In Arabidopsis, carbon dioxide uptake efficiency is enhanced more by increased cuticle permeability than by increased stomatal opening.
Abstract
Carbon dioxide (CO2) is an essential substrate for photosynthesis in plants. CO2 is absorbed mainly through the stomata in land plants because all other aerial surfaces are covered by a waxy layer called the cuticle. The cuticle is an important barrier that protects against extreme water loss; however, this anaerobic layer limits CO2 uptake. Simply, in the process of adapting to a terrestrial environment, plants have acquired drought tolerance in exchange for reduced CO2 uptake efficiency. To evaluate the extent to which increased cuticle permeability enhances CO2 uptake efficiency, we investigated the CO2 assimilation rate, carbon content, and dry weight of the Arabidopsis (Arabidopsis thaliana) mutant excessive transpiration1 (extra1), whose cuticle is remarkably permeable to water vapor. We isolated the mutant as a new allele of ACETYL-COA CARBOXYLASE1, encoding a critical enzyme for fatty acid synthesis, thereby affecting cuticle wax synthesis. Under saturated water vapor conditions, the extra1 mutant demonstrated a higher CO2 assimilation rate, carbon content, and greater dry weight than did the wild-type plant. On the other hand, the stomatal mutant slow-type anion channel-associated1, whose stomata are continuously open, also exhibited a higher CO2 assimilation rate than the wild-type plant; however, the increase was only half of the amount exhibited by extra1. These results indicate that the efficiency of CO2 uptake via a permeable cuticle is greater than the efficiency via stomata and confirm that land plants suffer a greater loss of CO2 uptake efficiency by developing a cuticle barrier.
To absorb carbon dioxide (CO2) for photosynthesis, land plants expose their wet surfaces to a dry atmosphere and suffer evaporative water loss as a consequence (Hall et al., 1993). As too much water loss would result in dehydration, plants cover most of their aerial surfaces with a relatively impermeable layer, called the cuticle, and take in CO2 mainly through stomatal pores, which make up only about 2% per a leaf area (Willmer and Fricker, 1996). In other words, the cuticle provides drought tolerance to plants in exchange for reduced efficiency in CO2 uptake.
The cuticle is a continuous membrane consisting of a polymer matrix (cutin), polysaccharides, and organic solvent‐soluble lipids (cuticular waxes; Holloway, 1982; Jeffree, 1996; Riederer and Schreiber, 2001). The cuticle is an important structure to protect plants against excess drought, high temperature, strong UV radiation, pathogens, and harmful insects (Kerstiens, 1996a, 1996b; Burghardt and Riederer, 2006; Riederer and Müller, 2006; Domínguez et al., 2011; Yeats and Rose, 2013). The cuticle limits the transpiration through plant surfaces other than through the stomatal pores to <10% of the total (Mohr and Schopfer, 1995). On the other hand, this impermeable layer also strongly restricts CO2 influx. Boyer et al. (1997) and Boyer (2015a, 2015b) reported a lower conductance for CO2 than for water vapor in cuticles of intact leaves of grape (Vitis vinifera) and sunflower (Helianthus annuus) due to the differences in molecular size and diffusion paths between the two gases. However, although many studies have explored the water permeability of cuticles in various conditions and species (Kerstiens, 1996a; Riederer and Müller, 2006; Kosma et al., 2009; Schreiber and Schönherr, 2009), much less attention has been directed to CO2, despite its substantial role in photosynthesis.
In this study, we verified the hypothesis that plants could absorb CO2 more efficiently under non-drought stress conditions if their cuticles are more permeable. In addition, we also investigated the extent to which a permeable cuticle can enhance CO2 uptake efficiency. To verify the hypothesis, we investigated whether the CO2 uptake efficiency is increased in a mutant with a high cuticle permeability. For this research, we isolated an Arabidopsis (Arabidopsis thaliana) mutant named excessive transpiration1 (extra1), which exhibited marked evaporative water loss due to an increased cuticle permeability caused by a new allele of ACETYL-COA CARBOXYLASE1 (ACC1). ACC1 encodes a critical enzyme for the synthesis of malonyl-CoA, an essential substrate for fatty acid synthesis (Baud et al., 2003). To evaluate CO2 uptake efficiency, we investigated CO2 assimilation rate, carbon content, and dry weight of the extra1 mutant and compared them to that of wild-type plants as well as that of another mutant, slow-type anion channel-associated1 (slac1) with continuously open stomata (Negi et al., 2008; Vahisalu et al., 2008). Our results reveal that the increased cuticle permeability strongly and constantly enhances CO2 uptake efficiency under non-drought stress conditions.
RESULTS
The extra1 Mutant Shows Excessive Transpiration Due to an Increased Cuticle Permeability Caused by a New Allele of ACC1
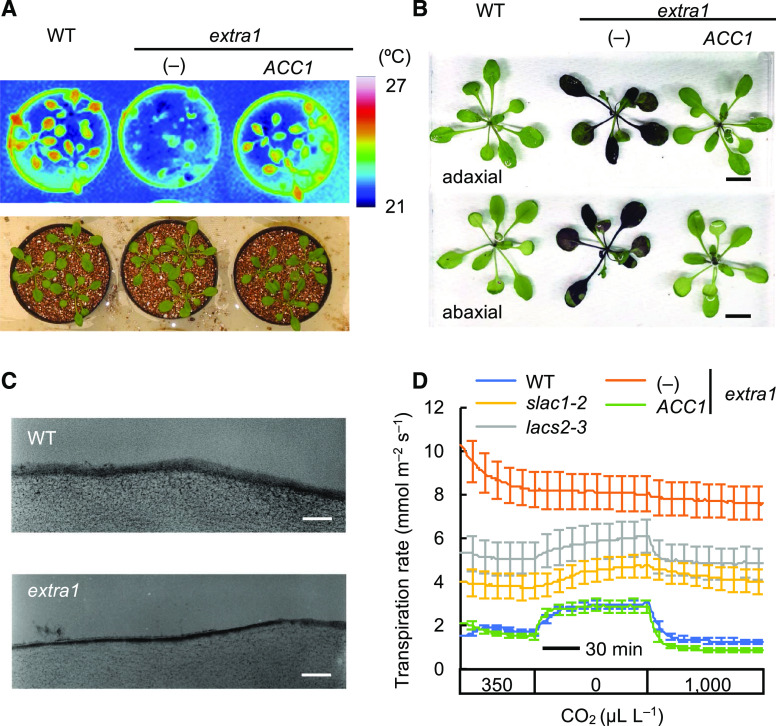
Leaf temperature provides a convenient indicator of transpiration (Negi et al., 2014). Using thermography, we isolated a mutant with a remarkably low leaf temperature (as indicated by an increased amount of transpiration), from the ∼8,000 ethyl methanesulfonate-mutagenized M2 population of Arabidopsis (Fig. 1A). We named this mutant extra1.
Figure 1.
The extra1 mutant shows excessive transpiration due to increased cuticle permeability. A, Thermal imaging of wild type (WT) of Col-0, extra1, and the extra1 transgenic lines expressing a normal, functioning ACC1 gene. B, Cuticle permeability analysis. Aerial parts of wild-type, extra1, and the extra1 transgenic lines expressing ACC1 were stained with 0.1% (w/v) toluidine blue for 5 min. Scale bars = 1 cm. C, TEM images of the cuticle layer of rosette leaves of wild type and extra1 mutant. Scale bars = 100 nm. D, Transpiration rate of the three accessions; slac1-2 (the mutant with stomata always open), and lacs2-3 (the mutant with a thin cuticle). Data presented are means ± se (n ≥ 7).
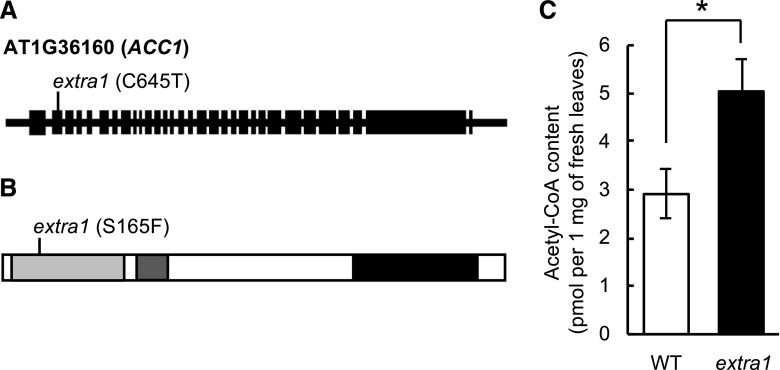
ACC1 (At1g36160) was identified as the causal gene of extra1 by gene mapping and next-generation sequencing. ACC1 is expressed in all parts in both young seedlings and in mature plants of Arabidopsis (Supplemental Fig. S1; Lü et al., 2011). The extra1 mutant has a single nucleotide substitution from C to T at position 645 of ACC1 gene (Fig. 2A) and a single amino acid substitution from Ser to Phe, which is within the ACC1 protein’s biotin carboxylase domain (Fig. 2B). ACC1 is a critical enzyme for the synthesis of malonyl-CoA, an essential substrate for fatty acid synthesis (Baud et al., 2003), and the malfunction of ACC1 affects the cuticle wax biosynthesis (Lü et al., 2011). So, we speculated that the higher transpiration in extra1 could be due to an abnormal cuticle caused by alterations in the malonyl-CoA synthetic pathway. The fact that the leaves of extra1 contained high levels of acetyl-CoA (Fig. 2C) suggested that the cytosolic acetyl-CoA in leaves is not carboxylated to malonyl-CoA by the mutated ACC1 in extra1.
Figure 2.
The causal gene of extra1 is ACC1. A, The structure of the ACC1 (At1g36160) gene and the positions of the point mutation identified in extra1. Black boxes represent exons and black lines stand for introns and untranslated regions. Nucleotide positions are relative to the translational start site (+1). B, Amino acid change in the conceptual translation product of the ACC1 gene. The light gray, gray, and black boxes show the biotin carboxylase domain, biotin-carboxyl carrier domain, and carboxyl transferase domain, respectively. C, Acetyl-CoA content of wild type (WT) and extra1. Data presented are means ± se (n ≥ 6). Asterisk indicates statistical significance by Student’s t test (*P < 0.05).
To determine the cause of the increased transpiration rate in extra1, we analyzed its cuticle permeability by toluidine blue staining (Fig. 1B). The rosette leaves of extra1 were stained clearly by toluidine blue, but those of wild type were not. This result shows that the cuticle of extra1 has very high permeability to water. Transmission electron microscopy (TEM) can be used to visualize the basic cuticle membrane ultrastructure (Lü et al., 2011), and so we used TEM to analyze the differences between extra1 and wild type. The TEM images showed that the extra1 cuticle membrane was thinner than that of wild type (Fig. 1C), suggesting that this could be the reason for the increased cuticle permeability in extra1.
We carried out a complementarity assay for confirming that the causal gene of extra1 was ACC1. The similarity we observed in leaf temperature, toluidine blue stain, and transpiration rate between wild type and the extra1 transduced with wild-type ACC1 allele (Fig. 1, A, B, and D) demonstrates that the causal gene of extra1 is indeed ACC1.
To examine the transpiration rates of extra1 in detail, we measured with a gas exchange system the response to [CO2] changes among three types of plants: Columbia (Col-0) wild-type plants; the slac1-2 mutant that suppresses stomatal closure due to an abnormal slow-type anion channel; and the long chain acyl-CoA synthetase2-3 (lacs2-3) mutant, a T-DNA insertion line with a high cuticle permeability caused by an abnormality in the long-chain acyl-CoA synthetase that is essential for cutin biosynthesis (Supplemental Fig. S2; Schnurr et al., 2004; Bessire et al., 2007). We then compared these lines with the extra1 and the extra1 transgenic lines expressing normal functioning ACC1 gene (Fig. 1D). We observed a 1.8-times increase in transpiration rate in response to the [CO2] decrease, from 350 to 0 μL L–1, and a 60% reduction in response to the [CO2] increase, from 0 to 1,000 μL L–1, in the wild-type plants. On the other hand, the transpiration rate of extra1 was hardly altered in response to [CO2] change and was consistently higher than that of wild type. The change in transpiration rate of slac1-2 mutant was also minimal in response to [CO2], and was higher than that of wild type, but lower than that of extra1. We observed a change in transpiration rate of lacs2-3 in response to [CO2], and the transpiration rate remained at a higher level than those of wild type and slac1-2, but lower than that of extra1, regardless of the [CO2] levels.
We next verified whether extra1 mutants have normal stomatal response because the transpiration rate hardly changed in response to [CO2] in extra1 (Fig. 1D). The plants or leaves used for the measurements were incubated under high-humidity conditions or floated on water, because extra1 has a low tolerance to low humidity. The stomata of extra1 showed normal responses to a change in [CO2] and light–dark transitions (Supplemental Fig. S3, A and B). In addition, we also measured the stomatal size and frequency in wild-type and extra1 plants. The stomatal density (SD), stomatal index (SI), and guard cell length (GCL) of extra1 were very similar to those of wild type (Supplemental Fig. S3, C–E). These results show that the stomata of extra1 mutants were not abnormal.
We also performed experiments comparing the water loss of wild type and extra1 mutants (Supplemental Fig. S4). Detached shoots of wild type treated by abscisic acid (ABA), which induces stomatal closure, showed decreased water loss rate compared with mock-treated shoots; however, there were almost no differences in water loss rate between ABA-treated extra1 and mock-treated extra1. These results suggest that the stomata are not responsible for the remarkably increased transpiration in extra1.
The Increased Cuticle Permeability Caused by ACC1 Mutation Enhances CO2 Uptake Efficiency
As previous studies have indicated a difference in cuticle conductance of CO2 and water vapor in grape and sunflower (Boyer et al., 1997; Boyer, 2015a, 2015b), we next investigated if there is indeed a difference in CO2 uptake efficiency compared to that of water vapor in extra1 mutants.
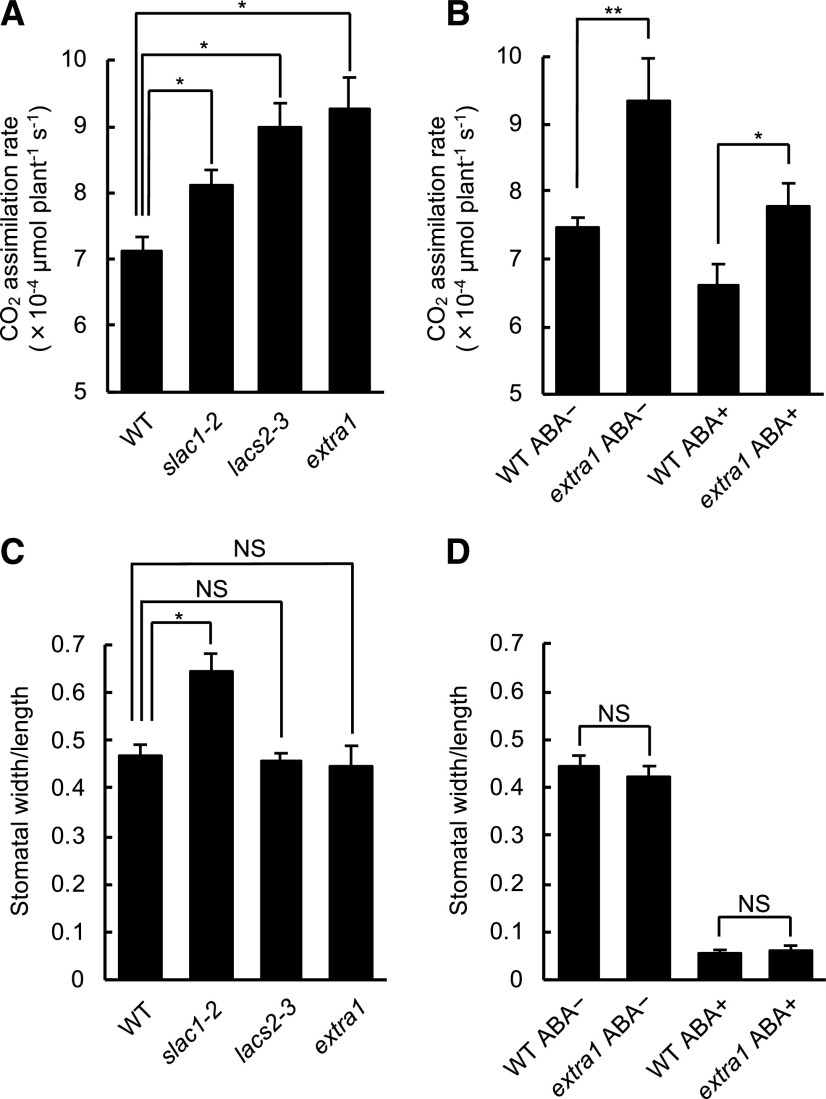
To evaluate the CO2 uptake efficiency in extra1, we compared the CO2 assimilation rate per individual plant among extra1, wild type, slac1-2, and lacs2-3, with a gas exchange system (Fig. 3). The measurement was performed at 400 μL L–1 [CO2], which is close to ambient CO2 concentration, and under a high-humidity condition (95% of relative humidity [RH]) and a relatively weak light condition (60 μmol m–2 s–1) to avoid drought and light stresses; we used the model no. GFS-3000 gas exchange system (Heinz Walz) for this precise regulation of environments within a measurement chamber. The extra1, lacs2-3, and slac1-2 mutants showed significantly higher CO2 assimilation rates than wild type (Fig. 3A; Dunnett’s test). We also performed a multiple comparison test (Tukey–Kramer test) among extra1, wild type, and slac1-2; each of the accessions showed a significantly different CO2 assimilation rate compared to the other accessions (P < 0.05). This tendency was also maintained in CO2 assimilation rate per projected leaf area (Supplemental Table S1). To see the differences in CO2 assimilation rates due to the increased stomatal aperture versus increased cuticle permeability, we measured the stomatal apertures just after measuring CO2 assimilation rate. The slac1-2 mutant showed a more significantly increased stomatal aperture than wild type, but extra1 and lacs2-3 did not (Fig. 3C; Dunnett’s test). These results indicate that the increased cuticle permeability caused by ACC1 mutation enhances CO2 uptake efficiency more than the increased stomatal aperture caused by SLAC1 mutation, under a non-drought stress condition.
Figure 3.
The extra1 mutant demonstrated a high CO2 assimilation rate. A and C, The CO2 assimilation rates per individual plant (A) and stomatal apertures as indicated by the ratio of width/length (C) of wild type (WT), slac1-2, lacs2-3, and extra1 are shown. Data presented are means ± se (n ≥ 6). Asterisks indicates statistical significance by Dunnett’s test (*P < 0.05). B and D, The CO2 assimilation rates per individual plant (B) and stomatal apertures as indicated by the ratio of width/length (D) of wild type and extra1, treated with 1 mm of ABA or mock (0.1% [v/v] DMSO). Data presented are means ± se (n ≥ 6). Asterisks indicates statistical significance by Student’s t test (*P < 0.05 and **P < 0.01). The CO2 concentration (400 μL L–1), light intensity (60 μmol m–2 s–1), and humidity condition (95%) were kept constant throughout the measurement of the CO2 assimilation rate. The stomatal apertures were measured just after measuring the CO2 assimilation rate. NS, Not significant.
Next, we analyzed the stomatal aperture size and CO2 assimilation rate in extra1 and wild-type plants treated with ABA to ensure that the observed differences in CO2 assimilation rate in extra1 are due to differences in cuticle permeability. ABA decreased stomatal apertures in both the extra1 and wild-type plants to the same extent, but extra1 showed a higher CO2 assimilation rate than wild type both under ABA treatment and non-ABA treatment (Fig. 3, B and D). This result demonstrated that the cause of the high CO2 assimilation rate in extra1 was cuticle permeability.
The Increased Cuticle Permeability Caused by ACC1 Mutation Increases Dry Weight and Carbon Content
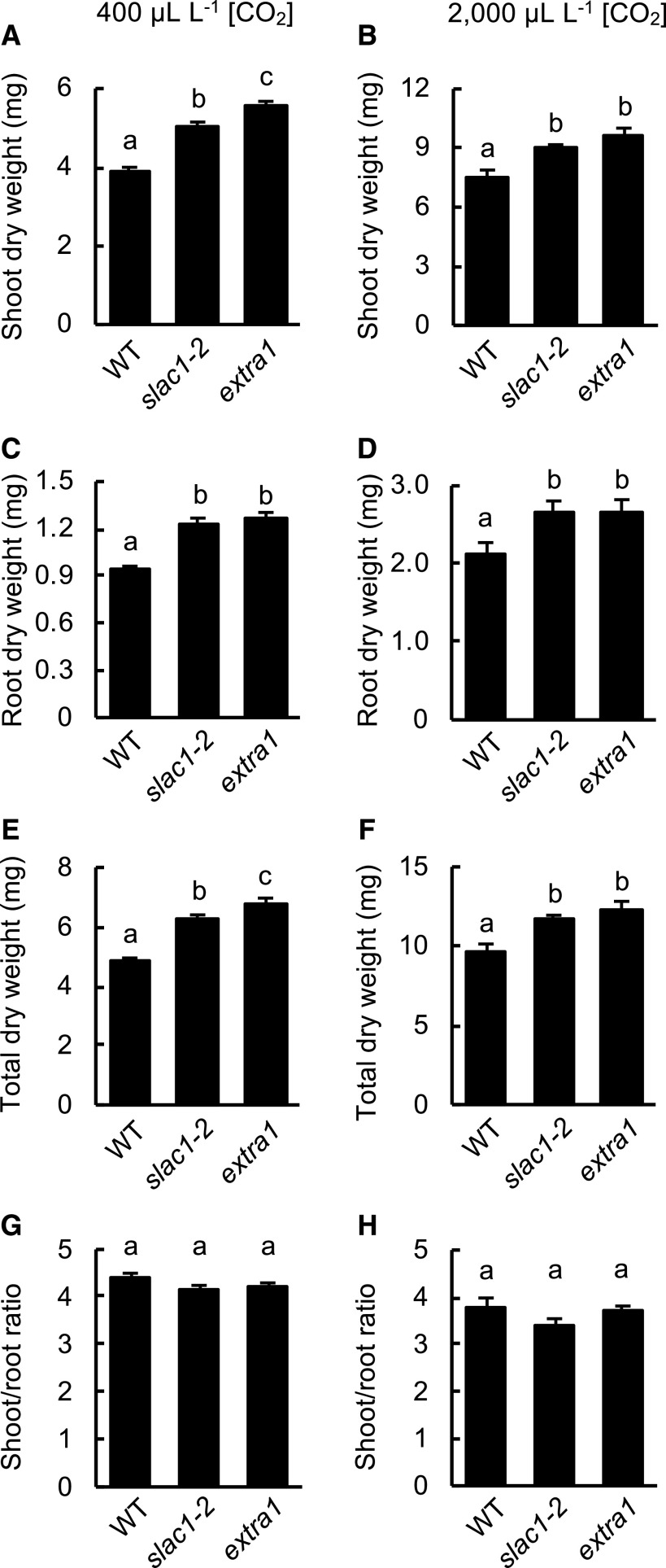
An increased CO2 assimilation rate may enhance plant growth. To verify this hypothesis, we compared the dry weight of shoots in extra1, wild type, and slac1-2 (Fig. 4). Plants were grown on culture medium in petri dishes (water vapor was saturated in the petri dishes) and under a relatively weak light condition (30 μmol m–2 s–1). The extra1 mutant had a larger shoot dry weight and total dry weight than wild type and slac1-2, when grown under 400 μL L–1 [CO2]. The root dry weight of extra1 was also larger than that of wild type, although it was not significantly different from that of slac1-2. This result suggests that the increased dry weight of extra1 was due to increased CO2 uptake. Next, we wanted to see if there is a difference in dry weight between plants grown under a high (2,000 μL L–1) and ambient (400 μL L–1) [CO2] to see if the extent of stomatal closure affects the CO2 uptake efficiency. The dry weight of extra1 was considerably higher than that of slac1-2 at 2,000 μL L–1 [CO2], but the difference was not statistically significant. We also observed that CO2 uptake efficiency affects photosynthesis at 400 μL L–1 [CO2] more than at 2,000 μL L–1 [CO2]. This seems to be because even slightly open stomata can absorb sufficient CO2 for photosynthesis at a high [CO2] condition.
Figure 4.
Increased cuticle permeability promoted growth in extra1. The dry weight of shoots (A and B), roots (C and D), the sum of shoot and root (E and F), and shoot/root ratio (G and H) in extra1, slac1-2, and wild type (WT) are shown. Plants were grown under 400 μL L–1 [CO2] (A, C, E, and G) or 2,000 μL L−1 [CO2] conditions (B, D, F, and H). The plants were also grown on medium in petri dishes under a constant light condition of 30 μmol m–2 s–1. Data presented are means ± se (n = 20). Values marked with different lowercase letters are significantly different among accessions by Tukey–Kramer test (P < 0.05).
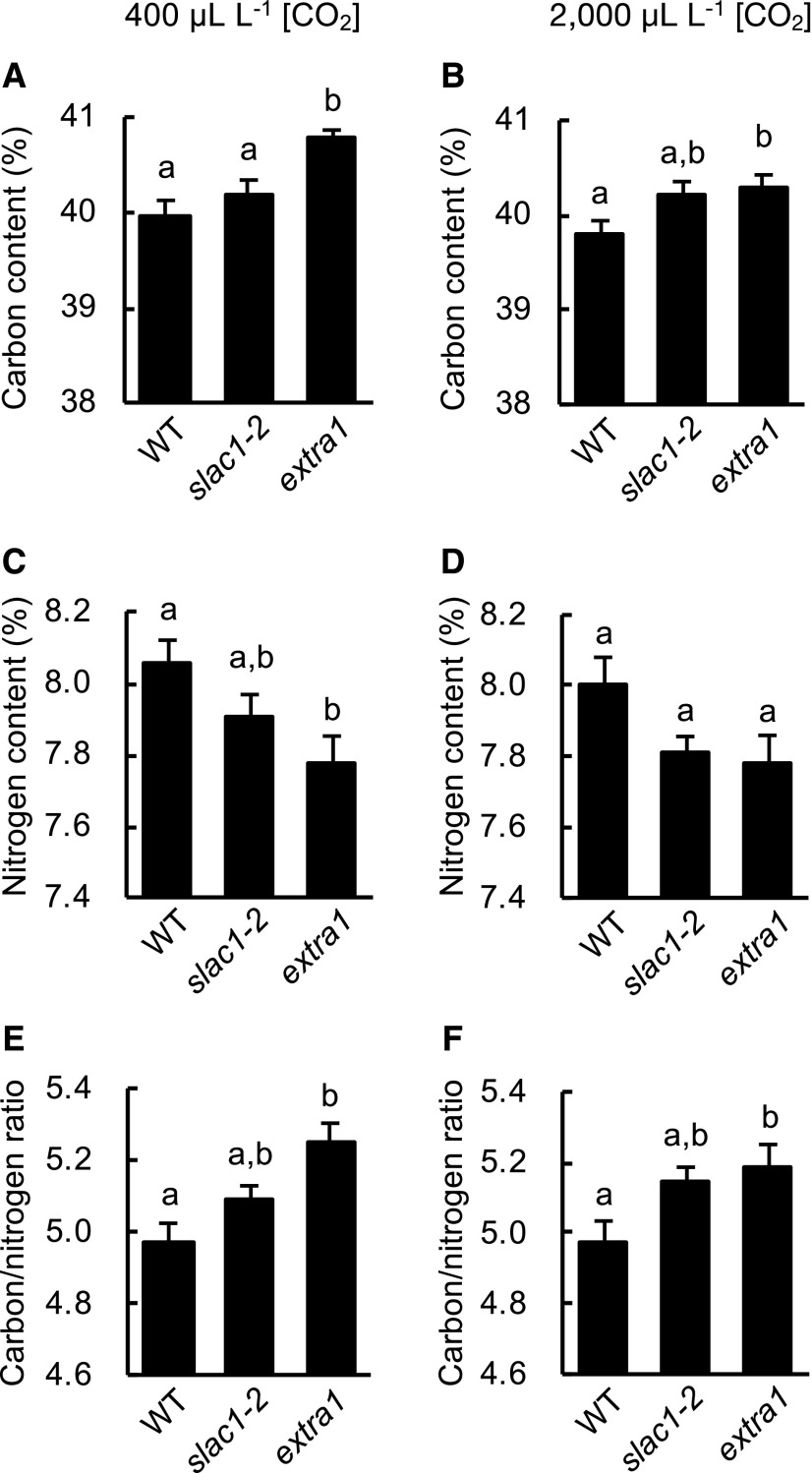
To investigate the CO2 uptake efficiency in extra1 from a different angle, we analyzed carbon and nitrogen contents in aerial parts of extra1, wild type, and slac1-2 after measuring their dry weight (Fig. 5). The extra1 mutant had significantly higher carbon content than slac1-2 and wild type at 400 μL L–1 [CO2]. In conjunction with that, the extra1 mutant had lower nitrogen content than wild type. The pattern of carbon and nitrogen content changes was similar in plants grown at 2,000 μL L–1 [CO2], but the difference was smaller or not statistically significant. This result was consistent with the result we observed on dry weight of the plants (Fig. 4).
Figure 5.
The extra1 mutant showed higher carbon content than wild type (WT). Percent Carbon content (A and B), percent nitrogen content (C and D), and carbon/nitrogen ratio (E and F) in aerial parts of extra1, slac1-2, and wild type are shown. Plants were grown under 400 μL L–1 [CO2] (A, C, and E) or 2,000 μL L–1 [CO2] conditions (B, D, and F). Plants for dry weight measurement (Fig. 4) were used for this experiment after the weight measurement. Data presented are means ± se (n = 12). Values marked with different lowercase letters are significantly different among accessions by Tukey–Kramer test (P < 0.05).
The extra1 Mutant Suppresses the Decrease in Photosynthesis Rate Caused by Fluctuating Light
Our results so far demonstrated that the high cuticle permeability in extra1 increases CO2 uptake efficiency and plant growth (Figs. 3, 4, and 5), which led us to suspect that extra1 also enhances photosynthesis efficiency. Therefore, we analyzed the quantum yield of PSII[Y(II)], an indicator of photosynthetic rate, at three different photosynthesis active radiations (PARs; Table 1). There was no significant difference in Y(II) between wild type and extra1 regardless of the PAR values (P > 0.05, paired t test).
Table 1. The quantum yields of PSII (Y[II]) of wild type and the extra1 mutant.
Data presented are means ± se (n = 18). NS, No significant difference in Y(II) between wild type and extra1 (P > 0.05, paired t test).
| Genotype | PAR | ||
|---|---|---|---|
| 20 | 80 | 145 | |
| μmol m–2 s–1 | |||
| Wild type | 0.632 ± 0.003 | 0.487 ± 0.003 | 0.411 ± 0.011 |
| extra1 | 0.634 ± 0.003NS | 0.489 ± 0.003NS | 0.411 ± 0.011NS |
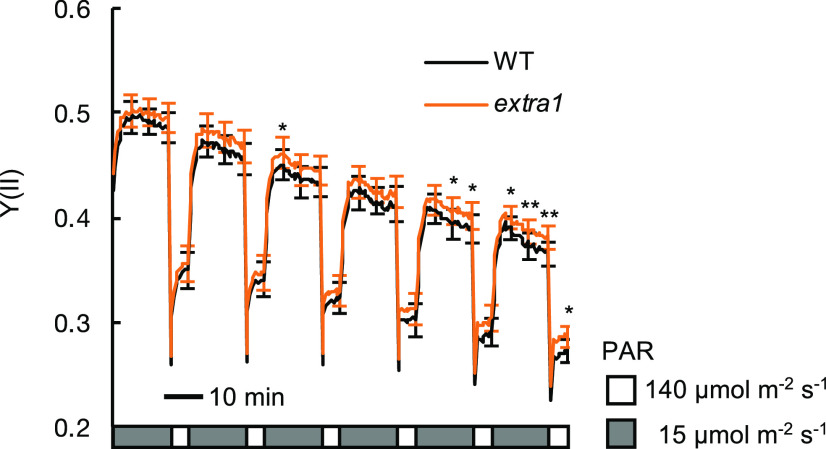
In general, the photosynthetic rate is lower under fluctuating light conditions than under constant light conditions (Lawson et al., 2012; Yamori et al., 2016; Kaiser et al., 2018; Faralli and Lawson, 2020). This is mainly because CO2 uptake efficiency is affected by the changes in stomatal aperture induced by the changes in light intensity. This is validated by the fact that the slac1-2 mutant exhibited a stay-open stomata phenotype and showed a higher rate of photosynthesis and plant growth under fluctuating light than wild type (Kimura et al., 2020). This fact led to the hypothesis that CO2 uptake through cuticles reduced the negative effect on photosynthesis caused by fluctuating light. To verify this hypothesis, we compared Y(II) in extra1 and wild type under fluctuating light conditions (mimicked natural fluctuations in light; Fig. 6). The Y(II) of extra1 was not statistically different from that of wild type in the beginning of the measurement, but it became significantly higher as the measurement proceeded (Fig. 6; Supplemental Fig. S5), supporting our hypothesis.
Figure 6.
Decrease in the quantum yields of PSII [Y(II)] induced by fluctuating light was suppressed in extra1. Data presented are means ± se (n = 5). Asterisks indicate significant differences in the Y(II) between wild type (WT) and extra1 by paired t test (*P < 0.05 and **P < 0.01).
DISCUSSION
Plants make constant tradeoffs between the need to photosynthesize and the need to prevent water loss. The development of cuticles enables prevention of water loss but reduces CO2 uptake efficiency under non-drought stress conditions. The ultimate purpose of our study was to evaluate the extent to which an increase in cuticle permeability enhances CO2 uptake efficiency under non-drought stress conditions—in other words, how much of the CO2 uptake efficiency in plants has been lost by developing a cuticle in exchange for drought tolerance. We evaluated the CO2 uptake efficiency in the extra1 mutant, which has a highly permeable cuticle caused by a new allele of ACC1 (Figs. 1 and 2), by measuring CO2 assimilation rate, carbon content, and dry weight. The extra1 mutant exhibited an increased CO2 assimilation rate, carbon content, and dry weight versus not only wild type but also slac1-2, which has permanently open stomata (Figs. 3, 4, and 5). These results show that the increased cuticle permeability in extra1 enhances CO2 uptake efficiency more than the stomatal opening, as shown by the slac1-2 mutants. Moreover, the Y(II) analysis results suggest that extra1 showed higher electron transport at PSII under fluctuating light conditions than wild type, indicating that CO2 uptake through cuticles reduced the negative effect on photosynthesis caused by fluctuating light (Fig. 6).
Lü et al. (2011) reported that ACC1 plays a principal role in the biosynthesis of cuticular waxes. In addition to this, ACC1 has been shown to be involved in several other functions. For example, Baud et al. (2003) reported that ACC1 is also essential for embryo development in Arabidopsis. Another study reported that an ACC1 mutation causes an increase in cold sensitivity (Amid et al., 2012). Thus, it is possible that higher CO2 uptake efficiency observed in extra1 is due to reasons other than cuticle malformation caused by ACC1. To understand if the higher CO2 uptake efficiency in extra1 is due to the highly permeable cuticle, we analyzed the CO2 assimilation rate of lacs2-3, one of the mutants with a high cuticle permeability. The CO2 assimilation of lacs2-3 was higher than that of wild type (Fig. 3A). This result is consistent with the data of extra1, strengthening our hypothesis that the increased cuticle permeability enhances CO2 uptake efficiency.
There are substantial differences in cuticle permeability to water vapor and CO2 in the extra1 mutant, consistent with the previous reports of grape and sunflower (Boyer et al., 1997; Boyer, 2015a, 2015b). The extra1 mutant showed such a remarkably higher transpiration rate (Fig. 1D) and water loss (Supplemental Fig. S4) that the effect of stomatal opening and closure seemed negligible. On the other hand, the CO2 assimilation rate of extra1 was affected by stomatal closure by ABA (Fig. 3, B and D). These results show that the CO2 uptake through cuticles is inefficient from the perspective of water use.
We investigated the stomatal aperture immediately after measuring the CO2 assimilation rate in each accession to evaluate, as concretely as possible, the extent of increase in cuticle permeability needed to affect the CO2 assimilation rate. The differences in CO2 assimilation rates were 2.13 × 10−4 μmol plant–1 s–1 (per individual plant) and 0.36 μmol m–2 s–1 (per projected leaf area) between wild type and extra1, and 1.01 × 10−4 μmol plant–1 s–1 (per individual plant) and 0.18 μmol m–2 s–1 (per projected leaf area) between wild type and slac1-2 (Fig. 3A; Supplemental Table S1). The difference of stomatal width/length between wild type and slac1-2 was 0.18 (Fig. 3C). These data imply that, under the present measurement conditions, the increase in CO2 assimilation rate caused by the high cuticle permeability in extra1 was approximately twice that caused by the increase in the stomatal width/length by the 0.18 difference. To evaluate the CO2 assimilation rates correctly, the data from an empty chamber was subtracted from all the data of CO2 assimilation rates. We also confirmed that slac1-2 has a normal cuticle permeability (Supplemental Fig. S6) and that there is no significant difference in SD among the accessions (Supplemental Fig. S7).
Our results showing that the CO2 uptake efficiency via a permeable cuticle is higher than that via the stomatal pore agreed with the previous reports on the difference in the ratio of conductances of water vapor and CO2 between open stomata and the cuticle (Boyer et al., 1997; Boyer, 2015a, 2015b). The ratio between conductances of water vapor and CO2 in open stomata was ∼1.6, and the ratio in the cuticle was between 20 and 40 in sunflower and grape; therefore, cuticle conductance of CO2 is lower by one order of magnitude than stomatal conductance of CO2 when using water diffusions as a reference, at least in these plants. Our study confirmed the previous reports in a different way and provided supplementary evidence to support the findings of these reports.
It is worth mentioning that the ABA-treated wild type had a considerable CO2 assimilation rate despite the stomata being hardly open (Fig. 3, B and D). This might have been caused by CO2 uptakes through immature stomata, slightly open stomata, or cuticles. Previous work by Kerstiens (1995) has also shown a possibility of gas leaks through stomata may exist even when stomata are closed. We must also consider the possibility that the cuticles of wild-type plants grown under higher-humidity conditions are more permeable to CO2 than those grown under lower-humidity conditions. For example, plants grown at high humidity usually develop softer cuticles and are less resistant to herbicide penetration (Varanasi et al., 2016). In addition, stomatal closure induces an increase in turgor, which increases cuticle conductance, as reported by Boyer (2015b).
CONCLUSION
Taken together, our results show that increased permeability of cuticle increases the efficiency of CO2 uptake more than increased stomatal opening in Arabidopsis. However, it is worth mentioning that our results have been obtained from plants grown under high-humidity conditions in the lab. Therefore, cuticle mutants are unlikely to increase agricultural production. However, this study serves as a proof of concept and provides insight into the extent to which cuticles inhibit CO2 uptake efficiency in plants. Increasing the permeability of cuticles is one way of increasing this efficiency. Further studies that explore this enhancement under varying environmental conditions could possibly aid in better understanding, and extend such insight to regular agricultural production.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) wild-type accessions Columbia (Col-0), Landsberg erecta (Ler), and slac1-2 mutant (Negi et al., 2008; Vahisalu et al., 2008), were obtained from our laboratory stock. The lacs2-3 mutant was supplied by the Arabidopsis Biological Resource Center (Ohio State University). The plants of extra1, slac1-2, and lacs2-3 were derived from the Col-0 ecotype.
The Arabidopsis seeds were surface-sterilized, plated on Murashige and Skoog (MS) medium (Murashige and Skoog, 1962) supplemented with 1.0% (w/v) Suc and 0.5% (w/v) gellan gum, and maintained at 4°C for 3 d. Next, the plants were grown in a growth chamber (LPH-1.5PH-NC; NK System) at 22°C, 60% of RH, and at continuous light condition with a light intensity of 30 μmol m–2 s–1. We confirmed that the water vapor was saturated in the MS medium plates where plants were grown using a hygrometer (the data logger TR-74Ui with a high precision temperature and humidity sensor THA-3151; T&D Corp.). The seedlings at 13 d after sowing (DAS) were transplanted into a new MS medium plate, and the seedlings at 19 to 20 DAS were transplanted into pots filled with a mixture of vermiculite and perlite supplemented with mineral nutrients. Plants at various DAS were used for different experiments as indicated: 20 DAS for the TEM observation; 24 DAS for thermal imaging, water loss analysis, and toluidine blue staining; 23 to 24 DAS for the measurements of stomatal morphological traits shown in Supplemental Figure S3, C to E; 23 to 25 DAS for the measurements of stomatal aperture; 22 to 27 DAS for the measurements of transpiration rate; and 24 to 28 DAS for the acetyl-CoA quantitative analysis. For the analyses of dry weight, carbon and nitrogen content, CO2 assimilation rate, and photosynthetic parameters, the Arabidopsis seeds were plated on MS medium without Suc. The seedlings at 10 DAS were transplanted into a new MS medium plate without Suc. Plants at 20 DAS were used for the SD measurement shown in Supplemental Figure S7; plants at 20 to 23 DAS were measured for the analyses of CO2 assimilation rate and stomatal aperture in Figure 3; 24-DAS plants were used for the measurements of dry weight and carbon and nitrogen content; and 22 to 25 DAS plants were used for the analysis of quantum yield of PSII. Unless otherwise noted, the extra1 mutant measured in this study was backcrossed with Col-0 wild type twice.
Gene Mapping and Cloning
First, the causal gene of extra1 was roughly mapped to chromosome 1 between two simple sequence length polymorphism markers, NGA63 and F5I14, using 20 Ler × extra1 F2 plants. Then, PCR-based genotyping was used for fine mapping of the causative mutation in extra1. Using further simple sequence length polymorphism and cleaved smplified polymorphic sequence or derived cleaved amplified polymorphic sequence markers and a population of 192 F2 plants, we were able to map the gene to within a 353-kb genomic region between 13,493 and 13,846 kb. Candidate genes in this region were sequenced with a HiSeq2000 (Illumina) and aligned with the Col-0 wild-type sequence (The Arabidopsis Information Resource 10, ftp://ftp.arabidopsis.org/home/tair/home/tair/Sequences/whole_chromosomes/) using the tool BWA V0.6.2 (BioWeb). The sequencing was performed by Macrogen Japan.
Construction of Binary Vectors for Plant Transformation
To construct a binary vector for a complementarity assay in extra1, the genomic region of ACC1, including a 2-kb promoter and 900-bp 3′UTR, was PCR-amplified from genomic DNA in halves using the oligonucleotide primers (the former half of the genomic region, 5′-ATCCCGGGCTATCATTTGCTTTGTTCGG-3′ and 5′-CATCAGGATCCTCACTTGTC-3′; and the latter half of the genomic region, 5′-TGTGACAAGTGAGGATCCTG-3′ and 5′-GCACGCACTAGTGCGTGATGTACAAACACAGG-3′) and was then inserted into the pBluescript KS(+) vector at the XmaI-SpeI sites. The XmaI-SpeI fragments were then inserted into the binary vector pPZP2H-lac. To construct a binary vector for GUS staining, the ACC1 promoter was PCR-amplified from genomic DNA using the oligonucleotide primers (5′-CGAGGATCCCCCAACCTTGTGTCAACGAC-3′ and 5′-GCCCGGGTGTCACTGCCCTTTAAGCTT-3′) and was inserted into the pBI221 vector at the BamHI-XmaI sites. The BamHI-XmaI fragments were then inserted into the GUS gene fusion vector pBI101. Transgenic Arabidopsis plants were generated by Agrobacterium tumefaciens-mediated transformation. The analyses were carried out using the homozygous T3 seeds (for the complementarity assay) or T4 seeds (for the GUS staining) of the transgenic plants.
Thermal Imaging
Plants were transferred to a custom-made growth cabinet equipped with an automatic CO2 control unit (TMC-LW1208A/K; TM Systems) and incubated under the conditions of constant white light of 100 μmol m–2 s–1 at 22°C and 400 μL L–1 [CO2] for 1 h. The measurement was under a relatively low RH (40%) because higher-humidity conditions suppress transpiration of plants. Thermal images were captured using a thermography camera (InfReC Thermography R300; NEC Avio Infrared Technologies) and the program InfRec Analyzer NS9500 Standard (NEC Avio Infrared Technologies).
Toluidine Blue Staining
Toluidine blue staining was performed as described in Tanaka et al. (2004), with minor modifications. After 5 min of staining by 0.1% (w/v) toluidine blue, Arabidopsis aerial parts were rinsed with water and then photographed floating on the water.
Quantitative Analysis of Acetyl-CoA
The acetyl-CoA content in leaves was determined with the PicoProbe Acetyl CoA Fluorometric Assay Kit (BioVision).
Measurements of Stomatal Morphological Traits
Stomatal morphological traits were analyzed as described in Monda et al. (2016). Abaxial leaf epidermis samples from the fifth or sixth leaves of plants were used for the measurements. The stomatal number per 1 mm2 was counted for the determination of SD. The SD for each individual plant was calculated from 10 or more of random epidermal parts (each measuring 0.15 mm2). The average of SD for each accession was calculated from 23 or more individual plants per accession (Supplemental Fig. S3 C) or from eight or more individual plants per accession (Supplemental Fig. S7). The SI (stomatal numbers/the total numbers of stomata and pavement cells) for each individual plant was calculated from five random epidermal parts (each measuring 0.15 mm2). The average SI for each accession was calculated from eight individual plants per accession. The GCL for each individual plant was calculated from 19 or more of stomata. The average GCL for each accession was calculated from 23 or more individual plants per accession. The SD, SI, and GCL values were analyzed from photographs of peels taken with an 8-inch tablet monitor attached to a light microscope (model no. d-EL401; Uchida Yoko).
Measurement of Stomatal Aperture
Plants used to measure the [CO2] response were incubated in the growth cabinet under constant white light conditions of 100 μmol m–2 s–1 at 22°C. The measurements were under high RH (80%) to avoid low-humidity stress. The plants were incubated at 700 μL L–1 [CO2] for 2 h after incubation at 400 μL L–1 [CO2] for 2 h. The abaxial epidermis of rosette leaves was peeled at each [CO2] condition by using Scotch tape and photographed. In the light response measurements, mature rosette leaves were detached and soaked in test medium containing 30 mm of KCl, 10 mm of MES-KOH at pH 6.15, and 0.1 mm of CaCl2. The plants were incubated in dark conditions for 2 h after incubation in light conditions of 80 μmol m−2 s–1 for 3 h. The peeling of epidermis and the measurement of stomatal aperture were performed in a way similar to that of the CO2 response measurement. Stomatal apertures were analyzed from photographs of peels taken with a digital camera attached to a microscope (model no. IX71; Olympus). The extra1 mutant used for the analyses was backcrossed with Col-0 wild type once. We also measured stomatal apertures just after measuring CO2 assimilation rate. The stomatal apertures were analyzed from photographs of peels taken with an 8-inch tablet monitor attached to a light microscope (catalog no. d-EL401; Uchida Yoko). All measurements were done within 15 min after peeling to avoid changing the stomatal apertures. Immature stomata were excluded from the measurements.
Water Loss Analysis
A stock solution of 100 mm of ABA in DMSO was added to the test medium containing 10 mm of KCl, 10 mm of MES-KOH at pH 6.15, and 0.1 mm of CaCl2. DMSO-only was added as a mock. The final concentrations of ABA and DMSO were 100 μm and 0.1% (v/v), respectively. The shoots of wild-type and extra1 plants were sprayed with ABA or mock. After 16 h, the shoots were detached from their roots and immediately weighed. Each shoot was then placed on a petri dish covered with filter paper. The petri dishes were incubated in the custom-made growth cabinet under conditions of constant white light of 80 μmol m–2 s–1 at 22°C, 60% RH, and 400 μL L–1 [CO2]. Each shoot was weighed at the designated times.
GUS Staining
Histochemical staining of GUS activity in the ACC1pro::GUS transformants was performed as described in Negi et al. (2008).
Measurement of Transpiration Rate and CO2 Assimilation Rate
Transpiration rate and CO2 assimilation rate were measured using a portable gas exchange fluorescence system (model no. GFS-3000; Heinz Walz) equipped with a model no. 3010-A Arabidopsis chamber. Measurements were performed on the whole aerial part of each plant. The leaf temperature throughout the measurements was regarded as very close to the cuvette temperature. Transpiration rates were measured under a relatively low RH (40%) because higher-humidity conditions suppress transpiration of plants. To avoid leakage of air, a plastic sheet was inserted between the aerial plant part and the pot, and the hole in the sheet was filled with petrolatum. The flow rates (750 μmol s–1), the cuvette temperature (22°C), and the light intensity (150 μmol m–2 s–1) were kept constant throughout the measurement of the transpiration rate.
To avoid drought and light stress, the CO2 assimilation rates were measured under higher-humidity conditions (95% RH), lower flow rates (600 μmol s–1), and a weaker light intensity (60 μmol m–2 s–1). As a wind cover, a plastic petri dish, which was turned over and tilted, covered each plant in the measuring chamber. The petri dish did not decrease the light intensity from the light box of GFS-3000. The data with an empty chamber was subtracted from all the data of CO2 assimilation rates. For the analysis of ABA response, a stock solution of 1 m of ABA in DMSO was added to distilled water. Only DMSO was added as a mock. The final concentrations of ABA and DMSO were 1 mm and 0.1% (v/v), respectively. The ABA or mock solution were added into the MS medium where plants were grown. The CO2 assimilation rate was measured 2 h after the beginning of the experiment.
TEM Observation
The samples of leaf tissues for TEM were fixed in phosphate-buffered 2% (w/v) glutaraldehyde, and subsequently postfixed in 2% (w/v) osmium tetra-oxide for 3 h in the ice bath. The specimens were then dehydrated in graded ethanol and embedded in epoxy resin. The sample preparation and TEM observations were performed by the Hanaichi Ultrastructure Research Institute.
Measurement of Dry Weight
As mentioned above, 24 DAS-plants grown in MS medium without Suc were used for the measurement. The plants were stripped from the MS medium and the shoots and roots were separated. They were weighed using a precision balance, after being oven-dried for 2 d.
Analyses of Carbon and Nitrogen Contents
Post dry-weight measurement, the plants were heated in a microwave oven for 1 min and in an oven set at 70°C for 2 h, and then incubated in a vacuum for 2 h. After incubation, the shoots were homogenized with stainless beads for 2 min using a model no. BMS-A15SR ShakeMaster (Bio Medical Science). The homogenized samples were used for subsequent analyses.
The carbon and nitrogen contents were measured using the elemental analyzers (Yanaco CHN Corder MT-5 and MT-6; Yanaco Apparatus Development Laboratory). The analyses of carbon and nitrogen contents were performed by The Service Center of the Elementary Analysis of Organic Compounds, Faculty of Science, Kyushu University.
Measurement of the Quantum Yield of PSII
The quantum yields of PSII [Y(II)] were measured using an Imaging-PAM M series MAXI version (Heinz Walz). The plants in plastic petri dishes were used for the analysis. The values of Y(II) were measured every 30 s. Room temperature (22°C) was kept constant throughout the measurement.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: ACC1, AT1G36160 (gene ID: 840521); SLAC1, AT1G12480 (gene ID: 837805); and LACS2, AT1G49430 (gene ID: 841367).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Tissues expressing the ACC1pro::GUS reporter gene fusion.
Supplemental Figure S2. The comparison of leaf temperature and cuticle permeability among wild type, extra1, and lacs2-3.
Supplemental Figure S3. The stomatal characteristics of extra1 are normal.
Supplemental Figure S4. The extra1 mutant shows excessive water loss independent of stomatal movement.
Supplemental Figure S5. The integrated value of Y(II) of wild type and extra1 for first or sixth fluctuating light cycles in Figure 6.
Supplemental Figure S6. The slac1-2 mutant has a normal cuticle impermeability.
Supplemental Figure S7. The stomatal densities are similar among wild type, slac1-2, lacs2-3, and extra1.
Supplemental Table S1. Projected leaf area and CO2 assimilation rate per projected leaf area.
Acknowledgments
We thank Hitoshi Sakakibara for quantitative analysis of ABA for mutant screening, Fumitaka Konishi and Misato Aikawa for their help in identifying the causal gene of the extra1 mutant, and Naomi Kawahara for her skillful assistance in sample preparation.
Footnotes
This work was supported by the Japan Society for the Promotion of Science (KAKENHI grant nos. JP20K15820 to K.M., JP18K06293 to J.N., JP17H05718 and JP19H04718 to I.T., JP16H06552, JP18H02185, JP20H05687, and JP18KK0170 to W.Y., and JP20H03279 to K.I.), the Japan Science and Technology Core Research for Evolutional Science and Technology (grant no. JPMJCR15O5 to K.I.), and the Kyushu University Qdai-jump Research Program (grant no. 01325 to A.M.).
References
- Amid A, Lytovchenko A, Fernie AR, Warren G, Thorlby GJ(2012) The sensitive to freezing3 mutation of Arabidopsis thaliana is a cold-sensitive allele of homomeric acetyl-CoA carboxylase that results in cold-induced cuticle deficiencies. J Exp Bot 63: 5289–5299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud S, Guyon V, Kronenberger J, Wuillème S, Miquel M, Caboche M, Lepiniec L, Rochat C(2003) Multifunctional acetyl-CoA carboxylase 1 is essential for very long chain fatty acid elongation and embryo development in Arabidopsis. Plant J 33: 75–86 [DOI] [PubMed] [Google Scholar]
- Bessire M, Chassot C, Jacquat AC, Humphry M, Borel S, Petétot JM, Métraux JP, Nawrath C(2007) A permeable cuticle in Arabidopsis leads to a strong resistance to Botrytis cinerea. EMBO J 26: 2158–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JS.(2015a) Impact of cuticle on calculations of the CO2 concentration inside leaves. Planta 242: 1405–1412 [DOI] [PubMed] [Google Scholar]
- Boyer JS.(2015b) Turgor and the transport of CO2 and water across the cuticle (epidermis) of leaves. J Exp Bot 66: 2625–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JS, Wong SC, Farquhar GD(1997) CO2 and water vapor exchange across leaf cuticle (epidermis) at various water potentials. Plant Physiol 114: 185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt M, Riederer M(2006) Cuticular transpiration In Riederer M, and Müller C, eds, Biology of the Plant Cuticle. Blackwell Publishing, Oxford, United Kingdom, pp 292–311 [Google Scholar]
- Domínguez E, Heredia-Guerrero JA, Heredia A(2011) The biophysical design of plant cuticles: An overview. New Phytol 189: 938–949 [DOI] [PubMed] [Google Scholar]
- Faralli M, Lawson T(2020) Natural genetic variation in photosynthesis: An untapped resource to increase crop yield potential? Plant J 101: 518–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DO, Scurlock JMO, Bolhar-Nordenkampf HR, Leegood RC, Long SP(1993) Photosynthesis and Production in a Changing Environment: A Field and Laboratory Manual. Springer Science & Business Media, Berlin, Germany [Google Scholar]
- Holloway PJ.(1982) Structure and histochemistry of plant cuticular membranes: An overview In Cutler DF, Alvin KL, and Price CE, eds, The Plant Cuticle. Academic Press, London, pp 1–32 [Google Scholar]
- Jeffree CE.(1996) Structure and ontogeny of plant cuticles In Kerstiens G, ed, Plant cuticles: An integrated functional approach. Bios Scientific Publishers, Oxford, United Kingdom, pp 33–82 [Google Scholar]
- Kaiser E, Morales A, Harbinson J(2018) Fluctuating light takes crop photosynthesis on a rollercoaster ride. Plant Physiol 176: 977–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstiens G.(1995) Cuticular water permeance of European trees and shrubs grown in polluted and unpolluted atmospheres, and its relation to stomatal response to humidity in beech (Fagus sylvatica L.). New Phytol 129: 495–503 [Google Scholar]
- Kerstiens G.(1996a) Cuticular water permeability and its physiological significance. J Exp Bot 47: 1813–1832 [Google Scholar]
- Kerstiens G.(1996b) Signalling across the divide: A wider perspective of cuticular structure-function relationships. Trends Plant Sci 1: 125–129 [Google Scholar]
- Kimura H, Hashimoto-Sugimoto M, Iba K, Terashima I, Yamori W(2020) Improved stomatal opening enhances photosynthetic rate and biomass production in fluctuating light. J Exp Bot 71: 2339–2350 [DOI] [PubMed] [Google Scholar]
- Kosma DK, Bourdenx B, Bernard A, Parsons EP, Lü S, Joubès J, Jenks MA(2009) The impact of water deficiency on leaf cuticle lipids of Arabidopsis. Plant Physiol 151: 1918–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson T, Kramer DM, Raines CA(2012) Improving yield by exploiting mechanisms underlying natural variation of photosynthesis. Curr Opin Biotechnol 23: 215–220 [DOI] [PubMed] [Google Scholar]
- Lü S, Zhao H, Parsons EP, Xu C, Kosma DK, Xu X, Chao D, Lohrey G, Bangarusamy DK, Wang G, et al. (2011) The glossyhead1 allele of ACC1 reveals a principal role for multidomain acetyl-coenzyme A carboxylase in the biosynthesis of cuticular waxes by Arabidopsis. Plant Physiol 157: 1079–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr H, Schopfer P(1995) Physiology of xylem transport In Mohr H, and Schopfer P, eds, Plant Physiology. Springer, Berlin, Heidelberg, Germany, pp 467–486 [Google Scholar]
- Monda K, Araki H, Kuhara S, Ishigaki G, Akashi R, Negi J, Kojima M, Sakakibara H, Takahashi S, Hashimoto-Sugimoto M, et al. (2016) Enhanced stomatal conductance by a spontaneous Arabidopsis Tetraploid, Me-0, results from increased stomatal size and greater stomatal aperture. Plant Physiol 170: 1435–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F(1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Negi J, Hashimoto-Sugimoto M, Kusumi K, Iba K(2014) New approaches to the biology of stomatal guard cells. Plant Cell Physiol 55: 241–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi J, Matsuda O, Nagasawa T, Oba Y, Takahashi H, Kawai-Yamada M, Uchimiya H, Hashimoto M, Iba K(2008) CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 452: 483–486 [DOI] [PubMed] [Google Scholar]
- Riederer M, Müller C(2006) Biology of the Plant Cuticle. Blackwell Publishing, Oxford, United Kingdom [Google Scholar]
- Riederer M, Schreiber L(2001) Protecting against water loss: Analysis of the barrier properties of plant cuticles. J Exp Bot 52: 2023–2032 [DOI] [PubMed] [Google Scholar]
- Schnurr J, Shockey J, Browse J(2004) The acyl-CoA synthetase encoded by LACS2 is essential for normal cuticle development in Arabidopsis. Plant Cell 16: 629–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber L, Schönherr J(2009) Water and Solute Permeability of Plant Cuticles: Measurement and Data Analysis. Springer, Berlin, Germany [Google Scholar]
- Tanaka T, Tanaka H, Machida C, Watanabe M, Machida Y(2004) A new method for rapid visualization of defects in leaf cuticle reveals five intrinsic patterns of surface defects in Arabidopsis. Plant J 37: 139–146 [DOI] [PubMed] [Google Scholar]
- Vahisalu T, Kollist H, Wang YF, Nishimura N, Chan WY, Valerio G, Lamminmäki A, Brosché M, Moldau H, Desikan R, et al. (2008) SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature 452: 487–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varanasi A, Prasad PVV, Jugulam M(2016) Impact of climate change factors on weeds and herbicide efficacy In Sparks DL, ed, Advances in Agronomy, Vol Vol 135 Elsevier Academic Press, San Diego, CA, pp 107–146 [Google Scholar]
- Willmer C, Fricker M(1996) Stomata, 2nd ed Springer Science & Business Media, Berlin, Germany [Google Scholar]
- Yamori W, Makino A, Shikanai T(2016) A physiological role of cyclic electron transport around photosystem I in sustaining photosynthesis under fluctuating light in rice. Sci Rep 6: 20147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeats T, Rose J(2013) The formation and function of plant cuticles. Plant Physiol 163: 5–20 [DOI] [PMC free article] [PubMed] [Google Scholar]