Abstract
Studies report inconsistent findings on the relationship between ASD and breastfeeding. We explored associations between ASD and breastfeeding initiation (yes/no) and duration (months categorized in tertiles) in the Study to Explore Early Development, a community-based case–control study in six sites in the Unites States. We adjusted for various child and mother demographic and pregnancy factors. Breastfeeding initiation was reported in 85.7% of mothers of children with ASD and 90.6% of mothers of controls. After adjustment, we found no significant difference in breastfeeding initiation (adjusted odds-ratio [aOR]: 0.88 and 95% confidence interval (CI) 0.60–1.28). However, mothers of children with ASD were less likely to report duration of breastfeeding in the high (≥12 months) versus low tertile (<6 months) (aOR and 95% CI: 0.61 [0.45–0.84]) or the middle (6–<12 months) versus low tertile (0.72: 0.54–0.98). The association of ASD and breastfeeding duration was slightly attenuated when the presence of the broader autism phenotype (BAP) in the mother was accounted for, but still remained for the highest tertile. This association does not appear to be totally explained by maternal BAP. We were unable to distinguish whether the difference in duration was due to difficulties breastfeeding children who later develop ASD, other factors not adjusted in our study, or greater ASD risk resulting from shorter breastfeeding duration. Longitudinal studies that compare reasons why mothers stop breastfeeding between ASD and controls and establish a temporal relation between ASD and breastfeeding are needed. Future studies should also evaluate interactions between ASD risk genes and breastfeeding.
Lay Summary:
In this study, we compared breastfeeding practices between mothers of children with and without autism spectrum disorder (ASD). We found that the percentage of mothers who started breastfeeding was similar between the two groups, but mothers of children with ASD breastfed for a shorter amount of time compared to mothers of children without ASD. Future studies are needed to evaluate the reasons why the duration of breastfeeding was shorter for mothers of children with ASD compared to those without ASD.
Introduction
The phenotypic profile of autism spectrum disorder (ASD) is heterogeneous (American Psychiatric Association, 2013), and ASD etiology likely involves genetic and nongenetic factors [Crawford, 2015]. Various nongenetic factors, including sododemographic characteristics, child diseases and conditions, pregnancy complications, maternal infections, breastfeeding, chemical exposures, and maternal diet have been evaluated in relation to ASD [Fernandes, 2015; Fujiwara, Morisaki, Honda, Sampei, & Tani, 2016; Mandy & Lai, 2016; Ng, Montigny, Ofner, & Do, 2017].
Breastfeeding is a dynamic, interactive, and bidirectional social behavior and “not simply a meal at the breast” [Kaye & Wells, 1980; Raju, 2011]. There are numerous benefits of breastfeeding for the child, such as providing key nutrients for physical and neurologic development, protecting from infections, and decreasing the risk for food allergies, asthma, and cardiovascular diseases [Bar, Milanaik, & Adesman, 2016; Brahm & Valdes, 2017]. The American Academy of Pediatrics (AAP) recommends exclusive breastfeeding for the first 6 months of life and continuation of breastfeeding for at least a year, or as mutually desired by the mother and the child [American Academy of Pediatrics, 1997]. Various prenatal (e.g., maternal age, race/ethnicity, education, parity, family income, area of residence, cigarette smoking, maternal marital, and employment statuses), perinatal (mode of delivery, birthweight, gestational age), and postnatal factors (mother’s ability to produce adequate milk, existence of lactation support programs) can influence breastfeeding initiation and duration [Atchan, Foureur, & Davis, 2011; Demirci, Sereika, & Bogen, 2013; Forste, Weiss, & Lippincott, 2001; Li, Fein, Chen, & Grummer-Starwn, 2008; Odom, Li, Scanlon, Perrine, & Grummer-Strawn, 2013; Patel et al., 2015; Wallwiener et al., 2016].
Past studies have reported inconsistent findings on the relationship between ASD and breastfeeding [Hong, Ziegler, & Brody, 2014; Tseng et al., 2017]. Most studies found lower breastfeeding initiation or shorter duration in children with ASD versus children without ASD, suggesting a possible protective effect of breastfeeding [Al-Farsi et al., 2012; Bittker & Bell, 2018; Boucher et al., 2017; Brown, Austin, & Busija, 2014; George, Padman, Nair, Leena, & Russell, 2014; Johnson et al., 2010; Lemcke, Pamer, Bjerrum, Thomsen, & Lauritsen, 2018; Ravi, Chandrasekaran, Kattimani, & Subramanian, 2016; Say, Karabekiroğlu, Babadaği, & Yüce, 2016; Schultz et al., 2006; Shafai, Mustafa, Hild, Mulari, & Curtis, 2017; Tseng et al., 2017], Likewise, a recent meta-analysis of seven studies, some of which were cited above, documented an overall significantly lower breastfeeding initiation in children with ASD versus those without ASD. However, the meaning of the lower breastfeeding initiation or shorter duration in ASD versus controls is not dearly understood since most of these studies use designs that preclude establishing a temporal relationship between ASD and breastfeeding. Breastfeeding may protect against ASD for several reasons. Breast milk contains various substances (e.g., essential fatty adds, insulin-like grow factor, oxytocin, melatonin) involved in brain development and maturation [Deoni et al., 2013; Hall, 2016; Herba et al., 2013; Koletzo et al., 2008; Steinman & Mankuta, 2013]. Breastfeeding promotes mother-child bonding through release of maternal hormones (e.g., prolactin, oxytocin), which can affect social behaviors in the mother and the child [Feldman et al., 2012; Kim et al., 2011; Vanya, Szucs, Vetro, & Bartfai, 2017; Zhang, Zhang, Han, & Han, 2017]. Breast milk contains immune factors that protect a child against various infections. Studies have reported that breastfed children are less likely than those not breastfed to contract ear, throat, and sinus infections [Li, Dee, Li, Hoffman, & Grummer-Strawn, 2014; Oddy, 2002], Infections can trigger chronic inflammation of the nervous system that could affect brain development and maturation [Ng et al., 2017]. Breast milk contributes to the development of the gut microbiome. It has been suggested that alterations of gut microbiome could play a role in the etiology of neurodevelopmental disorders, including ASD [Heijtz, 2016; Vuong & Hsiao, 2016].
Alternatively, it is also possible that less frequent or shorter-duration breastfeeding in ASD versus controls may indicate early manifestations of difficulties in social interaction; differences in characteristics of children who will later develop ASD, such as high prevalence of preterm birth or birth complications [Ng et al., 2017]; or the presence of emotion dysregulation, sleep, or gastrointestinal problems in the child [Bresnahan et al., 2015; Brian, Bryson, & Zwaigenbaum, 2015; Gomez & Baird, 2005; Lemcke et al., 2018; Tseng et al., 2017; Zwaigenbaum, Bryson, & Garon, 2013]. Less or shorter breastfeeding could also be due to some maternal factors, including the broader autism phenotype (BAP), which is a sub-clinical collection of quantitative autism traits seen in family members of children with ASD [Berthoz, Lalanne, Crane, & Hill, 2013]. BAP has a higher prevalence in mothers of children with ASD versus controls and may interfere with breastfeeding because of difficulties in mother–child social interaction [Berthoz et al., 2013; Henderson, Evans, Straton, Priest, & Hagan, 2003; Ingersoll & Hambrick, 2011].
Other studies have found no associations between ASD and breastfeeding initiation or duration [Burd, Kerbeshian, Vesely, Durgin, & Reep, 1988; Christian et al., 2018; Fernandes, 2015; Gore, Emerson, & Brady, 2015; Husk & Keim, 2015; Tanoue & Oda, 1989]. In a large and nationally representative sample, Husk and Keim [2015] found no association between breastfeeding and ASD after adjusting for child and family characteristics. These authors suggested that residual confounding might explain some of the associations reported by others. In contrast, two studies [Nishijo et al., 2014; Shamberger, 2011] found higher breastfeeding initiation in children with ASD. Shamberger [2011] used an ecological study in the United States and found a positive correlation between ASD prevalence in a state and the proportion of children exclusively breastfed. Nishijo et al. [2014] reported a positive correlation between the levels of a type of dioxin in breast milk and autistic traits among 3-year-old children of mothers living near a contaminated former air base in Vietnam. The high exposure to environmental chemical contaminants (i.e., dioxin) through breast milk [Nishijo et al., 2014] or the defidency in some nutrients in human breast milk, such as riboflavin or thiamin in children exclusively breastfed [Shamberger, 2011], were suggested as possible contributing factors for the aforementioned positive association between ASD and breastfeeding.
Past studies that assessed the relationship between ASD and breastfeeding had methodological limitations. For example, most of the cited studies, except Al-Farsi et al. [2012], Husk and Keim [2015], and Schultz et al. [2006], used small clinical samples. Therefore, these studies might have been subject to selection bias and insufficient power. Further, as suggested by Husk and Keim [2015], most studies did not adjust fully for potential confounding factors. In the majority of studies, the diagnosis of ASD was based on parental report or the use of screening instruments, which could result in misclassification of some children. To provide a more accurate assessment of the relationship between ASD and breastfeeding, we evaluated breastfeeding initiation and duration in a large, diverse, and well-characterized sample of preschoolers, the Study to Explore Early Development (SEED), and adjusted for a wide variety of child, mother, and family characteristics. Further, we also assessed whether the presence of the BAP in the mother affected any found association between ASD and breastfeeding.
Methods
Study Design
This is a cross-sectional analysis of data from the SEED case–control study. SEED is a multi-site, community-based study. We used data from SEED Phase 1 which was conducted in six sites in the United States (California, Colorado, Georgia, Maryland, North Carolina, and Pennsylvania). The SEED protocol was approved by the Centers for Disease Control and Prevention and each site’s institutional review board.
Recruitment and Data Collection Procedures
Detailed descriptions of SEED methodology have been published by others [Schendel et al., 2012; Wiggins et al., 2015]. Study steps included enrolment, a maternal interview, clinic visit with specimen collection (blood or saliva), and various self-administered questionnaires. For most enrolled children (98%), the biological mother provided information on the different questionnaires, and we limited this analysis to those mother–child pairs. In SEED Phase 1, children aged 30–68 months, born and residing in a given site’s catchment area, with a caregiver fluent in English or, at two sites (California and Colorado), English or Spanish, were eligible for enrollment. At each site, we recruited potential cases of ASD from special education programs, clinics, and other local providers of disabilities services. Controls were recruited from randomly selected birth certificates in the same birth cohort and geographic area as cases. After enrollment, the Social and Communication Questionnaire [SCQ; Rutter, Bailey, & Lord, 2003] was administered to the caregivers, and children received an in-person developmental evaluation during a clinic visit using the Mullen Scales of Early Learning [MSEL; Mullen, 1995]. Children who screened positive on the SCQ [score ≥11] [Wiggins, Bakeman, Adamson, & Robins, 2007] and/or had a previous ASD diagnosis received an in-depth ASD evaluation including the Autism Diagnostic Observation Schedule [ADOS; Lord et al., 2000] and the Autism Diagnostic Interview-Revised [ADI-R; Lord, Rutter, & Le Couteur, 1994], Final ASD classification was based on ADOS and ADI-R scores using an algorithm developed by SEED clinicians [Wiggins et al., 2015]. All participants included in this analysis were seen by a SEED clinician during a clinic visit.
Variables of Interest
The two variables of interest were breastfeeding initiation (yes/no) and breastfeeding duration collected on the “Pregnancy Reference Form” (PRF) during a telephone interview with a mother after enrollment. Mothers were asked: “Did you ever breastfeed your child even for a few days?” Mothers who responded “yes” were also asked: “How old was the child (days, weeks, months) when you completely stopped breastfeeding? Please include the time period when you continued to breastfeed while supplementing with other liquids or solids.” Among breastfed children, we derived a variable for breastfeeding duration (months) that was categorized in tertiles: high (≥12.0 months), middle (6.0–<12.0 months), and low (<6.0 months) based on the distribution of breastfeeding duration in the control group. Mother’s responses on breastfeeding initiation were later confirmed during a more in-depth interview (“Caregiver Interview”), which collected detailed information about the index pregnancy and early postnatal exposures. We limited this analysis to children whose mothers provided concordant responses on breastfeeding initiation in the PRF and the Caregiver Interview. A total of 11 children (0.7%) were excluded due to discordant answers between the two instruments.
The choice of confounders that we adjusted for was based on the published literature [e.g., Forste et al., 2001]. We included the following variables: child sex, gestational age, birth weight, 5-min Apgar score, birth plurality, mode of delivery; mother’s age at the time of child’s birth in years, parity with the index child, race and ethnicity, education at time of child’s birth, place of birth, employment status during the 3 months before the child’s birth until the end of breastfeeding period, marital status, smoking status, and presence of neuropsychiatric diagnoses (specifically, depression, anxiety, seizure disorder, and intellectual disability); family estimated income during the year preceding the index child’s birth, the presence of BAP in the mother, and study site. Except for maternal age and Apgar score, all variables were analyzed as categorical. Data on these potential confounders were obtained from the caregiver interview, maternal medical history form, and birth certificates. Marital status and Apgar score were only tested in sub-analyses that included the five sites that provided data on these two variables. The presence of the BAP in the mother was based on the Social Responsiveness Scale-Adult T scores [Constantino & Todd, 2005]. Mothers with T scores ≥60 were classified as having BAP and those with T scores <60 as not having BAP [Rubenstein et al., 2017 for details].
Analytic Strategy
We compared characteristics of children with ASD and controls using Chi-Square tests for categorical variables and t-tests for continuous variables in the entire sample and among children who were breastfed. We tested associations between ASD and breastfeeding initiation and duration first in all six sites and second in the five sites that reported data on marital status and Apgar score using all the preselected potentially confounding variables, including study site (full variables models). We used logistic regression with breastfeeding initiation as the dependent variable to compare breastfeeding initiation between ASD and controls, and among breastfed children, we used multinomial logistic regression with breastfeeding duration categorized in tertiles as the dependent variable to compare breastfeeding duration between ASD and controls using the low tertile as the reference. Next, we rerun the above analyses using only a subset of variables that were individually associated with both case status and breastfeeding initiation/duration at the P-value of ≤0.20 in bivariate analyses (reduced variables models). We included: maternal age, race, education, place of birth, family income, maternal psychiatric diagnoses, child sex, gestational age, mode of delivery, and study site in the reduced variables model for breastfeeding initiation. Maternal age, race, ethnicity, education, family income, maternal psychiatric diagnoses, child sex, gestational age, birth weight, birth plurality, mode of delivery, and study site were used in the reduced variables model for breastfeeding duration. To specifically assess the effect of the presence of the BAP, we rerun all the above analyses (full and reduced models) including BAP as a covariate and compared the changes in the effect estimates between the models with and without BAP. We primarily report findings based on the results using the full variables models in six sites. Results from the full variables models in the subset of five sites with data on marital status and Apgar score and those from the reduced variables models in six and five sites are presented for comparison purposes. As an alternative to analyzing duration of breastfeeding as a categorical variable, we compared breastfeeding duration as continuous (in months) using analysis of covariance (ANCOVA), and we also reported the unadjusted cumulative probabilities of breastfeeding (Kaplan-Meier curves) between children with ASD and controls. In order to assess the individual contribution of each preselected variable in the change of the unadjusted estimates of breastfeeding initiation and duration, we used multivariable logistic and multinomial logistic regression, respectively, including only case status, breastfeeding and the potentially confounding variable.
Results
We included a total of 673 children with ASD and 876 controls in breastfeeding initiation analyses. Of those, 577 children with ASD (85.7% of ASD) and 794 controls (90.6% of controls) were breastfed. Data on breastfeeding initiation/duration, the characteristics of the entire sample and of the subset of children who were breastfed by case-control status are presented in Table 1. Cases and controls differed on a number of sociodemographic and perinatal characteristics. For example, cases were more likely than controls to be male and born before 37 weeks of gestation. Further, cases were also more likely than controls to have a mother from a minority racial group, without a college degree, and born outside of the United States. The proportion of mothers with a BAP was higher in cases versus controls. Child’sex and gestational age; maternal age, race, education, marital status and neuropsychiatric diagnoses; mode of delivery of the child; family income, the presence of a BAP in the mother, and study site were associated with both breastfeeding initiation and duration at P-value ≤0.20 (Table 2).
Table 1.
Comparisons of Child and Family Characteristics Between Preschoolers With Autism Spectrum Disorder and Controls in the Study to Explore Early Development
| Total sample (n = 1549) |
Among breastfed children (n = 1371) |
|||||
|---|---|---|---|---|---|---|
| Variable | ASDa n = 673 |
Controls n = 876 |
P-value | ASDa (n = 577) |
Controls (n = 794) |
P-value |
| Breastfeeding initiation (n, %) | ||||||
| Yes | 577 (85.7) | 794 (90.6) | 577 (100) | 794 (100) | n/a | |
| No (ref) | 96 (14.3) | 82 (9.4) | 0.003 | 0 | 0 | |
| Breastfeeding duration (months) | ||||||
| Mean (SDb) | n/a | n/a | 7.3 (7.2) | 9.3 (7.2) | <0.0001 | |
| Median | n/a | n/a | n/a | 6.0 | 8.5 | <0.0001 |
| Breastfeeding duration (tertiles) | ||||||
| High (≥12 months) | n/a | n/a | n/a | 141 (24.5) | 277 (34.9) | |
| Middle (6.0 months-<12.0 months) | n/a | n/a | n/a | 152 (26.3) | 255 (32.1) | |
| Low (<6.0 months) | n/a | n/a | n/a | 284 (49.2) | 262 (33.0) | <0.0001 |
| Child sex (n, %) | ||||||
| Female | 124 (18.4) | 412 (47.0) | 108 (18.7) | 379 (47.7) | ||
| Male | 549 (81.6) | 464 (53.0) | <0.0001 | 469 (81.3) | 415 (52.3) | <0.0001 |
| Gestational age (n, %) | ||||||
| Preterm (<37 weeks) | 109 (16.2) | 81 (9.3) | 91 (15.8) | 72 (9.1) | ||
| Term (≥37 weeks) | 560 (83.2) | 792 (90.4) | 482 (83.5) | 721 (90.8) | ||
| Missing | 4 (0.6) | 3 (0.3) | 0.0001 | 4 (0.7) | 1 (0.1) | 0.0002 |
| Child birthweight (n, %) | ||||||
| Low (<2500 g) | 89 (13.2) | 61 (7.0) | 75 (13.0) | 54 (6.8) | ||
| Normal (≥2500 g) | 568 (84.4) | 799 (91.2) | 487 (84.4) | 727 (91.6) | ||
| Missing | 16 (2.4) | 16 (1.8) | 0.0001 | 15 (2.6) | 13 (1.7) | 0.0002 |
| Child Apgar score at the 5th minutec | ||||||
| Mean (SDb) | 8.8 (0.6) | 8.9 (0.6) | 8.8 (0.6) | 8.9 (0.6) | ||
| Median | 9.0 | 9.0 | 0.08 | 9.0 | 9.0 | 0.18 |
| Maternal age at child’s birth (years) | ||||||
| Mean (SDb) | 31.7 (5.5) | 32.1 (5.3) | 31.9 (5.4) | 32.3 (5.1) | ||
| Median | 32.2 | 32.5 | 0.15 | 32.3 | 32.6 | 0.16 |
| Parity of the index child (n, %) | ||||||
| Second or later birth | 353 (52.4) | 470 (53.7) | 302 (52.3) | 419 (52.8) | ||
| First birth | 318 (47.3) | 404 (46.1) | 274 (47.5) | 373 (47.0) | ||
| Missing | 2 (0.3) | 2 (0.2) | 0.87 | 1 (0.2) | 2 (0.2) | 0.94 |
| Birth plurality (n, %) | ||||||
| Multiple | 54 (8.0) | 32 (3.7) | 48 (8.3) | 29 (3.6) | ||
| Singleton | 617 (91.7) | 842 (96.1) | 528 (91.5) | 763 (96.1) | ||
| Missing | 2 (0.3) | 2 (0.2) | 0.0009 | 1 (0.2) | 2 (0.3) | 0.001 |
| Maternal ethnicity (n, %) | ||||||
| Hispanic | 80 (11.9) | 77 (8.8) | 71 (12.3 | 71 (8.9) | ||
| Non-Hispanic | 592 (88.0) | 799 (91.2) | 505 (87.5) | 723 (91.1) | ||
| Missing | 1 (0.1) | 0 (0.1) | 0.07 | 1 (0.2) | 0 | 0.06 |
| Maternal race (n, %) | ||||||
| Non-Hispanic African American | 134 (19.9) | 106 (12.1) | 102 (17.7) | 78 (9.8) | ||
| Others | 113 (16.8) | 105 (12.0) | 104 (18.0) | 96 (12.1) | ||
| Non-Hispanic White | 423 (62.9) | 661 (75.5) | 369 (63.9) | 616 (77.6) | ||
| Missing | 3 (0.4) | 4 (0.4) | <0.0001 | 2 (0.4) | 4 (0.5) | <0.0001 |
| Maternal education at child’s birth (n, %) | ||||||
| No college degree | 317 (47.1) | 290 (33.1) | 248 (43.0) | 237 (29.9) | ||
| College degree or higher | 354 (52.6) | 585 (66.8) | 327 (56.7) | 556 (70.0) | ||
| Missing | 2 (0.3) | 1 (0.1) | <0.0001 | 2 (0.3) | 1 (0.1) | <0.0001 |
| Maternal place of birth (n, %) | ||||||
| Outside the United States | 143 (21.2) | 119 (13.6) | 136 (23.6) | 113 (14.2) | ||
| In the United States | 530 (78.8) | 757 (86.4) | <0.0001 | 441 (76.4) | 681 (85.8) | <0.0001 |
| Mother employment status (n, %) | ||||||
| Employed | 489 (72.7) | 696 (79.4) | 424 (73.5) | 629 (79.2) | ||
| Not employed | 183 (27.2) | 176 (20.1) | 153 (26.5) | 161 (20.3) | ||
| Missing | 1 (0.1) | 4 (0.5) | 0.003 | 0 | 4 (0.5) | 0.007 |
| Mother marital status (n, %)c | ||||||
| Not married | 173 (30.2) | 165 (22.4) | 126 (26.0) | 133 (20.0) | ||
| Married | 398 (69.6) | 573 (77.6) | 357 (73.8) | 531 (80.0) | ||
| Missing | 1 (0.2) | 0 | 0.003 | 1 (0.2) | 0 | 0.03 |
| Mother smoking status (n, %) | ||||||
| Smoker | 262 (38.9) | 301 (34.4) | 218 (37.8) | 272 (34.3) | ||
| Not smoker | 409 (60.8) | 571 (65.2) | 358 (62.0) | 518 (65.2) | ||
| Missing | 2 (0.3) | 4 (0.4) | 0.16 | 1 (0.2) | 4 (0.5) | 0.26 |
| Maternal neuropsychiatric diagnosesd (n, %) | ||||||
| Yes | 130 (19.3) | 89 (10.2) | 108 (18.7) | 78 (9.8) | ||
| No | 543 (80.7) | 786 (89.7) | 469 (81.3) | 715 (90.1) | ||
| Missing | 0 (0.0) | 1 (0.1) | <0.0001 | 0 | 1 (0.1) | <0.0001 |
| Mother broader autism phenotype | ||||||
| Yes | 43 (7.5) | 33 (4.3) | 0.01 | 34 (6.9) | 25 (3.6) | 0.009 |
| No | 530 (92.50) | 735 (95.7) | 460 (93.1) | 675 (96.4) | ||
| Mode of delivery of the child (n, %) | ||||||
| All others | 418 (62.1) | 606 (69.2) | 364 (63.1) | 555 (69.9) | ||
| Cesarean | 255 (37.9) | 270 (30.8) | 0.004 | 213 (36.9) | 239 (30.1) | 0.008 |
| Family income (n, %) | ||||||
| $10,000–<30,000 | 156 (23.2) | 114 (13.0) | 118 (20.4) | 86 (10.8) | ||
| $30,000–<70,000 | 175 (26.0) | 184 (21.0) | 146 (25.3) | 164 (20.7) | ||
| $70,000–<110,000 | 161 (23.9) | 245 (27.9) | 143 (24.8) | 232 (29.2) | ||
| $>110,000 | 161 (23.9) | 307 (35.1) | 155 (26.9) | 290 (36.5) | ||
| Missing | 20 (3.0) | 26 (3.0) | <0.0001 | 15 (2.6) | 22 (2.8) | <0.0001 |
| Study site | ||||||
| California | 101 (15.0) | 138 (15.8) | 93 (16.1) | 130 (16.4) | ||
| Colorado | 138 (20.5) | 188 (21.5) | 121 (20.9) | 176 (22.2) | ||
| Georgia | 132 (19.6) | 169 (19.3) | 0.67 | 112 (19.4) | 147 (18.5) | 0.92 |
| Maryland | 103 (15.3) | 123 (14.0) | 86 (14.9) | 110 (13.8) | ||
| North Carolina | 102 (15.2) | 151 (17.2) | 92 (15.9) | 139 (17.5) | ||
| Pennsylvania | 97 (14.41) | 107 (12.2) | 73 (12.6) | 92 (11.6) | ||
Note. Bolds values are assessed at a P-value of ≤0.20.
ASD: autism spectrum disorder.
SD: standard deviation.
Available only in five sites.
Neuropsychiatrie diagnoses include depression, anxiety, seizure disorder, and intellectual disability.
Table 2.
Association Between Breastfeeding Initiation/Duration and Each Covariate in Preschoolers Enrolled in the Study to Explore Early Development
| Breastfeeding initiation (n = 1549) |
Breastfeeding duration among breastfed children in tertiles (n = 1371) |
||||||
|---|---|---|---|---|---|---|---|
| Variable | Yes | No | P-value | Low | Middle | High | P-value |
| Child sex (n, %) | |||||||
| Female | 487 (35.5) | 49 (27.5) | 169 (30.9) | 156 (38.3) | 162 (38.8) | ||
| Male | 884 (64.5) | 129 (72.5) | 0.03 | 377 (69.1) | 251 (61.7) | 256 (61.2) | 0.02 |
| Gestational age (n, %) | |||||||
| Preterm (<37 weeks) | 163 (11.9) | 27 (15.2) | 101 (18.5) | 39 (9.6) | 23 (5.5) | ||
| Term (≥37 weeks) | 1203 (87.8) | 149 (83.7) | 442 (80.9) | 368 (90.4) | 393 (94.0) | ||
| Missing | 5 (0.3) | 2 (1.1) | 0.16 | 3 (0.6) | 0 | 2 (0.5) | <0.0001 |
| Child birth weight (n, %) | |||||||
| Low (<2500 g) | 129 (9.4) | 21 (11.8) | 77 (14.1) | 32 (7.9) | 20 (4.8) | ||
| Normal (>2500 g) | 1214 (88.6) | 153 (86.0) | 453 (83.0) | 370 (90.9) | 391 (93.5) | ||
| Missing | 28 (2.0) | 4 (2.2) | 0.58 | 16 (2.9) | 5 (1.2) | 7 (1.7) | <0.0001 |
| Child Apgar score at the 5th minutea | |||||||
| Mean (SDb) | 8.9 (0.6) | 8.9 (0.4) | 8.8 (0.6) | 8.8 (0.6) | 8.9 (0.7) | ||
| Median | 9.0 | 9.0 | 0.92 | 9.0 | 9.0 | 9.0 | 0.23 |
| Maternal age at child’s birth (years) | |||||||
| Mean (SDb) | 32.2 (5.2) | 30.1 (6.5) | 31.4 (5.6) | 32.2 (4.9) | (4.7) | ||
| Median | 32.5 | 30.0 | <0.001 | 31.8 | 32.4 | 33.3 | <0.001 |
| Parity of the index child (n, %) | |||||||
| First birth | 647 (47.2) | 75 (42.1) | 275 (50.4) | 189 (46.4) | 183 (43.8) | ||
| Second or later birth | 721 (52.6) | 102 (57.3) | 269 (49.3) | 218 (53.6) | 234 (56.0) | ||
| Missing | 3 (0.2) | 1 (0.6) | 0.33 | 2 (0.3) | 0 | 1 (0.2) | 0.21 |
| Birth plurality (n, %) | |||||||
| Multiple | 77 (5.6) | 9 (5.1) | 53 (9.7) | 14 (3.4) | 10 (2.4) | ||
| Singleton | 1291 (94.2) | 168 (94.4) | 491 (89.9) | 393 (96.6) | 407 (97.4) | ||
| Missing | 3 (0.2) | 1 (0.6) | 0.67 | 2 (0.4) | 0 | 1 (0.2) | <0.0001 |
| Maternal ethnicity (n, %) | |||||||
| Hispanic | 142 (10.3) | 15 (8.4) | 70 (12.8) | 35 (8.6) | 37 (8.9) | ||
| Non-Hispanic | 1228 (89.6) | 163 (91.6) | 475 (87.0) | 372 (91.4) | 381 (91.1) | ||
| Missing | 1 (0.1) | 0 | 0.68 | 1 (0.2) | 0 | 0 | 0.11 |
| Maternal race (n, %) | |||||||
| Non-Hispanic African American | 180 (13.1) | 60 (33.7) | 108 (19.8) | 49 (12.0) | 23 (5.5) | ||
| Others | 200 (14.6) | 18 (10.1) | 80 (14.7) | 49 (12.0) | 71 (17.0) | ||
| Non-Hispanic White | 985 (71.9) | 99 (55.6) | 355 (65.0) | 307 (75.4) | 323 (77.3) | ||
| Missing | 6 (0.4) | 1 (0.6) | <0.0001 | 3 (0.5) | 2 (0.6) | 1 (0.2) | <0.0001 |
| Maternal education at birth (n, %) | |||||||
| No college degree | 485 (35.4) | 121 (68.0) | 264 (48.4) | 120 (29.5) | 101 (24.2) | ||
| College degree or higher | 883 (64.4) | 55 (30.9) | 280 (51.3) | 287 (70.5) | 316 (75.6) | ||
| Missing | 3 (0.2) | 2 (1.1) | <0.0001 | 2 (0.3) | 0 | 1 (0.2) | <0.0001 |
| Maternal place of birth (n, %) | |||||||
| Outside of the United States | 249 (18.2) | 13 (7.3) | 103 (18.9) | 75 (18.4) | 71 (17.0) | ||
| In the United States | 1122 (81.8) | 165 (92.7) | 0.0003 | 443 (81.1) | 332 (81.6) | 347 (83.0) | 0.74 |
| Mother employment status (n, %) | |||||||
| Employed | 1053 (76.8) | 132 (74.1) | 424 (77.7) | 318 (78.1) | 311 (74.4) | ||
| Not employed | 314 (22.9) | 45 (25.3) | 121 (22.1) | 88 (21.6) | 105 (25.1) | ||
| Missing | 4 (0.3) | 1 (0.6) | 0.64 | 1 (0.2) | 1 (0.3) | 2 (0.5) | 0.64 |
| Mother marital status (n, %)a | |||||||
| Not married | 259 (22.6) | 79 (48.8) | 146 (30.4) | 65 (18.9) | 48 (14.8) | ||
| Married | 888 (77.3) | 83 (51.2) | 333 (69.4) | 278 (81.1) | 277 (85.2) | ||
| Missing | 1 (0.1) | 0 | <0.0001 | 1 (0.2) | 0 | 0 | <0.001 |
| Mother smoking status (n, %) | |||||||
| Smoker | 490 (35.7) | 73 (41.0) | 216 (39.6) | 141 (34.6) | 133 (31.8) | ||
| Non Smoker | 876 (63.9) | 104 (58.4) | 327 (59.9) | 266 (65.4) | 283 (67.7) | ||
| Missing | 5 (0.4) | 1 (0.6) | 0.35 | 3 (0.5) | 0 | 2 (0.5) | 0.07 |
| Maternal neuropsychiatric diagnosesc (n, %) | |||||||
| Yes | 186 (13.6) | 33 (18.5) | 91 (16.7) | 37 (9.1) | 58 (13.9) | ||
| No | 1184 (86.4) | 145 (81.5) | 454 (83.1) | 370 (90.9) | 360 (86.1) | ||
| Missing | 1 (0.1) | 0(0) | 0.19 | 1 (0.2) | 0 | 0 | 0.01 |
| Mother broader autism phenotype | |||||||
| Yes | 59 (4.9) | 17 (11.6) | 0.001 | 28 (6.0) | 22 (6.2) | 9 (2.4) | 0.03 |
| No | 1135 (95.1) | 130 (88.4) | 440 (94.0) | 335 (93.8) | 360 (97.6) | ||
| Mode of delivery (n, %) | |||||||
| Others | 919 (67.0) | 105 (59.0) | 335 (61.4) | 270 (66.3) | 314 (75.1) | ||
| Cesarean | 452 (33.0) | 73 (41.0) | 0.03 | 211 (38.6) | 137 (33.7) | 104 (24.9) | <0.0001 |
| Family income (n, %) | |||||||
| 10,000–<30,000 | 204 (14.9) | 66 (37.1) | 108 (19.8) | 48 (11.8) | 48 (11.5) | ||
| $30,000–<70,000 | 310 (22.6) | 49 (27.5) | 134 (24.5) | 95 (23.3) | 81 (19.4) | ||
| $70,000–<110,000 | 375 (27.4) | 31 (17.4) | 142 (26.0) | 109 (26.8) | 124 (29.7) | ||
| $>110,000 | 445 (32.5) | 23 (12.9) | 148 (27.1) | 148 (36.4) | 149 (35.6) | ||
| Missing | 37 (2.7) | 9 (5.1) | <0.0001 | 14 (2.6) | 7 (1.7) | 16 (3.8) | 0.0002 |
| Study site | |||||||
| California | 223 (16.3) | 16 (9.0) | 66 (12.09) | 64 (15.72) | 93 (22.25) | ||
| Colorado | 297 (21.7) | 29 (16.3) | 115 (21.06) | 96 (23.59) | 86 (20.57) | ||
| Georgia | 259 (18.9) | 42 (23.6) | 0.0003 | 124 (22.71) | 74 (18.18) | 61 (14.59) | <0.0001 |
| Maryland | 196 (14.3) | 30 (16.8) | 85 (15.57) | 64 (15.72) | 47 (11.24) | ||
| North Carolina | 231 (16.8) | 22 (12.4) | 83 (15.20) | 61 (14.99) | 87 (20.81) | ||
| Pennsylvania | 165 (12.04) | 39 (21.9) | 73 (13.37) | 48 (11.79) | 44 (10.53) | ||
Note. Bolds values are assessed at a P-value of ≤0.20.
Only available in five sites.
SD: standard deviation.
Psychiatric diagnoses include depression, anxiety, seizure disorder, intellectual disability.
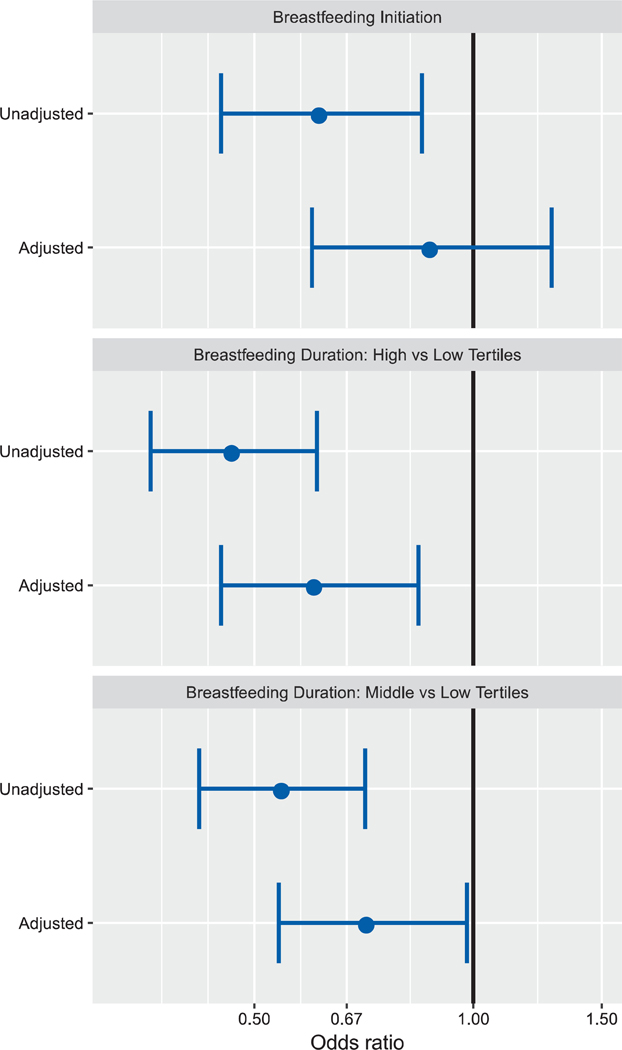
In six-site models (Fig. 1 and Table 3), the unadjusted odds of breastfeeding initiation were lower in children with ASD versus controls, but we found no significant difference after adjustment (adjusted odds ratio [aOR]: 0.88 [95% confidence interval (CI): 0.60–1.28]). Results were similar in the subset of five sites and when we used the reduced variable model (Table 3). Three variables were most influential in attenuating the estimates of breastfeeding initiation: maternal race, maternal education, and family income (Supporting Information Table S1).
Figure 1.
Odds of breastfeeding initiation and duration in cases of autism spectrum disorder versus controls among preschoolers enrolled in the Study to Explore Early Development.
Table 3.
Odds of Breastfeeding Initiation and Duration Using Full and Reduced Variables Models in Six and Five Sites in Pre-schoolers With Autism Spectrum Disorder Versus Controls Enrolled in the Study to Explore Early Development
| Full variables modela |
Reduced variables model |
|||
|---|---|---|---|---|
| Breastfeeding parameter | Unadjusted odds-ratios and 95% confidence interval | Adjusted odds-ratios and 95% confidence interval | Unadjusted odds-ratios and 95% confidence interval | Adjusted odds-ratios and 95% confidence interval |
| Breastfeeding initiation in six sitesb | 0.62 (0.45, 0.85) | 0.88 (0.60,1.28) | 0.62 (0.45, 0.85) | 0.89 (0.61, 1.28)b |
| Breastfeeding initiation in five sitesc | 0.61 (0.44, 0.85) | 0.87 (0.58, 1.30) | 0.61 (0.44, 0.85) | 0.90 (0.61, 1.33)c |
| Breastfeeding duration in six sites (high tertile vs. low tertile)d | 0.47 (0.36, 0.61) | 0.61 (0.45, 0.84) | 0.47 (0.36, 0.61) | 0.61 (0.44, 0.83)d |
| Breastfeeding duration in five sites (high tertile vs. low tertile)e | 0.46 (0.35, 0.62) | 0.60 (0.42, 0.85) | 0.46 (0.35, 0.62) | 0.60 (0.42, 0.84)e |
| Breastfeeding duration in six sites (middle tertile vs. low tertile)d | 0.55 (0.42, 0.71) | 0.72 (0.54, 0.98) | 0.55 (0.42, 0.71) | 0.73 (0.54, 0.98)d |
| Breastfeeding duration in five sites (middle tertile vs. low tertile)e | 0.50 (0.38, 0.66) | 0.64 (0.46, 0.89) | 0.50 (0.38, 0.66) | 0.64 (0.46, 0.88)e |
Note. Bold values are assessed at the P-value of <0.05.
Adjusted for the following variables: child sex, gestational age, birth weight, 5-min Apgar score, birth plurality, mode of delivery; mother’s age at the time of child’s birth in years, parity with the index child, race and ethnicity, education at time of child’s birth, place of birth, employment status during the 3 months before the child’s birth until the end of breastfeeding period, marital status, smoking status, and presence of neuropsychiatric diagnoses; family estimated income during the year preceding the index child’s birth, and study site.
Adjusted in the reduced variables model for maternal age, race, education, place of birth, family income, maternal psychiatric diagnoses, child sex, gestational age, mode of delivery.
Adjusted in the reduced variables model for all the variables in six site reduced variables model listed above plus mother’s marital status.
Adjusted in the reduced variables model for maternal age, race, ethnicity, education, family income, maternal psychiatric diagnoses, child sex, gestational age, birth weight, birth plurality, mode of delivery.
Adjusted in the reduced variables model for all the variables in six site reduced variables model listed above plus mother’s marital status.
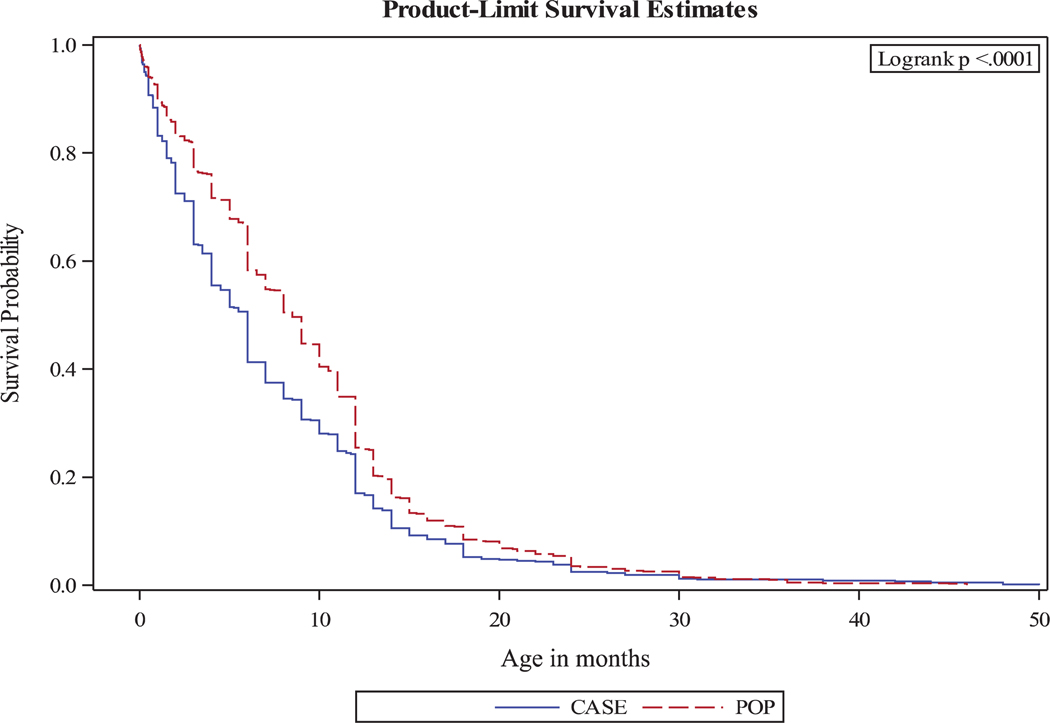
Both before and after adjustment, in those who breastfed, the odds of longer breastfeeding duration were lower in children with ASD versus controls when comparing high to low tertile ([aOR and 95% CI]: 0.61 [0.45–0.84]) and middle to low tertile ([aOR and 95% CI]: 0.72 [0.54–0.98]). Breastfeeding duration findings did not change considerably when we included only five sites or when we used a reduced variable model (Table 3). Maternal education strongly contributed to differences between unadjusted and adjusted breastfeeding duration estimates (Supporting Information Table S1). The difference in breastfeeding duration between the two groups was slightly attenuated after the presence of a BAP in the mother was added in the models. In the full models, the adjusted odds decreased by 9.8% in the comparison between high versus low tertile ([aOR and 95% CI]: 0.67 [0.48–0.94]). The adjusted odds decreased by 4.2% and no longer statistically significant in the comparison between middle versus low tertiles ([aOR and 95% CI]: 0.75 [0.54–1.04]). The survival curves of breastfeeding duration showed a greater decrease in the proportion of children who were still breastfed in cases versus controls during the first 6 months and a persistent difference between ASD and controls at 20 months after birth (Fig. 2). Similarly, a shorter breastfeeding duration in months among children with ASD versus controls was found in the ANCOVA analysis in both the full and reduced variables models (data not shown).
Figure 2.
Estimates adjusted for child sex, gestational age, birth weight, maternal age, party of the index child, birth plurality, maternal ethnicity, race, education, place of birth, maternal employment status, maternal smoking status, maternal psychiatric diagnoses, mode of delivery of the child, family income.
Discussion
Mothers of children with ASD were less likely to initiate breastfeeding than mothers of controls; however, this association was no longer significant after adjustment for socio-demographic and pregnancy characteristics. Among those who were breastfed, mothers of children with ASD were less likely to have a duration of breastfeeding in the high or in the middle fertile than mothers of controls and this finding remained significant after adjustment for confounding variables, whether breastfeeding duration was analyzed as a categorical or continuous variable. The association of ASD and breastfeeding duration was slightly attenuated when the presence of the BAP in the mother was accounted for, but still remained for the highest fertile. Thus, our data suggest that maternal BAP does not entirely explain the observed association.
Our finding that breastfeeding initiation was not significantly associated with ASD after adjustment for confounders is in line with the suggestion by Husk and Keim [2015] that some of the significant associations reported by others could have been due to residual confounding. Likewise, though a longitudinal study [Lind, Li, Perrine, & Schieve, 2014] found significantly lower odds in unadjusted analyses of social, emotional, and conduct problems at age 6 years among breastfed children than not breastfed; these findings lost statistical significance after controlling for maternal sociodemographic and child characteristics. We found that maternal race, maternal education, and family income contributed most to the attenuation in breastfeeding initiation estimates. Other researchers [e.g., Forste et al., 2001] have also documented these three factors as important predictors of breastfeeding initiation. This suggests that all studies of breastfeeding initiation should attempt to control for at least these variables. Our finding was different from those reported by Christian et al. [2018], Nishijo et al. [2014], and Shamberger [2011]; and partially different from the meta-analysis by Tseng et al. [2017]. In their meta-analysis, Tseng et al. [2017] reported an overall significantly lower initiation of breastfeeding in mothers of children with ASD compared to controls. There are methodological differences that may explain these differences. We used a well-defined sample of children with ASD whose classification was confirmed by a clinician, and we adjusted for numerous factors. Further, small sample size [Nishijo et al., 2014] or the reliance on an ecological design, which is subject to ecological fallacy [Shamberger, 2011], could explain differences between our finding and those of these two studies. Tseng et al. [2017] acknowledged important limitations in their meta-analysis, including the small number of included studies (n = 7), inability to adjust for confounding factors within the recruited studies and the use of a number of different diagnostic criteria to define ASD in the different studies.
In line with other studies [Al-Farsi et al., 2012; Bittker & Bell, 2018; Boucher et al., 2017; Lemcke et al., 2018; Shafai et al., 2017], we documented a shorter duration of breastfeeding in children with ASD versus controls, independent of sociodemographic and pregnancy factors. As we have acknowledged in the introduction, the meaning of this shorter breastfeeding duration in children with ASD versus controls is not clearly understood. It is possible that since children who were later diagnosed with ASD were breastfed for a shorter duration compared to controls, they did not receive the benefits of an optimal breastfeeding duration, including more key nutrients for brain development and maturation, longer mother–child bonding, better development of gut microbiota, and/or decrease in susceptibility to infections [Hall, 2016; Heijtz, 2016; Li et al., 2014]. As detailed in the introduction, children breastfed for a shorter duration may not receive the full protective effect of breastfeeding and may be at increased risk of ASD in the presence of other risk factors (e.g., genetic susceptibility) since ASD is a multifactorial condition [Krol, Monakhov, Lai, Ebstein, & Grossman, 2015].
It is also possible that this difference in breastfeeding duration between the two groups may be due to unmeasured confounding or, as acknowledged in the introduction, may be a correlate of the underlying developmental condition (ASD). Early manifestations of core ASD symptoms, other ASD-associated health conditions or symptoms in infants who later develop ASD (e.g., gastrointestinal, self-regulation, and sensory problems), or other maternal characteristics in mothers of children with ASD not adjusted in this study, may have influenced these mothers to discontinue breastfeeding earlier than mothers of controls. Other researchers have documented early developmental disturbances in emotion regulation, activity and motor development in children who later are diagnosed with ASD [Gomez & Baird, 2005; Lemcke et al., 2018] and these disturbances may affect breastfeeding duration. Further, co-occurring conditions, including sleep disturbances (which are more prevalent in children who later develop ASD [Zwaigenbaum et al., 2013], might also contribute to shorter breastfeeding duration. Like children with ADHD, as reported by Stadler, Musser, Holton, Shanon, and Nigg [2016], it is possible that children who later develop ASD may be more difficult to breastfeed resulting in shorter breastfeeding duration. Though studies have documented infant difficulties as one of the reasons for early breastfeeding discontinuation by mothers in the general population [Ahluwalia, Morrow, & Hsia, 2005; Li et al., 2008], data on breastfeeding behaviors in children later diagnosed with ASD are very limited. In a case series of seven children with ASD, Keen [2008] found two children with ASD who had poor suck, became distressed and refused to be breastfed if feeding was delayed. Likewise, a small study (n = 23) by Lucas and Cutler [2015] documented a deregulated breastfeeding pattern of sucking without stopping on their own volition in 56% of children with ASD. This pattern of sucking without stopping may lead to sore nipples, which is another reason for early breastfeeding discontinuation [Ahluwalia et al., 2005; Li et al., 2008]. We found that the estimates of the association between ASD and breastfeeding duration were slightly attenuated when the presence of the BAP in the mother was accounted for, but the relationship was still present in the comparison between the highest and lowest tertile. This suggests that this finding could not be totally explained by the presence of the BAP in the mother.
It is important to point out that our results in both breastfeeding initiation and duration were similar to those reported by Stadler et al. [2016] in a sample of well-characterized children with ADHD, a developmental condition that can co-occur with ASD. Like in our study, Stadler et al. reported no significant difference in breastfeeding initiation between children with ADHD and controls, but breastfeeding duration was shorter in children with ADHD. This similarity add to the validity of our findings.
This study has a number of strengths, including a large and diverse sample of children with ASD whose classification was confirmed by clinicians during in-person assessments using standardized instruments. Additionally, the comprehensive data collection in SEED allowed us to adjust for a variety of maternal, child, and family characteristics and assess the effect of the presence of the BAP in the mother on the association between ASD and breastfeeding duration. Breastfeeding initiation data were confirmed in two interviews, and we were able to evaluate both breastfeeding initiation and duration. However, this study also has limitations. First, we did not collect detailed information on breastfeeding practices, such as exclusive, predominant, or partial breastfeeding [Boue et al., 2017]. This impeded our ability to evaluate the AAP recommendations in our study. Although we found a shorter breastfeeding duration in children with ASD, we could not determine whether this may be attributed to breastfeeding difficulties due to the child, maternal factors not examined in this study or other factors, including the presence of lactation support programs, since we did not have information on why mothers stopped breastfeeding. While the control group was recruited from a random sample of each site’s population, our control sample included a considerable proportion of families with higher socioeconomic status (SES). In contrast to other studies, families of cases had lower SES in our study than the controls. However, this has been reported by other studies in and outside the United States [Bittker & Bell, 2018; He et al., 2018; Lemcke et al., 2018; Rai et al., 2012]. Further, our controls were different from the source population. We cannot discount the possibility of some degree of selection bias in our sample and its effects on our findings since we were not able to locate all families to whom we initially sent invitation letters to determine if they were eligible for the study. However, an analysis of responders and nonresponders at one study site with sufficient data on nonresponders was encouraging. At this site, it was found that although certain demographic factors, such as maternal age and education, were associated with nonresponse, biologic factors, such as preterm delivery, were not [Schieve et al., 2018]. Nevertheless, we adjusted for important sociodemographic characteristics that may influence breastfeeding such as maternal race and education and family income to account for the possibility of selection bias. SEED included only six sites in the United States that were not randomly selected, thus, caution is required when attempting to generalize these results to other areas. The accuracy of breastfeeding duration recall may be problematic since breastfeeding duration was assessed up to 5 years after birth. However, a study by Amissah, Kancherla, Ko, and Li [2017] found that maternal recall of breastfeeding duration was valid 6 years after childbirth with some variation based on sociodemographic characteristics of the respondents. Further, the presence of ASD may differentially affect the reporting of breastfeeding duration in mothers of children with ASD. While our sample size was large, it was not sufficiently large to examine associations in more homogeneous ASD subgroups (e.g., developmental regression, intellectual disability, gestational age, maternal age), and we plan to investigate these subgroups in subsequent phases of SEED after accrual of a larger study population.
In conclusion, our study documents a shorter breastfeeding duration in children with ASD compared to controls, even after accounting for various factors. While this may inform our understanding of ASD etiology in some children, it is important to acknowledge the need for future prospective studies in order to establish a temporal relationship between breastfeeding practices and ASD [Tseng et al., 2017]. Further, our findings suggest the importance for future large studies to assess the reasons why mothers of children with ASD stop breastfeeding earlier compared to controls, so specific interventions might be provided to support adequate breastfeeding duration as recommended by the AAP. As proposed by Stadler et al. [2016], future studies should consider providing detailed descriptions of breastfeeding practices, such as exclusive breastfeeding, partial breastfeeding, and the use of expressed breast milk or breast milk from milk banks. Given the large variation in breastfeeding initiation across population sub-groups, future studies of breastfeeding initiation should consider controlling for key sociodemographic factors. Because of the heterogeneity in ASD phenotype, potential gene-breastfeeding interactions in ASD [Krol et al., 2015], and the multifactorial nature of ASD, future studies should consider evaluating breastfeeding in more homogenous ASD subgroups and assessing ASD risk gene-breastfeeding interactions. Lastly, since the decision to breastfeed and its duration are influenced by multiple factors, the effects of other factors, not included in this study, such as pregnancy intention, intent to breastfeed the child, early developmental behavior disturbances in the child, and the existence of lactation support programs in the community should also be assessed in future studies.
Supplementary Material
Table S1. Changes in unadjusted estimates of breastfeeding initiation and duration in relation to ASD versus controls, including each covariate individually in models among preschoolers enrolled in the Study to Explore Early Development.
Acknowledgments
The authors acknowledge SEED principal investigators, scientists, project coordinators, clinicians, and research assistants for their contribution in data collection and cleaning during SEED 1. We are also grateful to all families and children who participated in SEED 1.
SEED I was funded by six cooperative agreements from the Centers for Disease Control and Prevention: Cooperative Agreement Number U10DD000180, Colorado Department of Public Health and Environment; Cooperative Agreement Number U10DD000181, Kaiser Foundation Research Institute (CA); Cooperative Agreement Number U10DD000182, University of Pennsylvania; Cooperative Agreement Number U10DD000183, Johns Hopkins University; Cooperative Agreement Number U10DD000184, University of North Carolina at Chapel Hill; and Cooperative Agreement Number U10DD000498, Michigan State University.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. A preliminary version of this work was presented as a poster presentation during the 2017 International Meeting for Autism Research in San Francisco, California.
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Compliance with Ethical Standards
All procedures performed in studies involving human participants were in accordance with ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All caregivers of children included in SEED provided written informed consent. All authors in this study do not have any conflict of interests to disclose
Supporting Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
References
- Ahluwalia IB, Morrow B, & Hsia J (2005). Why do women stop breastfeeding? Findings from the pregnancy risk assessment and monitoring system. Pediatrics, 116,1408–1412. [DOI] [PubMed] [Google Scholar]
- Al-Farsi YM, Al-Shabarti MM, Waly MI, Al-Farsi OA, Al-Shafaee MA, Al-Khaduri MM, et al. (2012). Effect of suboptimal breast-feeding on occurrence of autism: A case-control study. Nutrition, 28, 27–32. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics. (1997). Work Group on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics, 100,1035–1039. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5thed ed.). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Amissah EA, Kancherla V, Ko Y, & Li R (2017). Validation study of maternal recall on breastfeeding duration 6 years after childbirth. Journal of Human Lactation, 33, 390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchan M, Foureur M, & Davis D (2011). The decision not to initiate breastfeeding-women’s reasons, attitudes and influencing factors – a review of the literature. Breastfeeding Review, 19, 9–17. [PubMed] [Google Scholar]
- Bar S, Milanaik R, & Adesman A (2016). Long-term neurodevelopmental benefits of breastfeeding: A review. Current Opinion in Pediatrics, 28, 559–566. [DOI] [PubMed] [Google Scholar]
- Berthoz S, Lalanne C, Crane L, & Hill E (2013). Investigating emotional impairments in adults with autism spectrum disorder and the broader autism phenotype. Psychiatry Research, 208, 257–264. [DOI] [PubMed] [Google Scholar]
- Bittker SS, & Bell KR (2018). Acetaminophen, antibiotics, ear infection, breastfeeding, vitamin D drops, and autism: an epidemiological study. Neuropsychiatric Disease and Treatment, 14,1399–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher O, Julvez J, Guxens M, Arranz E, Ibarluzea J, DeMiguel MS, et al. (2017). Association between breastfeeding duration and cognitive development, autistic traits and ADHD symptoms: A multicenter study in Spain. Pediatric Research, 81, 434–442. [DOI] [PubMed] [Google Scholar]
- Boue G, Cummins E, Guillou S, Antignac JP, Le Bizec B, & Membre JM (2017). Public health risks and benefits associated with breast milk and infant formula consumption. Critical Reviews in Food Science and Nutrition, 58,126–145. [DOI] [PubMed] [Google Scholar]
- Brahm P, & Valdes V (2017). Benefits of breastfeeding and risks associated with not breastfeeding. Review of Child Pediatric, 88,15–21. [Google Scholar]
- Bresnahan M, Hornig M, Schultz A, Gunnes N, Hirtz D, Lie K, et al. (2015). Association of maternal report of infant and toddler gastrointestinal symptoms with autism: evidence from a prospective birth cohort. JAMA Psychiatry, 72, 466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brian J, Bryson S, & Zwaigenbaum L (2015). Autism spectrum disorder in infancy: developmental considerations in treatment targets. Current Opinion in Neurology, 28,117–123. [DOI] [PubMed] [Google Scholar]
- Brown CM, Austin DW, & Busija 1 (2014). Observable essential fatty acids deficiency markers and autism spectrum disorder. Breastfeeding Review, 22, 21–26. [PubMed] [Google Scholar]
- Burd L, Kerbeshian J, Vesely B, Durgin B, & Reep P (1988). A comparison of breastfeeding rates among children with pervasive developmental disorder and controls. Developmental and Behavioral Pediatrics, 9, 247–251. [PubMed] [Google Scholar]
- Christian MC, Samms-Vaughan M, Lee M, Bressler J, Hessabi M, Grove M, et al. (2018). Maternal exposures associated with autism spectrum disorder in Jamaican children. Journal of Autism and Developmental Disabilities, 48, 2766–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, & Todd RD (2005). Intergenerational transmission of subthreshold autistic traits in the general population. Biological Psychiatry, 57(6), 655–660. [DOI] [PubMed] [Google Scholar]
- Crawford S (2015). On the origins of autism: The quantitative threshold exposure. Medical Hypotheses, 85, 798–806. [DOI] [PubMed] [Google Scholar]
- Dernird J, Sereika S, & Bogen D (2013). Prevalence and predictors of early breastfeeding among late preterm mother-infants dyads. Breastfeeding Medicine, 8, 277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni SC, Dean DC III, Piryatinsky I, Waskiewicz N, Lehman K, Han M, et al. (2013). Breastfeeding and early white matter development: A cross-sectional study. Neuro-Image, 82, 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, Zagoory-Sharon O, Weisman O, Schneiderman I, Gordon I, Maoz I, et al. (2012). Sensitive parenting is associated with plasma oxytocin and polymorphisms in the OXTR and CD38 genes. Biological Psychiatry, 72,175–181. [DOI] [PubMed] [Google Scholar]
- Fernandes VN (2015). Non-genetic potential risk factors for autism spectrum disorders. Medical Science, 5, 25–30. [Google Scholar]
- Forste R, Weiss J, & Lippincott E (2001). The decision to breastfeed in the United States: Does race matter? Pediatrics, 108, 291–296. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Morisaki N, Honda Y, Sampei M, & Tani Y (2016). Chemicals, nutrition, and autism spectrum disorder: A Mini-review. Frontiers in Neuroscience, 10,1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George B, Padman RMS, Nair MKC, Leena ML, & Russell PSSR (2014). CDC kerala 14: Early child care practices at home among children (2–6y) with autism. A case-control study. Indian Journal of Pediatrics, 81(suppl 2), S138–S141. [DOI] [PubMed] [Google Scholar]
- Gomez CR, & Baird S (2005). Identifying early indicators for autism in self-regulation difficulties. Focus on autism and other developmental disabilities, 20,106–116. [Google Scholar]
- Gore N, Emerson E, & Brady S (2015). Rates of breastfeeding and exposure to socio-economic adversity amongst children with intellectual disability. Research in Developmental Disabilities, 39,12–19. [DOI] [PubMed] [Google Scholar]
- Hall B (2016). The role of brain lipids in the causal model of autism: Re-interpretation of the existing data. Hypothesis, 14,1–8. [Google Scholar]
- He P, Guo C, Wang Z, Chen G, Li N, & Zheng X (2018). Socioeconomic status and childhood autism: A population-based study in China. Psychiatry Research, 259,27–31. [DOI] [PubMed] [Google Scholar]
- Heijtz RD (2016). Fetal, neonatal, and infant microbiome: Perturbations and subsequent effects on brain development and behavior. Seminars in Fetal and Neonatal Medicine, 21, 410–417. [DOI] [PubMed] [Google Scholar]
- Henderson J, Evans S, Straton J, Priest S, & Hagan R (2003). Impact of postnatal depression on breastfeeding duration. Birth, 30, 175–179. [DOI] [PubMed] [Google Scholar]
- Herba CM, Roza S, Govaert P, Hofman A, Jaddoe V, Verhust FC, et al. (2013). Breastfeeding and early brain development: The generation R study. Maternal Child and Nutrition, 9, 332–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong L, Ziegler J, & Brody R (2014). Breastfeeding and autism spectrum disorders. Topics in Clinical Nutrition, 29, 278–285. [Google Scholar]
- Husk JS, & Keim SA (2015). Breastfeeding and autism spectrum disorder in the National Survey of Children’s Health. Epidemiology, 26, 451–457. [DOI] [PubMed] [Google Scholar]
- Ingersoll B, & Hambrick DZ (2011). The relationship between the broader autism phenotype, child severity, and stress and depression in parents of children with autism spectrum disorders. Research in Autism Spectrum Disorders, 5, 337–344. [Google Scholar]
- Johnson S, Holllis C, Kocchar P, Hennessy E, Wolke D, & Marlow N (2010). Autism spectrum disorders in extremely preterm children. The Journal of Pediatrics, 156, 525–531. [DOI] [PubMed] [Google Scholar]
- Kaye K, & Wells AJ (1980). Mother’s jiggling and the burst-pause pattern in neonatal feeding. Infant Behavior and Development, 3, 29–46. [Google Scholar]
- Keen DV (2008). Childhood autism, feeding problems, and failure to thrive in early infancy. Seven cases studies. European Child and Adolescent Psychiatry, 17, 209–216. [DOI] [PubMed] [Google Scholar]
- Kim P, Feldman R, Mayes LC, Eicher V, Thompson N, Leckman JF, & Swain JE (2011). Breastfeeding, brain activation to own infant cry, and maternal sensitivity. Journal of Child Psychology and Psychiatry, 52, 907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koletzo B, lien E, Agostoni C, Bohles H, Campoy C, Cetin I, et al. (2008). The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation, and infancy: Review of current knowledge and consensus recommendations. Journal of Perinatal Medicine, 36, 5–14. [DOI] [PubMed] [Google Scholar]
- Krol KM, Monakhov M, Lai SP, Ebstein RP, & Grossman T (2015). Genetic variation in CD38 and breastfeeding experience interact to impact infant’s attention to social eye cues. PNAS, 112, e5434–e5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemcke S, Pamer ET, Bjerrum M, Thomsen PH, & Lauritsen MB (2018). Early regulation in children who are later diagnosed with autism spectrum disorder: A longitudinal study within the Danish National Birth Cohort. Infant Mental Health Journal, 25,170–182. [DOI] [PubMed] [Google Scholar]
- Li R, Dee D, Li C-M, Hoffman HJ, & Grummer-Strawn LM (2014). Breastfeeding and risk of infections at 6 years. Pediatrics, 134, S13–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Fein SB, Chen J, & Grummer-Starwn LM (2008). Why mothers stop breastfeeding: Mother’s self-reported reasons for stopping breastfeeding during the first year. Pediatrics, 122, S69–S76. [DOI] [PubMed] [Google Scholar]
- Lind JN, Li R, Perrine CG, & Schieve L (2014). Breastfeeding and later psychosocial development of children at 6 years of age. Pediatrics, 134, S36–S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. (2000). The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30, 205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, & Le Couteur AL (1994). Autism diagnostic interview-revised: A revised version of the diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24, 659–685. [DOI] [PubMed] [Google Scholar]
- Lucas RF, & Cutler A (2015). Dysregulated breastfeeding behaviors in children later diagnosed with autism. The Journal of Perinatal Education, 24,171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandy W, & Lai M-C (2016). Annual research review: The role of environment in the developmental psychopathology of autism spectrum condition. Journal of Child Psychology and Psychiatry, 57, 271–292. [DOI] [PubMed] [Google Scholar]
- Mullen E (1995). Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service Inc. [Google Scholar]
- Ng M, Montigny JG, Ofner M, & Do MH (2017). Environmental factors associated with autism spectrum disorder: a scoping review for the years 2003–2013. Health Promotion and Chronic Disease Prevention in Canada, 37,1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijo M, Pham TT, Nguyen ATN, Tran NN, Nakagawa H, Hoang LV, ... Nishijo H (2014). 2, 3, 7, 8-Tetrachlorodibenzo-p-dioxin in breast milk increases autistics traits of 3-year-old children in Vietnam. Molecular Psychiatry, 19,1220–1226. [DOI] [PubMed] [Google Scholar]
- Oddy WH (2002). Long-term health outcomes and mechanisms associated with breastfeeding. Expert Review of Pharmacoeconomics & Outcomes Research, 2, 161–177. [DOI] [PubMed] [Google Scholar]
- Odom EC, Li R, Scanlon S, Perrine CG, & Grummer-Strawn L (2013). Reasons for earlier than desired cessation of breastfeeding. Pediatrics, 131, 726–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Bucher S, Pusdekar Y, Esamai F, Krebs N, Goudar S, et al. (2015). Rates and determinants of early initiation of breastfeeding and exclusive breastfeeding at 42 days postnatal in six low and middle-income countries: A prospective cohort study. Reproductive Health, 12, S1–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai D, Lewis G, Lundberg M, Araya R, Svenson A, Dalman C, et al. (2012). Parental socioeconomic status and risk of autism spectrum disorders in a Swedish population-based study. Journal of American Academy of Child and Adolescent Psychiatry, 51, 467–476. [DOI] [PubMed] [Google Scholar]
- Raju TNK (2011). Breastfeeding is a dynamic process—Not simply a meal at the breast. Breastfeeding Medicine, 6, 257–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi S, Chandrasekaran V, Kattimani S, & Subramanian M. (2016). Maternal and birth risk factors for children screening positive for autism spectrum disorders on M-CHAT-R. Asian Journal of Psychiatry, 22,17–21. [DOI] [PubMed] [Google Scholar]
- Rubenstein E, Pretzel E, Windham GC, Schieve LA, Wiggins LD, DiGuiseppi C, et al. (2017). The Broader Autism Phenotype in mothers is associated with increased discordance between maternal-reported and clinician-observed instruments that measure child autism spectrum disorder. Journal of Autism and Developmental Disorders, 47, 3253–3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Bailey A, & Lord C (2003). SCQ: Social Communication Questionnaire. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Say GN, Karabekiroğlu K, Babadaği Z, & Yüce M (2016). Maternal stress and perinatal features in autism and attention deficit/hyperactivity disorder. Pediatrics International, 58, 265–269. [DOI] [PubMed] [Google Scholar]
- Schendel D, DiGuiseppi C, Croen L, Fallin D, Reed P, Schieve L, et al. (2012). The Study to Explore Early Development (SEED): A multisite epidemiologic study of autism by the Centers for Autism and Developmental Disabilities Research and Epidemiology (CADDRE) Network. Journal of Autism and Developmental Disorders, 42, 2121–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieve LA, Harris S, Croen L, Maenner M, Alexander A, & Dowling N (2018). Assessment of demographic and perinatal predictors of non-response and impact of non-response on measures of association in a population-based control study: findings from the Georgia Study to Explore Early Development. Emerging Themes in Epidemiology, 15, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz ST, Klonoff-Cohen H, Wingard DL, Akshoomoff NA, Macera CA, Ji M, et al. (2006). Breastfeeding, infant formula supplementation, and autistic disorder: The results of a parent survey. International Breastfeeding Journal, 1,1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafai T, Mustafa M, Hild T, Mulari J, & Curtis A (2017). The impact of infant feeding methods on the development of autism spectrum disorder In Fitzgerald M & Yip J (Eds.), Autism–paradigms, recent research and clinical applications (pp. 70–83). Indianapolis: Intech. [Google Scholar]
- Shamberger R (2011). Autism rates associated with nutrition and the WIC program. Journal of American College of Nutrition, 30, 348–353. [DOI] [PubMed] [Google Scholar]
- Stadler DD, Musser ED, Holton KF, Shanon J, & Nigg JT (2016). Recalled initiation and duration of maternal breastfeeding among children with and without ADHD in a well characterized case-control sample. Journal of Abnormal Child Psychology, 44, 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman G, & Mankuta D (2013). Breastfeeding as a possible deterrent to autism—A clinical perspective. Medical Hypotheses, 81, 999–1001. [DOI] [PubMed] [Google Scholar]
- Tanoue Y, & Oda S (1989). Weaning time of children with infantile autism. Journal of Autism and Developmental Disorders, 19,1–10. [DOI] [PubMed] [Google Scholar]
- Tseng PT, Chen YW, Stubbs B, Carvalho AF, Whiteley P, Tang CH, ... Lin PY (2017). Maternal breastfeeding and autism spectrum disorder in children: A systematic review and meta-analysis. Nutritional Neuroscience, 18, epub ahead of print, 1–9. [DOI] [PubMed] [Google Scholar]
- Vanya M, Szucs S, Vetro A, & Bartfai G (2017). The potential role of oxytocin and perinatal factors in the pathogenesis of autism spectrum disorders—Review of the literature. Psychiatry Research, 247, 288–290. [DOI] [PubMed] [Google Scholar]
- Vuong HE, & Hsiao EY (2016). Emerging roles for the gut microbiome in autism spectrum disorder. Society of Biological Psychiatry, 81, 411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallwiener S, Muller M, Doster A, Plewniok K, Wallwiener CW, Fluhr H, et al. (2016). Predictors of impaired breastfeeding initiation and maintenance in a diverse sample: What is important? Archives of Gynecology and Obstetrics, 294, 455–466. [DOI] [PubMed] [Google Scholar]
- Wiggins L, Bakeman R, Adamson LB, & Robins DL (2007). The utility of the social communication questionnaire in screening for autism in children referred for early intervention. Focus on Autism and Other Developmental Disabilities, 22, 33–38. [Google Scholar]
- Wiggins L, Reynolds A, Rice C, Moody E, Bernal P, Blaskey L, et al. (2015). Using standardized diagnostic instruments to classify children with autism in the Study to Explore Early Development. Journal of Autism and Developmental Disorders, 45,1271–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Zhang HF, Han JS, & Han SP (2017). Genes related to oxytocin and arginine-vasopressin pathways: Associations with autism spectrum disorders. Neuroscience Bulletin, 33, 238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, & Garon N (2013). Review: Early identification of autism spectrum disorders. Behavioural Brain Research, 251,133–146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Changes in unadjusted estimates of breastfeeding initiation and duration in relation to ASD versus controls, including each covariate individually in models among preschoolers enrolled in the Study to Explore Early Development.