Abstract
AIM:
The aim of this study is to evaluate silk-fibroin electrospun nanofibers and blood-derived fibroblast-like cells for cytotoxicity and cell adhesion.
BACKGROUND:
Silk fibroin (SF) has emerged as a favorable and potential bio-material owing to its unique properties such as biocompatibility, biodegradability, the possibility of functional modifications, mechanical strength, and regenerative capability. Despite current advancements in tissue engineering technologies, delay wound healing and scar formation remain unresolved. Bioequivalent skin graft having human fibroblast and keratinocytes (Apligraft®) has proven to be beneficial, but the cost is a limiting factor.
OBJECTIVE:
The blood born fibroblast-like cells express several growth factors, extracellular matrix proteins, and these factors are crucial in the various steps of the wound-healing process. SF is an idea polymer by the virtue of its multifaceted characteristics such as mechanical strength, biodegradability, improved cell attachment, biocompatibility, good elasticity, having application in biomedical, tissue engineering, and medicine. The objective of the present study is to evaluate SF as a biomaterial for making nanofibers scaffold and culturing blood-derived fibroblast-like cells on it for the potential application to wound site.
MATERIALS AND METHODS:
Blood-derived fibroblast-like cells evaluated for cytotoxicity, collagen 1 expression, and cell adhesion on SF electrospun nanofibers. The silk nanofibers were fabricated by the electrospinning method using silk-derived fibroin solution and analyzed for protein composition, viscosity, and further characterized using the Fourier transformed infrared spectroscopy.
RESULTS:
The SF nanofibers were nontoxic to the blood-derived fibroblast-like cells. It improved cell adhesion with collagen 1 expression.
CONCLUSION:
The composite scaffold of SF nanofibers with blood-derived fibroblast-like cells would be a potential healing patch for many types of wounds.
Keywords: Blood-derived fibroblast-like cells, cell adhesion, cytotoxicity introduction, nanofibers, silk fibroin
Introduction
Silk cocoon composed of sericin, and fibroin, two proteins of a very different nature, and silk fiber is a secretion of these proteins as a continuous strand. These proteins protect and provide security to growing pupae from parasites, predators inside the cocoon. Seventy percent fiber strands form the major component of the firboin and serve as structural support of the cocoon. The fibers made up of two protein subunits having a molecular weight of 370 and 25 kDa in equimolar ratio and linked by disulfide covalent bonds. Sericin is another protein of silk to hold together the fibroin threads and form the closed structure of the cocoon.[1,2] The silk cocoons are made up of three major components, namely fibroin, sericin, and nonsericin, in the range of 60%–80%, 15%–30%, and 1%–5%, respectively.
Silk fibroin (SF) derived films, hydrogels, and scaffolds are used for various biomedical applications.[3] The salient features of SF such as (i) improved biocompatibility with minimal immunogenicity, (ii) ease of functionalities for various cellular interactions, and (iii) mechanical architectural and structural variability, imitating a range of tissue from soft to hard, are appealing and diverse due to the various morphologies of SF.[2,3,4,5,6,7]
Wound healing is a serious issue worldwide associated with the various types of wounds ranging from traumatic injuries, burns, diabetes-induced ulcers/wounds, chronic wounds, and cancer-related wounds. Chronic wounds represent the challenging wound type because these wounds do not follow an orderly healing process due to the several underlying causes. Despite current advancements in therapeutic strategies, delay wound healing, and scar formation, remain unresolved. Wound healing is a complex, dynamic process that is achieved through four coordinated and overlapping phases such as (i) hemostasis, (ii) inflammatory, (iii) proliferative, and (iv) remodeling.[8,9]
Wound healing is a multifactorial, multidimensional, complicated, physiological process involving cellular, molecular, and enzymatic pathways.[10] The use of synthetic and natural polymers scaffold for carrying cells or growth factors or other molecules have investigated extensively as wound dressings. Some natural polymers have excellent structural and physicochemical properties making them suitable material for different wound dressing applications. A variety of neutral polymers such as cellulose or basic polymer chitosan or acidic and sulfated polysaccharide polymers have been explored and utilized in biomedical/wound care applications.[11,12,13,14]
The dermal substitute like Biobrane® (Dow/Hickam Bertek, Sugar Land Pharmaceutics, TX, USA) made of nylon mesh with collagen fiber as a wound dressing. Apligraf® (Organogenesis Inc. Canton, MA, Novartis Pharmaceuticals, Corporation, NJ) is a bilayer bioengineered skin substitute composed of nylon mesh with collagen fiber seeded with neonatal keratinocytes and fibroblast for the treatment of foot ulcer and venous leg ulcers in diabetic patients. Similarly, Transcyte® (Advance Tissue Sciences, California, USA) is another commercial product available for burn wounds, made up of nylon mesh and collagen fibers with neonatal fibroblast. However, the cost, availability of donor tissue, and immune rejection are the limiting factors for the acceptance of this technology at a large scale.
Cell-based therapies for wound healing are gaining momentum in recent times. These therapies aim at repairing and replacing the damaged tissue with the release of various factors required for the wound-healing process. For example, adipose tissue or bone marrow-derived mesenchymal stem cells have been investigated in the experimental wound-healing models and found to be a promising therapy.[15,16]
The recruitment of blood-derived immunocompetent cells at the site of the wound is a common phenomenon during the inflammatory phase of wound healing and had been documented in the several models of tissue injury. Badiavas et al.[17,18] and group used green fluorescent protein (GFP) tagged bone marrow transplanted mice for wound-healing model and found the GFP positive cells differentiated toward various lineages. Similarly, Fathke et al. observed the presence of dermal fibroblast, differentiated from bone marrow progenitor cells, in cutaneous wounds.[19] These results suggest a major role of stem cells in the wound-healing process. Blood-derived circulating fibrocytes at the site of wound healing have been demonstrated earlier.[20]
In the present investigation, we have characterized the SF nanofibers and evaluated the suitability of silk nanofibers for the culturing of blood-derived fibroblast-like cells. The blood-derived fibroblast-like cells were stained for collagen1and found to express collagen1 at the protein level. The viability of blood-derived fibroblast-like cells was analyzed, and it did not show any cytotoxicity or cell death. The cell adhesion was significantly improved on silk nanofibers, as compared to the control surface without any cytotoxicity.
Materials and Methods
Isolation of silk fibroin
Silk purification
We purchased Bombyx mori cocoons (moisture content 6%–7% v/w) from Bharatiya Agro Industries Foundation, Development Research Foundation, Urulikanchan, Pune, Maharashtra. The silk fibroin isolation was carried out as described earlier.[21]
We cut the dried cocoons with scissors into 2–3 cm small squares and weighed 5 g of it for further processing. The sodium carbonate solution was made by weighing 4.24 g of sodium carbonate and dissolved it in 2 L of demineralized water with heating to get a clear solution. The cut, dried cocoon pieces were added to this solution and continued boiling for 30 min. The sodium carbonate solution discarded and transferred the degummed silk fibers in a beaker containing 1 L demineralized water. Afterward, the fibers cooled down to the room temperature and thoroughly rinsed 4–5 times with demineralized water to get rid of water-soluble sericin. At the end of the last rinse, silk fibers were taken out and squeezed to remove excess water. Degummed silk fibers were air-dried on the bench for at least 12 h for further processing.
Silk-based electrospun fibers fabricated as described by using two solvents.
Solubilization in formic acid containing calcium chloride
The degummed silk fibers directly dissolved in formic acid having calcium chloride (2%) to get the concentration of 8%–10% w/v of SF.
Solubilization in lithium bromide
The degummed silk fibers solubilized in lithium bromide (LiBr). Briefly, solution of the LiBr having 9.3 M concentration was poured on the top of the fibers and covered the beaker with the aluminum foil. The beaker was then placed in the oven at 50°–70°C, and silk fibers were dissolved completely for up to 4 h. The Silk-LiBr solution, thus obtained, was viscous, yellowish, and having honey-like consistency and appearance.
The resultant SF solution further subjected to the dialysis. The dialysis membrane with the molecular weight cutoff value of 12000–14000 was purchased from Himedia Pvt. Ltd., India. The dialysis membrane was placed in the water for 2–3 min for wetting. The SF solution in the dialysis bag was placed in the beaker containing 1 L of demineralized water. The water was changed after 1, 3, and 5 h on the 1st day followed by two water changes on a subsequent day. On the 3rd day, water was replaced with the fresh water for one time. A total of six numbers of water changes were done for 3 days.
After dialysis, a SF solution removed from the dialysis bag and centrifuged for 20 min at 4°C–6°C at 4000 rpm. The supernatant SF solution then transferred into a new clean centrifuge tube. The same step repeated, and after the second centrifugation, the silk solution collected in a new, clean tube. The silk solution obtained was clear yellowish liquid.
Viscosity measurement
Viscosity was measured by Brookfield viscometer (Viscometer model DV II + Pro, USA). First, the viscometer was calibrated using dill oil having specific viscosity. The consistency of dill oil was similar to the SF solution prepared in the LiBr solution. Three readings were taken for standard and compared with the specified value of dill oil. Based on the calibration study, the spindle number and rotation per minute (rpm) were selected for the viscosity measurement of the SF solution.
Protein estimation
Bradford protein assay was used to estimate the fibroin protein concentration.[22] Bovine serum albumin at five different concentrations was prepared separately in five test tubes. Then, Bradford reagent added in each tube mixed and kept aside for 5 min at 30°C. Similarly, the fibroin solution was taken in separate test tubes, mixed with Bradford reagent and kept aside along with BSA standard tubes. After 5 min of incubation, the absorbance of the solution of each test tube was measured at 595 nm.
The standard graph of bovine serum albumin plotted and the concentration of the fibroin solution estimated from the standard BSA graph.
Fourier-transform infrared spectroscopy
Fourier transforme infrared (FTIR) spectra of regenerated SF were obtained on the FTIR Spectrophotometer (Shimadzu, Japan) in reflection mode with a resolution of 2 cm−1. The SF solution was dried completely to get a thin film at 25°C. The film was crushed with potassium bromide. The mixture was placed in the die and kept under pressure into the QwikHandi Press to get a thin, transparent pellet for FTIR assessment.
Electrospinning
Silk-based electrospun fibers fabricated as described earlier[23,24] at Bharti Vidyapeeth, Department of Mechanical engineering, Pune, using ESPIN-NANO instrument. The electrospinning parameters like the concentration and the viscosity of the solution, the type of solvents used, flow rate, electrical potential, hypodermic syringe needle gauge, and its angle were optimized for fabrication of SF nanofibers. SF in formic acid (98%), and SF in LiBr (9.3 M) solution were prepared to get a concentration of 8% (w/v). The solution used for electrospinning was having 8% w/v SF concentration. Before electrospinning, to avoid any lumps and to get a clear, lump-free solution, the SF solution stirred continuously for 1 h at the room temperature, then filled into a 2.5 ml plastic syringe (18 G) and subjected to electrospinning. The solution was extruded at a rate of 0.3–0.5 ml/h from a distance of 8–12 cm from the collector. The collector speed of 200–2500 rpm and a spinning voltage of 18 (kV) were selected to fabricate the electrospun nanofibers.
Scanning electron microscopy
The nano silk fibers observed for the morphological features with a scanning electron microscopy (SEM) (Carl Zeiss, supra5, Germany) at Diya labs, Mumbai. The silk nanofibers air-dried overnight and transferred to SEM sample holder with the help of carbon tape. The nanofibers on the sample holder were coated with 20 nm thick platinum layer under vacuum. The coated nanofibers were observed and scanned at a voltage of 15 kV and room temperature.
Isolation of blood-born fibroblast-like cells
All healthy humans participated in studies voluntarily. They were informed about the research protocol, and research project and taken their written consent as per the local institutional ethical guidelines. Heparinized human blood (10 ml) collected from the donors. The heparinized blood was layered on the 5 ml of density gradient solution (Histopaque, Himedia Laboratories Pvt. Ltd., Mumbai, Maharashtra), and centrifuge at 1800 rpm for 30 min at 18°C.
The isolated cell then cultured in Dulbecco's Modified Eagle Medium containing 10% fetal calf serum, mixture of penicillin, and streptomycin at 10 U/μl and 10 μg/μl, respectively, as per method given in earlier report.[25]
Immunocytochemistry for collagen1 expression
The method was carried out as described earlier.[25] The 5 min fixation of cultured cells was carried out with frozen (−200°C) acetone: methanol (1:1 v/v) mixture at the room temperature. To block the nonspecific binding sites, fixed cells were incubated for 1 h with blocking solution prepared using 3% bovine serum solution and goat serum in 1:1 (v/v) ratio. The pan myeloid surface marker CD45 was detected using antibody CD45 (Chemicaon International). The enzyme, prolyl-4-hydroxylase (PH 4B), was detected with antibody prolyl 4-hydroxylase (DAKO, Hamburg, Germany). For intracellular collagen 1, cells were stained with antibody collagen 1 (Biodesign, New York, NY). All primary antibodies were used in the 1:100 dilution for staining. Then cells were stained with respective fluorochrome-conjugated secondary antibodies. Finally, cells were stained with nuclear dye, TO-PRO-3 iodide (Molecular Probes). The respective IgG served as isotype controls. Images were captured using a confocal microscope (Leica, Wetzler, Germany).
Cell adhesion assay on electrospun silk fibroin nanofibers
The isolated peripheral blood mononuclear cells (PBMC) were cultured on silk nanofibers for 7 days. The suspended PBMCs eliminated by aspiration and the viability of adherent cells evaluated on day 7th with trypan blue.
In addition, the adherent cells were fixed with acetone: methanol mixture and stained with hematoxylin and eosin stain (Himedia, Mumbai) and counted under × 100 oil immersion objective lens.
Statistics
Statistical analysis was performed with the help of statistical software, Graph Pad Prism statistical program version 7.04. (GraphPAd Software for windows, CA, USA). The data with more than two groups were analyzed using the two-way ANOVA statistical method. The P < 0.05 was considered statistically significant. The bar graph was generated using GraphPad Prism. The data are presented as means and standard deviations. In the figure, error bars indicate standard deviation.
Results and Discussion
The silk cocoons dried after heating in sodium carbonate solutions, and degummed silk fibers weighed for evaluating the percentage weight loss that indicates the sericin protein of silk cocoons.
Sixty-to-eighty percent of fibroin, 15%–35% sericin, and 1%–5% nonsericin make up the silk cocoon. Sericin is the outer water-soluble layer of the cocoon that protects the pupae from the external environment. The weight loss after degumming was found to be 29.02% [Table 1]. The percent weight loss represents the water-soluble sericine along with other matters. The SF found to be 70.98%, as denoted in Table 1, which is in line with reported data about the composition of the silk cocoon.[1]
Table 1.
Characteristics of silk fibroin isolated from the silk cocoon
| Properties | Mean±SD (n=6) |
|---|---|
| Silk fibroin (%) | 70.98±2.3 |
| Silk sericin (%) | 29.02±2.31 |
| Viscosity (cp) of silk firboin (8% w/v) | 11.17±0.69 |
| Protein concentration of silk fibroin solution (8% w/v) (mg/ml) | 1.17±0.15 |
SD: Standard deviation
The SF solution was prepared using two solvents, such as formic acid, and LiBr. The degummed silk fibers did not completely dissolve in formic acid to get 8%–10% w/v concentration. Different concentrations of calcium chloride were evaluated to facilitate the complete dissolution of degummed silk fibers in the formic acid. The formic acid with 2% w/v calcium chloride found to be optimum for nanofiber fabrication.
The second solvent used for SF solution was LiBr (9.3M) followed by the dialysis. The dissolution of degummed silk fibers was carried out at different temperatures (50°C–70°C). The optimum temperature for the complete dissolution of degummed silk fibres found to be 70°C giving a clear solution.
As the presence of LiBr is not desirable for the final application of silk nanofibers, extensive dialysis was done to remove the traces of LiBr from solubilized SF. There was an increase in SF volume postdialysis resulting in the SF solution having 10%–11% w/v concentration.
Viscosity
The viscosity of SF solution (8% w/v) found to be 11–12 cp (~ 0.012 Pascals), as shown in Table 1. The rheological studies with the same concentration of silk solution reported having approximately 10 cp of viscosity. When the silk solutions stored at the room temperature or 4°C, the silk solution transformed to the viscous solution followed by conversion to the gel state. The gelation time found to be 3–4 days at the room temperature as well as 4°C. After 3–4 days of gelation, the silk solution gets converted to a liquid state with low viscosity, indicating a sol-gel-sol property of SF.
Protein estimation
There was an increase in protein concentration, as the temperature increased from 50°C to 70°C, and at 70°C, it remained constant, i.e., 1.1 mg/ml. As the temperature increases, a disulfide bond between the heavy and light chains breaks, releasing protein. The concentration of protein of SF solution (8% w/v) was found to be 1.17 mg/ml, as shown in Table 1.
Fourier transformed infrared of silk fibroin
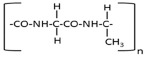
Degummed SF was further characterized using FTIR spectroscopy. The SF crystalline structure of SF extended/three chains alternate with –H and –CH3 groups, i.e., a co-polymer repeat of alanine and glycine as shown below.
The larger residues of co-polymer like tyrosine are associated with a non-crystalline part of SF. FTIR is a useful tool to study and characterize the secondary structure of degummed fibers. The absorptions bands observed [Figure 1] represent the functional groups of SF molecules. The peaks in the range of 1698 cm−1 and 1622 cm−1 represented as Amide I absorption reveals C = O stretching and β-sheet. These peaks indicate the beta-sheet crystallinity. The absorption peak observed at 1514 cm−1 indicates CN stretching, NH bending and β-sheet which is shown as Amide II. Amide III bands between 1258 cm−1 indicates the secondary structure having α-helix/random coils and bands seen at 1229 cm−1 shows β-sheet. A strong and intense amide A band demonstrating NH bending seen at 3282 cm−1 (β-sheet).
Figure 1.
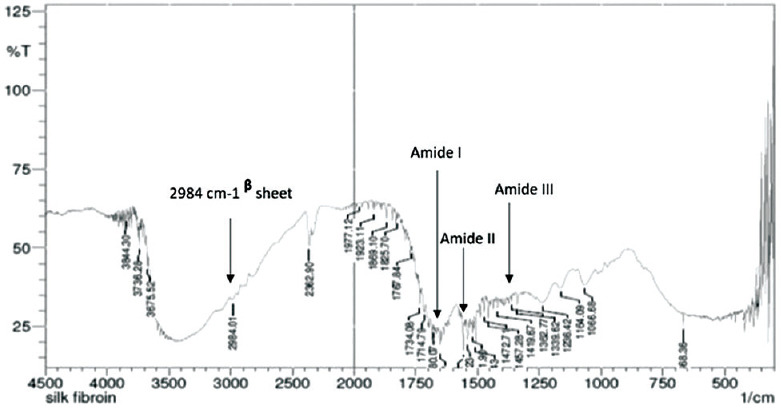
Fourier transform infrared spectroscopy spectra of silk fibroin
Scanning electron microscopy
The nanofibers obtained from SF solution made with LiBr were without aggregates, compared to the SF solution prepared in formic acid, as shown in Figure 2a and b. The SF solution prepared with LiBr exhibited smooth fine, uniform size fibers having approximately 300 nm of diameter [Figure 2a and b]. The nanofibers obtained from both solutions were of different morphology even though both solutions have 8% w/v SF concentration, as shown in Figure 2a-d. The plausible explanation could be the poor conductivity of the solution. The nanofibers derived from SF solution prepared in formic acid plus 2% w/v calcium chloride showed the presence of aggregates [Figure 2c and d]. Our results are in line with the findings of Zhou et al. and Han et al. studies.[26,27]
Figure 2.
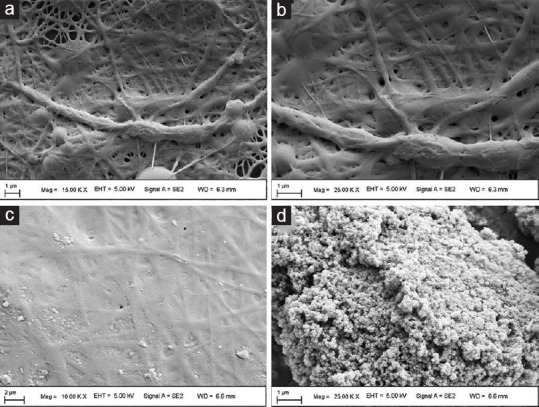
Scanning electron microscopy of silk fibroin fibers. (a) and (b) nanofibers prepared using lithium bromide; (c) and (d) nanofibers prepared using formic acid- CaCl2
The biomaterial used for culturing endogenous cells should possess the supporting capability, bioactive, biodegradability, and the most important one, the ability to modulate cell behavior and cell functionality.[28] The first step for cells cultured on the engineered scaffold is the establishment of cell-focal adhesion, followed by their proliferation, differentiation, and growth. The extracellular matrix (ECM) that supports endogenous cells is made up of different fibrous proteins. The collagen constitutes 30% of ECM and is associated with other fibrous proteins like fibronectin, elastin, and vimentin. The diameter of collagen, present in different tissue, has a wide range of sizes, for example, collagen fibrils present in the skin have a diameter of 30–300 nm.
The wound-healing process involves the number of cell types primarily being fibroblasts, and these are the major cell type that secret collagen fibers. We have optimized our electrospinning process to get the fibers of approximately 300 nm in diameter. We did not investigate the impact of varied dimensions of nanofibers on cell adhesion.
The nanofibers dimension, orientation, pore size and their impact on cell behavior, functionality for the wound-healing process are desired in further studies
Culturing of blood-derived fibroblast cells on the silk nanofibers
The blood-derived fibroblast-like cells expressed collagen1, and the enzyme PH4B essential in collagen synthesis, as shown in Figure 3a. PBMC seeded on the SF nanofibers using the fibroblasts cell culture media. We did not observe any cell toxicity or cell death for up to 7 days. The number of adherent cells was quantified in six different fields of view by randomly counting the cells under a ×100 objective lens. Cell adhesion to the nanofibers was significantly enhanced, in comparison to the control surface, as shown in Figure 3b.
Figure 3.
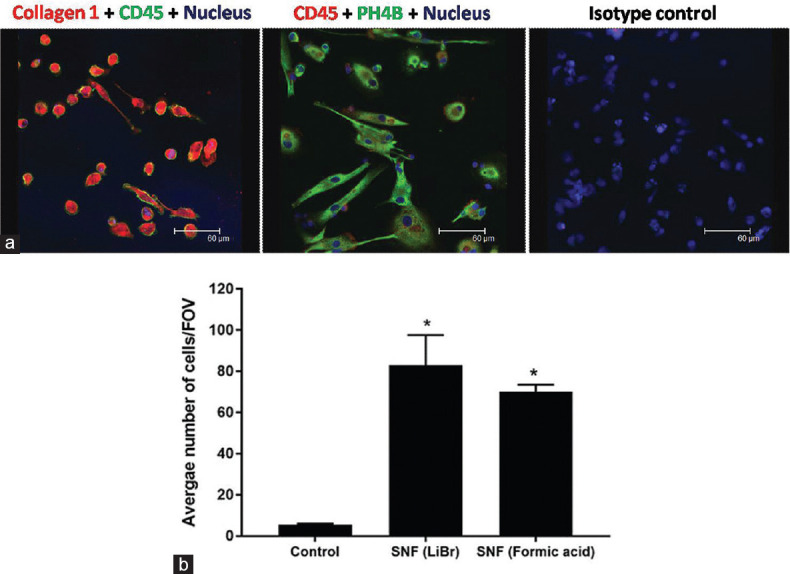
The blood-derived fibroblast-like cells evaluated for the expression of collagen 1 and cell adhesion. (a) The blood-derived fibroblast-like cells expressed collagen 1 and prolyl-4-hydroxylase showing spindle-shaped morphology. (b) The cell adhesion was significantly increased on silk fibroin electrospun nanofibers. (*P < 0.05 significant vs. control; n = 6)
There was a 40-fold increase in adherent cells on silk nanofibers (either made in formic acid or LiBr), compared to control (without nanofibers culture surface), as depicted in Figure 3b.
The cell adhesion on silk nanofibers prepared with LiBr was slightly high as compared to nanofibers derived from SF dissolved in formic acid.
Conclusion
Silk, as a biopolymer, is considered to be one of the ideal biopolymers having distinct features such as biocompatibility, biodegradability, excellent mechanical properties, elasticity, and wound-healing property.
The blood born fibroblast-like cells get recruited at the site of the wound and participate in the multiple steps of wound healing. The application of the wound dressings with live cells had demonstrated to augment the wound-healing capacity, especially in chronic wounds or delay wound-healing processes associated with diseases/disorders.
The blood-derived fibroblast-like cells have many advantages, like availability, ease of isolation, not so painful method of blood withdrawal, quantity, the possibility of autologous cells, and the expression of various markers useful for wound-healing application.
Impaired wound healing is a clinical problem in several disorders/diseases, and one of the prominent beings is diabetes mellitus. Wound healing is slow in the aged population as well as chronic and deep wounds.
The commercial products for wound care and management have proven to be beneficial. However, the cost, availability of donor tissue, and immune rejection (xenograft or allograft tissue) are the limiting factors for the acceptance of this technology at a large scale.
We did not observe cytotoxicity with silk nanofibers, and cell adhesion was significantly increased on nanofibers as compared to control. Our present data primarily suggest the suitability of “the silk nanofibers – blood-born fibroblast-like cells” for wound healing application.
However, further work on the evaluation of wound-healing activity in the experimental models for the composite is warranted.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Qi Y, Wang H, Wei K, Yang Y, Zheng RY, Kim IS, et al. A reviewof structure construction of silk fibroin biomaterials from singlestructures to multi-level structures. Int J Mol Sci. 2017;18:237. doi: 10.3390/ijms18030237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holland C, Numata K, Rnjak-Kovacina J, Seib FP. The biomedical use of silk: Past, present, future. Adv Healthc Mater. 2019;8:e1800465. doi: 10.1002/adhm.201800465. [DOI] [PubMed] [Google Scholar]
- 3.Zhang H, Li LL, Dai FY, Zhang HH, Ni B, Zhou W, et al. Preparation and characterization of silk fibroin as a biomaterial with potential for drug delivery. J Transl Med. 2012;10:117. doi: 10.1186/1479-5876-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jao D, Mou X, Hu X. Tissue regeneration: A silk road. J Funct Biomater. 2016;7:22. doi: 10.3390/jfb7030022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawrence BD, Marchant JK, Pindrus MA, Omenetto FG, Kaplan DL. Silk film biomaterials for cornea tissue engineering. Biomaterials. 2009;30:1299–308. doi: 10.1016/j.biomaterials.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li C, Vepari C, Jin HJ, Kim HJ, Kaplan DL. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials. 2006;27:3115–24. doi: 10.1016/j.biomaterials.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Yucel T, Lu Q, Hu X, Kaplan DL. Silk nanospheres and microspheres from silk/pva blend films for drug delivery. Biomaterials. 2010;31:1025–35. doi: 10.1016/j.biomaterials.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin P. Wound healing – Aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 9.Shaw TJ, Martin P. Wound repair at a glance. J Cell Sci. 2009;122:3209–13. doi: 10.1242/jcs.031187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: Molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514–25. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 11.Ahsan SM, Thomas M, Reddy KK, Sooraparaju SG, Asthana A, Bhatnagar I. Chitosan as biomaterial in drug delivery and tissue engineering. Int J Biol Macromol. 2018;110:97–109. doi: 10.1016/j.ijbiomac.2017.08.140. [DOI] [PubMed] [Google Scholar]
- 12.Saeidi N, Sander EA, Zareian R, Ruberti JW. Production of highly aligned collagen lamellae by combining shear force and thin film confinement. Acta Biomater. 2011;7:2437–47. doi: 10.1016/j.actbio.2011.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weigel PH, Schnaar RL, Roseman S, Lee YC. Preparation of polyacrylamide gels containing copolymerized omega-acrylamidoalkyl glycosides. Methods Enzymol. 1982;83:294–9. doi: 10.1016/0076-6879(82)83023-1. [DOI] [PubMed] [Google Scholar]
- 14.Silva TH, Alves A, Popa EG, Reys LL, Gomes ME, Sousa RA, et al. Marine algae sulfated polysaccharides for tissue engineering and drug delivery approaches. Biomatter. 2012;2:278–89. doi: 10.4161/biom.22947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu D, Chen B, Liang Z, Deng W, Jiang Y, Li S, et al. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: A double-blind, randomized, controlled trial. Diabetes Res Clin Pract. 2011;92:26–36. doi: 10.1016/j.diabres.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012;21:2724–52. doi: 10.1089/scd.2011.0722. [DOI] [PubMed] [Google Scholar]
- 17.Badiavas EV, Abedi M, Butmarc J, Falanga V, Quesenberry P. Participation of bone marrow derived cells in cutaneous wound healing. J Cell Physiol. 2003;196:245–50. doi: 10.1002/jcp.10260. [DOI] [PubMed] [Google Scholar]
- 18.Badiavas EV, Falanga V. Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol. 2003;139:510–6. doi: 10.1001/archderm.139.4.510. [DOI] [PubMed] [Google Scholar]
- 19.Fathke C, Wilson L, Hutter J, Kapoor V, Smith A, Hocking A, et al. Contribution of bone marrow-derived cells to skin: Collagen deposition and wound repair. Stem Cells. 2004;22:812–22. doi: 10.1634/stemcells.22-5-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: Differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–62. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 21.Rockwood DN, Preda RC, Yücel T, Wang X, Lovett ML, Kaplan DL. Materials fabrication from Bombyx mori silk fibroin. Nat Protoc. 2011;6:1612–31. doi: 10.1038/nprot.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 23.Park BK, Um IC. Effect of molecular weight on electro-spinning performance of regenerated silk. Int J Biol Macromol. 2018;106:1166–72. doi: 10.1016/j.ijbiomac.2017.08.115. [DOI] [PubMed] [Google Scholar]
- 24.Pignatelli C, Perotto G, Nardini M, Cancedda R, Mastrogiacomo M, Athanassiou A. Electrospun silk fibroin fibers for storage and controlled release of human platelet lysate. Acta Biomater. 2018;73:365–76. doi: 10.1016/j.actbio.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 25.Nikam VS, Wecker G, Schermuly R, Rapp U, Szelepusa K, Seeger W, et al. Treprostinil inhibits the adhesion and differentiation of fibrocytes via the cyclic adenosine monophosphate-dependent and Ras-proximate protein-dependent inactivation of extracellular regulated kinase. Am J Respir Cell Mol Biol. 2011;45:692–703. doi: 10.1165/rcmb.2010-0240OC. [DOI] [PubMed] [Google Scholar]
- 26.Zhou W, He J, Cui S, Gao W. Studies of electrospun cellulose acetate nanofibrous membranes open mater. Sci J. 2011;5:51. [Google Scholar]
- 27.Han SO, Youk JH, Min KD, Kang YO, Park WH. Electrospinning ofcellulose acetate nanofibers using a mixed solvent of acetic acid/water: Effects of solvent composition on the fiber diameter. Mater Lett. 2007;62:759–62. [Google Scholar]
- 28.Miyoshi H, Adachi T. Topography design concept of a tissue engineering scaffold for controlling cell function and fate through actin cytoskeletal modulation. Tissue Eng Part B Rev. 2014;20:609–7. doi: 10.1089/ten.teb.2013.0728. [DOI] [PMC free article] [PubMed] [Google Scholar]


