Abstract
Background
Peroxiredoxin2 (Prdx2) is an endogenous peroxidase and has been found to reduce the oxidative burden in cells and thereby reduce cell damage and apoptosis. Therefore, the purpose of this study was to investigate the effect of Prdx2 on the oxidative level and apoptosis of myocardial cells after acute myocardial infarction (AMI).
Material/Methods
We constructed an AMI model for Sprague-Dawley rats by ligating the left anterior descending coronary artery. We determined the effect of Prdx2 on AMI by detecting changes in Prdx2 in myocardial tissue via western blot and qRT-PCR. In addition, we used recombinant Prdx2 protein to treat rats and detect changes in oxidative stress and apoptosis in rat myocardial tissue to verify the protective effect of Prdx2 on the rat heart.
Results
The protein and mRNA expression of Prdx2 in myocardial tissue of rats in the AMI group was significantly lower than that in the control group. The oxidative and apoptotic levels of myocardial tissue in Prdx2-administered rats were significantly improved compared to the non-administered group, which was manifested by a decrease in reactive oxygen species (ROS) levels and a decrease in the expression of the caspase family. In addition, Prdx2 also inhibited p65 phosphorylation in myocardial tissues and inhibited TLR4/NF-κB signaling pathway activity.
Conclusions
The expression of Prdx2 was decreased in myocardial tissue after AMI. Prdx2 can reduce apoptosis and ROS caused by AMI by inhibiting the TLR4/NF-κB signaling pathway, thereby reducing myocardial injury caused by AMI.
MeSH Keywords: Apoptosis, Myocardial Infarction, Oxidative Stress, Peroxiredoxins
Background
Acute myocardial infarction (AMI) is a common ischemic heart disease, in which coronary artery blood supply is sharply reduced or interrupted on the basis of coronary artery disease, resulting in severe and persistent myocardial ischemic necrosis caused by acute ischemia [1]. In the United States, the incidence rate of AMI was 7% among men aged 35 to 84, and 2.2% among women. About 150 million people develop AMI each year [2]. On the basis of atherosclerotic stenosis, some triggers cause the rupture of atherosclerotic plaques. Platelets in the blood gather on the surface of the ruptured plaque and form thrombus that blocks the coronary lumen and leads to myocardial ischemia and necrosis [3]. When AMI occurs, persistent hypoxia and ATP deficiency in myocardial cells cause activation of the apoptotic cascade and myocardial cell necrosis. Necrotic cardiomyocytes activate the immune system and produce severe inflammatory response [4]. Both inflammatory response and apoptosis affect the development of MI and repair of myocardial injury. Proper inflammation facilitates myocardial repair, while excessive inflammatory responses cause secondary myocardial damage. Inhibition of excessive inflammation and apoptosis has become an important part of repair of MI, control of ventricular remodeling after MI, and improvement of cardiac function [5]. In the first few minutes of ischemia, the myocardium will suffer from strong oxidative stress response. This harmful oxidative stress response mediates myocardial injury and cardiomyocyte death through a variety of different mechanisms [6]. Therefore, antioxidant therapy may an effective method to reduce myocardial ischemia-reperfusion injury. In recent years, many new methods have been used to repair damaged cardiac tissue, including tissue engineering and regenerative nanomedicine applications. However, myocardial injury after AMI is still difficult to heal [7].
Peroxiredoxin (Prdx) protein is a newly discovered class of peroxidase, which belongs to the antioxidant protein superfamily and is widely present in prokaryotes and eukaryotes [8]. The Prdx family in mammals includes 6 members. Prdx2 is localized in the cytoplasm and is expressed in a variety of tissues [9]. Prdx2 protein has the ability to reduce hydrogen peroxide to water, and its expression is upregulated under oxygen emergency conditions to remove excess reactive oxygen molecules in the cell [10]. Prdx2 gene-deficient mice can develop and mature normally, but Heinz body can be detected in peripheral blood, and the number of abnormal morphological cells and the content of reactive oxygen species (ROS) in red blood cells increases [11]. In addition, Prdx2 can inhibit apoptosis caused by various factors. In thyroid cell line FRTL-5, thyroid hormone can induce the production of H2O2, which leads to cell apoptosis, but high expression of Prdx2 can reduce this apoptosis [12]. However, it is unclear whether the antioxidant and anti-apoptotic effects of Prdx2 can reduce the apoptosis of cardiomyocytes caused by AMI. Therefore, we used rats to make an AMI model to study the effect of Prdx2 on rat AMI.
Material and Methods
Animals
All animal experiments followed the ARRIVE guidelines 2.0. Sprague-Dawley (SD) rats were used in this study. A total of 80 8-week-old male SD rats were purchased and bred at Qinhuangdao Haigang Hospital Animal Center. This study was approved by the Experimental Animal Ethics Committee of Qinhuangdao Haigang Hospital (CN-HB-IACUC-2019-04-17136). The Prdx2 recombinant protein (Abcam, Cambridge, MA, USA) was used for subcutaneous injection into rats. NF-κB signaling pathway agonist, Betulinic acid (BA, 10 mg/kg) (MCE, Monmouth Junction, NJ, USA), was used to activate the NF-κB signaling pathway in rats. Rats in the Prdx2 and AMI+Prdx2 groups were injected subcutaneously with Prdx2 (50 μg/kg) daily after modeling.
AMI model
We used 1% sodium pentobarbital (50 mg/kg) to anesthetize rats. The rats were then fixed on the operating table in supine position [13]. Rats with stable heart rates were selected for modeling. A small-animal ventilator (ALC-V8S, Harvard Apparatus, Newbury Park, CA, USA) was used to maintain rat respiration (100 breaths/min of respiratory rate; 1: 2 of respiratory ratio; 8 mL of tidal volume). After wiping the rat’s chest with 75% alcohol, we used scissors to gently cut open the chest skin and parts of the ribs. After exposing the heart, we used forceps to gently detach and ligate the left anterior descending coronary artery. We then sutured the surgical incision, removed the ventilator, and placed the rats on an electric blanket to recover. After 28 days, we sacrificed the rats and took the heart for subsequent experiments. No antibiotics or analgesics were administered to the rats throughout the experiment.
Echocardiography
At 24 h after the last dose, rats were anesthetized with 1% sodium pentobarbital. Then, we fixed the rats on the operating table and used two-dimensional and M-mode echocardiography (1E33, Philips, Eindhoven, Netherlands) to test the heart function of each group of rats. Left ventricular end-systolic diameter (LVIDs), left ventricular end-diastolic diameter (LVIDd), left ventricular end-systolic volume (LVESV), and left ventricular end-diastolic volume (LVEDV) were measured. We calculated the left ventricular short-axis shortening rate (FS) and ejection fraction (EF) according to the formula:
Hematoxylin-eosin (HE) staining
The left ventricular anterior wall tissue of each group of rats was collected and immersed in 4% paraformaldehyde fixative for 24 h. The tissue was then dehydrated in gradient alcohol and placed in paraffin to make a paraffin mass. Then, we used a microtome (RM2235, Leica, Wetzlar, Germany) to make paraffin sections. We dewaxed and hydrated paraffin sections in xylene and gradient alcohol. The sections were then rinsed in running water for 3 min. The sections were placed in hematoxylin stain (Beyotime, Shanghai, China) for 1 min. After rinsing under running water, we placed the sections in hydrochloric acid alcohol (Beyotime, Shanghai, China) for another 3 s. The sections were then rinsed with running water, placed in eosin staining solution (Beyotime, Shanghai, China) for 1 min, and dehydrated to seal the sections.
Immunohistochemical (IHC) staining
After dewaxing and hydration, the sections were placed in citrate buffer and heated to 95°C for 20 min. After the sections cooled naturally, we treated the sections with 3% H2O2 for half an hour. After washing the sections with phosphate-buffered saline (PBS), we treated the sections with 10% goat serum for 1 h. We then treated the sections with SOD1 (ab13498, Abcam, Cambridge, MA, USA), SOD2 (ab13534, Abcam, Cambridge, MA, USA), NF-E2-realted factor2 (Nrf2) (ab137550, Abcam, Cambridge, MA, USA), caspase8 (ab25901, Abcam, Cambridge, MA, USA), and caspase9 (ab32539, Abcam, Cambridge, MA, USA) antibodies at 4°C overnight. The next day, we treated the sections with the universal secondary antibody from the IHC staining kit (GeneTech, Shanghai, China) for 1 h. Finally, we used the developer in the IHC staining kit for color development.
Detection of content of malondialdehyde (MDA) and superoxide dismutase (SOD)
After obtaining rat cardiac muscle tissue, we ground the cardiac muscle tissue at low temperature and dissolved it in PBS. We then used the MDA enzyme-linked immunosorbent assay (ELISA) kit (Invitrogen, Carlsbad, CA, USA) and the SOD ELISA kit (Invitrogen, Carlsbad, CA, USA) to measure MDA and SOD content according to the manufacturer’s instructions.
Western blot
We collected the left ventricular anterior wall tissue of each group of rats and extracted total protein using radioimmunoprecipitation assay (RIPA) lysate (Beyotime, Shanghai, China). A 1-mm-thick gel was used for western blot analysis. The bicinchoninic acid (BCA) kit (Beyotime, Shanghai, China) was used to detect protein concentrations. We transferred proteins to polyvinylidene fluoride (PVDF) membranes (Roche, Basel, Switzerland) (0.2 μm) by gel electrophoresis. We then used 5% skim milk to block non-specific antigens. Prdx2 (ab109367, Abcam, Cambridge, MA, USA), TLR4 (ab22048, Abcam, Cambridge, MA, USA), p65 (ab16502, Abcam, Cambridge, MA, USA), p-p65 (ab86299, Abcam, Cambridge, MA, USA), SOD1 (ab13498, Abcam, Cambridge, MA, USA), SOD2 (ab13534, Abcam, Cambridge, MA, USA), Bax (ab32503, Abcam, Cambridge, MA, USA), and Bcl-2 (ab59348, Abcam, Cambridge, MA, USA) antibodies were used to incubate PVDF membranes at 4°C overnight. After washing the PVDF membrane with phosphate-buffered saline-tween (PBST), we used the secondary antibody dilution (ab150077, Abcam, Cambridge, MA, USA) to incubate the PVDF membrane. Finally, we used luminescent fluid to detect protein bands. β-actin was used to normalize data.
RNA isolation and quantitative real-time polymerase chain reaction (qRT-PCR)
The left ventricular anterior wall tissue of each group of rats was used to extract total RNA. TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was used to lyse tissue and extract RNA. Then, we used a spectrophotometer (Mettler Toledo, Shanghai, China) to detect the RNA concentration and configured the reverse transcription system (2 μL 5×PrimeScript Buffer+0.5 μL PrimeScript Enzyme Mix+0.5 μL Oligo dT Primer+0.5 μL Random 6 mers+0.5 μL Total RNA+0.5 μL RNase Free DH2O2) (Invitrogen, Carlsbad, CA, USA). The complementary deoxyribose nucleic acid (cDNA) product after completion of reverse transcription was stored at −20°C. Then, we used different primers to amplify the cDNA to detect the expression of related indicators. Endogenous glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used to normalize data. The relative expression of RNA was expressed as 2−ΔΔCT. The primer sequences are shown in Table 1.
Table 1.
Primers for qRT-PCR.
| Name | Sense/anti-sense | Sequence (5′–3′) |
|---|---|---|
| Prdx2 | Sense | AGGGCATCGCTTACAGG |
| Anti-sense | GACTTCCCCATGCTCATCT | |
| TLR4 | Sense | GACTCCATTCAAGCCCAA |
| Anti-sense | TCTCCCAAGATCAACCGA | |
| SOD1 | Sense | CAATGTGGCTGCTGGAA |
| Anti-sense | TGATGGAATGCTCTCCTGA | |
| SOD2 | Sense | GCCGTGTTCTGAGGAGAG |
| Anti-sense | GTCGTAAGGCAGGTCAGG | |
| Caspase8 | Sense | CACATCCCGCAGAAGAAG |
| Anti-sense | GATCCCGCCGACTGATA | |
| Caspase9 | Sense | GGAGTTGACTGAGGTGGGA |
| Anti-sense | GGAGTTGACTGAGGTGGGA | |
| Bax | Sense | GAGGTCTTCTTCCGTGTGG |
| Anti-sense | GATCAGCTCGGGCACTTT | |
| Bcl-2 | Sense | AGGAACTCTTCAGGGATGG |
| Anti-sense | GCGATGTTGTCCACCAG | |
| GAPDH | Sense | ATGGCTACAGCAACAGGGT |
| Anti-sense | TTATGGGGTCTGGGATGG |
Flow cytometry
We took the left ventricular anterior wall tissue of each group of rats and dissolved it in lysate. The DCFH-DA kit (GeneChem, Shanghai, China) was then used to detect ROS levels in cardiomyocytes. DCFH is oxidized by ROS to the fluorescent substance DCF, so detecting the fluorescence of DCF can determine the level of ROS in the cell. We added 1 μL of DCFH-DA reagent to the lysate and detected the fluorescence intensity of DCF using a flow cytometer.
Statistical analysis
We used Graphpad Prism 7.0 (La Jolla, CA, USA) and Statistical Product and Service Solutions (SPSS) 21.0 statistical software (IBM, Armonk, NY, USA) for data analysis. All the normally distributed data obtained are expressed as mean±standard deviation. Comparisons between multiple groups was done using one-way ANOVA followed by a post hoc test (least significant difference). Differences between 2 groups were analyzed by using the t test. P<0.05 indicates that the difference is statistically significant.
Results
Expression of Prdx2 in AMI rats was lower than in the control group
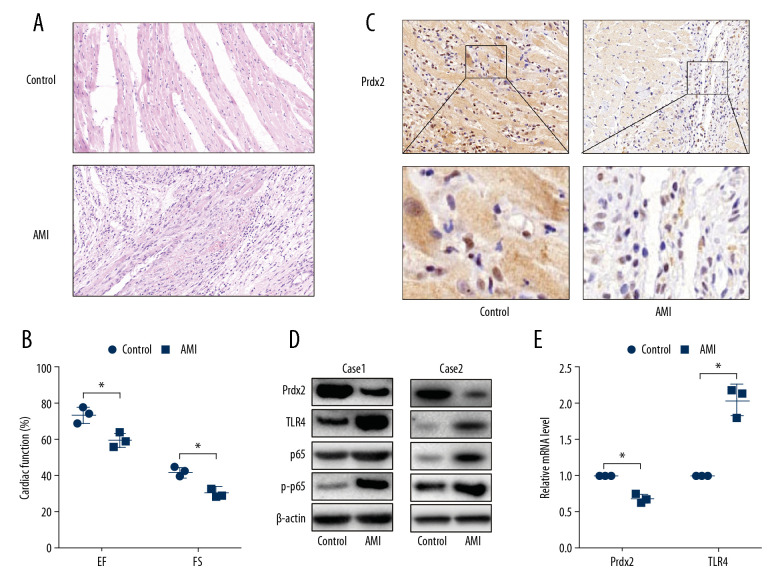
To demonstrate whether Prdx2 expression is related to AMI, we constructed the AMI model by ligating the left anterior descending coronary artery of the rats. HE staining (Figure 1A) was used to identify the effects of the AMI model. The cardiomyocytes in the anterior wall region of the left ventricle of the AMI group were disordered and decreased in number (n=3). Moreover, the EF and FS (Figure 1B) of the AMI rats were lower than that of the control group, indicating that the AMI model was successfully constructed (n=3). The expression of Prdx2 was detected by IHC staining (Figure 1C), and the results showed that the expression of Prdx2 in the myocardial necrosis area of the rats in the AMI group was significantly lower than that in the control group (n=3). In addition, we extracted proteins from infarcted myocardial tissues and normal tissues and examined the expression changes of Prdx2, TLR4, p65, and p-p65. The protein expression of Prdx2 was lower in the infarcted myocardial tissue, while TLR4 expression and p65 phosphorylation were higher (Figure 1D) (n=3). The results of qRT-PCR (Figure 1E) were similar to those of western blot (n=3).
Figure 1.
Expression of Prdx2 in AMI rats was lower than that in the control group. (A) HE staining of rat myocardial tissue (magnification: 200×) (n=3); (B) EF and FS results of cardiac function (n=3); (C) Expression of Prdx2 in rat myocardial tissue (n=3) (magnification: 200×); (D) Western blot results of Prdx2, TLR4, p65, and p-p65 in rat myocardial tissue (n=3); (E) mRNA expression of Prdx2 and TLR4 in rat myocardial tissue (n=3). (“*” means the difference between the 2 groups was statistically significant and P<0.05).
Prdx2 recombinant protein reduces myocardial oxidative stress in AMI rats
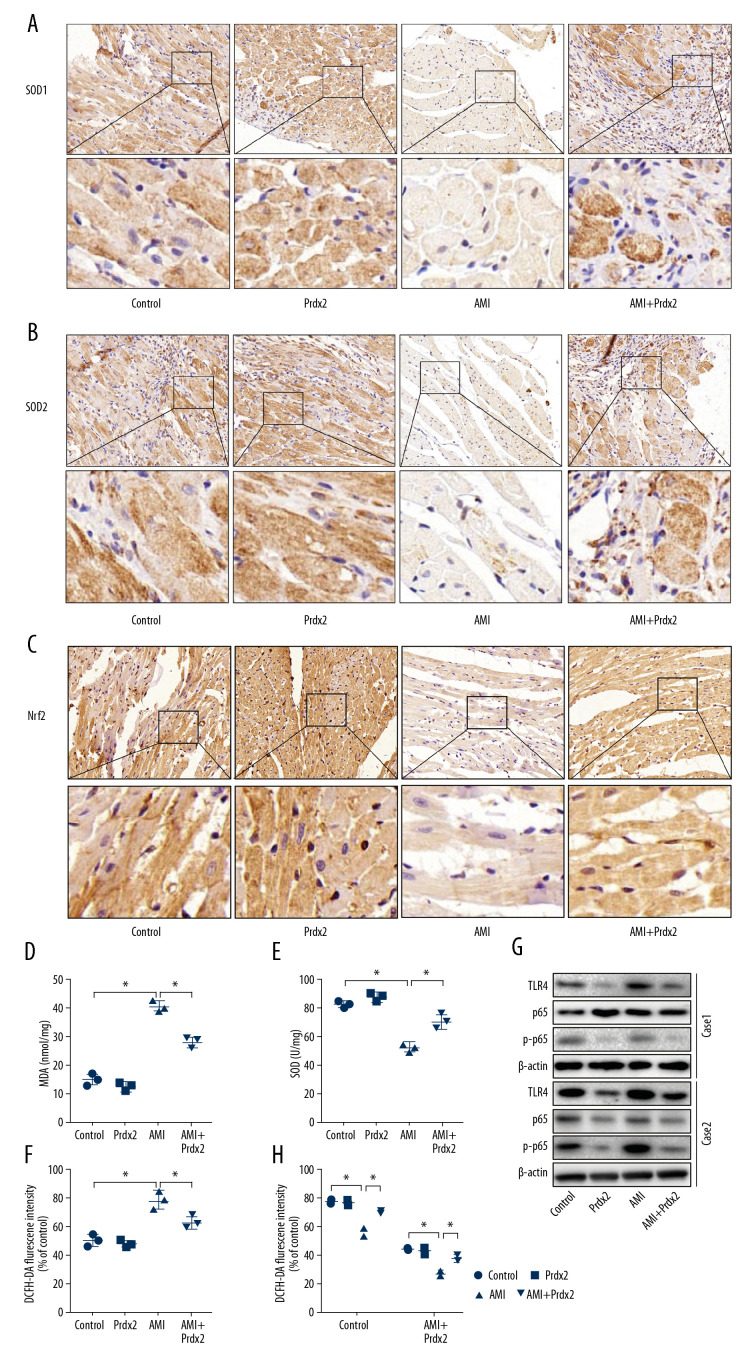
To determine the effect of Prdx2 on oxidative stress in the myocardium of AMI rats, we treated the rats with Prdx2 recombinant protein. Rats were randomly divided into a control group, Prdx2 group, AMI group, and AMI+Prdx2 group. We detected the expression of SOD1/2 and Nrf2 in rat cardiac tissue by IHC staining (Figure 2A–2C) (n=3). The expression of SOD1/2 and Nrf2 in the myocardial tissue of AMI rats was significantly lower than that of the control group, and the expression of SOD1/2 and Nrf2 in the myocardial tissue increased after the rats were treated with Prdx2. In addition, we isolated the anterior wall of the left ventricle of the rat and examined the activity of MDA (Figure 2D) and SOD (Figure 2E) and the level of ROS (Figure 2F) in myocardial tissue. Prdx2 was found to reduce the activity of MDA and increase the activity of SOD, effectively alleviating myocardial damage caused by AMI (n=3). In addition, ROS levels in cardiomyocytes of AMI rats increased significantly, while Prdx2 also decreased ROS levels (n=3). There was no significant difference between the control group and Prdx2 group. Western blot results (Figure 2G) were similar to those of IHC staining (n=3). In addition, Prdx2 also improved the EF and FS of rats after AMI (Figure 2H) (n=3).
Figure 2.
Prdx2 recombinant protein reduces myocardial oxidative stress in AMI rats. (A–C) IHC staining results of SOD1/2 and Nrf2 in rat myocardial tissue (n=3) (magnification: 200×); (D, E) ELISA results of MDA and SOD (n=3); (F) ROS level of rat myocardial tissue (n=3); (G) Western blot results of SOD1 and SOD2 (n=3); (H) EF and FS results of cardiac function (n=3). (“*” means the difference between the 2 groups was statistically significant and P<0.05).
Prdx2 recombinant protein reduced cardiomyocyte apoptosis in AMI rats
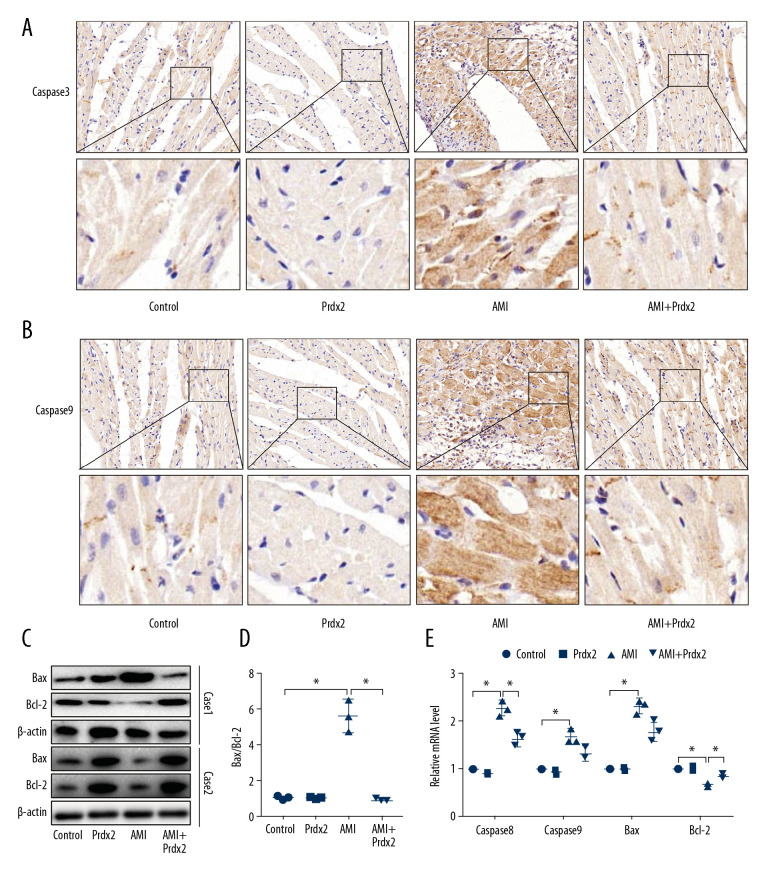
AMI can cause a high degree of cardiomyocyte apoptosis, so we examined the effect of Prdx2 on the apoptosis of cardiomyocytes in AMI rats. The caspase family is a class of important signaling molecules that mediate cell apoptosis. We examined the expression of caspase8 and caspase9 in rat myocardial tissue by IHC staining (Figure 3A, 3B). The expression of caspase8 and caspase9 in myocardial tissue of the AMI group was significantly upregulated, while Prdx2 reduced their expression (n=3). The expression of pro-apoptotic factor Bax and apoptotic inhibiting factor Bcl-2 was detected by western blot (Figure 3C) (n=3). The results showed that Prdx2 reduced the ratio of Bax and Bcl-2 (Figure 3D). There was no significant difference between the control group and Prdx2 group (n=3). qRT-PCR results (Figure 3E) were similar to those of IHC staining and western blot (n=3).
Figure 3.
Prdx2 recombinant protein reduces cardiomyocyte apoptosis in AMI rats. (A, B) IHC staining results of caspase8 and caspase9 in rat myocardial tissue (n=3) (magnification: 200×); (C) Western blot results of Bax and Bcl-2 (n=3); (D) Ratio of expression of Bax and Bcl-2 (n=3); (E) mRNA expression of caspase8, caspase9, Bax, and Bcl-2 (n=3). (“*” means the difference between the 2 groups was statistically significant and P<0.05).
Prdx2 inhibited the TLR4/NF-κB signaling pathway in rat cardiomyocytes
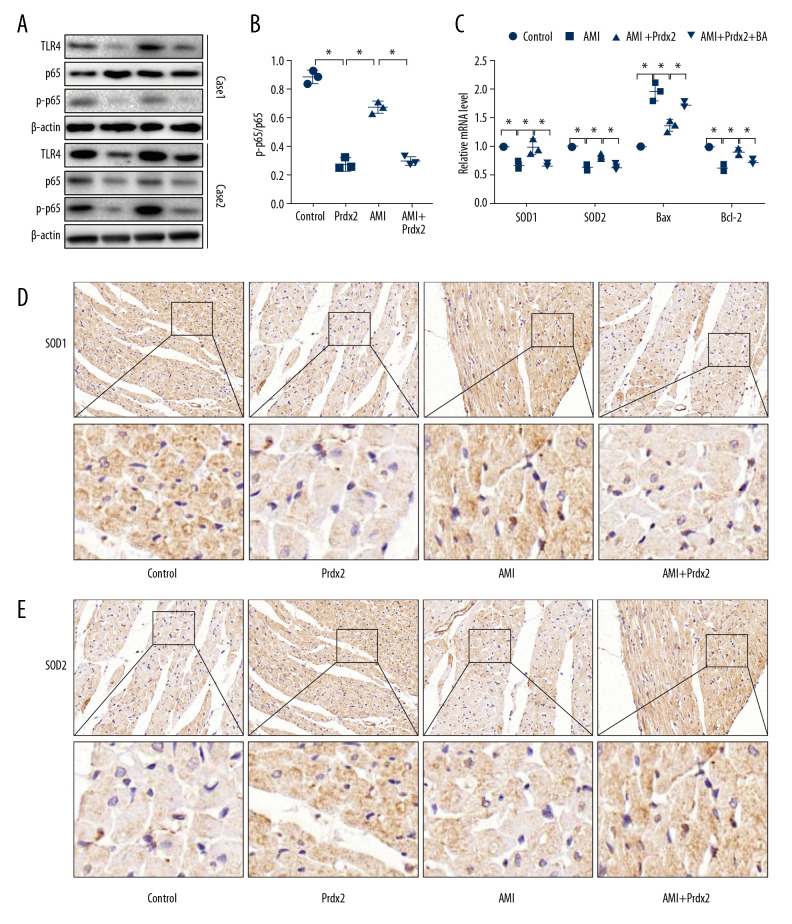
The TLR4/NF-κB signaling pathway plays an important role in mediating AMI-induced myocardial injury, so we examined the effect of Prdx2 on the TLR4/NF-κB signaling pathway. We determined the nuclear translocation of p65 by detecting the phosphorylation level of p65. Western blot results (Figure 4A) showed that the ratio of p-p65 and p65 in the AMI group increased, while the p-p65/p65 ratio in the AMI+Prdx2 group was lower than that in the AMI group (Figure 4B), indicating that Prdx2 inhibited p65 phosphorylation and thus inhibited the TLR4/NF-κB signaling pathway (n=3). In addition, we treated rats with TLR4/NF-κB signaling pathway agonists (BA) and detected changes in oxidative stress and apoptosis in rat myocardial tissues by IHC staining and qRT-PCR. The results of qRT-PCR (Figure 4C) and IHC staining (Figure 4D, 4E) showed that after treatment with BA, the antioxidant and antiapoptotic effects of Prdx2 were weakened, suggesting that the protective effect of Prdx2 on the myocardium is related to inhibition of the TLR4/NF-κB signaling pathway (n=3).
Figure 4.
Prdx2 inhibits TLR4/NF-κB signaling pathway in rat cardiomyocytes. (A) Western blot results of TLR4, p65, and p-p65 (n=3); (B) Ratio of expression of p-p65 and p65 (n=3); (C) mRNA expression of SOD1, SOD2, Bax, and Bcl-2 (n=3); (D, E) IHC staining results of SOD1 and caspase8 in rat myocardial tissue (n=3) (magnification: 200×). (“*” means the difference between the 2 groups was statistically significant and P<0.05).
Discussion
AMI is an acute cardiovascular disease with high mortality, disability, and high medical costs [14]. The incidence of AMI has been increasing year by year, and shows a trend of affecting a younger population [15]. It has become a prominent public health and social problem. Although there are currently clinical methods such as drug therapy and coronary intervention reperfusion therapy, myocardial necrosis and myocardial remodeling after myocardial infarction still lead to irreversible damage to cardiac function [16]. The pathogenesis of AMI is not completely clear. However, under the same environmental exposure, only a small number of individuals in the population develop the disease. Large-scale genomic studies have found multiple MI susceptibility sites and segments, suggesting that the occurrence and development of AMI is the result of multiple factors, including environmental factors and genetic factors [17]. Therefore, it is important to screen for specific markers of AMI and find new targets for the treatment of myocardial infarction. We found less Prdx2 protein and mRNA expression in myocardial tissue of AMI model rats, suggesting that the lack of Prdx2 may play a role in rat myocardial injury. We also found in rats with AMI that the level of oxidative stress in myocardial tissue was significantly increased and the apoptosis of myocardial cells was also increased. In rats treated with Prdx2 recombinant protein, the levels of oxidative stress and apoptosis in myocardial tissue were significantly reduced, indicating that the ingestion of Prdx2 significantly improved myocardial damage caused by AMI in rats. The TLR4/NF-κB signaling pathway was found to be highly expressed in myocardial tissue of AMI rats, suggesting that it is involved in the process of myocardial injury. Prdx2 shows a good effect of inhibiting the TLR4/NF-κB signaling pathway.
After blocking the left anterior descending coronary artery of rats, the SOD activity in rat myocardial tissue was significantly reduced and the MPO activity was increased. Decreased antioxidant capacity and increased levels of oxidative stress aggravated myocardial injury in rats. After the rats were treated with Prdx2, the activity of SOD in cardiac muscle tissue increased, indicating an increase in the antioxidant capacity. The ROS level also decreased with Prdx2 treatment. The physiological level of ROS plays a key role in cell signal transduction and stabilization of the intracellular environment. Some ROS, especially H2O2, are considered as local signaling molecules [18]. Therefore, completely quenching the ROS may interrupt the normal signal cascade and have serious adverse consequences. It is generally believed that ROS is suitable for compartmentalized signal transduction, and the antioxidant defense system prevents it from “leaking” into unwanted areas [19]. When the degradation of ROS is impaired, oxidative stress can occur. Oxidative stress refers to the pathological process in which the body produces too much of oxidative active substances and weakens the body’s antioxidant capacity, and the insufficient clearance of ROS results in the increase of ROS in the body, disrupts the normal balance of the body’s oxidation/reduction, and causes cell oxidative damage [20]. ROS can also activate phospholipase, degrade membrane phospholipids, damage mitochondrial structure, and decrease mitochondrial membrane potential. Mitochondrial permeability transition pores thus open, resulting in proton gradient destruction, mitochondrial swelling and rupture, and the release of apoptotic substances into the cytoplasm [21]. Our study also found that Prdx2 can reduce the production of ROS, thus alleviating oxidative damage of the myocardium. In the case of AMI, excess production of ROS occurs and the activity of antioxidant enzymes is decreased. Studies have shown that ROS levels are increased in animal models of heart failure, while the activities of SOD, CAT, and GSH-Px are significantly reduced [22]. Compared with wild-type mice, the protein oxidation product of nitrotyrosine in SOD knockout mice increased, and the incidence of myocardial fibrosis and myocardial hypertrophy significantly increased. GSH-Px deficiency can increase oxidative stress and induce vascular endothelial dysfunction and structural vascular abnormalities [23]. In addition, ROS can activate NF-κB, p53 gene, MAPK, and PARA-1 signal transduction pathways to induce apoptosis. Low levels of H2O2 are related to MAPK activation and protein synthesis, while higher levels of H2O2 stimulate MAPK, JNK, p38, and protein kinase B and promote apoptosis [24]. Therefore, reducing the level of oxidative stress in myocardial tissue during AMI is the key to delaying myocardial cell injury and promoting myocardial cell regeneration. The antioxidant effect of Prdx2 may be the key to alleviating myocardial damage caused by AMI.
MI is accompanied by a high degree of cardiomyocyte apoptosis. Caspase 8/9 in myocardial tissue of rats in the AMI group increased significantly and the ratio of Bax to Bcl-2 also increased. The ingestion of Prdx2 reduced the level of myocardial tissue apoptosis in rats, which indicated that the mechanism by which Prdx2 alleviates myocardial injury is also related to apoptosis. The NF-κB signaling pathway is involved in many diseases. We found that activation of the NF-κB signaling pathway aggravated myocardial injury and that the myocardial-protective effect of Prdx2 was also related to NF-κB signaling pathway regulation. NF-κB is closely related to the redox reaction [25]. Studies have shown that in a rat model of essential hypertension, the expression of NF-κB is significantly increased, which increases the mRNA levels of NOX-2 and NOX-4, members of the NADPH oxidase family, thereby increasing ROS production. After treatment with NF-κB inhibitors, the expression of NOX-2 and NOX-4 decreased, resulting in decreased production of ROS and increased expression of anti-inflammatory factors, thereby reducing the degree of myocardial damage [26]. In addition, Misra et al. [27] found that NF-κB was activated while reducing the expression of the c-Jun amino-terminal kinase in myocardial tissues when MI and local myocardial hypertrophy were induced by ligation of the left anterior descending coronary artery in mice. When nuclear translocation of NF-κB p65 was inhibited, the expression of apoptosis-inhibiting-related protein Bcl-2 was increased, and myocardial cell apoptosis was decreased. In our study, the inhibitory effect of Prdx2 on the TLR4/NF-κB signaling pathway in cardiomyocytes effectively reduced the levels of oxidative stress and apoptosis in cardiomyocytes. This is also how Prdx2 alleviates AMI in rats.
To the best of our knowledge, this is the first study to investigate the effect of Prdx2 on AMI. Prdx2 was found to be one of the key targets for mitigating AMI in this study. We hoped that through this research we can improve the clinical treatment of AMI.
Conclusions
AMI is accompanied by a large degree of apoptosis and rising levels of oxidative stress in myocardial tissue. Prdx2 plays a protective role in cardiomyocytes. Expression of Prdx2 is reduced in damaged myocardial tissues and supplementation of Prdx2 can alleviate oxidative stress and apoptosis of cardiomyocytes induced by AMI. In addition, Prdx2 can inhibit the TLR4/NF-κB signaling pathway, which is an important mechanism by which Prdx2 exerts myocardial protection.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Razvi S, Leng O, Jabbar A, et al. Sample timing, diagnosis of subclinical thyroid dysfunction and mortality in acute myocardial infarction: ThyrAMI1 study. J Clin Endocrinol Metab. 2020;105:dgz143. doi: 10.1210/clinem/dgz143. [DOI] [PubMed] [Google Scholar]
- 2.DeFilippis AP, Chapman AR, Mills NL, et al. Assessment and treatment of patients with type 2 myocardial infarction and acute nonischemic myocardial injury. Circulation. 2019;140:1661–78. doi: 10.1161/CIRCULATIONAHA.119.040631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dharma S, Sari NY, Parlautan A, et al. The 3q25 rs2305619 polymorphism is associated with coronary microvascular obstruction following primary angioplasty for acute ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv. 2019;12:e8228. doi: 10.1161/CIRCINTERVENTIONS.119.008228. [DOI] [PubMed] [Google Scholar]
- 4.Khodayari S, Khodayari H, Amiri AZ, et al. Inflammatory microenvironment of acute myocardial infarction prevents regeneration of heart with stem cells therapy. Cell Physiol Biochem. 2019;53:887–909. doi: 10.33594/000000180. [DOI] [PubMed] [Google Scholar]
- 5.Demidova MM, Carlson J, Erlinge D, Platonov PG. Early repolarization pattern on ECG recorded before the acute coronary event does not predict ventricular fibrillation during ST-elevation myocardial infarction. Heart Rhythm. 2020;17(4):629–36. doi: 10.1016/j.hrthm.2019.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Chi RF, Wang JP, Wang K, et al. Progressive reduction in myocyte autophagy after myocardial infarction in rabbits: association with oxidative stress and left ventricular remodeling. Cell Physiol Biochem. 2017;44:2439–54. doi: 10.1159/000486167. [DOI] [PubMed] [Google Scholar]
- 7.Amani H, Mostafavi E, Arzaghi H, et al. Webster: Three-dimensional graphene foams: Synthesis, properties, biocompatibility, biodegradability, and applications in tissue engineering. ACS Biomater Sci Eng. 2019;5:193–214. doi: 10.1021/acsbiomaterials.8b00658. [DOI] [PubMed] [Google Scholar]
- 8.Hurtado-Gallego J, Redondo-Lopez A, Leganes F, et al. Peroxiredoxin (2-cys-prx) and catalase (katA) cyanobacterial-based bioluminescent bioreporters to detect oxidative stress in the aquatic environment. Chemosphere. 2019;236:124395. doi: 10.1016/j.chemosphere.2019.124395. [DOI] [PubMed] [Google Scholar]
- 9.Peskin AV, Pace PE, Winterbourn CC. Enhanced hyperoxidation of peroxiredoxin 2 and peroxiredoxin 3 in the presence of bicarbonate/CO2. Free Radic Biol Med. 2019;145:1–7. doi: 10.1016/j.freeradbiomed.2019.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Wang S, Chen Z, Zhu S, et al. PRDX2 protects against oxidative stress induced by H. pylori and promotes resistance to cisplatin in gastric cancer. Redox Biol. 2020;28:101319. doi: 10.1016/j.redox.2019.101319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bayer SB, Low FM, Hampton MB, Winterbourn CC. Interactions between peroxiredoxin 2, hemichrome and the erythrocyte membrane. Free Radic Res. 2016;50:1329–39. doi: 10.1080/10715762.2016.1241995. [DOI] [PubMed] [Google Scholar]
- 12.Kim H, Lee TH, Park ES, et al. Role of peroxiredoxins in regulating intracellular hydrogen peroxide and hydrogen peroxide-induced apoptosis in thyroid cells. J Biol Chem. 2000;275:18266–70. doi: 10.1074/jbc.275.24.18266. [DOI] [PubMed] [Google Scholar]
- 13.Stekiel TA, Contney SJ, Bosnjak ZJ, et al. Chromosomal substitution-dependent differences in cardiovascular responses to sodium pentobarbital. Anesth Analg. 2006;102:799–805. doi: 10.1213/01.ane.0000195582.22822.e7. [DOI] [PubMed] [Google Scholar]
- 14.Ram E, Sternik L, Klempfner R, et al. Outcomes of different revascularization strategies among patients presenting with acute coronary syndromes without ST elevation. J Thorac Cardiovasc Surg. 2020;160(4):926–35.e6. doi: 10.1016/j.jtcvs.2019.08.130. [DOI] [PubMed] [Google Scholar]
- 15.Sheng Z, Zhou P, Liu C, et al. Relationships of coronary culprit-plaque characteristics with duration of diabetes mellitus in acute myocardial infarction: An intravascular optical coherence tomography study. Cardiovasc Diabetol. 2019;18:136. doi: 10.1186/s12933-019-0944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vinten-Johansen J, Shi W. Preconditioning and postconditioning: Current knowledge, knowledge gaps, barriers to adoption, and future directions. J Cardiovasc Pharmacol Ther. 2011;16:260–66. doi: 10.1177/1074248411415270. [DOI] [PubMed] [Google Scholar]
- 17.Ando T, Yoshihisa A, Kimishima Y, et al. Prognostic impacts of nutritional status on long-term outcome in patients with acute myocardial infarction. Eur J Prev Cardiol. 2019 doi: 10.1177/2047487319883723. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Eghbalzadeh K, Georgi L, Louis T, et al. Compromised anti-inflammatory action of neutrophil extracellular traps in PAD4-deficient mice contributes to aggravated acute inflammation after myocardial infarction. Front Immunol. 2019;10:2313. doi: 10.3389/fimmu.2019.02313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Z, Zhang S, Ding S, et al. Excessive neutrophil extracellular trap formation aggravates acute myocardial infarction injury in apolipoprotein E deficiency mice via the ROS-dependent pathway. Oxid Med Cell Longev. 2019;2019 doi: 10.1155/2019/1209307. 1209307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zou J, Fei Q, Xiao H, et al. VEGF-A promotes angiogenesis after acute myocardial infarction through increasing ROS production and enhancing ER stress-mediated autophagy. J Cell Physiol. 2019;234:17690–703. doi: 10.1002/jcp.28395. [DOI] [PubMed] [Google Scholar]
- 21.Sinning C, Westermann D, Clemmensen P. Oxidative stress in ischemia and reperfusion: Current concepts, novel ideas and future perspectives. Biomark Med. 2017;11:11031–40. doi: 10.2217/bmm-2017-0110. [DOI] [PubMed] [Google Scholar]
- 22.van Deel ED, Lu Z, Xu X, et al. Extracellular superoxide dismutase protects the heart against oxidative stress and hypertrophy after myocardial infarction. Free Radic Biol Med. 2008;44:1305–13. doi: 10.1016/j.freeradbiomed.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quidim A, Bruno T, Leocadio P, et al. The prognostic value of nitrotyrosine levels in coronary heart disease: Long-term evaluation in the Acute Coronary Syndrome Registry Strategy (ERICO study) Clin Biochem. 2019;66:37–43. doi: 10.1016/j.clinbiochem.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Ren J, Su D, Li L, et al. Anti-inflammatory effects of Aureusidin in LPS-stimulated RAW264.7 macrophages via suppressing NF-kappaB and activating ROS- and MAPKs-dependent Nrf2/HO-1 signaling pathways. Toxicol Appl Pharmacol. 2020;387:114846. doi: 10.1016/j.taap.2019.114846. [DOI] [PubMed] [Google Scholar]
- 25.Jiang Y, Feng YP, Tang LX, et al. The protective role of NR4A3 in acute myocardial infarction by suppressing inflammatory responses via JAK2-STAT3/NF-kappaB pathway. Biochem Biophys Res Commun. 2019;517:697–702. doi: 10.1016/j.bbrc.2019.07.116. [DOI] [PubMed] [Google Scholar]
- 26.Sung PH, Yin TC, Wallace CG, et al. Extracorporeal shock wave-supported adipose-derived fresh stromal vascular fraction preserved left ventricular (LV) function and inhibited LV remodeling in acute myocardial infarction in rat. Oxid Med Cell Longev. 2018;2018 doi: 10.1155/2018/7518920. 7518920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Misra A, Haudek SB, Knuefermann P, et al. Nuclear factor-kappaB protects the adult cardiac myocyte against ischemia-induced apoptosis in a murine model of acute myocardial infarction. Circulation. 2003;108:3075–78. doi: 10.1161/01.CIR.0000108929.93074.0B. [DOI] [PubMed] [Google Scholar]