Abstract
Live-attenuated rubella vaccine strain RA27/3 has been demonstrated to be safe and immunogenic in millions of children. The vaccine strain was used to insert SIV gag sequences and the resulting rubella vectors were tested in rhesus macaques alone and together with SIV gag DNA in different vaccine prime-boost combinations. We previously reported that such rubella vectors induce robust and durable SIV-specific humoral immune responses in macaques. Here, we report that recombinant rubella vectors elicit robust de novo SIV-specific cellular immune responses detectable for >10 months even after a single vaccination. The antigen-specific responses induced by the rubella vector include central and effector memory CD4+ and CD8+ T cells with cytotoxic potential. Rubella vectors can be administered repeatedly even after vaccination with the rubella vaccine strain RA27/3. Vaccine regimens including rubella vector and SIV gag DNA in different prime-boost combinations resulted in robust long-lasting cellular responses with significant increase of cellular responses upon boost. Rubella vectors provide a potent platform for inducing HIV-specific immunity that can be combined with DNA in a prime-boost regimen to elicit durable cellular immunity.
Keywords: Live attenuated viral vector, Rubella vaccine strain RA27/3, rhesus macaque, SIV Gag, de novo cellular responses, recall cellular responses, memory T cells, cytotoxic T cells, DNA vaccine, CM9 tetramer response, IFN-γ response, HIV vaccine, DNA prime rubella boost, rubella prime boost
1. Introduction
Several viral vectors are being pursued as potential vehicles to express HIV antigens including CMV, measles, mumps, rubella, adenovirus, vaccinia, yellow fever, VSV and varicella [1–26]. The live-attenuated rubella vaccine strain RA27/3 has a proven track record for safety and immunogenicity efficacy [27–29] with a single dose shown to induce humoral immunity and life-long protection against rubella infection. Rubella vaccine can also boost previously immunized persons, even in the presence of pre-existing rubella immunity [30]. The generation of rubella vectors carrying HIV genes has the potential to elicit durable immunity. The challenging task is the generation of viable recombinant rubella carrying immunogens of the required length. We previously demonstrated that rubella vaccine strain RA27/3 [31] can accommodate insertions up to ~300 amino acids of SIV Gag at the structural insertion site located between the envelope E2 and E1 [18, 21]. Gene expression at this site is controlled by the strong subgenomic promoter, which assures efficient expression of the insert.
Using recombinant rubella vectors in macaques, we reported the induction of robust SIV/HIV-specific humoral responses [21], which could be boosted upon re-exposure to the vector, indicating the development of memory B cells. Different prime-boost combinations including recombinant rubella vectors and DNA elicited even higher antibody responses with extended longevity (>9 months), indicating that the rubella-induced responses could be further augmented.
DNA as vaccine platform has several advantages related to its simplicity, scalability, and possibility of repeated applications due to lack of immunity against the vector (reviewed in [32]). HIV/SIV DNA vaccines administered by the intramuscular route (IM) followed by in vivo electroporation (EP) were shown to induce high cellular and humoral immune responses that persisted for >5 years after the last vaccination [33–35]. The potency of these cellular immune responses was demonstrated by their ability to greatly reduce viremia in macaques infected with pathogenic SIV or SHIV, in both preventive and therapeutic vaccine studies [32]. Humoral responses could be significantly augmented by combining DNA vaccine with different boosts (protein, recombinant viral vectors) [32].
In this report, we examined the Gag-specific cellular immune responses from macaques vaccinated with rubella vectors or with DNA and rubella vectors in different prime-boost combinations. We focused on Gag as antigen, because Gag-specific T cell responses were reported to correlate with control of viremia in infected individuals [36–39] and such responses are expected to reduce viremia in both preventive and therapeutic vaccination protocols.
2. Methods
2.1. Cellular immune response analysis in vaccinated macaques
Macaques were sequentially vaccinated via the IM route [19] with rubella vectors expressing four T cell epitopes (GY9, TE15, CM9 and ME11; vector type 3) or p27gag and part of p2gag (amino acids 135–391 of Gag; vector type 3*) of SIVmac239 Gag [19] and SIV gag DNAs [40]. Gag-specific cellular immune responses were measured at the day of vaccination and indicated time points thereafter using a Gag peptide pool [33] and Gag181–189 CM9 tetramer [41] in MamuA*01+ macaques as detailed in Supplementary Methods.
3. Results
3.1. Sequential vaccination regimens using recombinant rubella vectors and DNA
We tested the ability of recombinant rubella vectors to induce Gag-specific cellular immune responses in macaques. Figure 1 shows the vaccination regimens, which included rubella vectors and DNA expressing SIV gag in different prime-boost regimens. Rubella vector type 3 and type 3* differ by the size of the gag insert, comprising 4 epitopes located in the p19gag and p27gag regions (type 3) or the complete p27gag and p2gag (type 3*), and both elicited antibodies against Rubella and SIV Gag in vaccinated macaques [19]. Some of the macaques were recycled from a previous study where they received the rubella vaccine strain RA27/3 (rubella vaccine) or rubella vectors types 1 and 2 which did not replicate in vivo or show a vaccine “take” and did not develop humoral [19] or cellular immunity to Gag (not shown).
FIGURE 1. Vaccination protocols.
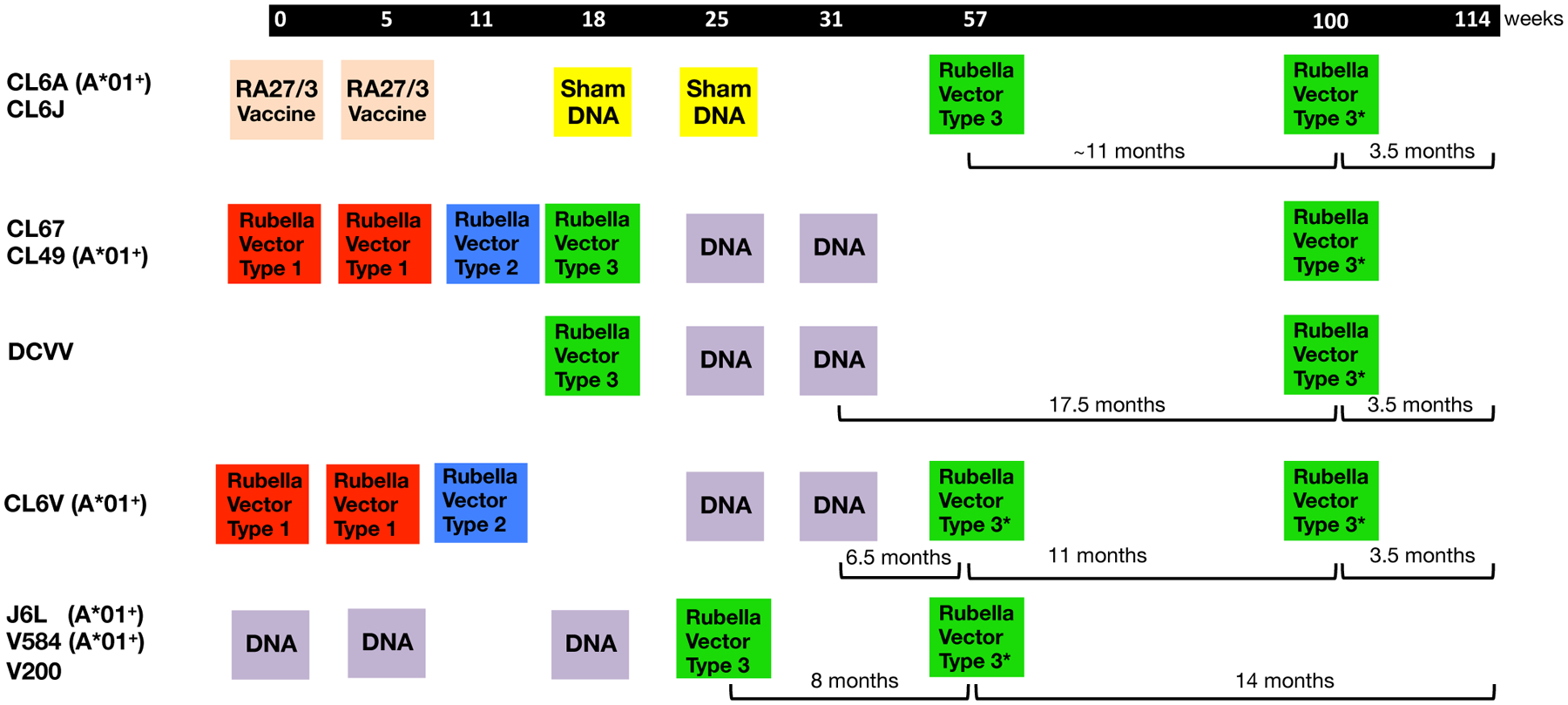
The cartoon is an adaptation from Virnik et al. [19] and details the vaccination timeline used for this Indian rhesus macaque study. Animals CL6A and CL6J had received RA27/3 rubella vaccine strains and sham DNA. Macaques CL67, CL49, CL6V had received rubella type 1 and type 2 which were “no takes” and did not induce antibodies to rubella and also did not induce Gag-specific T cell immunity. The animals received sequential vaccinations with DNA and rubella vector type 3 or type 3*, referred to as rubella vector herein, expressing SIV Gag. The animals were analyzed for Gag-specific IFN-γ+ T cell responses, and macaques positive for the MamuA*01 haplotype were also analyzed for Gag CM9 tetramer responses.
This report focuses on the induction and characterization of cellular immune responses addressing the following questions: (i) Can rubella vector vaccination induce de novo Gag-specific cellular immune responses (5 animals: CL6A, CL6J, DCVV, CL67, CL49)? (ii) Can DNA vaccination boost pre-existing rubella vector induced responses (3 animals: CL67, CL49, DCVV)? (iii) Can rubella vector vaccination boost pre-existing DNA induced responses (7 animals: CL67, CL49, DCVV, CL6V, J6L, V584, V200)? (iv) Can a 2nd rubella vector vaccination boost responses after rubella vector priming (all 9 animals: CL6A, CL6J, CL67, CL49, DCVV, CL6V, J6L, V584, V200)?
3.2. Vaccination with rubella vectors induces Gag-specific T cell responses in macaques
Two macaques (CL6A, CL6J), previously vaccinated with the live-attenuated rubella vaccine strain RA27/3, were immunized one year later, when the anti-rubella antibody titer had slightly declined, with the rubella vector expressing SIV Gag. A single vaccination with rubella vector was able to induce Gag-specific cellular responses in both macaques, reaching up to ~0.2% IFN-γ+ T cells (Figure 2A). In CL6A, the responses were mediated by similar levels of CD4+ and CD8+ T cells, while CL6J showed a primarily CD4+ T cell response. The MamuA*01+ CL6A also showed a robust CM9-specific response, reaching ~2.9% of CD8+ T cells (Figure 2B, upper panels).
FIGURE 2. Recombinant rubella vector vaccination induces de novo Gag-specific responses in macaques.
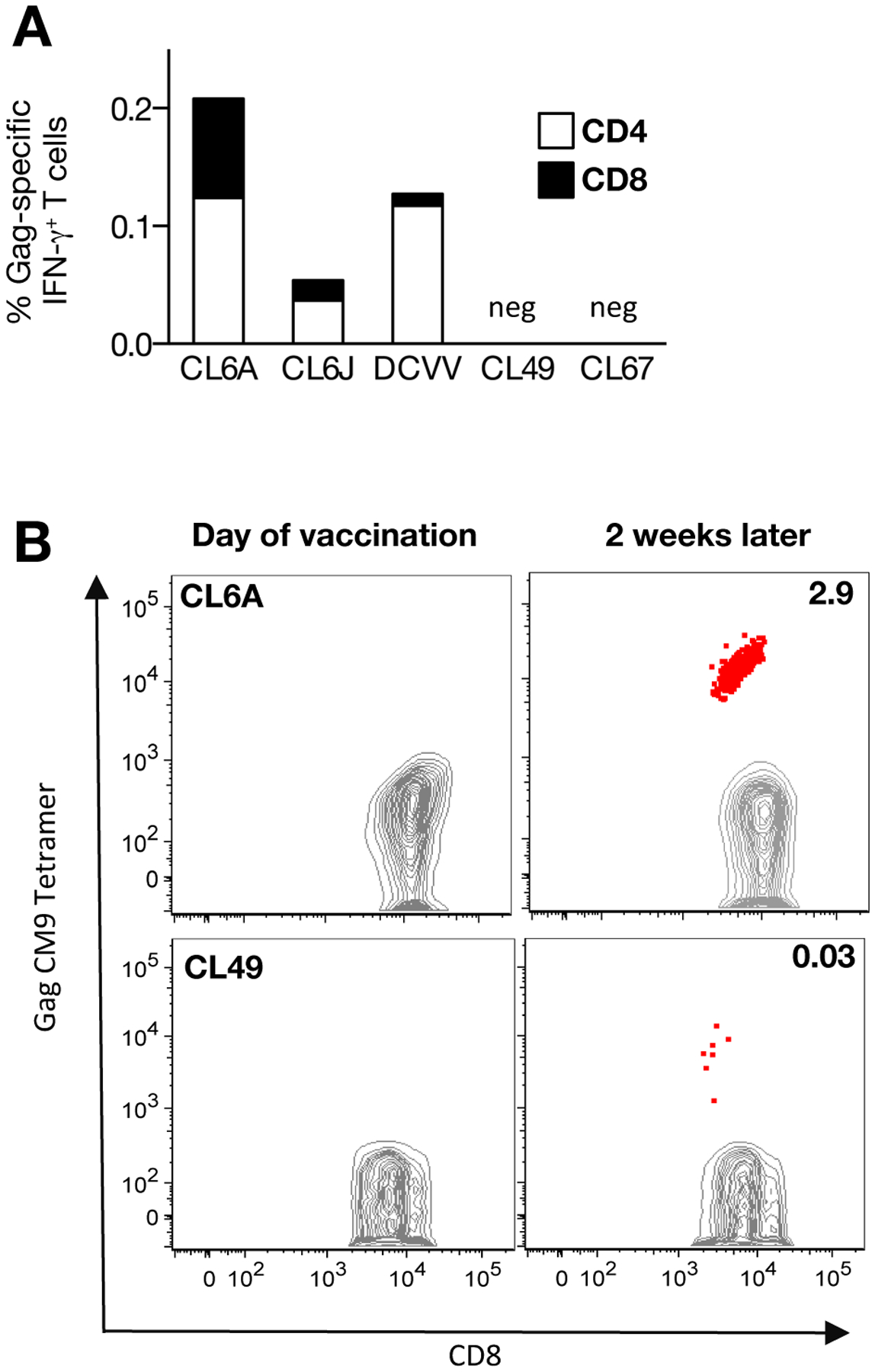
(A) CD4+ and CD8+ Gag-specific IFN-γ+ T cell responses measured 2 weeks after vaccination with the rubella vector in five animals (CL6A, CL6J, DCVV, CL49, CL67). (B) Dot plots show CM9 Gag tetramer responses in MamuA*01+ macaques (CL6A and CL49) at the day of vaccination and 2 weeks later.
Three other macaques (DCVV, CL49, and CL67) that developed anti-Rubella and anti-Gag antibody responses after a single immunization with the rubella vector [19], were also examined for Gag-specific IFN-γ+ cellular immune responses (Figure 2A). DCVV developed Gag-specific T cells (0.13%); CL49 did not develop a Gag-specific T cell response upon peptide stimulation, but showed low levels (0.03%) of Gag CM9+-specific T cells, while CL67 failed to show Gag-specific T cell immunity. The difference in responsiveness could reflect known animal-to-animal variation of outbred macaques as we observed in prior studies [33, 34, 40]. Together, these data show that a single vaccination with rubella vector induced Gag-specific T cell responses in 4 of the 5 animals tested (80% response rate). A single vaccination with rubella vector induced Gag-specific T cell responses comparable to those obtained upon a single gag DNA vaccination (1 mg dose) using the efficient EP delivery method with a mean response of 0.025% IFN-γ+ T cells (unpublished). These responses were also similar to those obtained upon IM delivery of the Vaxfectin® adjuvanted DNA (1 mg dose) which, although low, contributed to control of viremia [42].
3.3. gag DNA vaccination in rubella vector primed macaques
We examined the effect of gag DNA boost in three animals (CL49, CL67, DVCC) previously vaccinated with rubella vector (Figure 3A). Upon rubella vector vaccination, all animals were positive for anti-Rubella and anti-Gag antibody responses [19], and macaques DCVV and CL49 were also positive for Gag cellular responses (Figure 3A, week 20 of study; data from Figure 2). Upon a single DNA vaccination (week 25 of study), all three animals showed an increase of the Gag-specific T cells ranging from 0.02 to 0.5% of IFN-γ+ T cells (upper panels, week 27). Analysis of the MamuA*01+ CL49 also demonstrated an increase of the CM9 response to 0.7% of CD8+ T cells (lower panel). Pre-existing immunity to rubella did not prevent successful vaccination with gag DNA.
FIGURE 3. Responses after different prime boost regimens with rubella vector and gag DNA.
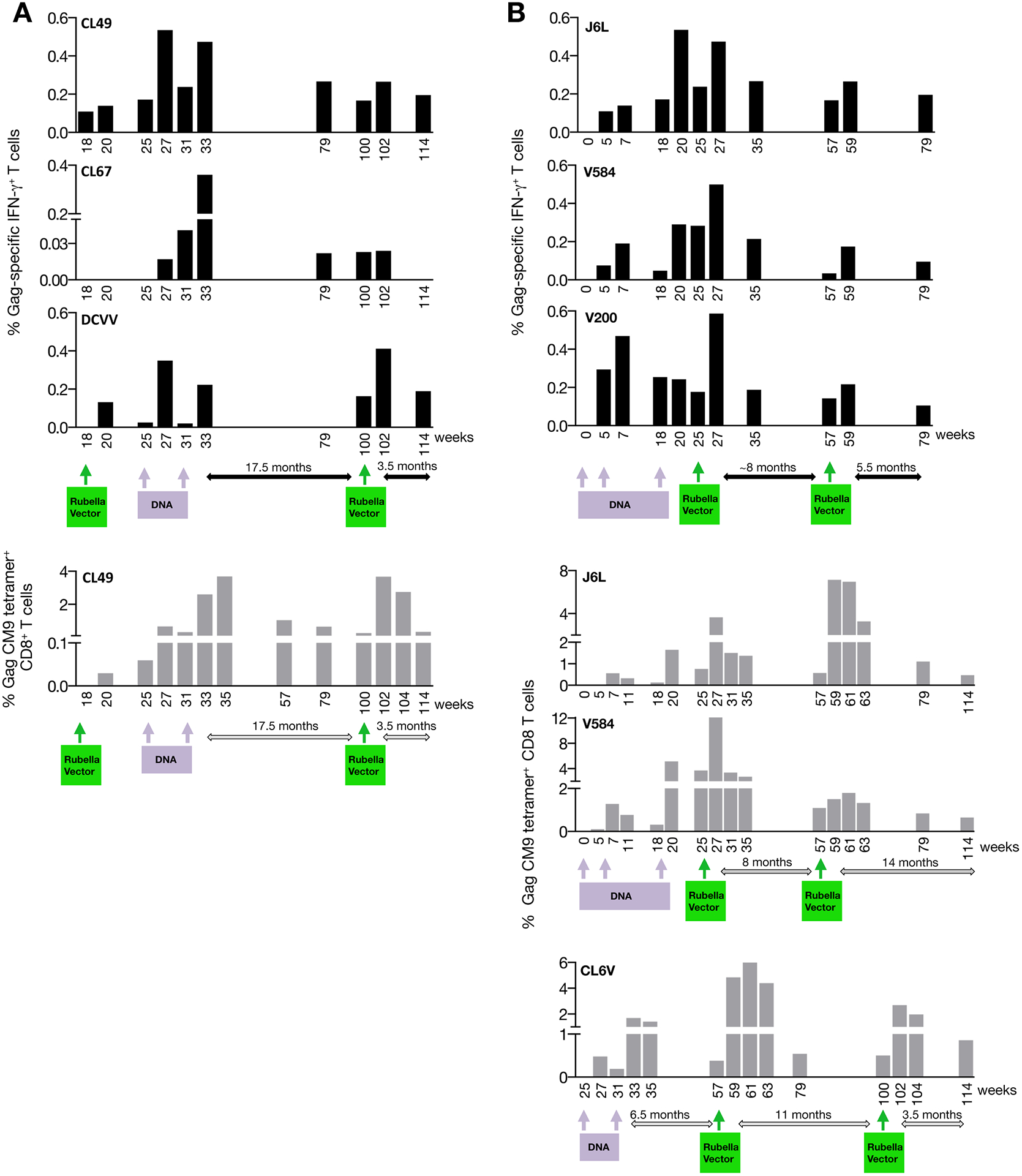
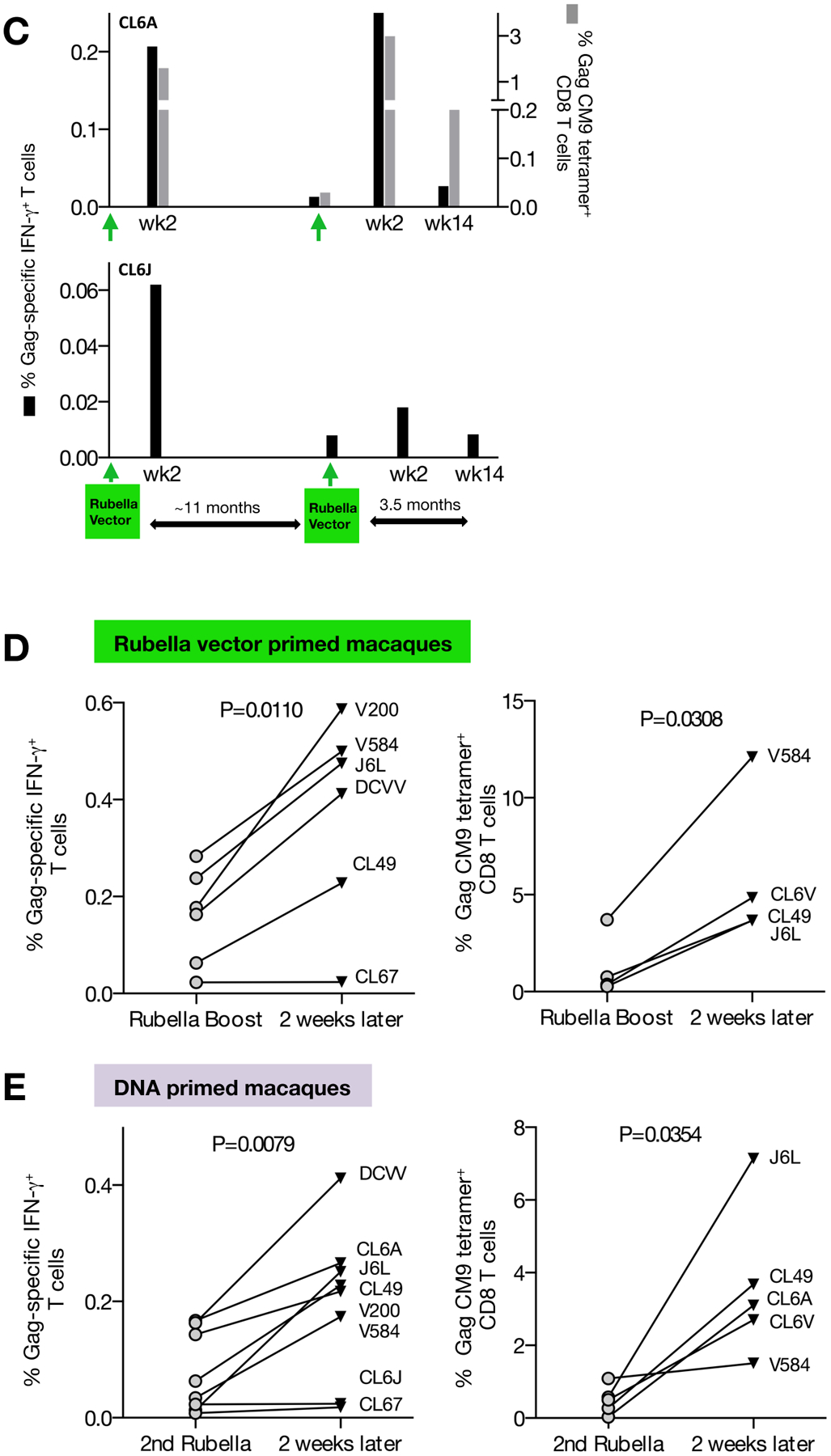
(A) DNA vaccination of rubella vector primed macaques, and a 2nd rubella vector boost. The Gag-specific IFN-γ+ T cell responses (upper panels) of macaques CL67, CL49, and DCVV and the Gag CM9 tetramer-specific CD8+ T cells of the MamuA*01+ CL49 (lower panel) were measured at the indicated time points. The data from weeks 18 and 20 are from Figure 2A. (B) Rubella vector boosts of DNA-primed macaques. The Gag-specific IFN-γ+ T cells of J6L, V584 and V200 (top panels), and the CM9 tetramer-specific CD8+ T cells of the MamuA*01+ J6L, V584 and CL6V (lower panels) were measured at the indicated time points. CL6V could not be analyzed for Gag-specific IFN-γ+ T cell responses due to high background levels in the unstimulated PBMC at all time points. (C) Gag-specific responses of two macaques (CL6A, CL6J) after vaccination with rubella vector only. The Gag-specific IFN-γ+ T cell responses (black bars) are shown for both macaques, and the CM9 tetramer responses (grey bars) are shown for the MamuA*01+ CL6A. Data after the 1st rubella vector vaccination are from Figure 2. (D) Changes in cellular immunity upon a 2nd rubella vector vaccination. Gag-specific IFN-γ+ T cells (left panel) and CM9 tetramer-specific CD8+ T cells (right panel) are shown at day of vaccination and 2 weeks later. P values were obtained using paired t test. Left panel includes data from 8 macaques shown in Figures 3A (CL49, CL67, DCVV; weeks 100–102), 3B (J6L, V584, V200, CL6V (weeks 57–59) and 3C (CL6A, CL6J; weeks 100–102). Right panel includes data from 5 macaques depicted in Figures 3A (CL49; weeks 100–102), 3B (J6L, V584, CL6V (weeks 57–59) and 3C (CL6A; weeks 100–102). (E) Changes in cellular immunity in DNA-primed macaques upon rubella vector boost comparing Gag-specific IFN-γ+ T cells (left panel) and CM9 tetramer-specific CD8+ T cells (right panel) responses at day of vaccination and 2 weeks after rubella vector boost. P values were obtained using paired t test. Left panel includes data from 6 macaques shown in Figures 3A (CL49, CL67, DCVV; weeks 100–102) and 3B (J6L, V584, V200 (weeks 27). Right panel includes data from 4 macaques shown in Figures 3A (CL49; weeks 100–102) and 3B (J6L, V584, at weeks 25–27, and CL6V at weeks 100–102).
3.4. Rubella vector boost of DNA-primed macaques
We tested the ability of rubella vector to boost pre-existing immunity induced by gag DNA vaccination in four macaques (Figure 3B). The gag DNA vaccine induced robust responses ranging from 0.25–0.5% of IFN-γ+ T cells (J6L, V584, V200, upper panels; note CL6V, could only be evaluated for the tetramer responses), similar to our previous results with IM/EP vaccinated macaques [33, 34, 40]. The MamuA*01+ J6L, V584 and CL6V also developed robust tetramer responses reaching ~2% to 5% of CD8+ T cells (lower panels). The DNA-primed animals received a rubella vector vaccination (week 25 for J6L, V584, V200; week 57 for CL6V), resulting in increased Gag-specific responses (~2-fold; reaching ~0.5 % of T cells) as well as an increase of the CM9 responses (3- to 5-fold; reaching ~4–12% CD8+ T cells). In all animals, the Gag-specific responses were higher after the rubella vector boost compared to the peak responses obtained upon DNA vaccination, indicating a benefit of DNA vaccine prime and rubella vector boost regimen.
3.5. Boosting of pre-existing rubella vector induced immunity by 2nd rubella vector vaccination
To examine whether the Gag-specific cellular responses in rubella vector-primed macaques could be boosted by a 2nd vector vaccination, we tested animals primed with rubella vector only (Figure 3C) or with DNA/rubella prime boost regimens (Figures 3A, 3B).
Two macaques (CL6A, CL6J) primed with rubella vector had detectable Gag-specific cellular immune responses 11 months later, when they received a 2nd rubella vector vaccine (Figure 3C). This boost led to an increase of the T cell responses in CL6A of both Gag-specific IFN-γ+ T cells (~0.2%) and the CM9+ CD8+ T cell responses (3%), and had a lesser effect in CL6J. The DNA primed-rubella vector boosted animals (J6L, V584, V200, CL6V; Figure 3B) were immunized with a 2nd dose of recombinant rubella vector after a rest period of 8 to 11 months [week 57 (J6L, V584, V200) or week 100 (CL6V) of study]. In all macaques, the Gag-specific responses increased reaching 0.1–0.2% of IFN-γ+ T cells (upper panels) and 1.5–7% of CM9+ CD8+ T cells (lower panels). Similarly, rubella vector and DNA-primed macaques (CL49, CL67, DCVV; Figure 3A) received a 2nd dose of recombinant rubella vector after a 17.5 months rest period (week 100 of study). Two (CL49, DCVV) of three animals showed an increase of the Gag-specific IFN-γ+ T cells (0.2 to 0.4%, week 102 of the study, upper panels), and CL49 showed an increase, reaching ~4%, of CM9+ CD8+ T cells (lower panel, week 102 of the study).
Together, we found that repeated vaccination with rubella vector is feasible and that a 2nd dose of rubella vector resulted in a statistically significant increase of Gag immune response (N=8, Figure 3D), including both the IFN-γ T cell (left panel) and tetramer responses (right panel). Our data further showed that DNA-primed macaques benefitted from a rubella vector boost given after various rest times (7 weeks and 6.5 months Figure 3B; 17.5 months, Figure 3A). Rubella vector vaccination significantly boosted the Gag cellular immunity in DNA-primed macaques (Figure 3E) for both the IFN-γ T cell (left panel) and the tetramer responses (right panel). Thus, rubella vector boosts could be administered a long time after the DNA priming, leading to a rapid and significant increase of Gag–specific cellular immunity. Together, these data demonstrate that a prime boost vaccine regimen combining rubella vectors and DNA vaccine greatly enhances the T cell responses to antigens such as SIV Gag.
3.6. Durability
We also monitored the durability of cellular immunity induced by the rubella vector. All macaques showed persistence of the Gag T cell responses monitored for ~4–14 months after the last rubella boost (Figure 3A–C). Figure 3A shows responses detectable for at least 3.5 months after the last rubella vector vaccination (~0.2% of Gag-specific IFN-γ+ T cells at week 114 of study; upper panel; ~0.3% of CM9+ CD8+ T cells; lower panel). Figure 3B shows responses detectable at 8–11 months after the rubella vector boost (~0.04% to 0.2% of Gag-specific IFN-γ+ T cells at week 57 of study; upper panel; ~0.6% to 1.1% of CM9+ CD8+ T cells; lower panel). Similarly, Gag T cell responses were found even 11 months after a single rubella vector vaccination, albeit at low levels (Figure 3C). Both Gag-specific IFN-γ+ T cell responses and tetramer responses showed persistence after the 2nd rubella vector vaccination. Two of the animals (J6L, V584) were examined at 14 months after the 2nd rubella vector boost and maintained ~0.5% of CM9+ T cells (Figure 3B). Thus, rubella vector boost was potent in inducing durable responses.
3.7. Characterization of the rubella vector vaccine induced Gag-specific T cell responses
Figure 4A shows the memory phenotype, central memory-like (CM, CD95+CD28+) and effector memory (EM, CD95+CD28−) in PBMC from a representative animal (macaque DCVV) with the Gag-specific T cells induced upon vaccination with rubella vector overlaid in red. The memory T cell subsets were analyzed upon priming with either rubella vector or DNA (Figure 4B). Macaques CL6A, CL6J and DCVV showed induction of CM-like and EM T cells producing IFN-γ, with more cells having a CM-like phenotype. Subsequent vaccinations with rubella vector (CL6A and CL6J), DNA only (CL67), or DNA followed by rubella vector (CCL49, and DCVV) showed a similar distribution of antigen-specific EM and CM T cells, except CL6A which preferentially accumulated Gag-specific effector cells upon boosting with the rubella vector. Similarly, we found that DNA priming vaccination (CL67, CL49, J6L, V584, V200) induced both CM-like and EM Gag-specific T cells, with a greater proportion of CM-like T cells. Subsequent vaccinations with rubella vectors (J6L, V584, V200) did not change the distribution of the antigen-specific memory T cells. Together, these data show that rubella vector vaccination was able to potently prime and boost Gag-specific T cells having CM-like and EM phenotypes.
FIGURE 4. Phenotypic analysis of Gag-specific memory T cells.
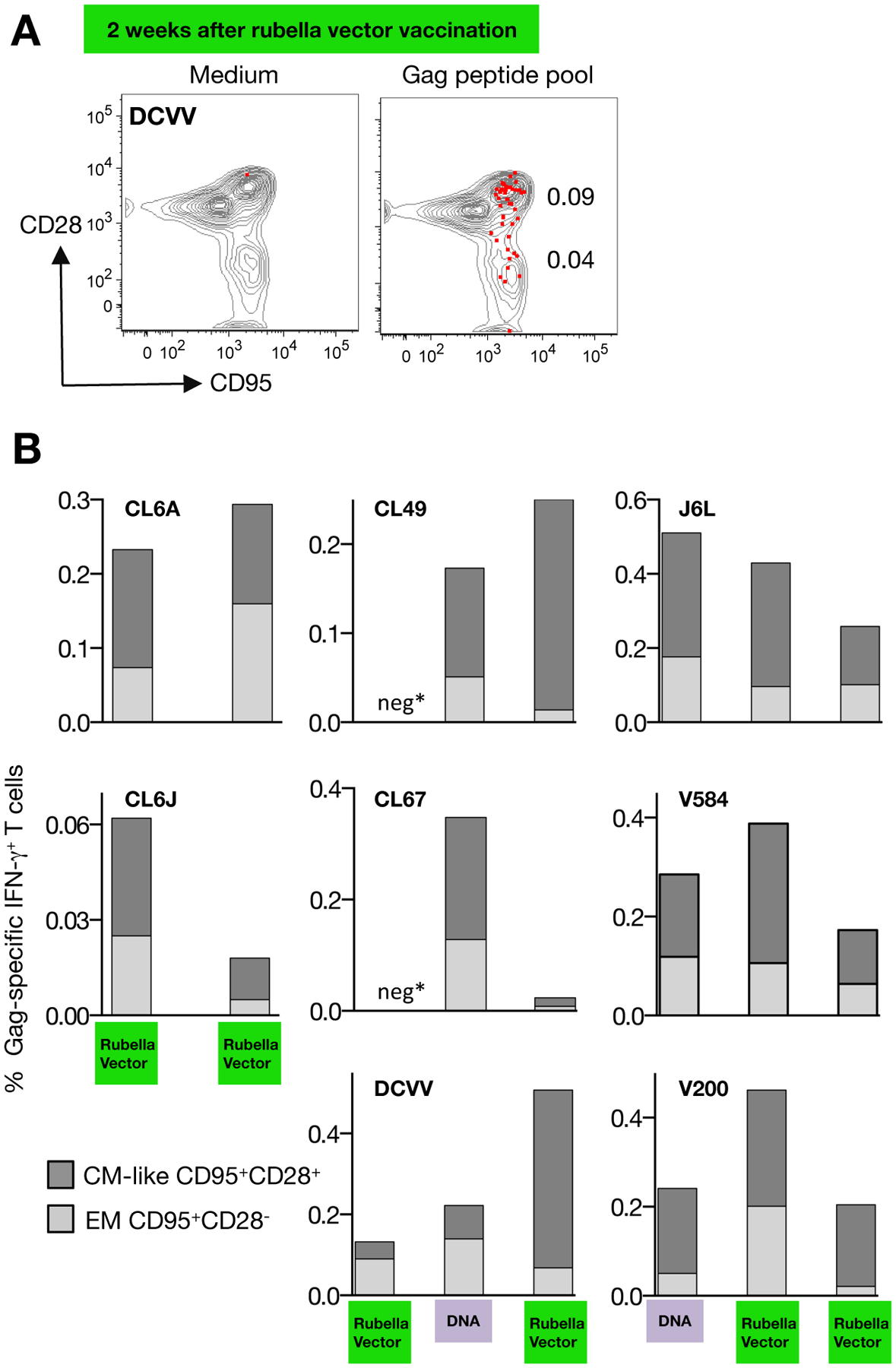
Gag-specific responses were analyzed by flow cytometry using an antibody cocktail for memory markers. (A) Dot plot overlays show the phenotypic characterization (CM-like: CD95+CD28+; effector memory EM: CD95+CD28−) of the Gag-specific T cells induced by rubella vector type 3 vaccination in macaque DCVV at the day of vaccination and 2 weeks later. (B) The percentage of Gag-specific IFN-γ+ T cells with CM-like and EM cells are shown 2 weeks after the indicated vaccinations. Responses in macaque CL6V could not be analyzed (see Fig. 3). Neg* indicates absence of detectable Gag-specific T cell responses.
Analysis of the cytotoxic potential of the Gag-specific T cells (Figure 5) after priming with rubella vector (CL6A, CL6J, CL49, CL67, DCVV) or with gag DNA (J6L, V854, V200) was performed 2 weeks post-immunization. After a single vaccination with rubella vector, three of the five macaques (CL6A, CL6J, DCVV) showed a subset of IFN-γ+ and GzmB+ double-positive T cells, demonstrating the presence of cytotoxic Gag-specific T cells. This T cell subset was also found upon boosting with either rubella vector (CL6A, CL6J) or with gag DNA followed by rubella vector (CL49, CL67, DCVV). As previously reported [33, 34, 40, 43], priming with DNA induced Gag-specific T cells with cytotoxic potential (macaques J6L, V584, V200). Monitoring the cellular responses upon subsequent heterologous boosting with rubella vectors confirmed that the antigen-specific cytotoxic T cell subsets were maintained. We also found the presence of TNF-α producing Gag-specific T cells and a subset of TNF-α+ and GzmB+ double-positive T cells after vaccination with vector type 3 (CL6A, DCVV) and after vaccination with vector type 3* (CL49, DCVV, J6L, V585, V200) (data not shown). Taken together, these data demonstrate that rubella vector vaccination induces strong Gag-specific responses with cytotoxic potential (GzmB+).
FIGURE 5. Analysis of the cytotoxic potential of the Gag-specific T cells.
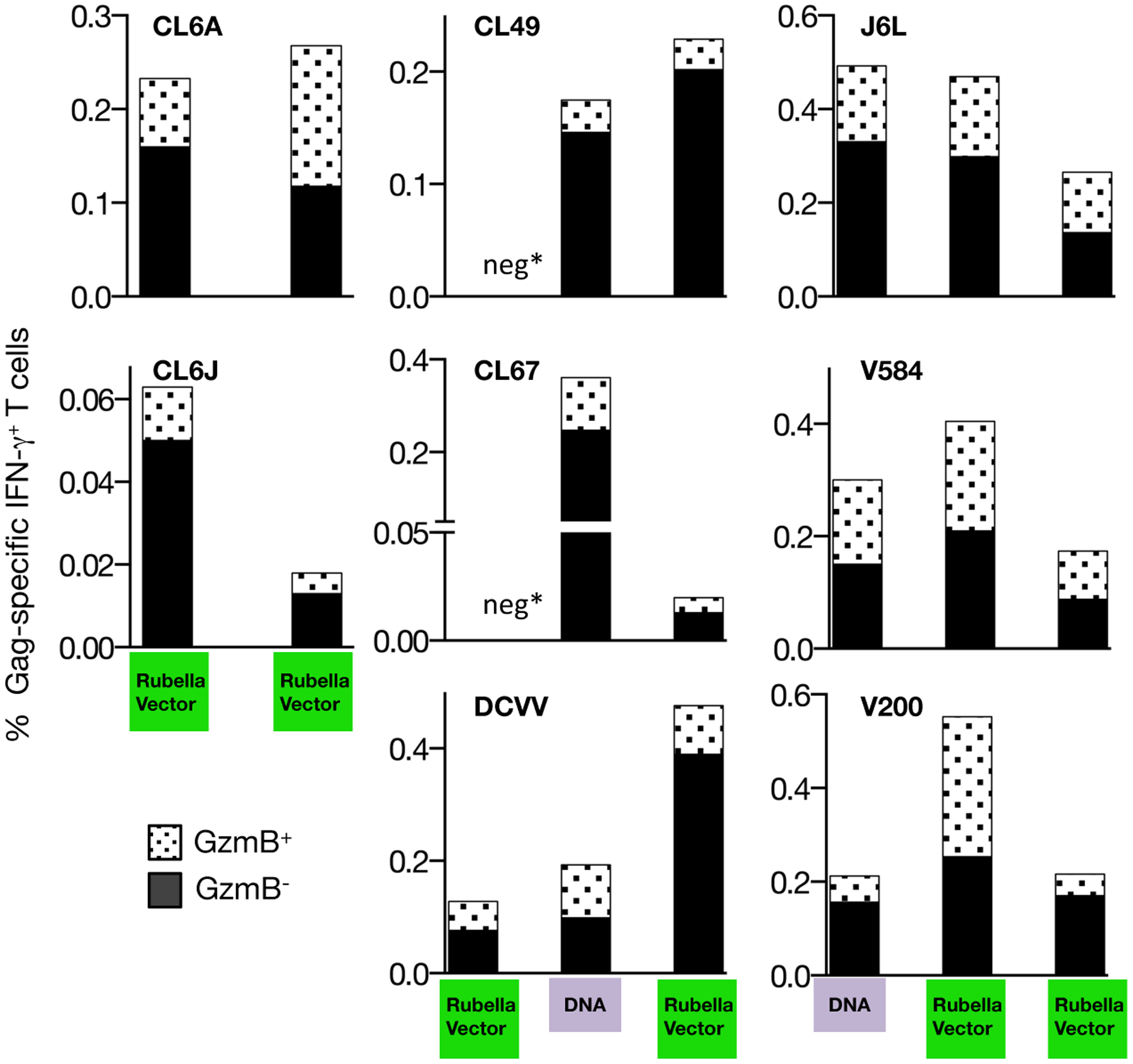
Gag-specific T cells were analyzed by flow cytometry for production of IFN-γ and the presence of granzyme B+ (GzmB) 2 weeks after the indicated vaccinations. The responses were analyzed using a Boolean gating strategy. The percentage of IFN-γ+ GzmB+ double positive antigen-specific T cells and IFN-γ+ GzmB− single positive cells are shown. Neg* indicates absence of detectable Gag-specific responses, as in Figure 4.
4. Discussion
We examined the cellular immune responses induced upon vaccination with recombinant rubella vectors expressing regions of SIV Gag and found that these vectors induce robust antigen-specific responses (up to ~0.2 % Gag-specific IFN-γ+ T cells; ~3% CM9+ CD8+ T cells). This vaccine modality was able to induce Gag-specific responses of a magnitude comparable to those obtained upon a single gag DNA vaccination using the efficient EP delivery method. Such levels are higher than those found using other viral vectors such as MVA, Adenovirus or VSV and which were found to greatly benefit from a DNA prime [5, 6, 11, 15–17, 22–25]. The rubella vector induced a balanced Gag-specific T cells immune responses mediated by CD4+ and CD8+ T cells with effector and central memory-like phenotype. In addition, the antigen-specific IFN-γ+ T cells harbor granzyme B, indicating cytotoxic potential.
Sequential vaccination showed that the rubella vector could also significantly boost preexisting responses, often reaching peak levels even higher than those induced by DNA vaccine alone. Remarkably, although the rubella vector boosts were given after prolonged rest periods (~12–18 months), the responses rapidly increased, supporting their potency. In spite of the presence of anti-rubella antibodies, a 2nd dose of rubella vectors was able to significantly boost T cell responses, regardless of the order of the primary immunization with rubella vector and DNA vaccines (Figures 3). Similarly, prior exposure to the rubella vaccine strain RA27/3 did not prevent induction by the rubella vector boost of Gag-specific antibodies, as reported previously [19], or Gag-specific T cell responses reaching up to 3% CM9+ CD8+ T cells (CL6A, Figure 2). This is in overall agreement with another report [30], which showed that rubella vaccine could boost pre-existing rubella immunity in previously immunized humans. Thus, priming and boosting of naïve subjects with rubella vectors containing vaccine inserts is a feasible application. Our data also show that once the vector has successfully primed a response, it can be used to boost the response, despite the presence of anti-Rubella antibodies.
Longevity of responses is another critical feature of any vaccine. The RV144 vaccine trial elicited immunity that waned over time, and the transient nature of the vaccine-induced immune responses is thought to be, at least partially, responsible for the limited vaccine efficacy [44]. Although we detected cellular responses induced by rubella vector alone after ~1 year in some animals (Figure 3C), the responses had waned considerably. Thus, these responses did not show the robustness reported using a recombinant CMV vector [45] or SIV DNA vaccine [33, 34, 43, 46]. On the other hand, it has been a common finding that DNA delivered by different methods has the remarkable ability to induce persistent immune responses in non-human primates [34, 35, 46]. As observed for other viral vector vaccines (MVA, VSV, vaccinia, adenovirus), the combination of rubella vectors with DNA prime augmented Gag immunogencity. Future studies will test the efficacy of recombinant rubella vector alone or in combination with DNA, two vaccine platforms that are safe in humans.
The current rubella vectors can accommodate vaccine inserts up to ~300 amino acids in length. Thus, rubella vectors could be used to express well-defined T cell epitopes, or chimeric molecules expressing a selection of the most conserved epitopes from different HIV clades ([47, 48] and references therein). Inclusion of HIV Gag conserved elements in DNA-based immunogens provided a critical step to focus the immune responses to such epitopes by avoiding decoy epitopes, and provided an effective strategy to broaden responses in vaccinated macaques, while others showed that responses to a few epitopes led to effective control of viremia [49]. Alternatively, since Gag-specific T cells have been associated with control of viremia in HIV-1 infected people [36–39], recombinant rubella vectors may provide an additional method to induce such responses. Recombinant rubella vectors expressing Gag display key features of an effective vaccine such as the induction of robust T cell responses with effector memory and central memory-like phenotype and cytotoxic potential (this work), and the stimulation of humoral responses [19]. Additional studies are needed to assess the efficacy of the recombinant Rubella vector vaccines as preventive and therapeutic vaccines.
Live-attenuated rubella vaccine has a number of advantages as a vaccine platform for SIV and HIV: the vector’s safety and potency have been established in millions of children, and the recombinant rubella vector presents the immunogen in the context of an ongoing infection. The immune response to the Gag insert in rubella vectors includes long-lasting cytotoxic T cells (this report) and antibody immunity [19]. A strong T cell response may target infected cells early, reducing peak viremia, and during the chronic phase of infection, resulting in lower viral set point. The overlapping host range (macaque, man) will allow us to test the protective efficacy of new rubella vectors in the macaque model.
Supplementary Material
Acknowledgments
The authors wish to thank L. Shankle of CBER and D. Weiss of Advanced BioScience Laboratories, Inc. for their essential contributions to this work and T. Jones for editorial assistance.
Funding: This work was supported in part by the Intramural Research Program of the National Cancer Institute, National Institutes of Health (NCI/NIH) (BKF, GNP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: GNP and BKF are inventors on US Government-owned patents and patent applications related to DNA vaccines and gene expression optimization. N.Y.S. is a full time employee of Inovio Pharmaceuticals and as such receives compensation in the form of salary and stock options. There are no further patents, products in development or marketed products to declare. This does not alter our adherence to all the policies on sharing data and materials. The remaining authors declare that no other competing interests exist.
References
- [1].Hansen SG, Piatak M, Jr., Ventura AB, Hughes CM, Gilbride RM, Ford JC, et al. Immune clearance of highly pathogenic SIV infection. Nature. 2013;502:100–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15:293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bolton DL, Santra S, Swett-Tapia C, Custers J, Song K, Balachandran H, et al. Priming T-cell responses with recombinant measles vaccine vector in a heterologous prime-boost setting in non-human primates. Vaccine. 2012;30:5991–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Martins MA, Bonaldo MC, Rudersdorf RA, Piaskowski SM, Rakasz EG, Weisgrau KL, et al. Immunogenicity of seven new recombinant yellow fever viruses 17D expressing fragments of SIVmac239 Gag, Nef, and Vif in Indian rhesus macaques. PLoS One. 2013;8:e54434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dai K, Liu Y, Liu M, Xu J, Huang W, Huang X, et al. Pathogenicity and immunogenicity of recombinant Tiantan Vaccinia Virus with deleted C12L and A53R genes. Vaccine. 2008;26:5062–71. [DOI] [PubMed] [Google Scholar]
- [6].Cottingham MG, Carroll MW. Recombinant MVA vaccines: dispelling the myths. Vaccine. 2013;31:4247–51. [DOI] [PubMed] [Google Scholar]
- [7].Bonaldo MC, Martins MA, Rudersdorf R, Mudd PA, Sacha JB, Piaskowski SM, et al. Recombinant yellow fever vaccine virus 17D expressing simian immunodeficiency virus SIVmac239 gag induces SIV-specific CD8+ T-cell responses in rhesus macaques. J Virol. 2010;84:3699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Liniger M, Zuniga A, Morin TN, Combardiere B, Marty R, Wiegand M, et al. Recombinant measles viruses expressing single or multiple antigens of human immunodeficiency virus (HIV-1) induce cellular and humoral immune responses. Vaccine. 2009;27:3299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Xu R, Nasar F, Megati S, Luckay A, Lee M, Udem SA, et al. Prime-boost vaccination with recombinant mumps virus and recombinant vesicular stomatitis virus vectors elicits an enhanced human immunodeficiency virus type 1 Gag-specific cellular immune response in rhesus macaques. J Virol. 2009;83:9813–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang Z, Hangartner L, Cornu TI, Martin LR, Zuniga A, Billeter MA, et al. Recombinant measles viruses expressing heterologous antigens of mumps and simian immunodeficiency viruses. Vaccine. 2001;19:2329–36. [DOI] [PubMed] [Google Scholar]
- [11].Lorin C, Delebecque F, Labrousse V, Da Silva L, Lemonnier F, Brahic M, et al. A recombinant live attenuated measles vaccine vector primes effective HLA-A0201-restricted cytotoxic T lymphocytes and broadly neutralizing antibodies against HIV-1 conserved epitopes. Vaccine. 2005;23:4463–72. [DOI] [PubMed] [Google Scholar]
- [12].Lorin C, Mollet L, Delebecque F, Combredet C, Hurtrel B, Charneau P, et al. A single injection of recombinant measles virus vaccines expressing human immunodeficiency virus (HIV) type 1 clade B envelope glycoproteins induces neutralizing antibodies and cellular immune responses to HIV. J Virol. 2004;78:146–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Stebbings R, Fevrier M, Li B, Lorin C, Koutsoukos M, Mee E, et al. Immunogenicity of a recombinant measles-HIV-1 clade B candidate vaccine. PLoS One. 2012;7:e50397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Traina-Dorge V, Pahar B, Marx P, Kissinger P, Montefiori D, Ou Y, et al. Recombinant varicella vaccines induce neutralizing antibodies and cellular immune responses to SIV and reduce viral loads in immunized rhesus macaques. Vaccine. 2010;28:6483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Malkevitch NV, Patterson LJ, Aldrich MK, Wu Y, Venzon D, Florese RH, et al. Durable protection of rhesus macaques immunized with a replicating adenovirus-SIV multigene prime/protein boost vaccine regimen against a second SIVmac251 rectal challenge: role of SIV-specific CD8+ T cell responses. Virology. 2006;353:83–98. [DOI] [PubMed] [Google Scholar]
- [16].Patterson LJ, Malkevitch N, Venzon D, Pinczewski J, Gomez-Roman VR, Wang L, et al. Protection against mucosal simian immunodeficiency virus SIV(mac251) challenge by using replicating adenovirus-SIV multigene vaccine priming and subunit boosting. J Virol. 2004;78:2212–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Patterson LJ, Daltabuit-Test M, Xiao P, Zhao J, Hu W, Wille-Reece U, et al. Rapid SIV Env-specific mucosal and serum antibody induction augments cellular immunity in protecting immunized, elite-controller macaques against high dose heterologous SIV challenge. Virology. 2011;411:87–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Spadaccini A, Virnik K, Ni Y, Prutzman K, Berkower I. Stable expression of a foreign protein by a replication-competent rubella viral vector. Vaccine. 2010;28:1181–7. [DOI] [PubMed] [Google Scholar]
- [19].Virnik K, Hockenbury M, Ni Y, Beren J, Pavlakis GN, Felber BK, et al. Live attenuated rubella vectors expressing SIV and HIV vaccine antigens replicate and elicit durable immune responses in rhesus macaques. Retrovirology. 2013;10:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Virnik K, Ni Y, Berkower I. Live attenuated rubella viral vectors stably express HIV and SIV vaccine antigens while reaching high titers. Vaccine. 2012;30:5453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Virnik K, Ni Y, Berkower I. Enhanced expression of HIV and SIV vaccine antigens in the structural gene region of live attenuated rubella viral vectors and their incorporation into virions. Vaccine. 2013;31:2119–25. [DOI] [PubMed] [Google Scholar]
- [22].Ourmanov I, Kuwata T, Goeken R, Goldstein S, Iyengar R, Buckler-White A, et al. Improved survival in rhesus macaques immunized with modified vaccinia virus Ankara recombinants expressing simian immunodeficiency virus envelope correlates with reduction in memory CD4+ T-cell loss and higher titers of neutralizing antibody. J Virol. 2009;83:5388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Melamed S, Wyatt LS, Kastenmayer RJ, Moss B. Attenuation and immunogenicity of host-range extended modified vaccinia virus Ankara recombinants. Vaccine. 2013;31:4569–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Goepfert PA, Elizaga ML, Sato A, Qin L, Cardinali M, Hay CM, et al. Phase 1 safety and immunogenicity testing of DNA and recombinant modified vaccinia Ankara vaccines expressing HIV-1 virus-like particles. J Infect Dis. 2011;203:610–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Egan MA, Chong SY, Rose NF, Megati S, Lopez KJ, Schadeck EB, et al. Immunogenicity of attenuated vesicular stomatitis virus vectors expressing HIV type 1 Env and SIV Gag proteins: comparison of intranasal and intramuscular vaccination routes. AIDS Res Hum Retroviruses. 2004;20:989–1004. [DOI] [PubMed] [Google Scholar]
- [26].Haglund K, Forman J, Krausslich HG, Rose JK. Expression of human immunodeficiency virus type 1 Gag protein precursor and envelope proteins from a vesicular stomatitis virus recombinant: high-level production of virus-like particles containing HIV envelope. Virology. 2000;268:112–21. [DOI] [PubMed] [Google Scholar]
- [27].Plotkin SA. Rubella vaccination. Lancet. 1979;1:382. [DOI] [PubMed] [Google Scholar]
- [28].Plotkin SA. Rubella vaccine. J Infect Dis. 1995;171:1690–2. [DOI] [PubMed] [Google Scholar]
- [29].Hilleman MR, Buynak EB, Weibel RE, Stokes J Jr. Live, attenuated rubella-virus vaccine. N Engl J Med. 1968;279:300–3. [DOI] [PubMed] [Google Scholar]
- [30].Diaz-Ortega JL, Bennett JV, Castaneda-Desales D, Quintanilla DM, Martinez D, de Castro JF. Booster immune response in children 6–7 years of age, randomly assigned to four groups with two MMR vaccines applied by aerosol or by injection. Vaccine. 2014;32:3680–6. [DOI] [PubMed] [Google Scholar]
- [31].Pugachev KV, Galinski MS, Frey TK. Infectious cDNA clone of the RA27/3 vaccine strain of Rubella virus. Virology. 2000;273:189–97. [DOI] [PubMed] [Google Scholar]
- [32].Felber BK, Valentin A, Rosati M, Bergamaschi C, Pavlakis GN. HIV DNA Vaccine: Stepwise improvements make a difference. Vaccines. 2014;2:354–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Patel V, Jalah R, Kulkarni V, Valentin A, Rosati M, Alicea C, et al. DNA and virus particle vaccination protects against acquisition and confers control of viremia upon heterologous SIV challenge. Proc Natl Acad Sci U S A. 2013;110:2975–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Patel V, Valentin A, Kulkarni V, Rosati M, Bergamaschi C, Jalah R, et al. Long-lasting humoral and cellular immune responses and mucosal dissemination after intramuscular DNA immunization. Vaccine. 2010;28:4827–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Jalah R, Kulkarni V, Patel V, Rosati M, Alicea C, Bear J, et al. DNA and protein co-immunization improves the magnitude and longevity of humoral immune responses in rhesus macaques. PLoS One. 2014;9:e91550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kiepiela P, Ngumbela K, Thobakgale C, Ramduth D, Honeyborne I, Moodley E, et al. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med. 2007;13:46–53. [DOI] [PubMed] [Google Scholar]
- [37].Masemola A, Mashishi T, Khoury G, Mohube P, Mokgotho P, Vardas E, et al. Hierarchical targeting of subtype C human immunodeficiency virus type 1 proteins by CD8+ T cells: correlation with viral load. J Virol. 2004;78:3233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mothe B, Ibarrondo J, Llano A, Brander C. Virological, immune and host genetics markers in the control of HIV infection. Dis Markers. 2009;27:105–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zuniga R, Lucchetti A, Galvan P, Sanchez S, Sanchez C, Hernandez A, et al. Relative dominance of Gag p24-specific cytotoxic T lymphocytes is associated with human immunodeficiency virus control. J Virol. 2006;80:3122–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Rosati M, Bergamaschi C, Valentin A, Kulkarni V, Jalah R, Patel V, et al. DNA vaccination in rhesus macaques induces potent immune responses and decreases acute and chronic viremia after SIVmac251 challenge. Proc Natl Acad Sci U S A. 2009;06:15831–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Valentin A, McKinnon K, Li J, Rosati M, Kulkarni V, Pilkington GR, et al. Comparative Analysis of SIV-specific Cellular Immune Responses Induced by Different Vaccine Platforms in Rhesus Macaques. Clin Immunol 2014;55:91–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kulkarni V, Rosati M, Valentin A, Jalah R, Alicea C, Yu L, et al. Vaccination with Vaxfectin® adjuvanted SIV DNA Induces Long-lasting Humoral Immune Responses Able to Reduce SIVmac251 Viremia. Hum Vaccin Immunother. 2013;9:2069–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Jalah R, Patel V, Kulkarni V, Rosati M, Alicea C, Ganneru B, et al. IL-12 DNA as molecular vaccine adjuvant increases the cytotoxic T cell responses and breadth of humoral immune responses in SIV DNA vaccinated macaques. Hum Vaccin Immunother. 2012;8:1620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–20. [DOI] [PubMed] [Google Scholar]
- [45].Hansen SG, Sacha JB, Hughes CM, Ford JC, Burwitz BJ, Scholz I, et al. Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science. 2013;340:1237874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Cristillo AD, Weiss D, Hudacik L, Restrepo S, Galmin L, Suschak J, et al. Persistent antibody and T cell responses induced by HIV-1 DNA vaccine delivered by electroporation. Biochem Biophys Res Commun. 2008;366:29–35. [DOI] [PubMed] [Google Scholar]
- [47].Kulkarni V, Valentin A, Rosati M, Alicea C, Singh AK, Jalah R, et al. Altered Immunodominance Hierarchy and Increased T-cell Breadth upon HIV-1 Conserved Element DNA Vaccination in Macaques. PLoS One. 2014;9:e86254.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kulkarni V, Valentin A, Rosati M, Rolland M, Mullins JI, Pavlakis GN, et al. HIV-1 Conserved Elements p24CE DNA Vaccine Induces Humoral Immune Responses with Broad Epitope Recognition in Macaques. PLoS One. 2014;9 e111085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Mudd PA, Martins MA, Ericsen AJ, Tully DC, Power KA, Bean AT, et al. Vaccine-induced CD8+ T cells control AIDS virus replication. Nature. 2012;491:129–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


