Abstract
Background
There is scarcity of data on outcome of COVID-19 in patients with hematological malignancies. Primary objective of study was to analyse the 14-day and 28-day mortality. Secondary objectives were to correlate age, comorbidities and remission status with outcome.
Methods
Retrospective multicentre observational study conducted in 11 centres across India. Total 130 patients with hematological malignancies and COVID-19 were enrolled.
Results
Fever and cough were commonest presentation. Eleven percent patients were incidentally detected. Median age of our cohort was 49.5 years. Most of our patients had a lymphoid malignancy (n = 91). One-half patients (52%) had mild infection, while moderate and severe infections contributed to one-fourth each. Sixty seven patients (52%) needed oxygen For treatment of COVID-19 infection, half(n = 66) received antivirals. Median time to RT-PCR COVID-19 negativity was 17 days (7–49 days). Nearly three-fourth (n = 95) of our patients were on anticancer treatment at time of infection, of which nearly two-third (n = 59;64%) had a delay in chemotherapy. Overall, 20% (n = 26) patients succumbed. 14-day survival and 28-day survival for whole cohort was 85.4% and 80%, respectively. One patient succumbed outside the study period on day 39. Importantly, death rate at 1 month was 50% and 60% in relapse/refractory and severe disease cohorts, respectively. Elderly patients(age ≥ 60) (p = 0.009), and severe COVID-19 infection (p = 0.000) had a poor 14-day survival. The 28-day survival was significantly better for patients in remission (p = 0.04), non-severe infection (p = 0.00), and age < 60 years (p = 0.05).
Conclusions
Elderly patients with hematological malignancy and severe covid-19 have worst outcomes specially when disease is not in remission.
Keywords: COVID-19, Hematology, Remission, Comorbidity, Survival
1. Introduction
Corona virus disease 2019 (COVID-19) is caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus which originated in Wuhan, China. With the 2nd wave of COVID being reported in a lot of countries, better understanding of disease is very important. The spectrum of COVID-19 varies from asymptomatic cases to acute respiratory distress syndrome (ARDS) and death [1].Secondary bacterial pneumonias, thrombotic complications, myocarditis, and gastrointestinal involvement are more prevalent in those with co morbidities such as hypertension, diabetes, cardiac disease, cancer and age > 70 years [2].
Patients with hematological malignancies present a group of vulnerable patients. Hirsch etal has demonstrated that patients with hematological malignancies are at a higher risk of respiratory tract infections and severe complications [3].These patients are often immunocompromised because of the disease, chemotherapy and hematopoietic stem cell transplant (HSCT) used for the treatment of these diseases. This further adds to the increased risk of complications and mortality [4]. Various studies have reported a higher mortality of 32–40% in patients with hematological malignancies and concomitant COVID infection [[5], [6], [7], [8]], as more and more cases are being reported our understanding of disease is improving. However, we are still in dilemma with regards to various aspects of COVID infections in hematological diseases. With 55 million people affected and 1.3 million mortalities as of 17 November 20, India has second highest number of patients affected worldwide [9]. We present the largest retrospective series from India with an aim to identify risk factors, associated with more severe disease and mortality.
1.1. Objectives
Primary objective of study was to analyse the 14-day and 28-day mortality in this population. Secondary objectives were to correlate age, comorbidities and remission status with severity and outcome.
2. Patients & methods
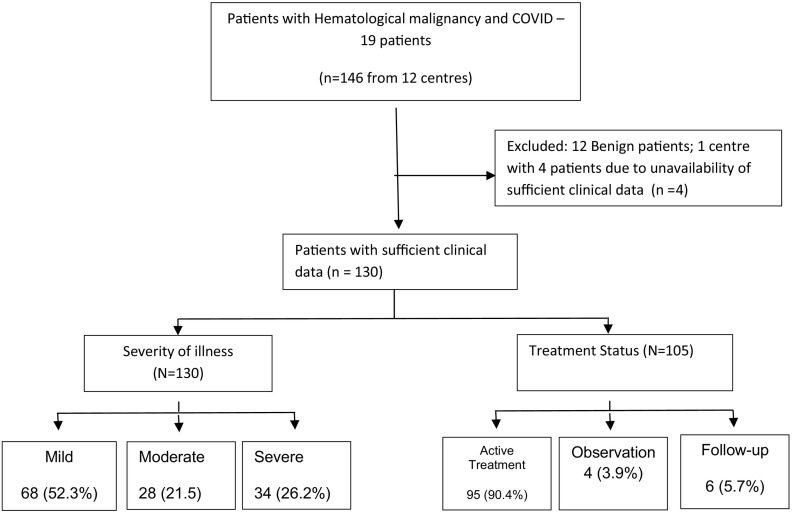
This was a retrospective multicentre observational study conducted in 11 centres treating hematological malignancies across India. Patients with malignant hematological disorders either newly diagnosed, or on ongoing therapy or follow-up at any of the participating centres were included. Patients were included irrespective of COVID-19 severity, need for hospitalisation or outpatient management and remission status of primary hematological disorder. Patients diagnosed with COVID 19 infection from 15 March to 30 Sep 2020 were enrolled in the study. A diagnosis of SARS-CoV-2infection was based on quantitative real- time reverse transcriptase-polymerase chain reaction (qRT- PCR) of nasal and/or oropharyngeal swabs. Repeat testing was performed based on institutional protocols. Baseline demographic data including comorbidities, severity of illness, remission status, ongoing therapy were extracted from electronic/manual health records and entered in common Microsoft Excel spreadsheet format. All centres submitted their data in anonymised form to central repositor (RN). Any follow-up queries were sent on email and sheet modified accordingly. One centre with 4 patients was excluded due to inability to respond to queries generated. Fig. 1 shows the CONSORT diagram. Data was coded centrally and sent to SM for statistical analysis. Patients received various treatment regimens as per physician discretion or institutional protocol in accordance with the national guidelines for the management of COVID-19, issued by the government from time to time [10]. A variety of medicines were used in these patients and included hydroxychloroquine (HCQ), remdesivir, favipiravir, other broad spectrum antibiotics, steroids (methylprednisolone or dexamethasone), tocilizumab and oxygen support/ventilation, convalescent plasma therapy as required [11]. Criteria for classifying patients into severe category were as per the clinical management protocols of government of India [7]. Outcomes including time to negativity and death were recorded.
Fig. 1.
CONSORT diagram of the study.
2.1. Inclusion criteria
Patients with hematological malignancies with COVID-19 infection confirmed with RT-PCR were included. Patients were required to have at least 14 days follow-up from first positive test.
2.2. Exclusion criteria
Patients with benign hematological disorders were excluded. Any patient lacking sufficient clinical information or less than 14 days follow-up was excluded. Patients diagnosed COVID-19 based on only radiological criteria or with indeterminate COVID status were excluded.
3. Statistical analysis
Data was described in percentages for categorical variables and as the mean ± standard deviation and median in case of continuous variables. For categorical data, comparisons were made by using the Chi square/Fisher exact-test, for quantitative data by t-test/F-test and for non-normally distributed quantitative variables by the Mann-Whitney/Kruskal Wallis test.
Data was analysed with SPSS v 23 software. P value ≤0.05 was considered significant in all statistical evaluations.
4. Results
One hundred and thirty patients with hematological malignancies diagnosed with COVID −19 were analysed. Baseline characteristics are enlisted in Table 1 . Median age of our cohort was 49.5 years (2–84) years, with a male predominance (2.5:1). Importantly, one-third of our patients with hematological cancers were elderly (≥65 years) and more than two-fifth patients had comorbidities. Most of our patients had a lymphoid malignancy (n = 91;70%). Common disorders observed were acute leukemias (42%) (ALL:23%, AML:18%), non-hodgkin lymphomas (26%), multiple myeloma (17%), followed by others [myelodysplastic syndromes (MDS), myeloproliferative neoplasms (MPN), MDS/MPN overlap, Hodgkin lymphoma, waldenstrom's macroglobulinemia]. A small proportion of patients (5%) were post HSCT. At the time of SARS-CoV2 infection, 73% were on active treatment. Active therapies included – chemotherapy (C) alone (73% (Induction, consolidation - intensive chemotherapy for AML, ALL; hypomethylating agents; CHOP backbone for NHL; ABVD for hodgkin's), immunotherapy (I) alone (1%) (rituximab, brentuximab, nivolumab, daratumumab), oral (O) targeted agents (13.6%) (imatinib, dasatinib, nilotinib, ibrutinib, lenalidomide, venetoclax), or combination therapies (11.6%) [chemo-immunotherapy (C + I), chemotherapy+oral (C + O), chemo-immunotherapy+oral (C + I + O)].
Table 1.
Demographic profile of study cohort.
| Patient characteristics | (N (%) |
|---|---|
| Baseline Characteristics (n = 130) | |
| Median age (Range) | 49.5 years (2–84) years |
|
47 (36.2) |
|
20 (15.4) |
| Gender | |
|
93 (71.5) |
|
37 (28.5) |
| Comorbidities | |
|
39(30) (42.3) |
|
32 (24.6) |
|
22 (17) |
|
7 (5.3) |
|
3 (2.3) |
|
4 (3.1) 3 (2.3) |
|
6 (4.6) |
|
14 (10.7) |
|
7 (5.4) |
| Hematological malignancies | |
| Diagnosis (N = 130) | |
|
55(42.3) |
| Acute myeloblastic leukemia | 24 (18.5) |
| Acute lymphoblastic leukemia | 31 (23.8) |
|
10 (7.7) |
|
4 (3) |
|
1 (0.7) |
|
3 (2.3) |
|
34 (26) |
| Chronic lymphocy | 13 (10) |
| Diffuse large B cell ly | 8 (6.1) |
| Follicular l | 4 (3.1) |
| Hairy cell leukemia | 4 (3.1) |
| Others (GZL, MCL, AITL, PTCL-NOS, BL, B-NHL) | 6 (4.6) |
|
23 (17.7) |
| Post HSCT | 6 (4.6) |
| Autologous HSCT | 4 (3.1) |
| Allogeneic HSCT | 2 (1.5) |
| Status of Hematological Malignancy (n = 126) | |
|
18 (14.3) |
|
36 (28.6) |
|
44 (35) |
|
4 (3.1) |
|
24 (19) |
| Therapy status (N = 105) (excluding 18 newly diagnosed, 7 – not available) |
|
|
95 (73.1) |
|
4 (3) |
|
6 (4.6) |
| Active treatment (n = 95) | |
|
70 (53.8) |
|
1 (0.7) |
|
13 (10) |
|
9 (6.9) |
|
2 (1.5) |
VTE-Venous thromboembolism, GZL-Gray zone lymphoma, MCL-mantle cell lymphoma, AITL-Angioimmunoblastic lymphoma, PTCL-NOS-Peripheral T cell lymphoma-not otherwise specified, BL-Burkitt lymphoma, B-NHL-non-hodgkin lymphoma, CR-complete remission, PR-prtial remission,
5. SARS-CoV2 infection
Most common symptom at time of presentation was fever (77%) followed by cough (50.7%) and breathlessness (49.2%). Fifteen (11%) asymptomatic patients with newly diagnosed hematological malignancy were incidentally detected prior to start of therapy (Table 2 ). Interestingly, one patient had repeat RT PCR positivity four weeks after prior documentation of negativity. Majority of patients were hospitalized (78%). Amongst all SARS-CoV2 infections, half patients (52%) had mild infection, while moderate and severe infections contributed to one-fourth each. Amongst HSCT recipients, both the allogeneic HSCT patients (n = 2) had severe COVID-19 infection, while only one of autologous HSCT recipients (n = 4) had severe disease. With respect to disease status, greater number of patients had moderate-severe COVID-19 whose disease was not in remission (51%), versus those in remission (CR/PR) (36%) (Table 3 ).High resolution computerised tomograms (HRCT) of chest were done in more than 50%(n = 74) patients. Nearly all of the (90.5%) HRCTs were suggestive of COVID-19 pneumonia, with radiological features similar to those described for immunocompetent patients. Sixty seven patients (52%) needed oxygen support [majority with oxygen by face-mask or nasal cannula (88%)]. Nearly one-third required oxygen by non-invasive ventilation(22%) and invasive ventilation (7%). For treatment of COVID-19 infection, half(n = 66) received antivirals. More than three-fourth of these antivirals included monotherapy with remdesivir (25%), favipiravir (11%), HCQ(6.2%), while a small fraction of patients (17%) received various combinations. More than 60% subjects received steroids (dexamethasone), and less than one-third received prophylactic anticoagulation. Convalescent plasma therapy was administered in 17/34 patients with severe COVID-19.In data available in 84 patients, median time to RT-PCR COVID-19 negativity was 17 days (7-49 days). Seven patients were still positive at last follow-up (range:14-105 days). Nearly three-fourth (n = 95) of our patients were on anticancer treatment at time of infection, of which nearly two-third (n = 59;64%) had a delay in their subsequent chemotherapy.
Table 2.
Clinical profile of COVID-19 infection.
| Symptoms (n = 130) | |
|---|---|
|
100 (77) |
|
66 (50.8) |
|
64 (49.3 |
|
15 (11.5) |
| Severity of COVID infection (n = 130) | |
|
68 (52.3) |
|
28 (21.5) |
|
34 (26.2) |
| Hospitalisation (n = 130) | |
|
102 (78.5) |
|
28 (21.5) |
| Oxygen support (n = 130) | 67 (51.5) |
|
59 (45.4) |
|
29 (22.3) |
|
9 (6.9) |
| HRCT done | 74 (57) |
|
67 (51.5) |
|
5 (3.8) |
|
2 (1.5) |
| Treatment received Antivirals | 65 (50) |
| HCQsa | 8 (6.2) |
| Favipiravir | 14 (10.8) |
| Remdesivir | 32 (25) |
| HCQs + Favipiravir | 2 (1.5) |
| HCQs + Remdesivir | 7 (5.4) |
| Favipiravir+Remdesivir | 2 (1.5) |
| Convalescent plasma | 17 (13.1) |
| Corticosteroids | 80 (61.5) |
| Anticoagulation | 39 (30) |
HCQs-Hydroxychloroquine,
Table 3.
Severity of infection in patients as per their disease status.
| Mild | Moderate | Severe | Total | |
|---|---|---|---|---|
| Remission (CR/PR)a | 24 | 7 | 7 | 38 |
| Relapsed/Refractory | 11 | 5 | 10 | 26 |
| Newly diagnosed | 30 | 14 | 13 | 57 |
| Stable disease | 3 | 2 | 3 | 8 |
| Not assessed /unclear | 0 | 0 | 1 | 1 |
CR-complete remission, PR-partial remission
6. Survival
Overall, 20% (n = 26) patients succumbed. 14-day survival and28-day survival for whole cohort was 85.4% and 80%, respectively (Table 4 ). One patient succumbed outside the study period on day 39. Importantly, death rate at 1 month was 50% and 60% in relapse/refractory and severe disease cohorts, respectively. One-month OS for mild, moderate and severe COVID-19 infection was 95.6%, 92.8% and 38.2% respectively. Elderly patients(age ≥ 60) (p = 0.009), and severe COVID-19 infection (p = 0.000) had a poor 14-day survival. The 28-day survival was significantly better for patients in remission (p = 0.04), non-severe infection (p = 0.00), and age < 60 years (p = 0.05).
Table 4.
– Survival in hematological patients with COVID-19.
| Survival parameters | N (%) | P value |
|---|---|---|
| N = 130 | ||
| 14-day death rate | 19 (14.6) | |
| 28-day death rate | 26 (20) | |
| 14-day mortality | ||
| Remission (CR/PR) (n = 38) | 3 (7.9) | 0.18 |
| Not in remission (SD/ R/R / not assessed) (n = 92) | 16(17.4) | |
| Mild (n = 68) | 2(2.9) | 0.000 |
| Moderate (n = 28) | 1(3.6) | |
| Severe (n = 34) | 16(47.1) | |
| Age < 60 Years (n = 83) | 7(8.4) | 0.009 |
| Age ≥ 60 Years (n = 47) | 12(25.5) | |
| Active treatment (n = 95) | 15(15.8) | 0.63 |
| Not on active treatment (n = 35) | 4(11.4) | |
| Comorbidities present (n = 54) | 9(16.6) | 0.61 |
| Comorbidities absent (n = 76) | 10(13.2) | |
| 28-day mortality | ||
| Remission (CR/PR) (n = 38)a | 3(7.9) | 0.04 |
| Not in remission (SD/ R/R / not assessed) (n = 92) | 23(25) | |
| Mild (n = 68) | 3(4.4) | 0.00 |
| Moderate (n = 28) | 2(7.1) | |
| Severe (n = 34) | 21(61.7) | |
| Age < 60 Years (n = 83) | 12(14.5) | 0.05 |
| Age > 60 Years (n = 47) | 14(29.8) | |
| Active treatment (n = 95) | 20(21.1) | 0.63 |
| Not on active treatment (n = 35) | 6(17.1) | |
| Comorbidities present (n = 54) | 11(20.4) | 1.0 |
| Comorbidities absent (n = 76) | 15(19.7) | |
CR-complete remission, PR-partial remission, SD-stable disease, R/R-relapse refractory
Amongst various hematological disorders, one-month survival was 67% in AML, 86% in ALL, 93% in MDS / MPN, and 78.4% in lymphoma, and plasma cell dyscrasias, respectively. Interestingly, amongst those on active treatment, 36% (n = 4) patients on immunotherapy alone/ combinations (n = 11) (I, C + I, C + I + O) succumbed, versus 19.7% of those on chemotherapy (n = 71) (C, C + O). Amongst those with severe disease, administration of remdesivir (55% vs 68%;p = 0.49) and/or steroids (66% vs 28.5%;p = 0.09) did not improve survival.
7. Discussion
Being a novel virus, there is scarcity of data on how corona virus impacts the management of patients with hematological malignancies. Patients with hematologic malignancies appear to have a greater risk of COVID-19 infection and severe disease due to myelosuppression and lymphopenia [12].In this study, 130 patients with various hematological malignancies were assessed retrospectively from 11 centres in India. About one-third of our patients were > 65 years age.
About 25–50% of people exposed to COVID-19 are asymptomatic [13]. Eleven percent of our patients were asymptomatic and were detected as part of routine screening for COVID-19 before starting chemotherapy. Fever was the most common manifestation of COVID-19 infection in our cohort, followed by cough and breathlessness. These patients may be screened for COVID-19, including a baseline computed tomography of the chest without contrast due to the potential for false-negative PCR from the nasopharyngeal swab, regardless of symptoms [14]. Ninety percent of patients in our cohort had radiological findings of COVID-19 pneumonia.
There is scare data on the prevalence of COVID-19in patients with hematological malignancies. One-hundred and twenty-eight patients with hematologic malignancies, hospitalized at two centers in Wuhan, China were evaluated in a cohort study; 13 (10%) developed COVID-19 [15].In a data from 3 Italian surveys the prevalence of COVID-19 was 0.4% (1 of 267 patients) in adult Ph + acute lymphoblastic leukemia patients, 0.5% (47 of 9339 patient) in chronic lymphocytic leukemia patients, and 0.17% (12 of 6883 patients) in chronic myeloid leukemia patients [[16], [17], [18]].
In a study from Italy, the prevalence of COVID-19 in hematologic patients, mainly affected by malignancies, was not significantly higher compared to that of the general population [19].However, another report from China suggested that patients with cancer had an estimated two-fold increased risk of contracting COVID-19 than the general population and, if infected, also had a higher risk of severe events (ICU admission, invasive ventilation, or death) compared to patients without cancer [15].Another study reported higher rates of severe illness (intensive care unit admissions, invasive ventilation, or death) in patients with cancer when compared with others (39 vs. 8%; p = 0.0003) (8). Patients with cancer also developed severe disease symptoms more rapidly compared with others (median 13 vs. 43 days; p < 0.001) [20].
In a Spanish multicenter retrospective observational study which included 367 pediatric and adult patients with hematological malignancies, prognostic factors identified for day 45 overall mortality included age > 70 years, uncontrolled hematological malignancy, ECOG 3–4, neutropenia (< 0.5 × 109/L) and a C-reactive protein (CRP) > 20 mg/dL (9) [14]. In a multicenter, retrospective, cohort study from Italy, 536 patients with COVID-19 and hematological malignancies were compared with the non-COVID-19 cohort with hematological malignancies, and the standardized mortality ratio was 41·3 (38·1–44·9) [21]. Older age, progressive disease status, diagnosis of AML, aggressive NHL or plasma cell neoplasms and severe COVID-19 were associated with worse overall survival [8,18] suggesting that hematological malignancies have worse outcomes than both the general population with COVID-19 and patients with hematological malignancies without COVID-19. In another retrospective metacentric cohort study from France studying the outcome in Covid-19 hospitalized patients with lymphoma, 30-day mortality was associated with being older (age > 70 years) and relapsed/refractory nature of lymphoma [22].In our cohort, outcome in patients with comorbidities was not different from those without. Median age in our cohort was much younger than other reported studies and half the patients had mild illness. This may have accounted for this lack of association. Similar to our results A European study documented higher mortality (45% vs 11%) in patients more than 60 years age [23]. Patients not in remission had higher mortality compared to patients in remission. Kuderer et al. have also demonstrated worse outcomes in elderly and people whose disease was not in remission [24].They also established increased all-cause mortality in patients with cancer vs general population. Similarily, Garcia-Suarez et al. observed higher mortalities in elderly patients >60 years age and with more than 2 comorbidities [25].
In spite the available literature till date, when to treat, how to treat, when to wait, how long to wait, how to predict and manage toxicities, and how to avoid compromising cure rates remains unknown [12]. There have been expert panel recommendations on how best to manage these patients in current pandemic [26]. In the absence of more specific data, potential risk factors for a severe course of the disease should be assumed as for other viral infections: severe immunodeficiency, lymphopenia, long and profound neutropenia, and older age [27,28]. Patients in remission for their hematological malignancy had better survival and recovery from COVID-19 compared to those patients who were not in remission.
Cancer patients generally shed respiratory viruses longer than immunocompetent people and this is probably true for this novel coronavirus as well [29]. Our median time to negative PCR was 17 days (7–49 days) while one patient was still positive till day 105 at last follow-up. This needs to be kept in mind while devising therapy for these patients. Such long waits may not be feasible sometimes.
Considering the risk of contracting COVID-19 infections, a risk-benefit evaluation should be considered, because the patients may be at a high risk of contracting the COVID-19 infection and dying from it and, on the other hand, that patients may be at a high risk of a fatal hematologic disease progression if not treated appropriately and timely. With the aim of reducing the risk of patient's exposure to the viral infection, alternative strategies have been considered like telemedicine services; a reduction of clinic visits; less intensive chemotherapy and immunotherapy regimens; switch to subcutaneous or oral therapies, rather than intravenous ones, when possible; and postponement of stem cell transplant procedures to a more favorable period [9,30].
8. Limitations
This is a retrospective study. Therefore, complete data was not available for all patients. COVID-19 being a new disease, national guidelines for testing and need for hospitalisation/home isolation were dynamic. Hence, many patients in this study were hospitalized, who at the time of writing the manuscript could have been managed as outpatients. Study duration was wide and treatment guidelines were dynamic over this time period. Data was collected from multiple centres with different expertise and facilities to handle complications.
These limitations not withstanding we are able to identify important prognostic markers in relatively large cohort of hemato-oncology patients across different regions of India.
9. Conclusions
Elderly patients with hematological malignancy and severe covid-19 have worst outcomes specially when disease is not in remission.
Declaration of competing interest
None.
Editor: Mohandas Narla
References
- 1.CDC Guidelines-Treatment Guidelines, Updated: July 30,2020;https://www.COVID-19treatmentguidelines.nih.gov.
- 2.Juan A., Siordia J.R. Epidemiology and clinical features of COVID-19: a review of current literature. J. Clin. Virol. 2020;127:104357. doi: 10.1016/j.jcv.2020.104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirsch H.H., Martino R., Ward K.N., Boeckh M., Einsele H., Ljungman P. Fourth European conference on infections in Leukaemia (ECIL-4): guidelines for diagnosis and treatment of human respiratory syncytial virus, parainfluenza virus, metapneumovirus, rhinovirus, and coronavirus. Clin. Infect. Dis. 2013;56:258–266. doi: 10.1093/cid/cis844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samuel M Rubinstein, Jeremy L Warner; COVID-19 and haematological malignancy: navigating a narrow strait; Thelancet.com; Haematology Vol 7 October 2020. [DOI] [PMC free article] [PubMed]
- 5.Malard F., Genthon A., Brissot E., van de Wyngaert Z., Marjanovic Z., Ikhlef S., Banet A., Lapusan S., Sestilli S., Corre E., et al. COVID-19 outcomes in patients with hematologic disease. Bone Marrow Transplant. 2020 doi: 10.1038/s41409-020-0931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scarfò L., Chatzikonstantinou T., Rigolin G.M., Quaresmini G., Motta M., Vitale C., Garcia-Marco J.A., Hernández-Rivas J.Á., Mirás F., Baile M., et al. COVID-19 severity and mortality in patients with chronic lymphocytic leukemia: a joint study by ERIC, the European research initiative on CLL, and CLL campus. Leukemia. 2020 doi: 10.1038/s41375-020-0959-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glenthøj A, Jakobsen LH, Sengeløv H, Ahmad SA, Qvist K, Rewes A et al.SARS-CoV-2 infection among patients with haematological disorders: Severity and one-month outcome in 66 Danish patients in a nationwide cohort study.Eur. J. Haematol. 2020 Sep 16. doi:10.1111/ejh.13519. Online ahead of print. [DOI] [PubMed]
- 8.Lattenist R., Yildiz H., De Greef J., Bailly S., Yombi J.C. COVID-19 in adult patients with hematological disease: analysis of clinical characteristics and outcomes. Indian J Hematol Blood Transfus. 2020 Jul;7:1–5. doi: 10.1007/s12288-020-01318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.https://www.worldometers.info/coronavirus/ accessed Nov 17, 2020.
- 10.Ministry of Health & family Welfare. Clinical Management Protocol: COVID-19 version 5 [Internet]. Mohfw.gov.in. 2020 [cited 23 October 2020]. Available from: https://www.mohfw.gov.in/pdf/UpdatedClinicalManagementProtocolforCOVID-19dated03072020.pdf.
- 11.Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P; PLACID Trial Collaborators. Convalescent plasma in the management of moderate COVID-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial).BMJ. 2020 Oct 22;371:m3939. doi: 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed]
- 12.Isidori A, de Leval L, Gergis U, Musto P, Porcu P. Management of patients with hematologic malignancies during the COVID-19 pandemic: practical considerations and lessons to be learned. Front Oncol Front Oncol 2020 Aug 14;10:1439.doi: 10.3389/fonc.2020.01439. [DOI] [PMC free article] [PubMed]
- 13.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 Apr 30;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piñana J.L., Martino R., García-García I., Parody R., Morales M.D., Benzo G., et al. Risk factors and outcome of COVID-19 in patients with hematological malignancies. Exp Hematol Oncol. 2020;9(1):1–16. doi: 10.1186/s40164-020-00177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He W., Chen L., Chen L., Yuan G., Fang Y., Chen W., et al. COVID-19 in persons with haematological cancers. Leukemia. 2020 Jun;34(6):1637–1645. doi: 10.1038/s41375-020-0836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foà R., Bonifacio M., Chiaretti S., Curti A., Candoni A., Fava C., et al. Ph+ acute lymphoblastic leukaemia in Italy during the Covid-19 pandemic: a campus ALL study. Br. J. Haematol. 2020 Jul;190(1):e3–e5. doi: 10.1111/bjh.16758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuneo A., Scarfò L., Reda G., Varettoni M., Quaglia F.M., Marchetti M., et al. Chronic lymphocytic leukemia management in Italy during the covid-19 pandemic: a campus CLL report. Blood. 2020 Aug 6;136(6):763–766. doi: 10.1182/blood.2020006854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breccia M., Abruzzese E., Bocchia M., Bonifacio M., Castagnetti F., Fava C., Campus CML working group, et al. Chronic myeloid leukemia management at the time of the COVID-19 pandemic in Italy. A campus CML survey [online ahead of print.] Leukemia. 2020 Aug;34(8):2260–2261. doi: 10.1038/s41375-020-0904-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paul S., Rausch C.R., Jain N., Kadia T., Ravandi F., DiNardo C.D., et al. Treating leukemia in the time of COVID-19. Acta Haematol. 2020 May;11:1–13. doi: 10.1159/000508199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang W., Guan W., Chen R., Wang W., Li J., Xu K., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020 Mar 1;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Passamonti F., Cattaneo C., Arcaini L., Bruna R., Cavo M., Merli F., et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020 Oct 1;7(10):e737–e745. doi: 10.1016/S2352-3026(20)30251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamure S, Duléry R, Blasi RD, Chauchet A, Laureana C, Deau-Fischer B, et al. Determinants of outcome in Covid-19 hospitalized patients with lymphoma: a retrospective multicentric cohort study. EClinicalMedicine 2020 Oct;27:100549. doi: 10.1016/j.eclinm.2020.100549. [DOI] [PMC free article] [PubMed]
- 23.van Doesum J., Chinea A., Pagliaro M., Pasquini M.C., van Meerten T., Bakker M., et al. Clinical characteristics and outcome of SARSCoV-2-infected patients with haematological diseases: a retrospective case study in four hospitals in Italy. Spain and the Netherlands. Leukemia. 2020;34(9):2536–2538. doi: 10.1038/s41375-020-0960-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuderer N.M., Choueiri T.K., Shah D.P., Shyr Y., Rubinstein S.M., Rivera D.R., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Julio García-Suárez, Javier de la Cruz, Ángel Cedillo, Pilar Llamas, Rafael Duarte, Víctor Jiménez-Yuste et al. Impact of hematologic malignancy and type of cancer therapy on COVID-19 severity and mortality: lessons from a large population-based registry study. J Hematol Oncol 2020 Oct 8;13(1):133. doi: 10.1186/s13045-020-00970-7. [DOI] [PMC free article] [PubMed]
- 26.Jain A, Singh C, Dhawan R, Jindal N, Mohindra R, Lad D, et al.How to use a prioritised approach for treating hematological disorders during the COVID-19 pandemic in India? Indian J Hematol Blood Transfus. 2020 Jun 6;36(4):1–11. [DOI] [PMC free article] [PubMed]
- 27.Wang Q., Berger N.A., Xu R. When hematologic malignancies meet COVID-19 in the United States: infections, death and disparities. Blood Rev. 2020 Nov;9:100775. doi: 10.1016/j.blre.2020.100775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeidan A.M., Boddu P.C., Patnaik M.M., Bewersdorf J.P., Stahl M., Rampal R.K., et al. Special considerations in the management of adult patients with acute leukaemias and myeloid neoplasms in the COVID-19 era: recommendations from a panel of international experts. Lancet Haematol. 2020 Aug 1;7(8):e601–e612. doi: 10.1016/S2352-3026(20)30205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lehners N., Tabatabai J., Prifert C., Wedde M., Puthenparambil J., Weissbrich B., et al. Long-term shedding of influenza virus, parainfluenza virus, respiratory syncytial virus and nosocomial epidemiology in patients with hematological disorders. PLoS One. 2016;11(2) doi: 10.1371/journal.pone.0148258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.https://ishbt.com/pdf_files/ISHBT%20COVID-19%20Resource_ver1_25.08.2020.pdf Accessed Nov 19, 2020.