Abstract
Objective:
Omphalocele is the second most common abdominal birth defect and often occurs with other structural and genetic defects. Little is known about rates, patterns and trends of mortality after the first year of life in diverse populations. The objective of this study was to determine the prevalence, time trends and mortality during early childhood for infants with omphalocele overall, by geographical region, and by presence of associated anomalies.
Methods:
We conducted a retrospective study with 23 birth defect surveillance systems in 18 countries who are members of the International Clearinghouse for Birth Defects Surveillance and Research, which submitted data on cases ascertained between 2000 and 2012. Approximately 16 million pregnancies that ended in livebirths, stillbirths, or elective terminations for fetal anomalies (ETOPFA) were surveyed and cases with omphalocele were included. Overall prevalence and mortality rates for specific time periods were calculated (24 hours, neonatal, infant and early childhood). We used Kaplan-Meier estimates with 95% confidence intervals (CI) to calculate cumulative mortality and joinpoint regression for time trend analyses.
Results:
Between 2000 and 2012, the prevalence of omphalocele was 2.6 per 10,000 births (95% CI: 2.5, 2.7). Prevalence decreased minimally during 2000-2012 (average annual percent change = −0.19%, P=0.52). The overall mortality rate for this period was 32.1% (95% CI: 30.2, 34.0). Most deaths occurred during the neonatal period and among children with multiple or syndromic omphalocele. Prevalence and mortality varied by registry type (e.g., hospital- vs. population-based) and inclusion or exclusion of ETOPFA.
Conclusions:
The prevalence of omphalocele did not change over time during the study period. Approximately one-third of children with omphalocele did not survive early childhood with most deaths occurring in the neonatal period.
Precis:
In our multi-country study, we found that the prevalence of omphalocele did not change over the study period and about thirty percent of these children will not survive early childhood, with most deaths occurring in the neonatal period.
INTRODUCTION
Omphalocele is the second most commonly occurring abdominal birth defect, with prevalence estimates ranging between 1.0 and 3.8 per 10,000 births globally.1-12 Characterized by a defect of the midline abdominal wall, a common feature is a thin membranous sac in which the organs (e.g., small intestine, liver, bladder, spleen, stomach, uterus, and ovaries) protrude into the base of the umbilical cord.13 Omphaloceles range in severity and are classified as small (involving herniation of the bowel and stomach), giant (involving herniation of the bowel, stomach, and liver) and ruptured.14-16
The specific etiology of omphalocele remains largely unknown; however, an oft cited hypothesis is failure of the abdomen to completely close at the umbilical ring that results from a defect in lateral folding during embryogenesis.17 Some risk factors have been identified, which include: advanced and very young maternal age,7,18,19 maternal pre-pregnancy overweight or obesity,18,20 nulliparity,21 multiparity,22 maternal prenatal alcohol use,23 maternal cigarette smoking,23,24 maternal asthma medication use,25 maternal selective serotonin reuptake inhibitor (SSRI) use,26 lack of periconceptional/prenatal multivitamin use,18 abnormality in vitamin B12 production, transport and metabolism,18 and the 677C–T mutation in the methylenetetrahydrofolate reductase (MTHFR) gene.18 Additionally, alterations in glycemic control,18 maternal history of febrile illness,18 multiple gestation pregnancies,18,21,23,27-30 and in vitro fertilization (IVF) treatments also increase risk.8,18,21,31,32 Folic acid fortification may decrease risk of omphalocele occurrence.18 In contrast to other abdominal defects,13,32 the occurrence of omphalocele has been reported as stable over time8,13,33-35 in developed countries. Omphalocele seems to occur more frequently in males than females,2,3,8,18,21,31,33,35,36 in Hispanic populations21,31 more than non-Hispanic (NH) white, and least frequently among US NH blacks.31
Omphalocele can occur in isolation in approximately one-third of all cases, but more often is associated with other major defects.8,9,37-39 Associated defects mainly occur in the heart, central nervous system, urogenital or musculoskeletal system.8,9,18,37,38 In about half of non-isolated omphalocele cases, chromosomal anomalies (mainly trisomy 13 and 18) or genetic defects (e.g., Beckwith-Wiedemann syndrome) are found.9,13,37,40
The number and type of associated anomalies may also influence the survival of infants with omphalocele. Prior studies show that survival rates for children with omphalocele depend upon the severity of the associated anomalies.8,41 Children born with isolated omphalocele usually have better survival than children born with omphalocele and other anomalies.42-44 A US-based study reported survival rates of 92% (overall) in liveborns; 100% in those with isolated omphalocele; 88% in those with non-isolated omphalocele,39 but estimates were based on a very small number of prenatally diagnosed cases. Surprisingly, little is known about mortality during early childhood among children with omphalocele. Most studies conducted to date focused on clinical populations and in-patient mortality, with small numbers of omphalocele cases.39,40,45-48 Few population-based studies have investigated early childhood mortality among children with omphalocele.8,9,49,50 One US study pooled data from several birth defects registries and reported infant mortality rates.8 Early and late neonatal mortality was studied in 493 omphalocele cases from Europe51 and a study from China which used data from a single registry, reported perinatal mortality rates.49 Thus, information about mortality beyond age one year or in countries outside the US is lacking. Therefore, the purpose of this study was to investigate (1) total and livebirth prevalence, (2) time trends, and (3) mortality related to omphalocele during early childhood (< age 5 years) overall, by country/geographical region and by presence of associated anomalies.
METHODS
Study Design
We conducted a retrospective study using data from 23 birth defects surveillance systems that are members of the International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR; http://www.icbdsr.org). The ICBDSR was established in 1974 as a not-for-profit volunteer organization affiliated with the World Health Organization. Its purpose is to conduct worldwide surveillance and research into the occurrence and possible causes of birth defects for prevention and reduction of their consequences. As of 2018, 42 birth defects surveillance programs from 36 countries were members and 27 programs submit aggregated data annually on 39 birth defects for the ICBDSR annual report.
Twenty-three member programs of the ICBDSR from 18 countries in North and South America, Europe, and the Middle East submitted data for this project. Surveillance programs were eligible to participate if they ascertained cases of omphalocele and could provide information on vital status. Programs included data on the surveillance method used (hospital- or population-based), year the program began surveillance, years of ascertainment of omphalocele cases, follow-up period for ascertainment of death, method of ascertaining death, program’s definition of stillbirth, program’s definition of elective terminations, national policy on elective terminations of pregnancy for fetal anomalies (ETOPFA) and availability of prenatal screening and diagnostic services.
Study Population
Each of the 23 programs submitted information on the annual number of omphalocele cases and pregnancy outcomes (live birth, stillbirth, and ETOPFA) from the earliest time period available in each registry until December 31, 2014, the end of the study period (or the most current available data for the registry). Omphalocele (exomphalos) was defined as “a congenital malformation characterized by herniation of abdominal contents through the umbilical insertion and covered by a membrane which may or may not be intact. Excludes: gastroschisis (para-umbilical hernia), aplasia or hypoplasia of abdominal muscles, skin-covered umbilical hernia”52 which corresponds to the International Classification of Disease (ICD)-10-Clinical Modification (CM) code “Q79.2” and ICD-9-CM code “756.70”. Omphalocele cases were classified based on clinical presentation (i.e., isolated, multiple congenital anomalies (MCA), and syndromic) by 16 programs with available data. South America - Latin American Collaborative Study of Congenital Malformations (ECLAMC) and Israel – Soroka Medical Center (SMC) had data on isolated and MCA cases and Czech Republic had data only on genetic cases. We defined isolated cases as pregnancies affected by an omphalocele with no other major malformation present based on the ICBDSR definition. We defined MCA cases as those having two or more major unrelated anomalies in different organ systems (e.g., a fetus/infant who had an omphalocele and craniosynostosis was defined as MCA). Syndromic cases were defined as omphalocele cases that have chromosomal or genetic abnormalities.
Ascertainment of Mortality
Surveillance programs ascertained the vital status of omphalocele cases using various methods, such as active or passive follow-up of cases by clinical or registry staff or linkage to death records (Supplementary Table 1 and 2); some programs used more than one mortality ascertainment method. Length of follow-up for vital status varied by program, e.g., from birth until hospital discharge, first week, first year or longer (Supplementary Table 2). We considered infants deceased if identified as such by the surveillance program through examination of medical files or reports from medical or government death records.
Statistical Analysis
We calculated descriptive statistics for the main study variables and covariates. Three-year rolling averages of overall, live birth, stillbirth and ETOPFA prevalence were calculated and graphed for all 23 programs and for the 17 programs that include data from 2000 to 2012. We examined the prevalence estimates and mortality rates from 2000 to 2012 since that was the time period that the majority of registries had the most complete data. We calculated total prevalence as the total number of omphalocele cases (all pregnancy outcomes combined) divided by the total number of live births and stillbirths per 10,000. The denominator of the prevalence formula did not include ETOPFA because of the lack of information on the total number of terminations for each program. We calculated the average annual percent change (AAPC) from Joinpoint regression analyses. Joinpoint regression analyses were used to assess statistically significant annual changes in prevalence and mortality for nonlinear time trends between 2000 and 2012.53 Each regression model began with zero joinpoints and up to four joinpoints were allowed in the model if statistically significant changes in rates or direction were noted using a Monte Carlo permutation test until an optimal-fitted model were selected.53 Among live birth cases with omphalocele, we calculated age-specific mortality as the number of deaths at different ages (day of birth, 1-6 days, 7-27 days, 28-364 days, 1-4 years, and ≥5 years of age), divided by the total number of live birth cases with omphalocele,. We also examined cumulative percent mortality with corresponding confidence intervals (CI) at specific ages using a modified Kaplan-Meier Product-Limit method for each program, registry type, and total to account for censoring. Survival probability was calculated by taking the cumulative proportion of cases that were still alive at different time periods after birth divided by the total number of live births with omphalocele. We display cumulative survival graphs (which adjust for differential follow-up time) for North American and European programs, since they had the highest number of participating programs and most complete follow-up of live births through linkage with death certificates. We examined mortality by clinical presentation: isolated, omphalocele cases with MCA and syndromic omphalocele cases for the programs where data was available (18 programs; 78.0%). SAS version 9.4 (SAS Inc., Cary, NC) and Joinpoint Regression Program version 4.7.0.0 were used for the analyses.
Human Subjects
We conducted the research in accordance with the prevailing ethical principles and the Office of Research Integrity and Compliance, Institutional Review Board (IRB) at the University of Arkansas for Medical Science determined this study exempt from IRB review.
RESULTS
Approximately 16 million births occurred during the 2000-2012 study period in the areas monitored by the 23 surveillance programs in 18 countries. Most programs which participated in the study were population-based (n=15, 65%). Sixty-five percent monitored regions of countries or states; only 13% of the programs monitored entire states or provinces, and 22% monitored an entire country. Registries also varied in inclusion of stillbirths and terminations in their case ascertainment methods. Although 22 of the 23 registries included stillbirths in their case ascertainment methods, the definition of stillbirth varied between registries (Supplementary Table 1). Seventy percent (16/23) of registries included ETOPFA in their case ascertainment methods during the entire study period (Supplementary Table 1) and all but one registry were in areas that had access to prenatal screening services.
Prevalence of Omphalocele
During 2000-2012, 4,157 cases of omphalocele were identified from 15,955,640 births, for an overall prevalence of 2.6 per 10,000 births (95% CI: 2.5, 2.7) based on all programs (Table 1). Of the 4,157 cases, 63.0% were live births, 11.5% were stillbirths, 25.2% were ETOPFA and 0.3% had an unknown outcome. The highest ETOPFA proportions were seen in Spain, France (Paris) and Italy (Tuscany) (83%, 71% and 66%, respectively). ETOPFA was more often performed in syndromic omphalocele cases (67%) compared to MCA omphalocele (23%) and isolated omphalocele (20%) (Supplementary Table 3). Total and livebirth prevalence varied by case ascertainment method: hospital-based programs had a higher prevalence of omphalocele cases than population-based programs (3.1 vs. 2.4 and 2.4 vs. 1.4 per 10,000 births, respectively, Table 1). France (Paris) and the UK (United Kingdom, Wales) showed the highest omphalocele prevalences (5.8 and 4.1, respectively), whereas the lowest prevalence were seen in Slovak Republic and Israel (0.8 and 0.9, respectively). The three-year rolling average prevalence between 2000 and 2012 for omphalocele cases by pregnancy outcomes is displayed in Supplementary Figure 1a and the 3-year rolling average prevalence between 2000 and 2012 for omphalocele cases by pregnancy outcomes for surveillance systems which included ETOPFA is displayed in Supplementary Figure 1b. Joinpoint regression analyses showed a negligible decline in the overall prevalence of omphalocele from 2000-2012 (AAPC = −0.19%; P=0.52) (data not shown). Time trends varied by registry type: population-based registries showed an overall modest linear decrease in prevalence over time (AAPC = −0.41%; P=0.31). In contrast, hospital-based registries showed a statistically significant increase in prevalence from 2000-2010 (AAPC = 3.19%) followed by a drastic decline from 2010-2012 (AAPC = −21.74%) (data not shown).
Table 1.
Total number of births, total number of omphalocele cases, prevalence per 10,000 births and pregnancy outcome proportions by registry type, 23 birth defects surveillance systems in 18 countries for surveillance period 2000-2012
| Country - registry | Surveillance period |
Total births | Number of cases |
Total prevalence3 per 10,000 births (95% CI) |
Livebirth prevalence (95% CI) |
Livebirth % (95% CI) |
Stillbirth % (95% CI) |
ETOPFA4 % (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Hospital-based registries | ||||||||
| Argentina – RENAC1 | 2009-2012 | 422,173 | 113 | 2.7 (2.2, 3.2) | 2.3 (1.9, 2.8) | 85.8% (78.0, 91.7) | 14.2% (8.3, 22.0) | - |
| Colombia – Bogotá1 | 2000-2012 | 356,649 | 53 | 1.5 (1.1, 1.9) | 1.4 (1.0, 1.8) | 94.3% (84.3, 98.8) | 5.7% (1.2, 15.7) | - |
| Colombia – Cali1 | 2011-2012 | 12,762 | 3 | 2.4 (0.5, 6.9) | 2.4 (0.5, 6.9) | 100% (29.2, 100) | 0.0% (0.0, 70.8) | - |
| South America – ECLAMC1 | 2000-2012 | 2,035,032 | 794 | 3.9 (3.6, 4.2) | 3.1 (2.9, 3.4) | 80.5% (77.6, 83.2) | 19.5% (16.8, 22.5) | - |
| Spain - ECEMC | 2000-2012 | 275,813 | 88 | 3.2 (2.6, 3.9) | 0.5 (0.3, 0.9) | 15.9% (9.0, 25.3) | 1.1% (0.03, 6.2) | 83.0% (73.5, 90.1) |
| Mexico – RYVEMCE1 | 2000-2012 | 287,674 | 58 | 2.0 (1.5, 2.5) | 1.7 (1.2, 2.2) | 84.5% (72.6, 92.7) | 15.5% (7.3, 27.4) | - |
| Iran – TROCA | 2004-2012 | 160,755 | 37 | 2.3 (1.6, 3.2) | 2.1 (1.4, 2.9) | 89.2% (74.6, 97.0) | 5.4% (0.7, 18.2) | 5.4% (0.7, 18.2) |
| Israel – SMC2 | 2000-2012 | 169,973 | 15 | 0.9 (0.5, 1.5) | 0.9 (0.5, 1.5) | 100% (78.2, 100) | - | - |
| Total | 2000-2012 | 3,720,831 | 1,161 | 3.1 (3.0, 3.3) | 2.4 (2.3, 2.6) | 77.5% (75.0, 79.9) | 16.0% (14.0, 18.3) | 6.5% (5.2, 8.0) |
| Population-based registries | ||||||||
| Czech Republic | 2000-2012 | 1,364,555 | 310 | 2.3 (2.0, 2.5) | 1.2 (1.1, 1.4) | 54.2% (48.5, 59.8) | 0.6% (0.1, 2.3) | 45.2% (39.5, 50.9) |
| France - Paris | 2000-2012 | 346,109 | 202 | 5.8 (5.1, 6.7) | 1.5 (1.1, 1.9) | 25.2% (19.4, 31.8) | 4.0% (1.7, 7.7) | 70.8% (64.0, 77.0) |
| Germany - Saxony Anhalt | 2000-2012 | 226,907 | 70 | 3.1 (2.4, 3.9) | 1.0 (0.6, 1.5) | 31.4% (20.9, 43.6) | 10.0% (4.1, 19.5) | 58.6% (46.2, 70.2) |
| Italy - Lombardy | 2003–2012 | 133,182 | 24 | 1.8 (1.2, 2.7) | 0.8 (0.4, 1.4) | 41.7% (22.1, 63.4) | 8.3% (1.0, 27.0) | 50.0% (29.1, 70.9) |
| Italy - Tuscany | 2000-2012 | 379,464 | 76 | 2.0 (1.6, 2.5) | 0.6 (0.4, 0.9) | 31.6% (21.4, 43.3) | 2.6% (0.3, 9.2) | 65.8% (54.0, 76.3) |
| Malta – MCAR1 | 2000-2012 | 52,474 | 14 | 2.7 (1.5, 4.5) | 2.3 (1.2, 4.0) | 85.7% (57.2, 98.2) | 14.3% (1.8, 42.8) | - |
| Netherlands - North | 2000-2012 | 242,341 | 59 | 2.4 (1.9, 3.1) | 1.2 (0.8, 1.7) | 49.2% (35.9, 62.5) | 6.7% (1.9, 16.5) | 44.1% (31.2, 57.6) |
| Slovak Republic | 2001-2012 | 667,992 | 55 | 0.8 (0.6, 1.1) | 0.8 (0.6, 1.0) | 92.7% (82.4, 98.0) | 1.8% (0.1, 9.7) | 5.5% (1.1, 15.1) |
| Sweden | 2000-2012 | 1,319,370 | 361 | 2.7 (2.5, 3.0) | 1.1 (1.0, 1.3) | 41.3% (36.2, 56.6) | 2.2% (1.0, 4.3) | 56.5% (51.2, 67.7) |
| UK - Wales | 2000-2012 | 435,834 | 179 | 4.1 (3.5, 4.8) | 1.7 (1.3, 2.1) | 40.2% (33.0, 47.8) | 3.9% (1.6, 7.9) | 55.9% (48.3, 63.3) |
| Ukraine - OMNI-Net5 | 2000-2012 | 372,434 | 136 | 3.7 (3.1, 4.3) | 1.3 (1.0, 1.7) | 35.3% (27.3, 44.0) | 6.6% (3.1, 12.2) | 52.9% (44.2, 61.6) |
| USA - Arkansas5 | 2000-2012 | 508,654 | 130 | 2.6 (2.1, 3.0) | 2.0 (1.6, 2.4) | 76.9% (68.7, 83.9) | 16.9% (10.9, 24.5) | 3.8% (1.3, 8.8) |
| USA - Atlanta5 | 2000-2008 | 479,379 | 137 | 2.9 (2.4, 3.4) | 1.5 (1.2, 1.9) | 51.8% (43.1, 60.4) | 20.4% (14.0, 28.2) | 26.3% (19.1, 34.5) |
| USA - Texas | 2000-2012 | 5,033,546 | 1,044 | 2.1 (2.0, 2.2) | 1.6 (1.4, 1.7) | 74.9% (72.2, 77.5) | 15.7% (13.6, 18.1) | 9.4% (7.7, 11.3) |
| USA - Utah | 2000-2012 | 672,568 | 199 | 3.0 (2.6, 3.4) | 1.9 (1.6, 2.2) | 63.3% (56.2, 70.0) | 14.1% (9.6, 19.7) | 22.6% (17.0, 29.1) |
| Total5 | 2000-2012 | 12,234,809 | 2,996 | 2.4 (2.3, 2.5) | 1.4 (1.3, 1.5) | 57.2% (55.5, 59.0) | 9.8% (8.8, 10.9) | 32.5% (30.9, 34.3) |
| All registries5 | 2000-2012 | 15,955,640 | 4,157 | 2.6 (2.5, 2.7) | 1.6 (1.5, 1.7) | 63.0% (61.6, 64.5) | 11.5% (10.6, 12.5) | 25.2% (23.9, 26.5) |
ETOPFA (elective termination of pregnancy for fetal anomalies) not allowed during (part of) surveillance period
data on live born children with congenital omphalocele from one hospital
denominator includes livebirths and stillbirths
ETOPFA= elective termination of pregnancy for fetal anomalies
percentages of live birth, stillbirth, and ETOFA do not add up to 100% due to unknown pregnancy outcome of some cases
ECEMC= Spanish Collaborative Study of Congenital Malformations; ECLAMC=Latin American Collaborative Study of Congenital Malformations; GP=General Practitioner; MCAR=Malta Congenital Anomalies Registry; OMNI-Net=Ukraine Birth Defects Prevention Program; RENAC=National Network of Congenital Anomalies of Argentina; RYVEMCE= Mexican Registry and Epidemiological Surveillance of Congenital Malformations; TROCA=Tabriz Registry of Congenital Anomalies; SMC=Soroka Medical Center; UK=United Kingdom; USA=United States of America
The prevalence of omphalocele cases during 2000-2012 by clinical presentation was 1.1 per 10,000 births (95% CI: 1.0, 1.2) for isolated cases, 1.2 per 10,000 births (95% CI: 1.1, 1.3) for omphalocele with MCA and 0.7 per 10,000 births (95% CI: 0.6, 0.8) for syndromic omphalocele (data not shown). Of the registries that reported clinical presentation (n=2,499), 37% were isolated, 42% had multiple congenital anomalies and 21% had chromosomal or genetic abnormalities (Supplementary Table 3).
Overall mortality
During 2000-2012, the overall mortality rate was 32.1% (95% CI: 30.2, 34.0) (Table 2); however, the rate varied by method of case ascertainment and age at death. For hospital-based surveillance systems, the overall mortality rate was 40.8% (95% CI: 37.4, 44.3), whereas for population-based surveillance systems the overall mortality rate was 27.8% (95% CI: 25.7, 30.0). The greatest percentage of deaths occurred within the first 24 hours of life (12.2% overall; 18.9% for hospital-based and 8.7% for population-based) followed by 1-6 days (8.7% overall; 11.5% for hospital-based and 7.5% for population-based). Overall, the largest proportion of deaths (25.9%; 39.7% for hospital-based and 19.9% for population-based) occurred during the neonatal period (0-27 days of life). About 6 percent of deaths occurred between 28 and 364 days of life. Our one-year mortality rate was 30.7%. Five-year mortality rates overall were higher for surveillance systems in countries that did not include ETOPFA/did not register ETOPFA than those that allowed ETOPFA (overall mortality: 42.4% vs. 27.3%) (Table 2).
Table 2.
Mortality rates and Kaplan-Meier Product-Limit estimates with 95% confidence intervals by age at death and registry type for infants born with omphalocele in 18 countries from 23 birth defects surveillance systems, 2000-2012
| Kaplan Meier Mortality Estimates for Age at Death3 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Country - registry | Surveillance period |
Number Livebirths |
< 1 day | 1-6 days | 7-27 days | 0-27 days | 28 - 364 days |
1-year | 1 - 4 years |
≥5 years | 5-year (95% CI)3 |
| Hospital-based registries | |||||||||||
| Argentina – RENAC1 | 2009-2012 | 97 | 43.3% | - | 43.3% | - | 43.3% | - | - | 43.3% (33.4, 53.2) | |
| Colombia – Bogotá1 | 2000-2012 | 50 | 20.0% | 0.0% | - | 20.0% | - | 20.0% | - | - | 20.0% (8.9, 31.1) |
| Colombia – Cali1 | 2011-2012 | 3 | 0.0% | 0.0% | - | 0.0% | - | 0.0% | - | - | 0.0% (0.0, 0.0) |
| South America – ECLAMC1 | 2000-2012 | 639 | 23.2% | 8.0% | 9.2%5 | 40.4% | 1.1%5 | 41.5% | - | - | 41.5% (37.7, 45.3) |
| Spain - ECEMC | 2000-2012 | 14 | 0.0% | 7.1% | - | 7.1% | - | 7.1% | - | - | 7.1% (0.0, 20.6)4 |
| Mexico – RYVEMCE1 | 2000-2012 | 49 | 18.4% | 4.1% | - | 22.5% | - | 22.5% | - | - | 22.5% (10.8, 34.1) |
| Iran - TROCA | 2004-2012 | 33 | 0.0% | 0.0% | - | 0.0% | - | 0.0% | - | - | 0.0% (0.0, 0.0) |
| Israel – SMC2 | 2000-2012 | 15 | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% (0.0, 0.0) |
| Total | 2000-2012 | 900 | 18.9% | 11.5% | 9.3% | 39.7% | 1.1% | 40.8% | 0.0% | 0.0% | 40.8% (37.4, 44.3) |
| Population-based registries | |||||||||||
| Czech Republic | 2000-2012 | 168 | 2.4% | 3.0% | 3.0% | 8.3% | 4.8% | 13.1% | 1.8% | 1.2% | 16.1% (10.5, 21.6) |
| France - Paris | 2000-2012 | 51 | 5.9% | 2.0% | 0.0% | 7.8% | - | 7.8% | - | - | 7.8% (0.5, 15.2) |
| Germany - Saxony Anhalt | 2000-2012 | 22 | 0.0% | 0.0% | 4.5% | 4.5% | 0.0% | 4.5% | - | - | 4.5% (0.0, 13.2)4 |
| Italy - Lombardy | 2003-2012 | 10 | 0.0% | 0.0% | 0.0% | 0.0% | 10.0% | 10.0% | 0.0% | 0.0% | 10.0% (0.0, 28.6)4 |
| Italy - Tuscany | 2000-2012 | 24 | 8.3% | 8.3% | 8.3% | 25.0% | 0.0% | 25.0% | 0.0% | 0.0% | 25.0% (7.7, 42.3) |
| Malta – MCAR1 | 2000-2012 | 12 | 16.7% | 0.0% | 16.6% | 33.3% | 16.7% | 50.0% | - | - | 50.0% (37.3, 62.7) |
| Netherlands - North | 2000-2012 | 29 | 3.4% | 13.8% | 6.9% | 24.2% | 3.4% | 27.6% | - | - | 27.6% (11.3, 43.9) |
| Slovak Republic | 2001-2012 | 51 | 0.0% | 13.7% | 2.0% | 15.7% | - | 15.7% | - | - | 15.7% (5.7, 25.7) |
| Sweden | 2000-2012 | 149 | 1.3% | 6.0% | 2.0% | 9.3% | 7.4% | 16.7% | 0.7% | 0.0% | 17.4% (11.4, 23.5) |
| UK - Wales | 2000-2012 | 72 | 5.6% | 12.5% | 0.0% | 18.1% | 9.7% | 27.8% | 1.4% | 0.0% | 29.2% (18.7, 39.7) |
| Ukraine - OMNI-Net | 2000-2012 | 48 | 6.3% | 6.2% | 6.2% | 18.7% | 2.1% | 20.8% | - | - | 20.8% (9.3, 32.3) |
| USA - Arkansas | 2000–2012 | 100 | 17.0% | 12.0% | 0.0% | 29.0% | 9.0% | 38.0% | 3.0% | 0.0% | 41.0% (31.4, 50.6) |
| USA - Atlanta | 2000-2008 | 71 | 9.9% | 11.3% | 4.2% | 25.4% | 4.2% | 29.6% | 1.4% | 0.0% | 31.0% (20.2, 41.7) |
| USA - Texas | 2000-2012 | 782 | 11.0% | 7.9% | 4.5% | 23.4% | 6.4% | 29.8% | 1.2% | 0.1% | 31.1% (27.8, 34.3) |
| USA - Utah | 2000-2012 | 126 | 15.9% | 5.6% | 4.0% | 25.4% | 6.3% | 31.7% | 1.6% | - | 33.3% (25.1, 41.6) |
| Total | 2000-2012 | 1,715 | 8.7% | 7.5% | 3.6% | 19.9% | 6.4% | 26.3% | 1.3% | 0.3% | 27.8% (25.7, 30.0) |
| All registries | 2000-2012 | 2,615 | 12.2% | 8.7% | 5.0% | 25.9% | 4.8% | 30.7% | 1.2% | 0.2% | 32.1% (30.2, 34.0) |
| ETOPFA not allowed or registered versus ETOPFA allowed | |||||||||||
| Not allowed/registered | 2000-2012 | 865 | 19.8% | 12.0% | 9.3% | 41.1% | 1.3% | 42.4% | 0.0% | 0.0% | 42.4% (38.9, 45.9) |
| Allowed | 2000-2012 | 1,750 | 8.5% | 7.4% | 3.6% | 19.5% | 6.3% | 25.8% | 1.3% | 0.2% | 27.3% (25.2, 29.5) |
ETOPFA (elective termination of pregnancy for fetal anomalies) not allowed during (part of) surveillance period
data on live born children with congenital omphalocele from one hospital
cumulative percent mortality was calculated using a modified Kaplan-Meier Product-Limit method to account for censoring and differential length of follow-up
Lower limit confidence intervals fitted to zero
incomplete follow-up, but deaths reported
ECEMC= Spanish Collaborative Study of Congenital Malformations; ECLAMC=Latin American Collaborative Study of Congenital Malformations; GP=General Practitioner; MCAR=Malta Congenital Anomalies Registry; OMNI-Net=Ukraine Birth Defects Prevention Program; RENAC=National Network of Congenital Anomalies of Argentina; RYVEMCE= Mexican Registry and Epidemiological Surveillance of Congenital Malformations; TROCA=Tabriz Registry of Congenital Anomalies; SMC=Soroka Medical Center; UK=United Kingdom; USA=United States of America
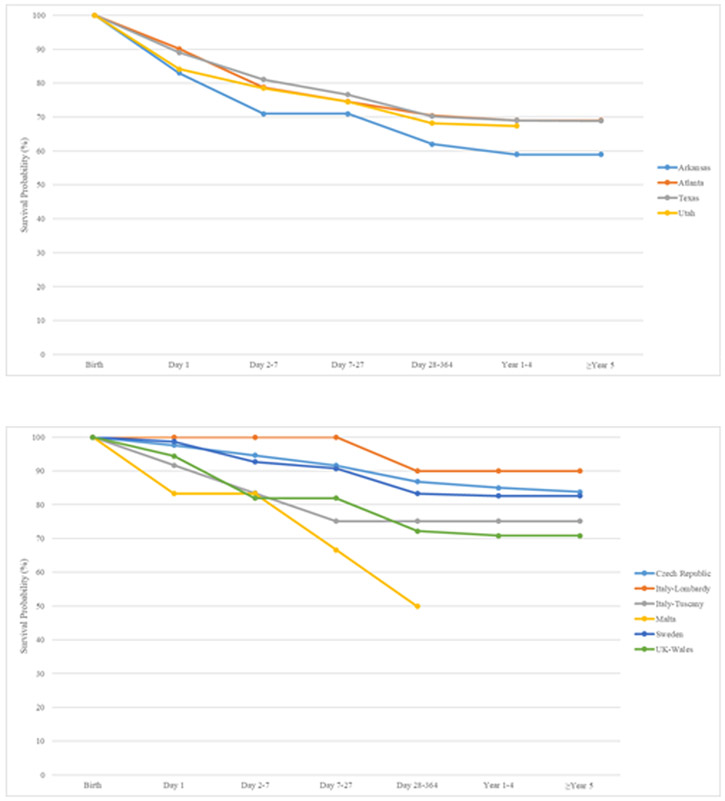
Figure 1 shows Kaplan-Meier survival curves up to age five years for all liveborn cases with omphalocele in ten surveillance systems with linkage to death certificates in North America and Europe. Overall, survival is somewhat lower in North America compared to Europe. Survival was highest in Italy (Lombardy) (90%, based on 10 cases) and the Czech Republic (83.3%, based on 168 cases) and lowest in Malta (49.9%, based on 12 cases) and Arkansas (59.0%, based on 100 cases).
Figure 1.
Kaplan-Meier survival curves up to age five years for all liveborn cases with omphalocele in ten birth defects surveillance systems with linkage to death certificates by continent, 2000-2012
1a: North America
1b Europe: Czech, Italy Lombardy, Italy Tuscany, Malta, Sweden, UK Wales
Time trend analyses for mortality rates between 2000 and 2012 showed an overall pattern of decline during the time period but was not constant. Between 2000 and 2004, mortality rates declined (AAPC = −5.51%; P=0.14) but then increased between 2004 and 2007 (AAPC = 8.59%; P=0.47) and then decreased again from 2007 to 2012 (AAPC = −10.47%; P=0.02). For population-based registries, no time trends in mortality rates were observed (AAPC = −0.01%; P=0.96) and hospital-based registries showed a very modest decline in mortality rates between 2000 and 2012 (AAPC = −2.15%; P=0.20) (data not shown).
Mortality by Geographic Location
The highest mortality for omphalocele in 2000-2012 was seen for Malta (50%, 12 liveborn cases), Argentina (43.3%, 97 liveborn cases), South America (41.5%, 639 liveborn cases) and USA (Arkansas) (41.0%, 100 liveborn cases) (Table 2). The lowest mortality (0%) was seen for Colombia (Cali) (3 liveborn cases), Iran (33 liveborn cases) and Israel (15 liveborn cases).
Mortality by Clinical Presentation
Mortality rates varied by clinical presentation (Supplementary Tables 4). Overall, only 17.2% of children with isolated omphalocele died during the 2000-2012 period. For hospital-based surveillance systems the mortality rate for cases of isolated omphalocele was higher than the population-based programs (23.7% vs. 8.9%). Similar to the pattern observed for all cases of omphalocele, the majority of deaths of isolated omphalocele occurred within the first 24 hours, days 1-6 and days 7-27 (6.1%, 4.6% and 4.6%, respectively)(data not shown). Forty-eight percent of children with omphalocele with MCA died during the 2000-2012 period. Hospital-based surveillance systems also had higher mortality rates of omphalocele with MCA than population-based systems (56.7% vs. 28.5%). The pattern of mortality by age at death for omphalocele with MCA remained similar to the pattern observed for all cases and isolated cases of omphalocele with the majority of deaths occurring within the first 7 days of life and the neonatal period. Children with syndromic omphalocele had the highest mortality rates (55.8%). However, hospital-based programs had a lower mortality rate for syndromic omphalocele cases than population-based programs (46.4% vs. 54.7%, respectively). Unlike the pattern observed for age at death for other clinical presentations of omphalocele, a higher proportion of deaths in syndromic omphalocele occurred during days 1-6 (17.5%) rather than within the first 24 hours of birth (15.6%). In addition, rather than a steady decline in mortality rates as age increases, mortality rates in syndromic omphalocele almost doubled between the 7-27 days and 28-364 days period, from 6.7% to 13.2% (data not shown).
Mortality by clinical presentation and geographic location
The highest mortality in isolated omphalocele was seen in South America (23.4%, 282 liveborn cases) and Argentina (23.3%, 43 liveborn cases). In Italy (Lombardy) and Malta high mortality was observed (33.3%), but each reported only 3 liveborn cases. Also for omphalocele associated with MCA, the highest mortality was seen in Argentina (60.8%, 51 liveborn cases) and South America (55.7%, 357 liveborn cases). In Italy (Tuscany), mortality was 66.7%, but this was based on only 3 liveborn cases. In contrast, among syndromic cases highest mortality was registered in and Malta (100%, only 2 cases), the Czech Republic (78.6%, 14 cases), Italy (Tuscany) (75%, 4 liveborn cases) and Sweden (73.7%, 19 cases).
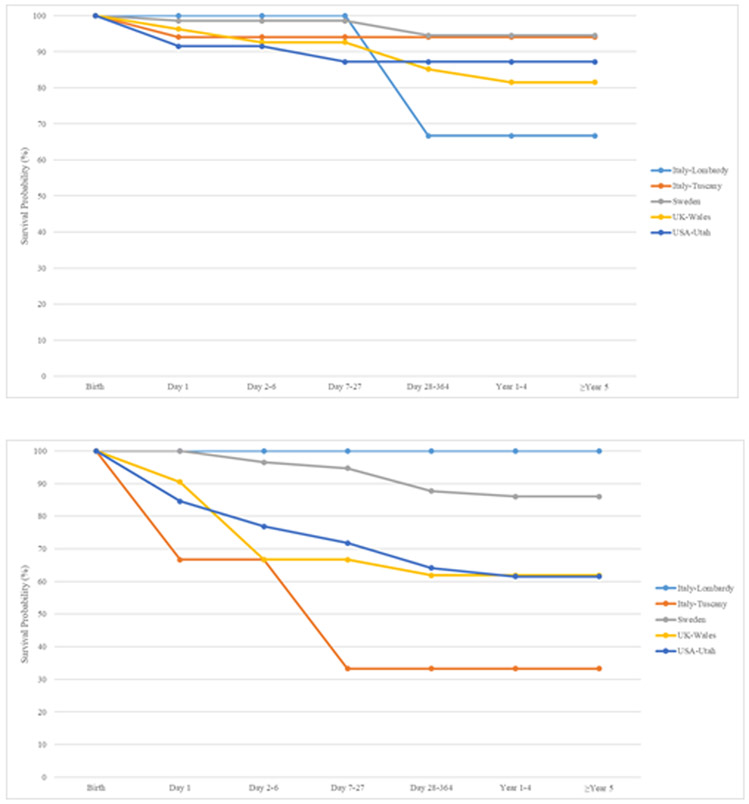
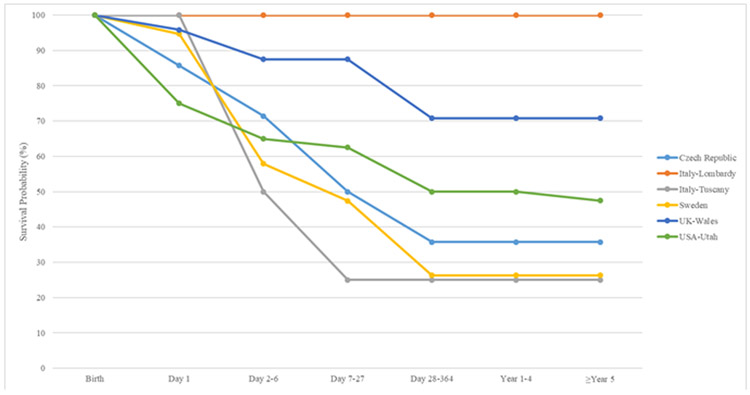
Figure 2 displays Kaplan-Meier curves up to age five years for isolated, MCA and syndromic omphalocele cases. Survival for omphalocele cases with isolated defects ranged from 66.7% (Italy-Lombardy) to 94.5% (Sweden). For omphalocele cases with MCA, survival ranged from 33.3% (Italy-Tuscany) to 86% (Sweden) and 100% (Italy-Lombardy) based on 66 MCA cases. Syndromic omphalocele cases had survival estimates which ranged from 25.0% (Italy-Tuscany) to 70.8% (UK-Wales) and 100% (Italy-Lombardy, based on 1 case).
Figure 2.
Kaplan-Meier survival curves up to age five for isolated, multiple congenital anomalies (MCA) and syndromic omphalocele in six surveillance systems with available data, 2000-2012
2a Isolated Cases: Italy Lombardy, Italy Tuscany, Sweden, UK Wales, USA Utah
2b Omphalocele with Multiple Congenital Anomalies: Italy Lombardy, Italy Tuscany, Sweden, UK Wales, USA Utah
2c. Syndromic omphalocele: Czech Republic1, Italy Lombardy, Italy Tuscany, Sweden, UK Wales, USA Utah
1Czech Republic only provided data on syndromic cases
DISCUSSION
The prevalence of omphalocele in our multi-country retrospective study from 2000-2012 was 2.6 per 10,000 births. Approximately one-third of children born with omphalocele died before age 5 years. Most deaths occurred during the first 24 hours after delivery, followed by the first week of life. Once children survived to one year of age, few deaths occurred at older ages. Children born with omphalocele and chromosomal or genetic abnormalities had higher mortality rates than children born with omphalocele alone or those with MCA.
Our prevalence estimate of 2.6 per 10,000 births is consistent with the published literature which reports prevalence estimates that range from 2.8 to 3.8 per 10,000 for studies conducted in the US, United Kingdom and Australia.7,9,10,54 Our livebirth prevalence of 1.6 per 10,000 births is also consistent with previous reports (1.3 to 1.92 per 10,000 live births).7-9,31,54 In agreement with prior studies,8,33,34 we observed no decline in the overall prevalence from 2000-2012 (AAPC = −0.19%; P=0.52). Forty-two percent of our omphalocele cases were MCA and 21% had chromosomal or other genetic abnormalities. Comparing our results to the published literature is somewhat challenging since the prevalence of MCA and chromosomal anomalies among infants with omphalocele varied greatly. Springett et al. observed that 31% of their cases had MCA and 32% were chromosomal.9 A New York-based study reported that 8% of their infants had chromosomal anomalies.7 In a US multi-state study, 16.7% of infants had a chromosomal anomaly and more than 50% had MCA.8 Conner et al. (2018) found that 48% of cases had associated structural anomalies and 19% of cases had a genetic disorder. Agopian et al. reported that 17.4% of their cases had chromosomal anomalies.21
Our study demonstrated an overall five-year mortality rate of 32.1%, a one-year mortality rate of 30.7% and an overall mortality rate of 17.2% for isolated cases. We observed that children with omphalocele and chromosomal or genetic abnormalities had the highest mortality rates (55.8%). Most studies in the literature focused on infant mortality and thus only reported first-year mortality rates. Our first-year mortality rate is generally consistent with the published literature. A study based on 1992-1999 data from New York reported a 23% mortality rate.7 A 2005-2011 study from six British Isles Network of Congenital Anomaly Regional (BINOCAR) registries in England and Wales reported an overall mortality rate of 16%.9 Marshall et al8 used 1995-2005 data from twelve state birth defect registries in the US National Birth Defects Prevention Network and reported a 28.7% first year mortality rate. An Australian study using 1980-1990 data reported a 15.6% first-year mortality rate.54 Corey et al40 conducted a hospital-based study that included data from 1997-2012 and reported a mortality rate of 18%.
We also demonstrated that in most cases, hospital-based surveillance systems had higher prevalence and mortality among omphalocele cases compared to population-based surveillance systems. Furthermore, surveillance systems in countries which do not include ETOPFA also had higher prevalence and mortality of omphalocele cases. A possible explanation could be that the most severe omphalocele cases (with MCA or syndromic cases) in these countries are not terminated during pregnancy, leading to a higher mortality in liveborns, compared to countries where ETOPFA is included.
One of the main strengths of our study is its large study population. It is the largest study to date of omphalocele prevalence and mortality with more than 16 million births and over 4,000 cases. Another strength is its ethnic diversity; it includes cases from 18 countries in Europe, North and South America, and the Middle East. Another major strength of the study is that cases were ascertained from hospital- and population–based surveillance systems, which allowed us to examine differences between types of surveillance systems in their prevalence and mortality estimates. Livebirths, stillbirths and terminations of pregnancy for fetal anomalies were included, which allowed us to assess the impact of ETOPFA on prevalence and mortality estimates. In addition, for most registries data was available on the clinical presentation, allowing us to compare mortality between isolated, MCA and syndromic cases of omphalocele.
Notwithstanding, our study has potential limitations that should be considered. The main limitation of our study is the lack of individual level information on patient characteristics, clinical presentation of the defect (e.g., size of the omphalocele), sociodemographic factors and co-morbidities. Another limitation is the varying methodologies that were used by the different programs, especially for ascertainment of mortality. For example, the length of follow up that differed between registries, with six registries only having information on first week mortality and nine registries having follow-up available through age five years. Moreover, our 5-year mortality rates were based on nine registries, one in Israel, five in Europe and three in the US and is therefore not representative for the worldwide mortality of omphalocele. The other registries did have longer follow up, but not all had linked to death certificates and it remains possible that some deaths will have been missed, leading to an underestimation of the mortality. The overall cumulative mortality percentages are based on different follow-up times and should, therefore, be interpreted as the minimum cumulative mortality (with longer follow-up times, the mortality is expected to increase). Also, we did not have information on the exact cause of death, on risk factors for a poor prognosis, on time of diagnosis (pre- or postnatal), or on type of treatment. Variability in the data is present due to limitations with the consistency in data collection for many registries in multiple countries. However, our results are similar to previously published studies and we have described the characteristics of each registry in detail.
Based on a very large multi-country sample of pregnancies and children affected by omphalocele, one-third of livebirths will not survive the first five years of life, with most deaths occurring in the neonatal period. Mortality varied by region of the world, ascertainment method and inclusion or exclusion of ETOPFA. Considerations for future studies may include clinical factors to elucidate the factors associated with mortality and how they might vary by region. It seems clear that omphalocele is quantitatively and qualitatively important and deserves attention. This study provides valuable information for clinicians and public health professionals around the world in planning and providing obstetric and pediatric services. It also makes data available for use in future comparisons in the follow-up of mortality linked to omphalocele.
Supplementary Material
Acknowledgements
We would like to thank Dr. Wei Fan and Yevgenia Gokun for their advice and assistance with the statistical methods for this manuscript.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Parker SE, Mai CT, Canfield MA, et al. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004-2006. Birth Defects Res A Clin Mol Teratol. 2010;88(12):1008–1016. [DOI] [PubMed] [Google Scholar]
- 2.Tan KH, Kilby MD, Whittle MJ, Beattie BR, Booth IW, Botting BJ. Congenital anterior abdominal wall defects in England and Wales 1987-93: retrospective analysis of OPCS data. Bmj. 1996;313(7062):903–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hemminki K, Saloniemi I, Kyyronen P, Kekomaki M. Gastroschisis and omphalocele in Finland in the 1970s: prevalence at birth and its correlates. J Epidemiol Community Health. 1982;36(4):289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryon-Scott R, Haan E, Chan A, Bower C, Scott H, Clark K. A population-based study of abdominal wall defects in South Australia and Western Australia. Paediatric and Perinatal Epidemiology. 1998;12:136–151. [DOI] [PubMed] [Google Scholar]
- 5.Forrester MB, Merz RD. Epidemiology of abdominal wall defects, Hawaii, 1986-1997. Teratology. 1999;60(3):117–123. [DOI] [PubMed] [Google Scholar]
- 6.Rankin J, Dillon E, Wright C. Congenital anterior abdominal wall defects in the north of England, 1986-1996: occurrence and outcome. Prenat Diagn. 1999;19(7):662–668. [DOI] [PubMed] [Google Scholar]
- 7.Salihu HM, Pierre-Louis BJ, Druschel CM, Kirby RS. Omphalocele and gastroschisis in the State of New York, 1992-1999. Birth Defects Res A Clin Mol Teratol. 2003;67(9):630–636. [DOI] [PubMed] [Google Scholar]
- 8.Marshall J, Salemi JL, Tanner JP, et al. Prevalence, Correlates, and Outcomes of Omphalocele in the United States, 1995-2005. Obstet Gynecol. 2015;126(2):284–293. [DOI] [PubMed] [Google Scholar]
- 9.Springett A, Draper ES, Rankin J, et al. Birth prevalence and survival of exomphalos in England and Wales: 2005 to 2011. Birth Defects Res A Clin Mol Teratol. 2014;100(9):721–725. [DOI] [PubMed] [Google Scholar]
- 10.Goldkrand JW, Causey TN, Hull EE. The changing face of gastroschisis and omphalocele in southeast Georgia. J Matern Fetal Neonatal Med. 2004;15(5):331–335. [DOI] [PubMed] [Google Scholar]
- 11.European Network of Population-Based Registries for the Epidemiological Surveillance of Congenital Anomalies. Prevalence Charts and Tables. 2019; https://eu-rd-platform.jrc.ec.europa.eu/eurocat/eurocat-data/prevalence. Accessed February 4, 2020. [Google Scholar]
- 12.St Louis AM, Kim K, Browne ML, et al. Prevalence trends of selected major birth defects: A multi-state population-based retrospective study, United States, 1999 to 2007. Birth defects research. 2017;109(18):1442–1450. [DOI] [PubMed] [Google Scholar]
- 13.Prefumo F, Izzi C. Fetal abdominal wall defects. Best practice & research Clinical obstetrics & gynaecology. 2014;28(3):391–402. [DOI] [PubMed] [Google Scholar]
- 14.Kamata S, Ishikawa S, Usui N, et al. Prenatal diagnosis of abdominal wall defects and their prognosis. J PediatrSurg. 1996;31(2):267–271. [DOI] [PubMed] [Google Scholar]
- 15.Tsakayannis DE, Zurakowski D, Lillehei CW. Respiratory insufficiency at birth: a predictor of mortality for infants with omphalocele. J Pediatr Surg. 1996;31(8):1088–1090; discussion 1090-1081. [DOI] [PubMed] [Google Scholar]
- 16.Biard JM, Wilson RD, Johnson MP, et al. Prenatally diagnosed giant omphaloceles: short- and long-term outcomes. Prenat Diagn. 2004;24(6):434–439. [DOI] [PubMed] [Google Scholar]
- 17.Vermeij-Keers C, Hartwig NG, van der Werff JF. Embryonic development of the ventral body wall and its congenital malformations. Semin Pediatr Surg. 1996;5(2):82–89. [PubMed] [Google Scholar]
- 18.Frolov P, Alali J, Klein MD. Clinical risk factors for gastroschisis and omphalocele in humans: a review of the literature. Pediatric surgery international. 2010;26(12):1135–1148. [DOI] [PubMed] [Google Scholar]
- 19.Reefhuis J, Honein MA. Maternal age and non-chromosomal birth defects, Atlanta--1968-2000: teenager or thirty-something, who is at risk? Birth Defects Res A Clin Mol Teratol. 2004;70(9):572–579. [DOI] [PubMed] [Google Scholar]
- 20.Waller DK, Shaw GM, Rasmussen SA, et al. Prepregnancy obesity as a risk factor for structural birth defects. ArchPediatrAdolescMed. 2007;161(8):745–750. [DOI] [PubMed] [Google Scholar]
- 21.Agopian A, Marengo L, Mitchell LE. Descriptive epidemiology of nonsyndromic omphalocele in Texas, 1999-2004. Am J Med Genet A. 2009;149a(10):2129–2133. [DOI] [PubMed] [Google Scholar]
- 22.Duong HT, Hoyt AT, Carmichael SL, et al. Is maternal parity an independent risk factor for birth defects? Birth Defects Res A Clin Mol Teratol. 2012;94(4):230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mac Bird T, Robbins JM, Druschel C, Cleves MA, Yang S, Hobbs CA. Demographic and environmental risk factors for gastroschisis and omphalocele in the National Birth Defects Prevention Study. J Pediatr Surg. 2009;44(8):1546–1551. [DOI] [PubMed] [Google Scholar]
- 24.Feldkamp ML, Srisukhumbowornchai S, Romitti PA, et al. Self-reported maternal cigarette smoke exposure during the periconceptional period and the risk for omphalocoele. Paediatric and perinatal epidemiology. 2014;28(1):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin S, Munsie JPW, Herdt-Losavio ML, et al. Maternal asthma medication use and the risk of selected birth defects. Pediatrics. 2012;129(2):e317–e324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alwan S, Reefhuis J, Rasmussen SA, Olney RS, Friedman JM. Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. The New England journal of medicine. 2007;356(26):2684–2692. [DOI] [PubMed] [Google Scholar]
- 27.Riley MM, Halliday JL, Lumley JM. Congenital malformations in Victoria, Australia, 1983-95: an overview of infant characteristics. J Paediatr Child Health. 1998;34(3):233–240. [DOI] [PubMed] [Google Scholar]
- 28.Doyle PE, Beral V, Botting B, Wale CJ. Congenital malformations in twins in England and Wales. J Epidemiol Community Health. 1991;45(1):43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mastroiacovo P, Castilla EE, Arpino C, et al. Congenital malformations in twins: An international study. American Journal of Medical Genetics. 1999;83:117–124. [DOI] [PubMed] [Google Scholar]
- 30.Hwang PJ, Kousseff BG. Omphalocele and gastroschisis: an 18-year review study. Genet Med. 2004;6(4):232–236. [DOI] [PubMed] [Google Scholar]
- 31.Kirby RS. The prevalence of selected major birth defects in the United States. Semin Perinatol. 2017;41(6):338–344. [DOI] [PubMed] [Google Scholar]
- 32.Kirby RS, Marshall J, Tanner JP, et al. Prevalence and correlates of gastroschisis in 15 states, 1995 to 2005. Obstetrics and gynecology. 2013;122(2 Pt 1):275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bugge M, Holm NV. Abdominal wall defects in Denmark, 1970-89. PaediatrPerinatEpidemiol. 2002;16(1):73–81. [DOI] [PubMed] [Google Scholar]
- 34.Allman R, Sousa J, Walker MW, Laughon MM, Spitzer AR, Clark RH. The epidemiology, prevalence and hospital outcomes of infants with gastroschisis. Journal Of Perinatology. 2016;36:901. [DOI] [PubMed] [Google Scholar]
- 35.Bugge M, Drachmann G, Kern P, et al. Abdominal Wall Defects in Greenland 1989-2015. Birth defects research. 2017;109(11):836–842. [DOI] [PubMed] [Google Scholar]
- 36.Calzolari E, Bianchi F, Dolk H, Milan M, Group EW. Omphalocele and gastroschisis in Europe: a survey of 3 million births 1980-1990. Am J Medical Genetics. 1995;58:187–194. [DOI] [PubMed] [Google Scholar]
- 37.Benjamin B, Wilson GN. Anomalies associated with gastroschisis and omphalocele: analysis of 2825 cases from the Texas Birth Defects Registry. J Pediatr Surg. 2014;49(4):514–519. [DOI] [PubMed] [Google Scholar]
- 38.Stoll C, Alembik Y, Dott B, Roth MP. Omphalocele and gastroschisis and associated malformations. Am J Med Genet A. 2008;146a(10):1280–1285. [DOI] [PubMed] [Google Scholar]
- 39.Conner P, Vejde JH, Burgos CM. Accuracy and impact of prenatal diagnosis in infants with omphalocele. Pediatric surgery international. 2018;34(6):629–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corey KM, Hornik CP, Laughon MM, McHutchison K, Clark RH, Smith PB. Frequency of anomalies and hospital outcomes in infants with gastroschisis and omphalocele. Early human development. 2014;90(8):421–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brantberg A, Blaas HG, Haugen SE, Eik-Nes SH. Characteristics and outcome of 90 cases of fetal omphalocele. Ultrasound Obstet Gynecol. 2005;26(5):527–537. [DOI] [PubMed] [Google Scholar]
- 42.Heider AL, Strauss RA, Kuller JA. Omphalocele: clinical outcomes in cases with normal karyotypes. Am J Obstet Gynecol. 2004;190(1):135–141. [DOI] [PubMed] [Google Scholar]
- 43.Porter A, Benson CB, Hawley P, Wilkins-Haug L. Outcome of fetuses with a prenatal ultrasound diagnosis of isolated omphalocele. Prenat Diagn. 2009;29(7):668–673. [DOI] [PubMed] [Google Scholar]
- 44.Cohen-Overbeek TE, Tong WH, Hatzmann TR, et al. Omphalocele: comparison of outcome following prenatal or postnatal diagnosis. Ultrasound Obstet Gynecol. 2010;36(6):687–692. [DOI] [PubMed] [Google Scholar]
- 45.Raymond SL, Downard CD, St Peter SD, et al. Outcomes in omphalocele correlate with size of defect. J Pediatr Surg. 2018. [DOI] [PubMed] [Google Scholar]
- 46.Akinkuotu AC, Sheikh F, Olutoye OO, et al. Giant omphaloceles: surgical management and perinatal outcomes. J Surg Res. 2015;198(2):388–392. [DOI] [PubMed] [Google Scholar]
- 47.Sakonidou S, Ali K, Farmer I, Hickey A, Greenough A. Mortality and short-term morbidity in infants with exomphalos. Pediatr Int. 2018;60(5):438–441. [DOI] [PubMed] [Google Scholar]
- 48.Fratelli N, Papageorghiou AT, Bhide A, Sharma A, Okoye B, Thilaganathan B. Outcome of antenatally diagnosed abdominal wall defects. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2007;30(3):266–270. [DOI] [PubMed] [Google Scholar]
- 49.Deng K, Qiu J, Dai L, et al. Perinatal mortality in pregnancies with omphalocele: data from the Chinese national birth defects monitoring network, 1996–2006. 2014;14(1):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hijkoop A, Peters NCJ, Lechner RL, et al. Omphalocele: from diagnosis to growth and development at 2 years of age. Archives of disease in childhood Fetal and neonatal edition. 2019;104(1):F18–f23. [DOI] [PubMed] [Google Scholar]
- 51.Groen H, Bouman K, Pierini A, et al. Stillbirth and neonatal mortality in pregnancies complicated by major congenital anomalies: Findings from a large European cohort. Prenatal diagnosis. 2017;37(11):1100–1111. [DOI] [PubMed] [Google Scholar]
- 52.The Centre of the International Clearinghouse for Birth Defects Surveillance and Research. Annual Report 2014:10. [Google Scholar]
- 53.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Statistics in medicine. 2000;19(3):335–351. [DOI] [PubMed] [Google Scholar]
- 54.Byron-Scott R, Haan E, Chan A, Bower C, Scott H, Clark K. A population-based study of abdominal wall defects in South Australia and Western Australia. Paediatr Perinat Epidemiol. 1998;12(2):136–151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.