ABSTRACT
Cervical cancer is the fourth-most prevalent malignancy in women. For advanced cervical cancer, radiotherapy is a major treatment. Micro RNAs (miRNAs) are small, noncoding RNAs that negatively regulate the target gene expression posttranscriptionally. miR-22 is frequently downregulated in various cancers including cervical cancer, and is associated with a poor prognosis in cervical cancer. Exosomes are small endosomally secreted vesicles that carry components such as proteins, messenger RNA (mRNA), DNA and miRNA. We investigated whether or not exosomes can efficiently deliver miR-22 to recipient cervical cancer cells and affect the gene expression in the cells, as well as assessed the role of exosomal miR-22 in radiosensitivity. Exosomes containing high levels of miR-22 were extracted by ultracentrifugation and then characterized by Western blotting, a nanoparticle tracking analysis and electron microscopy. The high presence of miR-22 in the exosome was confirmed by real-time polymerase chain reaction. After the administration of the collected exosomal miR-22 to SKG-II and C4-I cervical cancer cells, the level of miR-22 in the cells was significantly increased, indicating the absorption of the exosomal miR-22. When miR-22 encapsulated in exosomes was administered to the SKG-II cells, the level of c-Myc binding protein (MYCBP) and human telomerase reverse transcriptase (hTERT) was significantly decreased in correlation with increased radiosensitivity determined by a clonogenic assay. Taken together, these results suggest that the administration of exosomal miR-22 may be a novel drug delivery system for cervical cancer radiotherapy.
KEYWORDS: cervical cancer, exosome, miR-22, radiotherapy, drug delivery system
Introduction
Over the years, cervical cancer screening and early treatment have reduced the incidence and mortality of cervical cancer.1,2 However, the prognosis of advanced cervical cancer remains poor. Because advanced cervical cancers receive radiotherapy or chemoradiotherapy, radiotherapy is a critical component of the standard treatment regimen.3–5 However, radiation therapy is associated with issues, such as radioresistance and recurrence after radiation therapy that induces cancer cell repopulation.6 Furthermore, radiotherapy for advanced cervical cancer often causes long-term side effects on the bladder and bowel function. Therefore, a novel therapeutic approach to induce radiosensitivity is required. While targeted and biologic therapy has attracted attention in recent years, few dramatic improvements have been seen.4
Exosomes are nanometer-sized extracellular vesicles secreted from many cell types7 and also isolated from body fluids, such as semen, urine, plasma, and saliva.8–12 An increasing number of researchers have focused on the potential utility of exosomes as a novel drug delivery system (DDS) transferring their components to recipient cells in the body.8,13,14 Several clinical trials using exosomes for immunotherapy have been started, and the safety of exosome management in humans has been demonstrated.14–16
The Myc family of transcription factors (c-Myc, N-Myc and L-Myc) has an overall influence on cell proliferation and growth as an important carcinogenic transcription factor, modulating approximately 15% of all genes.17–20 The c-Myc gene has been reported to be overexpressed and genetically amplified much more frequently in advanced tumors than in early cervical cancer. Furthermore, c-Myc is involved in the high-risk human papilloma virus (HPV)-18 carcinogenic activity.21 c-Myc gene overexpression is a poor prognostic factor regarding the risk of distant metastases in patients with invasive cervical cancer.22
Micro RNAs (miRNAs) are short non-coding RNAs of about 22 nucleotides and are known to be key regulators of a wide range of biological processes such as cell differentiation, development and homeostasis by recognizing the 3ʹuntranslated region (UTR) of its target gene mRNA and suppressing the expression of the genes.23 miRNAs not only help maintain normal cellular processes but also act as tumor suppressors or promoters, depending on the targeting gene.24 However, miRNAs that regulate the c-Myc expression and their regulatory mechanisms are poorly understood.
A recent study showed that miR-22 directly inhibits MYCBP expression by targeting the 3ʹUTR of the gene and subsequently reducing the activity of c-Myc.17 In previous studies, miR-22 was identified as a tumor suppressor. miR-22 is frequently downregulated in tumors, such as those of the lung, liver and colorectal region, compared with normal tissues.25–27 In cervical cancer, cell proliferation was reported to be attenuated by miR-22 via the inhibition of ATP citrate lyase, which is a key enzyme influencing metabolic activity.28 Furthermore, a previous report showed that the decreased level of miR-22 in cervical cancer correlates with a poor prognosis.29 However, the relevance of miR-22 in cancer radiotherapy has not been reported in detail.
Human telomerase reverse transcriptase (hTERT), a target gene of c-Myc, plays an important role in the immortality of cancer cells by catalyzing the synthesis of the telomeric DNA.7,30,31 Telomerase has been reported to be involved in the regulation of radiosensitivity via the downregulation of hTERT in cervical cancer cells.7 Our preliminary data showed that the forced expression of miR-22 by gene transfection resulted in the suppression of the MYCBP gene expression and subsequent reduction of hTERT, thereby increasing the radiosensitivity in cervical cancer cells.
In the present study, we examined the potential therapeutic role of exosomal miR-22 on cervical cancer radiotherapy.
Materials and methods
Cell culture
Human embryonic kidney cell line 293 (HEK293) cells were purchased from RIKEN BioResource Research Center (Ibaraki, Japan). The cells were cultured in Minimum Essential Medium Eagle (Sigma-Aldrich, St Louis, MO, USA) with MEM Non-Essential Amino Acids Solution (100X; Thermo Fisher Scientific, Waltham, MA, USA) and 10% fetal bovine serum (FBS) (Gibco, Grand Island, NY, USA) in an atmosphere of 5% CO2 at 37°C. C4-I cells were obtained from American Type Culture Collection (Manassas, VA, USA). SKG-II was kindly provided by Keio University. The cells were cultured in DMEM medium (Sigma-Aldrich) supplemented with 10% FBS in an atmosphere of 5% CO2 at 37°C.
Transient transfection
HEK293 cells were transfected with Lipofectamine 2000 reagent (Life Technologies, Carlsbad, CA, USA). One day before transfection, 0.25 × 106 cells were seeded into 6-well plates. Pre-miR-22 (100 pmol, AM17101; Ambion, Waltham, MA, USA) and 5 μl of Lipofectamine 2000 reagent (Invitrogen) were mixed in Opti-MEM (Gibco) and added to the cells as described previously.32 After 4–6 h of incubation, the medium was renewed and incubated overnight. The medium was then renewed again to DMEM supplemented with 10% exosome-depleted FBS (System Biosciences, Palo Alto, CA, USA). After 72 h, the medium was collected.
Western blot analyses
Western blotting was performed as described previously.33,34 In brief, total protein was lysed by Pierce RIPA Buffer (Thermo Fisher Scientific). Equal amounts of cell proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to PVDF membranes. The membranes were blocked in 10% bovine serum albumin in 1X Tris-buffered saline and incubated with specific primary antibody of CD63(1:1000 dilution; Santa Cruz Biotechnology, Dallas, TX, USA), TSG101(1:1000 dilution; Abcam, Cambridge, UK), MYCBP (1:200 dilution; Sigma-Aldrich, St. Louis, MO, USA), Bax (1:1000 dilution; Cell Signaling, Boston, MA, USA) and Bcl-2 (1:1000 dilution, Cell Signaling) overnight at 4°C. After washing, the membranes were incubated with secondary antibody of mouse immunoglobulin for 1 h. Finally, the bands were visualized using enhanced chemiluminescence (ECL Plus; GE Healthcare Life Sciences, Pittsburgh, PA, USA). The density of the bands was quantified using ImageJ.35
Reverse transcription polymerase chain reaction (RT-PCR)
miRNA was extracted from SKG-II cells and C4-1 cells using the miRNA isolation kit (Ambion) according to the manufacturer’s protocol, and cDNA was synthesized using SuperScript II reverse transcriptase kit (Invitrogen). PCR was carried out using the Platinum PCR SuperMix (Invitrogen) system according to the manufacturer’s protocol. The following primers were used for PCR: miR-22 (Assay ID: 000398; Applied Biosystems, Carlsbad, CA, USA), hTERT (Hs00972650; Applied Biosystems), MYCBP (Hs00429315; Applied Biosystems). The expression of miR-16 and GADPH was assessed as an internal control in reactions.
Preparation of exosomes
Cell supernatants were centrifuged at 2000 g for 10 minutes and then filtered through a 0.22-μm filter (Merck Millipore, Bedford, MA, USA) to remove cell debris. To pellet exosomes, ultracentrifugation was carried out at 100,000 g for 70 minutes using an Optima XE-100 (BECKMAN COULTER, Brea, CA, USA), SW41 T1(BECKMAN COULTER) and Ultra-Clear tube (BECKMAN COULTER). The exosomes were collected with phosphate-buffered saline (PBS).
The nanoparticle tracking analysis (NTA)
NTA measurement was performed using NanoSight LM 10 (NanoSight, Wiltshire, UK). Collected EV pellets were resuspended in 1 ml of PBS and diluted to 1:100 before the analysis. The samples were loaded in the instrument and analyzed according to the manufacturer’s instructions using the NTA software program, version 2.3 (NanoSight).
Scanning electron microscopy
Exosomes were incubated with poly-L-lysine solution coated beads (φ3.10 μm; Merck Millipore). After drying, the beads were washed and fixed in 1.25% glutaraldehyde in 0.1 M phosphate buffer (PB; pH 7.4). The beads were then washed again with PB and fixed in 1% osmium tetroxide for 40 min. After washing with PB, the beads were gradually dehydrated in a graded series of ethanol washes. The platinum palladium was evaporated, and vapor deposition was performed. We performed scanning electron microscopy (S-5000; HITACHI, Tokyo, Japan).
The clonogenic assay (2D)
A clonogenic assay was performed using the technique described previously.36 In brief, cultured SKG-II and C4-1 cells were trypsinized to produce a single-cell suspension, and then the desired number of cells was seeded in 6-well dish. Four hours later, each well was sprinkled with exosomes (1 μg/cell). Twenty-four hours later, cultures were irradiated using an X-ray machine M-150WE (SOFTEX, Tokyo, Japan) with 0, 2, 4, 6 and 8 Gy. The dishes were placed in an incubator at 37°C in 5% CO2 for about 2 weeks until the cells formed sufficiently large colonies (≥ 50 cells).
The clonogenic assay (3D)
Measurement of 3D cell survival in Matrigel-based cultures was accomplished as reported before.37,38 The 3D clonogenic survival assay was performed as described previously.38 In brief, each exosome was administered to SKG-II cells, then the cells were irradiated using an X-ray machine (M-150WE; SOFTEX) with 0, 3 and 6 Gy. Twenty-four hours later, single cells were seeded in Matrigel/DMEM (1:1) at a concentration of 2 × 103/well. The dishes were placed in an incubator at 37°C in 5% CO2 for 5 days. The spheres were counted using an all-in-one microscope (BZ-X700; KEYENCE, Osaka, Japan). Sphere numbers and diameter sizes were assessed for each sphere with a minimum diameter of 40 µm.
Statistical analyses
The statistical analyses were performed using the StatView software program (SAS Institute, Cary, USA). The data represent the mean ± standard deviation of three independent experiments. The statistical analysis was performed using Student’s t-test at a significance level of p < .05.
Results
Characterization of exosomes released from HEK293 cells
We performed Western blotting, an NTA and electron microscopy (Figure 1). Western blotting showed that the exosomal inclusive markers CD63 and TSG101 were expressed in the exosomes (Figure 1a). To obtain exosomes containing high levels of miR-22, precursor miR-22 (Pre-miR-22) or control (control miR) was transfected into the HEK293 cells. The culture supernatants were then collected, and ultracentrifugation was performed. The exosome size distribution was characterized by an NTA at a diameter between 118- and 129 nm. The mode of the exosomes secreted from Pre-miR-22-transfected HEK293 cells was 129 nm, and the mode of exosomes secreted from control miR-transfected HEK293 cells was 110 nm, indicating no marked differences in the size distribution between transfection of miR-22 and control miRNA (Figure 1b). On scanning electron microscopy, exosomes were visualized and confirmed to have a spherical shape (Figure 1c).
Figure 1.
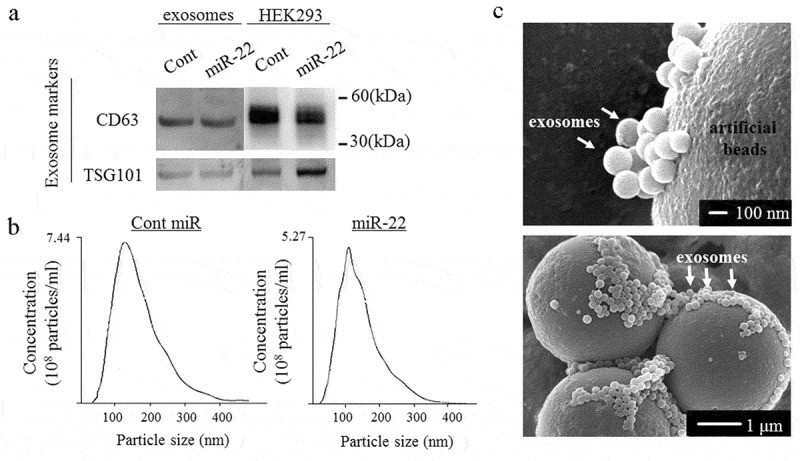
The exosome confirmation analysis. The exosomes were isolated from the culture medium of HEK293 cells transfected with either precursor miR-22 (miR-22) or control (cont miR). (a) Western blot analyses were performed to detect the exosomal marker proteins (CD63 and TSG101) in vesicles released by HEK293 cells. Representative examples of bands from three independent experiments are shown. (b) The particle size distributions and concentrations of exosomes were measured using a nanoparticle tracking system. (c) A representative image of exosomes using transmission electron microscopy
The miR-22 levels in exosomes secreted from Pre-miR-22-transfected HEK293 cells were significantly increased compared to those secreted from control miR-transfected HEK293 cells
To confirm the levels of miR-22 in the exosomes, real-time PCR was performed. It showed that the levels of miR-22 in the exosomes were significantly higher in those derived from Pre-miR-22-transfected HEK293 cells (exo-miR22) than in those derived from control miR-transfected HEK293 cells (exo-cont miR) (Figure 2; p < .01).
Figure 2.
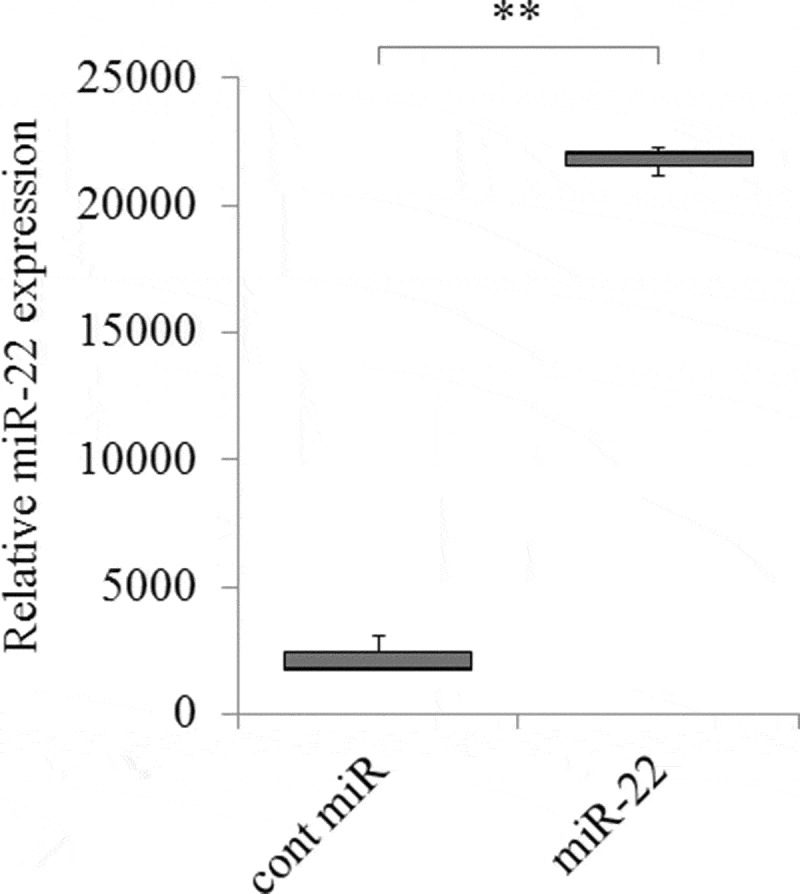
The increased expression of miR-22 in exosomes secreted by HEK293 cells. HEK293 cells were transfected with either precursor miR-22 (miR-22) or control (cont miR). The relative abundance of miR-22 in exosomes was calculated by qRT-PCR, and the relative fold difference compared with cont miR was presented (** p < .01)
The administration of exosomes derived from HEK 293 altered the expression of MYCBP and hTERT in the recipient cervical cancer cells
To examine the effect of miR-22 encapsulated in exosomes on the cervical cancer cells, we treated SKG-II cervical cancer cells with exo-miR22 or exo-cont miR. After treatment with these exosomes, we first evaluated the expression of miR-22 in the treated SKG-II cells to confirm the absorption of the exosomes. The administration of exo-miR22 to the cervical cancer cells induced a significant increase in miR-22 in the treated cells compared to treatment with exo-cont miR (Figure 3a; p < .01).
Figure 3.
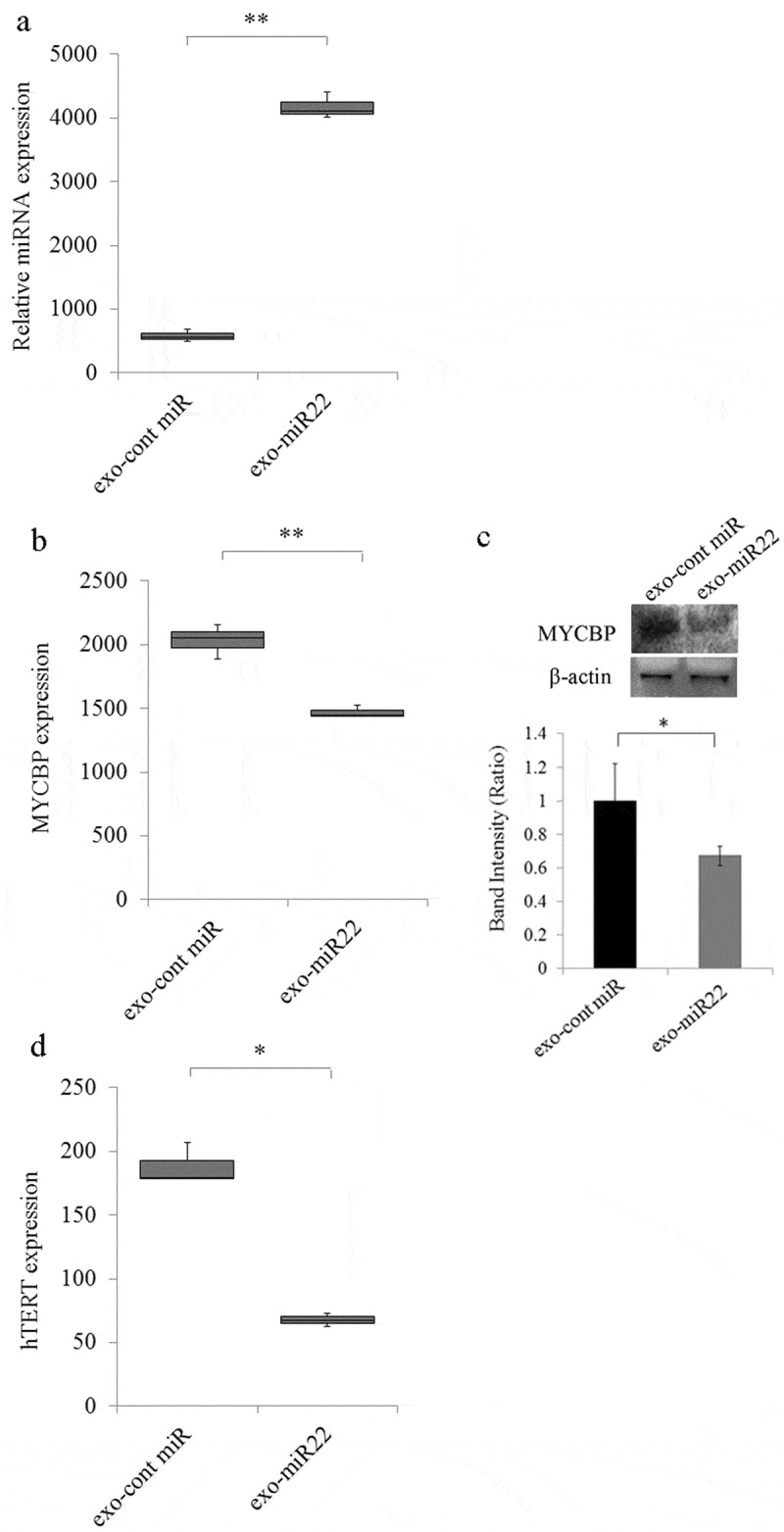
The uptake of miR-22-highly-containing exosomes by cervical cancer SKG-II cells. Exosomes containing high levels of miR (exo-miR22) or cont miR exosomes derived from HEK293 cells were administered to SKG-II cells. (a) The relative abundance of miR-22 in the recipient SKG-II cells was calculated by qRT-PCR. The relative fold difference compared with exo-cont miR-treatment was presented (** p < .01). (b) The administration of exo-miR22 inhibited MYCBP mRNA in SKG-II cells. The relative fold difference compared with exo-cont miR-administration was presented (** p < .01). (c) Western blot analysis of MYCBP. The administration of exo-miR22 suppressed MYCBP protein expression in SKG-II cells. Bar graph showing the ratio of MYCBP/β-actin in each group (* p < .05). (d) The administration of exo-miR22 decreased hTERT mRNA in SKG-II cells. The relative fold difference compared with exo-cont miR-administration was presented (* p < .05)
Our previous findings and recent reports17 showed that miR-22 directly targeted c-Myc binding protein (MYCBP) through binding to the MYCBP 3ʹUTR. We therefore examined whether or not treatment with exo-miR22 of SKG-II cells repressed the expression of MYCBP in the cells. The level of MYCBP mRNA in SKG-II cells was significantly decreased after the administration of exo-miR22 compared to exo-cont miR (Figure 3b; p < .01). The MYCBP protein expression was also inhibited by exo-miR22 compared to exo-cont miR (Figure 3c; p < .05). MYCBP promotes the activation of the c-Myc target gene including hTERT via E-box.39 We therefore next examined the effect of exo-miR22 treatment on the hTERT repression. As expected, the hTERT mRNA expression was significantly decreased by the administration of exo-miR22 (Figure 3d).
Radiosensitivity was enhanced by exosomal miR-22 in vitro
We examined the effect of miR-22-containing exosomes on radiation therapy. The clonogenic assay is widely used to determine cell reproductive death after treatment with ionizing radiation. In 2D models, SKG-II cells and C-4I cells were treated with exo-miR22 or exo-cont miR, and irradiated with various radiation doses (2, 4, 6 and 8 Gy). Compared to the treatment of exo-cont miR, the survival fraction of the exo-miR22-treated group was lower in both SKG-II cells and C4-I cells, indicating increased radiosensitivity after treatment of exo-miR22 (Figure 4). We further examined the impact of exo-miR22 on the 3D clonogenic survival in SKG-II cells. The cell survival in 2D was found to be associated with the 3D result as well (Figure 5).
Figure 4.
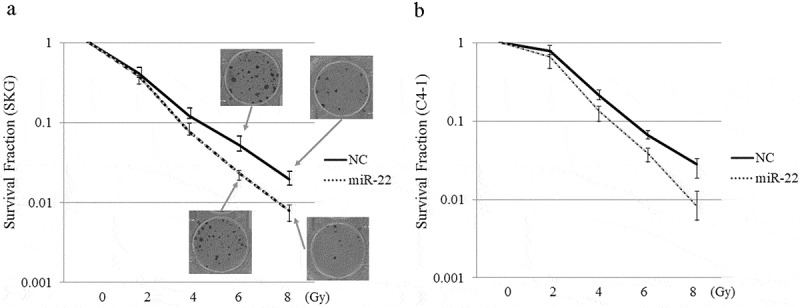
The effect of exosomal miR-22 on radiosensitivity of cervical cancer cells in 2D. HEK293-derived exosomes, either exo-miR22 or exo-cont miR, were administered to SKG-II (a) and C-4I (b) cells. After the administration of the exosomes, the cells were irradiated with various doses of X-rays
Figure 5.
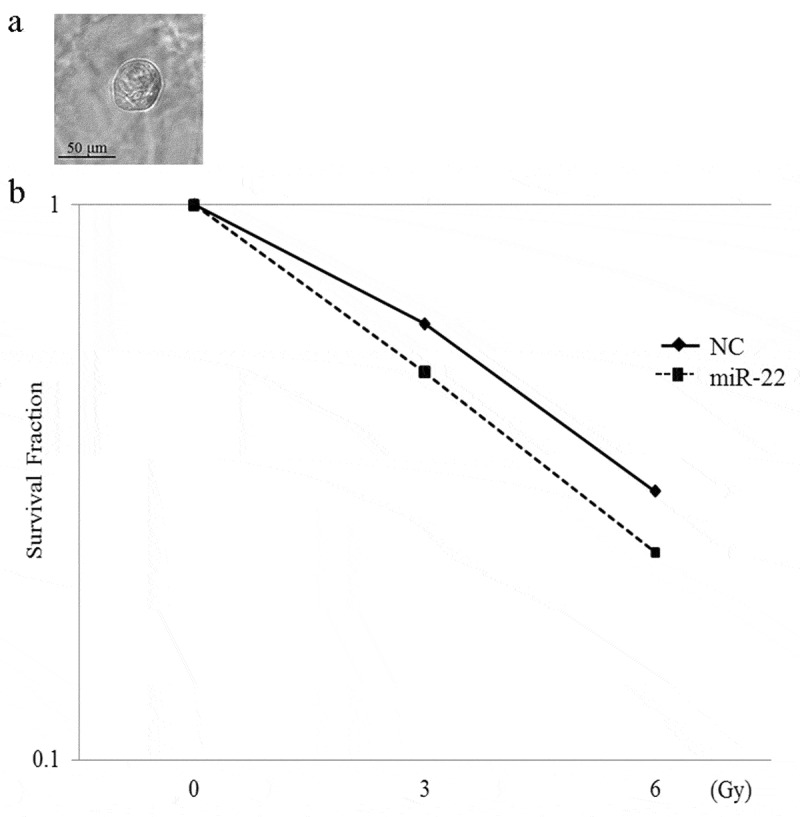
The effect of exosomal miR-22 on the radiosensitivity of cervical cancer cells in 3D. (a) Morphology of SKG-II cells in 3D (magnification, ×20). Scale = 50 µm. (b) HEK293-derived exosomes, either exo-miR22 or exo-cont miR, were administered to SKG-II cells. After the administration of the exosomes, the cells were irradiated with various doses of X-rays
Exosomal miR-22 upregulated apoptotic pathway in cervical cancer cells
To elucidate the mechanism underlying the increased radiosensitivity induced by exosomal miR-22, we then investigated the effect of exosomal miR-22 on apoptosis. We found that treatment of exosomal miR-22 increased the expression of Bax, whereas the expression of Bcl-2 was decreased after the treatment of SKG-II cells with exosomal miR-22 (Figure 6; p < .05). These results collectively indicated that exosomal miR-22 administration on irradiation increases the apoptotic pathway.
Figure 6.
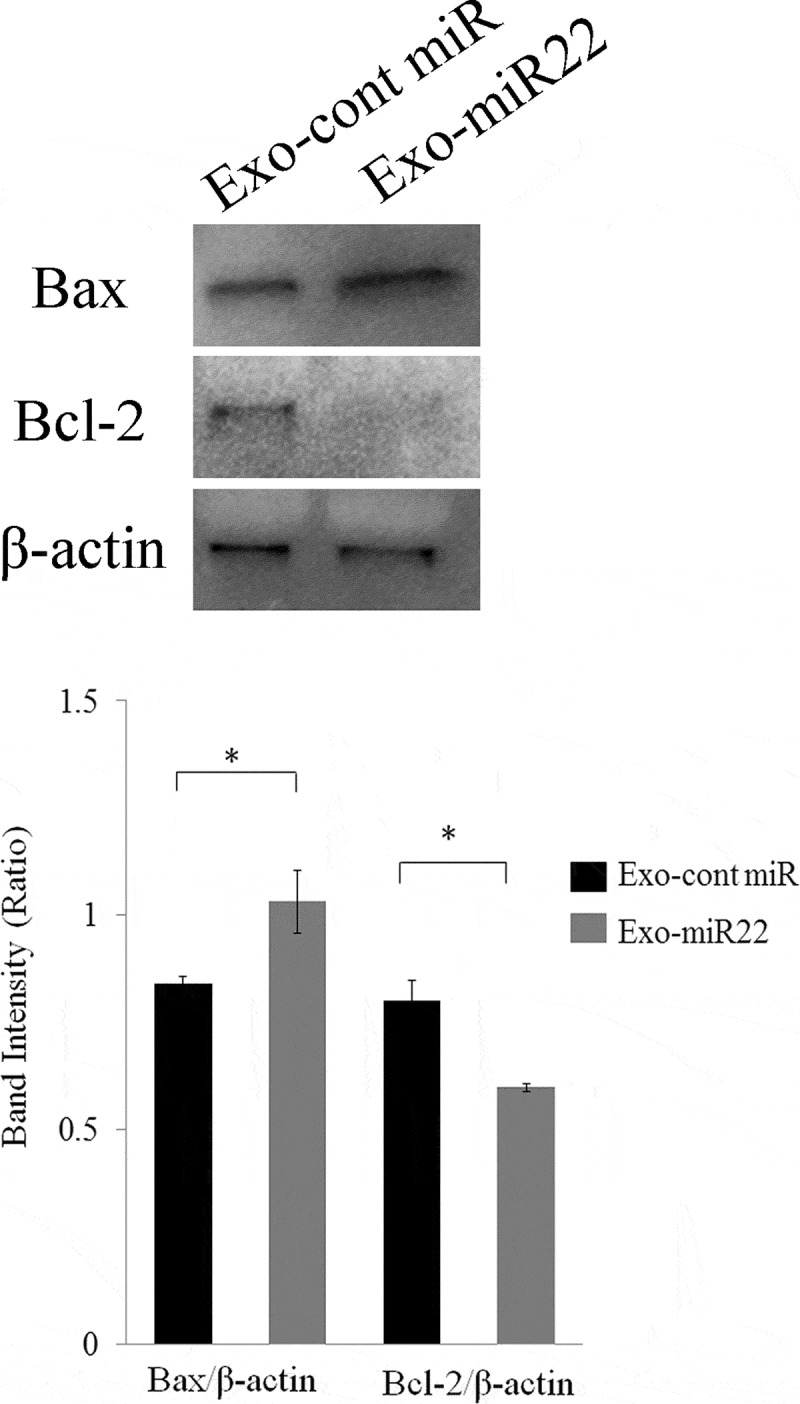
The effect of exosomal miR-22 on cell apoptosis. Proapoptotic Bax expression and antiapoptotic Bcl-2 expression were assessed by Western blot analysis. HEK293-derived exosomes, either exo-miR22 or exo-cont miR, were administered to SKG-II cells. The cells were then irradiated with 4 Gy of X-rays, and protein samples were harvested. Bar graph showing the ratio of Bax/β-actin and Bcl-2/β-actin in each group (* p < .05)
Discussion
Exosomes have received increasing attention for their role in cell-to-cell communication and their biological functions such as in angiogenesis, proliferation and cell cycle regulation and apoptosis through their protein and gene cargoes including miRNAs. In the present study, we explored whether or not exosomes can efficiently deliver miRNA to target recipient cancer cells and alter the gene expression in the cells. We found that miR-22 encapsulated in exosomes did indeed alter the MYCBP and hTERT gene expression in the recipient cervical cancer cells. We further showed that the administration of the exosomal miR-22 led to the increased radiosensitivity of the recipient cervical cancer cells in vitro.
Several materials have been developed as DDS carriers. Polyethylene glycol-coated liposomes have been frequently used as DDS carriers because of their simple preparation techniques and acceptable toxicity profiles. These liposomes have either single or multiple lipid bilayers. The structural features of exosomes are similar to those of liposomes, making exosomes attractive as a DDS. In addition, since exosomes are produced by the cells themselves, they consist of only biogenic substances, an advantage over liposomes.40 In recent years, preclinical strategies concerning exosomes encapsulating miRNAs as DDS have been vigorously employed. Katakowski et al. showed that mesenchymal stem cell (MSC)-derived exosomes loaded with miR-146b inhibited glioma growth.41 Ohno et al. recently showed that HEK293-derived exosomes containing let-7a miRNA inhibited breast cancer progression in in vivo mouse model.41 Bigagli et al. showed that colon cancer cell-derived exosomes containing miR-210 regulate epithelial-mesenchymal transition and metastasis.42
Radiotherapy is a major treatment modality in advanced cervical cancer. However, radioresistance poses a major barrier to the advanced cervical cancers, which undermines the efficacy of radiotherapy. Hence, additional therapeutic approaches that enhance the effects of radiation treatment are essential. A recent report showed that MSC-derived exosomes, combined with radiotherapy, enhanced the effects of radiation on melanoma cells, suggesting the potential utility of exosomes as a DDS.43 However, the detailed mechanisms underlying how the exosomes contribute to the increased radiosensitivity have not been examined.
hTERT is reportedly very important in radioresistance through the PI3K/AKT pathway.44 In cervical cancer patients, the increased expression of hTERT is related to radiation resistance and poor prognosis.45 In the current study, we treated cervical cancer cells with HEK293-derived exosomes containing miR-22 and observed a reduced hTERT expression and increased radiosensitivity of the recipient cervical cancer cells in vitro. Although the present study has a limitation in that the role of exosomal miR-22 was evaluated only in in vitro experiments, this is the first report to show that exosomal miR-22 induced radiosensitization through hTERT reduction. Recently, a few studies have described the effect of decreased TERT on apoptosis. Ling et al. showed that TERT knockdown induced apoptosis in mouse germ cells.46 In human osteosarcoma cells, increased TERT expression has been shown to inhibit cisplatin-induced apoptosis, whereas knockdown of TERT promoted cisplatin-induced apoptosis.47 In human glioma cells, Wang et al. reported silencing of the hTERT gene induced apoptosis by decreasing Bax protein and increasing Bcl-2 protein.48 Consistent with these previous studies, we found that the treatment of exosomal miR-22 with irradiation increased the Bax protein expression and decreased Bcl-2 protein expression, indicating that exosomal miR-22-promoted radiosensitivity was accompanied by increased apoptosis.
In conclusion, our results show that the administration of miR-22-enriched exosomes can sensitize cervical cancer radiotherapy in vitro, which may be partly mediated by promoted apoptosis. Further studies should attempt to increase exosome production and reduce the antigenicity in order to establish exosomal miRNA as a novel DDS in clinical settings.
Funding Statement
This work was supported by JSPS KAKENHI under Grant [No. 16K11164 to M.H. and No. 17K11304 to M.O].
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed
Author contributions
H.K. and M.H. made substantial contributions to the study concept and design of the study, acquisition of the data and/or statistical analyses and drafting of the manuscript. K.T. contributed to the preparation of exosomes. M. N. was involved in the transfection experiments and radiation treatment. Y.I. and Y.K. contributed to the electron microscopic analysis. H.S. and Y.T. critically reviewed the paper. Y.K. and Y.A. conducted the nanoparticle tracking analysis. M.O. edited the manuscript and approved the final version to be published.
References
- 1.Siegel RL, Miller KD, Jemal A.. 2018. Cancer statistics, 2018. CA Cancer J Clin. 68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Chan JK, Chow S, Bhowmik S, Mann A, Kapp DS, Coleman RL. 2018. Metastatic gynecologic malignancies: advances in treatment and management. Clin Exp Metastasis. 35:521–533. doi: 10.1007/s10585-018-9889-7. [DOI] [PubMed] [Google Scholar]
- 3.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. 2015. Global cancer statistics, 2012. CA Cancer J Clin. 65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 4.Crafton SM, Salani R. 2016. Beyond chemotherapy: an overview and review of targeted therapy in cervical cancer. Clin Ther. 38(3):449–458. doi: 10.1016/j.clinthera.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Monk BJ, Sill MW, McMeekin DS, Cohn DE, Ramondetta LM, Boardman CH, Benda J, Cella D. 2009. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a gynecologic oncology group study. J Clin Oncol. 27:4649–4655. doi: 10.1200/JCO.2009.21.8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang W, Xing L. 2013. RNAi gene therapy of SiHa cells via targeting human TERT induces growth inhibition and enhances radiosensitivity. Int J Oncol. 43:1228–1234. doi: 10.3892/ijo.2013.2051. [DOI] [PubMed] [Google Scholar]
- 7.Simons M, Raposo G. 2009. Exosomes–vesicular carriers for intercellular communication. Curr Opin Cell Biol. 21:575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Borges FT, Reis LA, Schor N. 2013. Extracellular vesicles: structure, function, and potential clinical uses in renal diseases. Braz J Med Biol Res = Revista Brasileira De Pesquisas Medicas E Biologicas. 46:824–830. doi: 10.1590/1414-431X20132964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ronquist G, Brody I. The prostasome: its secretion and function in man. Biochim Biophys Acta. 1985;822:203–218. [DOI] [PubMed] [Google Scholar]
- 10.Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17:879–887. [DOI] [PubMed] [Google Scholar]
- 11.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A. 2004;101:13368–13373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogawa Y, Miura Y, Harazono A, Kanai-Azuma M, Akimoto Y, Kawakami H, Yamaguchi T, Toda T, Endo T, Tsubuki M, et al. Proteomic analysis of two types of exosomes in human whole saliva. Biol Pharm Bull. 2011;34:13–23. [DOI] [PubMed] [Google Scholar]
- 13.Raposo G, Stoorvogel W. 2013. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vader P, Mol EA, Pasterkamp G, Schiffelers RM. 2016. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev. 106:148–156. doi: 10.1016/j.addr.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Escudier B, Dorval T, Chaput N, André F, Caby MP, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J Transl Med. 2005;3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, Valente N, Shreeniwas R, Sutton MA, Delcayre A, et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med. 2005;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiong J, Du Q, Liang Z. 2010. Tumor-suppressive microRNA-22 inhibits the transcription of E-box-containing c-Myc target genes by silencing c-Myc binding protein. Oncogene. 29:4980–4988. doi: 10.1038/onc.2010.241. [DOI] [PubMed] [Google Scholar]
- 18.Cole MD, McMahon SB. The Myc oncoprotein: a critical evaluation of transactivation and target gene regulation. Oncogene. 1999;18:2916–2924. [DOI] [PubMed] [Google Scholar]
- 19.Nesbit CE, Tersak JM, Prochownik EV. MYC oncogenes and human neoplastic disease. Oncogene. 1999;18:3004–3016. [DOI] [PubMed] [Google Scholar]
- 20.Meyer N, Penn LZ. 2008. Reflecting on 25 years with MYC. Nat Rev Cancer. 8:976–990. doi: 10.1038/nrc2231. [DOI] [PubMed] [Google Scholar]
- 21.Wang YW, Chang HS, Lin CH, Yu WC. HPV-18 E7 conjugates to c-Myc and mediates its transcriptional activity. Int J Biochem Cell Biol. 2007;39:402–412. [DOI] [PubMed] [Google Scholar]
- 22.Bourhis J1, Le MG, Barrois M, Gerbaulet A, Jeannel D, Duvillard P, Le Doussal V, Chassagne D, Riou G. Prognostic value of c-myc proto-oncogene overexpression in early invasive carcinoma of the cervix. J Clin Oncol. 1990;8:1789–1796. [DOI] [PubMed] [Google Scholar]
- 23.Gebert LFR, MacRae IJ. 2019. Regulation of microRNA function in animals. Nat Rev Mol Cell Biol. 20:21–37. doi: 10.1038/s41580-018-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou X, Natino D, Zhai X, Gao Z, He X. 2018. MicroRNA22 inhibits the proliferation and migration, and increases the cisplatin sensitivity, of osteosarcoma cells. Mol Med Rep. 17:7209–7217. doi: 10.3892/mmr.2018.8790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xia SS, Zhang GJ, Liu ZL, Tian HP, He Y, Meng CY, Li LF, Wang ZW, Zhou T. 2017. MicroRNA-22 suppresses the growth, migration and invasion of colorectal cancer cells through a Sp1 negative feedback loop. Oncotarget. 8:36266–36278. doi: 10.18632/oncotarget.16742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiao DD, Yang J, Lei XF, Mi GL, Li SL, Li K, Xu CQ, Yang HL. Expression of microRNA-122 and microRNA-22 in HBV-related liver cancer and the correlation with clinical features. Eur Rev Med Pharmacol Sci. 2017;21:742–747. [PubMed] [Google Scholar]
- 27.Chen J, Wu FX, Luo HL, Liu JJ, Luo T, Bai T, Li LQ, Fan XH. Berberine upregulates miR-22-3p to suppress hepatocellular carcinoma cell proliferation by targeting Sp1. Am J Transl Res. 2016;8:4932–4941. [PMC free article] [PubMed] [Google Scholar]
- 28.Xin M, Qiao Z, Li J, Liu J, Song S, Zhao X, Miao P, Tang T, Wang L, Liu W, et al. miR-22 inhibits tumor growth and metastasis by targeting ATP citrate lyase: evidence in osteosarcoma, prostate cancer, cervical cancer and lung cancer. Oncotarget. 2016;7:44252–44265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L, Chen B, Ding D. Decreased microRNA-22 is associated with poor prognosis in cervical cancer. Int J Clin Exp Pathol. 2017;10:9515–9520. [PMC free article] [PubMed] [Google Scholar]
- 30.Satra M, Tsougos I, Papanikolaou V, Theodorou K, Kappas C, Tsezou A. Correlation between radiation-induced telomerase activity and human telomerase reverse transcriptase mRNA expression in HeLa cells. Int J Radiat Biol. 2006;82:401–409. [DOI] [PubMed] [Google Scholar]
- 31.Qi DL, Ohhira T, Fujisaki C, Inoue T, Ohta T, Osaki M, Ohshiro E, Seko T, Aoki S, Oshimura M, et al. 2011. Identification of PITX1 as a TERT suppressor gene located on human chromosome 5. Mol Cell Biol. 31:1624–1636. doi: 10.1128/MCB.00470-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, Fujita K, Mizutani T, Ohgi T, Ochiya T, et al. 2013. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 31:185–191. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura K, Terai Y, Tanabe A, Ono YJ, Hayashi M, Maeda K, Fujiwara S, Ashihara K, Nakamura M, Tanaka Y, et al. 2017. CD24 expression is a marker for predicting clinical outcome and regulates the epithelial-mesenchymal transition in ovarian cancer via both the Akt and ERK pathways. Oncol Rep. 37:3189–3200. doi: 10.3892/or.2017.5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ono YJ, Hayashi M, Tanabe A, Hayashi A, Kanemura M, Terai Y, Ohmichi M. 2015. Estradiol-mediated hepatocyte growth factor is involved in the implantation of endometriotic cells via the mesothelial-to-mesenchymal transition in the peritoneum. Am J Physiol Endocrinol Metab. 308:E950–E959. doi: 10.1152/ajpendo.00573.2014. [DOI] [PubMed] [Google Scholar]
- 35.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franken NAP, Rodermond HM, Stap J, Haveman J, van Bree C. 2006. Clonogenic assay of cells in vitro. Nature Protoc. 1:2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 37.Bahmad HF, Cheaito K, Chalhoub RM, Hadadeh O, Monzer A, Ballout F, El-Hajj A, Mukherji D, Liu YN, Daoud G, et al. 2018. Spere-formation assay: theree-dimensional in vitro culturing of prostate cancer stem/progenitor sphere-forming cells. Front Oncol. 8:347. doi: 10.3389/fonc.2018.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bodgi L, Bahmad HF, Araji T, Al Choboq J, Bou-Gharios J, Cheaito K, Zeidan YH, Eid T, Geara F, Abou-Kheir W. 2019. Assessing radiosensitivity of bladder cancer in vitro: A 2D vs. 3D approach. Front Oncol. 9:153. doi: 10.3389/fonc.2019.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakamuro D, Prendergast GC. New Myc-interacting proteins: a second Myc network emerges. Oncogene. 1999;18:2942–2954. [DOI] [PubMed] [Google Scholar]
- 40.Liu C, Gao H, Lv P, Liu J, Liu G. 2017. Extracellular vesicles as an efficient nanoplatform for the delivery of the therapeutics. Hum Vaccin Immunother. 13:2678–2687. doi: 10.1080/21645515.2017.1363935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katakowski M, Buller B, Zheng X, Lu Y, Rogers T, Osobamiro O, Shu W, Jiang F, Chopp M. 2013. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 335:201–204. doi: 10.1016/j.canlet.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bigagli E, Luceri C, Gausti D, Cinci L. 2016. Exosomes secreted from human colon cancer cells influence the adhesion of neighboring metastatic cells: role of microRNA-210. Cancer Biol Ther. 17:1062–1069. doi: 10.1080/15384047.2016.1219815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Araujo Farias V, O’Valle F, Serrano-Saenz S, Anderson P, Andrés E, López-Peñalver J, Tovar I, Nieto A, Santos A, Martín F, et al. 2018. Exosomes derived from mesenchymal stem cells enhance radiotherapy-induced cell death in tumor and metastatic tumor foci. Mol Cancer. 17:122. doi: 10.1186/s12943-018-0867-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ram R, Uziel O, Eldan O, Fenig E, Beery E, Lichtenberg S, Nordenberg Y, Lahav M. Ionizing radiation in mammals. J Exp Med. 2000;192:1625–1636.11104804 [Google Scholar]
- 45.Moreno-Acosta P, Vallard A, Carrilo S, Gamboa O, Romero-Rojas A, Molano M, Acosta J, Mayorga D, Rancoule C, Garcia MA, et al. 2017. Biomarkers of resistance to radiation therapy: a prospective study in cervical carcinoma. Radiat Oncol. 12:120. doi: 10.1186/s13014-017-0856-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ling X, Yang W, Zou P, Zhang G, Wang Z, Zhang X, Chen H, Peng K, Han F, Liu J, et al. 2018. TERT regulates telomere-related senescence and apoptosis through DNA damage response in male germ cells exposed to BPDE in vitro and to B[a]P in vivo. Environ Pollut. 235:836–849. doi: 10.1016/j.envpol.2017.12.099. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Z, Yu L, Dai G, Xia K, Liu G, Song Q, Tao C, Gao T, Guo W. 2017. Telomerase reverse transcriptase promotes chemoresistance by suppressing cisplatin-dependent apoptosis in osteosarcoma cells. Sci Rep. 7:7070. doi: 10.1038/s41598-017-07204-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang T, Xue Y, Wang M, Sun Q. 2012. Silencing of the hTERT gene through RNA interference induces apoptosis via bax/bcl-2 in human glioma cells. Oncol Rep. 28:1153–1158. doi: 10.3892/or.2012.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]


