Abstract
Contrast-induced encephalopathy (CIE) is a rare complication following percutaneous carotid and coronary interventions, and important diagnostic radiological signs include brain edema and cortical enhancement. In this report, we detail a case of probable CIE in an 84-year-old woman following a normal diagnostic coronary angiography (CAG) that involved 20 mL of the low-osmolar, non-ionic monomeric, iodine-based contrast agent iopromide (Ultravist 370). The patient was unconscious and presented with hemiparesis, hemianopia, recurrent seizures, and cardiac and respiratory arrest within minutes to hours following the procedure. Non-contrast computed tomography (CT) of the head showed increased subarachnoid density, cortical enhancement, and brain edema in the right hemisphere. Three days of rehydration, reduction in cranial pressure, and treatment with an anticonvulsant and dexamethasone resulted in a gradual recovery with no neurological deficits. This case highlights that severe neurotoxic symptoms may occur in response to low doses of low-osmolar, non-ionic, monomeric contrast agents. This finding is of importance to interventional cardiologists for diagnostic considerations and development of treatment plans.
Keywords: contrast-induced encephalopathy, coronary angiography, percutaneous carotid and coronary interventions
Introduction
Contrast-induced encephalopathy (CIE) is a rare complication of percutaneous carotid and coronary interventions.1,2 Contrast media, which include ionic, non-ionic, low osmolarity, iso-osmolar, and high osmolarity solutions have been reported to induce CIE.3 Clinical manifestations of CIE include encephalopathy, seizures, motor and sensory disturbances, disturbances in vision, and focal neurological deficits,1,3,4 which fully resolve within 48–72 hours. Heterogeneity of clinical presentation necessitates evaluation of radiological signs associated with contrast encephalopathy, such as brain edema and cortical enhancement, to allow for differentiation between hemorrhagic and thromboembolic complications of angiography.5 Contrast media-induced neurotoxic effects result from the disruption of the blood brain barrier (BBB).6 Hypertension, diabetes mellitus, renal impairment, administration of large volumes of iodinated contrast, percutaneous coronary intervention or selective angiography of internal mammary grafts, and previous adverse reaction to iodinated contrast are common risk factors for development of CIE.2 In this manuscript, we present a case of a patient who presented with severe symptoms of CIE following treatment with a small amount of low-osmolar, non-ionic, monomeric contrast.
Case Report
An 84-year-old woman with a history of hypertension, paroxysmal atrial fibrillation, and chronic bronchitis presented with intermittent chest distress, palpitation, and shortness of breath for several minutes-duration for 3 months. Coronary angiography (CAG) was performed through the right radial approach, which revealed mild plaque disease in the left anterior descending coronary artery (LAD) only. Twenty milliliters of iopromide (Ultravist 370, Bayer Healthcare, Pittsburgh, PA, USA), a low-osmolar, non-ionic, monomeric, iodine-based contrast agent, was administered during the procedure. Local anesthetic (1% lignocaine) was administered prior to CAG. The patient also received 3000 IU of heparin intra-arterially during CAG and did not complain of discomfort during the operation.
Within minutes of radial sheath removal, the patient lost consciousness, and exhibited left limb hemiplegia with muscle strength level 0 and eyes staring to the right. The patient was seen by the stroke team to determine whether she had cerebrovascular disease. Emergency computed tomography (CT) was performed, which showed no acute pathological findings (Figure 1A). Cerebral angiography showed no stenosis, thrombosis, or hemorrhage in the bilateral carotid artery, the middle cerebral artery, or the anterior cerebral artery, which indicated absence of any cerebrovascular events (Supplementary Figure S1). The patient regained consciousness spontaneously and left limb muscle strength returned to level 4, which is considered safe. The patient was then returned to the coronary care unit (CCU) for further treatment.
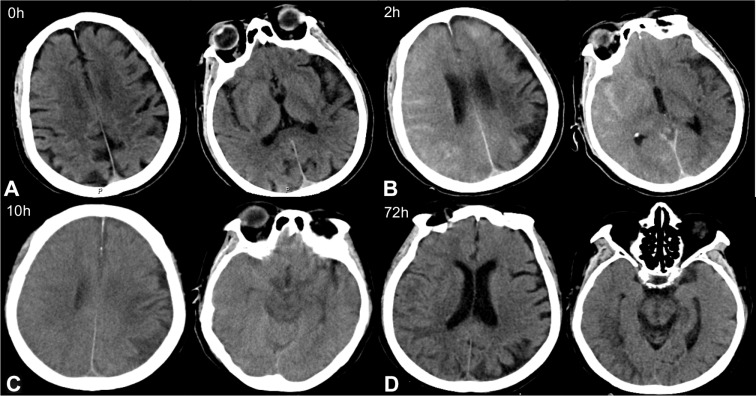
Figure 1.
Contrast-induced encephalopathy in brain computed tomography (CT) scans. (A) Emergency brain CT showed no acute pathological findings. (B) Brain CT 2 hours after surgery indicated multiple high-density regions in the subarachnoid space. (C) Brain CT 10 hours after surgery showed significantly swollen brain tissue, particularly in the right hemisphere, the left frontal lobe, and the left occipital lobe. The high-density shading in the subarachnoid cavity was significantly denser than in previous scans. (D) Brain CT 72 hours after surgery showed the sulci of the right hemisphere became shallow, and low-density shadows could be seen in the frontal parietal lobe. But cerebral edema was significantly improved.
Ten minutes after transfer to the CCU, the patient experienced limb convulsions, jaw chomping, and lost consciousness. Physical examination revealed no response to pain stimulation, general unresponsiveness, eyes gazing to the right, and weak pupillary light reflex. The patient exhibited positive bilateral Babinski signs and neck resistance. The symptoms gradually improved after 2–3 minutes. Brain CT examination 2 hours after surgery showed multiple high-density regions in the subarachnoid space (Figure 1B), which indicated potential subarachnoid hemorrhage or contrast agent leakage. Brain computed tomography angiography (CTA) did not show evidence of macrovascular embolism (Figure 2), and emergency brain magnetic resonance imaging (MRI) showed hyperintense areas could be seen on T2-weighted image and fluid-attenuated inversion recovery (FLAIR) in frontal, parietal, temporal and occipital of right cortex, and no clear signs of subarachnoid hemorrhage were observed in diffusion-weighted imaging (DWI) (Figure 3). The patient was given fluids to accelerate excretion of contrast agent, sodium valproate 60 mg per hour to treat epilepsy, mannitol 125 mL every 6 hours to dehydrate and reduce intracranial pressure, nimodipine 10 mg once a day to prevent vasospasm, and other drugs to improve clinical symptoms. Following this treatment course, the patient suffered from seizures twice and exhibited a steady decline in cardiac rhythm, blood pressure, and blood oxygen level. Brain CT examination 10 hours after surgery showed significantly swollen brain tissue, particularly in the right hemisphere, the left frontal lobe, and the left occipital lobe, with more obvious swelling in the right hemisphere (Figure 1C). The high-density shading in the subarachnoid cavity was significantly more pronounced than that in previous scans. Neurologists excluded cerebral hemorrhage, which suggested that CIE was a possibility.
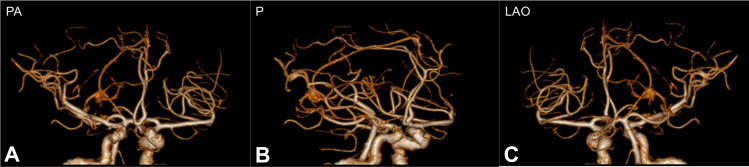
Figure 2.
Computed tomography angiography (CTA) showed no evidence of macrovascular embolism in (A) posteroanterior (PA), (B) posterior (P) and (C) left anterior oblique (LAO) view.
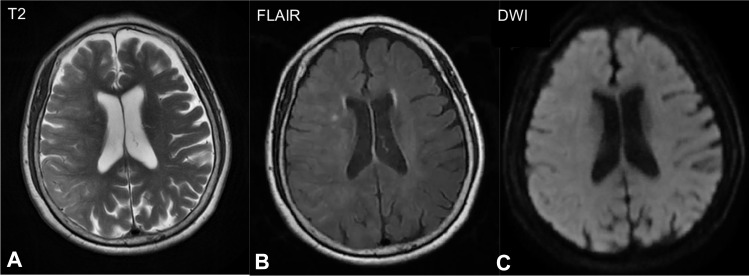
Figure 3.
Brain magnetic resonance imaging (MRI) 2 hours after surgery showed hyperintense areas in the right cortex in (A) T2-weighted image and (B) fluid-attenuated inversion recovery (FLAIR) images, and no clear signs of subarachnoid hemorrhage were observed on (C) diffusion-weighted imaging (DWI).
The patient experienced another seizure 18 hours post CAG, then developed cardio-respiratory arrest. Physical examination showed an absence of carotid pulse and pupil dilation with weak light reflex. Cardiopulmonary resuscitation and tracheal intubation were performed immediately, and one milligram of epinephrine was administered intravenously. Following this treatment course, the patient’s heart rate and blood pressure recovered. The cause of cardio-respiratory arrest was determined to be by mild cerebral hernia preceded by cerebral edema in CIE. Clinical experience and a literature search resulted in administration of 10 mg of dexamethasone once per day for 2 days in addition to treatment with anti-epilepsy drugs, reduction of cranial pressure, and fluid replacement. Following treatment, the patient’s condition gradually improved. Consciousness was restored 72 hours after surgery, and the tracheal tube was removed. Brain CT reexamination showed the sulci of the right hemisphere became shallow, and low-density shadows could be seen in the frontal parietal lobe. However, cerebral edema was significantly improved compared with that observed 3 days prior (Figure 1D). These findings resulted in a decrease in drug dosage that nimodipine and dexamethasone were discontinued, sodium valproate was discontinued and changed to levetiracetam 500 mg twice a day, and mannitol was changed to 125 mL once every 12 hours. Multiple complications such as rib, shoulder blade, and lumbar vertebrae fractures, hemothorax, and loss of front teeth occurred during rescue, each of these injuries improved following active treatment. The patient was discharged after her condition improved. Follow-up 2 months after the operation showed short-term memory loss, but neurological symptoms were absent and limb muscle strength had returned to normal. Brain MRI showed that the sulci and gyri had returned to normal in frontal, parietal, temporal and occipital, cerebral edema had disappeared, and no residual cerebral infarction or hemorrhage was observed. These findings indicated a good prognosis.
Discussion
Contrast-induced encephalopathy is a known uncommon complication associated with use of intravascular radiocontrast media during percutaneous carotid and coronary interventions.1,7 The incidence of CIE ranges between 0.3% and 1.0%. However, use of hyperosmolar iodinated contrast agents results in an incidence of CIE of up to 4%.8,9 Although non-ionic, low-osmolar agents are relatively less neurotoxic,10,11 these agents are associated with pharmacological side effects such as confusion, seizure, altered cerebral function, mental aberrations, and ophthalmoplegia, all of which can induce CIE.2,3,5 Since the first clinical description of CIE in 1970, there have been 36 cases of CIE following cardiac catheterization with non-ionic, low-osmolar contrast agents in a total of 41 patients reported in the literature (Table 1).2,3,5,7,12–43 In this report, we presented a highly probable case of post-CAG CIE following administration of a small amount of non-ionic, low-osmolar contrast agent, with severe symptoms and spontaneous recovery.
Table 1.
Cases of CIE Following Cardiac Catheterization with Non-Ionic, Low-Osmolar Contrast Agents from 1970-Present
| Reference | Age | Gender | Risk Factor | Procedure | Contrast Agent | Contrast Type | Contrast Volume (mL) | Presentation | Neuroimaging | Treatment Provided | Symptom Duration | Complete Symptom Resolution |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eleftheriou et al (2018)12 | 57 | F | CKD, HT, DM | Diag+PCI | Iodixanol | Non-ionic, dimer, low osmolar | 130 | Tonic–clonic seizures | Right-sided relatively widespread edema of the parenchyma (CT) | Supportive care, acyclovir, meropenem, betamethasone. | 72 h | Yes |
| Yen et al (2018)13 | 64 | M | CKD, HT, DM | Diag+PCI | Iohexol | Non-ionic, monomer, low osmolar | 100 | Disorientation with raves | Normal (CT, MRI) | Hemodialysis | N/A | Yes |
| Dattani et al (2018)3 | 6 | M | HT, DM | Diag | Iohexol | Non-ionic, monomer, low osmolar | 120 | Confused, aggressive, expressing verbal profanities | Normal (CT) | Supportive care | 9 d | Yes |
| Hamra et al (2017)14 | 62 | M | HT | Diag+PCI | Iohexol | Non-ionic, monomer, low osmolar | 300 | Right-sided homonymous hemianopia | Slight enhancement of the venous sinuses (CT) | Supportive care | 48 h | Yes |
| Spina et al (2017)15 | 65 | M | Previous CIE | Diag | Iopromide | Non-ionic, monomer, low osmolar | 120 | Global aphasia, bilateral limb weakness | Normal (CT, MRI) | Supportive care | 24 h | Yes |
| Gollol Raju et al (2015)16 | 44 | F | ESKD, HT, DM | Diag+PCI | Iohexol | Non-ionic, monomer, low osmolar | 190 | Left-sided weakness, seizure activity | Contrast enhancement of right cerebral hemisphere(CT) | Anticonvulsants, hemodialysis | 72 h | Yes |
| Kocabay et al (2014)2 | 68 | M | HT | Diag+PCI | Iopromide | Non-ionic, monomer, low osmolar | 250 | Monoplegia | Normal (CT and MRI) | Supportive care | 12 h | Yes |
| 58 | F | HT | Diag+PCI | Iopromide | Non-ionic, monomer, low osmolar | 220 | Bilateral oculomotor opthalmoplegia | Normal (CT and MRI) | Supportive care | >30 d | No | |
| Kocabay et al (2014)2 | 70 | M | HT | Diag+PCI | Iopromide | Non-ionic, monomer, low osmolar | 130 | Cerebellar dysfunction | Normal (CT and MRI) | Supportive care | 14 h | Yes |
| 68 | M | HT, DM | Diag+PCI | Iopromide | Non-ionic, monomer, low osmolar | 180 | Unilateral oculomotor monoplegia | Normal (CT and MRI) | Supportive care | 1 h | Yes | |
| Sridhar et al (2014)17 | 63 | F | HT, DM | Diag (IMA graft) | Iopamidol | Non-ionic, monomer, low osmolar | 250 | Cortical blindness | Contrast enhancement of occipital lobes (CT), no ischemia or hemorrhage on MRI | Supportive care | 72 h | Yes |
| Ting et al (2013)18 | 49 | M | – | Diag | Iopromide | Non-ionic, monomer, low osmolar | 205 | Confusion, decrease in level of consciousness | Normal (CT and MRI/A) | Supportive care | 12 h | Yes |
| Liao et al (2013)19 | 76 | F | HT, DM | Diag | Ioversol | Non-ionic, monomer, low osmolar | 125 | Aphasia, cortical blindness, rightsided weakness | Hyperintensity high frontoparietal regions (MRI) | Supportive care | 48 h | Yes |
| Terlecki et al (2013)20 | 32 | M | – | Diag | Iopromide | Non-ionic, monomer, low osmolar | 100 | Cortical blindness | Normal (CT) | Supportive care | 24 h | Yes |
| Law et al (2012)21 | 69 | F | CKD, DM, PCR | Diag+PCI | Iodixanol | Non-ionic, dimer, low osmolar | 320 | Partial seizure, homonymous hemianopia, hemisensory loss, hemiparesis | Cerebral edema (CT) | Intravenous benzodiazepines, thrombolysis | 24 h | Yes |
| Jiang et al (2012)22 | 64 | M | HT, DM | Diag+PCI | Iopromide | Non-ionic, monomer, low osmolar | 160 | Confusion, irritability, limb paralysis, aphasia | Hyperdensity of sagittal sinus (CT). Slowing in a range occipital lobe (EEG) | Supportive care | 28 h | Yes |
| Aykan et al (2012)23 | 68 | M | HT | Diag+PCI | Iopromide | Non-ionic, monomer, low osmolar | 250 | Left lower extremity weakness and sensory loss | Contrast enhancement sagittal sinus and occipital lobe (CT) | Supportive care | 12 h | Yes |
| Kocabay et al (2011)7 | 47 | M | – | Diag+PCI | Iopromide | Non-ionic, monomer, low osmolar | 150 | Confusion, agitation, nausea, headache | Contrast enhancement of occipital lobes (CT) | Supportive care | 8 h | Yes |
| 70 | M | DM | Diag+PCI | Iopromide | Non-ionic, monomer, low osmolar | 120 | Confusion, agitation, nausea, headache | Contrast enhancement of occipital lobes (CT) | Supportive care | 12 h | Yes | |
| Gürer et al (2011)24 | 69 | M | – | Diag+PCI | Iohexol | Non-ionic, monomer, low osmolar | 100 | Confusion, headache, vomiting, left hemiplegia, | Focal hyperdense lesions | Supportive care | 6 h | Yes |
| Chisci et al (2011)25 | 76 | M | CKD, DM | PCI+CAS | Iodixanol | Non-ionic, dimer, low osmolar | 200 | Stupor, aphasia, hemiparesis | Hyperdensity of cerebral sulci and subarachnoid spaces | Intravenous mannitol, methylprednisone, | 48 h | Yes |
| Akhtar et al (2011)26 | 39 | F | – | Diag | Iopamidol | Non-ionic, monomer, low osmolar | 80 | Cortical blindness | Normal (CT, vertebral angiogram) | Antiplatelet therapy, intravenous heparin | 1 h | Yes |
| Borghi et al (2008)27 | 74 | M | HT | Diag+aorto-gram | Iomeprol | Non-ionic, monomer, low osmolar | 320 | Cortical blindness | Normal (CT) | Supportive care | 24 h | Yes |
| Sawaya et al (2007)5 | N/A | M | – | Diag+PCI | Iohexol | Non-ionic, monomer, low osmolar | 120 | Agitation, confusion, convulsions, slurred speech, loss of consciousness | Normal (CT) Diffuse slowing in the theta range over both hemispheres (EEG) | Orotracheal intubation and ventilation, intravenous benzodiazepines, hydration | 18 h | Yes |
| Tatli et al (2007)28 | 52 | F | – | Diag | Iomeprol | Non-ionic, monomer, low osmolar | 150 | Cortical blindness | Contrast enhancement of occipital lobes (CT) | Supportive care | 5 h | Yes |
| Yazici et al (2007)29 | 70 | F | DM, HT | Diag | Iobitridol | Non-ionic, monomer, low osmolar | 75 | Cortical blindness | Contrast enhancement of occipital lobes (CT) | Supportive care | 72 h | Yes |
| Frye et al (2005)30 | 18 mo | M | – | Diag | Ioversol | Non-ionic, monomer, low osmolar | 7mL/kg | Myoclonus | Right holohemispheric parenchymal and subarachnoid hyperdensity cerebral edema | Intravenous benzodiazepines | 24 h | Yes |
| Schulte A et al (2004)31 | 56 | M | – | Diag | Iopromide | Non-ionic, monomer, low osmolar | 135 | Confusion, dysarthria, cortical blindness | Contrast enhancement right occipital lobe | Supportive care | 24 h | Yes |
| Velden et al (2003)32 | 82 | F | CKD, HT | Diag+PCI | Iomeprol | Non-ionic, monomer, low osmolar | 500 | Aphasia, right-sided hemiparesis | Hyperdensities filling the sulci of both cerebral hemispheres | Supportive care | 40 h | Yes |
| Foltys et al (2003)33 | 82 | M | CKD, DM, HT | Diag (IMA graft) | Iopromide | Non-ionic, monomer, low osmolar | 130 | Right-sided hemiparesis, aphasia | Cerebral edema and extravascularly localized contrast media left hemisphere | Supportive care | 6 h | Yes |
| Gellen et al (2003)34 | 52 | N/A | CKD | Diag (IMA graft) | Iopamidol | Non-ionic, monomer, low osmolar | 400 | Cortical blindness | Contrast enhancement occipital lobes | Supportive care | 72 h | Yes |
| Yildiz et al (2003)35 | 63 | M | – | Diag+Aortogram | Iomeprol | Non-ionic, monomer, low osmolar | 450 | Amnesia, numbness, right upper extremity numbness | Contrast enhancement right occipital lobe | Intravenous dexamethasone | 12 h | Yes |
| Lim et al (2002)36 | 63 | F | DM, HT | Diag (IMA graft) | Iopromide | Non-ionic, monomer, low osmolar | 160 | Cortical blindness, right homonymous hemianopia | Contrast enhancement of occipital lobes (CT, MRI) | Supportive care, intravenous heparin | 48 h | Yes |
| Zwicker et al (2002)37 | 52 | F | HT | Diag+PCI | Ioversol | Non-ionic, monomer, low osmolar | 280 | Cortical blindness | Contrast enhancement of occipital lobes (CT) | Supportive care, antihypertensives | 36 h | Yes |
| Kwok et al (2000)38 | 53 | M | – | Diag | Ioversol | Non-ionic, monomer, low osmolar | 100 | Cortical blindness, catatonia | Normal (CT, MRI) | Antiplatelet therapy, low molecular weight heparin | 12 h | Yes |
| Vranckx et al (1999)39 | 68 | M | – | Diag (IMA graft)+PCI | Iohexol | Non-ionic, monomer, low osmolar | 180 | Cortical blindness, aphasia, periodic alternating gaze with nystagmus | Contrast enhancement of occipital lobes, temporal lobes, thalami (CT) | CPR, temporary transvenous pacemaker | 6 d | No |
| Sticherling et al (1998)40 | 55 | M | – | Diag (graft) | Iomeprol | Non-ionic, monomer, low osmolar | 280 | Cortical blindness | Contrast enhancement of occipital lobes (CT) | Supportive care | 5 d | Yes |
| Kamata et al (1995)41 | 62 | M | CKD, HT | Diag (IMA graft)+PCI | Iopamidol | Non-ionic, monomer, low osmolar | 170 | Headache, confusion, cortical blindness | Contrast enhancement cerebellum, thalamus | Thrombolysis, intravenous dexamethasone, glycerine, plasma expander | 12 h | Yes |
| Rama et al (1993)42 | 59 | M | HT | Diag+PCI | Ioversol | Non-ionic, monomer, low osmolar | 220 | Cortical blindness | Normal (CT) | Supportive care | 12 h | Yes |
| 45 | M | HT | Diag+PCI | Ioversol | Non-ionic, monomer, low osmolar | 167 | Cortical blindness | Normal (CT) | Supportive care | 24 h | Yes | |
| 68 | M | HT | Diag+PCI | Ioversol | Non-ionic, monomer, low osmolar | 262 | Homonymous hemianopia | Normal (CT) | Supportive care | 15 min | Yes | |
| Parry et al (1993)43 | 62 | M | HT | Diag (IMA graft) | Iopamidol | Non-ionic, monomer, low osmolar | 270 | Cortical blindness, loss of coordination right arm | Contrast enhancement of occipital lobes (CT) | Supportive care | 72 h | Yes |
Abbreviations: HT, hypertension; DM, diabetes mellitus; CKD, chronic kidney disease; ESKD, end stage kidney disease; CT, computed tomography; MRI, magnetic resonance imaging; Diag, diagnostic; PCI, primary coronary intervention; CAS, carotid artery stenting; EEG, electroencephalogram; IMA, internal mammary artery.
The mechanism and causes of iodine-based contrast-induced neurotoxicity are unclear. Compared to lower osmolality or iso-osmolar solutions, hypertonic contrast agents are more likely to disrupt the BBB, resulting in increased entry into the brain, which can result in edema and direct neuronal toxicity.44,45 However, many studies have shown that CIE can occur in response to high or low osmolality compounds.21,25 We identified 23 of 41 (56.09%) cases that resulted from percutaneous coronary interventions (PCI), and the remaining 18 occurred during diagnostic CAG. The median volume of contrast media administered was 168.5 mL (range from 75 to 500 mL). In our case, the patient developed CIE following administration of 20 mL of iopromide, a low-osmolar, non-ionic, monomeric, iodine-based contrast agent during CAG. In addition to use of contrast agents, male gender, hypertension, diabetes mellitus, impaired renal function, impaired cerebral autoregulation, and transient ischemic attack (TIA) are risk factors for development of CIE.46,47 In patients with renal failure, renal excretion of contrast media is delayed and brain concentrations of contrast agent can remain high, resulting in increased risk of developing CIE.11 Furthermore, chronic hypertension impairs cerebral autoregulation and TIA may increase permeability of the BBB, which may contribute to contrast extravasation.25,48 Review of the literature showed that the median age of patients with CIE was 63 years (range from 1.5 to 82 years), and 72.50% of patients were male. Most of the patients had diabetes, hypertension, chronic kidney disease, or other comorbidities. Our 84-year-old female patient had chronic hypertension, which is typically associated with poor control of blood pressure, which may contribute to increased blood-brain barrier permeability. Advanced age and high blood pressure may have resulted in increased risk of contrast-induced encephalopathy in response to 20 mL of contrast medium.
Clinical presentations of CIE are highly variable and include localized cortical and subcortical deficits such as hemiparesis, hemianopia, cortical blindness, speech changes, parkinsonism, and global syndromes such as confusion, seizure, and coma.6,49 Transient cortical blindness (TCB) is the most common manifestation of CIE. Literature review indicated that symptoms of neurological dysfunction presented within minutes to hours after contrast agent administration, and most patients fully recovered within 48–72 hours.2,3,5,7,12–43,50,51 In our case, the patient presented with hemiparesis, hemianopia, and unconsciousness within minutes of radial sheath removal, and more extreme features developed, such as recurrent seizures, and cardiac and respiratory arrest. Her symptoms resolved completely within 72 hours, which was consistent with the clinical course of CIE. We suspect that the severe symptoms of this patient were related to advanced age, hypertension, and other risk factors, which may have increased BBB permeability. This increased permeability may have allowed for contrast agent exudation and brain tissue colloid osmotic pressure changes, resulting in cerebral edema and neurotoxicity and contrast-induced encephalopathy.
Typical head CT imaging findings associated with CIE typically include local cortical enhancement, increased subarachnoid density, and brain edema,16 which are important for differentiation of CIE from other neurological pathologies such as thromboembolism and hemorrhage following angiography. Moreover, abnormalities observed during emergency CT as measured by Hounsfield units (HU) can help to differentiate blood from contrast, with the higher attenuation (100 to 300 HU) of contrast compared with blood (40 to 60 HU).52 On MRI, patients with CIE showed hyperintense areas on spin-spin relaxation time, FLAIR, and DWI imaging, and no change in apparent diffusion coefficient (ADC) maps, which differs from observations consistent with cerebral ischemia.48 However, some patients with CIE have no radiological features. In addition, CSF examination is useful to rule out subarachnoid hemorrhage through absence of xanthochromia or red blood cells. Simultaneous high concentrations of iodine contrast in the CSF and serum support contrast extravasation rather than hemorrhage.32 Brain CT of our patient immediately following the first symptom showed no acute pathological findings, but indicated multiple high-density subarachnoid regions after 2 hours. Cerebral edema occurred 10 hours after surgery. Brain MRI showed hyperintense areas in the right cortex via FLAIR imaging, which further supported a diagnosis of CIE.
Most patients with CIE have a good prognosis and recover quickly with supportive treatment including intravenous fluids and close observation. In some cases, anticonvulsive drugs can be used to treat seizures and mannitol can be used to decrease pressure in the brain. Steroid hormones such as dexamethasone can be used to reduce inflammatory reactions as necessary.2,3,5,7,12–43 Neurological toxicity typically occurs within minutes or hours after angiography, and resolves spontaneously within 72 hours. Although the prognosis of most cases of CIE is good, some cases with persistent deficits have been reported.4 Eight cases of autopsy-confirmed fatal cerebral edema due to contrast neurotoxicity in the early stage of angiography have been observed in response to administration of ionic high osmolar contrast agents.53–55 Recently, a small body of literature has shown that low osmotic pressure contrast agents can induce permanent neurological dysfunction4 and fatal cerebral edema.56 In our case, the patient developed recurrent seizures and cardiac and respiratory arrest, but her neurological symptoms gradually improved after 3 days in response rehydration, anticonvulsant therapy, reduction of cranial pressure, and treatment with dexamethasone.
There is clinical and radiological overlap between CIE and posterior reversible encephalopathy syndrome (PRES), which is a reversible clinicoradiological subcortical vasogenic edema in patients with acute neurological symptoms (eg, seizures, encephalopathy, visual disturbances, and focal neurological deficits) that occurs more frequently in patients with uncontrolled hypertension and chronic kidney disease.57 Magnetic resonance imaging findings in CIE with hyperintensity on DWI and FLAIR, and no change in ADC, can overlap with PRES radiological features.58 Moreover, the pathophysiological theory underlying PRES supports endothelial dysfunction related to abrupt blood pressure changes and/or direct effects of cytokines. This results in breakdown of the blood-brain barrier and subsequent brain edema. Therefore, the pathophysiological mechanism of PRES following CAG, similar to that in CIE, may be endothelial dysfunction indirectly induced by contrast media through excessive circulating cytokines, such as endothelin-1, which have been shown to be increased by administration of moderate levels of contrast media.59–61 In our case, CIE could also be considered a case of PRES triggered by contrast media administration.
Conclusion
Contrast-induced encephalopathy following cardiac catheterization is a rare but complex neurological disturbance. Manifestations of CIE vary, but the prognosis is typically good in conjunction with supportive treatment. Although many studies have suggested that the risk of developing CIE is higher in response to high osmolality agents, our review demonstrates that severe symptoms of CIE can also occur in response to small amounts of low-osmolar, non-ionic, monomeric iodine-based contrast agents. Contrast-induced encephalopathy must be considered in differential diagnosis of stroke following cardiac catheterization. Early clinical suspicion of CIE might alter therapeutic considerations and may help to avoid potentially harmful interventions such as thrombolysis.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (grant number 81673815).
Ethics Approval
The Institutional Review Board of China-Japan Friendship Hospital approved the publication of the case details.
Consent for Publication
A signed informed consent was obtained from the patient for publication of the case details and any accompanying images.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Fang HY, Kuo YL, Wu CJ. Transient contrast encephalopathy after carotid artery stenting mimicking diffuse subarachnoid hemorrhage: a case report. Catheter Cardiovasc Interv. 2009;73(1):123–126. doi: 10.1002/ccd.21779 [DOI] [PubMed] [Google Scholar]
- 2.Kocabay G, Karabay C, Kalayci A, et al. Contrast-induced neurotoxicity after coronary angiography. Herz. 2014;39(4):522–527. doi: 10.1007/s00059-013-3871-6 [DOI] [PubMed] [Google Scholar]
- 3.Dattani A, Au L, Tay KH, Davey P. Contrast-induced encephalopathy following coronary angiography with no radiological features: a case report and literature review. Cardiology. 2018;139(3):197–201. doi: 10.1159/000486636 [DOI] [PubMed] [Google Scholar]
- 4.Leong S, Fanning NF. Persistent neurological deficit from iodinated contrast encephalopathy following intracranial aneurysm coiling. Interv Neuroradiol. 2012;18(1):33–41. doi: 10.1177/159101991201800105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawaya RA, Hammoud R, Arnaout S, Alam S. Contrast-induced encephalopathy following coronary angioplasty with iohexol. South Med J. 2007;100(10):1054–1055. doi: 10.1097/SMJ.0b013e3181540086 [DOI] [PubMed] [Google Scholar]
- 6.Dangas G, Monsein LH, Laureno R, et al. Transient contrast encephalopathy after carotid artery stenting. J Endovasc Ther. 2001;8(2):111–113. doi: 10.1177/152660280100800202 [DOI] [PubMed] [Google Scholar]
- 7.Kocabay G, Karabay CY. Iopromide-induced encephalopathy following coronary angioplasty. Perfusion. 2011;26(1):67–70. doi: 10.1177/0267659110385511 [DOI] [PubMed] [Google Scholar]
- 8.Potsi S, Chourmouzi D, Moumtzouoglou A, Nikiforaki A, Gkouvas K, Drevelegas A. Transient contrast encephalopathy after carotid angiography mimicking diffuse subarachnoid haemorrhage. Neurol Sci. 2012;33(2):445–448. doi: 10.1007/s10072-011-0765-3 [DOI] [PubMed] [Google Scholar]
- 9.De Bono D. Complications of diagnostic cardiac catheterization: results from 34,041 patients in the United Kingdom confidential enquiry into cardiac catheterization complications. Br Heart J. 1993;70:297–300. doi: 10.1136/hrt.70.3.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hershkowitz N, Bryan RN. Neurotoxic effects of water-soluble contrast agents on rat hippocampus: extracellular recordings. Invest Radiol. 1982;17(3):271–275. [PubMed] [Google Scholar]
- 11.Junck L, Marshall WH. Neurotoxicity of radiological contrast agents. Ann Neurol. 1983;13(5):469–484. doi: 10.1002/ana.410130502 [DOI] [PubMed] [Google Scholar]
- 12.Eleftheriou A, Rashid AS, Lundin F. Late transient contrast-induced encephalopathy after percutaneous coronary intervention. J Stroke Cerebrovasc Dis. 2018;27(6):e104–e106. doi: 10.1016/j.jstrokecerebrovasdis.2017.12.051 [DOI] [PubMed] [Google Scholar]
- 13.Yen CC, Sung SF, Hsu YH. Clinical presentations of contrast-induced encephalopathy in end-stage renal disease. Intern Med J. 2018;48(5):604–605. doi: 10.1111/imj.13776 [DOI] [PubMed] [Google Scholar]
- 14.Hamra M, Bakhit Y, Khan M, Moore R. Case report and literature review on contrast-induced encephalopathy. Future Cardiol. 2017;13(4):331–335. doi: 10.2217/fca-2016-0075 [DOI] [PubMed] [Google Scholar]
- 15.Spina R, Simon N, Markus R, Muller DW, Kathir K. Recurrent contrast-induced encephalopathy following coronary angiography. Intern Med J. 2017;47(2):221–224. doi: 10.1111/imj.13321 [DOI] [PubMed] [Google Scholar]
- 16.Raju NSG, Joshi D, Daggubati R, Movahed A. Contrast induced neurotoxicity following coronary angiogram with Iohexol in an end stage renal disease patient. World J Clin Cases. 2015;3:942–945. doi: 10.12998/wjcc.v3.i11.942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sridhar GS, Sadiq MA, Wan Ahmad WA, et al. Transient cortical blindness: a benign but devastating complication after coronary angiography and graft study. J Pak Med Assoc. 2014;64:1195–1197. [PubMed] [Google Scholar]
- 18.Ting F, Bhat A, Markus R, Ortega M, Ting S. Contrast-induced encephalopathy post-angiography. Internet J Neurol. 2013;16:1–3. [Google Scholar]
- 19.Liao MT, Lin TT, Lin YL, Hwang JJ, Tseng CD. Contrast-induced encephalopathy after percutaneous coronary intervention. Acta Cardiol Sin. 2013;29:277–280. [PMC free article] [PubMed] [Google Scholar]
- 20.Terlecki M, Wojciechowska W, Rajzer M, et al. Transient cortical blindness after coronary artery angiography. Adv Interv Cardiol. 2013;9:105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Law S, Panichpisal K, Melaku D, et al. Contrast-induced neurotoxicity following cardiac catheterization. Case Rep Med. 2012;2012:267860. doi: 10.1155/2012/267860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang X, Li JL, Chen X. Contrast-induced encephalopathy following coronary angioplasty with iopromide. Neurosciences. 2012;17:378–379. [PubMed] [Google Scholar]
- 23.Aykan A, Zehir R, Karabay CY, Kocabay G. Contrast-induced monoplegia following coronary angioplasty with iopromide. Kardiol Pol. 2012;70:499–500. [PubMed] [Google Scholar]
- 24.Gürer B, Yilmaz ER, Kahveci R, Sekerci Z. Non-ionic contrast media neurotoxicity mimicking intracerebral hematoma. Acta Neurochir. 2011;153:419–420. doi: 10.1007/s00701-010-0780-9 [DOI] [PubMed] [Google Scholar]
- 25.Chisci E, Setacci F, Donato G, De, Setacci C. A case of contrast-induced encephalopathy using iodixanol. J Endovasc Ther. 2011;18:540–544. doi: 10.1583/11-3476.1 [DOI] [PubMed] [Google Scholar]
- 26.Akhtar N, Khatri IA, Naseer A, Ikram J, Ahmed W. Transient cortical blindness after coronary angiography: a case report and literature review. J Pak Med Assoc. 2011;61:295–297. [PubMed] [Google Scholar]
- 27.Borghi C, Saia F, Marzocchi A, Branzi A. The conundrum of transient cortical blindness following coronary angiography. J Cardiovasc Med. 2008;9:1063–1065. doi: 10.2459/JCM.0b013e3282fe1718 [DOI] [PubMed] [Google Scholar]
- 28.Tatli E, Buyuklu M, Altun A. An unusual but dramatic complication of coronary angiography: transient cortical blindness. Int J Cardiol. 2007;121:e4–e6. doi: 10.1016/j.ijcard.2007.04.129 [DOI] [PubMed] [Google Scholar]
- 29.Yazici M, Ozhan H, Kinay O, et al. Transient cortical blindness after cardiac catheterization with Iobitridol. Tex Heart Inst J. 2007;34:373–375. [PMC free article] [PubMed] [Google Scholar]
- 30.Frye RE, Newburger JW, Nugent A, Sahin M. Focal seizure and cerebral contrast retention after cardiac catheterization. Pediatr Neurol. 2005;32:213–216. doi: 10.1016/j.pediatrneurol.2004.07.012 [DOI] [PubMed] [Google Scholar]
- 31.Schulte-Altedorneburg G, Rub K, Scheglmann K. Simultaneous ischemic and neurotoxic brain damage after coronary angiography. Neurol Res. 2004;26:79–82. doi: 10.1179/016164104773026570 [DOI] [PubMed] [Google Scholar]
- 32.Velden J, Milz P, Winkler F, Seelos K, Hamann GF. Nonionic contrast neurotoxicity after coronary angiography mimicking subarachnoid hemorrhage. Eur Neurol. 2003;49:249–251. doi: 10.1159/000070198 [DOI] [PubMed] [Google Scholar]
- 33.Foltys H, Krings T, Block F. Cerebral contrast medium extravasation after coronary angioplasty. Nervenarzt. 2003;74:892–895. doi: 10.1007/s00115-003-1574-6 [DOI] [PubMed] [Google Scholar]
- 34.Gellen B, Remp T, Mayer T, Milz P, Franz WM. Cortical blindness: a rare but dramatic complication following coronary angiography. Cardiology. 2003;99:57–59. doi: 10.1159/000068443 [DOI] [PubMed] [Google Scholar]
- 35.Yildiz A, Yencilek E, Apaydin FD, Duce MN, Ozer C, Atalay A. Transient partial amnesia complicating cardiac and peripheral arteriography with nonionic contrast medium. Eur Radiol. 2003;13:L113–L115. doi: 10.1007/s00330-003-1975-8 [DOI] [PubMed] [Google Scholar]
- 36.Lim KK, Radford DJ. Transient cortical blindness related to coronary angiography and graft study. Med J Aust. 2002;177:43–44. doi: 10.5694/j.1326-5377.2002.tb04636.x [DOI] [PubMed] [Google Scholar]
- 37.Zwicker JC, Sila CA. MRI findings in a case of transient cortical blindness after cardiac catheterization. Catheter Cardiovasc Interv. 2002;57:47–49. doi: 10.1002/ccd.10246 [DOI] [PubMed] [Google Scholar]
- 38.Kwok BW, Lim TT. Cortical blindness following coronary angiography. Singapore Med J. 2000;41:604–605. [PubMed] [Google Scholar]
- 39.Vranckx P, Ysewijn T, Wilms G, Heidb€uchel H, Herregods MC, Desmet W. Acute posterior cerebral circulation syndrome accompanied by serious cardiac rhythm disturbances: a rare but reversible complication following bypass graft angiography. Catheter Cardiovasc Interv. 1999;48:397–401. doi: [DOI] [PubMed] [Google Scholar]
- 40.Sticherling C, Berkefeld J, Auch-Schwelk W, Lanfermann H. Transient bilateral cortical blindness after coronary angiography. Lancet. 1998;351:570. doi: 10.1016/S0140-6736(05)78557-3 [DOI] [PubMed] [Google Scholar]
- 41.Kamata J, Fukami K, Yoshida H, et al. Transient cortical blindness following bypass graft angiography: a case report. Angiology. 1995;46:937–946. doi: 10.1177/000331979504601009 [DOI] [PubMed] [Google Scholar]
- 42.Rama BN, Pagano TV, DelCore M, Knobel KR, Lee J. Cortical blindness after cardiac catheterization: effect of re-challenge with dye. Cathet Cardiovasc Diagn. 1993;28:149–151. doi: 10.1002/ccd.1810280211 [DOI] [PubMed] [Google Scholar]
- 43.Parry R, Russell Rees J, Wilde P. Transient cortical blindness after coronary angiography. Br Heart J. 1993;70:563–564. doi: 10.1136/hrt.70.6.563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Torvik A, Walday P. Neurotoxicity of water-soluble contrast media. Acta Radiol Suppl. 1995;36:221–229. doi: 10.1177/0284185195036S39927 [DOI] [PubMed] [Google Scholar]
- 45.Velaj R, Drayer B, Albright R, Fram E. Comparative neurotoxicity of angiographic contrast media. Neurology. 1985;35:1290–1298. doi: 10.1212/WNL.35.9.1290 [DOI] [PubMed] [Google Scholar]
- 46.Muruve DA, Steinman TI. Contrast-induced encephalopathy and seizures in a patient with chronic renal insufficiency. Clin Nephrol. 1996;45(6):406–409. [PubMed] [Google Scholar]
- 47.Frantz WM. Cortical blindness following coronary angiography in a patient with LIMA bypass graft and end stage renal failure. Proc EuroPCR. 2006;21–24. [Google Scholar]
- 48.Guimaraens L, Vivas E, Fonnegra A, et al. Transient encephalopathy from angiographic contrast: a rare complication in neurointerventional procedures. Cardiovasc Intervent Radiol. 2010;33:383–388. doi: 10.1007/s00270-009-9609-4 [DOI] [PubMed] [Google Scholar]
- 49.Lantos G. Cortical blindness due to osmotic disruption of the blood-brain barrier by angiographic contrast material: CT and MRI studies. Neurology. 1989;39:567–571. doi: 10.1212/WNL.39.4.567 [DOI] [PubMed] [Google Scholar]
- 50.Nagamine Y, Hayashi T, Kakehi Y, et al. Contrast-induced encephalopathy after coil embolization of an unruptured internal carotid artery aneurysm. Intern Med. 2014;53(18):2133–2138. doi: 10.2169/internalmedicine.53.2380 [DOI] [PubMed] [Google Scholar]
- 51.Pagani-Estévez GL, Nasr DM, Brinjikji W, Perry A, Fugate JE. Dual-energy CT to diagnose pseudoedema in contrast-induced encephalopathy following cerebral angiography. Neurocrit Care. 2017;27(2):261–264. doi: 10.1007/s12028-017-0394-7 [DOI] [PubMed] [Google Scholar]
- 52.Phan CM, Yoo AJ, Hirsch JA, Nogueira RG, Gupta R. Differentiation of hemorrhage from iodinated contrast in different intracranial compartments using dual-energy head CT. AJNR Am J Neuroradiol. 2012;33(6):1088–1094. doi: 10.3174/ajnr.A2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lalli AF. Contrast media reactions: data analysis and hypothesis. Radiology. 1980;134(1):1–12. doi: 10.1148/radiology.134.1.6985735 [DOI] [PubMed] [Google Scholar]
- 54.Shrivastava S, Mohan JC, Chopra P. Fatal cerebral edema following angiocardiography: a case report. Int J Cardiol. 1985;8(4):490–491. doi: 10.1016/0167-5273(85)90127-5 [DOI] [PubMed] [Google Scholar]
- 55.Junck L, Marshall WH. Fatal brain edema after contrast-agent overdose. AJNR Am J Neuroradiol. 1986;7(3):522–525. [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao W, Zhang JP, Song Y, et al. Irreversible fatal contrast-induced encephalopathy: a case report. BMC Neurol. 2019;19(1):46. doi: 10.1186/s12883-019-1279-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015;14:914–925. doi: 10.1016/S1474-4422(15)00111-8 [DOI] [PubMed] [Google Scholar]
- 58.Gao B, Lyu C, Lerner A, McKinney AM. Controversy of posterior reversible encephalopathy syndrome: what have we learnt in the last 20 years? J Neurol Neurosurg Psychiatry. 2018;89(1):14–20. doi: 10.1136/jnnp-2017-316225 [DOI] [PubMed] [Google Scholar]
- 59.Saigal G, Bhatia R, Bhatia S, Wakhloo AK. MR Findings of cortical blindness following cerebral angiography: is this entity related to posterior reversible leukoencephalopathy. Am J Neuroradiol. 2004;25:252–256. [PMC free article] [PubMed] [Google Scholar]
- 60.Ulas T, Buyukhatipoglu H, Dal MS, et al. Urotensin- II and endothelin-I levels after contrast media administration in patients undergoing percutaneous coronary interventions. J Res Med Sci. 2013;18(3):205–209. [PMC free article] [PubMed] [Google Scholar]
- 61.de Falco A, De Simone M, d’Onofrio F, Spitaleri D, de Falco FA. Posterior reversible encephalopathy syndrome overlapping contrast-induced encephalopathy after coronary angiography. Neurol Sci. 2019;40:1951–1953. doi: 10.1007/s10072-019-03810-w [DOI] [PubMed] [Google Scholar]