; 3E2 versus Ctrl, **P < 0.001.) F Tube formation assay of HUVECs treated with pFN, FN-EDA or rEDA (40 μg/ml) for 6 h (n = 3). (Data are shown as mean ± SEM; rEDA versus Ctrl: *P < 0.01; FN-EDA versus Ctrl: **P < 0.001; pFN versus Ctrl: ns. Scale bar = 100 μm.) G Migration assay of HUVECs treated with pFN, FN-EDA or rEDA (40 μg/ml) for 24 h. (Data are shown as mean ± SEM, n = 3. rEDA versus Ctrl: ****P < 0.00001; FN-EDA versus Ctrl: ****P < 0.00001; pFN versus Ctrl: **P < 0.001. Scale bar = 100 μm.) H Tube formation assay of HSECs treated with pFN, FN-EDA or rEDA (40 μg/ml) for 6 h (n = 3). (Data are shown as mean ± SEM; rEDA versus Ctrl: **P < 0.001; FN-EDA versus Ctrl: **P < 0.001; pFN versus Ctrl: ns. Scale bar = 100 μm.) I Migration assay of HSECs treated with pFN, FN-EDA or rEDA (40 μg/ml) for 24 h. FN-EDA and rEDA significantly promote HSECs tube formation (n = 3). (Data are shown as mean ± SEM; rEDA versus Ctrl: *P < 0.01; FN-EDA versus Ctrl: **P < 0.001; pFN versus Ctrl: ns. Scale bar =100 μm.) J Immunoblot result of VEGFR2 related pathway in HUVECs treated with pFN, FN-EDA or rEDA (40 μg/ml) for 6 h. (Data are shown as mean ± SEM; pVEGFR2: rEDA versus Ctrl: *P < 0.01; FN-EDA versus Ctrl: ***P < 0.0001; pFN versus Ctrl: ns; pPLCγ rEDA versus Ctrl: **P < 0.001; FN-EDA versus Ctrl: **P < 0.001; pFN versus Ctrl: ns; pPI3K rEDA versus Ctrl: ***P < 0.0001; FN-EDA versus Ctrl: ***P < 0.0001; pFN versus Ctrl: ns; pAKT rEDA versus Ctrl: *P < 0.01; FN-EDA versus Ctrl: ***P < 0.0001; pFN versus Ctrl: *P < 0.01; pERK rEDA versus Ctrl: *P < 0.01; FN-EDA versus Ctrl: **P < 0.001; pFN versus Ctrl: ns.) K Immunoblot result of pVEGFR2 in HUVECs. HUVECs were treated with FN-EDA (40 μg/ml) and/or ZM323881 (5 μM) for 6 h (n = 3). (Data are shown as mean ± SEM; FN-EDA versus FN-EDA + ZM323881, **P < 0.001; FN-EDA + ZM versus ZM323881, ****P < 0.00001).
FN-EDA promoted VEGFR2 phosphorylation by activating integrin receptors
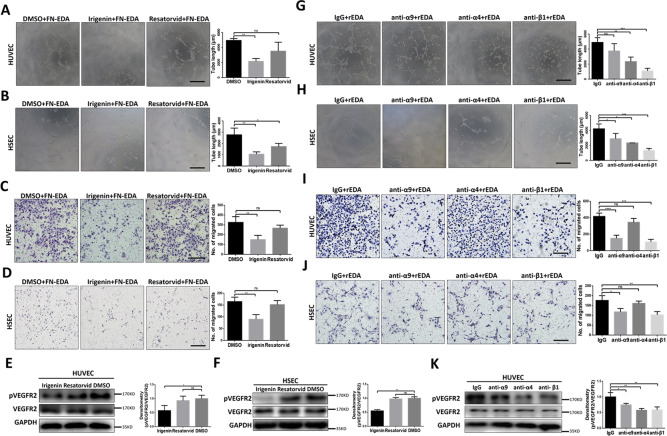
FN-EDA can bind integrins and Toll-like receptor 4 (TLR4)19,36. To further explore the mechanism by which FN-EDA promotes the phosphorylation of VEGFR2, we used the small molecular inhibitor irigenin (specifically blocking the EDA segment and integrin conjunction37) or resatorvid (a specific TLR4 inhibitor19) to specifically inhibit the interaction of FN-EDA and these potential receptors. Irigenin showed an inhibitory effect in both migration and tube formation assays on HUVECs and HSECs after stimulation by FN-EDA while resatorvid showed only a limited inhibitory effect (Fig. 4A–D). Meanwhile, phosphorylation of VEGFR2 was significantly weakened by irigenin but not resatorvid which was consistent with the above results (Fig. 4E, F).
Fig. 4. FN-EDA promoted angiogenesis through Integrins.
A, B Tube formation assay of HUVECs and HSECs treated with Irigenin or Resatorvid (5 μM) along with FN-EDA (40 μg/ml) for 6 h (n = 3). (Data are shown as mean ± SEM; HUVEC: Irigenin versus Ctrl: **P < 0.001; Resatorvid versus Ctrl: ns. HSEC, Irigenin versus Ctrl: **P < 0.001; Resatorvid versus Ctrl: *P < 0.01; scale bar = 100 μm.) C, D Migration assay of HUVECs and HSECs treated with Irigenin or Resatorvid (5 μM) along with FN-EDA (40 μg/ml) for 24 h (n = 3). (Data are shown as mean ± SEM; HUVEC, Irigenin versus Ctrl: **P < 0.001; Resatorvid versus Ctrl: ns. HSEC, Irigenin versus Ctrl: **P < 0.001; Resatorvid versus Ctrl: ns; scale bar = 100 μm.) E, F Immunoblot result of HUVECs and HSECs treated with Irigenin or Resatorvid (5 μM) along with FN-EDA (40 μg/ml) for 6 h (n = 3). (Data are shown as mean ± SEM; HUVEC: Irigenin versus Ctrl: *P < 0.01; Restorvid versus Ctrl: ns; HSEC: Irigenin versus Ctrl: **P < 0.001; Restorvid versus Ctrl: ns.) G, H Tube formation assay of HUVECs and HSECs pretreated with varies of integrin neutralizing antibodies anti-α9, anti-α4, anti-β1 or IgG (40 μg/ml) 20 minutes before rEDA (40 μg/ml) stimulation for 6 h (n = 3). (Data are shown as mean ± SEM; HUVEC: anti-α9 versus Ctrl: ns; anti-α4 versus Ctrl: **P < 0.001; anti-β1 versus Ctrl: ***P < 0.0001. HSEC, anti-α9 versus Ctrl: *P < 0.01; anti-α4 versus Ctrl: *P < 0.01; anti-β1 versus Ctrl: ***P < 0.0001; scale bar = 100 μm.) I, J Migration assay of HUVECs and HSECs treated with varies of integrin neutralizing antibodies anti-α9, anti-α4, anti-β1 or mouse IgG (40 μg/ml) 20 minutes before rEDA (40 μg/ml) stimulation for 24 h (n = 3). (Data are shown as mean ± SEM; HUVEC, anti-α9 versus Ctrl: ***P < 0.0001; anti-α4 versus Ctrl: ns; anti-β1 versus Ctrl: ***P < 0.0001. HSEC, anti-α9 versus Ctrl: **P < 0.001; anti-α4 versus Ctrl: ns; anti-β1 versus Ctrl: *P < 0.01; scale bar = 100 μm.) K Immunoblot result of HUVECs pretreated with varies of integrin neutralizing antibodies anti-α9, anti-α4, anti-β1 or IgG (40 μg/ml) 20 min before rEDA (40 μg/ml) stimulation for 6 h (n = 3). (Data are shown as mean ± SEM; HUVEC, anti-α9 versus Ctrl: *P < 0.01; anti-α4 versus Ctrl: **P < 0.001; anti-β1 versus Ctrl: **P < 0.001).
Therefore, we focused on integrin and used a series of integrin neutralizing antibodies, including anti-α4, anti-α9, and anti-β1 to explore which integrin subunit plays the core role. We used rEDA to stimulate endothelial cells that were pretreated with the above integrin neutralizing antibodies. In HUVECs and HSECs, after blocking integrin β1, both migration and tube formation were dramatically inhibited while after blocking integrin α4 only tube formation ability was significantly attenuated, and after blocking integrin α9 only migration ability was significantly decreased (Fig. 4G–J). Furthermore, we detected phosphorylation of VEGFR2 after blocking the above integrins before treated with rEDA. We observed that after blocking integrin α4 and β1, phosphorylation of VEGFR2 was significantly decreased (Fig. 4K). These results indicated that integrin receptors, especially integrin β1, were crucial for FN-EDA-promoted angiogenesis and VEGFR2 phosphorylation.
CD63 mediated integrin and VEGFR2 coaggregation is crucial for FN-EDA induced angiogenesis
Integrins are important mediators in angiogenesis and are often coactivated by receptor tyrosine kinases (RTKs), including VEGFRs38,39. Therefore, we detected the integrin downstream pathway and found that phosphorylation of Src and FAK was significantly increased after FN-EDA stimulation and was attenuated after inhibition by irigenin (Fig. 5A). Several studies have demonstrated transactivation of RTKs in a ligand independent manner through integrin and its downstream kinases especially Src40–42. Thus, we pretreated HUVECs with SU6656 (a specific Src inhibitor) before FN-EDA stimulation, and it was observed that the phosphorylation of VEGFR2 enhanced by FN-EDA was decreased (Fig. 5B).
Fig. 5. CD63 participated in FN-EDA induced angiogenesis.
A Immunoblot result of HUVECs treated with FN-EDA and/or irigenin for 6 h. (Data are shown as mean ± SEM; pFAK: FN-EDA versus Irigenin+FN-EDA: **P < 0.001, FN-EDA versus Ctrl: **P < 0.001; pSrc: FN-EDA versus Irigenin+FN-EDA: **P < 0.001, FN-EDA versus Ctrl: **P < 0.001.) B Immunoblot result of HUVECs treated with FN-EDA and/or Src inhibitor SU6656 for 6 h. (Data are shown as mean ± SEM; pVEGFR2: FN-EDA + SU6656 versus FN-EDA: *P < 0.01; pSrc: FN-EDA versus Ctrl: ns; pSrc: FN-EDA + SU6656 versus FN-EDA: **P < 0.001; pSrc: FN-EDA versus Ctrl: ns.) C Immunoblot result of HUVECs lysis immunoprecipitated by anti-flag antibody. HUVEC were treated with rEDA (40 μg/ml) for 6 h before harvest. D Tube formation assay of HUVECs transfected with CD63-siRNA alone or both CD63-siRNA and CD63-plasmid before treated with FN-EDA (40 μg/ml) for 6 h (n = 3). (Data are shown as mean ± SEM; CD63-siRNA versus CD63-siRNA+ CD63-plasmid: *P < 0.01 CD63-siRNA versus Ctrl-siRNA: *P < 0.01; scale bar = 100 μm.) E Migration assay of HUVECs transfected with CD63-siRNA alone or both CD63-siRNA and CD63-plasmid before treated with FN-EDA (40 μg/ml) for 24 h (n = 3). (Data are shown as mean ± SEM; CD63-siRNA versus CD63-siRNA+ CD63-plasmid: **P < 0.001 CD63-siRNA versus Ctrl-siRNA: **P < 0.001. Scale bar = 100 μm.) F Immunoblot result of HUVECs transfected with CD63-siRNA alone or both CD63-siRNA and CD63-plasmid before treated with FN-EDA (40 μg/ml) for 6 h. (Data are shown as mean ± SEM; pVEGFR2: CD63-siRNA versus CD63-siRNA+CD63-plasmid: *P < 0.01; CD63-siRNA versus Ctrl: **P < 0.001; pFAK: CD63-siRNA versus CD63-siRNA+CD63-plasmid: ns; CD63-siRNA versus Ctrl: ns; pSrc: CD63-siRNA versus CD63-siRNA+CD63-plasmid: ns; CD63-siRNA versus Ctrl: ns; CD63: CD63-siRNA versus CD63-siRNA+CD63-plasmid: ***p < 0.0001; CD63-siRNA versus Ctrl: ***p < 0.0001.) G Immunohistochemical staining of CD63 in mice fibrosis hepatic tissues. Immunofluorescence shows CD63 (red) and CD31 (green) were co-stained.
Src is recruited to activated integrins39, if recruited kinases straightforward activate VEGFR2, the neighborship between integrins and VEGFR2 must be important. Tetraspanins are a family of proteins that form tetraspanin-enriched microdomains within the plasma membrane and simultaneously bind receptors, including RTKs and integrins43. CD63 is among the most highly expressed tetraspanins in endothelial cells44. Therefore, we performed co-immunoprecipitation to confirm the combination of FN-EDA with β1-CD63-VEGFR2 complex. Integrin, CD63 and VEGFR2 were co-immunoprecipitated with rEDA (Fig. 5C). Next, we knocked down CD63 expression in HUVECs and found that tube formation and migration stimulated by FN-EDA were significantly decreased, and angiogenesis abilities were recovered when CD63 was reoverexpressed (Fig. 5D, E). Similar changes were also observed in the phosphorylation of VEGFR2 while the phosphorylation of FAK and Src were barely impaired (Fig. 5F). In addition, CD63 was highly expressed in the neovessel areas and colocalized with CD31 in mouse experimental liver fibrosis models (Fig. 5G). The above results indicate that CD63, the bridge linking integrin β1 with VEGFR2 to maintain their spatial contiguity, plays an indelible role.
Blocking of EDA/integrin combination suppresses hepatic angiogenesis and fibrosis in vivo
Irigenin is an active ingredient of the herbal medicine Rhizoma Belamcanda, which is officially listed in the Chinese pharmacopoeia and is widely used45. Considering its specificity in targeting the FN-EDA C-Cʹ loop37 and its known safety as a bioactive constituent of an officially-approved drug, irigenin could be a potential anti-angiogenesis drug for hepatic fibrosis. Therefore, we treated mice with irigenin via intragastric gavage every 2 days 2 weeks after the first treatment with CCl4 and collected liver tissue every two weeks thereafter (Fig. 6A). Immunohistochemical analysis showed that the irigenin-treated group had less CD31 and α-SMA expression and lower Masson stain scores than the control group in the early stage of fibrosis after treatment with irigenin. However, this protective effect was attenuated in the tenth weeks, and the differences in Masson stain and α-SMA expression were reduced (Fig. 6B–D). We also examined the fenestration of HSECs in six weeks CCl4-treated mouse hepatic tissues using transmission electron microscopy. In CCl4-treated mice, HSECs were capillarized with a thick basement membrane and shrunken fenestration, while the irigenin-treated group had little basement membrane and more fenestrations (Fig. 6E). The results above indicated that irigenin may be a potential targeted candidate drug to inhibit angiogenesis by blocking the binding of FN-EDA and integrin in hepatic fibrosis.
Fig. 6. Irigenin relived mouse CCl4-induced hepatic fibrosis.
A Schematic representation of hepatic fibrosis induction in C57BL/6 mice and treated with irigenin. B Masson staining in mouse CCl4-induced fibrotic livers treated with irigenin (n = 3). (Analysis relative area of Masson staining. Data are shown as mean ± SEM; week 2: ns; week 4: *P = 0.0207; week 6: ***P = 0.0003; week 8: **P = 0.0087; week 10: ns; scale bar = 200 μm.) C Immunohistochemical analysis of α-SMA expression in mouse CCl4-induced fibrotic livers treated with irigenin (n = 3). (Data are shown as mean ± SEM. Week 2: ns; week 4: ***P = 0.0003; week 6: **P = 0.0019; week 8: *P = 0.0436; week 10: ns; scale bar = 200 μm.) D Immunohistochemical analysis of CD31 expression in mouse CCl4-induced fibrotic livers treated with irigenin. Irigenin reduced CD31 expression in liver during hepatic fibrosis (n = 3). (Data are shown as mean ± SEM; week 2: ns week 4: *P = 0.0235; week 6: *P = 0.0169; week 8: *P = 0.0160; week 10: ns; scale bar = 200 μm.) E Transmission electron microscope (TEM) showing the hepatic sinusoids in the liver tissues. (Short arrows: LSEC fenestrae; long arrows: basement membrane; scale bar = 10 μm).
Discussion
In this study, we focused on FN-EDA, a special splicing variant of fibronectin, and provided evidence to clarify its role in pathological angiogenesis and the cross-talk between HSCs and HSECs in hepatic fibrosis. We first verified the positive correlation between FN-EDA and pathological angiogenesis, and then demonstrated that FN-EDA promote angiogenesis in vitro and vivo. We observed that FN-EDA itself could promote the phosphorylation of VEGFR2, and further demonstrated the promotion effect occurred through integrin receptors and was CD63 dependent (Fig. 7). Moreover, we preliminarily verified the irigenin, which specifically blocks the conjunction of FN-EDA and integrin, as a potential anti-hepatic fibrosis therapy.
Fig. 7. Schematic figure illustrating the mechanism of FN-EDA promoting angiogenesis in hepatic fibrosis.
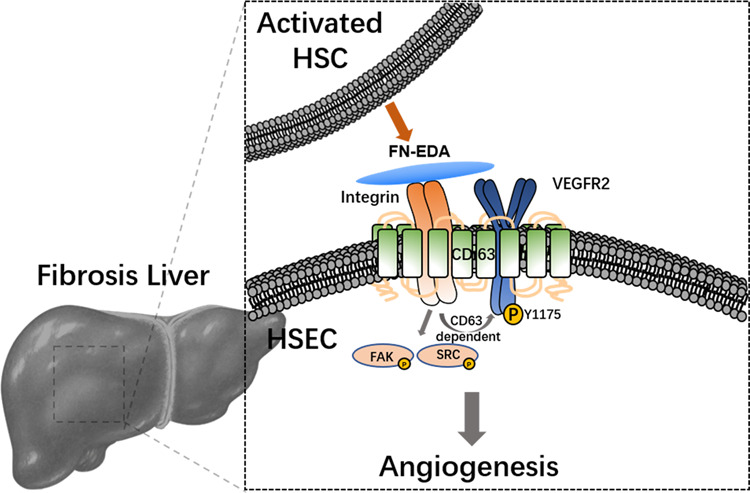
In hepatic fibrosis, FN-EDA is over secreted by activated HSCs to HSECs and stimulates activation of HSECs via integrin-CD63-VEGFR2 receptor complex which leading to pathological angiogenesis.
Hepatic fibrosis is characterized by excessive ECM deposition and increased intrahepatic angiogenesis which is induced by activation of HSCs and HSECs. However, the intricate interplays have not been fully understood yet. HSCs and HSECs maintain co-activation during hepatic fibrosis that not only do capillarized HSECs secret fibroblast growth factor (FGF) and transforming growth factor-β1 (TGF-β1) to facilitate the activation and ECM deposition of HSCs, but also activated HSCs paracrine pro-angiogenic factors such as VEGF, platelet-derived growth factor (PDGF) and angiopoietins to promote angiogenesis of HESCs at the same time46–48. Over-deposited ECM help those pro-angiogenic factors to combine with their receptors and provide scaffold for pathological angiogenesis. Previous studies indicated that ECM-anchored VEGF prolonged activation of VEGFR49, and ECM components provide a binding scaffold for endothelial cell anchorage and migration during angiogenesis50. Moreover, further studies revealed the mechanobiological mechanism of ECM in hepatic fibrosis that mechanical strain generated during ECM remodeling has been shown to mediate intrahepatic angiogenesis51,52.
In spite of collagen is the quantitatively dominant matrix component in many fibrosis diseases, fibronectin is an early and important component which is upregulated in many fibrotic diseases and even considered as a fibrosis marker, there were limited investigation of its specific isoform and function. FN-EDA is reportedly upregulated and participated in fibrosis process, but most of the studies focus its role on activating fibroblasts19,20,33. A delicate designed research indicated that FN-EDA could promote only HSC motility not activation or differentiation while CCl4-induced EDA KO male mice did have less fibrosis and α-SMA expression27. In fact, the aforementioned studies focused on the effect of exogenous FN-EDA on HSCs; however, our data, including some unpublished data, suggested that HSCs were the dominant source of FN-EDA during hepatic fibrosis and that interfering with FN-EDA expression in vitro decreased the expression of α-SMA and VEGFA but not collagen, indicating that HSC derived FN-EDA, rather than exogenous FN-EDA, is important to maintain HSC activation. A recent study reported that specific deletion of FN-EDA in smooth muscle cells, but not in endothelial cells, reduced smooth muscle cell phenotypic switching highlighting the importance of fibroblast endogenous derived FN-EDA33. Traditionally, FN-EDA is known as a component of extra matrix that assembles into insoluble fibrils53. In current study, we confirmed that FN-EDA functions as paracrine factors that promotes the phosphorylation of VEGFR2. It was an interesting finding that maybe there is a serum level change of circulating FN-EDA which could indicated hepatic fibrosis or some vascular related complications and put us a clue of local inflammation and systemic response as FN-EDA was demonstrated as a proinflammatory factor19. To the best of our knowledge, no previous studies have reported the promotion of pathological angiogenesis by FN-EDA in fibrotic diseases.
As there is no evidence of FN-EDA directly binding with VEGFR2, we targeted the reported receptors of FN-EDA to screen. The EDA segment has been recognized as a ligand for integrins including α4, α9 and β136,54 and an established agonist for TLR4 that considered as a damage-associated molecular pattern molecule promoting fibroinflammatory19. Although both types of receptors are reportedly involved in angiogenesis, integrins are more likely to be related with VEGFR2, which mediates endothelial cell adhesion and migration, as well as signaling coreceptors of the receptor tyrosine kinases38,39. FN is a high-molecular-weight protein that has many regions other than the EDA segment that can bind with integrins15. To eliminate this interference, we used rEDA as previous reported19 to stimulate endothelial cells along with integrin neutralizing antibodies. Preliminary screening results supported our hypothesis. Further study, we systematically measured various integrin receptors and screened out the core integrin subunit β1. We also determined two auxiliary integrin subunits α4, α9, the former of which was closely related to tube formation, while the latter were more correlated with migration in our results.
The compact spatial structure of membrane proteins is critical important for signal transduction. the transmembrane-4 glycoprotein superfamily are well known for interacting among themselves and with other transmembrane proteins to form membrane microdomains55. CD63 is the most highly expressed tetraspanin in endothelial cells and was reported for integrin β1-VEGFR2 complex formation56. Therefore, to confirm the necessity of the neighborship of β1 and VEGFR2, we knocked down CD63 expression and observed attenuation of VEGFR2 phosphorylation, which was recovered after reoverexpressing CD63. These results indicated the importance of spatial relationships for transmembrane proteins cooperating with each other.
Although the importance of intrahepatic angiogenesis in hepatic fibrosis has been reported and several anti-angiogenic treatments have even been reported on hepatic fibrosis7,9, none has been approved for clinical use due to their low specificity and potential adverse effects. FN-EDA is expected to be a potential therapeutic target due to its strict expression in healthy adults. Some studies even identified FN-EDA as a drug delivery target and obtained good feedback which indirectly reflects the high specificity of FN-EDA to the pathological context57. Irigenin is one of the most abundant bioactive ingredients of isoflavones extracted from Rhizoma Belamcanda and specifically targets integrin α9β1 and α4β1 binding sites on FN-EDA in its C-Cʹ loop, inhibiting FN-EDA-induced metastasis in lung cancer37. Our results suggested irigenin could relieve intrahepatic angiogenesis and fibrosis in the early stage while these curative effects were weakened in the later stage, which was consistent with early studies that anti-angiogenesis therapy was more effective in the early stage of hepatic fibrosis51. Collectively, these data highlight the significance of FN-EDA as a potential anti-angiogenesis therapeutic target for the treatment of liver fibrosis.
Materials and methods
Human specimens
Liver tissue specimens with histologically diagnosed fibrosis were obtained from surgical surplus or biopsies. Normal liver tissues from the surgical surplus of liver trauma or hepatic hemangioma specimens were collected as the control. The demographic and clinical characteristics corresponding to the two groups are shown in Table 1. The authors obtained informed consent from each participant to conduct this study. All procedures were approved by the Ethics Committee of Shandong Provincial Hospital, Cheeloo College of Medicine, Shandong University (Jinan, China).
Table 1.
Demographic and clinical characteristics of hepatic fibrosis patients and healthy individuals.
| Hepatic fibrosis patients | Healthy individuals | |
|---|---|---|
| # | 30 | 15 |
| age | 55.57 ± 3.40 | 49.40 ± 3.58 |
| BP (mmHg) | 82.00 ± 3.13 ~ 123.27 ± 5.01 | 78.87 ± 3.36 ~ 122.00 ± 4.62 |
| BMI | 25.14 ± 1.21 | 22.60 ± 0.72 |
| PLT (109/L) | 147.97 ± 29.08 | 233.87 ± 20.08 |
| ALB(g/L) | 39.09 ± 3.28 | 43.51 ± 1.71 |
| PT (s) | 14.81 ± 1.15 | 13.29 ± 0.27 |
Mouse experiments
Six-week-old male wild-type C57BL/6 mice were purchased from the Shandong University Laboratory Animal Centre. For hepatic fibrosis model, mice were administered CCl4 (2 ml/kg, CCl4: olive oil = 1:4) or olive oil twice a week by intraperitoneal injection. AAV9-FN-EDA-shRNA or AAV9-Ctrl (Genechem, Shanghai, China) were injected into mice through tail vein every four weeks for eight weeks. Irigenin (5 mg/kg, Selleck, Shanghai, China) or water was administered through gavage after two weeks CCl4 administration. The animal study protocol was approved by the Ethics Committee of Shandong Provincial Hospital.
Cell culture
LX-2 cells (HSC cell line) (Procell, Wuhan, CN) were cultured in DMEM with 10% FBS and 1% penicillin/streptomycin. Human umbilical vein endothelial cells (HUVECs) (ATCC, Rockville, MD, USA) were cultured in complete 1640 medium. SK-hep1 cells (Procell, Wuhan, CN) were cultured in complete MEM. Primary HSECs (ScienCell, Carlsbad, CA, USA) were cultured in complete ECM with 10% endothelial cell growth supplement (ScienCell, Carlsbad, CA, USA). For in vitro knockdown, FN-EDA-siRNAs, CD63-siRNA and control siRNA (Biosune, Jinan, CN) were transfected into HUVEC or LX-2 at 30 pmol/ml (12 well plate). For in vitro reoverexpression of CD63, the ORF of CD63 in pEnter and vector plasmid control (Biosune, Jinan, CN) were transfected at 1.5 μg/ml (12 well plate) one day after CD63-siRNA transfection each group were harvested 84 hours in total. Transient transfection was performed using Lipofectamine 3000 (Life Technologies; Gaithersburg, MD) according to the manufacturer’s protocol. The target interfering sequences are listed in Table 2.
Table 2.
Target sequences for RNAi.
| Target sequence | |
|---|---|
| FN-EDA-siRNA1 | GGGACUCCUACCUUAGGUA |
| FN-EDA-siRNA2 | CCGUAAGUGACUACACCUA |
| CD63-siRNA | GCUGCCUCGUGAAGAGUAU |
RNA isolation and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from human samples with TRIzol reagent, and cDNA was generated with a reverse transcription kit (Takara, Japan). cDNA was amplified by qRT-PCR on Roche 480 Real Time PCR System instrument using SYBR Green PCR kit (Takara, Japan). mRNAs were normalized to GAPDH. The primers for qRT-PCR are listed in Table 3.
Table 3.
Primers for quantitative real-time PCR.
| Forward | Reverse | |
|---|---|---|
| EDA | GTAACCAACATTGAT | GGCTCGAGTAGGTCAC |
| EDB | AGTTAGTTGCGGCAGGAGAAG | CCGCCATTAATGAGAGTGAT |
| CD31 | CCGCATATCCAAGGTCAGCA | CACCTTGGTCCAGATGTGTGAA |
| GAPDH | GCACCGTCAAGGCTGAGAAC | TGGTGAAGACGCCAGTGGA |
Immunohistochemistry
Immunohistochemistry was performed as previously described8. Briefly, liver tissues were fixed with 4% paraformaldehyde and embedded in 5-μm-thick paraffin sections. After deparaffinization, hydration, citrate incubation, and 3% H2O2 blocking, slides were confined with 5% goat serum and incubated with primary antibodies. Following incubation with horseradish peroxidase second antibodies then reacted with a diaminobenzidine solution and counterstained with hematoxylin. The staining score was quantified by the mean integral optical density (IOD) using Image-Pro Plus 6 software (Baltimore, USA).
Immunofluorescence
Briefly, Liver tissues were fixed with 4% paraformaldehyde and cut into 5-μm-thick sections. After being incubated with 0.01 M citrate, sections were blocked with 5% goat serum and incubated with appropriate primary antibodies. After washing by PBS, the sections were incubated with secondary antibodies conjugated with CoraLite594 or FITC, and were then counterstained with DAPI. Fluorescence score was quantified by the mean integral optical density using Image-Pro Plus 6 software.
Immunoblot
Mouse hepatic samples were harvested in and lysed with RIPA lysis buffer supplemented with a protease and phosphatase inhibitor cocktail. After determining the protein concentration by using a BCA Protein Assay Kit (Solarbio, Beijing, CN), equal sample quantities were electrophoresed on SDS–PAGE gels and transferred onto PVDF membranes. The membrane was blocked for 1 h with 5% BSA and incubated with primary antibodies (Table 4), followed by incubation with horseradish peroxidase conjugated secondary antibodies for 1 h at room temperature. GAPDH was used as an internal control. All the antibody bands were normalized to their expression.
Table 4.
Antibodies.
| Antibody | Identifier | Application |
|---|---|---|
| FN-EDA | sc-59826, Santa Cruz | WB, IHC, Blocking, IF |
| FN-EDA | F6140, Sigma | Blocking |
| CD31 | sc-376764, Santa Cruz | WB, IHC, IF |
| α-SMA | ab5694, Abcam | WB, IHC, IF |
| CD63 | SC5275, Santa Cruz | WB, IHC |
| CD63 | ab134045, Abcam | WB |
| Albumin | 16475-1-AP, Proteintech | IF |
| pVEGFR2 | ab194806, Abcam | WB |
| pVEGFR2 | #2478, CST | WB |
| VEGFR2 | #9698, CST | WB |
| VEGFR2 | 26415-1-AP, Proteintech | WB |
| PI3K p85 | ab191606, Abcam | WB |
| pPI3K p85/p55 | ab226842, Abcam | WB |
| AKT | #4685, CST | WB |
| pAKT | #4060, CST | WB |
| PLCγ1 | #5640, CST | WB |
| pPLCγ1 | #8713, CST | WB |
| ERK | #4695, CST | WB |
| pERK | #4370, CST | WB |
| Src | #2109, CST | WB |
| pSrc | #6943, CST | WB |
| FAK | #3285, CST | WB |
| pFAK | #3281, CST | WB |
| integrin β1 | 26918-1-AP, Proteintech | WB |
| GAPDH | 60004-1-Ig, Proteintech | WB |
| integrin α9 | ab27947, abcam | Blocking |
| integrin α4 | ab25247, Abcam | Blocking |
| integrin β1 | ab24693, Abcam | Blocking |
| flag | #14793, CST | IP |
Tube formation assay
The tube formation assay was performed as previously described8. Briefly, endothelial cells were seeded at a density of 1–2 × 104 cells/well for 6 h in plates precoated with Matrigel at 37 °C and 5% CO2. For coculturing experiment, LX-2 cells were seeded into the upper chambers of a transwell plate (Corning, Costar 3422) for 24 h and then transferred to other plates with endothelial cells. Experiments were performed in the presence or absence of various fibronectins, including pFN (40 μg/ml, ECM001, Sigma-Aldrich; St. Louis, MO, USA), FN-EDA (40 μg/ml, F2518, Sigma-Aldrich; St. Louis, MO, USA), rEDA (40 μg/ml Flag-tagged, Daian, Wuhan, China) or inhibitors including irigenin (5 μM Selleck, Shanghai, China) and, resatorvid (5 μM, MCE, USA), or neutralizing antibodies (Table 4). Tube formation was photographed using inverted microscope and quantified by calculating the average tube length using ImageJ software (National Institutes of Health, Bethesda, MD, USA)58.
Transwell migration assay
Cell migration was measured by transwell assays as previously described8. Briefly, for coculturing experiment, we first transferred the upper chambers of the transwell plate (Corning, Costar 3422) into a 24-well plate in which LX-2 cells were seeded first, and then endothelial cells were added into the upper chambers. A total of 104 cells were added to the upper side of each insert and incubated for 24 h at 37 °C and 5% CO2. The number of cells that migrated to the lower surface of the chamber was evaluated. Experiments were performed in the presence or absence of various factors as described above. An average of three individual wells was quantified using Image-Pro Plus software (Media Cybernetics, Madrid, USA).
Co-immunoprecipitation
Cells were pretreated with rEDA (Flag-tagged, Daian, Wuhan, China) in 6-well plates and lysed using 1 ml of lysis buffer for IP (Beyotime) on ice for 30 min. After centrifuging at 10,000 × g for 15 min at 4 °C, the supernatant was incubated with flag antibody for 1 h, followed by incubation with protein A/G PLUS-Agarose beads (sc-2003, Santa Cruz) overnight, and then beads were collected by centrifugation at 2500 × g for 5 min at 4 °C. After washing four times with the above cell lysis buffer, beads were boiled in 5× loading buffer for 5 min followed by immunoblotting.
Transmission electron microscopy (TEM)
Mouse liver tissues were cut into 1mm3 section and immersed in precooled 2.5% glutaraldehyde immediately after being separated from enterocoelia for 4 h at 4 °C and, fixed with 1% OsO4 in 0.1 M PBS (pH 7.4) for 2 h in the dark. After dehydrating by a gradient concentration of ethanol and resin penetration and embedded, the embedding models with resin and samples were moved into a 65 °C oven to polymerize for 48 h. Resin blocks were cut into 60 nm thick blocks followed by 2% uranium acetate saturated alcohol solution avoid light staining for 8 min and 2.6% lead citrate to avoid CO2 staining for 8 min. Images were obtained using Hitachi HT7800 transmission electron microscope.
Statistical analysis
All statistical analyses were performed with GraphPad Prism 7.0 (La Jolla, CA). Experiments were repeated at least three independent times. Data for each group were expressed as the means ± SEM. The differences between groups were analyzed by paired and unpaired two-tailed Student’s t test or by ANOVA, as deemed appropriate. The Spearman rank correlation coefficients of determination were used to analyze the degree of correlation among parameters. For all analyses, the p-value reported was two-tailed, and p-values < 0.05 were considered statistically significant.
Acknowledgements
This work was supported by funds from National Natural Science Foundation of China (No. 81770607; 81570551; 81600469). These funding sources had no role in the study design, data collection, data analysis, interpretation, or writing of this manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Edited by Ivano Amelio
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jianni Qi, Email: slqijn@126.com.
Qiang Zhu, Email: zhuqiang@sdu.edu.cn.
References
- 1.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838–851. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim YS, Kim WR. The global impact of hepatic fibrosis and end stage liver disease. Clin. Liver Dis. 2008;12:733–746. doi: 10.1016/j.cld.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Thabut D, Shah V. Intrahepatic angiogenesis and sinusoidal remodeling in chronic liver disease: new targets for the treatment of portal hypertension? J. Hepatol. 2010;53:976–980. doi: 10.1016/j.jhep.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Ehling J, et al. CCL2-dependent infiltrating macrophages promote angiogenesis in progressive liver fibrosis. Gut. 2014;63:19601971. doi: 10.1136/gutjnl-2013-306294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez M, et al. Angiogenesis in liver disease. J. Hepatol. 2009;50:604–620. doi: 10.1016/j.jhep.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez M. Molecular pathophysiology of portal hypertension. Hepatology. 2015;61:1406–1415. doi: 10.1002/hep.27343. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez M, et al. Anti-VEGF receptor-2 monoclonal antibody prevents portal-systemic collateral vessel formation in portal hypertensive mice. Gastroenterology. 2004;126:886–894. doi: 10.1053/j.gastro.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, et al. Neuropilin-1 aggravates liver cirrhosis by promoting angiogenesis via VEGFR2-dependent PI3K/Akt pathway in hepatic sinusoidal endothelial cells. EBioMedicine. 2019;43:525–536. doi: 10.1016/j.ebiom.2019.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan, Z., et al. CD147 promotes liver fibrosis progression via VEGF-A/VEGFR2 signaling-mediated cross-talk between hepatocytes and sinusoidal endothelial cells. Clin. Sci. (Lond) 129, 699–710 (2015). [DOI] [PubMed]
- 10.Huang Y, et al. Bevacizumab attenuates hepatic fibrosis in rats by inhibiting activation of hepatic stellate cells. PLoS ONE. 2013;8:e73492. doi: 10.1371/journal.pone.0073492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshiji H, et al. Vascular endothelial growth factor and receptor interaction is a prerequisite for murine hepatic fibrogenesis. Gut. 2003;52:1347–1354. doi: 10.1136/gut.52.9.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamane A, et al. A new communication system between hepatocytes and sinusoidal endothelial cells in liver through vascular endothelial growth factor and Flt tyrosine kinase receptor family (Flt-1 and KDR/Flk-1) Oncogene. 1994;9:2683–2690. [PubMed] [Google Scholar]
- 13.Maurer LM, et al. Dynamic structure of plasma fibronectin. Crit. Rev. Biochem Mol. Biol. 2015;51:213–227. doi: 10.1080/10409238.2016.1184224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhanesha N, et al. Genetic ablation of extra domain a of fibronectin in hypercholesterolemic mice improves stroke outcome by reducing thrombo-inflammation. Circulation. 2015;132:2237–2247. doi: 10.1161/CIRCULATIONAHA.115.016540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White ES, et al. New insights into form and function of fibronectin splice variants. J. Pathol. 2008;216:1–14. doi: 10.1002/path.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peters JH, Hynes RO. Fibronectin isoform distribution in the mouse. I. The alternatively spliced EIIIB, EIIIA, and V segments show widespread codistribution in the developing mouse embryo. Cell Adhes. Commun. 1996;4:103–125. doi: 10.3109/15419069609010766. [DOI] [PubMed] [Google Scholar]
- 17.Astrof S, Crowley D, Hynes RO. Multiple cardiovascular defects caused by the absence of alternatively spliced segments of fibronectin. Dev. Biol. 2007;311:11–24. doi: 10.1016/j.ydbio.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.George EL, Baldwin HS, Hynes RO. Fibronectins are essential for heart and blood vessel morphogenesis but are dispensable for initial specification of precursor cells. Blood. 1997;90:3073–3081. doi: 10.1182/blood.V90.8.3073. [DOI] [PubMed] [Google Scholar]
- 19.Kelsh-Lasher RM, et al. Integrin α4β1 and TLR4 cooperate to induce fibrotic gene expression in response to fibronectin’s EDA domain. J. Invest. Dermatol. 2017;137:2505–2512. doi: 10.1016/j.jid.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muro AF, et al. An essential role for fibronectin extra type III domain A in pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2008;177:638–645. doi: 10.1164/rccm.200708-1291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malara A, et al. EDA fibronectin-TLR4 axis sustains megakaryocyte expansion and inflammation in bone marrow fibrosis. J. Exp. Med. 2019;216:587–604. doi: 10.1084/jem.20181074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muro AF, et al. Regulated splicing of the fibronectin EDA exon is essential for proper skin wound healing and normal lifespan. J. Cell Biol. 2003;162:149–160. doi: 10.1083/jcb.200212079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glukhova MA, et al. Expression of extra domain A fibronectin sequence in vascular smooth muscle cells is phenotype dependent. J. Cell Biol. 1989;109:357–366. doi: 10.1083/jcb.109.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chorawala MR, et al. Deletion of extra domain A of fibronectin reduces acute myocardial ischaemia/reperfusion injury in hyperlipidaemic mice by limiting thrombo-inflammation. Thromb. Haemost. 2018;118:1450–1460. doi: 10.1055/s-0038-1661353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gondokaryono SP, et al. The extra domain A of fibronectin stimulates murine mast cells via toll-like receptor 4. J. Leukoc. Biol. 2007;82:657–665. doi: 10.1189/jlb.1206730. [DOI] [PubMed] [Google Scholar]
- 26.George J, et al. Transforming growth factor-initiates wound repair in rat liver through induction of the EIIIA-fibronectin splice isoform. Am. J. Pathol. 2000;156:115–124. doi: 10.1016/S0002-9440(10)64711-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olsen AL, et al. Fibronectin extra domain-A promotes hepatic stellate cell motility but not differentiation into myofibroblasts. Gastroenterology. 2012;142:928–937 e923. doi: 10.1053/j.gastro.2011.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moriya K, et al. A fibronectin-independent mechanism of collagen fibrillogenesis in adult liver remodeling. Gastroenterology. 2011;140:1653–1663. doi: 10.1053/j.gastro.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarnagin WR, et al. Expression of variant fibronectins in wound healing: cellular source and biological activity of the EIIIA segment in rat hepatic fibrogenesis. J. Cell Biol. 1994;127:2037–2048. doi: 10.1083/jcb.127.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu Q, et al. Intestinal decontamination inhibits TLR4 dependent fibronectin-mediated cross-talk between stellate cells and endothelial cells in liver fibrosis in mice. J. Hepatol. 2012;56:893–899. doi: 10.1016/j.jhep.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turner CJ, et al. Endothelium-derived fibronectin regulates neonatal vascular morphogenesis in an autocrine fashion. Angiogenesis. 2017;20:519–531. doi: 10.1007/s10456-017-9563-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chauhan AK, Iaconcig A, Baralle FE, Muro AF. Alternative splicing of fibronectin: a mouse model demonstrates the identity of in vitro and in vivo systems and the processing autonomy of regulated exons in adult mice. Gene. 2004;324:55–63. doi: 10.1016/j.gene.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 33.Jain M, et al. Smooth muscle cell–specific fibronectin-EDA mediates phenotypic switching and neointimal hyperplasia. J. Clin. Investig. 2019;130:295–314. doi: 10.1172/JCI124708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calderone V, et al. Sequential functions of CPEB1 and CPEB4 regulate pathologic expression of vascular endothelial growth factor and angiogenesis in chronic liver disease. Gastroenterology. 2016;150:982–997 e930. doi: 10.1053/j.gastro.2015.11.038. [DOI] [PubMed] [Google Scholar]
- 35.Xiang L, et al. The extra domain A of fibronectin increases VEGF-C expression in colorectal carcinoma involving the PI3K/AKT signaling pathway. PLoS ONE. 2012;7:e35378. doi: 10.1371/journal.pone.0035378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shinde AV, et al. Identification of the peptide sequences within the EIIIA (EDA) segment of fibronectin that mediate integrin α9β1-dependent cellular activities. J. Biol. Chem. 2008;283:2858–2870. doi: 10.1074/jbc.M708306200. [DOI] [PubMed] [Google Scholar]
- 37.Amin A, et al. Irigenin, a novel lead from Western Himalayan chemiome inhibits fibronectin-extra domain a induced metastasis in lung cancer cells. Sci. Rep. 2016;6:37151. doi: 10.1038/srep37151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malinin NL, et al. Integrin signaling in vascular function. Curr. Opin. Hematol. 2012;19:206–211. doi: 10.1097/MOH.0b013e3283523df0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahabeleshwar GH, et al. Mechanisms of integrin-vascular endothelial growth factor receptor cross-activation in angiogenesis. Circ. Res. 2007;101:570–580. doi: 10.1161/CIRCRESAHA.107.155655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zou L, et al. Fibronectin induces endothelial cell migration through beta1 integrin and Src-dependent phosphorylation of fibroblast growth factor receptor-1 at tyrosines 653/654 and 766. J. Biol. Chem. 2012;287:7190–7202. doi: 10.1074/jbc.M111.304972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin Z, et al. Ligand-independent activation of vascular endothelial growth factor receptor 2 by fluid shear stress regulates activation of endothelial nitric oxide synthase. Circ. Res. 2003;93:354–363. doi: 10.1161/01.RES.0000089257.94002.96. [DOI] [PubMed] [Google Scholar]
- 42.García-Martín A, et al. Src kinases mediate VEGFR2 transactivation by the osteostatin domain of PTHrP to modulate osteoblastic function. J. Cell. Biochem. 2013;114:1404–1413. doi: 10.1002/jcb.24482. [DOI] [PubMed] [Google Scholar]
- 43.Delos Santos R, et al. Charming neighborhoods on the cell surface: plasma membrane microdomains regulate receptor tyrosine kinase signaling. Cell. Signal. 2015;27:1963–1976. doi: 10.1016/j.cellsig.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 44.Bailey RL, et al. The emerging role of tetraspanin microdomains on endothelial cells. Biochem. Soc. Trans. 2011;39:1667–1673. doi: 10.1042/BST20110745. [DOI] [PubMed] [Google Scholar]
- 45.Zhang WD, et al. Simultaneous determination of tectorigenin, irigenin and irisflorentin in rat plasma and urine by UHPLC-MS/MS: application to pharmacokinetics. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011;879:3735–3741. doi: 10.1016/j.jchromb.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 46.Arany Z, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 47.Lefere S, et al. Angiopoietin-2 promotes pathological angiogenesis and is a therapeutic target in murine nonalcoholic fatty liver disease. Hepatology. 2019;69:1087–1104. doi: 10.1002/hep.30294. [DOI] [PubMed] [Google Scholar]
- 48.Poisson J, et al. Liver sinusoidal endothelial cells: physiology and role in liver diseases. J. Hepatol. 2017;66:212–227. doi: 10.1016/j.jhep.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 49.Chen TT, et al. Anchorage of VEGF to the extracellular matrix conveys differential signaling responses to endothelial cells. J. Cell Biol. 2010;188:595–609. doi: 10.1083/jcb.200906044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bishop PN. The role of extracellular matrix in retinal vascular development and preretinal neovascularization. Exp. Eye Res. 2015;133:30–36. doi: 10.1016/j.exer.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 51.Liu L, et al. Mechanotransduction-modulated fibrotic microniches reveal the contribution of angiogenesis in liver fibrosis. Nat. Mater. 2017;16:1252–1261. doi: 10.1038/nmat5024. [DOI] [PubMed] [Google Scholar]
- 52.Kilarski WW, et al. Biomechanical regulation of blood vessel growth during tissue vascularization. Nat. Med. 2009;15:657664. doi: 10.1038/nm.1985. [DOI] [PubMed] [Google Scholar]
- 53.Hynes RO, Yamada KM. Fibronectins: multifunctional modular glycoproteins. J. Cell Biol. 1982;95:369–377. doi: 10.1083/jcb.95.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liao Y, et al. The EIIIA segment of fibronectin is a ligand for integrins alpha 9beta 1 and alpha 4beta 1 providing a novel mechanism for regulating cell adhesion by alternative splicing. J. Biol. Chem. 2002;277:14467–14474. doi: 10.1074/jbc.M201100200. [DOI] [PubMed] [Google Scholar]
- 55.Yunta M, Lazo PA. Tetraspanin proteins as organisers of membrane microdomains and signalling complexes. Cell. Signal. 2003;15:559–564 10. doi: 10.1016/S0898-6568(02)00147-X. [DOI] [PubMed] [Google Scholar]
- 56.Tugues S, et al. Tetraspanin CD63 promotes vascular endothelial growth factor receptor 2-β1 integrin complex formation, thereby regulating activation and downstream signaling in endothelial cellsin vitroandin vivo. J. Biol. Chem. 2013;288:19060–19071. doi: 10.1074/jbc.M113.468199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumra H, Reinhardt D. Fibronectin-targeted drug delivery in cancer. Adv. Drug Deliv. Rev. 2016;97:101–110. doi: 10.1016/j.addr.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 58.Xu M, et al. LECT2, a Ligand for Tie1, Plays a Crucial Role in Liver Fibrogenesis. Cell. 2019;178:1478–1492.e1420. doi: 10.1016/j.cell.2019.07.021. [DOI] [PubMed] [Google Scholar]