Abstract
Background:
A retained foreign object (RFO) is a devastating surgical complication that typically results in additional surgeries, increased length of stay, and risk of infections and is potentially fatal. Memorial Sloan Kettering Cancer Center (MSKCC) convened a multidisciplinary task force to undertake an improvement initiative to reduce the frequency of RFO incidents.
Methods:
A needs assessment was undertaken using focus group interviews, review of past RFOs, and operating room (OR) observations, and a comprehensive intervention plan was initiated. Items at risk of retention were reclassified and new tracking sheets were developed. A probabilistic risk model was developed based on aviation industry methodology, an RFO risk projection, and the retention risk classification of surgical items. Training initiatives were launched to shift organizational culture and staff behaviors toward greater awareness of RFO risk and proactive prevention.
Results:
Since the implementation of our task force’s recommendations on March 24, 2014, there have been no RFO incidents at our institution to this day. The last RFO occurred in August 2013—more than 1,300 days ago (as of March 28, 2017). The RFO incident frequency was reduced from 1.69 per year to a risk model estimate of 1 in 22 years. Ongoing training maintains the staff’s behavioral changes as well as the improved OR and organizational culture.
Conclusion:
Implementation of a multidisciplinary approach to preventing RFOs was successful at MSKCC. The use of an RFO risk model enabled the creation of a robust system for RFO prevention. Support from leadership, participation by all stakeholders, education, training, and cooperation from frontline staff are all important contributors to RFO prevention success.
A retained foreign object (RFO) is any item used during an operative procedure that is unintentionally left inside the patient. They are devastating surgical complications that typically require additional surgeries, increase the length of stay and risk of infections, and are potentially fatal.1-3 These events can be demoralizing to the surgical team involved in the procedure. Moreover, RFOs are a significant concern for government,4 regulators,5 and accreditors.6,7 and their occurrence can adversely affect Medicare reimbursement.4,8 Optimizing a strategy for preventing RFOs is of paramount importance to all stakeholders providing care for surgical patients. Varied risk factors leading to RFOs have been studied and discussed extensively in the literature, but the infrequency of these events has hampered efforts to design comprehensive and effective prevention strategies.2,9-12 This is a complex issue due to the overlap of systems design, human behavior, and mechanical failures. Many hospitals strive to eliminate all RFOs by focusing on only one of these three aspects. Instead, we focused on a redesign of our system—which entailed consideration of all three—to bolster the idea of constant improvement and to strive for a rate as close to zero as possible. Many hospitals have tackled the reduction of RFOs by examining near misses and miscounts to identify contributing factors.10,13 As new procedures and surgical techniques have been introduced, methods of accounting for sterile supplies during surgery have evolved gradually but without a concerted effort to evaluate these processes holistically.
The World Health Organization,7 the Association of periOperative Registered Nurses (AORN),1 The Joint Commission,6 and the Agency for Healthcare Research and Quality,4 have published guidelines and recommended practices for prevention of RFO incidents. The recommendations include the use of standardized, transparent, verifiable, and reliable practices in surgical counts.1,11,14 A Joint Commission Sentinel Alert, published in 2013, highlighted several strategies that hospitals could use to reduce the likelihood of an RFO, including specific recommendations for individual team members, as well as organizationwide risk reduction approaches that may be adopted.6
Over the years, Memorial Sloan Kettering Cancer Center (MSKCC; New York City), in response to RFO occurrences, developed and implemented strategies to prevent new incidents with the specific surgical item involved. An internal review of previously implemented strategies revealed that, in some instances, new steps that were incorporated into operating room (OR) processes were not value-added and appeared to complicate the process of counting and tracking various items during surgery. As a result of our internal review, we launched an institutional process improvement project to identify the RFO risk level of various surgical items, evaluate surgical staff behaviors that might increase the risk of RFOs, examine current count processes to identify areas of improvement, and, ultimately, reduce the number of RFOs at our institution. In this article, we describe this project and report its results. Our experience illustrates strategies that may be effective for RFO prevention at other institutions.
SETTING
MSKCC, a private, nonprofit organization, specializes in all types of oncologic surgery and medical treatment. The facility has 28 state-of-the-art ORs, and approximately 22,000 surgical procedures are performed annually.
METHODS
We describe the process improvement project in terms of its four phases, which extended from July 2013 through December 2015.
Phase I: Task Force Formation and Decision to Use a Risk Model–Based System of Analysis (July 2013–September 2013)
A multidisciplinary team was created to help identify and evaluate techniques used in aviation that could be used in the OR setting. To assess our current count processes in the surgical setting and develop team-focused approaches to prevent future RFOs, a task force was formed, which was headed by our institution’s former patient safety officer and included nurse leaders [including L.M.S.], clinical nurse specialists [including M.M.S.], RNs [M.N., T.M. S.], surgical technologists [including D.L.M.], colleagues from the Division of Quality and Safety (DQS) [E.G.D., J.F.], and an external consultant, as described below. A physician champion [including L.T.] was also designated to support the task force’s efforts. All recommendations required approval of the OR Safety Executive Committees, which was imperative to gain support and ensure effective monitoring during implementation and for subsequent sustainability. The task force believed that support from senior leadership was paramount and that the greatest impact would be achieved if all efforts were made within an organizational culture to promote and prioritize patient safety and ongoing improvement. In two articles, James Reason proposed a “Swiss cheese” model of human error wherein multiple layers of barriers within a system must fail in order for errors to occur. He suggests that these human errors can be addressed using either (a) a person approach, which examines actions of individuals and is typical in the field of medicine, or (b) a system approach, which acknowledges the reality of human fallibility and is commonly used in fields such as aviation,15,16 Our institution’s previous RFO prevention strategies had limited success in reducing the frequency of such incidents. On the basis of Reason’s insights, our task force decided on a new approach, involving recruitment of a systems expert from aviation, another industry in which high reliability is critical for safety. As a member of our task force, this systems expert brought extensive experience with evaluating system issues, organizational processes, and human factors, as well as predictive modeling to improve management of human errors that can occur in high reliability organizations, including health care. Based on 2014 data reported by the International Civil Aviation Organization, the aviation industry has had a high rate of reliability: 3 accidents per 1,000,000 departures.17 Our institution endeavored to achieve the same level of high reliability with RFOs. Specifically, the goal of our task force was to reduce the rate of RFO incidents from 1.69 per year to 1 in 50 years, which would equate to one RFO for every 1,000,000 surgeries.
Socio-Probabilistic Risk Model: Fault Trees.
As part of the redesign, the consultant and the system engineer used the principles of high reliability that have been traditionally used in aviation. Fault trees represent possible pathways through which systems can break down, and fault tree analysis software has historically been used in safety engineering to determine various combinations of system and human errors that could lead to an adverse event.12,18 For RFO prevention, we reviewed our internal guidelines and conducted observations within the OR followed by roundtable discussions to identify the various ways an RFO could happen. As a first step, every possible path leading to an RFO for each class of surgical tool or accessory item was documented and reviewed; for example, sponges (laparotomy pads, raytex, and towels), accessories with a counting cue (peanut and gastric sponges), and instruments (for example, clamps, forceps). Identified modes of failure were documented in a fault tree. We created two fault trees for each category of items—one tree for items introduced at the beginning of the case and another tree for items introduced during the case. Each tree included one branch per specific item, and each branch was divided into all basic events (modes of failure) that could possibly lead to the outcome of an RFO involving that item. The identification of basic and intermediate events was undertaken multiple times over the life span of our overall improvement project.
Within the model developed by our task force, the staff’s behavior was defined as either a choice or a human error, where choice is defined as a conscious decision to not follow the policy, while a human error is a mental lapse by a person who intended to follow a given policy but inadvertently failed to do so. For each behavior, a probability representing the percentage of times the staff engaged in a specific behavior was calculated. The probabilities were gathered through consensus from a group of frontline staff (RNs, surgical technologists [STs], and surgeons) who worked in the perioperative settings. The staff was asked for the number of times they engaged in a specific behavior. If there were disagreements about the frequency, the staff discussed their reasoning until consensus was achieved. For each possible mechanical failure, we inserted either the actual rate of failure experienced by our institution or the failure rate provided by the manufacturer if we had no historical reference data. After the initial evaluation of systems in the OR environment, the team recalculated the probabilities in the fault trees to reflect the new OR processes. The fault trees depicted the potential modes of failure and assumed a thorough adoption of the critical behaviors (see Critical Behavior in the Phase IV section) with the intended goal of a 1-in-50-year occurrence.
An adjustment period of six months was allowed, after which observations in the OR were conducted to monitor the updated processes. On the basis of those observations and discussions with the frontline staff, the task force revisited the fault tree model and incorporated any necessary updates.
Phase II: Needs Assessment and Information Gathering (July 2013–September 2013)
Using multiple information-gathering techniques, the task force identified current risk factors in the OR setting.
Information-Gathering Approaches.
To analyze the hospital’s existing OR processes and identify areas for improvement, the task force used multiple information-gathering approaches: (1) small focus groups of involved stakeholders (for example, perioperative RNs, STs, surgeons, quality assurance committee members) were convened for roundtable discussions of perceptions and experiences; (2) live observations of OR processes; and (3) a review of past RFO incidents. The task force identified seven major findings (Sidebar 1).
Sidebar 1.
Potential Factors Contributing to RFO Incidents Prior to Task Force Action
| Summary of Findings from the Information-Gathering Approach |
|---|
|
RFO, retained foreign object; OR, operating room.
Identification of RFO Risk Factors and Counting Cues.
Observations were conducted to determine the factors that affect RFO risk. The AORN guidelines include strategies for counting processes and tracking the various classes of surgical items.1 The guidelines, however, acknowledge that there is still room for improvement because count discrepancies can occur despite the use of detailed count sheets,10,19,20 and RFOs may occur due to factors other than count inaccuracies.2,11,21 The task force, in an effort to enhance the AORN–recommended counting processes, examined the RFO risk potential for surgical items; that is, it conducted a comprehensive review of different surgical items and their use during surgical procedures and identified four different risk groups (Sidebar 2). In addition to these risk categories, the task force identified and observed mechanisms that often prevent an RFO. It identified three types of detection systems for identifying a count discrepancy. First, the manufacturer’s package design for certain items offers stronger counting cues for nursing staff1. For example, gastric or laparotomy sponges are supplied in packets of five. Thus, the standard package design is a counting aid because staff will expect a package with a specific number of items. A second method of detection is the visual cue, which involves using the manufacturer’s package or visual aid to serve as a detection aid in identifying a discrepancy. An example is a peanut sponge and its associated receptacle, which has five spaces; a visual scan revealing one or more empty spaces facilitates detection of a missing peanut. The third type of detection is the use of a mechanical or technological device as an adjunct to the manual count. For example, our institution has, for quite some time, incorporated the use of radio frequency (RF) technology to verify and detect sponges.22,23
Sidebar 2.
Categories of RFO Risk According to Item Size/Usage
| Item Usage | RFO Category |
|---|---|
| Held by surgeon throughout task | Low Risk |
| Too large to fit entirely inside cavity | Moderate Risk |
| Fits inside cavity, but clearly within visual field | High Risk |
| Inside cavity, outside visual field | Extreme Risk |
RFO, retained foreign object.
Phase III: Project Implementation—Process Changes (January 2014–December 2014)
As a result of the observations, areas of improvement were identified and risk mitigation strategies were applied.
Item Counts.
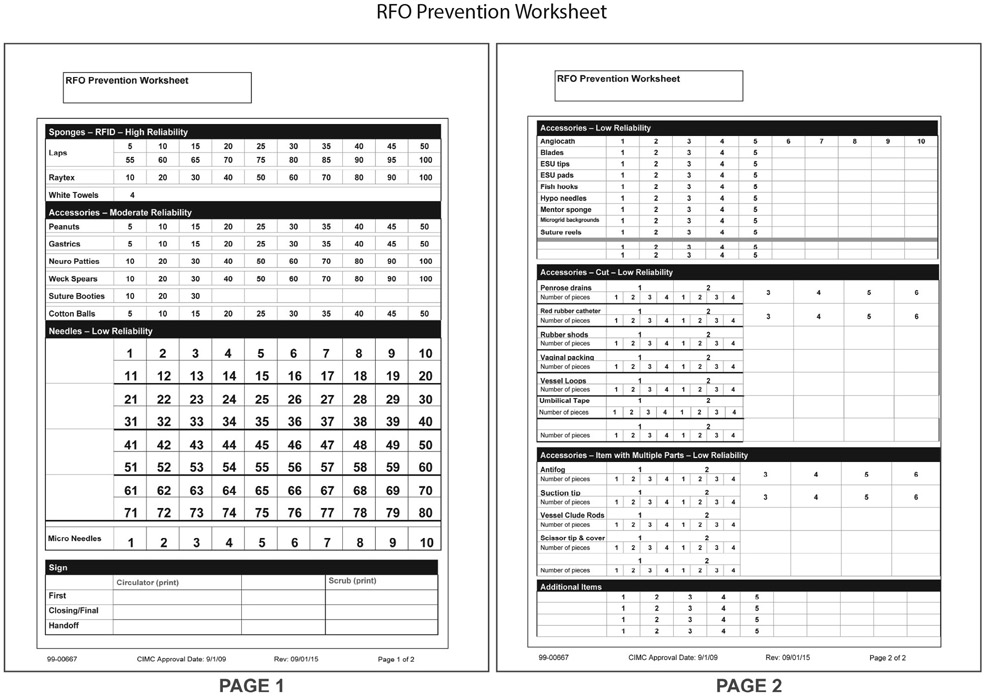
One of the first changes involved the modification of the count sheet, which was renamed the RFO Prevention Worksheet (Figure 1). A major element of the redesign was the grouping of items based on a “reliability” scale, wherein increasing reliability confers decreasing risk of an RFO incident. Items were classified as low, moderate, or high reliability. Items in the low-reliability grouping included, for example, needles and cut items. Items in the moderate reliability section included items with a count or visual cue, such as peanut sponges, suture booties, gastric sponges, neurology patties, and cotton balls. The final grouping included such high-reliability items as RF–tagged sponges and towels. These items have the highest reliability because, in addition to the use of visual and count cues, we use a detection system before the end of each case to ensure that no sponges are left behind. The categorization of items in this manner provided staff with a map and mental framework to heighten awareness of which items made them more vulnerable to error. Introducing the new RFO Prevention Worksheet also enabled staff to transition their focus from “just counting” to an overall mind-set of RFO prevention.
Figure 1:
The redesigned worksheet includes a listing of common surgical items grouped according to our reliability classification. An enlarged needle section facilitates increased count accuracy, and sections for cut items, items with multiple parts, and any additional or nonstandard items have also been incorporated.
The task force also identified a need for specific processes to account for items that are cut (for example, vessel loops, Penrose drain) and introduced into the surgical procedure. Historically, cut items were compared to uncut items to verify that they have been retrieved in their entirety. However, some items, such as vessel loops, can stretch and cannot be accurately measured against another intact item. As a result, the worksheet was modified to easily document cut items. A section was also added for items that are manufactured in multiple pieces, such as Poole suction (two pieces).
Surgical needles were categorized as having the lowest levels of reliability because they can be used in large quantities and are supplied in package counts ranging from a single needle to eight needles per package. Initial efforts were focused on modifying the needle section of the RFO Prevention Worksheet to facilitate easier tracking. Ultimately, the needle section of the worksheet was enlarged to maximize visibility and minimize documentation errors resulting from inappropriate spacing. In addition, a needle read-back method was developed after numerous attempts were made to minimize errors related to documentation and communication. The literature suggests that certain safety measures, such as the surgical team’s documentation of the counts and the surgical team’s attestation that the count is correct, can increase the team’s accountability and awareness of RFO prevention.11
Other items with low reliability were those that were not counted as part of the RFO Prevention Worksheet (for example, pens, syringes, blue towels) because they are rarely introduced into the surgical field. The task force determined that well-defined processes should exist to specifically prevent RFOs involving this item class. In the past, location control of these items depended entirely on the memory of the circulator (RN) or scrub (either RN or ST). Consequently, an “In/Out Sheet” was developed to track these items (Figure 2). Nursing staff developed a detailed list of items that were not counted or regularly accounted for and incorporated them as listed items on the In/Out Sheet. When an item listed on the In/Out Sheet is introduced to the surgical field, the item is documented as being “In” the surgical field and later documented as “Out” upon its return to the surgical table.
Figure 2:
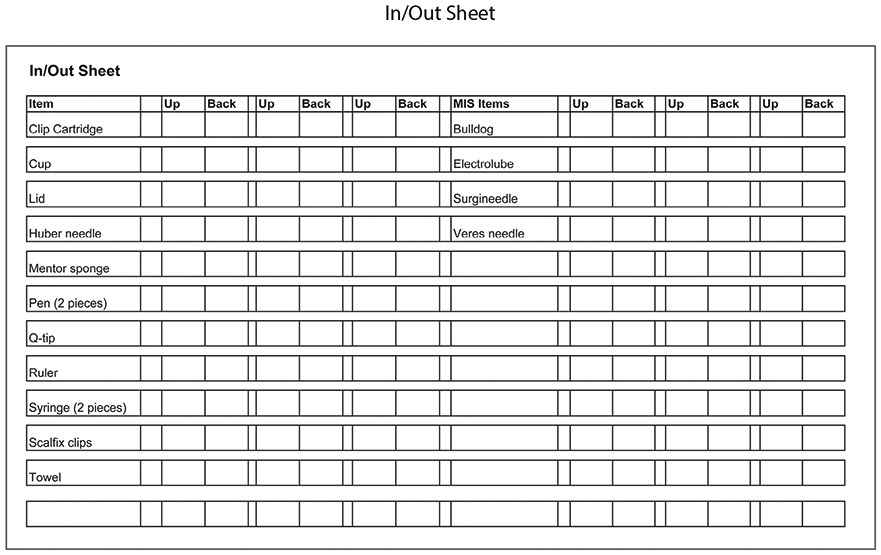
Implementation of Task Force recommendations required creation of an additional tracking sheet for items not typically included in the RFO Prevention Worksheet and instrument tray.
Dependent vs. Independent Counts.
Small focus groups reviewed the existing counting processes. After several discussions, the task force concluded that dependent counts, in which both the circulator and scrub personnel engaged in the count process, were laden with false security. The circulator’s participation in counting instruments and sponges before the procedure was not well focused due to numerous competing priorities that require completion before the surgical incision can commence (for example, ensuring that all surgical supplies, implants, and equipment are available, as well as measures to ensure strict adherence to Universal Protocol processes).24 Observations also led to the conclusion that dependent counts introduced many biases related to who was leading the count (circulator or scrub). Often, the individual observing the active counter would see whatever was presented by the active counter, but the announced item or quantity would unconsciously influence the observer’s perception of the count. This realization prompted the team to challenge the use of dependent counts. After much discussion and research into other industries’ visual checks, the task force decided to convert this instrument count from a dependent count to an independent count to model a concept of individual checks, which is frequently used in aviation. In aviation, individuals independently perform reviews and complete safety checklists prior to completing a task.25 In our revised process, the instruments are independently counted by the Central Processing Department (CPD) during tray assembly (Figure 3) before the containers are sealed and sterilized. The process of counting the instruments is completed in the OR through a second independent count by the scrub. This allows each person to review for errors without being influenced by the other. Implementing this new practice would ensure that two independent counts, or safety checks, were performed. If the scrub identified a discrepancy with information entered on the CPD instrument count sheet, the error was escalated to the circulator who would subsequently perform a dependent count with the scrub for validation of that specific item. This change was the most difficult to implement in our new RFO prevention process. Although this change allowed the scrub’s attention to be solely devoted to the counts while the circulator attended to the patient’s needs, the staff was initially uncomfortable with the new approach because, historically, two people had counted the items. After the team became adjusted to the new process, its members gained confidence in the ability of a single person to catch errors without dependence on a second counter. Keeping in mind Reason’s Swiss cheese theory of human error, the task force built a system with multiple layers of defense to establish more robust RFO prevention conditions wherein RFOs can occur only if there are multiple failures within the system of barriers (Figure 4).
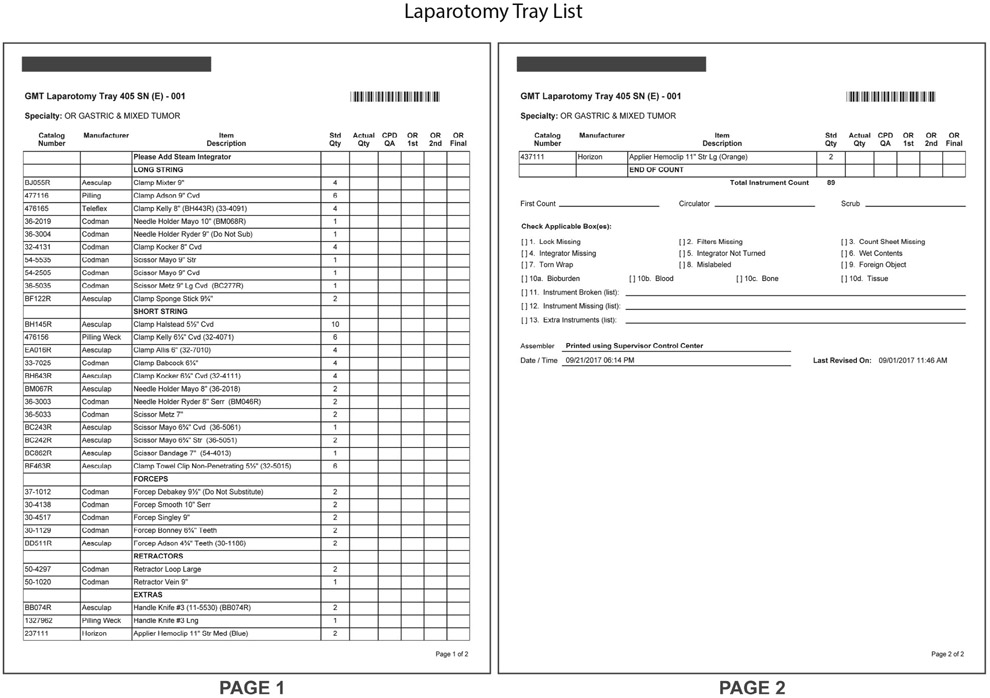
Figure 3:
A laparotomy tray list used by our institution’s gastric and mixed tumor surgical service is shown. The operating room central processing department maintains specialty-specific tray assembly lists to track the expected complement of surgical instruments for each surgical procedure.
Figure 4:
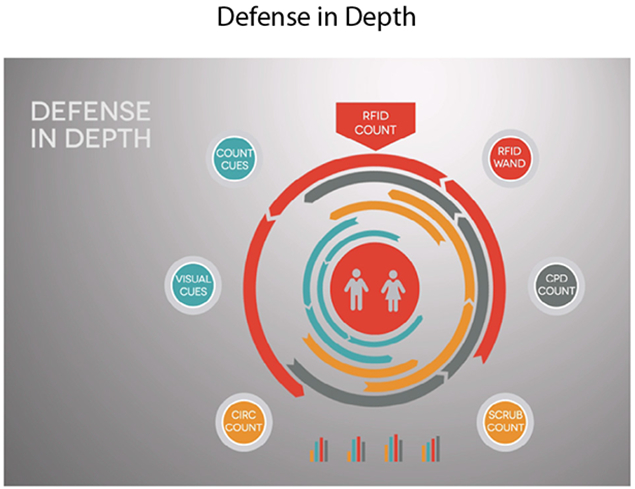
In order to prevent holes in our system, we made sure that our surgical items had multiple layers of defense while in normal use. These defense layers include counting barriers, visual barriers, and mechanical barriers.
Phase IV: Implementation—Changing the Focus From the Count to RFO Prevention (January 2014–December 2015)
As a result of the evaluation performed after the observations, the task force identified best practices, with a focus on the critical behaviors.
Sponges.
The scrub independently counts all RF–tagged sponges and towels before the procedure begins, then notifies the circulator of the quantities counted. During the procedure, the circulator and scrub dependently count all RF sponges and towels because the combination of assisting during the case and counting tends to divide the scrub’s attention. At the end of the procedure, the scrub and circulator dependently count all RF sponges. Visual cues are used throughout the case. Only multiples of 5 or 10 are discarded into the sponge bucket and then placed into the sponge bag counter. When placing the RF sponges into the sponge bag, the radio-opaque strip must be visible to ensure that the sponge is intact and to facilitate the visual count.
Instruments.
The scrub independently counts all instruments using the tray list from CPD. He or she visually inspects all instruments for broken or separated pieces that could potentially dislodge and be left behind during surgery. The scrub counts the instrument pieces and parts in the same manner as the CPD personnel. The scrub engages the circulator only if there is a count discrepancy. To prevent RFO incidents involving common instruments, our institution designates “preferred” designs for certain surgical instruments. For instance, we only use a malleable retractor that has been modified at our institution to include a hole (Figure 5). Before the closure of fascia, the scrub inserts one laparotomy pad through the hole to prevent the retractor from slipping inside the cavity during closure. The nursing staff counting the tray should be familiar with the preferred instruments specific to each surgical subspecialty, with escalation of the issue to the charge nurse if both scrub and circulator are unfamiliar with any particular instrument. This facilitates accurate identification during the counting process.
Figure 5:
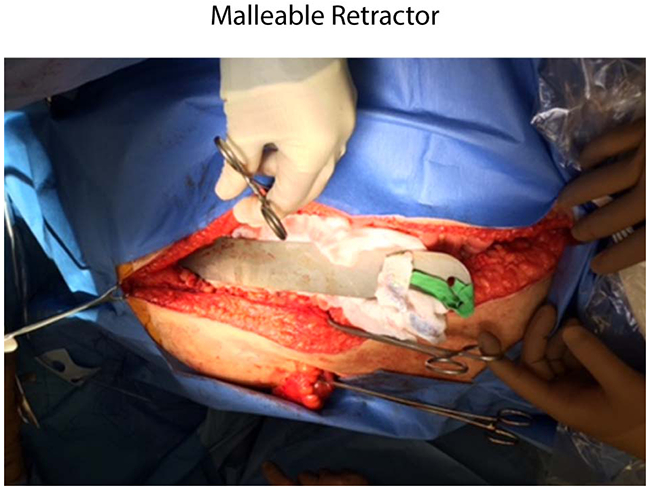
A modified malleable retractor is shown. An institutional modification of the traditional design incorporates a hole through which a laparotomy pad can be threaded to enhance the capacity to detect and remove the item before patient closure.
Needles and Accessories.
Needles and other accessories are counted before, during, and at the end of the surgical procedure. We use the needle read-back method to prevent documentation error in the counting of needles (Sidebar 3). To prevent accumulation of used needles on the field, we also use a 20-needle counter for open procedures and 10 for minimally invasive procedures. Multipart accessories and items cut into pieces during the procedure are documented according to the number of pieces. This information is entered on the RFO Prevention Worksheet so that the information can be clearly communicated when OR nursing staff are temporarily or permanently relieved, thus preventing a miscount and possible RFO incident.
Sidebar 3.
Oral Read-Back Method for Needles Added during the Case
| Step | Activity |
|---|---|
| 1 | The RN circulator provides the needles to the scrub. |
| 2 | The scrub (RN or ST) announces the number of needles received. |
| 3 | The RN circulator announces the number of needles recorded. |
ST, surgical technologist.
Cavity Sweep.
The surgical team must perform a cavity sweep before the end of the case and must respect the count process. The scrub reminds the surgeon of the need for the cavity sweep and holds the closing suture until the sweep is completed.
Count Discrepancy.
In the event of any discrepancies during the final count, the circulator notifies the surgeon. The surgical team then searches the operative field and, if necessary, the entire OR suite for the missing item. Complete closure of the incision is suspended until count discrepancies are resolved. An independent reviewer is also requested in the OR suite to help in a thorough search of the room. If the missing item is not located with a visual search of the operative field and OR suite, an x-ray of the operative field is requested.
Critical Behaviors.
On the basis of our knowledge of the system, we were able to identify behaviors that are least likely to be performed but are extremely important for RFO prevention, which we called critical behaviors. The task force identified nine critical behaviors that each surgical team must complete to prevent an RFO and concluded that compliance with these nine critical behaviors would achieve a higher level of reliability (Sidebar 4).
Sidebar 4.
Nine Critical Behaviors to Prevent RFOs
| Behavior | Description |
|---|---|
| 1 | RN Circulator and scrub (RN/ST) count accessories together (preexisting behavior). |
| 2 | Scrub escalates initial instrument discrepancy to the circulator (new behavior). |
| 3 | Scrub documents using the In/Out Sheet for items not listed on the RFO Prevention Worksheet and instrument tray list that are leaving the sterile table and passed to the surgical field (new behavior). |
| 4 | Scrub visually inspects instruments for broken/ separated pieces (preexisting behavior). |
| 5 | Oral read-back for added needles after the initial count (new behavior). |
| 6 | Surgeon uses a lap pad with a malleable retractor for closing surgical cavity (preexisting behavior). |
| 7 | Scrub uses clear count bag for lap pads (preexisting behavior). |
| 8 | Scrub and circulator use instrument list for final count (preexisting behavior). |
| 9 | Surgeon performs cavity sweep or visual inspection (preexisting behavior). |
ST, surgical technologist; RFO, retained foreign object.
Task force members were committed to observing and coaching staff through the change to encourage the adoption of the new behaviors. There were opportunities to offer positive reinforcement when the desired behaviors were performed, as well as opportunities to correct unsatisfactory behaviors. Some of the task force members performed weekly observations to document compliance with the critical behaviors. During these observations, staff members were informed, in real time, of any deviations from desired behaviors and practices. In addition, specific metrics were developed to assess compliance with critical behaviors.
RESULTS
Efficacy Projection with Our Socio-Probabilistic Risk Model
In January 2015, after the critical behaviors had been in place for approximately six months, the task force modified the fault trees to reflect changes in the surgical environment. New impartial observations were performed to gather new behavioral data. Based on the new observations, the structure of many of the parts of the risk model changed. We revised the model to incorporate the replacement of the sponge count machine, which was discontinued by the manufacturer. We also included the implementation of the needle read-back procedure. In addition to these changes, the model was revised to more accurately differentiate between the various types of items used. New fault trees were created for items that were originally grouped with other classes of items. We combined the existing fault trees from two trees per class of items to one tree per class of items. Through these revisions, we were able to produce a model that reflects the current processes and incorporates the current behaviors.
To validate the updated model, the DQS staff met with members of the task force, with additional representation from frontline staff, in October 2015 to review its validity and adjust it to exactly reflect the updated practices. After the update, another round of meetings was convened to identify the probabilistic data needed to evaluate the expected interval (in years) between RFOs. On the basis of observations in the OR and discussions with OR staff, the group provided information on the frequency of certain process failures within the framework of the new RFO prevention plan. After the final risk model was developed, the revised probability of an RFO was calculated at one in 22 years.
Training, Education, and Ongoing Quality Assurance
Education and training are important in any system change. Individualized and small-group training programs were established to teach staff the new counting process. A two-hour boot camp was developed to ensure that nursing staff members understand the new RFO prevention principles of changing the mental focus from counts to prevention and understanding each surgical item’s risk category. The training also included recommendations on how to speak up when the count process is uncontrolled. RFO prevention data, which include compliance with critical behaviors, are presented at weekly in-service meetings and sent to the nursing staff via e-mail. Case scenarios are provided to better understand how to incorporate the new risk model into the daily routine of counting. In addition, our program incorporated a system of renewed accountability for all members of the surgical team caring for our patients (Sidebar 5).
Sidebar 5.
RFO Roles in the Operating Room
| OR Member | Role/Responsibility |
|---|---|
| Nursing Staff (Scrub and Circulator) |
|
| Surgical Staff (Attending Surgeon/ Surgical Fellow/ Residents) |
|
| Anesthesia Staff (Attending Anesthesiologist/ CRNA/Residents |
|
| OR and Nursing Leadership |
|
RFO, retained foreign object; OR, operating room; CRNA, certified registered nurse anesthetist.
Each month, 20 observations on critical behaviors are randomly conducted by the Nursing Quality Assurance Council. Information gathered during the observations is shared with frontline perioperative staff during monthly in-service meetings. During these meetings, cause-and-effect diagrams are discussed to illustrate how instances of behavioral drift led to near-miss events. Critical behavior data are also reported to the OR Executive Committee to demonstrate compliance and, at times, to enlist the committee’s support in enforcing the desired RFO prevention practices among the physician group. As of March 28, 2017, we had achieved a period of 1,300 days since our last RFO incident, and no such incident had occurred almost a year later.
DISCUSSION
A major objective of this process improvement project was to achieve a reliability level as high as the airline industry. By building a risk model, we easily identified our vulnerabilities. Other published studies relied on analysis of near misses.10,13 We learned not just from our near misses but also from the risks inherent in every class of surgical items and staff behaviors. We were able to identify the categories of items that necessitated a strengthening of our layers of defense. For example, to improve tracking of needles in the surgical field, we created the needle read-back method. The In/Out Sheet is another example of an additional layer of defense for uncounted items. By reviewing the risk model, we were also able to identify areas of risk based on staff behavior. Our system takes into account a certain amount of human error, recognizing that, although we may have minimized the Swiss-cheese type of hazard risk, it is impossible to achieve zero RFOs. By engaging the staff in the identification of new strategies, we learned that we can achieve a higher rate of adoption of those strategies. It was also important to identify the critical behaviors that must be maintained to prevent RFOs.
Implementation Success: Contributing Factors
Through the use of a comprehensive risk modeling system widely used in the aviation industry, we developed a socio-probabilistic risk model designed to identify every possible scenario that could result in an RFO. Through this project we were able to develop a robust system for RFO prevention. This takes into account the system assessment, a safety culture, and an evaluation of the near misses. The safety culture starts with a change in behaviors and leads to the successful adoption of new strategies. The final part of our robust system requires the tracking of near misses to ensure that the system is working as intended. These near misses provide us with the ability to investigate, identify trends, and take action when needed.
An evaluation of the entire system involved in RFO prevention is crucial to obtaining excellent outcomes for patients undergoing a surgical procedure. Members of a multidisciplinary team must work together to prevent such complications. Support from leadership and positive participation by all stakeholders is important to sustain the program. Education and training are also necessary for achieving positive results. Ongoing discussions and training in RFO prevention strategies and principles are conducted, with an emphasis on changing the focus from item counts to the overall prevention of RFOs. The principles also include the use of technology (for example, sponge detection systems), count and visual cues, and fostering the development of a persistently vigilant state among surgical team members to prevent RFOs. Each OR staff member should fully understand each item’s risk level and its potential to become an RFO. The use of reliable reporting systems and a partnership with the DQS enabled us to maintain long-term compliance. After establishing the critical behaviors, continuous monitoring of compliance is vital to detect behavioral drift and implement training to reinforce the RFO prevention processes.
LIMITATIONS
The risk model that we created remains accurate as long as there is no new technology introduced into the environment and the processes remain unchanged. In addition, the critical behaviors need to be closely monitored to ensure that behavioral drift does not occur. If behavioral drift occurs, the model may require revision to determine the impact of the change in the critical behaviors. In our case, we decided to revise the model on the basis of changes to our sponge count equipment and the introduction of a new critical behavior for needles. When the model was revised to incorporate these changes, we also made structural changes to the fault trees, as we described. When structural changes are made to the fault trees, probability comparisons with previous trees are no longer valid. In our case, we grouped fault trees and created new ones after our new OR processes were implemented. Because of this change, we could not directly compare our newly calculated probabilities with the original ones. However, through these revisions, we were able to produce a more accurate model that reflects the current processes and incorporates the current behaviors.
CONCLUSION
As far as we can determine, we report the first time a socio-probabilistic risk model was applied to the counting of sterile supplies and instruments in the surgical field. This process improvement project has changed our institution’s approach to RFOs, leading to the design of a highly reliable system that has prevented new RFOs since its implementation. Collecting evidence based on our current practice enabled us to develop reliable and efficient counting practices that have helped us make the transition from the examination of past incidents to proactively identifying potential errors and building an appropriate system to effectively prevent their occurrence.
Acknowledgments
The authors thank Aileen Killen, PhD, RN, former Patient Safety Officer, Memorial Sloan Kettering Cancer Center, for leading the RFO Task Force, and David Marx, JD, Chief Executive Officer, Outcome Engenuity, LLC, for contributing his expertise to our risk model development and fault tree analysis. The authors also acknowledge all members of the RFO Task Force for their time and contributions, as well as Memorial Sloan Kettering’s operating room (OR) staff, Nursing Quality Assurance Council, OR Executive Leadership, Department of Quality and Safety, and Department of Nursing leadership for their support of the task force and its efforts.
Funding. Research at Memorial Sloan Kettering Cancer Center is supported in part by a grant from the National Institutes of Health/National Cancer Institute (#P30CA008748).
Footnotes
Conflicts of Interest. All authors report no conflicts of interest.
Contributor Information
Erika G. Duggan, Operational Excellence, Hospital Administration and Clinical Operations, Memorial Sloan Kettering Cancer Center, New York City..
Jimmy Fernandez, Quality and Safety, Division of Quality and Safety, Memorial Sloan Kettering Cancer Center..
Mary May Saulan, Department of Nursing, Memorial Sloan Kettering Cancer Center..
Dave L. Mayers, Department of Nursing, Memorial Sloan Kettering Cancer Center..
Mira Nikolaj, Department of Nursing, Memorial Sloan Kettering Cancer Center..
Tamara M. Strah, Department of Nursing, Memorial Sloan Kettering Cancer Center..
Lystra M. Swift, Perioperative Nursing, University of Texas MD Anderson Cancer Center, Houston..
Larissa Temple, Division of Colorectal Surgery, University of Rochester Medical Center, Rochester, New York..
References
- 1.Association of periOperative Registered Nurses (AORN), et al. Guideline for Prevention of Retained Surgical Items In: Guidelines for Perioperative Practice, Vol. 1 Denver: AORN, 2018. [Google Scholar]
- 2.Gawande AA, et al. Risk factors for retained instruments and sponges after surgery. N Engl J Med. 2003;348:229–235. January 16. [DOI] [PubMed] [Google Scholar]
- 3.Wan W, et al. Improving safety in the operating room: a systematic literature review of retained surgical sponges. Curr Opin Anaesthesiol. 2009;22:207–214. [DOI] [PubMed] [Google Scholar]
- 4.Agency for Healthcare Research and Quality (AHRQ). Selected Best Practices and Suggestions for Improvement: PSI 05: Retained Surgical Item or Unretrieved Device Fragment Count In: Toolkit for Using the AHRQ Quality Indicators. Rockville, MD: AHRQ, 2016, 1–6 Accessed Mar 2, 2018 https://www.ahrq.gov/sites/default/files/wysiwyg/professionals/systems/hospital/qitoolkit/combined/combined_toolkit.pdf. Tool D.4b. [Google Scholar]
- 5.Centers for Medicare & Medicaid Services. Press Release: Affordable Care Act Gives States Tools to Improve Quality of Care in Medicaid, Save Taxpayer Dollars. June 1, 2011. Accessed Mar 2, 2018 https://www.cms.gov/newsroom/MediaReleaseDatabase/Press-releases/2011-Press-releases-items/2011-06-01.html.
- 6.The Joint Commission. Preventing unintended retained foreign objects. Sentinel Event Alert. 2013;51:1–5. Accessed Mar 2, 2018 https://www.jointcommission.org/assets/1/6/SEA_51_URFOs_10_17_13_FINAL.pdf. [PubMed] [Google Scholar]
- 7.World Health Organization (WHO). WHO Guidelines for Safe Surgery 2009 In: Safe Surgery Saves Lives. Geneva: WHO, 2009. Accessed Mar 2, 2018 http://whqlibdoc.who.int/publications/2009/9789241598552_eng.pdf. [PubMed] [Google Scholar]
- 8.Mehtsun WT, et al. Surgical never events in the United States. Surgery. 2013;153:465–472. [DOI] [PubMed] [Google Scholar]
- 9.Hariharan D, Lobo DN. Retained surgical sponges, needles and instruments. Ann R Coll Surg Engl. 2013;95:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Judson TJ, et al. Miscount incidents: a novel approach to exploring risk factors for unintentionally retained surgical items. Jt Comm J Qual Patient Saf. 2013;39:468–A2. [DOI] [PubMed] [Google Scholar]
- 11.Stawicki SP, et al. Retained surgical items: a problem yet to be solved. J Am Coll Surg. 2013;216:15–22. [DOI] [PubMed] [Google Scholar]
- 12.Marx DA, Slonim AD. Assessing patient safety risk before the injury occurs: an introduction to sociotechnical probabilistic risk modelling in health care. Qual Safe Health Care. 2003;12(Suppl 2):ii33–ii38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cima RR, et al. A multidisciplinary team approach to retained foreign objects. Jt Comm J Qual Patient Safe. 2009;35:123–132. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg JL, Feldman DL. Implementing AORN recommended practices for prevention of retained surgical items. AORN J. 2012;95:205–219. [DOI] [PubMed] [Google Scholar]
- 15.Reason J Human error: models and management. BMJ. 2000;320:768–770. March 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reason J Safety in the operating theatre—part 2: human error and organizational failure. Qual Saf Health Care. 2005;14:56–60. [PMC free article] [PubMed] [Google Scholar]
- 17.International Civil Aviation Organization. Safety Report: 2016 Edition. 2016. Accessed Mar 2, 2018 http://www.icao.int/safety/Documents/ICAO_SR%202016_final_13July.pdf.
- 18.Lee WS, et al. Fault tree analysis, methods, and applications: a review. IEEE Trans Reliability. 1985;R-34:194–203. [Google Scholar]
- 19.Egorova NN, et al. Managing the prevention of retained surgical instruments: what is the value of counting? Ann Surg. 2008;247:13–18. [DOI] [PubMed] [Google Scholar]
- 20.Greenberg CC, et al. The frequency and significance of discrepancies in the surgical count. Ann Surg. 2008;248:337–341. [DOI] [PubMed] [Google Scholar]
- 21.Moffatt-Bruce SD, et al. Risk factors for retained surgical items: a meta-analysis and proposed risk stratification system. J Surg Res. 2014;190:429–436. [DOI] [PubMed] [Google Scholar]
- 22.Rogers A, Jones E, Oleynikov D. Radio frequency identification (RFID) applied to surgical sponges. Surg Endosc. 2007;21:1235–1237. [DOI] [PubMed] [Google Scholar]
- 23.Kranzfelder M, et al. Real-time instrument detection in minimally invasive surgery using radiofrequency identification technology. J Surg Res. 2013;185:704–710. [DOI] [PubMed] [Google Scholar]
- 24.The Joint Commission. Comprehensive Accreditation Manual for Hospitals (E-dition). Oak Brook, IL: Joint Commission Resources, 2018, 2017. [Google Scholar]
- 25.Turner JW, Huntley MS US Department of Transportations, Federal Aviation Administration, Office of Aviation Medicine, et al. The Use and Design of Flightcrew Checklists and Manuals. April 1991. Accessed Mar 2, 2018 https://rosap.ntl.bts.gov/view/dot/8631.