The microtubule-associated protein CLASP is regulated at the translational level when root meristem growth is inhibited in dark-grown plants.
Abstract
The ability for plant growth to be optimized, either in the light or dark, depends on the intricate balance between cell division and differentiation in specialized regions called meristems. When Arabidopsis (Arabidopsis thaliana) seedlings are grown in the dark, hypocotyl elongation is promoted, whereas root growth is greatly reduced as a result of changes in hormone transport and a reduction in meristematic cell proliferation. Previous work showed that the microtubule-associated protein CLASP sustains root apical meristem size by influencing microtubule organization and by modulating the brassinosteroid signaling pathway. Here, we investigated whether CLASP is involved in light-dependent root growth promotion, since dark-grown seedlings have reduced root apical meristem activity, as observed in the clasp-1 null mutant. We showed that CLASP protein levels were greatly reduced in the root tips of dark-grown seedlings, which could be reversed by exposing plants to light. We confirmed that removing seedlings from the light led to a discernible shift in microtubule organization from bundled arrays, which are prominent in dividing cells, to transverse orientations typically observed in cells that have exited the meristem. Brassinosteroid receptors and auxin transporters, both of which are sustained by CLASP, were largely degraded in the dark. Interestingly, we found that despite the lack of protein, CLASP transcript levels were higher in dark-grown root tips. Together, these findings uncover a mechanism that sustains meristem homeostasis through CLASP, and they advance our understanding of how roots modulate their growth according to the amount of light and nutrients perceived by the plant.
At the beginning of their lives, plants face a precarious situation. Resources stored in the seed will only sustain life for a limited time, and thus it is critical that plants quickly emerge from the soil to begin the process of photosynthesis and sugar production. Plants must therefore use their initial resources strategically during the first days of development to ensure survival. It is known that when seedlings are germinated and grown in complete darkness (skotomorphogenesis), the hypocotyl grows rapidly, whereas root elongation is inhibited. When grown in light conditions (photomorphogenesis), Suc derived from aerial organs is sufficient to promote root elongation in Arabidopsis (Arabidopsis thaliana; Kircher and Schopfer, 2012) and reduce hypocotyl growth. One fundamental question is how growth is promoted in some organs and inhibited in others to meet the needs of a plant during developmental transitions.
Several signaling pathways are necessary for plants to withstand stress due to lack of nutrients, which is the case in extended periods of darkness. Autophagy is a conserved degradation pathway in eukaryotes that is activated during cellular starvation. When Glc is available, the formation of autophagosomes is inhibited by increased reactive oxygen species (Huang et al., 2019). Several proteins are targeted for degradation through the Target Of Rapamycin (TOR) kinase, which is a highly conserved eukaryotic protein that integrates environmental signals with downstream developmental and metabolic pathways, such as translation, protein degradation, and cell division (for review, see Dobrenel et al., 2016). Previous work has found that sugar, which activates TOR, inhibits autophagy-mediated degradation of the Brassinosteroid-Resistant1 (BZR1) transcription factor that normally regulates thousands of genes through the brassinosteroid (BR) signaling pathway. In times of cellular starvation and growth arrest, TOR is inactive and BZR1 is consequently degraded (Zhang et al., 2016). Similarly, a related BR transcription factor, Brassinosteroid-Insensitive1 (BRI1) EMS Suppressor1 (BES1) forms a complex with Dominant Suppressor of KAR2 (DSK2) and Autophagy8 (ATG8), which directs cargo to autophagosomes to be degraded in times of stress (Nolan et al., 2017). Thus, it is clear that plants respond to stress through cross talk of BR and autophagy signaling pathways, but these mechanisms likely differ between hypocotyls and roots to control organ-specific growth.
Research into the function of Cytoplasmic Linker Associated Protein (CLASP) revealed its pivotal function in maintaining root meristem size through control of microtubule (MT) organization. Mutants that lack CLASP are dwarf, with fewer cells in the root division zone, indicating a role for CLASP in cell division (Ambrose et al., 2007). In plants, CLASP is involved in anchoring MTs to the cell cortex and is considered to be a stabilizing factor for MTs (Ambrose and Wasteneys, 2008). Through confocal imaging, CLASP has been found to distribute to the sharp transverse edges of newly divided root apical meristem (RAM) cells, which normally present a barrier to growing MTs and cause them to undergo catastrophe upon encounter (Ambrose et al., 2011). The presence of CLASP at these edges, however, enables formation of transfacial MT bundles (TFBs) that span the periclinal and transverse faces of the root meristematic cells. TFBs are associated with maintaining the capacity for cell division and are not present in elongating cells. Recent work has concluded that without CLASP, and consequently TFBs, meristem size is reduced because cells prematurely start to elongate instead of continuing to divide.
Two recent studies demonstrated the involvement of CLASP in both auxin and BR hormone signaling. CLASP directly interacts with the retromer component sorting nexin1 (SNX1) and tethers SNX1-associated endosomes to MTs (Ambrose et al., 2013). Stabilization of SNX1 along MTs fosters recycling of the auxin efflux carrier PIN-formed2 (PIN2) to the plasma membrane (pm), thereby reducing PIN2 transport to vacuoles for degradation (Ambrose et al., 2013). In mutants lacking CLASP expression, PIN2 is depleted at the PM, resulting in auxin accumulation in the root tip, consistent with PIN2’s function in directing auxin away from the quiescent center via the epidermis and cortex tissues (Ambrose et al., 2013). The CLASP-SNX1 interaction also promotes recycling of the BR receptor BRI1 to the PM, thus enhancing BR signaling. Ruan et al. (2018) identified a negative feedback loop whereby the BR-activated transcription factors BZR1 and BZR2/BES1 bind to the CLASP promoter and repress CLASP gene expression. The downregulation of CLASP, through application of exogenous BR or in mutants with constitutively active BR signaling, is strongly associated with premature exit of cells from the division zone, producing a smaller meristem phenotype similar to that observed in clasp-1 mutants.
To determine how root growth is regulated in early plant development, we compared the role of CLASP in actively proliferating light-grown meristems to those grown in dark conditions, when meristem growth is largely inhibited. Notably, in the absence of BZR1 in the dark, CLASP transcript levels were elevated, yet despite this, CLASP protein levels were greatly reduced. In addition, many CLASP-dependent processes such as MT organization and hormone signaling pathways were disrupted in dark-grown meristems. Our work reveals that CLASP is regulated posttranscriptionally based on light signals that control when root growth is desirable for a plant.
RESULTS
CLASP Is Required for Increased Cell Proliferation in Response to Light and/or Suc
To investigate the involvement of CLASP in light-dependent root meristem activity, we compared root growth of clasp-1 null-transcript mutants and the wild type in light and dark conditions. Although it is well known that hypocotyl expansion is stimulated and root growth is inhibited in the dark, we noted that most previous dark-growth investigations with the model plant Arabidopsis included some Suc in the media. Considering the likelihood that Suc, a product of photosynthesis, signals successful germination and stimulates root growth, we compared organ growth in culture media either lacking or supplemented with Suc.
Root growth responses indicated that CLASP’s function is strongly associated with the rapid root growth stimulated in the light. Whereas light stimulated a 7-fold length increase in 6-d-old wild-type roots, from 5.23 ± 0.40 to 37.50 ± 3.03 mm, it only caused clasp-1 root length to double from 3.47 ± 0.24 to 7.33 ± 0.94 mm (Fig. 1, A and C). We also noted that dark-grown wild-type roots were of similar length to those of clasp-1 mutants, suggesting that dark-grown wild-type meristems are deficient in CLASP.
Figure 1.
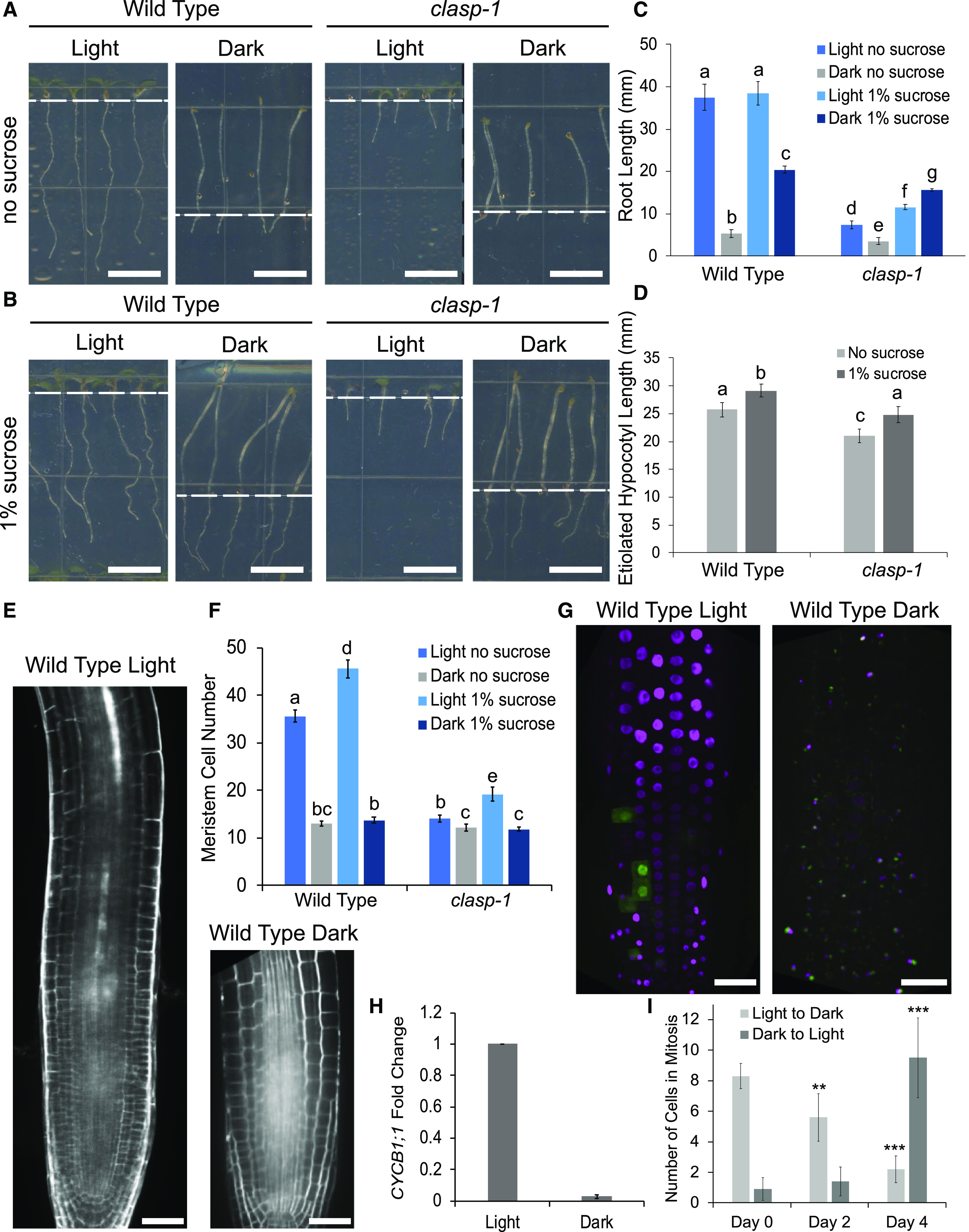
Light and Suc control growth of the RAM. A and B, Seedling morphology of the wild type and clasp-1 mutants grown in either 24-h light or 24-h dark conditions for 6 d with no Suc (A) or in the presence of 1% (w/v) Suc (B). Scale bars = 0.5 cm. Dashed white lines represent the boundary between roots and hypocotyls. C, Quantification of root lengths in the seedlings depicted in A and B. D, Quantification of etiolated hypocotyl lengths in dark-grown seedlings depicted in A and B. Different letters indicate significance (P < 0.01) assessed by a two-way ANOVA with interaction. Error bars indicate 95% confidence intervals. n = 30 for each growth condition. E, Wild-type root meristems stained with propidium iodide to show cell outlines. Scale bars = 30 μm. F, Quantification of meristem cell number in wild-type and clasp-1 roots without Suc in light and dark growth conditions. Different letters indicate significance assessed by two-way ANOVA. Error bars indicate 95% confidence intervals. n = 30 for each genotype and growth condition. G, Confocal z-projection of Cytrap-labeled roots grown in the light or dark. Nuclei lacking fluorescence are in the G1 growth phase, magenta-labeled nuclei are cells in the S phase, and green fluorescent nuclei represent cells undergoing mitosis. Scale bars = 30 μm. H, RT-qPCR of the CYCB1;1 expression fold change in light- and dark-grown root tips. MUSE3 was used as a reference gene. Error bars indicate the mean ± se of n = 3 biological replicates. I, Quantification of cells in mitosis after transfer from light to dark and from dark to light. Asterisks indicate significance by Student’s t test with equal variance with respect to day 0 (**P < 0.01 and ***P < 0.001). Error bars indicate 95% confidence intervals. n = 15 seedlings for each condition.
We next determined that supplementing the culture medium with a moderate (1% [w/v]) amount of Suc caused a major increase in the length of dark-grown roots, but that this effect was independent of CLASP. Treatments with 1% Suc increased dark-grown wild-type root length from 5.23 ± 0.40 to 20.42 ± 0.88 mm and mean clasp-1 root length from 3.47 ± 0.24 to 15.59 ± 0.83 mm (Fig. 1, B and C), an ∼4-fold increase for both genotypes. This indicates that the Suc-dependent growth stimulation is CLASP independent. By comparison, Suc had no significant effect on root length for light-grown wild-type seedlings and a moderate increase (from 7.33 ± 0.94 mm to 11.56 ± 0.60 mm) for clasp-1.
Based on the fact that hypocotyl expansion is entirely dependent on cell elongation, we compared the etiolated hypocotyl responses of dark-grown wild type and clasp-1 mutants to Suc. Although clasp-1 hypocotyls were significantly shorter than those of the wild type under both conditions, clasp-1 and wild-type hypocotyls responded in the same manner to 1% Suc, with an ∼10% length increase (Fig. 1D). These findings demonstrate that although Suc has relatively little effect on dark-grown hypocotyls, the increase is CLASP independent.
The observation that dark-grown wild-type and clasp-1 mutant root lengths are similar prompted us to investigate whether the light-induced changes in root elongation can be attributed to CLASP-dependent changes in cell proliferation. The number of cells in dark-grown wild-type RAMs was similar to that for clasp-1 mutants (Fig. 1, E and F). Growing seedlings in the light more than doubled the number of cells in wild-type root meristems but only increased the number of cells in clasp-1 mutant root meristems by 10% (Fig. 1, E and F). The addition of 1% Suc to dark-grown seedlings did not affect cell proliferation in either genotype (Fig. 1F), suggesting that the Suc-dependent increase in root length (Fig. 1C) was mainly due to cell elongation. These data indicate that the light-induced increase in root cell proliferation is largely dependent on CLASP.
To investigate the basis for light-dependent increases in cell proliferation, we used the Cell Cycle Tracking in Plants (Cytrap) fluorescent marker line to identify cells in S-phase with cyclin-dependent kinase type1a-red fluorescent protein (CDT1a-RFP) and mitosis with cyclin B1-GFP (CYCB1-GFP; Yin et al., 2014). Both reporters showed a dramatic loss of fluorescence in dark-grown roots without Suc (Fig. 1G). Consistent with the lack of CYCB1-GFP protein (Fig. 1G), CYCB1;1, which is upregulated specifically in G2/mitosis, showed very low levels of expression in dark-grown root tips, as measured by reverse transcription quantitative PCR (RT-qPCR; Fig. 1H). In plants transferred from light to dark, the number of cells in mitosis per root tip gradually decreased from 8.3 ± 0.83 to 5.6 ± 1.55 cells after 2 d and to 2.2 ± 0.87 cells after 4 d (Fig. 1I). In the reverse experiment (plants grown in the dark and transferred to light), there was no significant difference in the number of cells exhibiting CYCB1-GFP fluorescence after 2 d (0.9 ± 0.74 to 1.4 ± 0.93 cells). At 4 d of exposure to light, the number of cells in mitosis significantly increased to 9.5 ± 2.62 cells (Fig. 1I). This suggests that exposure of light-grown plants to complete darkness results in a gradual cessation of meristematic cell division, while dark-grown plants placed in light conditions take about 4 d to reestablish a typical number of dividing cells.
Taken together, the data shown in Figure 1 clearly demonstrate that the inhibition of root growth in the dark is associated with reduced cell proliferation, and the finding that clasp-1 knockout mutants are less affected by these conditions indicates that CLASP mediates the cell proliferation stimulated when seedlings are exposed to light.
CLASP Protein Levels Are Diminished under Dark Conditions
To determine whether CLASP protein levels are diminished under dark conditions, we used a GFP-CLASP fluorescent reporter driven by its endogenous promoter (Ambrose et al., 2011) to assess subcellular distribution patterns and measure protein levels by relative fluorescence intensity. In light-grown root meristems, GFP-CLASP was abundant along the transverse edges of cells in the division zone (Fig. 2A). In dark-grown root meristems, by contrast, only a few GFP-CLASP puncta were observed around the cell edges (Fig. 2B), and fluorescence intensity was reduced by about half (Fig. 2C, day 0). It is important to note that autofluorescent bodies were also observed in dark-grown root cells using microscope settings equivalent to those used for GFP fluorescence (Supplemental Fig. S1) and should not be confused with the GFP-CLASP puncta seen in Figure 2B. To further investigate the light dependence of GFP-CLASP protein levels, we transferred plants grown in dark conditions to growth chambers with 24 h light, and plants grown in light conditions to dark conditions (Fig. 2, D and E). In plants transferred from dark to light, GFP-CLASP protein levels steadily increased over a 4-d period (Fig. 2, C and D), and they gradually disappeared over the same time period in plants transferred from light to dark (Fig. 2, C and E).
Figure 2.
CLASP protein levels are diminished in dark-grown root meristems. A and B, Confocal z-projection of root division zone epidermal cells expressing CLASPpro:GFP-CLASP grown in the light (A) and dark (B) for 6 d. Insets show magnified views of GFP-CLASP on cell edges. Scale bars = 30 μm for large images and 10 μm for insets. C, Quantification of fluorescence intensity in the root division zone of CLASPpro:GFP-CLASP/clasp-1-expressing plants transferred from light to dark conditions and vice versa. Asterisks indicate significance assessed by Student’s t test with equal variance, performed to compare days 2 and 4 samples to those on day 0 (***P < 0.001). NS, Not significantly different. Error bars indicate 95% confidence intervals. n > 15 roots for each treatment. D, Confocal z-projection of CLASPpro:GFP-CLASP/clasp-1 plants germinated and grown in the dark for 4 d (day 0), then transferred to 24-h light conditions. Images show CLASPpro:GFP-CLASP 2 and 4 d following transfer to light. E, CLASPpro:GFP-CLASP/clasp-1 plants germinated and grown in the light for 4 d (day 0) then transferred to 24-h dark conditions. Images show CLASPpro:GFP-CLASP 2 and 4 d following transfer to the dark. Scale bars = 30 μm.
MTs Do Not Form TFBs in Dark-Grown Meristems
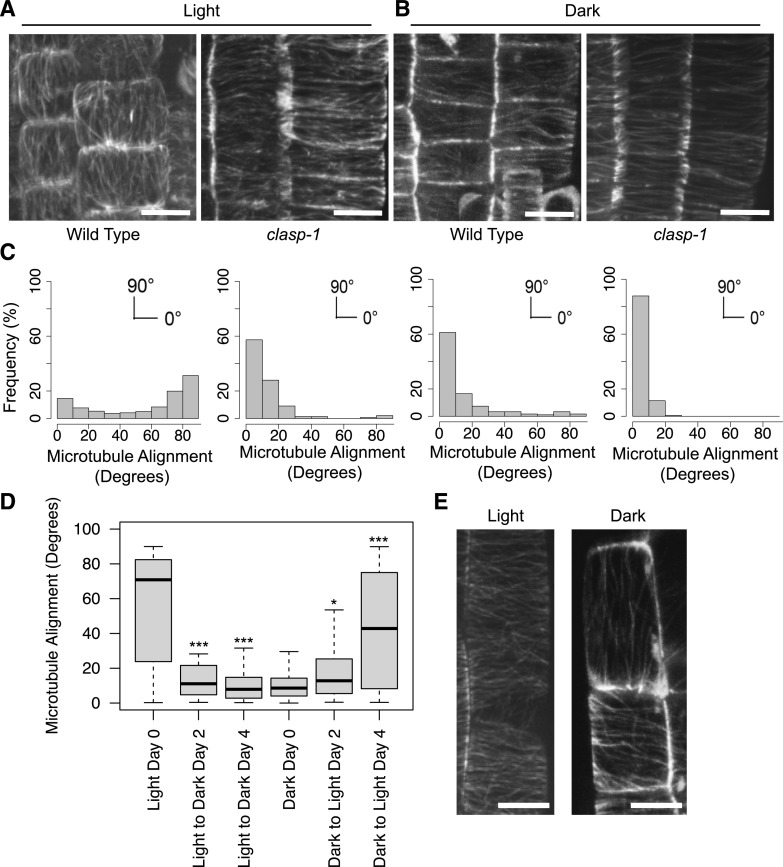
Since CLASP binds to MTs and influences their organization, we hypothesized that CLASP-dependent MT arrays would be altered in dark-grown roots. In roots grown in the light, CLASP mediates the formation of TFBs specific to cells in the division zone (Fig. 3, A and C; Ambrose et al., 2011). TFBs were completely absent in dark-grown root tips, and instead, MTs displayed a predominately transverse array characteristic of cells in the clasp-1 knockout mutant (Fig. 3, B and C). Plants transferred from light to dark growth conditions showed a significant increase in transverse MT alignment in division zone cells 2 d after transfer, while the reciprocal effect was observed in plants transferred from dark to light (Fig. 3D). When increasing amounts of Suc were added to the growth medium of dark-grown plants, the angle of MT alignment in division-zone cells had a tendency toward more longitudinal than transverse, but was not fully restored to light-grown conditions (Supplemental Fig. S2). The addition of Suc did not have a significant effect on MT alignment in light-grown seedlings (Supplemental Fig. S2). In the elongation zone of light-grown roots, MTs are organized transverse to the axis of growth. In contrast, in cells of the equivalent zone of dark-grown roots, MTs were sparse and oriented parallel to the long axis (Fig. 3E; Supplemental Movie S1). These results show that MTs have a dramatically different organization when roots are not actively growing in the dark and that Suc can slightly change MT alignment.
Figure 3.
MTs in the root have different array organization in the absence of light. A and B, MTs in light- (A) and dark-grown (B) wild type and clasp-1 labeled with GFP-MBD driven by a ubiquitin promoter in the division zone of the RAM. Scale bars = 5 μm. C, Distribution frequency of MT orientation angles from cells in the division zone of roots. Data correspond to the images in A and B shown above each graph. MTs at 0° are transverse and those at 90° are longitudinal. n = 500 cells from 10 roots for light-grown samples and 143 cells from 10 roots for dark-grown plants. D, Boxplot of MT orientation in division zone cells of roots transferred to either light or dark conditions. The dark horizontal lines mark the median, the box is the range from the 25th to the 75th percentile, and the whiskers show the highest and lowest data points within 1.5 times the interquartile range. Asterisks indicate significance compared to day 0 for each condition, as assessed by the Mardia-Watson-Wheeler test (*P < 0.05 and ***P < 0.001). n > 60 cells from at least five independent roots for each treatment. E, GFP-MBD-labeled MTs in elongating cells of plants grown in the light and dark. Scale bars = 5 μm.
We noted a considerable reduction in the number of MTs, which indicates a reduction in tubulin levels. In a previous study, GAs were identified as regulators of MT organization through the interaction of DELLA proteins and the prefoldin complex, which promotes proper tubulin folding (Locascio et al., 2013). This work showed that when GA is absent, the prefoldin complex is sequestered in the nucleus and decreases the amount of free tubulin heterodimers available for incorporation into the MT. Treatment with GA results in degradation of the DELLA proteins and movement of the prefoldin complex to the cytoplasm. Based on the sparse MT population observed in dark-grown roots, we hypothesized that GA treatment would induce the prefoldin complex to become functional in the cytoplasm and increase the assembly of MTs, which in turn would result in greater accumulation of CLASP at cell edges. In all cases examined, application of GA did not affect MT density or organization (Supplemental Fig. S3). This suggests that the lack of TFBs and CLASP protein cannot be rescued by a more active prefoldin complex in dark-grown roots.
Auxin Transporters and BR Receptors Accumulate in Large Central Vacuoles in Dark-Grown Roots
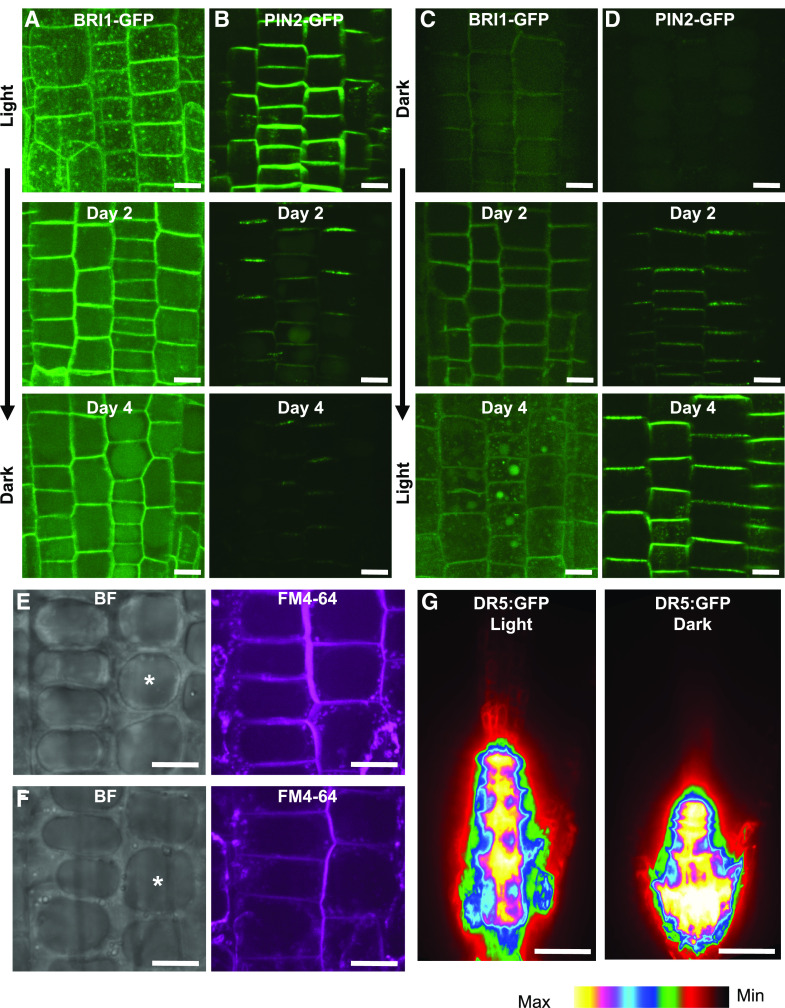
Previous work has shown that CLASP promotes recycling of the BRI1 receptor (Ruan et al., 2018) and PIN2 transporter (Ambrose et al., 2013) to the PM. Since CLASP protein levels were depleted in dark-grown seedlings, we hypothesized that the distribution and abundance of BRI1 and PIN2 would be affected in light- versus dark-grown seedlings. GFP-tagged BRI1 and PIN2 were observed in seedlings grown in the light and then transferred to dark conditions and in those grown in the dark and transferred to light conditions (Fig. 4, A–D). In light-grown seedlings, fluorescence intensity was highest at the PM (Fig. 4, A and B). After a period of 4 d in the dark, BRI1-GFP was detected in intracellular vacuoles (Fig. 4A), which appeared at 2 d in the dark for PIN2-GFP (Fig. 4B). In seedlings germinated and grown in the dark, both BRI1-GFP and PIN2-GFP were initially detected within large vacuoles, indicating that these proteins were undergoing degradation (Fig. 4, C and D). Fluorescence returned to the PM gradually over 4 d of exposure to 24-h light conditions. For seedlings grown in the dark with 1% Suc, PIN2-GFP and BRI1-GFP were still detected at the PM, although the fluorescence intensity was considerably reduced (Supplemental Fig. S4). The formation of large central vacuoles in meristematic cells of dark-grown BRI1-GFP- and PIN2-GFP-expressing seedlings (which were absent from cells of the same developmental stage exposed to light) are shown in Figure 4, E and F. Additionally, visualizing the synthetic auxin-responsive reporter DR5:GFP in light- and dark-grown roots indicated an accumulation of auxin in the meristem of dark-grown roots compared to light-grown roots (Fig. 4G). The transport of BRI1 and PIN2 to the vacuole in dark-grown plants, as well as the pooling of auxin in the root tip, is consistent with reduced recycling of proteins to the PM when CLASP levels are depleted.
Figure 4.
PIN2 and BRI1 are not maintained at the PM in dark-grown plants. Representative images are confocal z-projections. A to D, BRI1-GFP (A and C) and PIN2-GFP (B and D) in light-grown (A and B) and dark-grown (C and D) division-zone cells of roots transferred to dark (A and B) and light (C and D) conditions and imaged at days 0, 2, and 4. Scale bars = 5 μm. E and F, Large central vacuoles (asterisks in brightfield [BF] images) in dark-grown BRI1-GFP (E) and PIN2-GFP (F) plants that are absent in light-grown samples. FM4-64 staining was used to show the PM and the tonoplast of the vacuole. Scale bars = 5 μm. G, DR5:GFP in light- and dark-grown root tips. Scale bars = 30 μm.
The CLASP Protein Is Not Degraded Via the Proteasome or Autophagy in the Dark
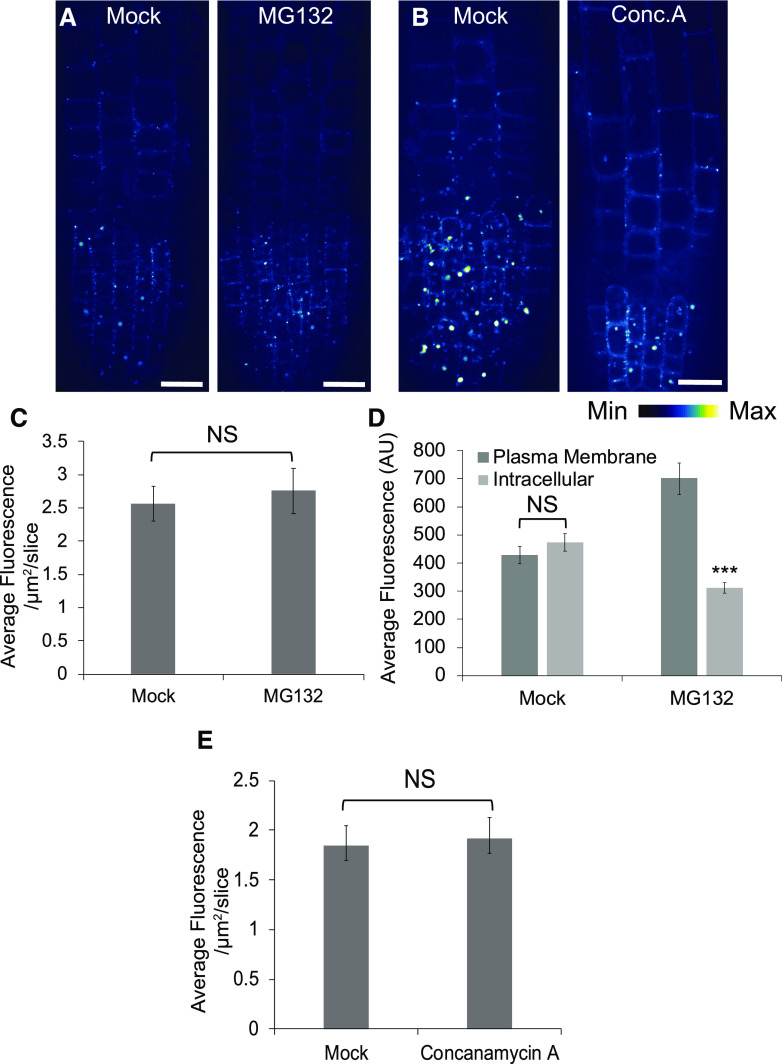
To investigate the cause of the reduction of CLASP in dark-grown roots, we first considered the possibility that CLASP protein was actively degraded. To test this, we inhibited two pathways involved in protein degradation and assessed protein abundance using the GFP-CLASP fluorescent reporter (Fig. 5, A and B). Plants were treated with 50 μm MG132 for 3 h and 1 μm concanamycin A for 6 h to inhibit the 26S proteasome and autophagy, respectively. No increase in GFP-CLASP was observed with MG132 (Fig. 5, A and C). As a positive control, we observed PIN2-GFP after treatment with 50 μm MG132 for 3 h, because this protein is known to be degraded by the 26S proteasome pathway (Laxmi et al., 2008). PIN2 fluorescence increased at the PM and decreased in the vacuole, demonstrating that the MG132 drug treatment was functioning as expected (Fig. 5D). GFP-CLASP levels were also unaffected by Concanamycin A treatments (Fig. 5, B and E). These results demonstrate that CLASP is not actively degraded in the dark, which suggests instead that the protein levels are regulated by suppression of either transcription or translation.
Figure 5.
CLASP is not degraded by the proteasome or autophagy in dark-grown roots. A, Confocal z-projection of CLASPpro:GFP-CLASP/clasp-1 plants treated with either a mock solution or 50 μm MG132 for 3 h. Scale bars = 10 μm. B, Confocal z-projection of CLASPpro:GFP-CLASP/clasp-1 plants treated with either a mock solution or 1 μm concanamycin A. Scale bars = 10 μm. C, Quantification of mean GFP-CLASP fluorescence intensity in the mock and MG132-treated plants shown in A. n = 20 roots for each treatment. D, Quantification of average PIN2-GFP PM and intracellular fluorescence intensity under the same treatment conditions as in A. n = 65 cells for mock and 58 cells for MG132. E, Quantification of mean GFP-CLASP fluorescence intensity in the mock and concanamycin A-treated plants in D. n = 20 roots for each treatment. Error bars in C to E indicate 95% confidence intervals. Asterisks indicate significance assessed by Student’s t test with unequal variance for each treatment (***P < 0.001). NS, No significant.
CLASP Transcript Levels Are Strongly Upregulated in the Dark, for Which the Dampened BR Pathway Is Only Partly Responsible
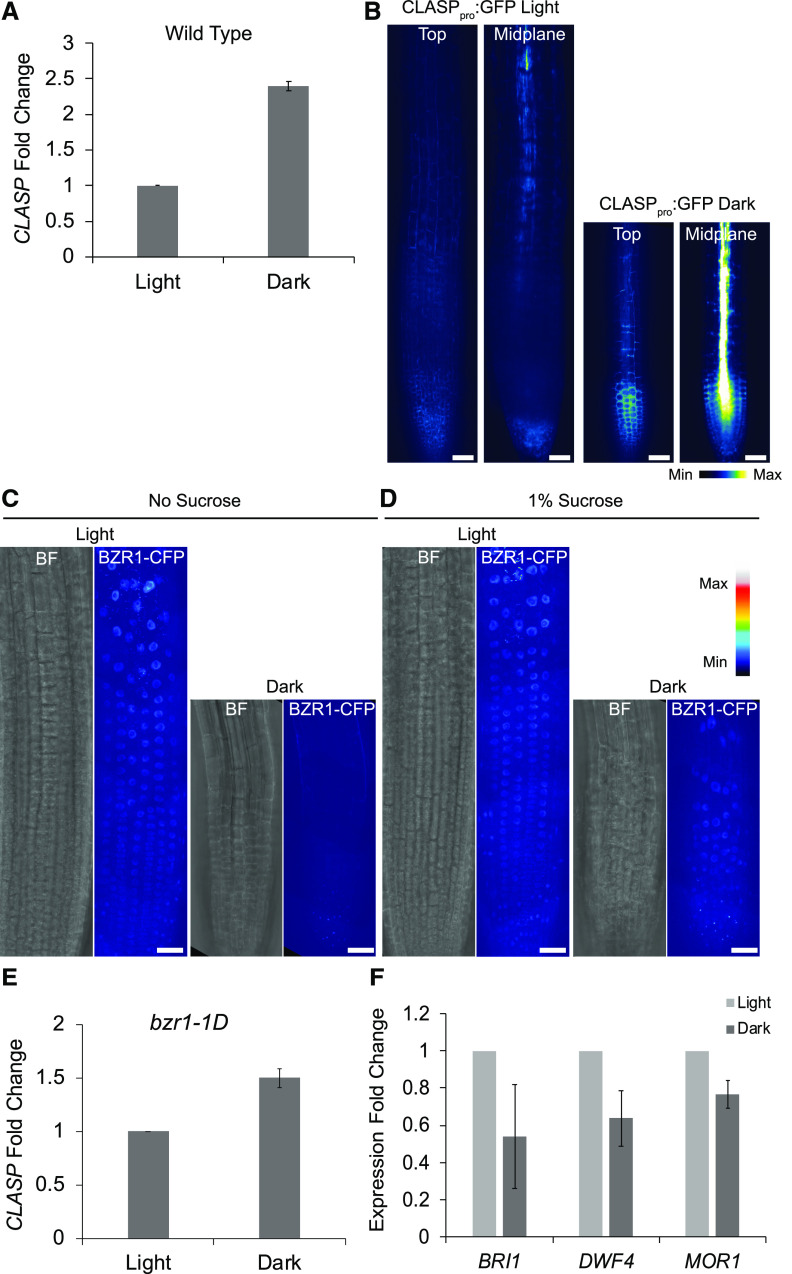
Given the lack of evidence for CLASP protein degradation, we considered the possibility that CLASP transcription is suppressed in dark-grown roots. Using RT-qPCR from root-tip RNA, we found an almost 2.5-fold increase in CLASP gene expression in the dark (Fig. 6A), which is inconsistent with, and in stark contrast to, the decrease in GFP-CLASP levels under identical conditions (Fig. 2). To see whether the increase in CLASP transcript in dark-grown roots reflects increased activity of the CLASP promoter, we measured fluorescence in a line in which the CLASP promoter was used to drive expression of free GFP (CLASPpro:GFP). Consistent with the RT-qPCR results, fluorescence was increased in dark-grown roots compared to light-grown roots (Fig. 6B). Notably, the level of free GFP in dark-grown roots was especially high in the stele (Fig. 6B).
Figure 6.
CLASP transcript levels are elevated in the dark, for which the BR pathway is only partly responsible. A, RT-qPCR analysis of CLASP expression in light- and dark-grown root tips. MUSE3 was used as a reference gene. Error bars indicate the mean ± se of n = 3 biological replicates. B, CLASPpro:GFP in light- and dark-grown root tips. Confocal images are shown of the top (epidermal) layer of tissue as well as in the midplane of the root. Scale bars = 30 μm. C and D, Confocal z-projections of light- and dark-grown root meristems grown with no Suc (C) and 1% Suc (D), showing brightfield (BF) and BZR1-CFP channels pseudo colored with a lookup table. Scale bars = 30 μm. E, RT-qPCR analysis of CLASP expression in bzr1-1D root tips. MUSE3 was used as a reference gene. Error bars indicate the mean ± se of n = 3 biological replicates. F, RT-qPCR of two genes involved in the BR pathway, BRI1 and DWF4, and a MT-associated protein, MOR1, in light- and dark-grown root tips. MUSE3 was used as a reference gene. Error bars indicate the mean ± se of n = 3 biological replicates.
Ruan et al. (2018) determined that CLASP transcription can be partially suppressed by the BR signaling pathway via direct targeting of the CLASP promoter by the BZR1 and BES1/BZR2 transcription factors. We considered the possibility that the elevation of CLASP transcript is a consequence of a shut-down of BR signaling in dark-grown roots. Previous work (Zhang et al., 2016) showed that dark-induced cellular starvation led to BZR1 degradation by autophagy. Using a BZR1-CFP (cyan fluorescent protein) fluorescent reporter, we confirmed that the BZR1 transcription factor accumulates in the nuclei of light-grown root cells with and without Suc, but was absent when plants were grown in media lacking Suc in the dark (Fig. 6C). In contrast, dark-grown roots with 1% Suc in the media still showed BZR1-CFP in the nuclei (Fig. 6D). Since germinating seedlings grown in dark conditions do not yet have a source of Suc, and the BR-activated transcription factor BZR1 was only degraded under these conditions, our remaining analyses focused on roots grown in the absence of Suc.
Given that BZR1 is a negative regulator of CLASP transcription, we predicted that CLASP expression would be greater in dark-grown root meristems in which BZR1 is degraded, and that the elevated expression would be absent if BZR1 remains active. Since previous studies have shown that the constitutively active bzr1-1D mutant protein is not degraded under nutrient-limiting conditions (Zhang et al., 2016), we measured CLASP expression in the bzr1-1D mutant background. Compared to the 2.5-fold increase of CLASP expression in dark-grown wild type plants (Fig. 6A), CLASP expression was still elevated 1.5-fold in the bzr1-1D mutant (Fig. 6E), suggesting that the collapse of BR signaling only partially accounts for the elevated expression of CLASP in dark-grown roots.
We next determined that upregulation of CLASP in dark-grown roots is in contrast to expression of other BR-regulated genes. Expression levels of genes encoding the BR receptor BRI1 and the BR-biosynthetic enzyme DWARF4 (DWF4) were reduced by ∼45% and 35%, respectively (Fig. 6F), indicating a general loss of the BR signaling pathway in dark-grown roots. Importantly, DWF4, like CLASP, is normally suppressed by BR signaling. The reduced expression of DWF4 in dark-grown roots, in contrast to the dramatic increase in CLASP expression, is further evidence that the elevated expression of CLASP is not simply the consequence of the loss of negative regulation by the BR signaling pathway.
To determine whether genes encoding other MT-associated proteins were affected in a similar manner to CLASP in dark-grown roots, we evaluated transcript levels of Microtubule Organization1 (MOR1), which encodes a protein that affects MT dynamics and has domain similarity to CLASP. RT-qPCR analysis showed that plants grown in the dark had a 25% decrease in MOR1 expression (Fig. 6F).
Recognizing that hypocotyl expansion is stimulated in the dark, we measured CLASP transcript and protein levels in hypocotyls. In rapidly growing dark-grown hypocotyls, GFP-CLASP remained abundant and could be seen distributed along MTs (Supplemental Movie S2). RT-qPCR from dark-grown seedlings revealed that CLASP expression was ∼30% higher in the hypocotyls compared to the root tips (Supplemental Fig. S5). This demonstrates that in the dark-grown hypocotyls, both GFP-CLASP protein and CLASP transcript levels remain high, in contrast to the dark-grown root tips, in which transcript levels increased 2.5-fold (Fig. 6A) but protein levels fell (Fig. 2B). To determine whether uncoupling of transcript and protein levels is a general phenomenon for BR-associated components, we measured the expression of DWF4. In contrast to the 30% lower expression of CLASP in the root tip compared to the hypocotyl, we found that that DWF4 expression was reduced by ∼80% (Supplemental Fig. S5).
CLASP Transcript Is Not Processed in the Dark
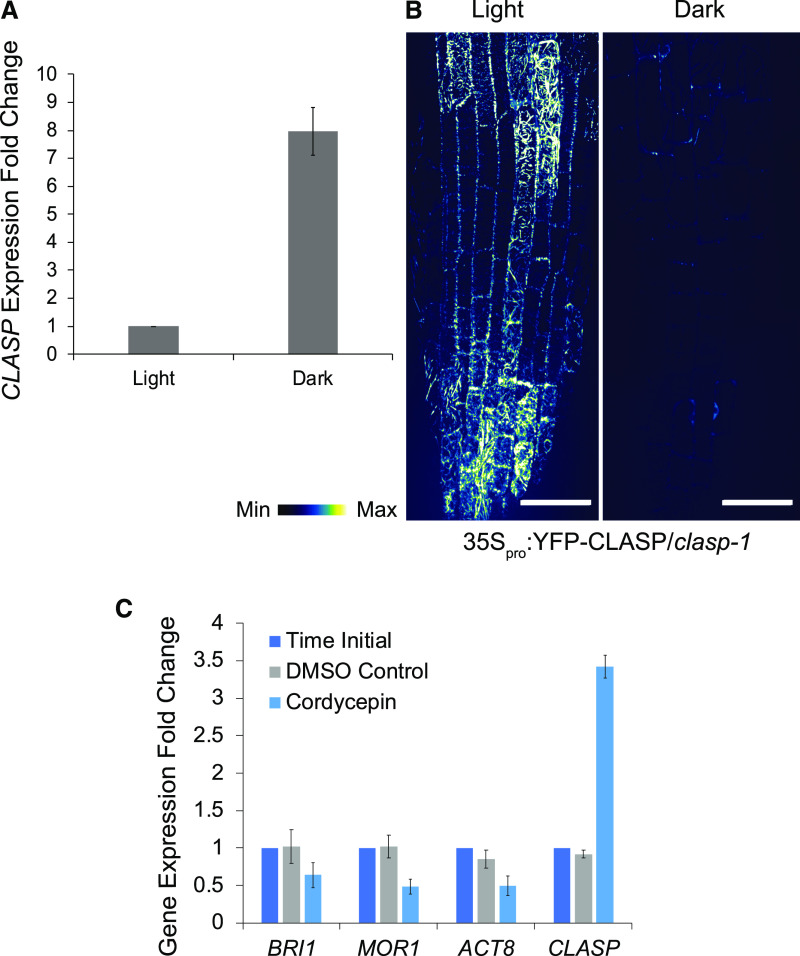
To test whether posttranscriptional control of CLASP is disconnected from its gene expression regulation, we used a line with YFP-CLASP driven by the constitutive 35S promoter to overexpress CLASP and compared root-tip transcript and protein levels in the light and dark. According to RT-qPCR analysis, CLASP gene expression in the 35Spro:YFP-CLASP line was about 8-fold higher in the dark compared to the light (Fig. 7A). As shown in Figure 7B, although 35Spro:YFP-CLASP had greatly increased fluorescence in the light compared to the CLASPpro:GFP-CLASP line (see Fig. 2A), there was no such increase in the dark, and in fact fluorescence was greatly diminished. Taken together with the results using the native CLASP promoter, these findings indicate that while CLASP protein levels are reduced in dark-grown roots, CLASP-specific transcripts accumulate.
Figure 7.
CLASP transcript is not processed in the dark. A, RT-qPCR of CLASP expression in 35Spro:YFP-CLASP root tips grown in the light or dark for 6 d. MUSE3 was used as a reference gene. Error bars denote the mean ± se of n = 3 biological replicates. B, Confocal z-projection of 35Spro:YFP-CLASP plants grown in the light and dark for 6 d. Scale bars = 30 μm. C, RT-qPCR of BRI1, MOR1, ACT8, and CLASP at an initial time point and after 6 h of treatment with 0.1% (v/v) DMSO (control) or 40 μm cordycepin. 6-d-old dark-grown root tips were used to obtain RNA. E1F4A-2 (AT1G54270) was used as a reference gene. Error bars denote the mean ± se of n = 3 biological replicates.
To test whether CLASP production is blocked at the translational level, we used the drug cordycepin to inhibit transcription. For transcripts that are efficiently translated into proteins, gene transcript (RNA) levels should decrease after cordycepin treatment compared to the initial time point, because no new transcript is being synthesized. RNA was extracted from dark-grown root tips at an initial time point and again following 6 h of exposure to 40 μm cordycepin or dimethyl sulfoxide (DMSO) as a solvent control (Fig. 7C). RT-qPCR was used to analyze CLASP gene expression under these conditions, along with that of three other genes, BRI1, MOR1, and actin 8 (ACT8). All four of these genes showed little variation in transcript level between the initial time point and the DMSO control (Fig. 7C, dark blue and gray bars). In cordycepin-treated samples, there was a ∼40%, 50%, and 50% decrease in transcript levels of BRI1, MOR1, and ACT8, respectively, compared to the initial time point (Fig. 7C, dark blue and light blue bars). CLASP, however, deviated from this trend and instead showed higher (∼3.5-fold) gene expression levels with the cordycepin treatment. Thus, the high CLASP expression in the dark is likely the result of an accumulation of transcript that is selectively excluded from being translated into protein.
DISCUSSION
Root meristem growth is essential for plant development and can be readily adjusted based on light availability, nutrients, or abiotic stress. Here, we show how modulation of CLASP protein levels is coincident with either rapid root growth in light conditions, or a cessation of growth in dark conditions (Fig. 8). We showed through fluorescence imaging that BZR1, a negative regulator of CLASP, is degraded in dark-grown root meristems. As expected, due to the loss of negative regulation by BZR1, CLASP transcript levels were increased in dark-grown root tips. Despite the 2.5-fold-elevated transcript level, we detected greatly reduced CLASP protein abundance both when CLASP was expressed under its endogenous promoter and when it was overexpressed with the 35S promoter. We also determined that CLASP protein is not actively degraded in dark-grown roots. The cordycepin transcriptional inhibitor treatments revealed a defect in translational processing of CLASP transcript. These results led us to conclude that there is a translational checkpoint that maintains low levels of CLASP when root cell division and elongation are not necessary, such as in the dark when plants must prioritize hypocotyl expansion. It is important to note that in early seedling development and prior to becoming photosynthetically active, plants must ration sugar reserves. Our work also clearly demonstrates that supplementing growth media with Suc while growing plants in the dark obscures the dramatic phenotypes associated with cellular starvation, which plants would likely experience when photosynthetic output is limited.
Figure 8.
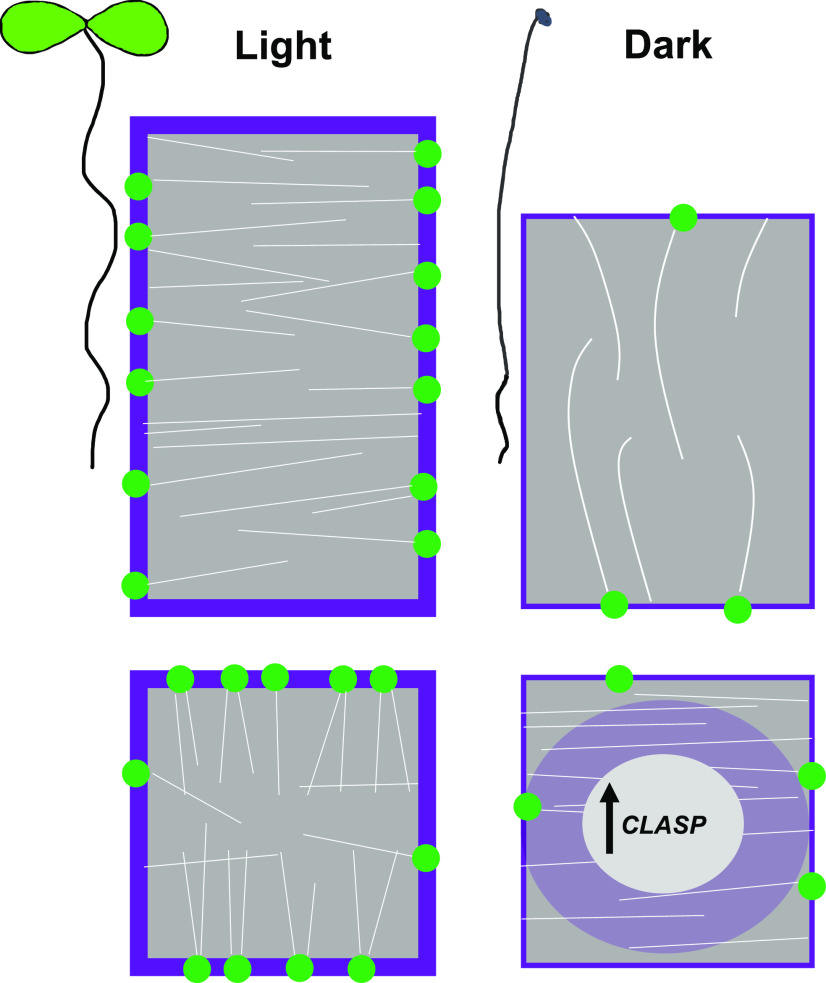
Summary schematic to illustrate how CLASP is implicated in the response to light or dark growth conditions. CLASP (green dots) localizes to the sharp transverse cell edges in dividing cells and mediates the formation of TFBs (white lines). CLASP also fosters the recycling of PIN2 and BRI1 (purple) to the PM. In elongating cells, CLASP is found along the longitudinal edges and MTs display a transverse pattern. In Suc-deprived roots grown in the dark, CLASP levels are diminished, resulting in the disappearance of MT bundles and PIN2 and BRI1 in the large central vacuole. CLASP transcript levels remain high in the dark, suggesting a translational mode of regulation.
This study has identified a very specific and specialized regulatory system that controls CLASP protein levels in response to light/dark conditions, and it further underlines the importance of CLASP as a modulator of meristem size in the Arabidopsis root tip (Ambrose et al., 2007). CLASP was previously shown to be transcriptionally downregulated by the BR signaling pathway (Ruan et al., 2018), but regulation at the translational level has not yet been demonstrated. Our results indicate a general dampening of the BR signaling pathway in root meristems under dark conditions, which is consistent with a study showing BZR1 degradation when carbon availability was limited (Zhang et al., 2016) and another study that found that DWF4 transcript was reduced in dark-grown root tips (Sakaguchi and Watanabe, 2017). Like CLASP, DWF4 is also suppressed by BZR1 (He et al., 2005), but it does not show the same effect of increased transcript as CLASP in dark-grown root meristems. Transcript accumulation under these conditions therefore does not appear to be a ubiquitous response, since genes such as BRI1, DWF4, MOR1, and the mitosis checkpoint regulator CYCB1 showed low expression compared to light-grown root tips. In the dark, GFP driven by the CLASP promoter showed high levels of fluorescence intensity compared to the light, suggesting that free GFP, which does not have a function in plants, is readily translated, whereas the CLASP protein is not. Live cell imaging confirmed that GFP-CLASP was abundant along MTs in expanding etiolated hypocotyls, suggesting that translational regulation of CLASP is organ specific.
When CLASP protein levels were diminished in dark-grown root tips, MT organization was impacted. TFBs did not form, and cells in the meristem division zone displayed transverse MT arrays, similar to the clasp-1 knockout mutant. The role of TFBs in root interphase cells is currently unknown, but these arrays are specific to proliferating cells and dependent on CLASP. The MTs in dark-grown cells were sparse, suggesting that the overall amount of tubulin was reduced, though at this point we can rule out involvement of the GA-dependent prefoldin activity (Locascio et al., 2013), since GA treatments did not lead to an increase in MTs. MTs in elongated cells of dark-grown roots displayed a longitudinal pattern, which is in stark contrast to the hyperparallel transverse array seen in rapidly elongating cells. SCAR1, a component of the actin-nucleation complex, is degraded by the COP1 E3 ligase and the 26S proteasome in the dark, leading to the loss of longitudinal F-actin and reduced root growth (Dyachok et al., 2011). It remains to be determined whether components of the root MT cytoskeletal machinery are affected in a similar manner by growing plants in the dark.
By depriving plants of light and a source of Suc, we showed that the auxin transporter PIN2 and BR receptor BRI1 were less abundant at the plasma membrane and accumulated in the vacuole. This redistribution is consistent with a lack of CLASP, which normally promotes recycling of PIN2 and BRI1 to the PM through tethering of SNX1 vesicles (Ambrose et al., 2013; Ruan et al., 2018). Cells in the division zone of roots normally have several small vacuoles, whereas an enlarged central vacuole is characteristic of elongating and differentiated cells. It is possible that cell wall properties play a role in vacuolar expansion in these cells, as mutants of extracellular Leu-rich repeat extensins and the receptor-like kinase FERONIA displayed enlarged vacuoles in root meristematic cells (Dünser et al., 2019), similar to what we observed in our study.
There is a clear association between the levels of CLASP protein and MT organization, recycling of PIN2 and BRI1, and cell proliferation in the RAM in response to either eliminating light from light-grown seedlings, or restoring light to dark-grown seedlings. Analysis of the sequence of events, however, makes it difficult to conclude with confidence that changes in CLASP protein levels precede and therefore cause the other events. With current imaging technology, it is challenging to quantify changes in CLASP protein at the cellular level. Taking previously published results into account, we note that a BR-mediated 25% to 30% reduction in GFP-CLASP fluorescence is sufficient to generate a massive change in microtubule orientation (Ruan et al., 2018) equivalent to what is observed in the complete absence of CLASP (Ambrose et al., 2011). Similarly, the redistribution of PIN2 (Ambrose et al., 2013) and BRI1 (Ruan et al., 2018) from PM to vacuole takes place both in the complete absence and moderate reduction in CLASP protein levels. These reported findings suggest that there is a critical concentration below which CLASP cannot sustain formation of TFBs, recycling of PIN2 and BRI1, and proliferation of cells in the RAM.
What mechanism controls translation in Arabidopsis root tips during carbon-limited conditions? Plants maintain elaborate nutrient-sensing signaling pathways that impinge on transcriptional and translational network remodeling. Carbon derived from the shoot activates TOR signaling to induce meristem growth in the root (Xiong et al., 2013). In addition to activating genes related to amino acid synthesis, the cell cycle, and RNA synthesis and processing, TOR phosphorylates the E2Fa transcription factor that activates S-phase-related genes (Xiong et al., 2013). When these processes are inhibited under nutrient starvation, mature ribosomes are degraded by autophagy (a process termed ribophagy) that has so far been described in yeast (Kraft et al., 2008) and mammals (An and Harper, 2018; Wyant et al., 2018). Work on ribophagy has been limited in plants, although Floyd et al. (2015) demonstrated ribosomal RNA degradation in the vacuole in a RNase mutant even under sufficient nutrient conditions. If ribophagy is occurring in dark-grown root meristems, it is possible that the buildup of CLASP mRNA is due to a reduction in translational machinery to efficiently process transcripts.
Plants must ensure a strategic partitioning of resources to survive before they can become photosynthetic and produce sugar. The minimal levels of CLASP protein and other components necessary for cell proliferation are consistent with an inhibition of division in the root meristem when nutrients are limited. It remains unknown whether this CLASP-specific response occurs in other systems. The only other evidence is from fission yeast in which, upon Glc starvation, the CLASP homolog Cls1 was negatively regulated by Protein Kinase A, causing MT destabilization and delayed cell division (Kelkar and Martin, 2015). Since GFP-CLASP fluorescence did not increase when protein degradation mechanisms were inhibited, it is not likely that CLASP is being synthesized and degraded, which would be a costly energetic investment. Based on our evidence from the constitutive overexpression of CLASP and the experiments with the transcriptional inhibitor cordycepin, the most logical explanation for the elevated CLASP transcript is the selective exclusion of CLASP mRNA from the translational machinery. It could be advantageous for plants to accumulate CLASP transcripts so that root growth can be readily promoted under the appropriate environmental circumstances. Given that plants undergo daily cycles of light and dark exposure, it would be of interest to determine whether these transcriptional, translational, and cellular modifications follow a diurnal pattern. The regulation of CLASP in response to environmental signals supports a role for this gene in modulating root growth when plants are subjected to stress during their development.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis (Arabidopsis thaliana) plants used in this study were all from the Columbia-0 ecotype background. Seeds were surface sterilized in a solution of 50% (v/v) ethanol and 3% (v/v) hydrogen peroxide and grown on solid one-half strength Murashige and Skoog (MS) medium (pH 5.8) with 1% (w/v) bacto agar and different concentrations of Suc as indicated in the experimental data. The seeds were kept in the dark at 4°C for 2 to 3 d, then germinated in growth chambers under continuous light at 22°C for 6 d. For dark-grown seedlings, the samples were exposed to 8 h of light to promote germination, after which the petri dishes were wrapped in foil for the remainder of the growth period.
The CLASPpro:GFP-CLASP construct fully rescued the clasp-1 null mutant, as described in Ambrose et al. (2011). The Cytrap marker lines, described by Yin et al. (2014), were kindly given to us by Dr. Masaaki Umeda, the BZR1-CFP lines, described in Wang et al. (2002), by Dr. Zhi-Yong Wang, and the 35Spro:YFP-CLASP reporter, described in Kirik et al. (2007), by Dr. Viktor Kirik. MTs were visualized with lines expressing UBQ1pro:GFP-MBD (Ruan et al., 2018). The BRI1pro:BRI1-GFP, described in Geldner et al. (2007), was from Dr. Joanne Chory. PIN2pro:PIN2-GFP is described in Xu and Scheres (2005) and DR5:GFP in Benková et al. (2003).
Propidium Iodide Staining
To quantify meristem cell number, root tips were stained in a 10 μg mL−1 solution of propidium iodide dissolved in distilled water for 1 min. This was followed by three 1-min rinses in distilled water before mounting on a glass slide for microscopy.
Confocal Microscopy
Six-day-old seedlings were mounted in either one-half strength MS (no Suc) liquid or drug solutions on glass slides for imaging. Fluorescent protein reporters were imaged using a spinning-disk confocal microscope (Leica DMi8 inverted microscope, Perkin-Elmer UltraView spinning-disk system, and Hamamatsu 9100-02 camera) with either a 40×/NA 1.25 oil or 63×/NA 1.3 glycerol lens. CFP was imaged with a 440-nm laser and 480/40 nm emission filter, GFP was detected using a 488-nm laser and 525/36 nm emission filter, and YFP was imaged using a 514-nm laser and 540/30 nm emission filter. For propidium iodide and FM4-64, excitation was with a 561-nm laser and 595/50 nm emission filter. The slice thickness for z-projections was 0.3 μm. Images were captured using the Volocity 6.3 software package (Perkin Elmer).
Image Analysis
Confocal images were processed using ImageJ (https://imagej.nih.gov/ij/). Root length was quantified using the NeuronJ plugin (Meijering et al., 2004). To measure the number of cells in mitosis, cells of the epidermal layer in Cytrap-expressing seedlings exhibiting green fluorescence were counted. Fluorescence intensity of GFP-CLASP in root meristems was measured by drawing regions that encompassed epidermal cells from the quiescent center to the beginning of the elongation zone from compressed z-stacks. The “corrected fluorescence” was calculated by subtracting the mean background fluorescence and then dividing by the area of the measured region and the number of z slices to facilitate comparisons between light and dark samples. MT orientation within meristematic cells was calculated using the ImageJ plugin FibrilTool (Boudaoud et al., 2014). BRI1 and PIN2 fluorescence was measured in two cell files from six independent roots in cells extending from the quiescent center to the elongation zone. Briefly, the sum slices tool was used to generate z-projections that extended from the topmost part of the cell to 8 to 9 μm deep. The PM fluorescence was measured by tracing the area using the segmented line tool (pixel width 3), while the intracellular space fluorescence was measured with the polygon tool.
RNA Extraction and RT-qPCR
Six-day-old seedlings were used for gene expression analysis. About 400 root tips for each treatment were excised directly beneath the differentiation zone where root hairs were visible (a length of ∼2 and 0.5 mm for light- and dark-grown roots, respectively). The root tips were ground in TRIZOL reagent (Invitrogen, Life Technologies) and RNA was extracted. The quality of the RNA was assessed on an agarose gel before proceeding. Samples were treated with DNaseI (Amplification Grade, Invitrogen) to remove any residual DNA, and then subjected to reverse transcription with SuperScript III Reverse Transcriptase (Invitrogen, Life Technologies) to obtain complementary DNA. RT-qPCR was carried out with SensiFAST SYBR & Fluorescein Mix (BIOLINE) in a Bio-Rad iQ5 thermal cycler. The Pfaffl method (Pfaffl, 2001) was used to calculate relative expression levels. The primers used are listed in Supplemental Table S1.
GA3 Treatment
Plants were grown in the dark for 6 d, as described in “Plant Material and Growth Conditions”. The samples were either germinated on media containing 10 μm GA for 6 d, or grown on one-half strength MS media (no Suc) for 5 d, then transferred to media containing 10 μm GA for 24 h. The mock treatment was 0.1% (v/v) ethanol.
Drug Treatments
Seedlings were transferred to one-half strength MS medium containing 50 μm MG132 (Sigma-Aldrich) for 3 h or 1 μm Concanamycin A (Sigma-Aldrich) for 6 h to inhibit the proteasome and autophagy, respectively. The mock treatment was one-half strength MS medium with 0.5% (v/v) DMSO. For transcription inhibition experiments, 6-d-old dark-grown seedlings were transferred to one-half strength MS medium containing 40 μm cordycepin (Sigma-Aldrich) or 0.1% (v/v) DMSO for 6 h. Small pieces of filter paper soaked in liquid solutions of either 40 μm cordycepin or 0.1% (v/v) DMSO were also placed atop the roots for the incubation period.
Statistical Analyses
Statistical analyses were performed in R (version 3.3.3; R Core Team, 2013). The Mardia-Watson-Wheeler test was performed using the Circular Statistics package in R (https://r-forge.r-project.org/projects/circular/).
Accession Numbers
Sequence data for the main genes from this article can be found in the GenBank/EMBL data libraries under accession numbers AT1G49240 (ACT8), AT1G27450 (APT1), AT4G39400 (BRI1), AT1G75080 (BZR1), AT2G20190 (CLASP), AT4G37490 (CYCB1;1), AT3G50660 (DWF4), AT1G54270 (E1F4A-2), AT2G35630 (MOR1), AT5G15400 (MUSE3), and AT5G57090 (PIN2).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Dark-grown root meristems have autofluorescent properties.
Supplemental Figure S2. Boxplot of MT orientation in division zone cells of roots grown in either light or dark on varying amounts of Suc.
Supplemental Figure S3. GA3 treatment does not change microtubule organization in dark-grown root meristems.
Supplemental Figure S4. BRI1 and PIN2 localization in root epidermal cells of light- and dark-grown plants with 1% (w/v) Suc.
Supplemental Figure S5. CLASP and DWF4 transcript levels in the hypocotyl and root of dark-grown plants.
Supplemental Table S1. Primer sequences used for RT-qPCR analysis.
Supplemental Movie S1. Time-lapse of MTs labeled with GFP-tubulin in elongating cells of dark-grown roots (related to Fig. 3).
Supplemental Movie S2. Time-lapse of GFP-CLASP in 6-d-old etiolated hypocotyls (related to Fig. 6).
Acknowledgments
The authors thank the University of British Columbia for the use of their Bioimaging Facility to conduct microscopy.
Footnotes
This work was supported by the Natural Sciences and Engineering Research Council (discovery grant no. RGPIN–2019–05432 to G.W. and a CGS–D graduate fellowship to L.H.), the Canada Research Chairs Program, and the Canada Foundation for Innovation.
Articles can be viewed without a subscription.
References
- Ambrose C, Allard JF, Cytrynbaum EN, Wasteneys GO(2011) A CLASP-modulated cell edge barrier mechanism drives cell-wide cortical microtubule organization in Arabidopsis. Nat Commun 2: 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose C, Ruan Y, Gardiner J, Tamblyn LM, Catching A, Kirik V, Marc J, Overall R, Wasteneys GO(2013) CLASP interacts with sorting nexin 1 to link microtubules and auxin transport via PIN2 recycling in Arabidopsis thaliana. Dev Cell 24: 649–659 [DOI] [PubMed] [Google Scholar]
- Ambrose JC, Shoji T, Kotzer AM, Pighin JA, Wasteneys GO(2007) The Arabidopsis CLASP gene encodes a microtubule-associated protein involved in cell expansion and division. Plant Cell 19: 2763–2775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose JC, Wasteneys GO(2008) CLASP modulates microtubule-cortex interaction during self-organization of acentrosomal microtubules. Mol Biol Cell 19: 4730–4737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An H, Harper JW(2018) Systematic analysis of ribophagy in human cells reveals bystander flux during selective autophagy. Nat Cell Biol 20: 135–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J(2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602 [DOI] [PubMed] [Google Scholar]
- Boudaoud A, Burian A, Borowska-Wykręt D, Uyttewaal M, Wrzalik R, Kwiatkowska D, Hamant O(2014) FibrilTool, an ImageJ plug-in to quantify fibrillar structures in raw microscopy images. Nat Protoc 9: 457–463 [DOI] [PubMed] [Google Scholar]
- Dobrenel T, Caldana C, Hanson J, Robaglia C, Vincentz M, Veit B, Meyer C(2016) TOR signaling and nutrient sensing. Annu Rev Plant Biol 67: 261–285 [DOI] [PubMed] [Google Scholar]
- Dünser K, Gupta S, Herger A, Feraru MI, Ringli C, Kleine-Vehn J(2019) Extracellular matrix sensing by FERONIA and Leucine-Rich Repeat Extensins controls vacuolar expansion during cellular elongation in Arabidopsis thaliana. EMBO J 38: e100353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyachok J, Zhu L, Liao F, He J, Huq E, Blancaflor EB(2011) SCAR mediates light-induced root elongation in Arabidopsis through photoreceptors and proteasomes. Plant Cell 23: 3610–3626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd BE, Morriss SC, MacIntosh GC, Bassham DC(2015) Evidence for autophagy-dependent pathways of rRNA turnover in Arabidopsis. Autophagy 11: 2199–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldner N, Hyman DL, Wang X, Schumacher K, Chory J(2007) Endosomal signaling of plant steroid receptor kinase BRI1. Genes Dev 21: 1598–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He JX, Gendron JM, Sun Y, Gampala SS, Gendron N, Sun CQ, Wang ZY(2005) BZR1 is a transcriptional repressor with dual roles in brassinosteroid homeostasis and growth responses. Science 307: 1634–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Yu L-J, Zhang X, Fan B, Wang F-Z, Dai Y-S, Qi H, Zhou Y, Xie L-J, Xiao S(2019) Autophagy regulates glucose-mediated root meristem activity by modulating ROS production in Arabidopsis. Autophagy 15: 407–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelkar M, Martin SG(2015) PKA antagonizes CLASP-dependent microtubule stabilization to re-localize Pom1 and buffer cell size upon glucose limitation. Nat Commun 6: 8445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher S, Schopfer P(2012) Photosynthetic sucrose acts as cotyledon-derived long-distance signal to control root growth during early seedling development in Arabidopsis. Proc Natl Acad Sci USA 109: 11217–11221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik V, Herrmann U, Parupalli C, Sedbrook JC, Ehrhardt DW, Hülskamp M(2007) CLASP localizes in two discrete patterns on cortical microtubules and is required for cell morphogenesis and cell division in Arabidopsis. J Cell Sci 120: 4416–4425 [DOI] [PubMed] [Google Scholar]
- Kraft C, Deplazes A, Sohrmann M, Peter M(2008) Mature ribosomes are selectively degraded upon starvation by an autophagy pathway requiring the Ubp3p/Bre5p ubiquitin protease. Nat Cell Biol 10: 602–610 [DOI] [PubMed] [Google Scholar]
- Laxmi A, Pan J, Morsy M, Chen R(2008) Light plays an essential role in intracellular distribution of auxin efflux carrier PIN2 in Arabidopsis thaliana. PLoS One 3: e1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locascio A, Blázquez MA, Alabadí D(2013) Dynamic regulation of cortical microtubule organization through prefoldin-DELLA interaction. Curr Biol 23: 804–809 [DOI] [PubMed] [Google Scholar]
- Meijering E, Jacob M, Sarria JC, Steiner P, Hirling H, Unser M(2004) Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry A 58: 167–176 [DOI] [PubMed] [Google Scholar]
- Nolan TM, Brennan B, Yang M, Chen J, Zhang M, Li Z, Wang X, Bassham DC, Walley J, Yin Y(2017) Selective autophagy of BES1 mediated by DSK2 balances plant growth and survival. Dev Cell 41: 33–46.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW.(2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi J, Watanabe Y(2017) Light perception in aerial tissues enhances DWF4 accumulation in root tips and induces root growth. Sci Rep 7: 1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna [Google Scholar]
- Ruan Y, Halat LS, Khan D, Jancowski S, Ambrose C, Belmonte MF, Wasteneys GO(2018) The microtubule-associated protein CLASP sustains cell proliferation through a brassinosteroid signaling negative feedback loop. Curr Biol 28: 2718–2729.e5 [DOI] [PubMed] [Google Scholar]
- Wang ZY, Nakano T, Gendron J, He J, Chen M, Vafeados D, Yang Y, Fujioka S, Yoshida S, Asami T, et al. (2002) Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev Cell 2: 505–513 [DOI] [PubMed] [Google Scholar]
- Wyant GA, Abu-Remaileh M, Frenkel EM, Laqtom NN, Dharamdasani V, Lewis CA, Chan SH, Heinze I, Ori A, Sabatini DM(2018) NUFIP1 is a ribosome receptor for starvation-induced ribophagy. Science 360: 751–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, McCormack M, Li L, Hall Q, Xiang C, Sheen J(2013) Glucose-TOR signalling reprograms the transcriptome and activates meristems. Nature 496: 181–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Scheres B(2005) Dissection of Arabidopsis ADP-RIBOSYLATION FACTOR 1 function in epidermal cell polarity. Plant Cell 17: 525–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin K, Ueda M, Takagi H, Kajihara T, Sugamata Aki S, Nobusawa T, Umeda-Hara C, Umeda M(2014) A dual-color marker system for in vivo visualization of cell cycle progression in Arabidopsis. Plant J 80: 541–552 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhu JY, Roh J, Marchive C, Kim SK, Meyer C, Sun Y, Wang W, Wang ZY(2016) TOR signaling promotes accumulation of BZR1 to balance growth with carbon availability in Arabidopsis. Curr Biol 26: 1854–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]