Abstract
Investigations of plasma amino acids in early psychosis and their unaffected siblings are rare. We measured plasma amino acids involved in the co-activation of dopaminergic, GABAergic, glutamatergic, and serotoninergic neurotransmitters in first-episode psychosis (FEP) patients (n = 166), unaffected siblings (n = 76), and community-based controls (n = 166) included in a cross-sectional study. Plasma levels of glutamic acid (GLU), glutamine, glycine, proline (PRO), tryptophan (TRP), tyrosine, serine and GABA were quantified by gas-chromatography-mass spectrometry. We used the generalized linear model adjusted by sex, age, and body mass index for group comparison and paired t-test for FEP-Sibling pairs. FEP had reduced GABA plasma levels compared to siblings and controls (p < 0.05 for both). Siblings had lower GLU, Glx and PRO (p < 0.05 for all) but increased TRP compared to patients and controls (p < 0.05 for both). FEP patients with longer duration of pharmacological treatment and medicated only with antipsychotics had increased GLU compared to FEP with shorter periods, or with those treated with a combination of medications (p < 0.05 for both). Finally, FEP patients treated only with antipsychotics presented higher Glx compared to those with mixed medications (p = 0.026). Our study suggests that FEP have low a GABA plasma profile. Unaffected siblings may be a possible risk group for metabolic abnormalities.
Subject terms: Molecular neuroscience, Biomarkers, Psychosis, Schizophrenia
Introduction
Schizophrenia and other psychosis have a multi-factorial biological background1,2. Among the different mediating mechanisms, evidence indicates that abnormal amino acid levels, which underlie changes in the metabolic profile, are correlated with psychosis3,4.
Twenty-one intracellular alpha-amino acids are present in the human proteins. Twelve amino acids, named non-essential, are synthesized by molecules provided by the organism and attend the cells necessity, such as glutamic acid (GLU), glutamine (GLN), glycine (GLY), proline (PRO), serine (SER), and tyrosine (TYR); the remaining, which are known as essential amino acids, cannot be synthesized in the body and therefore are supplied by the diet intake, such as tryptophan (TRP)5,6. Studies have identified that both nonessential and essential amino acids play an important role in energy metabolism7,8, including being the main precursors of neurotransmitters9, such as dopamine, glutamate, gamma Amino-n-butyric acid (GABA), and serotonin.
Abnormal levels of amino acids involved in the co-activation of dopaminergic, GABAergic, glutamatergic, and serotoninergic neurotransmitters in the brain of patients with psychosis are well-described findings10,11. Nevertheless, these investigations mainly rely on the post-mortem brains of chronic schizophrenia patients. This constitutes an important limitation, given the long exposure to pharmacological treatment12 and disease comorbidities (diabetes mellitus type 2, dyslipidemia, obesity)13,14, which are well-known conditions associated with metabolic profile changes in these patients15,16. For these reasons, characterizing the profile of amino acids in the peripheral blood of patients in their first-episode psychosis (FEP) can be a reasonable strategy to overcome these caveats, facilitating the comprehension of the pathophysiological processes of psychosis17 and the tailoring of preventive strategies.
In fact, the identification of molecular markers in the peripheral blood of FEP patients is a promising strategy in early intervention services, since it may configure future biomarkers of prognosis and treatment response with less association with confounding factors18,19. Even though studies investigating the metabolomic profile of drug-naïve and medicated psychotic patients have associated the deregulated peripheral blood amino-acids levels with the onset and course of the disease20–22, all these studies were conducted in small samples of early-onset psychosis, and the different methodologies employed do not permit the generalization of the findings. Furthermore, no studies have yet investigated amino-acid dysregulation in unaffected siblings of FEP patients. The inclusion of individuals that share a common genetic- and environmental background with psychotic patients can facilitate the understanding of the role of familial liability to psychosis.
In this study, we aimed to characterize the profile of amino acids related to the: (i) glutamatergic [glutamic acid (GLU), glutamine (GLN), glycine (GLY), glutamic acid + glutamine (Glx), GLN/GLU ratio, proline (PRO), serine (SER)]; (ii) dopaminergic [tyrosine (TYR)]; (iii) serotoninergic [tryptophan (TRP)], and (iv) GABAergic systems [y-Amino-n-butyric acid (GABA)] in the plasma of FEP patients, non-psychotic siblings and community-based controls as possible biomarkers for early-onset and familial risk for psychosis and to explore the role of antipsychotics in the amino acids profiles.
We selected the aforementioned amino acids for the following reasons: (i) serine and glycine are co-agonists of N-methyl-d-aspartate receptor (NMDAR)23,24; glutamine is a non-essential amino acid and the precursor of glutamic acid25; proline is a multifunctional amino acid that can modulate the function of glutamic acid decarboxylase26 and the glutamic acid itself; (ii) regarding the dopaminergic system, we explored the tyrosine amino acid, which is the precursor of dopamine27; (iii) we also investigated the amino acid tryptophan, which is the precursor of serotonin28; and finally (iv) we evaluated the GABAergic system, by measuring the GABA plasma levels.
The first hypothesis of the study was that amino acid plasma levels related to the dopaminergic system would be increased, whereas those associated with the glutamatergic, GABAergic, and serotoninergic systems would be decreased in FEP patients compared with non-psychotic siblings and matched community-based controls. The second hypothesis was that these abnormal amino acid plasma levels would be more prominent in subgroups of patients with longer exposure to pharmacological treatment. Finally, the third hypothesis was that siblings would present a profile congruent to an intermediate group between FEP patients and controls.
Methods
Subjects
This case-sibling-control study is part of an incidence study of mental disorders with psychotic symptoms named STREAM (Schizophrenia and Other Psychoses Translational Research: Environment and Molecular Biology) conducted in the Ribeirão Preto catchment area, Brazil, between 1st of April 2012 and 31st of March 201529. This Brazilian incidence study is part of the consortium European Network of National Schizophrenia Networks Studying Gene-Environment Interactions (EU-GEI)30,31.
We classified FEP patients as individuals who contacted mental health services due to their first manifestation of psychosis and who were treated with antipsychotic medications for the first time. One hundred and sixty-six FEP patients, aged between 16 and 64 years old, took part in the study. We included all FEP patients during the study period who were diagnosed with the spectrum of schizophrenia, such as brief psychotic disorder, schizophreniform disorder, schizophrenia, delusional disorder, and schizoaffective disorder; and affective disorders, such as psychotic bipolar disorder and major depressive disorder with psychotic features.
After the inclusion of patients in the study, we asked their permission also to include their non-affected siblings from which 76 accepted to participate. Furthermore, we recruited 166 age- and sex- matched community-based controls who agreed to take part in the blood collection, as detailed described before32. The community-based controls were recruited based on the census tracts, defined by the Brazilian census bureau of representative municipalities of the catchment area, stratified by sex and age of the population at risk32.
We included siblings and controls residing at the same catchment area of FEP patients, who had not presented psychotic symptoms lifelong confirmed using a standard diagnostic tool33,34. Biological siblings were included considering the genetic and environmental factors shared with their peers, as supported by our recent findings35,36.
All participants were given a diagnosis according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR), assessed by the Structured Clinical Interview for the DSM-IV, clinical version (SCID-CV)33,34, applied by trained mental health professionals supervised by senior psychiatrists. Moreover, we evaluated the medical records and the report of family members. The exclusion of psychotic symptoms in siblings and controls was also performed using the SCID-CV.
Exclusion criteria for patients were psychotic symptoms due to another medical or neurological illness, or drug intoxication. Additional exclusion criteria for the siblings and controls were a current or previous history of psychotic disorders.
For all participants, an extensive clinical and sociodemographic characterization was performed, as described previously36. For patients, the severity of psychiatric symptoms was assessed using the Brief Psychiatric Rating Scale (BPRS)37,38 and the duration of untreated psychosis (DUP) was evaluated using the Nottingham Onset Schedule39.
The power analysis based on our previous NMDAR proteins study32 indicates that the sample size (166 FEP patients and 166 controls) was adequate to provide a power of 90% (to detect a mean difference of 30% in the variance of the outcome variable) at a two-sided 0.05 significance level (Stata Corp).
Our study was approved by the Clinical Hospital, Ribeirão Preto Medical School, University of São Paulo Ethic committee from Brazil, and all participants (FEP patients and/or their legal guardians, community controls) provided written informed consent for participation in the study (Process number 12606/2012). Parents or a first-degree relative were often considered by the clinician investigators when FEP patients showed reduced capacity to determine data or participate in the study.
We guarantee that all methods were carried out in accordance with relevant guidelines and regulations.
Plasma samples
All blood samples were collected as closer as possible to the date of FEP patients’ inclusion in the study. Peripheral blood was drawn by venous puncture from all subjects during the day and collected into 4 mL EDTA-coated tubes. We then centrifuged the blood (3500 × g for 10 min at 4 °C), and the plasma was transferred to 1.5 mL eppendorf tubes and stored at − 80 °C until undergoing gas chromatography analysis. The details of the blood sample collection were previously described32.
Amino acids profile
We used 100 µL from the obtained plasma samples for amino acid quantification using the gas chromatography mass spectrometry technology (GC–MS), as recommended by the most standardized protocol40.
The amino acid analysis procedure of EZ:Faast Amino Acid Analysis (EZ:FAAST, PHENOMENEX, CA, USA) consists of a solid phase extraction followed by a by-pass and a liquid extraction. Chromatographic analyses were performed in a GC–MS QP2010 Ultra (SHIMADZU, KYOTO, JAPAN). The determination of amino acids plasma levels involved an initial pre-treatment of the samples with dithiothreitol to release protein-bound these molecules.
One standard amino acid, namely GABA, had its EZ:Faast Kit purchased separately from Sigma-Aldrich (Denmark A/S, DK) at the highest available purity. The standard solution was prepared separately to obtain a curve for GABA: 5–260 nmol/mL to an appropriate volume of milliQ-water. Amino acids plasma samples with high concentrations were diluted until these concentrations reached the range of the calibration curve.
Helium at a flow rate of 1 mL/min was used as the carrier gas. The injector and detector temperatures were maintained at 280 °C and 250 °C, respectively. The column temperature program was initialized at 80 °C and held for two minutes, and then increased to 320 °C at a ramp rate of 20 °C/min. The mass spectrometer was operated in electron ionization mode, and SIM modes were applied to detect and quantify these amino acids. The total run time was 15 min.
A calibration curve at concentrations of 25, 50, 100, and 200 ng/mL was prepared by fortifying blank blood with corresponding analytical working solutions. The linearity of the method was investigated by evaluating the coefficient of determination and was achieved with a minimal of 0.99.
Statistical analysis
Data were analyzed using SPSS version 20.0 for Windows (SPSS Inc) and Statistical Analysis System (SAS/STAT) software version 9.441. We described the groups’ raw data as mean, standard deviation (SD) and frequencies. Before the analysis, the amino acid plasma levels were transformed to a logarithmic scale to improve the normality and reduce the heterogeneity of variance, as often observed in published metabolic studies42–44.
We analyzed the sociodemographic and clinical variables among the groups (FEP patients, siblings, and controls) using Pearson’s chi-square and one-way ANOVA with Bonferroni correction. Controls were sex- and age-matched to the FEP patients.
Firstly, we investigated plasma amino acids differences among the three groups by using the generalized linear model (GLM)45, including the values of each amino acid as the outcome variables and groups as fixed factor, while adjusting for the effects of age, sex, and body mass index (BMI). Next, using the GLM model, we analyzed differences between amino acid plasma levels within the FEP group and their association with clinical variables while adjusting for the effects of age, sex, and BMI. Multiple comparisons were performed by orthogonal contrasts, and for each of the GLM models performed, the normality of the residuals was checked using normal probability plots. We calculated the effect size among the three groups based on ANOVA and we used the effect size index η2 for regression models and analysis of variance (η2 values, small: 0.01–0.05, medium: 0.06–0.13, large: ≥ 0.14)46,47.
Moreover, we performed an additional analysis in a sub-sample of FEP-Siblings correspondent pairs in relation to amino acid plasma levels using paired T-test and considered sex, age, and BMI as covariates. The effect size calculation was based on differences between the means for FEP-Sibling paired samples, using the effect size of Cohen’s d (d values, small: 0.20–0.49, medium: 0.50–0.79; large: ≥ 0.80)46,47. As a common language for the effect size statistics (variance η2; three groups) and means (d; FEP-Sibling pairs), we used an equivalence based on z-score probabilities48.
For all analyses, BMI data was recorded as a categorical variable according to the World Health Organization classification49, and for DUP and duration of treatment we used the same categorization as published in our previous study32.
Finally, in the FEP group, we tested for potential associations among the amino acids analyzed and some patients’ clinical features using GLM models. We considered Glx as a combination of GLU and GLN, as well as the GLN/GLU ratio.
In this study, no missing data were found for any participant in relation to each variable of interest.
Values of p < 0.05 were considered significant for all the analyses.
Results
Sociodemographic features of the sample
In this study, 166 FEP patients, 76 biological siblings and 166 controls matched for sex and age, accepted the invitation for peripheral blood collection, and the respective samples were included in all amino acids analysis. Our results (Table 1) showed that the sibling group was composed of a higher percentage of women when compared to both FEP patients and controls (p < 0.001). Moreover, FEP patients had a lower education level (less than 9 years of schooling), and reported less relationship bound in relation to siblings and controls (p < 0.001 for both). The percentage of FEP patients who were not currently working was higher than siblings and controls (p = 0.015).
Table 1.
Sociodemographic and clinical variables from FEP patients, non-psychotic siblings and matched community-based controls.
| Control group (n = 166) | Sibling group (n = 76) | FEP patients (n = 166) | Test | p-valuec,d | |
|---|---|---|---|---|---|
| Sociodemographic data: n (%), mean (SD) | |||||
| Age, years | 31.4 (12.0) | 31.5 (11.0) | 30.3 (12.2) | F(2,407) = 0.395 | 0.674 |
| Sex, male | 106 (63.9)b | 23 (30.3)a | 106 (64.0)b | χ2 = 28.574 | < 0.001 |
| Education, ≤ 9 years | 40 (24.1)b | 23 (30.3)b | 95 (57.2)a | χ2 = 41.217 | < 0.001 |
| Ethnicity, white | 113 (68.1)a | 39 (51.3)b | 81 (50.0)b | χ2 = 12.519 | 0.002 |
| Marital status, single | 83 (50.0)b | 32 (42.1)b | 123 (74.1)a | χ2 = 29.947 | < 0.001 |
| Occupation, currently not working | 67 (40.4)b | 24 (31.6)b | 84 (50.6)a | χ2 = 8.434 | 0.015 |
| Clinical data: mean (SD) | |||||
| Body mass index, kg/m2 | 26.5 (5.5)a | 24.9 (4.9)a,b | 24.7 (5.0)b | F(2,407) = 4.825 | 0.013 |
| Abdominal circumference, cm | 89.1 (15.1)a | 82.9 (14.4)b | 86.1 (13.3)a,b | F(2,331) = 4.286 | 0.016 |
| Use of psychoactive substances: n (%)e | χ2 = 87.107 | < 0.001 | |||
| Cannabis only | 15 (9.0) | 4 (5.3) | 9 (5.4) | ||
| Cannabis combined with others | 20 (12.0)b | 2 (2.6)b | 76 (45.8)a | ||
| Other substances | 11 (6.6) | 0 (0.0) | 12 (7.2) | ||
| None | 120 (72.4) | 70 (92.1) | 69 (41.6) | ||
| Cigarette smoking | 31 (18.7)b | 13 (17.1)b | 65 (39.2)a | χ2 = 22.191 | < 0.001 |
| Only FEP patients: mean (SD) | |||||
| DUP, weeks | 59.9 (173.6) | ||||
| Duration of treatment, weeks | 32.5 (42.1) | ||||
| BPRS total score | 9.1 (6.9) | ||||
| Median of psychosis until blood collection | 35.5 | ||||
| Median of treatment until blood collection | 12.5 | ||||
FEP first-episode psychosis, SD standard deviation, DUP Duration untreated psychosis, BPRS brief psychiatric rating score.
Bold: p ≤ 0.05.
a,bMeans followed by the same letter did not differ statistically from each group in the table. Different subscript letters represent the significant differences between groups.
cOne-way ANOVA with Bonferroni correction.
dChi-square test.
eUse of psychoactive substances (current or lifetime): Cannabis; alcohol; cocaine/crack; inhalants; amphetamine.
Regarding ethnicity, taking as a proxy the self-declared skin color, the percentage of white subjects was higher in controls than FEP patients and siblings (p = 0.002). Controls presented higher BMI than FEP patients (p = 0.013) and higher abdominal circumference measurements than siblings (p = 0.016). For life-time psychoactive substance use, FEP patients reported more cannabis use in combination with other illegal substances and more tobacco smoking than siblings and controls (p < 0.001 for both).
The median of the duration of psychosis until the blood collection was 35.5 weeks, while the median of pharmacological treatment in relation to the blood collection was 12.5 weeks. The majority of the patients were followed in an early intervention service, with a good response to the initial treatment (median BPRS = 9.1). More details of other sociodemographic and clinical variables were described previously32.
Amino acid plasma levels: group comparison
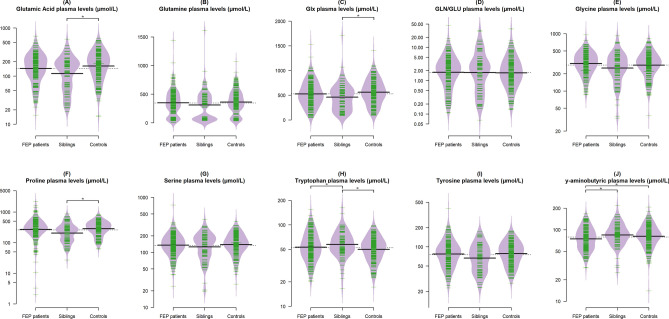
The Fig. 1A–J and Table 2 describe the (A) GLU, (B) GLN, (C) Glx, (D) GLN/GLU ratio, (E) GLY, (F) PRO, (G) SER, (H) TRP, (I) TYR, and (J) GABA plasma levels of FEP patients, non-affected siblings, and community-based controls.
Figure 1.
Beanplot of glutamic acid (A), glutamine (B), Glx (C), GLN/GLU ratio (D), glycine (E), proline (F), serine (G), tryptophan (H), tyrosine (I), and ÿ-aminobutyric (J) plasma levels (µmol/L, raw values) of FEP, siblings and controls (n = 408). *Group comparison; p < 0.05. The data was analyzed by the generalized linear model adjusted by sex, age and BMI.
Table 2.
The effect sizes and the common language effect size statistic correspondent between FEP-sibling-controls in relation to amino acid plasma profile.
| Amino acids | FEP | Siblings | Controls | FEP-sibling-controls | |||
|---|---|---|---|---|---|---|---|
| Mean (SD) | F | p-value | η2 (95% CI) | CL (%)* | |||
| GLU | 178.03 (110.80) | 149.20 (104.74) | 200.83 (123.05) | 3.43 | 0.002 | 0.06 (0.01, 0.12) | 60.0 |
| GLN | 349.22 (228.50) | 310.74 (268.68) | 360.99 (210.81) | 1.45 | 0.062 | 0.03 (0.01, 0.06) | 51.0 |
| GLY | 331.99 (141.39) | 298.61 (165.95) | 316.68 (143.92) | 2.64 | 0.198 | 0.05 (0.01, 0.09) | 59.0 |
| Glx | 527.25 (253.65) | 459.94 (275.84) | 561.83 (249.79) | 2.36 | 0.004 | 0.05 (0.01, 0.08) | 59.0 |
| GLN/GLU | 3.10 (4.38) | 3.97 (5.82) | 3.04 (4.54) | 0.34 | 0.953 | 0.01 (0.00, 0.01) | 54.0 |
| PRO | 365.29 (298.61) | 255.47 (170.42) | 324.93 (158.56) | 3.20 | 0.005 | 0.06 (0.02, 0.10) | 60.0 |
| SER | 150.30 (76.33) | 145.20 (72.28) | 152.05 (62.42) | 3.03 | 0.317 | 0.06 (0.02, 0.10) | 60.0 |
| TRP | 57.70 (23.75) | 61.42 (23.54) | 52.81 (17.11) | 3.18 | 0.022 | 0.06 (0.02, 0.10) | 60.0 |
| TYR | 86.41 (45.81) | 74.80 (36.05) | 85.09 (33.72) | 2.62 | 0.214 | 0.05 (0.02, 0.08) | 59.0 |
| GABA | 79.29 (28.30) | 90.15 (32.78) | 86.23 (29.84) | 1.70 | 0.023 | 0.04 (0.01, 0.06) | 57.0 |
η2 eta-squared (small: 0.01–0.05, medium: 0.06–0.13, large: ≥ 0.14), CI confidence interval, *CL common language effect size statistic.
Bold: p ≤ 0.05.
FEP patients showed reduced GABA plasma levels in comparison to both siblings (p = 0.006) and controls (p = 0.048). In addition, siblings had lower plasma levels of GLU (p = 0.006), Glx (p = 0.005) and PRO (p = 0.021), but increased TRP plasma levels in comparison to patients (p = 0.021) and controls (p < 0.001).
Finally, we did not find significant differences between the groups in relation to GLN (p = 0.062), GLN/GLU ratio (p = 0.953), GLY (p = 0.198), SER (p = 0.317), and TYR (p = 0.214).
In the secondary analysis of FEP-Siblings pairs comparison, we did not find any statistical significant differences in any amino acids profile (GLU: p = 0.852; GLN: p = 0.556; GLY: p = 0.773; Glx: p = 0.388; GLN/GLU: p = 0.607; PRO: p = 0.619; SER: p = 0.561; TRP: p = 0.091; TYR: p = 0.384; GABA: p = 0.124). Detailed information is described in Supplementary Table S1.
Associations between amino acid plasma levels and socio-demographic and clinical characteristics in FEP patients
We did not find significant differences in amino acid plasma levels in relation to sex, age, psychoactive substances use, and tobacco smoking. However, FEP patients with BMI between 25.0 to 35.9 kg/m2 had higher Glx plasma levels in comparison to FEP patients with BMI between 18.5 to 24.9 kg/m2 (p = 0.010) and BMI higher than 31.0 kg/m2 (p = 0.006). No other significant associations were observed.
FEP patients with up to 12 weeks of DUP had lower GLU (p = 0.005) and TYR (p = 0.002) plasma levels in comparison to those with more than 13 weeks of DUP [13 to 24 weeks, GLU (p = 0.032) and TYR (p = 0.044); and with DUP higher than 53 or more weeks, TYR (p = 0.009)].
In addition, GLU plasma levels changed in relation to different durations of treatment. FEP patients with up to 11 weeks of pharmacological treatment presented decreased GLU plasma levels in relation to FEP patients with 12 or more weeks (p = 0.009). Moreover, FEP patients treated with antipsychotics only had significantly increased GLU plasma levels when compared to FEP patients treated with a combination of medications (p = 0.010). FEP patients treated only with antipsychotics presented higher Glx plasma levels when compared to FEP patients with antipsychotic associated with other medications (p = 0.026).
Finally, we analyzed the FEP patients according to three categories of antipsychotic treatments: none (n = 9), atypical (n = 74) and typical (n = 83); the use or type of antipsychotics did not change any amino acid plasma concentrations (Table 3).
Table 3.
Differences between amino acid plasma levels in relation to clinical variables in FEP patients.
| Clinical variables | Amino acids plasma levels (µmol/L)c | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GLU | GLN | Glx | GLY | GLN/GLU | PRO | TRP | TYR | SER | GABA | |
| Mean (SD) | ||||||||||
| Sex | p = 0.387 | p = 0.436 | p = 0.387 | p = 0.652 | p = 0.735 | p = 0.693 | p = 0.984 | p = 0.464 | p = 0.794 | p = 0.467 |
| Female (n = 60) | 164.0 (101.9) | 335.0 (228.6) | 499.0 (263.6) | 318.1 (148.7) | 3.0 (3.2) | 368.4 (348.8) | 57.1 (22.5) | 78.9 (33.8) | 139.8 (59.6) | 75.9 (26.3) |
| Male (n = 106) | 186.0 (115.3) | 357.3 (229.1) | 543.2 (247.7) | 339.8 (137.2) | 3.1 (4.9) | 363.6 (267.8) | 58.1 (24.5) | 90.7 (51.1) | 156.2 (84.0) | 81.2 (29.3) |
| Age | p = 0.967 | p = 0.386 | p = 0.844 | p = 0.072 | p = 0.838 | p = 0.410 | p = 0.766 | p = 0.726 | p = 0.057 | p = 0.896 |
| 16 to 24 (n = 66) | 176.9 (96.2) | 369.4 (226.5) | 546.3 (249.8) | 362.4 (122.6) | 2.9 (2.6) | 412.3 (324.6) | 56.9 (24.2) | 88.6 (37.1) | 163.4 (57.5) | 79.5 (25.0) |
| 25 to 34 (n = 47) | 177.3 (117.4) | 355.3 (218.6) | 532.7 (243.0) | 315.0 (140.8) | 3.2 (3.4) | 309.3 (207.2) | 58.5 (22.8) | 85.9 (59.4) | 135.0 (50.6) | 79.0 (25.7) |
| 35 or more (n = 53) | 180.1 (123.2) | 318.7 (240.3) | 498.8 (269.4) | 309.2 (158.5) | 3.3 (6.5) | 356.4 (327.9) | 58.1 (24.4) | 84.1 (42.6) | 147.5 (107.9) | 79.3 (34.3) |
| BMI (kg/mn) | p = 0.233 | p = 0.264 | p = 0.001 | p = 0.078 | p = 0.437 | p = 0.597 | p = 0.919 | p = 0.942 | p = 0.210 | p = 0.223 |
| Up to 18.4 (n = 11) | 155.1 (66.5) | 435.3 (227.4) | 548.1 (213.2) | 366.2 (153.8) | 3.4 (2.9) | 303.6 (123.8) | 59.4 (22.5) | 86.0 (33.1) | 153.1 (59.4) | 74.7 (23.8) |
| 18.5 to 24.9 (n = 101) | 194.9 (119.5) | 339.2 (242.1) | 594.6 (289.5) | 349.8 (143.6) | 3.5 (6.4) | 385.9 (246.5) | 58.1 (21.7) | 83.7 (32.7) | 151.6 (60.9) | 78.3 (24.8) |
| 25.0 to 30.9 (n = 42) | 158.4 (91.2) | 334.3 (197.8) | 451.0 (217.4) | 296.9 (130.5) | 2.5 (2.2) | 382.4 (365.8) | 57.4 (26.4) | 88.5 (54.5) | 156.4 (90.6) | 83.7 (31.9) |
| 31 or more (n = 12) | 228.0 (159.5) | 407.3 (210.8) | 648.0 (214.1) | 273.5 (122.4) | 4.1 (4.6) | 262.2 (116.1) | 56.7 (19.2) | 85.8 (47.5) | 117.2 (50.0) | 65.2 (18.1) |
| DUP (weeks) | p = 0.032 | p = 0.673 | p = 0.169 | p = 0.624 | p = 0.404 | p = 0.658 | p = 0.164 | p = 0.002 | p = 0.356 | p = 0.806 |
| Up to 12 (n = 89) | 148.7 (92.6)a | 345.1 (245.7) | 493.8 (260.0) | 319.7 (141.3) | 3.7 (5.6) | 342.1 (250.3) | 53.9 (21.7) | 74.8 (34.3)a | 141.3 (63.4) | 78.0 (30.0) |
| 13 to 24 (n = 28) | 223.3 (137.3)b | 322.0 (187.8) | 545.2 (222.2) | 351.6 (165.6) | 2.2 (2.2) | 367.9 (307.1) | 62.4 (24.5) | 95.1 (34.9)b | 156.0 (57.1) | 78.4 (24.7) |
| 25 to 52 (n = 24) | 217.4 (114.4) b | 401.8 (232.6) | 619.2 (268.6) | 344.0 (130.7) | 2.7 (2.6) | 413.9 (412.3) | 63.0 (28.4) | 95.8 (40.6) | 152.1 (57.9) | 80.1 (24.1) |
| 53 or more (n = 25) | 194.0 (108.7) | 343.8 (206.0) | 537.8 (237.9) | 342.4 (125.2) | 2.2 (1.8) | 398.3 (329.8) | 60.9 (24.3) | 109.0 (77.0)b | 174.1 (132.2) | 84.0 (30.4) |
| Duration of treatment (weeks) | p = 0.009 | p = 0.801 | p = 0.213 | p = 0.433 | p = 0.599 | p = 0.379 | p = 0.432 | p = 0.191 | p = 0.380 | p = 0.636 |
| None (n = 9) | 171.1 (109.5) | 358.4 (165.1) | 529.5 (191.9) | 367.9 (131.4) | 3.0 (2.0) | 486.7 (389.0) | 59.1 (22.3) | 85.7 (44.3) | 188.2 (83.6) | 89.2 (37.0) |
| Up to 11 (n = 71) | 153.6 (105.6)a | 338.8 (217.9) | 492.4 (260.4) | 348.6 (148.8) | 3.5 (5.6) | 356.0 (304.9) | 56.2 (26.0) | 79.5 (37.2) | 147.8 (56.3) | 80.6 (29.6) |
| 12 or more (n = 86) | 198.9 (112.1)b | 356.9 (244.0) | 555.8 (252.5) | 314.6 (137.8) | 2.7 (3.2) | 360.2 (284.0) | 58.8 (22.1) | 92.2 (51.7) | 148.4 (88.8) | 77.2 (26.2) |
| Type of antipsychotic | p = 0.141 | p = 0.100 | p = 0.900 | p = 0.781 | p = 0.990 | p = 0.375 | p = 0.676 | p = 0.161 | p = 0.050 | p = 0.661 |
| None (n = 9) | 172.4 (109.5) | 352.7 (165.1) | 529.5 (191.9) | 354.7 (131.4) | 3.0 (2.0) | 472.9 (389.0) | 58.7 (22.3) | 85.7 (44.3) | 184.4 (83.6) | 89.0 (37.0) |
| Atypical (n = 74) | 199.3 (119.4) | 312.1 (232.9) | 540.7 (272.0) | 337.2 (142.4) | 3.1 (2.7) | 362.2 (228.5) | 59.8 (25.5) | 93.8 (53.2) | 162.4 (91.4) | 80.5 (28.8) |
| Typical (n = 83) | 159.7 (100.5) | 381.9 (227.8) | 511.9 (240.4) | 324.8 (142.5) | 3.1 (5.9) | 356.4 (341.2) | 55.8 (22.4) | 79.9 (37.5) | 135.8 (55.7) | 77.1 (26.9) |
| Current treatment | p = 0.010 | p = 0.257 | p = 0.031 | p = 0.500 | p = 0.440 | p = 0.428 | p = 0.979 | p = 0.489 | p = 0.371 | p = 0.644 |
| None (n = 9) | 171.1 (109.5) | 358.4 (165.1) | 529.5 (191.9) | 367.9 (131.4) | 3.0 (2.0) | 486.7 (389.0) | 59.1 (22.3) | 85.7 (44.3) | 188.2 (83.6) | 89.2 (37.0) |
| Antipsychotics only (n = 66) | 206.4 (111.3)a | 378.9 (238.7) | 585.3 (264.6) | 315.6 (111.1) | 2.5 (2.5) | 365.6 (253.4) | 56.3 (19.1) | 92.0 (52.3) | 143.9 (52.7) | 77.6 (25.7) |
| Antipsychotics and/or others (n = 91) | 158.1 (107.2)b | 326.8 (225.8) | 484.9 (244.6) | 340.3 (160.6) | 3.5 (5.5) | 353.1 (319.4) | 58.6 (26.9) | 82.4 (40.7) | 151.2 (88.8) | 79.5 (29.3) |
| Psychoactive substances | p = 0.606 | p = 0.677 | p = 0.940 | p = 0.381 | p = 0.668 | p = 0.438 | p = 0.502 | p = 0.269 | p = 0.770 | p = 0.522 |
| None (n = 100) | 173.9 (111.6) | 347.5 (242.2) | 521.4 (261.0) | 309.1 (138.9) | 3.4 (5.3) | 384.1 (348.9) | 59.0 (24.4) | 80.3 (38.5) | 140.0 (65.3) | 79.4 (30.1) |
| Cannabis only (n = 27) | 167.6 (107.0) | 368.3 (193.6) | 535.9 (239.7) | 361.0 (124.2) | 3.2 (2.4) | 388.4 (219.4) | 54.1 (21.2) | 86.5 (38.0) | 166.9 (57.6) | 79.5 (27.6) |
| Cannabis and othersd (n = 20) | 205.8 (138.0) | 322.7 (208.7) | 528.5 (237.4) | 355.9 (93.4) | 2.3 (2.2) | 347.1 (192.5) | 56.4 (24.7) | 93.4 (38.0) | 161.8 (52.2) | 84.9 (25.9) |
| *Others only (n = 19) | 185.3 (78.9) | 359.0 (233.1) | 544.3 (268.4) | 385.9 (194.2) | 2.4 (2.6) | 252.7 (150.4) | 57.7 (23.9) | 110.9 (81.1) | 168.9 (144.1) | 72.4 (21.7) |
| Use of nicotine | p = 0.746 | p = 0.377 | p = 0.701 | p = 0.710 | p = 0.907 | p = 0.148 | p = 0.515 | p = 0.380 | p = 0.382 | p = 0.570 |
| Yes (n = 65) | 173.0 (96.1) | 370.0 (215.8) | 543.0 (248.5) | 341.7 (128.1) | 3.0 (2.5) | 317.6 (211.4) | 56.9 (23.7) | 81.9 (32.9) | 147.1 (63.6) | 80.9 (25.8) |
| No (n = 101) | 181.3 (119.6) | 335.8 (236.4) | 517.1 (257.6) | 325.8 (149.6) | 3.2 (5.3) | 396.0 (340.7) | 58.2 (23.9) | 89.3 (52.4) | 152.4 (83.7) | 78.3 (29.9) |
GLU glutamic acid, GLN glutamine, GLY glycine, PRO proline, TRP tryptophan, TYR tyrosine, SER serine, GABA y-aminobutyric, SD standard deviation, BMI Body Mass Index, DUP duration of untreated psychosis.
Bold: ≤ 0.05.
a,bSuperscript letters were used for the mean values in the columns, in which the means followed by different letters differ statistically from each other. Multiple comparisons and orthogonal contrasts performed in the GLM with 5% probability.
cGeneralized Linear Model adjusted by sex, age and BMI analysed with log transformed.
dAlcohol, cocaine/crack and inhalants.
Discussion
We aimed to characterize the amino acid plasma profile related to the dopaminergic, glutamatergic, serotoninergic, and GABAergic systems in the early stages of psychosis. FEP patients did not differ from community-based controls, except by decreased GABA plasma levels, which was also observed in relation to their unaffected siblings. On the other hand, the non-affected siblings showed significant differences from controls in the plasma levels of amino acids, specifically related to the glutamatergic and serotoninergic systems. Altered amino acids plasma profile in unaffected siblings may be related to familial risk to psychosis since they share a similar genotype and environmental factors with FEP patients.
Plasma amino acids profile in FEP patients in relation to non-psychotic siblings and community-based controls
Our findings of lower GABA plasma levels in FEP patients compared to non-psychotic siblings and community-based controls are consistent with the literature suggesting decreased GABA in post-mortem brain tissues50,51, as well as in the plasma of patients with chronic schizophrenia52,53. However, a recent study reported higher levels of GABA in drug-naïve schizophrenia patients in comparison to controls17. Considering that, in our sample, the decreased GABA was independent of pharmacological treatment; the discrepant results could be justified based on the technique used for the amino acid quantification. Cao et al.17 applied the hydrophilic interaction liquid chromatography that has been used for polar metabolites; however, this method has disadvantages when compared to the method used in our study, especially due to its longer retention time drifts and extensive re-balance runs. To overcome that, we used the GC–MS system that has shown a higher capacity of separation, sensitivity and selectivity of amino acids. This methodology has been reported to be useful for metabolomics studies for providing quick screening approaches54. To the best of our knowledge, our study is the first that investigated GABA amino acid plasma levels using the GC–MS system in FEP patients.
The GABAergic system seems to play a central role in the neurobiology of schizophrenia and other psychoses, and disturbances in this system are suggested to contribute to core psychotic symptoms55,56. The decreased GABA levels in FEP patients is thought to be a secondary dysfunction from the well-known NMDAR hypofunction on inhibitory interneurons in schizophrenia57,58, or may also result from the interaction among this neurotransmitter with others, such as those related to the glutamatergic and dopaminergic systems58,59. Although speculative, the reduced GABA levels in the peripheral blood could be reflecting the GABA low concentration observed in the brain of FEP patients60,61, potentially suggesting concomitant blood alterations in GABA as a biomarker for psychosis52,62.
Contrasting our hypothesis, no differences were found in FEP patients compared with controls regarding their GLU, GLN, GLY, Glx, GLN/GLU ratio, SER, TRP, and TYR plasma levels. However, the findings regarding amino acid plasma levels in psychosis are still contradictory. For instance, while some studies did not show differences in GLU serum levels between schizophrenia patients and healthy controls63, other recent investigations found higher20,42 or lower64 GLU serum/plasma levels in psychotic patients.
Moreover, GLY and SER have been investigated as possible clinical markers for schizophrenia, with some studies demonstrating increased GLY and SER plasma levels in patients after antipsychotic treatment23,65; no differences in patients medicated with different antipsychotics3, or lower GLY and SER plasma levels in patients in comparison to controls66,67. A recent study demonstrated high plasma GLN levels in drug-naïve FEP patients, which was negatively correlated with negative symptoms; however no differences in plasma GLN levels were found after treating these patients68.
The discrepant findings could be justified by the distinct methodologies used for amino acid measurement, as well as by specificities of the samples, such as the duration of disease, severity of symptoms, duration of treatment and type of antipsychotics, dietary habits, and the amino acid measurement made by in the peripheral access22. In our study, we can justify the absence of the differences between FEP patients and controls by some features of our study (the case-sibling-control design, inclusion of affective and non-affective psychosis, not fasting plasma samples, and the heterogeneity in the duration of treatment and psychosis).
Particularly, in our study, treatment with antipsychotics may have brought the amino acid plasma levels closer to the normal range and may be one of the reasons why we did not find differences between FEP patients, siblings, and controls22. Corroborating with this explanation, we found that low DUP, short-duration of antipsychotic treatment, and use of antipsychotics combined with other medication were associated with low GLU plasma levels. Low DUP was also associated with reduced TYR plasma levels.
Plasma amino acids profile in non-psychotic siblings in comparison to community-based controls
Our data showed decreased GLU, Glx and PRO plasma but higher TRP plasma levels in non-psychotic siblings when compared to community-based controls.
We identified three studies investigating the metabolic profile in the brain regions of patients’ siblings using the proton magnetic resonance. One, in agreement with our findings, found lower prefrontal GLU in unaffected twins of schizophrenia patients when compared to controls69. In the same direction, a recent study showed lower cortical GLU in first-degree relatives compared with healthy controls, but no differences were observed between the relatives and schizophrenia patients50. However, a third study, did not find differences in GLU in adult siblings of schizophrenia patients compared with healthy volunteers, which can be justified by the small sample size and the use of unsegmented metabolite values70. Different from our study, these previous studies have not investigated the TRP and PRO plasma levels in unaffected siblings. Taken together, reduced peripheral GLU, Glx, and PRO levels and increased TRP levels may be reflecting imbalances in the amino acids plasma profile related to familial risk to psychosis, which may potentially be considered as an indicator of the biological vulnerability associated with psychosis, since non-affected siblings share a similar genotype and early-life environment with FEP patients.
Limitations and strengths
Our results should be interpreted with some caution. First, we measured the amino acids at one-time point and did not control for the participants’s diet. We cannot exclude the influence of fasting condition at the time of blood sampling in the amino acid plasma levels, given the fact that some of the amino acids are taken by diet, and that previous evidence demonstrated positive associations between food consumptions and altered profile of amino acids5,71. In addition, we do not have data concerning the physical activity of the participants, which can also impact the amino acids profile72,73 similar to information about metabolic diseases as dyslipidemia and diabetes mellitus. Third, our patients were not drug-naïve and were heterogeneous regarding the type and duration of pharmacological treatment. Fourth, we were not able to investigate a brain-blood correspondence of the amino acid profile, using methods such as resonance imaging spectroscopy. It is important to highlight that our data is related to plasma amino acids levels, which may not correlated with amino acids levels in the brain. Interestingly, evidence has demonstrated that some human brain amino acids, such as GLU, is correlated to the peripheral pathway in schizophrenia patients74. Furthermore, findings have suggested that TRP and TYR peripheral alterations may reflect brain changes due to their transportation by neutral amino acid carrier system75,76. Thus, although speculative, the altered amino acid profiles in the peripheral blood might, to some extent, reflect those changes observed in the brain76. However, future studies measuring blood and brain amino acid activities are needed to conclude their correlation and potential role as biomarkers in psychosis. Fifth, we did not perform an analysis of amino acids according to FEP heterogeneity. Future studies are necessary to evaluate the amino acid plasma levels considering the FEP patients in different diagnostic categories (affective and non-affective psychoses) since the diagnostic stability in first-episode psychosis is low, considering other clinical diagnoses and compared to controls. Sixth, we were only able to measure the racemic serine (DL-serine) using GC–MS in the peripheral blood, given that the Phenomenex lab Kit does not separate their chiral forms. D-serine that bind to GLY, a co-agonist of NMDAR, has an important role in the NMDAR function as the synaptic plasticity77. Finally, the assessment of metabolic diseases relied only on self-reported data collection, which can underestimate the formal clinical diagnoses.
Despite these important limitations, our paper has several strengths. We included FEP patients with a detailed clinical characterization allowing exploration of the association between psychotropic treatment and other clinical variables with the amino acid plasma levels. Furthermore, we included their biological siblings, considered a risk group that shares the same environment and similar genetic profile of the patients, instead of the traditional case–control design; we also included community-based controls representative of the general population. Finally, our study used the GC–MS, a technique that provides a robust profile of amino acid on their molecular level. This approach has often been considered a gold standard tool to explore amino acid profiling in metabolomic studies78,79, which had not yet been employed by other psychosis studies. We can also consider the generalizability of our findings as satisfactory. Our metabolite approach offers a low complexity of amino acids data acquisition, with high-sensitivity, fast time and low-cost to screening the metabolite features involved in predefined pathways implicated in the pathophysiology of psychosis.
Conclusions
Our study suggests that FEP patients may be characterized by a reduced GABA plasma profile comparedto community-based controls and their unaffected siblings. Furthermore, we were able to show the influence of patients’ pharmacological treatment in the GLU plasma profile. Our results also suggest that metabolic abnormalities, especially those associated with decreased GLU, Glx and PRO, are not restricted to psychosis, bringing the unaffected siblings as a possible risk group for metabolic abnormalities. Finally, the GC–MS technique should be considered a useful tool for the screening of peripheral amino acids, contributing to the comprehension of the pathophysiological processes in psychosis and their healthy first-relatives. Altogether, the peripheral blood alterations seen herein might reflect an imbalance of amino acids in psychosis. Further studies, especially metabolomics, are needed to confirm our findings and improve the understanding of psychosis pathogenesis.
Supplementary Information
Acknowledgements
CML is recipient of a scholarship from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior); AAJ (level 2, 302231/2018-8), RD (level 2, 305131/2018-4), PRM (level 1B, 303815/2015-9), CMD-B (level 1B, 308751/2019-1) and PL-Jr (level 2, 305797/2016-6) are recipients of fellowships from CNPq; DLR, FC-Z, HAF and RS are recipients of fellowships from FAPESP (grants 2018/07581-2; 2019/13229-2 and 2016/12195-9; 2015/02948-7 and 2017/00624-5; 2013/11167-3, respectively). The authors thank are indebted to the Nutrition and Metabolism Laboratory, Faculty of Medicine of Ribeirão Preto, University of São Paulo.
Author contributions
C.M.L. and P.L.-J. conceived the study. D.L.R., A.A.J.-J., R.D., P.R.M. and C.M.D.-B. contributed to the study design. P.R.M., C.M.D.-B. and P.L.-J. obtained funding. R.S. obtained ethical approval. C.M.L., F.C.-Z., L.M.C.S.-A. and R.D. managed gas chromatography analysis. C.M.L., D.L.R., R.S., R.D. and C.M.D.-B. analyzed the data. All authors collaborated in the interpretation of the data. C.M.L. wrote the first draft of the manuscript. R.S., F.C.-Z., H.A.F., A.A.J., R.D., C.M.D.-B. and P.L.-J. critically revised the manuscript. All the authors approved the final version of the manuscript.
Funding
This study was supported by São Paulo Research Foundation (Fundação de Amparo à Pesquisa do Estado de São Paulo, Brasil, FAPESP Grants 2012/05178-0, and 2013/08216-2 (Center for Research in Inflammatory Disease, CRID), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES—Finance Code 001).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-020-78559-w.
References
- 1.Davis J, et al. A review of vulnerability and risks for schizophrenia: beyond the two hit hypothesis. Neurosci. Biobehav. Rev. 2016;65:185–194. doi: 10.1016/j.neubiorev.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halldorsdottir T, Binder EB. Gene × environment interactions: from molecular mechanisms to behavior. Annu. Rev. Psychol. 2017;68:215–241. doi: 10.1146/annurev-psych-010416-044053. [DOI] [PubMed] [Google Scholar]
- 3.Domingues DS, et al. Simultaneous determination of amino acids and neurotransmitters in plasma samples from schizophrenic patients by hydrophilic interaction liquid chromatography with tandem mass spectrometry. J. Sep. Sci. 2015;38:780–787. doi: 10.1002/jssc.201400943. [DOI] [PubMed] [Google Scholar]
- 4.Saleem S, Shaukat F, Gul A, Arooj M, Malik A. Potential role of amino acids in pathogenesis of schizophrenia. Int. J. Health Sci. (Qassim) 2017;11:63–68. [PMC free article] [PubMed] [Google Scholar]
- 5.Bröer S, Bröer A. Amino acid homeostasis and signalling in mammalian cells and organisms. Biochem. J. 2017;474:1935–1963. doi: 10.1042/BCJ20160822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffer LJ. Human protein and amino acid requirements. J. Parenter. Enter. Nutr. 2014;40:460–474. doi: 10.1177/0148607115624084. [DOI] [PubMed] [Google Scholar]
- 7.Shimomura Y, Kitaura Y. Physiological and pathological roles of branched-chain amino acids in the regulation of protein and energy metabolism and neurological functions. Pharmacol. Res. 2018;133:215–217. doi: 10.1016/j.phrs.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37:1–17. doi: 10.1007/s00726-009-0269-0. [DOI] [PubMed] [Google Scholar]
- 9.Choi YK, Tarazi FI. Alterations in dopamine and glutamate neurotransmission in tetrahydrobiopterin deficient spr-/-mice: relevance to schizophrenia. BMB Rep. 2010;43:593–598. doi: 10.5483/BMBRep.2010.43.9.593. [DOI] [PubMed] [Google Scholar]
- 10.Zhang R, et al. Metabolomic profiling of post-mortem brain reveals changes in amino acid and glucose metabolism in mental illness compared with controls. Comput. Struct. Biotechnol. J. 2016;14:106–116. doi: 10.1016/j.csbj.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nuzzo T, et al. Decreased free d-aspartate levels are linked to enhanced d-aspartate oxidase activity in the dorsolateral prefrontal cortex of schizophrenia patients. NPJ Schizophr. 2017;3:16. doi: 10.1038/s41537-017-0015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaddurah-Daouk R, et al. Metabolomic mapping of atypical antipsychotic effects in schizophrenia. Mol. Psychiatry. 2007;12:934–945. doi: 10.1038/sj.mp.4002000. [DOI] [PubMed] [Google Scholar]
- 13.Anjum S, Bathla M, Panchal S, Singh GP, Singh M. Metabolic syndrome in drug naïve schizophrenic patients. Diabetes Metab. Syndr. Clin. Res. Rev. 2018;12:135–140. doi: 10.1016/j.dsx.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Liu M-L, et al. Severe disturbance of glucose metabolism in peripheral blood mononuclear cells of schizophrenia patients: a targeted metabolomic study. J. Transl. Med. 2015;13:226. doi: 10.1186/s12967-015-0540-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohan V, Subashini R, Padmavati R, Deepa M, Thara R. Prevalence of diabetes, obesity, and metabolic syndrome in subjects with and without schizophrenia (CURES-104) J. Postgrad. Med. 2011;57:272. doi: 10.4103/0022-3859.90075. [DOI] [PubMed] [Google Scholar]
- 16.Leppik L, et al. Profiling of amino acids and their derivatives biogenic amines before and after antipsychotic treatment in first-episode psychosis. Front. Psychiatry. 2018;9:155. doi: 10.3389/fpsyt.2018.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao B, et al. Characterizing amino-acid biosignatures amongst individuals with schizophrenia: a case-control study. Amino Acids. 2018;50:1013–1023. doi: 10.1007/s00726-018-2579-6. [DOI] [PubMed] [Google Scholar]
- 18.Davison J, O’Gorman A, Brennan L, Cotter DR. A systematic review of metabolite biomarkers of schizophrenia. Schizophr. Res. 2018;195:32–50. doi: 10.1016/j.schres.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 19.Li C, et al. Metabolomics in patients with psychosis: a systematic review. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2018;177:580–588. doi: 10.1002/ajmg.b.32662. [DOI] [PubMed] [Google Scholar]
- 20.Nagai T, et al. Reduced mismatch negativity is associated with increased plasma level of glutamate in first-episode psychosis. Sci. Rep. 2017;7:2258. doi: 10.1038/s41598-017-02267-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gong L, et al. Sumoylation differentially regulates Sp1 to control cell differentiation. Proc. Natl. Acad. Sci. U.S.A. 2014;111:5574–5579. doi: 10.1073/pnas.1315034111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madeira C, et al. Blood levels of glutamate and glutamine in recent onset and chronic schizophrenia. Front. Psychiatry. 2018;9:713. doi: 10.3389/fpsyt.2018.00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohnuma T, et al. Changes in plasma glycine, l-serine, and d-serine levels in patients with schizophrenia as their clinical symptoms improve: results from the Juntendo University Schizophrenia Projects (JUSP) Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2008;32:1905–1912. doi: 10.1016/j.pnpbp.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 24.Zafra F, et al. Glycine transporters and its coupling with NMDA receptors. Adv. Neurobiol. 2017;16:55–83. doi: 10.1007/978-3-319-55769-4_4. [DOI] [PubMed] [Google Scholar]
- 25.Walls AB, Waagepetersen HS, Bak LK, Schousboe A, Sonnewald U. The glutamine–glutamate/GABA cycle: function, regional differences in glutamate and GABA production and effects of interference with GABA metabolism. Neurochem. Res. 2015;40:402–409. doi: 10.1007/s11064-014-1473-1. [DOI] [PubMed] [Google Scholar]
- 26.Cappelletti P, et al. Proline oxidase controls proline, glutamate, and glutamine cellular concentrations in a U87 glioblastoma cell line. PLoS ONE. 2018;13:e0196283. doi: 10.1371/journal.pone.0196283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bjerkenstedt L, et al. Support for limited brain availability of tyrosine in patients with schizophrenia. Int. J. Neuropsychopharmacol. 2005;9:247–255. doi: 10.1017/S1461145705005638. [DOI] [PubMed] [Google Scholar]
- 28.Badawy AA-B. Modulation of tryptophan and serotonin metabolism as a biochemical basis of the behavioral effects of use and withdrawal of androgenic-anabolic steroids and other image- and performance-enhancing agents. Int. J. Tryptophan Res. 2018;11:1178646917753422. doi: 10.1177/1178646917753422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Del-Ben CM, et al. Urbanicity and risk of first-episode psychosis: incidence study in Brazil. Br. J. Psychiatry. 2019;215:726–729. doi: 10.1192/bjp.2019.110. [DOI] [PubMed] [Google Scholar]
- 30.Jongsma HE, et al. Treated incidence of psychotic disorders in the multinational EU-GEI study. JAMA Psychiatry. 2018;75:36. doi: 10.1001/jamapsychiatry.2017.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.European Network of National Networks studying Gene-Environment Interactions in Schizophrenia (EU-GEI) et al. Identifying Gene-environment interactions in Schizophrenia: contemporary challenges for integrated, large-scale investigations. Schizophr. Bull. 2014;40:729–736. doi: 10.1093/schbul/sbu069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loureiro CM, et al. Low plasma concentrations of N -methyl-d-aspartate receptor subunits as a possible biomarker for psychosis. Schizophr. Res. 2018;202:55–63. doi: 10.1016/j.schres.2018.06.037. [DOI] [PubMed] [Google Scholar]
- 33.Del-Ben CM, et al. Confiabilidade da ‘Entrevista Clínica Estruturada para o DSM-IV—Versão Clínica’ traduzida para o português. Rev. Bras. Psiquiatr. 2001;23:156–159. doi: 10.1590/S1516-44462001000300008. [DOI] [Google Scholar]
- 34.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Clinical Version (SCID-CV) New York: Biometrics Research, New York State Psychiatric Institute; 1997. [Google Scholar]
- 35.Fachim HA, et al. GRIN2B promoter methylation deficits in early-onset schizophrenia and its association with cognitive function. Epigenomics. 2019;11:401–410. doi: 10.2217/epi-2018-0127. [DOI] [PubMed] [Google Scholar]
- 36.Corsi-Zuelli F, et al. Cytokine profile in first-episode psychosis, unaffected siblings and community-based controls: the effects of familial liability and childhood maltreatment. Psychol. Med. 2019;50:1139–1147. doi: 10.1017/S0033291719001016. [DOI] [PubMed] [Google Scholar]
- 37.Crippa JA, Sanches RF, Hallak JE, Loureiro SR, Zuardi AW. A structured interview guide increases brief psychiatric rating scale reliability in raters with low clinical experience. Acta Psychiatr. Scand. 2001;103:465–470. doi: 10.1034/j.1600-0447.2001.00185.x. [DOI] [PubMed] [Google Scholar]
- 38.Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol. Rep. 1962;10:799–812. doi: 10.2466/pr0.1962.10.3.799. [DOI] [Google Scholar]
- 39.Singh SP, et al. Determining the chronology and components of psychosis onset: the Nottingham onset schedule (NOS) Schizophr. Res. 2005;80:117–130. doi: 10.1016/j.schres.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 40.Gelpi E, Koenig WA, Gibert J, Oro J. Combined gas chromatography-mass spectrometry of amino acid derivatives. J. Chromatogr. Sci. 1969;7:604–613. doi: 10.1093/chromsci/7.10.604. [DOI] [Google Scholar]
- 41.SAS Institute, Statistical Analysis System Institute (Cary, & NC) SAS Language: Reference: Version 94. Cary: SAS Institute; 2013. [Google Scholar]
- 42.Orešič M, et al. Metabolome in schizophrenia and other psychotic disorders: a general population-based study. Genome Med. 2011;3:19. doi: 10.1186/gm233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parksepp M, et al. Metabolomics approach revealed robust changes in amino acid and biogenic amine signatures in patients with schizophrenia in the early course of the disease. Sci. Rep. 2020;10:13983. doi: 10.1038/s41598-020-71014-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Labaki WW, et al. Serum amino acid concentrations and clinical outcomes in smokers: SPIROMICS metabolomics study. Sci. Rep. 2019;9:1–9. doi: 10.1038/s41598-019-47761-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCullagh P, Nelder JA. Generalized Linear Models. 2. London: Chapman & Hall; 1989. [Google Scholar]
- 46.Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 47.Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front. Psychol. 2013;4:863. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McGraw KO, Seok PW. A common language effect size statistic. Psychol. Bull. 1992;361:111. [Google Scholar]
- 49.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser.894:i-xii, 1–253 (2000). [PubMed]
- 50.Thakkar KN, et al. 7T proton magnetic resonance spectroscopy of gamma-aminobutyric acid, glutamate, and glutamine reveals altered concentrations in patients with schizophrenia and healthy siblings. Biol. Psychiatry. 2017;81:525–535. doi: 10.1016/j.biopsych.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 51.İlhan Atagün M, et al. Perisylvian GABA levels in schizophrenia and bipolar disorder. Neurosci. Lett. 2018;637:70–74. doi: 10.1016/j.neulet.2016.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cai H-LL, et al. Elevated plasma γ-aminobutyrate/glutamate ratio and responses to risperidone antipsychotic treatment in schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2010;34:1273–1278. doi: 10.1016/j.pnpbp.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 53.Arrúe A, et al. GABA and homovanillic acid in the plasma of schizophrenic and bipolar i patients. Neurochem. Res. 2010;35:247–253. doi: 10.1007/s11064-009-0048-z. [DOI] [PubMed] [Google Scholar]
- 54.Wang Y, Liu S, Hu Y, Li P, Wan J-B. Current state of the art of mass spectrometry-based metabolomics studies-a review focusing on wide coverage, high throughput and easy identification. RSC Adv. 2015;5:78728–78737. doi: 10.1039/C5RA14058G. [DOI] [Google Scholar]
- 55.Gaskin PL, Toledo-Rodriguez M, Alexander SP, Fone KC. Down-regulation of hippocampal genes regulating dopaminergic, gabaergic, and glutamatergic function following combined neonatal phencyclidine and post-weaning social isolation of rats as a neurodevelopmental model for schizophrenia. Int. J. Neuropsychopharmacol. 2016;19:062. doi: 10.1093/ijnp/pyw062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat. Rev. Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 57.Cannon TD. How schizophrenia develops: cognitive and brain mechanisms underlying onset of psychosis. Trends Cogn. Sci. 2015;19:744–756. doi: 10.1016/j.tics.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang JJ, et al. Reduced γ-aminobutyric acid and glutamate+glutamine levels in drug-naive patients with first-episode schizophrenia but not in those at ultrahigh risk. Neural Plast. 2016;2016:1–9. doi: 10.1155/2016/3915703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de la Fuente-Sandoval C, et al. Prefrontal and striatal gamma-aminobutyric acid levels and the effect of antipsychotic treatment in first-episode psychosis patients. Biol. Psychiatry. 2018;83:475–483. doi: 10.1016/j.biopsych.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koshiyama D, et al. Electrophysiological evidence for abnormal glutamate-GABA association following psychosis onset. Transl. Psychiatry. 2018;8:211. doi: 10.1038/s41398-018-0261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cohen SM, Tsien RW, Goff DC, Halassa MM. The impact of NMDA receptor hypofunction on GABAergic neurons in the pathophysiology of schizophrenia. Schizophr. Res. 2015;167:98–107. doi: 10.1016/j.schres.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chiapponi C, Piras F, Piras F, Caltagirone C, Spalletta G. GABA system in schizophrenia and mood disorders: a mini review on third-generation imaging studies. Front. Psychiatry. 2016;7:61. doi: 10.3389/fpsyt.2016.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alfredsson G, Wiesel FA. Monoamine metabolites and amino acids in serum from schizophrenic patients before and during sulpiride treatment. Psychopharmacology. 1989;99:322–327. doi: 10.1007/BF00445551. [DOI] [PubMed] [Google Scholar]
- 64.Palomino A, et al. Decreased levels of plasma glutamate in patients with first-episode schizophrenia and bipolar disorder. Schizophr. Res. 2007;95:174–178. doi: 10.1016/j.schres.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 65.Yamamori H, et al. Changes in plasma d-serine, l-serine, and glycine levels in treatment-resistant schizophrenia before and after clozapine treatment. Neurosci. Lett. 2014;582:93–98. doi: 10.1016/j.neulet.2014.08.052. [DOI] [PubMed] [Google Scholar]
- 66.Neeman G, et al. Relation of plasma glycine, serine, and homocysteine levels to schizophrenia symptoms and medication type. Am. J. Psychiatry. 2005;162:1738–1740. doi: 10.1176/appi.ajp.162.9.1738. [DOI] [PubMed] [Google Scholar]
- 67.Sumiyoshi T, et al. Plasma glycine and serine levels in schizophrenia compared to normal controls and major depression: relation to negative symptoms. Int. J. Neuropsychopharmacol. 2004;7:1–8. doi: 10.1017/S1461145703003900. [DOI] [PubMed] [Google Scholar]
- 68.Garip B, Kayir H. Alteration in NMDAR-related amino acids in first episode psychosis. Synapse. 2019;73:22127. doi: 10.1002/syn.22127. [DOI] [PubMed] [Google Scholar]
- 69.Lutkenhoff ES, et al. Proton MRS in twin pairs discordant for schizophrenia. Mol. Psychiatry. 2010;15:308–318. doi: 10.1038/mp.2008.87. [DOI] [PubMed] [Google Scholar]
- 70.Purdon SE, Valiakalayil A, Hanstock CC, Seres P, Tibbo P. Elevated 3T proton MRS glutamate levels associated with poor continuous performance test (CPT-0X) scores and genetic risk for schizophrenia. Schizophr Res. 2008;99:218–224. doi: 10.1016/j.schres.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 71.Włodarczyk A, Wiglusz MS, Cubała WJ. Ketogenic diet for schizophrenia: nutritional approach to antipsychotic treatment. Med. Hypotheses. 2018;118:74–77. doi: 10.1016/j.mehy.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 72.Gracia-Marco L, et al. Amino acids intake and physical fitness among adolescents. Amino Acids. 2017;49:1041–1052. doi: 10.1007/s00726-017-2393-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morris C, et al. The relationship between aerobic fitness level and metabolic profiles in healthy adults. Mol. Nutr. Food Res. 2013;57:1246–1254. doi: 10.1002/mnfr.201200629. [DOI] [PubMed] [Google Scholar]
- 74.De Luca V, et al. Peripheral amino acid levels in schizophrenia and antipsychotic treatment. Psychiatry Investig. 2008;5:203. doi: 10.4306/pi.2008.5.4.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ruddick JP, et al. Tryptophan metabolism in the central nervous system: medical implications. Expert Rev. Mol. Med. 2006;8:1–27. doi: 10.1017/S1462399406000068. [DOI] [PubMed] [Google Scholar]
- 76.Zaragozá R. Transport of amino acids across the blood-brain barrier. Front. Physiol. 2020;11:973. doi: 10.3389/fphys.2020.00973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cho SE, Na KS, Cho SJ, Kang SG. Low D-serine levels in schizophrenia: a systematic review and meta-analysis. Neurosci. Lett. 2016;634:42–51. doi: 10.1016/j.neulet.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 78.Fiehn O, Edu O. Metabolomics by gas chromatography-mass spectrometry: the combination of targeted and untargeted profiling. Curr. Protoc. Mol. Biol. 2016;114:1–32. doi: 10.1002/0471142727.mb3004s114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nair H, Clarke W. Mass Spectrometry for the Clinical Laboratory. 1. Cambridge: Academic Press; 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.