Abstract
In female squirrel monkeys (Saimiri sciureus), the reproductive period normally extends from ~2.5 years to the mid-teens. In the present study, we examined the age-associated cytological changes in the ovaries of 24 squirrel monkeys ranging in age from newborn to ~20 years. We found a significant, age-related decline in the number of primordial follicles, with the most pronounced loss occurring between birth and 5 years. After ~8 years of age, relatively few primordial follicles were evident in the ovarian sections examined. An unusual feature of the aging squirrel monkey ovary is the emergence of highly differentiated, encapsulated clusters of granulosa cells that increase in size and number, particularly after the age of 8 years. Many of these cells express anti-Müllerian hormone, and, histologically, the clusters resemble granulosa cell tumors in humans. However, granulosa cell clusters (GCCs) are present in both ovaries of all older squirrel monkeys, and they display no obvious signs of malignancy, suggesting that they are a normal feature of ovarian aging in this species. Our findings indicate that reproductive senescence in female squirrel monkeys, as in other primates, involves the inexorable depletion of ovarian follicles. In addition, the consistent appearance of abundant, well-differentiated clusters of granulosa cells in older squirrel monkeys, prior to the cessation of reproduction, suggests that these structures may influence the later stages of reproductive potential in this species. Analysis of GCCs in older squirrel monkeys also could yield insights into the pathophysiology of granulosa cell tumors in humans.
Keywords: aging, squirrel monkey, primordial follicle, granulosa cell tumor, hormones, ovary, menopause, reproduction
Introduction
Squirrel monkeys (Saimiri sciureus) are small New World primates with a maximum lifespan of ~30 years (Helvacioglu et al. 1994). Female squirrel monkeys reach reproductive maturity between 2.5 and 3.5 years of age (Taub 1980, Coe et al. 1981, Dukelow 1985); they breed seasonally, have a short ovarian cycle (7–12 days; Lang 1967, Gould et al. 1973, Travis & Holmes 1974, Wolf et al. 1977), and give birth approximately once every 2 years (Garber & Leigh 1997). Female squirrel monkeys can remain reproductively active up to the age of ~16 years (Mendoza 1999). However, with advancing age, they exhibit diminished rates of conception and increased rates of abortion (Diamond et al. 1985). In older animals (>12 years), progesterone levels fall, peak estrogen concentrations increase, and ovulation, as inferred from LH peaks, becomes increasingly infrequent (Helvacioglu et al. 1994).
Unlike many primate species (Walker & Herndon 2008), squirrel monkeys show no externally visible evidence of menstrual bleeding (Dukelow 1985), and their idiosyncratic hormonal profile distinguishes them from other primates (Yeoman et al. 1991), especially Old World species (Coe et al. 1985). In particular, squirrel monkeys have significantly higher circulating ovarian steroid hormone levels than do humans, rhesus monkeys, and marmosets (Hearn & Lunn 1975), and the high concentrations of these hormones appear to be associated with a compensatory decrease in the number (Chrousos et al. 1984) and sensitivity (Chrousos et al. 1982) of steroid receptors.
The ovary is a critical regulator of mammalian reproductive senescence (vom Saal & Finch 1988). In humans and other primates, the number of primordial follicles (the vesicles containing primary oocytes) reaches a maximum during prenatal development (Baker 1972), after which, through the process of atresia (Nichols et al. 2005), follicles are inexorably depleted with age (Gosden 2004, Jones et al. 2007). Follicle number is thus a strong predictor of fertility and, ultimately, sterility (Nicosia 1987). Other morphological changes that have been described include gross shrinkage of the ovary, a relative decrease in the volume of the cortex and an increase in medullary volume, an expansion of the stromal cell compartment, and progressively fewer evolving follicles and corpora lutea (Nicosia 1987).
Although the unusual ovarian hormonal profile of squirrel monkeys has been well documented, little is known about the microstructure of the ovary in Saimiri, and no studies have systematically examined ovarian changes that occur with age in this species. The goal of the present study was to assess the trajectory of primordial follicle depletion and the general histological features of ovaries in squirrel monkeys from birth through the entire reproductive age span.
Materials and Methods
Subjects
Ovarian tissue samples from 24 squirrel monkeys (Saimiri sciureus), ranging in age from birth (0.03 years) to ~20 years, were collected, processed for histological analysis, and archived over a 10-year period as part of routine necropsies at the Yerkes National Primate Research Center (Table 1). The subjects had not been used previously in studies that might have altered their reproductive function, and no animal was pregnant at the time of death (two animals died shortly after giving birth; Table 1). The deaths of the subjects occurred throughout the year, with no predilection for any particular season. The ages of all monkeys less than 8 years of age are known, whereas the ages of those 8 years old and over were estimated by experienced primate veterinarians based on the animals’ physical characteristics at the time of arrival at Yerkes. These latter subjects all were obtained as juveniles or young adults, and had been housed at the Yerkes facility for a minimum of 7 years (Mean years in residence = 12.66 years). This study was conducted in accordance with the Principles for the Ethical Treatment of Non Human Primates, and has been approved by the Yerkes National Primate Research Center, which is fully accredited by AAALAC. The animals at the Yerkes Center are maintained in compliance with all local and federal laws governing animal research.
Table 1.
Age (years), mean number of primordial follicles per section, follicle density, and prevalence of granulosa cell clusters (GCCs; % of total area) for 24 squirrel monkeys between birth and ~20 years of age.
| Subject # | Age (yrs) | Primordial Follicles | Follicle Density/mm2 | GCCs (%) (0–3) |
|---|---|---|---|---|
| 93–239 | 0.03 | 1082.00 | 109.60 | 0.00 |
| 91–245 | 0.12 | 333.50 | 236.52 | 0.00 |
| 92–226 | 0.18 | 1325.50 | 313.35 | 0.00 |
| 91–14 | 1.60 | 702.00 | 73.63 | 3.00 |
| 93–293 | 2.11 | 762.00 | 56.13 | 7.70 |
| 90–255* | 3.02 | 458.75 | 24.65 | 0.00 |
| 86–143 | 4.17 | 108.00 | 9.92 | 3.50 |
| 85–140 | 4.23 | 45.75 | 1.05 | 0.00 |
| 91–79 | 5.74 | 342.50 | 17.79 | 1.00 |
| 86–37 | 8+ | 1.75 | 0.09 | 12.0 |
| 86–207 | 9+ | 6.75 | 0.18 | 63.3 |
| 87–158* | 10+ | 11.25 | 0.54 | 27.2 |
| 86–174 | 13+ | 14.75 | 1.26 | 28.9 |
| 87–166 | 13+ | 6.50 | 0.34 | 51.7 |
| 87–183 | 13+ | 27.00 | 2.30 | 29.6 |
| 89–289 | 15+ | 0.50 | 0.01 | 20.0 |
| 90–166 | 15+ | 45.00 | 2.52 | 26.8 |
| 90–176 | 15+ | 0.50 | 0.05 | 20.5 |
| 90–36 | 16+ | 0.00 | 0.00 | 67.9 |
| 90–54 | 16+ | 58.00 | 2.63 | 13.3 |
| 93–43 | 18+ | 1.25 | 0.05 | 54.5 |
| 94–92 | 19+ | 1.25 | 0.07 | 40.9 |
| 94–114 | 19+ | 1.25 | 0.04 | 25.2 |
| 93–64 | 20+ | 0.00 | 0.00 | 16.8 |
Estimated ages;
Animal gave birth shortly before death
Histology and Quantitation
Ovaries obtained at necropsy were fixed in 10% formalin, embedded in paraffin wax, and sectioned at 5-mm thickness. Sections were mounted onto glass microscope slides, stained with hematoxylin and eosin and coverslipped. In addition, archived, paraffin-embedded tissue blocks were available from seven of the monkeys. Sections from these blocks were stained with Masson’s trichrome stain, which enables the simultaneous identification of cells and connective tissue. Additional sections were immunostained with a mouse MAB to human AMH (MCA2246TAMH; AbD Serotec, Raleigh, NC, USA), which, in the ovary, is specific for granulosa cells (Rajpert-De Meyts et al. 1999, Weenen et al. 2004). Briefly, the sections were de-paraffinized and endogenous peroxidase was inactivated with 3% H2O2 in methanol (1 h, room temperature). Nonspecific reagent binding was blocked with 2% normal serum in 0.2% Tween (1 h, room temperature), and antigenic sites were exposed by heating the tissue at 95 degrees C for 30 min in citrate buffer (pH 6). The sections then were incubated in primary antibody (1:100, diluted in buffer with blocking serum) overnight at 4 degrees C. After rinsing, they were incubated for 1 h at room temperature in biotinylated secondary antibody, rinsed again, immersed for 30 min in avidin–biotin complex, and then developed with diaminobenzidine (Vectastain Elite ABC Kit, Vector Laboratories, Burlingame, CA, USA). A hematoxylin counterstain was applied after immunostaining. Photographs were taken with a Spot Flex digital camera (Diagnostic Instruments, Sterling Heights, MI, USA) attached to a Leica DMLB microscope (Wetzlar, Germany).
Quantitation of follicles and GCCs
Two investigators independently examined the hematoxylin and eosin-stained slides from each of the 24 cases. In 20 of the subjects, two sections per monkey were analyzed, one from each ovary; in four subjects, only one slide was available from a single ovary. Because only archived tissues were available for analysis, the orientation and plane of section could not be systematically controlled, and stereological quantitation was not possible (Myers et al. 2004). Nevertheless, previous studies have demonstrated the value of single tissue sections in assessing ovarian aging and follicular loss both in human (Westhoff et al. 2000) and nonhuman primates (Jones et al. 2007).
Primordial follicles were systematically counted at 200X magnification using an ocular grid in a Leica DMLS microscope. A primordial follicle was defined as an ‘oocyte surrounded by a single layer of flattened granulosa cells’ (Miller et al. 1997). The follicular counts made by the two observers showed a highly significant correlation (r(22)=0.97, P<0.0001); therefore, the mean value of the counts per subject made by the two observers were used for all data analyses. To control for variability in the size of the ovaries and the area of the tissue samples, the planar area of ovarian tissue on each slide was calculated by stereological point-counting methods (Mouton 2002). Briefly, all tissue sections were photographed, and a grid of crosses, each defining an area of known dimensions (1mm2), was superimposed over the section. The total tissue area (in mm2) was calculated from the number of intersection points between the tissue and a defined point on the crosses. The planar area (mm2) of ovarian GCCs in the tissue sections was also determined using stereological methods. We noted cytological evidence of active folliculogenesis, as evidenced by the presence of maturing and atretic follicles (Table 1), with the caveat that ovulatory mechanisms can vary across the year in seasonally reproducing primates (Walker et al. 1983, Hutz et al. 1985, Zheng et al. 2001).
Data Analysis
The data were analyzed using Pearson’s product-moment correlations (r). To enable age comparisons of follicle loss, log transformations of the mean number of primordial follicles and follicle density for each subject were calculated. These numbers were used for subsequent, relevant data analyses.
Results
General ovarian histology
At birth, the ovaries in squirrel monkeys are small and the cortex is densely packed with primordial follicles (Fig. 1A). In postmenarcheal females (~3–6 years of age), the ovaries are larger and show signs of active folliculogenesis, as characterized by follicular growth and an increased presence of vesicular follicles (Fig. 1B) and corpora lutea. Most granulosa cells of developing follicles are immunoreactive for anti-Müllerian hormone (AMH; Fig. 2A). Granulosa cells in primordial follicles are negative for AMH, as are nearly all granulosa cells in atretic follicles. As the animals age from birth into adulthood, the diminishing stores of primordial follicles are progressively confined to a relatively thin, peripheral zone of the ovary, and the medullary compartment expands. In addition, especially after the age of ~8 years, both ovaries are increasingly occupied by well-circumscribed clusters of granulosa cells, each delimited by a thin shell of connective tissue (Fig. 1C–E) that also is demonstrable in trichrome-stained sections (not shown). Many cells in these clusters are immunoreactive for AMH (Fig. 2B; see below). The clusters often are separated by stromal cells, although they can directly abut one another (Fig. 1E), particularly when they are abundant. The size of the stromal compartment varies among adult animals. Hemosiderin is present in some adult ovaries, indicative of past bleeds, but there was little evidence of significant hemorrhage in any case, including the older animals with profuse granulosa cell clusters (GCCs). Based on this archival sample of tissue sections, maximum ovarian size is reached by ~5 years of age and remains relatively stable thereafter.
Figure 1.
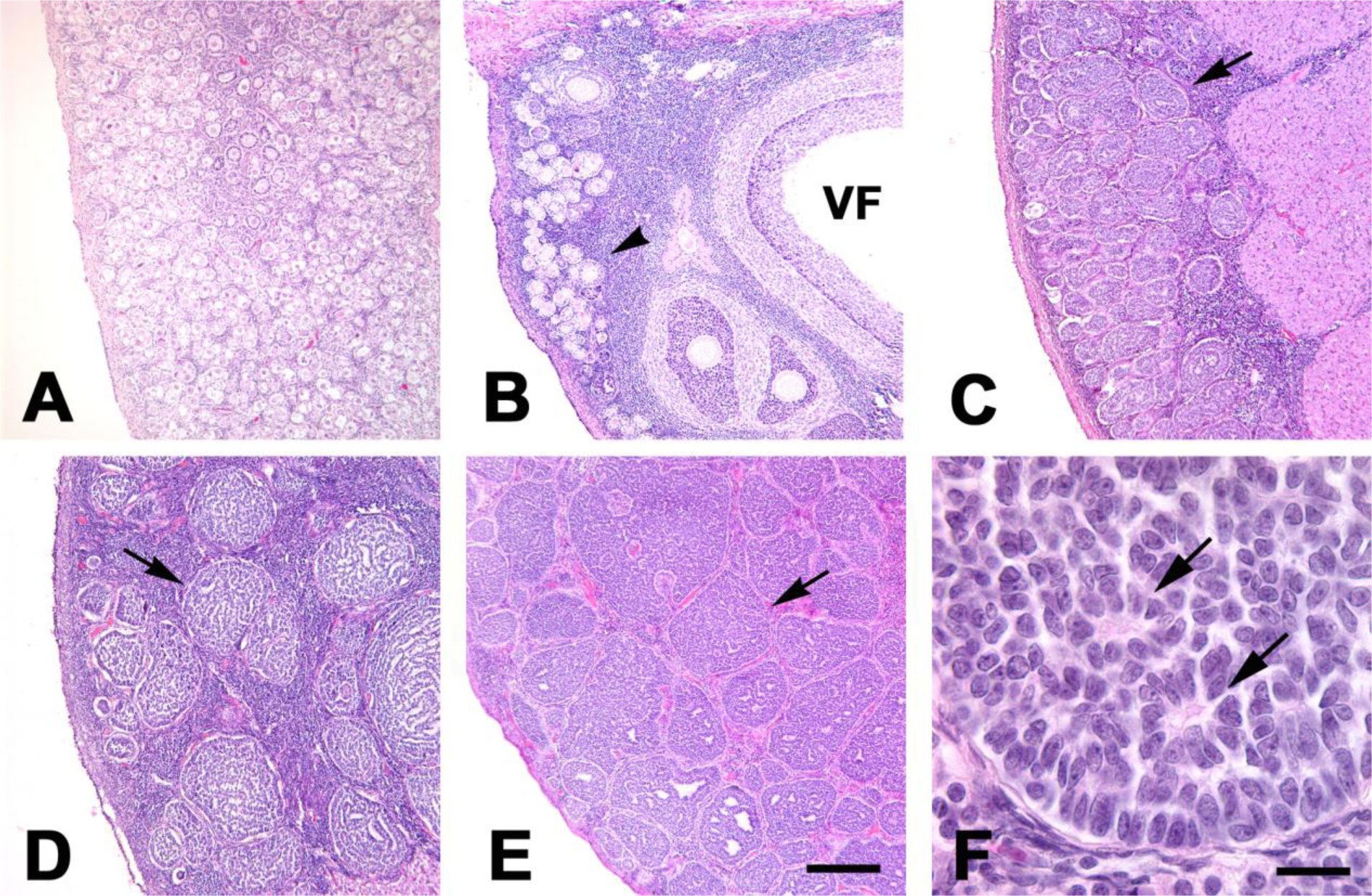
(A–F) Photomicrographs of ovarian sections from squirrel monkeys at five different ages: (A) 0.18 years; (B) 5.74 years; (C) ~8 years; (D) ~13 years; (E) ~18 years. (A) Shortly after birth, much of the ovary is occupied by primordial follicles. (B) In a young adult female, follicles of all stages are present. Primordial and early stage follicles (arrowhead) are mainly located in the cortex just beneath the surface of the ovary, whereas more developed follicles are more deeply situated. A vesicular (antral) follicle is indicated (VF). In older squirrel monkeys, granulosa cell clusters, each surrounded by a thin shell of connective tissue, become increasingly prominent (arrows in C, D, and E). In D, the cells in the clusters line profuse lacunae and channels, whereas in C and E these spaces are less apparent. Note the relative abundance of stromal cells in the spaces separating the clusters in D compared to E. Panel F is a high-magnification photomicrograph of a granulosa cell cluster in the ovary of a ~15-year-old squirrel monkey with characteristic Call-Exner bodies (arrows). Hematoxylin and eosin stain. Bar in E, 200 microns for panels A–E; Bar in F, 20 microns.
Figure 2.
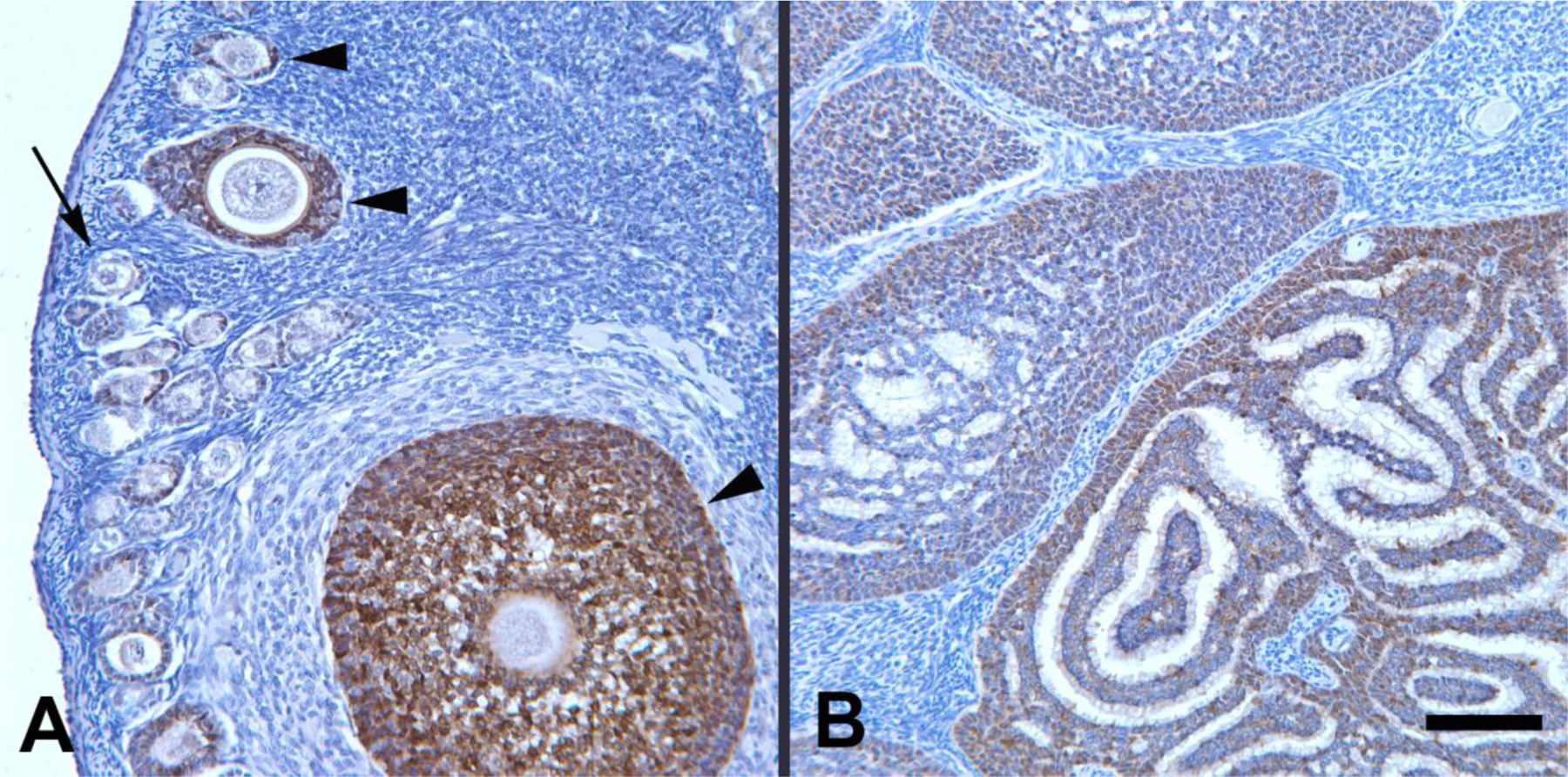
Anti-Müllerian hormone (AMH) immunoreactivity (brown) in ovarian granulosa cells of two squirrel monkeys. (A) AMH-immunoreactive follicles (arrowheads) at different stages of development in a 5.74-year-old squirrel monkey (91–79). Most granulosa cells in the developing follicles are intensely immunoreactive, as compared to the granulosa cells in a primordial follicle (arrow), which are negative for AMH. (B) Several AMH-immunoreactive granulosa cell clusters in a ~10-year-old squirrel monkey (87–158); AMH-immunoreactivity sometimes is more apparent in the cells of the periphery of the structures. Hematoxylin counterstain. Bar, 100 microns for both A and B.
Primordial follicle counts
At birth, primordial follicles occupy much of the ovarian volume, but as the animals age and the ovary grows, the relative size of the region occupied by primordial follicles diminishes, and they become increasingly sparse in the cortical shell (Fig. 1). The number of primordial follicles per section declines significantly with age, whether the data are analyzed using log-transformed follicle counts (r(22)= −0.854, P<0.001; Fig. 3) or raw counts (r(22)= −0.744, P<0.001; Table 1). An initially rapid decline in the number of primordial follicles is evident, with the asymptote reached near the age of 8 years, followed thereafter by consistently low numbers of follicles.
Figure 3.
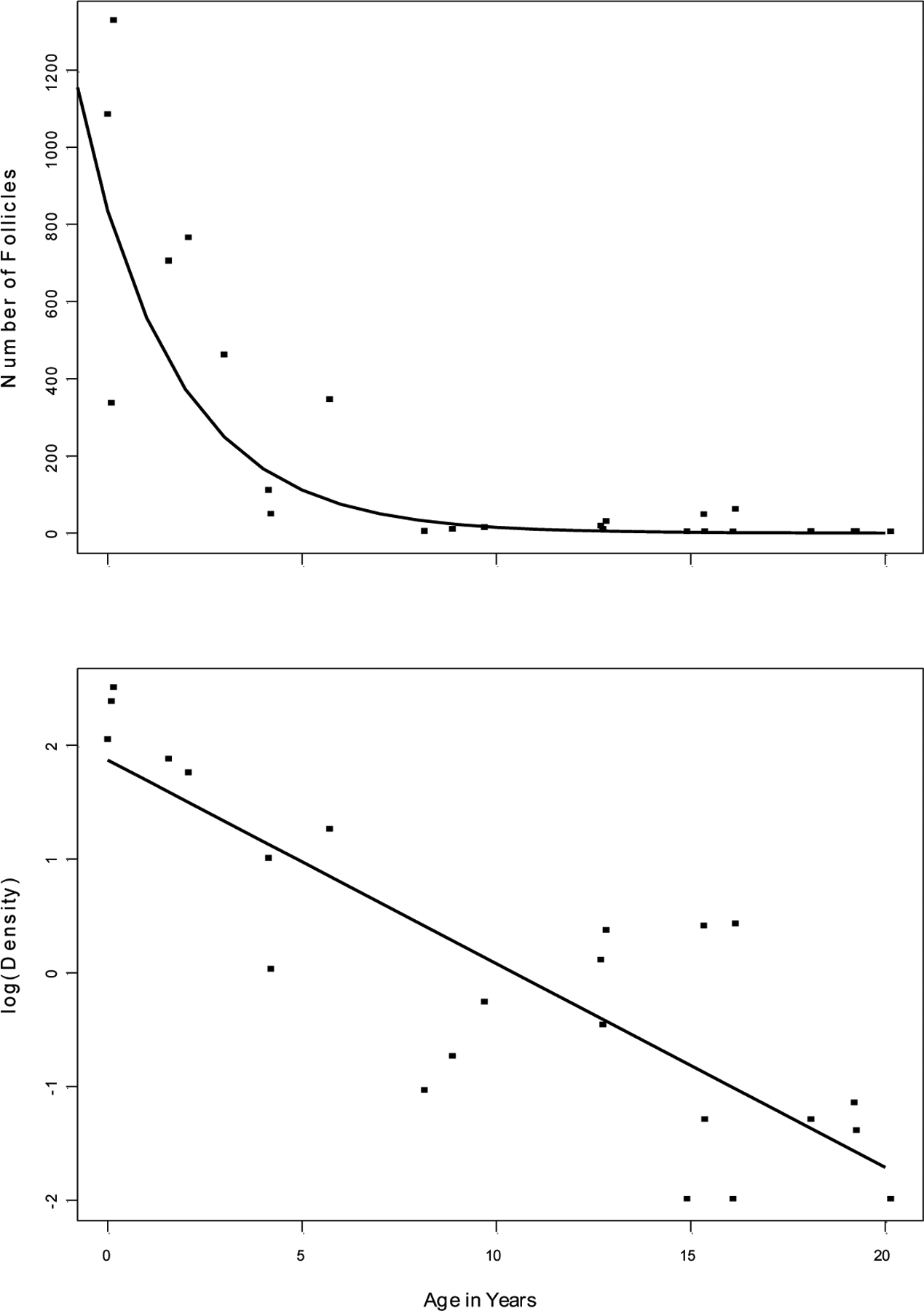
(Top panel) The number of primordial follicles in the ovaries of 24 female squirrel monkeys ranging in age from 0.03 to ~20 years of age. The data are fitted with a regression line based on the log-transformed density values (r = −0.854). (Lower panel) Log- transformed primordial follicular density (follicles/mm2) in ovaries from 24 squirrel monkeys ranging in age from 0.03 to ~20 years. The data are fitted with a linear regression line of best fit (r = −0.856).
To account for tissue size as a potential confounding variable in the assessment of primordial follicle number, we also determined the density of follicles based on the planar area of each section. Follicle density (number of follicles/mm2) for each animal is shown in Table 1. As was the case with absolute follicle number, follicle density was highest in the youngest monkeys and showed a significant, inverse correlation with age (r= −0.632, P<.001; Fig. 3).
Granulosa Cell Clusters
In older animals, numerous clusters of cells (Fig. 1C–F), encapsulated by a shell of connective tissue, were distributed throughout both ovaries. The arrangement and appearance of cells within these clusters varied somewhat among animals (and, to some extent, among the clusters within individual ovaries). Many of these cells express AMH (Fig. 2B), as is typical of the granulosa cells in developing follicles (Fig. 2A). The prevalence of GCCs correlated directly with age (r(22)=0.639, P<0.001 (Fig. 4 and Table 1). In examining hundreds of clusters in the older monkeys, we found little evidence of intact ova or Graafian-type vesicles that would indicate that the clusters are engaged in active folliculogenesis. In some instances, the clusters included cellular rosettes known as Call-Exner bodies (Fig. 1F), which have been described in normal developing follicles (Harrison 1962) and in human granulosa cell tumors (Orr 1962). As in granulosa cell tumors (Fox & Buckley 1992), there was a degree of variation in the cellular organization within the clusters in Saimiri. For instance, the cells sometimes were organized around apparent channels, reminiscent of secretory tissues (Figs 1D and 2B). In the depths of some clusters, a degenerate region was present in which cells appeared to be undergoing apoptosis. However, evidence of mitotic activity (mitotic figures) was rare. Because the clusters occurred bilaterally in all adult squirrel monkeys, consisted of spatially confined, well-differentiated cells, displayed no evidence of excessive mitosis, and did not cause obvious swelling of the ovaries, we conclude that they are not malignant in nature, but rather are a normal feature of aging in this species. Hence, we refer to them in squirrel monkeys as “granulosa cell clusters”, rather than “granulosa cell tumors”.
Figure 4.
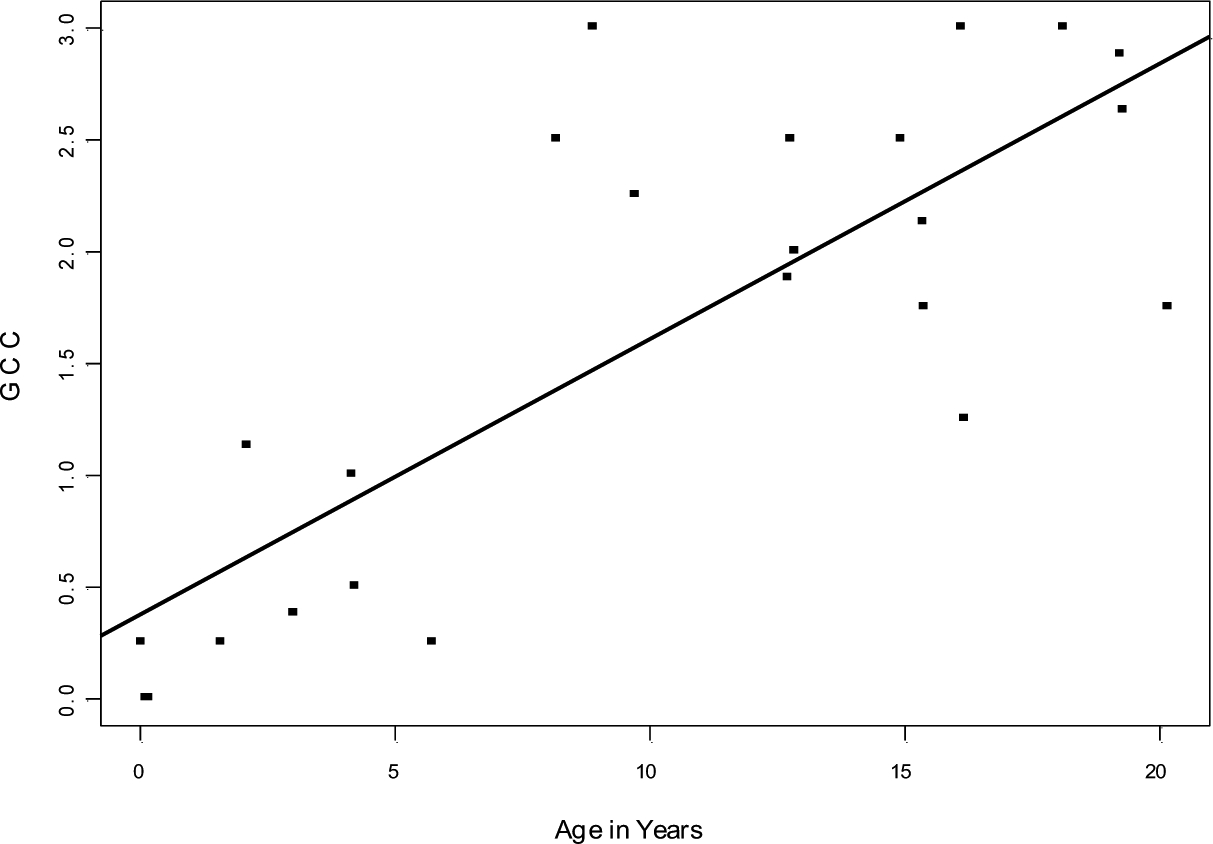
Prevalence (% of total area) of granulosa cell clusters (GCC) in the ovaries of 24 squirrel monkeys ranging in age from 0.03 to ~20 years of age. The data are fitted with a rectilinear line of best fit (r = 0.639).
Seasonality and Ovarian Histology.
Although no breeding season data were available for these animals, the month of death was recorded for each subject. Every month of the year was represented in our sample, and no relationship between the month of death and follicle number or the incidence of granulosa cell clusters was found.
Discussion
The ovaries of squirrel monkeys undergo conspicuous microstructural changes with age. At birth, the relatively abundant ovarian cortex is replete with primordial follicles, in the absence of follicular maturation. As the animals advance into adulthood, the primordial follicles are increasingly confined to the outer cortical region while the medulla increases in relative volume, similar to the changes in developing human ovaries (Nicosia 1983). In reproductively viable female squirrel monkeys (>2.5 years of age), the ovaries show signs of active follicular growth and maturation, atresia, and luteinization. At the same time, there is an inexorable depletion of primordial follicles and, in older animals (>8 years) in particular, a proliferation of unusual clusters of granulosa cells throughout much of the ovarian substance. The number of stromal cells varies among adult animals. Stromal cell hyperplasia is a characteristic of the postmenopausal human ovary (Boss et al. 1965), and is thought to be a normal, nonpathologic correlate of aging (Mossman & Duke 1973).
The loss of ovarian follicles with increasing age is consistent with findings in other primate species (Green & Zuckerman 1954, Nozaki et al. 1997, Miller et al. 1999, Nichols et al. 2005, Jones et al. 2007). Unlike humans, in which the rate of follicular depletion may accelerate in the late stages of reproductive viability (Faddy & Gosden 1996), the most marked loss of follicles postnatally in Saimiri occurs during the first 5 years of life. The number of follicles in our sample bore no obvious relationship to the time of year during which the ovaries were collected, or to the immediate postpartum status of the two females who had recently given birth (animal 90–255: 3.02 years old, mean of 458.75 follicles per ovarian section; animal 87–158: ~10 years old, mean of 11.25 follicles per ovarian section). As in other mammals (Franchi et al. 1962), the number of follicles varied among individual squirrel monkeys within a given age range (Fig. 3). Evidence of some degree of follicular cycling (maturation and atresia) was present even in several older monkeys.
Although detailed breeding records were not available for the animals in this study, archival data showed that fertility was maintained in some of the older females, consistent with reports of continued fertility in squirrel monkeys into the mid-teens (Mendoza 1999). In human females, follicular reserves number ~300,000 at menarche (Block 1952, Richardson et al. 1987) and continue to decline with age until the supply of follicles reaches a critical threshold at menopause (Ottolenghi et al. 2004). Even when the number of follicles in humans falls to just a few hundred, however, pregnancy is still possible (Richardson et al. 1987). In the present study, the oldest squirrel monkeys (~13–20 years of age) had nearly 1000 times fewer follicles than did the youngest animals (0.03–2 years of age), and the oldest female (~20 years old) had neither primordial follicles nor evidence of folliculogenesis in the sections examined. As observed by vom Saal & Finch (1988), ‘there is little doubt that ovarian exhaustion of follicles is the pacemaker of reproductive senescence,’ and total follicular depletion in humans often is viewed as a structural hallmark of menopausal status (Richardson et al. 1987). Based on analyses of humans and other primates (Green & Zuckerman 1954, Walker 1995, Nozaki et al. 1997, Miller et al. 1999, Nichols et al. 2005), the present study suggests that, from the standpoint of ovarian follicular reserve and maturation, squirrel monkeys may reach menopause sometime in the mid-to-late teens. A prospective study of ovarian structure and function, in relation to behavior, fertility and endocrine parameters, would be invaluable in more fully characterizing reproductive aging in squirrel monkeys.
A striking anomaly in aging squirrel monkeys is the emergence of multiple, encapsulated GCCs in the ovaries. In younger, postmenarcheal females, small, scattered aggregates of such cells were present, but in older females, nearly the entire ovary often was filled with prominent clusters (Fig. 1C–E). The arrangement of granulosa cells displayed remarkable variation, from a somewhat haphazard distribution with occasional lacunes and rosettes, to the presence of well-organized arrays of cells adjoining extensive lacunes and channels, suggestive of secretory activity. A large proportion of these cells were immunoreactive with an antibody to AMH. In the ovaries of other species, AMH occurs only in granulosa cells, and then only in developing follicles, and not in primordial or atretic follicles (Rajpert-De Meyts et al. 1999, Weenen et al. 2004). Experimental evidence indicates that AMH acts to suppress the evolution of primordial follicles into developing follicles (Durlinger et al. 2001). As a result, a paucity of AMH accelerates the depletion of ovarian reserves and advances the onset of age-associated infertility (see La Marca et al. 2009 for review). Contrariwise, an increase in the expression of ovarian AMH may inhibit ovarian maturation and thereby preserve the follicular supply and lengthen the period of reproductive viability (Mulders et al. 2004, La Marca et al. 2009). In this light, we suggest that AMH produced by the GCCs may function to preserve the small residual population of primordial follicles in aging squirrel monkeys, thereby extending the period of life during which the females are reproductively competent.
It will also be informative to establish whether the clusters of granulosa cells are sustained by age-related changes in gonadotropins, and whether they influence the species-typical hormonal profile of squirrel monkeys. Of the primate species that have been examined to date, squirrel monkeys are remarkable with respect to the natural appearance of ovarian GCCs with age, although the possible presence of such structures in other primates, particularly New World monkeys and prosimians, remains to be determined. A comparative analysis might also assess possible commonalities between the clusters and the ‘anovular follicles’ that have been reported in various mammalian species (League & Hartman 1925, Tamura 1927, Harrison 1962).
GCCs were described in a single squirrel monkey older than 20 years by Rewell (1954), who referred to them as ‘granulosa-cell tumors’. Indeed, they bear a conspicuous resemblance to some types of human granulosa cell tumors, which usually have a low degree of malignancy (Fox & Buckley 1992). However, unlike in squirrel monkeys, human tumors grow to a large size and typically occur only in one ovary (Stenwig et al. 1979). Our observations indicate that the cell clusters in Saimiri consist of well-differentiated cells, are regularly present in both ovaries of all mature squirrel monkeys, and do not cause excessive tumescence; thus, they appear to be a normal, nonpathologic feature of aging in this species. In this light, a comparative analysis of the ovaries of aging squirrel monkeys and humans might yield useful insights into the pathobiology of human granulosa cell tumors.
Acknowledgments
We gratefully acknowledge helpful discussions with Professor Rolf Warzok of the University of Greifswald, and the expert technical assistance of Eileen Breding, Evan Dessasau and Jeromy Dooyema.
Funding
This work was supported by National Institutes of Health (NIH) grant P51 OD011132 to the Yerkes National Primate Research Center.
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported, nor are there any financial or other potential conflicts of interest.
References
- Baker TG 1972. Primordial germ cells In Germ Cells and Fertilization, pp 1–13. Eds Austin CR and Short RV. Cambridge: Cambridge University Press. [Google Scholar]
- Block E 1952. Quantitative morphological investigations of the follicular system in women: variations at different ages. Acta Anatomica 14 pp 108–123. [DOI] [PubMed] [Google Scholar]
- Boss JH, Scully RE, Wegner KH & Cohen RB 1965. Structural variations in the adult ovary: clinical significance. Obstetrics and Gynecology 25 pp 747–764. [PubMed] [Google Scholar]
- Chrousos GP, Renquist DM, Brandon D, Barnard D, Fowler D, Loriaux DL & Lipsett MB 1982. The squirrel monkey: receptor-mediated end-organ resistance to progesterone? Journal of Clinical Endocrinology and Metabolism 55 pp 364–368. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Brandon D, Renquist DM, Tomita M, Johnson E, Loriaux DL & Lipsett MB 1984. Uterine estrogen and progesterone receptors in an estrogen- and progesterone “resistant” primate. Journal of Clinical Endocrinology and Metabolism 58 pp 516–520. [DOI] [PubMed] [Google Scholar]
- Coe CL, Chen J, Lowe EL, Davidson JM & Levine S 1981. Hormonal and behavioral changes at puberty in the squirrel monkey. Hormones and Behavior 15 pp 36–53. [DOI] [PubMed] [Google Scholar]
- Coe CL, Smith ER & Levine S 1985. The endocrine system of the squirrel monkey In Handbook of Squirrel Monkey Research, pp 191–218. Eds Rosenblum LA and Coe CL. New York: Plenum Press. [Google Scholar]
- Diamond EJ, Aksel S, Hazelton JM, Barnet SB, Williams LE & Abee CR 1985. Serum hormone patterns during abortion in the Bolivian squirrel monkey. Laboratory Animal Science 35 pp 619–622. [PubMed] [Google Scholar]
- Dukelow WR 1985. Reproductive cyclicity and breeding in the squirrel monkey In Handbook of Squirrel Monkey Research, pp 169–190. Eds Rosenblum LA and Coe CL. New York: Plenum Press. [Google Scholar]
- Durlinger AL, Gruijters MJ, Kramer P, Karrels B, Kumar TR, Matzuk MM, Rose UM, de Jong FH, Uilenbroek JT, Grootegoed JA et al. 2001. Anti- Müllerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology 142 4891–4899. [DOI] [PubMed] [Google Scholar]
- Faddy MJ & Gosden RG 1996. A model confirming the decline in follicle numbers to the age of menopause in women. Human Reproduction 11 pp 1484–1486. [DOI] [PubMed] [Google Scholar]
- Fox H & Buckley CH 1992. The female genital tract and ovaries In Oxford Textbook of Pathology 2a, pp 1565–1639. Eds McGee JO’D, Isaacson PG and Wright NA. Oxford: Oxford University Press. [Google Scholar]
- Franchi LL, Mandl AM & Zuckerman S 1962. The development of the ovary and the process of oogenesis In The Ovary, pp 1–88. Ed Zuckerman S. New York: Academic Press. [Google Scholar]
- Garber PA & Leigh SR 1997. Ontogenetic variations in small-bodied new world primates: implications for patterns of reproduction and infant care. Folia Primatologica 68 pp 1–22. [DOI] [PubMed] [Google Scholar]
- Gosden RG 2004. Germline stem cells in the postnatal ovary: is the ovary more like a testis? Human Reproduction Update 10 pp 193–195. [DOI] [PubMed] [Google Scholar]
- Gould KG, Cline EM & Williams WL 1973. Observations on the induction of ovulation and fertilization in vitro in the squirrel monkey (Saimiri sciureus). Fertility and Sterility 24 pp 260–268. [PubMed] [Google Scholar]
- Green SH & Zuckerman S 1954. Further observations on oocyte numbers in mature rhesus monkeys (Macaca mulatta). Journal of Endocrinology 10 pp 284–290. [DOI] [PubMed] [Google Scholar]
- Harrison RJ 1962. The structure of the ovary In The Ovary: C. Mammals 1, pp 143–187. Ed Zuckerman S. New York: Academic Press. [Google Scholar]
- Hearn JP & Lunn SF 1975. The reproductive biology of the marmoset monkey (Callithrix jacchus). Laboratory Animal Handbook 6 pp 191–202. [Google Scholar]
- Helvacioglu A, Aksel S, Yeoman RR, Williams LE & Abee CR 1994. Age-related hormonal differences in cycling squirrel monkeys (Saimiri boliviensis boliviensis). American Journal of Primatology 32 pp 207–213. [DOI] [PubMed] [Google Scholar]
- Hutz RJ, Dierschke DJ & Wolf RC 1985. Seasonal effects on ovarian folliculogenesis in rhesus monkeys. Biology of Reproduction 33 pp 653–659. [DOI] [PubMed] [Google Scholar]
- Jones K, Walker L, Anderson D, Lacreuse A, Robson SL & Hawkes K 2007. Depletion of ovarian follicles with age in chimpanzees: Similarities to humans. Biology of Reproduction 77 pp 247–251. [DOI] [PubMed] [Google Scholar]
- Lang CM 1967. The estrous cycle of the squirrel monkey (Saimiri sciureus). Laboratory Animal Care 17 pp 442–451. [PubMed] [Google Scholar]
- League B & Hartman CG 1925. Anovular Graafian follicles in mammalian ovaries. The Anatomical Record 30 pp 1–13. [Google Scholar]
- La Marca A, Broekmans FJ, Volpe A, Fauser BC & Macklon NS 2009. Anti-Müllerian hormone (AMH): what do we still need to know? Human Reproduction 24 2264–2275. [DOI] [PubMed] [Google Scholar]
- Mendoza SP 1999. Squirrel monkeys In The UFAW Handbook on the Care and Management of Laboratory Animals I, pp 591–600. Ed Poole TB. Oxford: Blackwell Science Ltd. [Google Scholar]
- Miller PB, Charleston JS, Battaglia DE, Klein NA & Soules MR 1997. An accurate, simple method for unbiased determination of primordial follicle number in the primate ovary. Biology of Reproduction 56 pp 909–915. [DOI] [PubMed] [Google Scholar]
- Miller PB, Charleston JS, Battaglia DE, Klein NA & Soules MR 1999. Morphometric analysis of primordial follicle number in pigtailed monkey ovaries: symmetry and relationship with age. Biology of Reproduction 61 pp 553–556. [DOI] [PubMed] [Google Scholar]
- Mossman HW & Duke KL 1973. Comparative Morphology of the Mammalian Ovary. Madison: University of Wisconsin Press. [Google Scholar]
- Mouton PR 2002. Principles And Practices Of Unbiased Stereology: An Introduction For Bioscientists. Baltimore: The Johns Hopkins University Press. [Google Scholar]
- Mulders AG, Laven JS, Eijkemans MJ, de Jong PH, Themmen AP & Fauser BC 2004. Changes in anti-Müllerian hormone serum concentrations over time suggest delayed ovarian ageing in normogonadotrophic anovulatory infertility. Human Reproduction 19 2036–2042. [DOI] [PubMed] [Google Scholar]
- Myers M, Britt KL, Wreford NG, Ebling FJ & Kerr JB 2004. Methods for quantifying follicular numbers within the mouse ovary. Reproduction 127 pp 569–580. [DOI] [PubMed] [Google Scholar]
- Nichols SM, Bavister BD, Brenner CA, Didier PJ, Harrison RM & Kubisch HM 2005. Ovarian senescence in the rhesus monkey (Macaca mulatta). Human Reproduction 20 pp 79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicosia SV 1983. Morphological changes of the human ovary throughout life In The Ovary, pp 57–81. Ed Serra GB. New York: Raven Press. [Google Scholar]
- Nicosia SV 1987. The aging ovary. Medical Clinics of North America 71 pp 1–9. [PubMed] [Google Scholar]
- Nozaki M, Yamashita K & Shimuzu K 1997. Age-related changes in ovarian morphology from birth to menopause in the Japanese monkey, Macaca fuscata fuscata. Primates 38 pp 89–100. [Google Scholar]
- Orr JW 1962. Tumours of the ovary and the role of the ovary and its hormones in neoplasia In The Ovary II, pp 533–565. Eds Mandl AM and Eckstein P. New York: Academic Press. [Google Scholar]
- Ottolenghi C, Uda M, Hamatani T, Crisponi L, Garcia J-E, Ko M, Pilia G, Sforza C, Schlessinger D & Forabosco A 2004. Aging of oocyte, ovary and human reproduction. Annals of the New York Academy of Science 1034 pp 117–131. [DOI] [PubMed] [Google Scholar]
- Rajpert-De Meyts E, Jørgensen N, Græm N, Müller J, Cate RL & Skakkebæk NE 1999. Expression of anti-Müllerian hormone during normal and pathological gonadal development: association with differentiation of Sertoli and granulosa cells. Journal of Clinical Endocrinology and Metabolism 84 3836–3844. [DOI] [PubMed] [Google Scholar]
- Rewell RE 1954. Uterine fibromyomas and bilateral ovarian granulosa-cell tumours in a senile squirrel monkey, Saimiri sciurea. Journal of Pathology & Bacteriology LXVIII pp 291–293. [DOI] [PubMed] [Google Scholar]
- Richardson SJ, Senikas V & Nelson JF 1987. Follicular depletion during the menopausal transition: evidence for accelerated loss and ultimate exhaustion. Journal of Clinical Endocrinology and Metabolism 65 pp 1231–1237. [DOI] [PubMed] [Google Scholar]
- Stenwig JT, Hazekamp JT & Beecham JB 1979. Granulosa cell tumors of the ovary. A clinicopathological study of 118 cases with long-term follow-up. Gynecologic Oncology 7 pp 136–152. [DOI] [PubMed] [Google Scholar]
- Tamura Y 1927. On anovular follicles in the ovaries of the sterile dingo and the aged mouse. Journal of Anatomy 61 pp 325–328.1. [PMC free article] [PubMed] [Google Scholar]
- Taub DM 1980. Age at first pregnancy and reproductive outcome among colony-born squirrel monkeys (Saimiri sciureus, Brazilian). Folia Primatologica 33 pp 262–272. [DOI] [PubMed] [Google Scholar]
- Travis JC & Holmes WN 1974. Some physiological and behavioral changes associated with oestrus and pregnancy in the squirrel monkey (Saimiri sciureus). Journal of Zoology. London 174 pp 41–66. [DOI] [PubMed] [Google Scholar]
- vom Saal FS & Finch CE 1988. Reproductive senescence: phenomena and mechanisms in mammals and selected vertebrates In The Physiology of Reproduction 2, pp 2351–2413. Eds Knobil E and Neill J. New York: Raven Press. [Google Scholar]
- Walker ML 1995. Menopause in female rhesus monkeys. American Journal of Primatology 35 pp 59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker ML & Herndon JG 2008. Menopause in nonhuman primates? Biology of Reproduction 79 pp 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker ML, Gordon TP & Wilson ME 1983. Menstrual cycle characteristics of seasonally breeding rhesus monkeys. Biology of Reproduction 29 pp 842–848. [DOI] [PubMed] [Google Scholar]
- Weenen C, Laven JSE, von Bergh ARM, Cranfield M, Groome NP, Visser JA, Kramer P, Fauser BCJM & Themmen APN 2004. Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Molecular Human Reproduction 10 77–83. [DOI] [PubMed] [Google Scholar]
- Westhoff C, Murphy P & Heller D 2000. Predictors of ovarian follicle number. Fertility and Sterility 74 pp 624–628. [DOI] [PubMed] [Google Scholar]
- Wolf RC, O’Connor RF & Robinson JA 1977. Cyclic changes in plasma progestins and estrogens in squirrel monkeys. Biology of Reproduction 27 pp 228–231. [DOI] [PubMed] [Google Scholar]
- Yeoman RR, Williams LE, Aksel S & Abee CR 1991. Mating-related estradiol fluctuations during the estrous cycle of the Bolivian squirrel monkey (Saimiri boliviensis boliviensis). Biology of Reproduction 44 pp 640–647. [DOI] [PubMed] [Google Scholar]
- Zheng P, Si W, Wang H, Zou R, Bavister BD & Ji W 2001. Effect of age and breeding season on the developmental capacity of oocytes from unstimulated and follicle-stimulating hormone-stimulated rhesus monkeys. Biology of Reproduction 64 pp 1417–1421. [DOI] [PubMed] [Google Scholar]


