Abstract
Charged particle radiation such as iron ions and their secondary fragmentation products are of particular concern to the skeleton due to their high charge and energy deposition. However, little is known about the long-term effects of these particles on trabecular and cortical bone morphology when applied at relatively low levels. We hypothesized that even a 4.4 cGy dose of a complex secondary iron ion radiation field will compromise skeletal quantity and architecture in adult mice. One year after radiation exposure and compared to age-matched controls, 4.4 cGy irradiated mice had 51 % more trabecular bone, 56 % greater trabecular bone volume fraction, 16 % greater trabecular number, and 17 % less trabecular separation in the distal metaphysis of the femur. Similar to the metaphysis, trabecular bone of the distal femoral epiphysis in 4.4 cGy mice had 33 % more trabecular bone, 31 % greater trabecular bone volume fraction, and a 33 % smaller structural model index. Cortical bone morphology, whole bone mechanical properties, and lower leg muscle mass were unaffected. When compared to two additional groups, irradiated at either 8.9 or 17.8 cGy, a (negative) dose response relationship was observed for trabecular bone in the metaphysis but not in the epiphysis. In contrast to our original hypothesis, these data indicated that a secondary field of low-level, high-linear energy transfer iron radiation may cause long-term augmentation, rather than deterioration, of trabecular bone in the femoral metaphysis and epiphysis of mice.
Keywords: Trabecular bone, Radiation, Space flight, Mechanical testing, MicroCT
Introduction
Manned space exploration towards long-term orbital and interplanetary missions involves major health risks for space travelers including the exposure to radiation and microgravity [1, 2]. While the deleterious consequences of microgravity on the skeletal system are well documented in carefully controlled ground-based and spaceflight studies [3, 4], the risks of radiation are less well understood. Generally, radiation exposure imposes major health risks including metabolic changes, loss of reproductive ability, cell death, organ dysfunction, and increased risk of cancer. Most studies reporting these issues, however, targeted clinical, occupational, or environmental radiation hazards.
It is difficult to extrapolate from these well characterized risks to space flight radiation risks due to the different nature of ionizing radiation encountered in space [1, 5, 6]. The exo-magnetospheric space and low earth orbit environments are characterized by the presence of complex mixed radiation fields, arising from solar disturbances and galactic cosmic rays. The components of cosmic radiation are high-linear energy transfer (LET) energetic charged particles, protons, and fully ionized nuclei of all elements [7]. Due to their high charge and energy deposition, high-Z and -energy particles with broad energy spectra at low fluence rates, such as (56)Fe and its secondary fragmentation products, are of particular concern.
In bone, occupational and clinical studies demonstrate that low-LET radiation using X-rays or gamma rays induce multiple pathologies that target osteoblasts, osteocytes, osteoclasts, marrow stromal cells, marrow hematopoietic cells, and blood vessels [8, 9]. At least some of these detrimental effects are negated at very low doses (1–10 cGy) [10]. The effects of high-LET radiation are less well established [10–15]. Carbon-ion appears to be well tolerated at therapeutic doses in radiotherapy, but results have been more equivocal in experimental systems. Exposure to 2 Gy of gamma, proton, carbon-ion, iron-ion, or X-ray radiation induced substantial bone loss in mice [13, 16, 17], consistent with earlier reports that used equivalent doses of 290 MeV carbon-ion [18]. Unexpectedly, higher doses (15–30 Gy) can cause greater bone volume [14], possibly due to the interference of radiation with osteoclast maturation [15]. Nevertheless, the preponderance of data suggests that both low- and high-LET radiation is damaging to specific aspects of the skeleton and, therefore, pose a health risk to space flight missions.
Most studies inferring radiation damage to the skeleton exposed cells and tissues to moderate to high doses of radiation, levels unlikely to be encountered in spaceflight where low-fluence and low-dose radiation dominates the radiation spectrum (with the exception of solar particle events) [1]. In the adult mouse, mixed particle exposure as low as 20 cGy can have negative effects on trabecular and cortical bone [19], but the effect of even lower doses of high-LET irradiation is essentially unknown. Here, lowfluence, low-dose 1 GeV iron-ion radiation was used to produce a complex secondary particle field via collimators that comprised different materials. Specifically, we hypothesized that a 4.4 cGy dose of this ionizing radiation field will detrimentally influence the appendicular musculoskeletal system of adult mice.
Materials and methods
Experimental design
All procedures were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC). This study resulted from tissue sharing of a larger study that focused on consequences of radiation on the brain and central nervous system under the direction of Dr. Marcelo Vasquez. To investigate the effect of low-level irradiation on femoral morphology, male C57BL/6 mice were weight-matched and divided into 4 groups: control (0 cGy, n = 11), 4.4 cGy (n = 10), 8.9 cGy (n = 10), and 17.8 cGy (n = 6). Mice were irradiated at 19 weeks of age. The 8.9 and 17.8 cGy groups were included for qualitative investigation of dose-response relationships for bone morphology, but were not directly compared to the 0 or 4.4 cGy groups because a priori power calculations for the given sample sizes indicated insufficient statistical power for multi-group comparisons.
Mice were group-housed and provided with free access to standard chow and water. They were sacrificed 11.5 months after radiation exposure. The soleus, tibialis anterior, and gastrocnemius muscles were harvested and weighed fresh immediately after sacrifice. Right femurs were stored at −20 °C for micro-computed tomography and whole bone mechanical testing.
Irradiation
Irradiation was performed at the NASA Space Radiation Laboratory (NSRL) at Brookhaven National Laboratory. At 19 weeks of age, the four groups of mice received a total of 0, 60, 120, and 240 cGy of single dose head-only iron ion radiation at dose rates of 30, 50, and 90 cGy/min, respectively. As detailed elsewhere [19, 20], the leakage dose that the mouse femur received in our experiments was 7.4 % of the dose provided to the head of the mouse with a track-averaged LET of 14.9 keV/mm for unattenuated fragments. Thus, the appendicular musculo-skeleton of the mice was subjected to a complex field of secondary particles containing protons, helium ions, and neutrons [19] at levels of 0, 4.4, 8.9, and 17.8 cGy across the four groups, respectively.
The irradiation procedure has been described in detail previously [19]. Briefly, mice were anesthetized (IP) with a mix of ketamine (50 mg/kg) and xylazine (5 mg/kg) and placed within polyethlyene shielded restraints into the 1 GeV/n (56)Fe beamline. Four mice were irradiated at a time while customized bite-bar cradles stabilized their heads. A collimator was placed between the mice and radiation source [21] that consists of layers of poly-methylmethacrylate, aluminum, and high-density polyethylene, effectively attenuating primary particles as well as attenuating and decreasing the velocity of primary ions with low-Z material. Dosimetry was performed with an ion chamber upstream of the collimator blocking all but 4 holes through which 4 ion beams passed through and irradiated mice head on. The chamber was calibrated against a small NIST-traceable calibrated thimble chamber placed in one of the 4 holes during the calibration. The beam was assumed to be uniform to a degree that the dose is uniform within 2–3 % from hole to hole. The beam was cut off when the desired dose was reached in the upstream ion chamber. The cutoff error was a small fraction of 1 % of the total dose. At the target surface, the beam had an energy of 969 MeV/nucleon and an LET of 151 keV/mm [19]. LET spectra were determined via nuclear track detectors in front and behind the collimator as well as inside and at the edge of the target column [19].
Micro-computed tomography
Micro-computed tomography was used to quantify trabecular and cortical bone morphology in the femur of the 4 groups of mice (MicroCT 40, Scanco, SUI). The diaphysis, distal metaphysis, and distal epiphysis of each femur were scanned at 55 kV, 145 μA, and 10 μm resolution (Fig. 1). The metaphyseal and epiphyseal volume of interest (VOI), chosen to maximize the amount of trabecular bone available, contained 130 and 35 transverse slices, respectively. The diaphyseal VOI contained 20 slices and was selected at the midpoint of each femur. Trabecular bone was separated from cortical bone with contour lines and segmented. For metaphyseal and epiphyseal trabecular bone, trabecular bone volume fraction (BV/TV), trabecular connectedness (Conn.D), structure model index reflecting the ratio of rod-to plate-like trabeculae (SMI), number of trabeculae (Tb.N), trabecular thickness (Tb.Th), and trabecular separation reflecting the average distance between trabeculae (Tb.Sp) were determined. Cortical bone morphology was assessed in the mid-diaphysis as well as in the epiphyseal and metaphyseal cortical shell surrounding the trabecular VOI. Cortical area (Ct.Ar), periosteal area (Ps.Ar), and marrow area (Ma.Ar) were determined for all cortical regions.
Fig. 1.
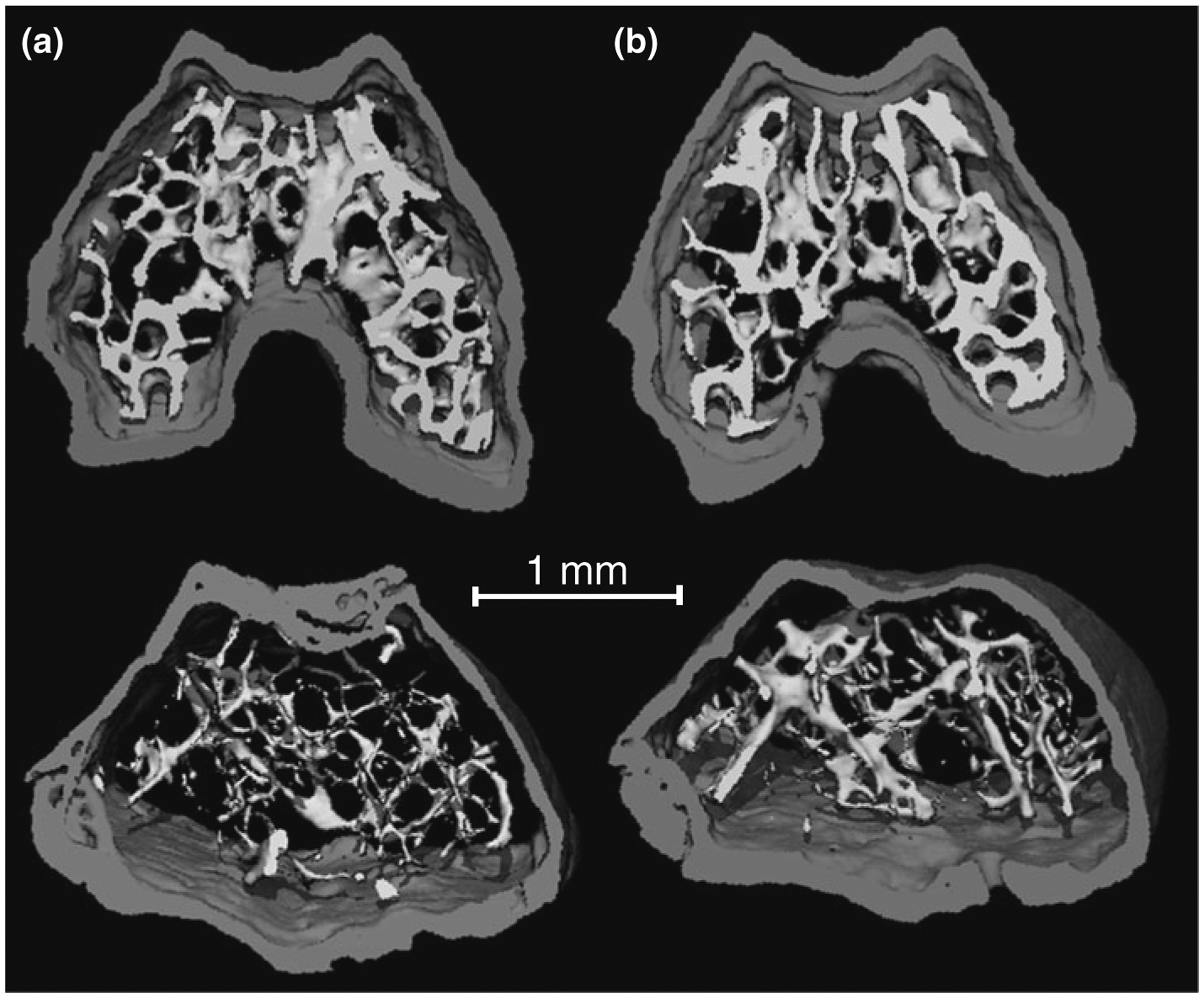
Three-dimensional μCT images of the epiphysis (top) and metaphysis (bottom) in the distal femur of a a B6 age-matched control mouse or b a mouse that was exposed to 4.4 cGy of a single dose of radiation. The 4.4 cGy irradiated mice showed more, but not thicker, trabeculae
Mechanical testing
To determine bone’s mechanical integrity after low-level radiation treatment, whole bone mechanical properties of the 0 and 4.4 cGy groups were determined by four-point bending mechanical tests (MTS 858 Mini Bionix II, Eden Prairie, MN, USA). Each bone was placed on a loading jig with the posterior side in tension. A load was applied at 0.1 mm/s until the bone fractured. Data were sampled at 205 Hz. Linear trend lines were fit to the elastic region of each loading curve to calculate the stiffness of each sample [22]. Additionally, ultimate load and deformation were calculated.
Statistical analyses
Bone morphology, whole bone mechanical properties, and muscle mass were compared between 0 and 4.4 cGy groups with 2-tailed independent t-tests. As noted above, we expected only a relatively small musculoskeletal response because of the very low radiation dose and, therefore, did not include the other two groups in multi-group ANOVA comparisons in this study. The bone morphological parameters that showed significant differences between 0 and 4.4 cGy mice were plotted as a dose-response curve across the 4 groups of mice. Statistical significance was set at p ≤ 0.05.
Results
There were no differences in body mass between the groups prior to sacrifice. At 11.5 months after receiving a 4.4 cGy dose at age 19 weeks, trabecular bone in the femoral metaphysis of irradiated mice was markedly different from control mice in terms of both quantity and architecture. Compared to age-matched controls, irradiated mice had 51 % more (p ≤ 0.05) trabecular bone (BV), 56 % greater (p ≤ 0.05) trabecular BV/TV, and 16 % greater (p ≤ 0.05) Tb.N while Tb.Sp was decreased (p ≤ 0.05) by 17 % (Fig. 2). Conn.D, SMI, and Tb.Th did not show significant differences between the groups (Table 1).
Fig. 2.
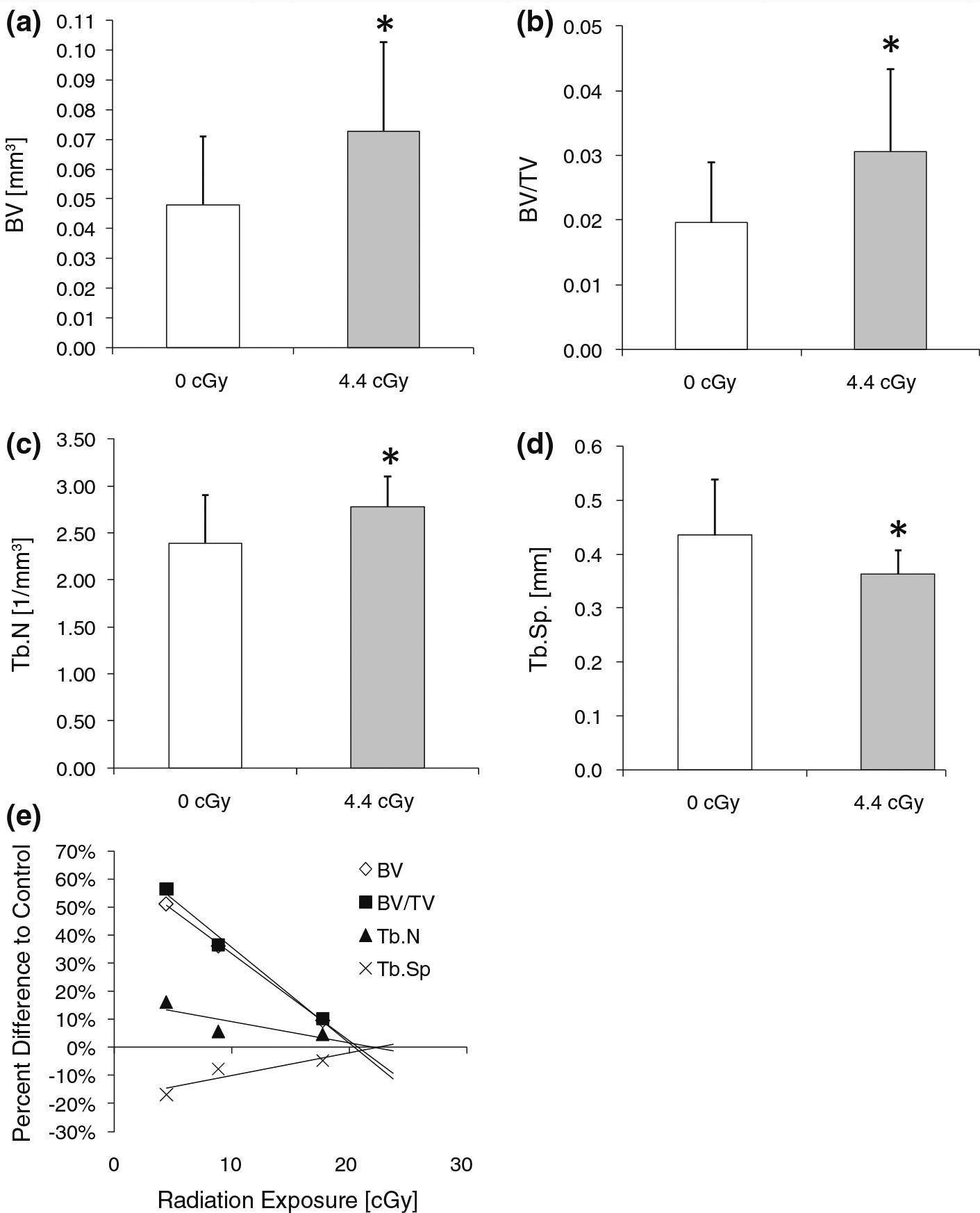
Bone morphology (mean + SD) was significantly enhanced in the trabecular metaphysis of mice exposed to 4.4 cGy of ionizing radiation including a trabecular bone volume (BV), b trabecular bone volume fraction (BV/TV), c trabecular number (Tb.N), and d trabecular separation (Tb.Sp). e Dose-response relation of tibial trabecular morphology of mice subjected to either 4.4, 8.9, or 17.8 cGy. Extrapolation from the regression line suggests an anabolic threshold at approximately 20 cGy. *p ≤ 0.05 against age-matched controls (0 cGy)
Table 1.
Trabecular architectural properties in the metaphysis and epiphysis, cortical morphological properties of the diaphysis, metaphysis, and epiphysis, or mechanical properties that did not show significant differences between control (0 cGy) and low-level irradiated (4.4 cGy) mice (mean ± SD)
| 0 cGy | 4.4 cGy | |
|---|---|---|
| Diaphysis | ||
| Cortical morphology | ||
| Ct.Ar (mm2) | 1.83 ± 0.26 | 1.94 ± 0.11 |
| Ps.Ar (mm2) | 5.11 ± 0.27 | 5.08 ± 0.21 |
| Ma.Ar (mm2) | 3.28 ± 0.23 | 3.13 ± 0.17 |
| Mechanical properties | ||
| Stiffness (N/mm2) | 90.7 ± 33.6 | 107.6 ± 31.1 |
| Ultimate load (N) | 20.6 ± 5.9 | 22.4 ± 5.1 |
| Ultimate deformation (mm) | 0.42 ± 0.10 | 0.55 ± 0.30 |
| Metaphysis | ||
| Trabecular microarchitecture | ||
| Conn.D (1/mm3) | 5.24 ± 6.59 | 10.03 ± 7.56 |
| SMI [1] | 3.48 ± 0.23 | 3.35 ± 0.31 |
| Tb.Th (mm) | 0.043 ± 0.007 | 0.044 ± 0.005 |
| Cortical morphology | ||
| Ct.Ar (mm2) | 0.90 ± 0.18 | 0.94 ± 0.05 |
| Ps.Ar (mm2) | 3.69 ± 0.21 | 3.59 ± 0.22 |
| Ma.Ar (mm2) | 2.79 ± 0.23 | 2.65 ± 0.19 |
| Epiphysis | ||
| Trabecular microarchitecture | ||
| Conn.D (1/mm3) | 137 ± 43 | 155 ± 0.34 |
| Tb.N (1/mm3) | 5.95 ± 0.20 | 5.94 ± 0.36 |
| Tb.Th (mm) | 0.047 ± 0.007 | 0.051 ± 0.003 |
| Tb.Sp (mm) | 0.18 ± 0.02 | 0.18 ± 0.01 |
| Cortical morphology | ||
| Ct.Ar (mm2) | 1.37 ± 0.19 | 1.47 ± 0.17 |
| Ps.Ar (mm2) | 4.36 ± 0.34 | 4.48 ± 0.32 |
| Ma.Ar (mm2) | 2.99 ± 0.26 | 3.01 ± 0.31 |
| Lower limb | ||
| Muscle | ||
| Gastrocnemius (g) | 0.115 ± 0.014 | 0.117 ± 0.023 |
| Tibialis anterior (g) | 0.005 ± 0.001 | 0.004 ± 0.001 |
| Soleus (g) | 0.017 ± 0.009 | 0.019 ± 0.008 |
Similar to the metaphysis, positive effects of the low radiation dose were apparent in trabecular bone of the epiphysis where 4.4 cGy mice had 33 % more (p ≤ 0.05) trabecular bone and 31 % greater (p ≤ 0.05) trabecular BV/TV (Fig. 3) than controls. In addition, SMI, a measure indicating whether the average trabecula within the structure is sphere-like (SMI 4), rod-like (SMI 3) or plate-like (SMI 0), was 33 % smaller (p ≤ 0.05) in irradiated than in control mice. All other architectural properties did not significantly differ between groups (Table 1).
Fig. 3.
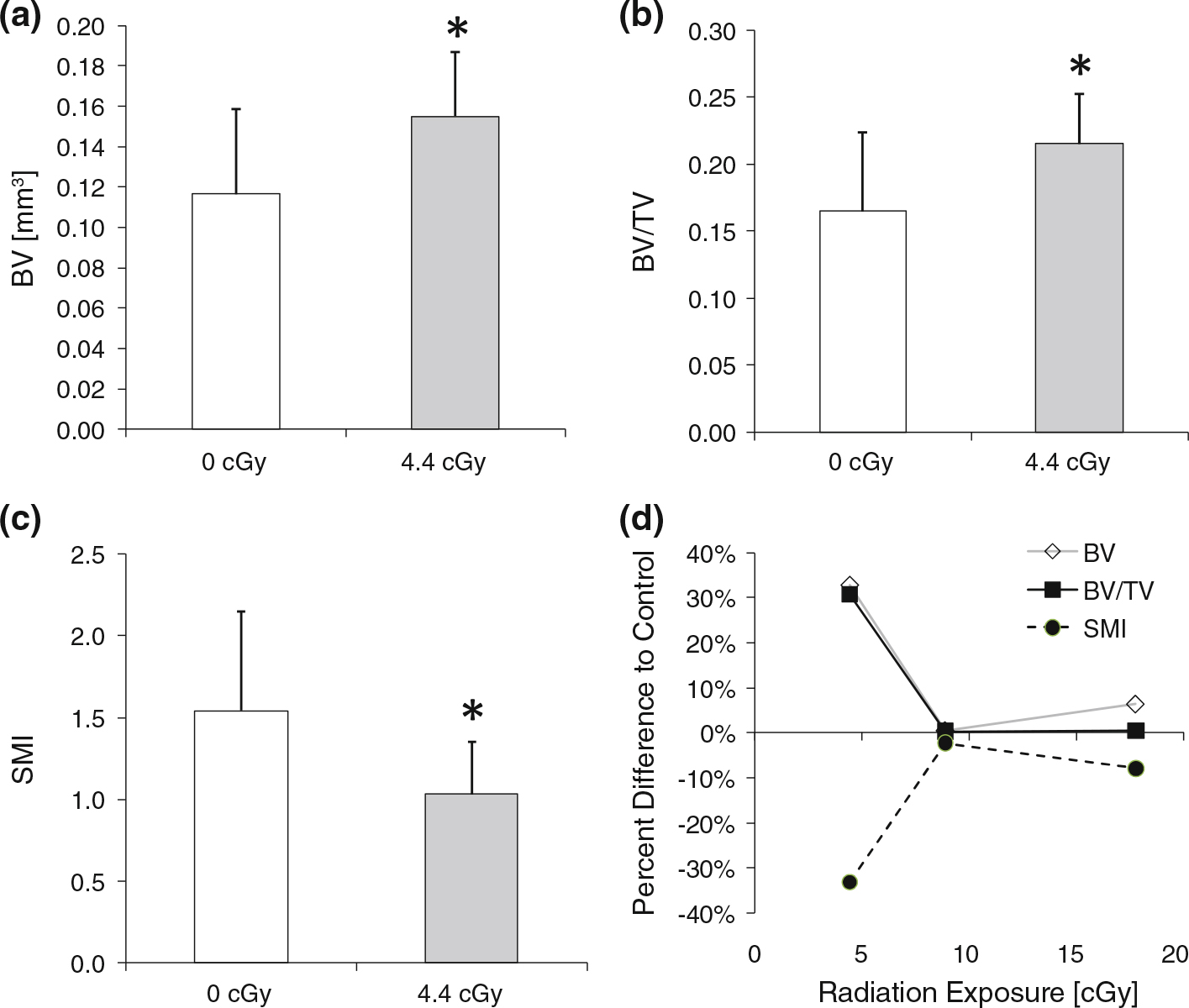
Bone morphology (mean + SD) was significantly enhanced in the trabecular epiphysis of mice exposed to 4.4 cGy of ionizing radiation including a trabecular bone volume (BV), b trabecular bone volume fraction (BV/TV), and c the structural model index (SMI). d No linear dose-response relation of tibial trabecular morphology of mice subjected to either 4.4, 8.9, or 17.8 cGy was found in epiphyseal trabecular bone. *p ≤ 0.05 against age-matched controls (0 cGy)
In contrast to trabecular bone, there was no statistically significant impact of 4.4 cGy irradiation in cortical bone of the mid-diaphysis, metaphysis, or epiphysis as all outcome measures of bone quantity and geometry were not significantly different between the two groups (Table 1). The mechanical properties of cortical bone tested in mid-diaphysis including stiffness, ultimate load, and ultimate deformation were also not significantly different between irradiated mice and controls (Table 1). Similar to cortical bone, no significant differences in muscle mass of the gastrocnemius, soleus, or tibialis anterior were detected (Table 1).
The morphological bone variables that showed significant differences between control and 4.4 cGy mice were further evaluated for dose-response relations by qualitative comparisons with data from those mice that received a single dose of 8.9 or 17.8 cGy radiation. For morphological variables pertaining to the trabecular metaphysis, a linear dose response relation was identified (Fig. 2). Extrapolation from this linear regression indicated that the threshold at which radiation became effective was approximately 20 cGy. In epiphyseal bone, no linear dose-response relation across the radiation doses was observed and, therefore, no radiation threshold could be estimated for the epiphysis (Fig. 3).
Discussion
We investigated the long term effects of low-dose high-LET radiation on the murine appendicular skeleton. In contrast to our initial hypothesis that low-dose radiation exposure would, over the course of a year, establish a catabolic response in bone, a one-time 4.4 cGy dose radiation did not deteriorate bone quantity, morphology, or mechanical properties in the femur of adult mice. Our novel results demonstrate an anabolic (and/or anti-catabolic) effect of low dose radiation on trabecular bone in the distal femur which responded by an increase in tissue quantity and enhanced trabecular microarchitecture. While trabecular bone in the femur benefited from the particle field caused by head-only iron radiation, no significant effects were observed in cortical bone or muscle. Together, these data demonstrate that a single dose of low-level, high-LET radiation can give rise to trabecular bone compartments, which compared to age-matched controls, are characterized by an enhanced morphology and microarchitecture.
Unlike most previous studies showing deleterious effects of ionizing radiation in bone, the experiment here used very low doses of radiation. Generally, in diagnostic and nuclear medicine imaging, the doses of ionizing radiation delivered to bone are considered to be relatively harmless. For example, the effective dose (assuming 1 Sv = 1 Gy for gamma/X-ray photons) is <0.0004, <0.01, and <0.3 cGy in a typical dental X-ray, chest X-ray, and radioisotopic bone scan, respectively. In contrast, the levels of ionizing radiation are orders of magnitude greater for radiation therapy because the intent is to kill cells. The administration of multiple fractions of 1–2 Gy with cumulative doses of greater than 70 Gy are common. At the highest levels, complications can include reduced bone density, osteoradionecrosis, and resulting fractures [23]. The doses used here fell between these two extremes.
Comparisons with higher doses of low- and high-LET radiation studies that resulted in bone loss [13, 14, 19, 24, 25] suggest that the dose-response relationship is complex in bone. Here, the dose-response trendline between the level of radiation and the difference in bone morphology compared to controls suggests an effect in trabecular bone of the metaphysis for doses less than 20 cGy. This contrasts, at least in part, with data from a recent study using the same mode of radiation delivered at 18 cGy to the proximal tibia in which erosion of metaphyseal trabecular bone was observed 4 months after radiation exposure [19]. A principal difference between the two investigations is the 3-fold difference in study duration and it is possible that mice subjected to 18 cGy in our study may also have initially experienced a loss of bone before recovering. More detailed future studies determining the time course by which low level radiation can be beneficial to bone will establish the physiologic processes underlying the novel phenomenon observed here.
While unclear at this point, the mechanism of the complex radiation dose-response relationship may result from biological and/or physical phenomena. Biologically, the processes of cell cycle arrest, apoptosis, premature senescence, and cytotoxicity—all modulators of radiation effects—can respond very differently and in a cell-type specific manner to a given radiation dose (30–33). Physically, large single or cumulative doses (>30 Gy) consistently caused bone atrophy, increased bone fragility, impaired fracture healing, and accelerated the onset of osteonecrosis. The significance of the absorbed, rather than the applied local dose, is illustrated by the decline in the rate of osteonecrosis when kilovoltage therapy is supplanted by megavoltage therapy because lower energy photons are more readily absorbed by bone. These relationships have not been observed for low-level radiation. For example, at the palliative doses employed for bone metastases (8–30 Gy, single or fractionated), radiation does not necessarily impair healing and ossification [26, 27]. While bone growth is attenuated by radiation [8], there is no clearly defined relationship between local dose and reduced bone density in pediatric patients [23]. Thus, the eventual determination of radiation thresholds, anabolic or catabolic, will likely depend on very detailed definitions of radiation identity and dose as well as its application.
Whether the benefit to trabecular morphology in our study was modulated by changes in osteoblast or osteoclast number and activity levels is unclear. It could be speculated that altered levels of bone resorption primarily determined the outcome as, in vitro, hematopoeitic cells and osteoclast precursors can be more radiosensitive than stromal and osteoblastic cells [28, 29]. Radiation effects on osteoblasts have been studied predominantly in cell culture differentiation models. Exposure of pre-osteoblastic cells to X-ray radiation of more than 5 Gy caused a cessation of cell proliferation and an increase in alkaline phosphatase activity, an early osteodifferentiation marker [30]. Cellular changes were accompanied by a decrease in the release of TGF-β and VEGF released by these cells [31]. In less differentiated C2C12 cells that were triggered towards osteo-differentiation, similar doses of radiation (4 or 8 Gy) showed no measurable effect on osteodifferentiation [32]. Together, these studies suggest that radiation effects on cells are complex and possibly biphasic. Future determination of the cellular effect of low-level radiation both in vivo and in vitro will provide critical clues towards the identification of the cells sensing and driving the response.
Our results should be interpreted in light of a few limitations. The relevant radiation field of the femur represented the leakage dose from the greater primary iron-ion dose supplied to the head. Due to its complex mixture, it is difficult to conclude which specific particles caused the enhanced trabecular structure in this study. Nevertheless, the cosmic radiation field to which astronauts are exposed in space is similarly complex and, ultimately, the effects of mixed fields will have to be compared to those of singleion fields. As the head was exposed to greater radiation levels than the appendicular skeleton, it is entirely possible that the results observed here were influenced by neurotransmitters originating in the brain. However, the same mode of head-only iron-ion radiation previously caused a site-specific response rather than a systemic response expected from a neurological signal, which argues against such a hypothesis [19]. Also, this study was kindly facilitated by a tissue sharing agreement and, therefore, outcome variables and study details could not be selected prior to the design of the experiment.
In summary, we report findings of a novel anabolic and/or anti-catabolic trabecular long-term response to a single 4.4 cGy dose of high-LET radiation. The radiation dose employed here may be closer to conditions encountered in space and, therefore, more relevant in assessing health risks for space travelers. It may also have relevance to diagnostic radiology, which has even lower radiation exposures. The enhanced trabecular morphology and architecture observed in this study is consistent with radiation hormesis [33–35], a controversial concept that argues against the generally accepted radiation protection Linear No Threshold standard because of potentially adaptive responses at low radiation levels [36, 37]. As the skeletal assessment here focused only on a small aspect of a complex biologic system at a single time point, negative outcomes in other sub-systems or at other time points are possible and need to be investigated. Regardless, our data emphasize that the radiation response of bone is complex and dependent on dose and radiation quality.
Acknowledgments
We thank Dr. Marcelo Vasquez for generously sharing the tissues that were used for this study. Deep gratitude is expressed to Drs. Ted Bateman, Laura Loudenslager, Louis Pena, and Adam Rusek. Funding by NASA and NIH was greatly appreciated.
Footnotes
Conflict of interest All authors have no conflicts of interest.
References
- 1.Hellweg CE, Baumstark-Khan C (2007) Getting ready for the manned mission to Mars: the astronauts’ risk from space radiation. Naturwissenschaften 94:517–526 [DOI] [PubMed] [Google Scholar]
- 2.Willey JS, Lloyd SA, Nelson GA, Bateman TA (2011) Space radiation and bone loss. Gravit Space Biol Bull 25:14–21 [PMC free article] [PubMed] [Google Scholar]
- 3.Orwoll ES, Adler RA, Amin S, Binkley N, Lewiecki EM, Petak SM, Shapses SA, Sinaki M, Watts NB, Sibonga JD (2013) Skeletal health in long-duration astronauts: nature, assessment, and management recommendations from the NASA bone summit. J Bone Miner Res 28:1243–1255 [DOI] [PubMed] [Google Scholar]
- 4.Judex S, Zhang W, Donahue LR, Ozcivici E (2013) Genetic loci that control the loss and regain of trabecular bone during unloading and reambulation. J Bone Miner Res 28:1537–1549 [DOI] [PubMed] [Google Scholar]
- 5.Schimmerling W (2010) Accepting space radiation risks. Radiat Environ Biophys 49:325–329 [DOI] [PubMed] [Google Scholar]
- 6.Kim MHY, De Angelis G, Cucinotta FA (2011) Probabilistic assessment of radiation risk for astronauts in space missions. Acta Astronaut 68:747–759 [Google Scholar]
- 7.Adriani O, Barbarino GC, Bazilevskaya GA, Bellotti R, Boezio M et al. (2011) PAMELA measurements of cosmic-ray proton and helium spectra. Science 332:69–72 [DOI] [PubMed] [Google Scholar]
- 8.Williams HJ, Davies AM (2006) The effect of X-rays on bone: a pictorial review. Eur Radiol 16:619–633 [DOI] [PubMed] [Google Scholar]
- 9.Green DE, Adler BJ, Chan ME, Rubin CT (2012) Devastation of adult stem cell pools by irradiation precedes collapse of trabecular bone quality and quantity. J Bone Miner Res 27:749–759 [DOI] [PubMed] [Google Scholar]
- 10.Alwood JS, Kumar A, Tran LH, Wang A, Limoli CL, Globus RK (2012) Low-dose, ionizing radiation and age-related changes in skeletal microarchitecture. J Aging Res 2012:481983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lloyd SA, Bandstra ER, Travis ND, Nelson GA, Bourland JD, Pecaut MJ, Gridley DS, Willey JS, Bateman TA (2008) Space-flight-relevant types of ionizing radiation and cortical bone: potential LET effect? Adv Space Res 42:1889–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamada T, Tsujii H, Tsuji H, Yanagi T, Mizoe JE, Miyamoto T, Kato H, Yamada S, Morita S, Yoshikawa K, Kandatsu S, Tateishi A, Working Group for the Bone and Soft Tissue Sarcomas (2002) Efficacy and safety of carbon ion radiotherapy in bone and soft tissue sarcomas. J Clin Oncol 20:4466–4471 [DOI] [PubMed] [Google Scholar]
- 13.Hamilton SA, Pecaut MJ, Gridley DS, Travis ND, Bandstra ER, Willey JS, Nelson GA, Bateman TA (2006) A murine model for bone loss from therapeutic and space-relevant sources of radiation. J Appl Physiol 101:789–793 [DOI] [PubMed] [Google Scholar]
- 14.Sawajiri M, Mizoe J (2003) Changes in bone volume after irradiation with carbon ions. Radiat Environ Biophys 42:101–106 [DOI] [PubMed] [Google Scholar]
- 15.Sawajiri M, Mizoe J, Tanimoto K (2003) Changes in osteoclasts after irradiation with carbon ion particles. Radiat Environ Biophys 42:219–223 [DOI] [PubMed] [Google Scholar]
- 16.Kondo H, Searby ND, Mojarrab R, Phillips J, Alwood J, Yumoto K, Almeida EA, Limoli CL, Globus RK (2009) Total-body irradiation of postpubertal mice with (137)Cs acutely compromises the microarchitecture of cancellous bone and increases osteoclasts. Radiat Res 171:283–289 [DOI] [PubMed] [Google Scholar]
- 17.Willey JS, Livingston EW, Robbins ME, Bourland JD, Tirado-Lee L, Smith-Sielicki H, Bateman TA (2010) Risedronate prevents early radiation-induced osteoporosis in mice at multiple skeletal locations. Bone 46:101–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukuda S, Iida H, Yan X (2002) Preventive effects of running exercise on bones in heavy ion particle irradiated rats. J Radiat Res 43:S233–S238 [DOI] [PubMed] [Google Scholar]
- 19.Bandstra ER, Thompson RW, Nelson GA, Willey JS, Judex S, Cairns MA, Benton ER, Vazquez ME, Carson JA, Bateman TA (2009) Musculoskeletal changes in mice from 20–50 cGy of simulated galactic cosmic rays. Radiat Res 172:21–29 [DOI] [PubMed] [Google Scholar]
- 20.Zeitlin C, Heilbronn L, Miller J (1998) Detailed characterization of the 1087 MeV/nucleon iron-56 beam used for radiobiology at the alternating gradient synchrotron. Radiat Res 149:560–569 [PubMed] [Google Scholar]
- 21.Wilson JW, Townsend LW, Nealy JE, Chun SY, Hong BS, Buck WW, Lamkin SL, Ganapol BD, Khan F, Cucinotta FA (1989) Bryntrn: a baryon transport model, Washington, DC [Google Scholar]
- 22.Turner CH, Burr DB (1993) Basic biomechanical measurements of bone: a tutorial. Bone 14:595–608 [DOI] [PubMed] [Google Scholar]
- 23.Hopewell JW (2003) Radiation-therapy effects on bone density. Med Pediatr Oncol 41:208–211 [DOI] [PubMed] [Google Scholar]
- 24.Bandstra ER, Pecaut MJ, Anderson ER, Willey JS, De Carlo F, Stock SR, Gridley DS, Nelson GA, Levine HG, Bateman TA (2008) Long-term dose response of trabecular bone in mice to proton radiation. Radiat Res 169:607–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willey JS, Grilly LG, Howard SH, Pecaut MJ, Obenaus A, Gridley DS, Nelson GA, Bateman TA (2008) Bone architectural and structural properties after 56Fe26+ radiation-induced changes in body mass. Radiat Res 170:201–207 [DOI] [PubMed] [Google Scholar]
- 26.Frassica DA (2003) General principles of external beam radiation therapy for skeletal metastases. Clin Orthop Relat Res 415:S158–S164 [DOI] [PubMed] [Google Scholar]
- 27.Garmatis CJ, Chu FC (1978) The effectiveness of radiation therapy in the treatment of bone metastases from breast cancer. Radiology 126:235–237 [DOI] [PubMed] [Google Scholar]
- 28.FitzGerald TJ, McKenna M, Rothstein L, Daugherty C, Kase K, Greenberger JS (1986) Radiosensitivity of human bone marrow granulocyte-macrophage progenitor cells and stromal colony-forming cells: effect of dose rate. Radiat Res 107:205–215 [PubMed] [Google Scholar]
- 29.Kolesnikova AI, Konoplyannikov AG, Hendry JH (1995) Differential sensitivity of two predominant stromal progenitor cell subpopulations in bone marrow to single and fractionated radiation doses. Radiat Res 144:342–345 [PubMed] [Google Scholar]
- 30.Matsumura S, Hiranuma H, Deguchi A, Maeda T, Jikko A, Fuchihata H (1998) Changes in phenotypic expression of osteoblasts after X irradiation. Radiat Res 149:463–471 [PubMed] [Google Scholar]
- 31.Dudziak ME, Saadeh PB, Mehrara BJ, Steinbrech DS, Greenwald JA, Gittes GK, Longaker MT (2000) The effects of ionizing radiation on osteoblast-like cells in vitro. Plast Reconstr Surg 106:1049–1061 [DOI] [PubMed] [Google Scholar]
- 32.Ikeda S, Hachisu R, Yamaguchi A, Gao YH, Okano T (2000) Radiation retards muscle differentiation but does not affect osteoblastic differentiation induced by bone morphogenetic protein-2 in C2C12 myoblasts. Int J Radiat Biol 76:403–411 [DOI] [PubMed] [Google Scholar]
- 33.Calabrese EJ, Baldwin LA (2000) Radiation hormesis: its historical foundations as a biological hypothesis. Hum Exp Toxicol 19:41–75 [DOI] [PubMed] [Google Scholar]
- 34.Zhang L, Tian Y, Wu Y, Zhang H, Wang Z, Huo H, Zhang Y, Zhang M, Ning P, Jiang J (2010) Low-dose radiation-induced hormetic effect on hematopoietic reconstitution. Int J Radiat Biol 86:329–333 [DOI] [PubMed] [Google Scholar]
- 35.Song XS, Zhou XZ, Zhang G, Dong QR, Qin L (2010) Low-dose X-ray irradiation promotes fracture healing through up-regulation of vascular endothelial growth factor. Med Hypotheses 75:522–524 [DOI] [PubMed] [Google Scholar]
- 36.Feinendegen LE (2005) Evidence for beneficial low level radiation effects and radiation hormesis. Br J Radiol 78:3–7 [DOI] [PubMed] [Google Scholar]
- 37.Mitchel RE (2007) Cancer and low dose responses in vivo: implications for radiation protection. Dose Response 5:284–291 [DOI] [PMC free article] [PubMed] [Google Scholar]


