The auxin-like metabolite melatonin regulates the production of citrus phytohormones upon infection with the huanglongbing pathogen and infestation with its vector.
Abstract
Huanglongbing (HLB) is a devastating citrus disease worldwide that is putatively caused by Candidatus Liberibacter asiaticus and transmitted by Diaphorina citri. Melatonin is a ubiquitously distributed auxin-like metabolite found in both prokaryotes and eukaryotes. In this study, we used integrative metabolomic and transcriptomic approaches to investigate the potential role of melatonin in citrus response against HLB and to understand the relationships between melatonin and the stress-associated phytohormones at molecular and metabolic levels. Melatonin was detected in the leaves of Valencia sweet orange (Citrus sinensis) after derivatization with N-methyl-N-trimethylsilyltrifluoroacetamide using a targeted gas chromatography-mass spectrometry running in selective ion monitoring mode-based method. Ca. L. asiaticus infection and D. citri infestation significantly increased endogenous melatonin levels in Valencia sweet orange leaves and upregulated the expression of its biosynthetic genes (CsTDC, CsT5H, CsSNAT, CsASMT, and CsCOMT). However, infection with Ca. L. asiaticus had a greater effect than did infestation with D. citri. Melatonin induction was positively correlated with salicylic acid content, but not that of trans-jasmonic acid. Moreover, melatonin supplementation enhanced the endogenous contents of the stress-associated phytohormones (salicylates, auxins, trans-jasmonic acid, and abscisic acid) and the transcript levels of their biosynthetic genes. Furthermore, melatonin supplementation diminished the Ca. L. asiaticus titer within the infected leaves, which suggests that melatonin might play an antibacterial role against this bacterium and gram-negative bacteria in general. These findings provide a better understanding of the melatonin-mediated defensive response against HLB via modulation of multiple hormonal pathways. Understanding the role of melatonin in citrus defense to HLB may provide a novel therapeutic strategy to mitigate the disease.
Melatonin (N-acetyl-5-methoxytryptamine) is a ubiquitously distributed compound, found in both prokaryotes such as bacteria and eukaryotes including fungi, algae, plants, and animals (Hardeland et al., 2011; Lee et al., 2014; Arnao and Hernández-Ruiz, 2015). Melatonin was discovered first in the kingdom Animalia in 1958 (Lerner et al., 1958) and then in Plantae in 1995 (Dubbels et al., 1995; Hattori et al., 1995). Melatonin plays pleiotropic biological roles in both animals and plants. In mammals, it acts as a neurohormone, secreted by the pineal gland, which regulates numerous physiological processes (Jan et al., 2009; Carrillo-Vico et al., 2013; Cipolla-Neto et al., 2014). It acts as a sleep regulator (Jan et al., 2009), an immunomodulatory molecule (Galano et al., 2011; Carrillo-Vico et al., 2013), a regulator of the circadian temporal internal order (Cipolla-Neto et al., 2014), and a natural antioxidant (Galano et al., 2011). In Plantae, melatonin (also known as phytomelatonin) modulates many physiological functions. For example, it stimulates plant growth and development in dicots (Hernández-Ruiz et al., 2004) and monocots (Hernández-Ruiz et al., 2005). Melatonin promotes adventitious and lateral root regeneration and elongation (Arnao and Hernández-Ruiz, 2007; Sarropoulou et al., 2012; Zhang et al., 2013). Additionally, it has a key role in seedling growth enhancement (Byeon et al., 2014), flowering delay (Kolar et al., 2003; Byeon and Back, 2014), and fruit ripening and fruit quality improvement (Sun et al., 2015).
As a secondary metabolite, melatonin is implicated in tolerance to abiotic stresses such as drought (Zhang et al., 2013; Wei et al., 2015), salinity (Zhang et al., 2014; Wei et al., 2015), freezing (Uchendu et al., 2013), and high temperature (Tiryaki and Keles, 2012). On the contrary, the protective role of melatonin against biotic stresses in plants is still unclear and not well studied. A few reports discussed the mechanisms of melatonin-dependent pathways in the plant response to phytopathogen infection (Yin et al., 2013; Lee et al., 2014, 2015; Shi et al., 2015; Lee and Back, 2016). For example, in apple trees (Malus prunifolia), exogenous melatonin treatment improved resistance to Diplocarpon mali, the fungal pathogen of Marssonina apple blotch (Yin et al., 2013). Additionally, melatonin and its derivatives, N-acetylserotonin and 2-hydroxymelatonin, improved plant resistance against the bacterial pathogen Pseudomonas syringae pv tomato strain DC3000 (Pst-DC3000) in Arabidopsis (Arabidopsis thaliana; Lee et al., 2014, 2015; Shi et al., 2015; Lee and Back, 2016) and Nicotiana benthamiana (Lee et al., 2014; Lee and Back, 2016). The protective role of melatonin is associated with the pathogenesis-related proteins (PRs; Yin et al., 2013; Lee et al., 2014; Shi et al., 2015), mitogen-activated protein kinases (MAPKs; Lee and Back, 2016), C-REPEAT-BINDING FACTORS/DROUGHT RESPONSE ELEMENT-BINDING1 FACTORS (Shi et al., 2015, 2016), nitric oxide (NO; Shi et al., 2015), and phytohormones such as salicylic acid (SA; Lee et al., 2014, 2015)
Phytohormones play key roles in host responses to a wide range of pathogen infections, insect attacks, and abiotic stresses (Bari and Jones, 2009). SA is associated with plant defense responses to biotrophic and hemibiotrophic pathogens and plays a key role in the establishment of systemic acquired resistance (Hatcher et al., 2004; Glazebrook, 2005; Bari and Jones, 2009). NPR1, a key signaling molecule in the SA-mediated pathway, acts as a receptor for SA (Wu et al., 2012). On the other hand, the jasmonic acid/ethylene (JA/ET)-mediated pathway is involved in defense responses against necrotrophic pathogens and insect herbivory (Mur et al., 2006; Bari and Jones, 2009). Many signaling molecules are implicated in the JA/ET-mediated pathways, such as MAPKs (Turner et al., 2002; Meldau et al., 2012) and ETHYLENE INSENSITIVE2 (which interacts with ETHYLENE RECEPTOR1; Bisson et al., 2009).
Huanglongbing (HLB) is a devastating disease causing great losses in citrus industries worldwide (Bové, 2006). HLB is associated with Candidatus Liberibacter species, a fastidious, gram-negative, phloem-limited, and nonculturable α-proteobacterium (Bové, 2006; Gottwald, 2010). Taxonomically, three bacterial species have been identified as causal pathogens of HLB based on the characteristic 16S rDNA sequences and their geographic distribution (Bové and Ayres, 2007; Tatineni et al., 2008; Gottwald, 2010; Wang and Trivedi, 2013). Candidatus Liberibacter asiaticus is a heat-tolerant species spreading in the Arabian Peninsula, Africa, Asia, Brazil, and North America; Candidatus Liberibacter americanus is a heat-tolerant species spreading only in Brazil; and Candidatus Liberibacter africanus is a heat-sensitive species spreading in numerous countries in Africa (Bové and Ayres, 2007; Tatineni et al., 2008; Gottwald, 2010; Wang and Trivedi, 2013). The three Candidatus Liberibacter species are transmitted by two species of citrus psyllids. Both Ca. L. asiaticus and Ca. L. americanus are typically transmitted by the Asian citrus psyllid Diaphorina citri, while Ca. L. africanus is transmitted by Trioza erytreae (Bové and Ayres, 2007; Gottwald, 2010; Wang and Trivedi, 2013).
Citrus plants have developed an innate immune system with multilayered complex defense responses to mediate the harmful effects of Ca. L. asiaticus infection and its vector, D. citri. These defense mechanisms are not only mechanical but also include the accumulation of defense molecules, alteration of the plant signaling system, changes in primary and secondary metabolites, and other biochemical and physiological modifications. Our previous studies showed that several leaf metabolites are involved directly or indirectly in plant defense response against HLB. These metabolites include volatile organic compounds (Hijaz et al., 2013), proteinogenic amino acids (Killiny and Hijaz, 2016; Killiny and Nehela, 2017a), nonproteinogenic amino acids and polyamines (Nehela and Killiny, 2019), organic and fatty acids (Killiny and Nehela, 2017a), tricarboxylic acid-associated compounds and γ-aminobutyrate shunt (Nehela and Killiny, 2019), phytohormones (Nehela et al., 2018), leaf pigments (Killiny and Nehela, 2017b), and other secondary metabolites. However, the interaction between these metabolites is poorly understood. We believe that the identification of these compounds and understanding the relationships between them will further help us to clarify the underlying molecular mechanisms of the citrus immunity system. Although the role of phytohormones, such as SA and JA, in citrus response to HLB is well reported, the potential role of melatonin in citrus response against HLB is poorly studied, and its role in citrus immunity/response remains unclear. Furthermore, the mechanisms behind the melatonin-mediated defensive response are inadequately explored.
Although some previous studies reported that exogenous melatonin application may trigger the plant defense responses against phytopathogenic bacteria (Lee et al., 2014, 2015; Qian et al., 2015; Shi et al., 2015; Zhao et al., 2015; Lee and Back, 2016, 2017a; Wei et al., 2018a; Chen et al., 2019b; Xian et al., 2020), none of these studies have investigated its relationships with the major groups of stress-associated phytohormones, particularly SA and trans-jasmonic acid (tJA). In this study, we used integrative metabolomic and transcriptomic approaches to (1) investigate the potential role(s) of melatonin in citrus response to different biotic stressors, including the phytopathogenic bacterium Ca. L. asiaticus and its insect vector D. citri; (2) provide a better understanding of the melatonin-mediated defensive response and its relationships with the major groups of stress-associated phytohormones, including salicylates (SAs), auxins, tJA, and abscisic acid (ABA), and the gene expression patterns of their biosynthetic genes; and (3) elucidate the antibacterial role of exogenous melatonin against Ca. L. asiaticus within HLB-infected Valencia sweet orange (Citrus sinensis) plants. We believe that melatonin is not only involved in the induction of both the SA-mediated pathway against Ca. L. asiaticus and the JA-mediated pathway against D. citri but also might be involved with other hormonal pathways, such as auxins and ABA, to enhance the disease resistance for HLB. Understanding the role of melatonin in citrus defense to HLB may provide a novel therapeutic strategy to mitigate the disease.
RESULTS
Detection of Melatonin in Valencia Sweet Orange Leaves Using GC-MS-SIM
Melatonin was detected in the leaves of Valencia sweet orange after derivatization with 50 μL of N-methyl-N-trimethylsilyltrifluoroacetamide (MSTFA) containing 1% (v/v) chlorotrimethylsilane at 85°C using targeted gas chromatography-mass spectrometry running in selective ion monitoring mode (GC-MS-SIM). The typical total ion current chromatograms obtained for a prepared melatonin standard of known concentration (20 ppm) and endogenous melatonin from Valencia sweet orange leaves (healthy, Ca. L. asiaticus infected, D. citri infested, and double attacked) are presented in Figure 1, A and B, respectively. The GC results for the melatonin standard in scan mode showed that the derivatization procedure gives the bis-trimethyl silyl (bis-TMS) derivative of melatonin as a final product. The mass spectrum of the bis-TMS-melatonin standard is presented in Figure 1C (in full scan mode) and Figure 1D (in SIM mode). The constituent mass-to-charge ratio (m/z) ions for melatonin are as follows: m/z M+ = 376, 304, 246, 245, 232, 202, and 160. In SIM mode, the MS spectrum profile of melatonin in Valencia sweet orange leaves (Fig. 1E) matches the MS spectrum profile of the melatonin standard (Fig. 1D). Seven ions were used for the identification of melatonin in Valencia sweet orange leaves, while the base ion 232 was used for quantification purposes. Although m/z 73 was the most abundant ion in the scan mode, it was not used for the identification or quantification of melatonin because it is a signature ion fragment for TMS derivatives and does not represent melatonin.
Figure 1.
Chromatographic characterizations of phytomelatonin detected in Valencia sweet orange leaves after infection with Ca. L. asiaticus and/or infestation with D. citri using targeted GC-MS-SIM. A, Chromatogram of melatonin authentic standard (20 ppm). B, Phytomelatonin from healthy (control), Ca. L. asiaticus-infected, D. citri-infested, or double-attacked (Ca. L. asiaticus infected and D. citri infested at the same time) Valencia sweet orange leaves. C to E, Mass spectra of melatonin authentic standard (200 ppm) in full scan mode, melatonin authentic standard (20 ppm) in SIM mode, and phytomelatonin from Valencia sweet orange leaves in SIM mode, respectively.
Ca. L. asiaticus Infection Altered the Melatonin, SA, and tJA Content of Valencia Sweet Orange Leaves
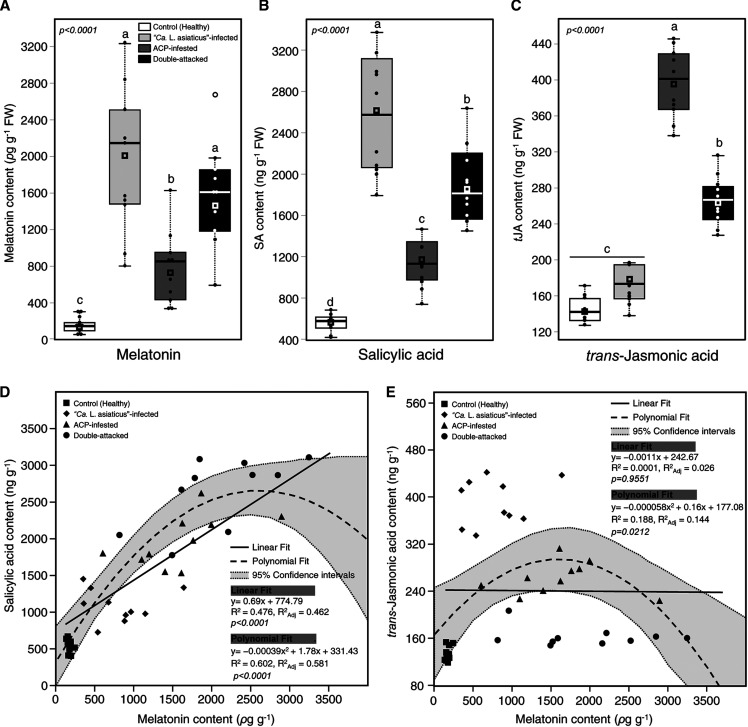
Our GC-MS results showed that both Ca. L. asiaticus infection and/or D. citri infestation significantly increased the endogenous melatonin content of Valencia sweet orange leaves, with a greater effect of Ca. L. asiaticus alone (Fig. 2A). Both Ca. L. asiaticus-infected and double-attacked trees had high concentrations of melatonin (1,922.69 ± 797.21 and 1,591.68 ± 612 pg g−1 fresh weight, respectively) without significant differences between them, followed by D. citri-infested trees (777.67 ± 404.41 pg g−1 fresh weight), which were lower in melatonin content than Ca. L. asiaticus-infected trees but higher than the control (164.50 ± 30.25 pg g−1 fresh weight; Fig. 2A).
Figure 2.
Endogenous contents of phytomelatonin, SA, and tJA detected in Valencia sweet orange leaves after infection with Ca. L. asiaticus and/or infestation with D. citri using GC-MS-SIM. A to C, Endogenous contents of phytomelatonin (pg g−1 fresh weight [FW]; n = 10), SA (ng g−1 fresh weight; n = 10), and tJA (ng g−1 fresh weight; n = 10), respectively, from healthy (control), Ca. L. asiaticus-infected, D. citri-infested, or double-attacked Valencia sweet orange leaves. Whiskers indicate the minimum and maximum values of the data, horizontal thick lines show the medians, black or white open squares signify the means, black or white dots indicate the raw data, and boxes show the interquartile ranges (25th to 75th percentiles of the data). Different letters indicate statistically significant differences among treatments, while the same letter signifies no significant differences between them using the Tukey-Kramer honestly significant difference test (Tukey’s HSD; P < 0.05). D and E, Simple linear regression and quadratic polynomial regression analyses between endogenous phytomelatonin and SA (D) and tJA (E). Black shapes represent the raw data (n = 10; see the key at the top left corner of the graph). The fitted regression line is presented as a solid line, while polynomial regression models are presented as dashed lines. The 95% confidence intervals for the estimated regression are gray shaded and edged by dotted lines. Regression equations, R2, R2adj, and P value based on the F test (P ≤ 0.05) were also obtained and are presented within the graph.
Likewise, SA has almost the same profile of melatonin, and it has been associated with citrus defense response to Ca. L. asiaticus infection (Fig. 2B). Ca. L. asiaticus-infected trees had the highest concentration of SA (2,601.22 ± 609.36 ng g−1 fresh weight), followed by double-attacked and D. citri-infested trees (1,896.37 ± 370.08 and 1,106.22 ± 227.44 ng g−1 fresh weight, respectively), which were also higher in SA content than the control (554.28 ± 88.91 ng g−1 fresh weight; Fig. 2B).
On the other hand, tJA has been associated with citrus defense response against D. citri. Infestation with D. citri significantly increased the tJA content (395.56 ± 39.38 ng g−1 fresh weight), followed by double-attacked trees (265.29 ± 28.08 ng g−1 fresh weight), while it did not affect the tJA levels in control or Ca. L. asiaticus-infected trees (138.67 ± 12.46 and 166.22 ± 16.62 ng g−1 fresh weight, respectively; Fig. 2C).
Furthermore, to understand the relationship between the endogenous melatonin content and the endogenous levels of SA and tJA, data were fitted using a simple linear regression (SLR) model and a second-degree polynomial regression model. Interestingly, the SLR showed that the endogenous melatonin content was positively correlated with the level of SA (y = 0.69x + 774.79; R2 = 0.476, R2adj = 0.462, and P < 0.0001; Fig. 2D), while no significant correlation was observed between endogenous melatonin content and tJA levels (y = −0.0011x + 242.67; R2 = 0.0001, R2adj = 0.026, and P = 0.9551; Fig. 2E).
Furthermore, to study the nonlinear phenomena between melatonin content and the levels of SA and tJA, data were fitted with a second-degree polynomial regression model. Data presented in Figure 2, D, and E, show the polynomial fit models and their 95% confidence curves for the estimated regression for both SA and tJA, respectively. Generally, the relationship between endogenous melatonin content and SA levels followed a positive and quadratic model, which were described by the equation y = −0.00039x2 + 1.78x + 331.43 (R2 = 0.602, R2adj = 0.581, and P < 0.0001; Fig. 2D). On the other hand, although the relationship between endogenous melatonin content and tJA levels followed a positive and quadratic model (y = −0.000058x2 + 0.16x + 177.08), this relationship was very weak (R2 = 0.188, R2adj = 0.144, and P < 0.0212; Fig. 2D).
Ca. L. asiaticus Infection Altered the Gene Expression of Melatonin Biosynthetic Genes in Valencia Sweet Orange Leaves
We investigated the transcript levels of 15 melatonin biosynthetic genes of citrus (Fig. 3A). Four reference/housekeeping genes, CsEF1, CsF-box, CsGAPDH, and CsSAND (Supplemental Table S1), were used for gene expression data normalization, which previously showed high stability for transcript normalization in citrus under biotic stress (Mafra et al., 2012; Wei et al., 2014a, 2014b). There were no significant differences between the normalized transcript levels using the four housekeeping genes. The differential relative expression levels of the 15 melatonin biosynthetic genes are visualized as a heat map combined with a nonstandardized two-way HCA and presented in Figure 3A. Generally, the presence of Ca. L. asiaticus induced the expression levels of all investigated genes (Fig. 3A).
Figure 3.
Relative expression of genes involved in the melatonin biosynthesis pathway in Valencia sweet orange. Two-way hierarchical cluster analysis (HCA) and its associated heat map diagram of differential nonstandardized gene expression patterns of the predicted melatonin biosynthetic genes in Valencia sweet orange leaves after infection with Ca. L. asiaticus and/or infestation with D. citri (A) or after melatonin supplementation (B) are shown. Rows represent the genes, whereas columns represent different treatments. Low expression levels are colored light blue and high expression levels are colored dark blue (see the scale at the right bottom corner of the heat map). Treatments and genes in the HCA were organized using Ward’s minimum variance method (Ward, 1963). The full list of expressed genes, names, accession numbers, and primers is available in Supplemental Table S1.
The total HCA dendrogram among treatments (presented at the bottom of Fig. 3A) showed that the transcript levels of Ca. L. asiaticus-infected and double-attacked trees were closer to each other and clustered together (the half-square Euclidean distance was less than 3; Fig. 3A). In contrast, D. citri-infested treatment was clustered with the control (healthy) trees (the half-square Euclidean distance was approximately 6).
Furthermore, the total HCA dendrogram among the tested melatonin biosynthetic genes showed that all 15 genes separated into two distinct clusters. Cluster I included the majority of studied genes (nine genes), which were expressed at higher levels in both Ca. L. asiaticus-infected and double-attacked trees, with a relative fold change greater than 4 (Fig. 3A). These genes included CsTDC1 (or CsAADC-like), CsSNAT-2, and seven CsASMT genes. Cluster II included only six genes, which were expressed at higher levels in both Ca. L. asiaticus-infected and double-attacked trees, but with a relative fold change less than 4 (Fig. 3A). Genes in cluster II included CsSNA-1, CsT5H (or CsCYP71P1), CsTDC2, and three CsCOMT genes. According to these findings, the gene expression results supported our findings from the GC-MS work.
Melatonin Supplementation Altered the Gene Expression of Melatonin Biosynthesis Genes in Valencia Sweet Orange Trees
We investigated the impact of exogenous melatonin supplementation on the transcript levels of melatonin biosynthesis genes of citrus. In general, the 15 genes studied were upregulated (up to 5.2-fold) at 72 h posttreatment (hpt) in all melatonin treatments compared with control trees (Fig. 3B). The transcript levels of melatonin biosynthesis genes were elevated by the low melatonin concentrations (0.05 and 0.1 mm), which clustered together and separately from other treatments (the half-square Euclidean distance was less than 6; Fig. 3B). Furthermore, the total HCA dendrogram among the melatonin biosynthesis genes showed that the different melatonin concentrations separated the 15 genes into four distinct clusters. Cluster I includes five genes (CsTDC1 [or CsAADC-like], CsCOMT-1, and three CsASMT1 genes), which were expressed at higher levels after the treatment of 0.1 mm melatonin (Fig. 3B). Likewise, Cluster II includes five genes (CsTDC2, CsT5H [or CsCYP71P1], CsASMT2, and two CsSNAT genes), which were expressed similarly in all treatments, but higher than the control (Fig. 3B). Cluster III includes three CsASMT genes, which were expressed similarly when the trees were treated with 0.05 or 0.1 mm melatonin, but much higher than other treatments. Cluster IV includes two CsCOMT-like genes, which were slightly induced (approximately twofold increases) after melatonin treatment (Fig. 3B). Generally, the expression levels of most, if not all, of the melatonin biosynthetic genes were increased after melatonin supplementation, particularly after the treatment with a low concentration (0.05 or 0.1 mm melatonin), which supported our GC-MS findings.
Melatonin Supplementation Enhanced the SAs Content of Valencia Sweet Orange Leaves
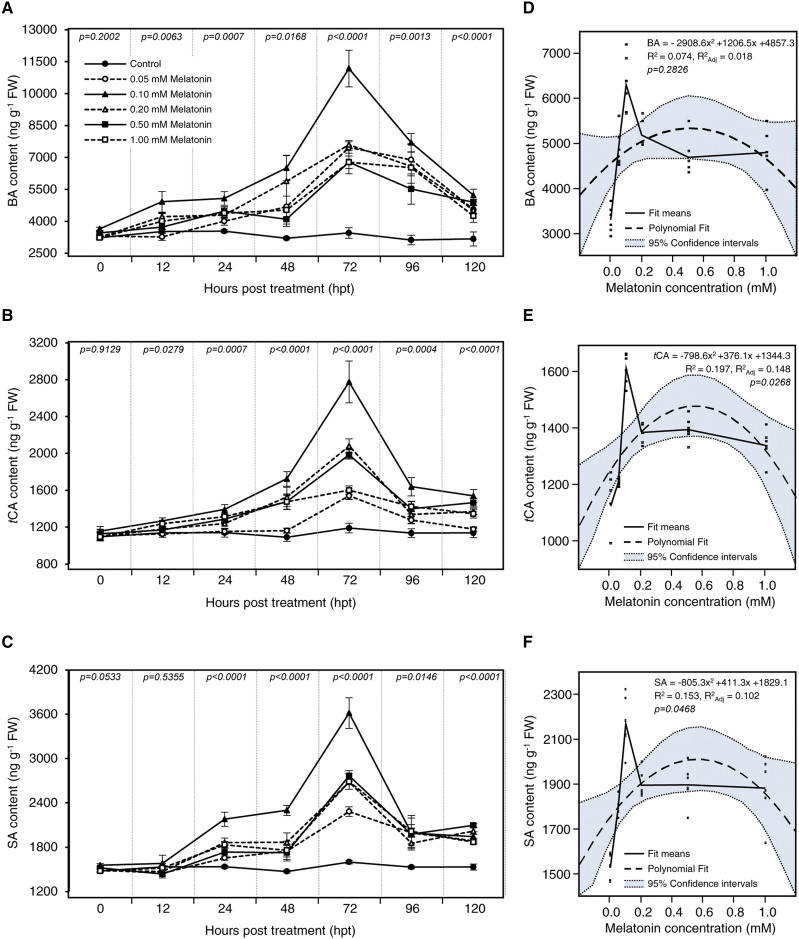
The impact of treatment with melatonin on SAs, including benzoic acid (BA), trans-cinnamic acid (tCA), and SA, was investigated. In general, at 12 hpt, the application of 0.1 mm melatonin induced the accumulation of both BA (Fig. 4A) and tCA (Fig. 4B) but not SA (Fig. 4C). In all melatonin treatments, BA, tCA, and SA began to rise markedly at 48 hpt until they reached their highest peak at 72 hpt, then plummeted when measured at 96 and 120 hpt. The trees treated with 0.1 mm melatonin had the highest BA, tCA, and SA levels throughout the experiment, particularly at 72 hpt (3.23-, 2.33-, and 2.26-fold higher, respectively; Fig. 4).
Figure 4.
Effects of exogenous melatonin supplementation on the endogenous SAs content of Valencia sweet orange. A to C, Endogenous levels of BA (A), tCA (B), and SA (C) after the exogenous melatonin treatments. Valencia sweet orange trees were treated with 150 mL of Milli-Q water (control; 0 mm melatonin) or 0.05, 0.1, 0.2, 0.5, or 1 mm melatonin. Data shown are means ± sd (n = 5). P < 0.05 indicates statistically significant differences among treatments, while P > 0.05 indicates no significant differences among them using Tukey’s HSD. D to F, Fit means models and quadratic polynomial regression analysis between exogenous melatonin concentrations and endogenous contents of BA (D), tCA (E), and SA (F). Black dots represent the raw data (n = 6). The fit means line is presented as a solid line, while polynomial regression models are presented as dashed lines. The 95% confidence intervals for the estimated regression are gray shaded and edged by dotted lines. Regression equations, R2, R2adj, and P value based on the F test (P ≤ 0.05) were also obtained and are presented within the graphs. FW, Fresh weight.
To understand the relationship between the additive melatonin and the endogenous content of different SAs, data were fitted with a second-degree polynomial regression model, because our data follow nonlinear phenomena. Data presented in Figure 4, D to F, show the fit means models, polynomial regression models, and the 95% confidence curves for the estimated regression for BA, tCA, and SA, respectively. Generally, the relationship between supplementary melatonin rates and different SAs followed a positive and quadratic model. The relationship between exogenous melatonin rates and BA is described by the equation BA = −2,908.6x2 + 1,206.5x + 4,857.3 (R2 = 0.074, R2adj = 0.018, and P = 0.2826), while for tCA it is described by tCA = −798.6x2 + 376.1x + 1,344.3 (R2 = 0.197, R2adj = 0.148, and P = 0.0268) and for SA it is described by SA = −805.3x2 + 411.3x + 1,829.1 (R2 = 0.153, R2adj = 0.102, and P = 0.0468). In addition, the fit means model for each value showed that the treatment with 0.1 mm melatonin induced the accumulation of BA (Fig. 4D), tCA (Fig. 4E), and SA (Fig. 4F) to higher levels compared with nontreated trees and other treatments, regardless of the sampling time.
Melatonin Supplementation Increased the Auxin Content of Valencia Sweet Orange Leaves
We investigated the effect of supplementary melatonin on three auxins, indole-3-acetic acid (IAA; Fig. 5A), indole-3-propionic acid (IPA; Fig. 5B), and indole-3-butyric acid (IBA; Fig. 5C). Like SA, the application of exogenous melatonin did not significantly affect any of the auxin contents at 12 hpt, while the auxin concentrations began to rise markedly at 24 hpt until the end of the experiment. Interestingly, the trees treated with 0.1 or 0.2 mm melatonin had the highest IAA and IBA levels during the experiment (Fig. 5, A and C, respectively), without significant differences between either treatment in most sampling points. On the other hand, treatment with 0.05 or 0.1 mm melatonin led to the highest IPA levels (Fig. 5B), particularly at the end of the experiment (72, 96, and 120 hpt), without significant differences between the two treatments. No noticeable reduction was recorded in the contents of the three auxins during the experiment until after 96 hpt, although some treatments showed a slight reduction after 120 hpt.
Figure 5.
Effects of exogenous melatonin supplementation on the endogenous auxins content of Valencia sweet orange. A to C, Endogenous levels of IAA (A), IPA (B), and IBA (C) after the exogenous melatonin treatments. Valencia sweet orange trees were treated with 150 mL of Milli-Q water (control; 0 mm melatonin) or 0.05, 0.1, 0.2, 0.5, or 1 mm melatonin. Data shown are means ± sd (n = 5). P < 0.05 indicates statistically significant differences among treatments, while P > 0.05 indicates no significant differences among them using Tukey’s HSD. D to F, Fit means models and quadratic polynomial regression analysis between exogenous melatonin concentrations and endogenous contents of IAA (D), IPA (E), and IBA (F). Black dots represent the raw data (n = 6). The fit means line is presented as a solid line, while polynomial regression models are presented as dashed lines. The 95% confidence intervals for the estimated regression are gray shaded and edged by dotted lines. Regression equations, R2, R2adj, and P value based on the F test (P ≤ 0.05) were also obtained and are presented within the graphs. FW, Fresh weight.
Because of the nonlinear relationship between exogenous melatonin concentrations and the endogenous content of different auxins, data were fitted using a second-degree polynomial regression model and are presented in Figure 5, D to F. Generally, the relationship between supplementary melatonin rates and different auxins followed a positive and quadratic model. The relationship between exogenous melatonin rates and IAA is described by the equation IAA = −166.6x2 + 76.4x + 283.5 (R2 = 0.139, R2adj = 0.086, and P = 0.0358), while for IPA it is described by IPA = 6x2 + 2.6x + 340.4 (R2 = 0.022, R2adj = 0.037, and P = 0.6911) and for IBA it is described by IBA = −28.3x2 + 10.9x + 346.7 (R2 = 0.173, R2adj = 0.123, and P = 0.0432). In addition, the fit means model for each value showed that the accumulation of IAA was induced by treatment with 0.1 or 0.2 mm melatonin, without significant differences between them (Fig. 5D). Similarly, the accumulation of IPA was induced by treatment with 0.05 or 0.1 mm melatonin, without significant differences between them (Fig. 5E), and IBA was induced by treatment with 0.1 mm melatonin (Fig. 5F), regardless of the sampling time.
Melatonin Supplementation Induced the Accumulation of tJA in Valencia Sweet Orange Leaves
The impact of treatment with melatonin on tJA was investigated and is presented in Figure 6A. The endogenous tJA contents increased slightly at 12 hpt, then began to increase at 24 hpt until they reached their highest peak at 72 hpt (for the lowest melatonin treatment [0.05 mm]), but it decreased thereafter. The trees treated with 0.05 mm melatonin had the highest tJA levels throughout the experiment, particularly at 72 hpt (1.52-fold increase; Fig. 6A).
Figure 6.
Effects of exogenous melatonin supplementation on the endogenous tJA content of Valencia sweet orange. A, Endogenous levels of tJA after the exogenous melatonin treatments. Valencia sweet orange trees were treated with 150 mL of Milli-Q water (control; 0 mm melatonin) or 0.05, 0.1, 0.2, 0.5, or 1 mm melatonin. Data shown are means ± sd (n = 6). P < 0.05 indicates statistically significant differences among treatments, while P > 0.05 indicates no significant differences among them using Tukey’s HSD. B, Fit means models and quadratic polynomial regression analysis between exogenous melatonin concentrations and endogenous tJA content. Black dots represent the raw data (n = 6). The fit means line is presented as a solid line, while polynomial regression models are presented as dashed lines. The 95% confidence intervals for the estimated regression are gray shaded and edged by dotted lines. Regression equations, R2, R2adj, and P value based on the F test (P ≤ 0.05) were also obtained and are presented within the graph. FW, Fresh weight.
The nonlinear relationship between exogenous melatonin concentrations and the endogenous tJA content was fitted using a second-degree polynomial regression model and is presented in Figure 6B. Generally, the relationship between supplementary melatonin rates and tJA content followed a positive and quadratic model and could be described by the equation tJA = −50.5x2 + 32.2x + 337.6 (R2 = 0.061, R2adj = 0.004, and P = 0.3537). In addition, the fit means model for each value showed that the accumulation of tJA was induced by treatment with 0.05 mm melatonin (Fig. 6B), regardless of the sampling time.
Melatonin Supplementation Increased the ABA Content of Valencia Sweet Orange Leaves
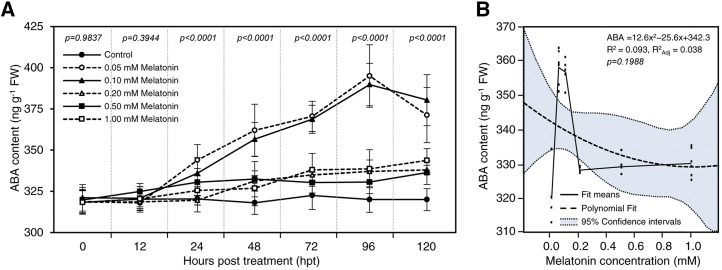
The effect of supplementary melatonin on ABA content was studied and is presented in Figure 7A. Like SA and auxins, the application of exogenous melatonin did not affect the ABA content at 12 hpt; however, the ABA endogenous levels increased significantly at 24 hpt, reaching the highest peak at 96 hpt. The trees treated with 0.05 or 0.1 mm melatonin had the highest ABA levels during the experiment (Fig. 7A), without significant differences between the treatments in all sampling points. At 120 hpt, a slight reduction in ABA content was recorded in the trees treated with 0.05 or 0.1 mm melatonin.
Figure 7.
Effects of exogenous melatonin supplementation on the endogenous ABA content of Valencia sweet orange. A, Endogenous levels of ABA after exogenous melatonin treatments. Valencia sweet orange trees were treated with 150 mL of Milli-Q water (control; 0 mm melatonin) or 0.05, 0.1, 0.2, 0.5, or 1 mm melatonin. Data shown are means ± sd (n = 5). P < 0.05 indicates statistically significant differences among treatments, while P > 0.05 indicates no significant differences among them using Tukey’s HSD. B, Fit means models and quadratic polynomial regression analysis between exogenous melatonin concentrations and endogenous ABA content. Black dots represent the raw data (n = 6). The fit means line is presented as a solid line, while polynomial regression models are presented as dashed lines. The 95% confidence intervals for the estimated regression are gray shaded and edged by dotted lines. Regression equations, R2, R2adj, and P value based on the F test (P ≤ 0.05) were also obtained and are presented within the graph. FW, Fresh weight.
Results from the second-degree polynomial regression analysis of the nonlinear relationship between exogenous melatonin concentrations and the endogenous ABA content showed a positive and quadratic relationship (Fig. 7B). The relationship between exogenous melatonin rates and ABA is described by the equation ABA = 12.6x2 − 25.6x + 342.3 (R2 = 0.093, R2adj = 0.038, and P = 0.1988). In addition, the fit means model for each value showed that the accumulation of ABA was induced by treatment with 0.05 mm melatonin (Fig. 7B), regardless of the sampling time.
Melatonin Supplementation Differentially Altered the Expression of the Phytohormone Biosynthetic Genes
In total, we investigated the transcript levels of 38 genes involved in four phytohormone biosynthesis pathways in Valencia sweet orange trees at 72 hpt with melatonin. These genes included 11 genes of SAs biosynthesis (Fig. 8A), 11 genes of auxin biosynthesis (Fig. 8B), 10 genes of tJA biosynthesis (Fig. 8C), and six genes involved in the ABA biosynthesis pathway (Fig. 8D). Gene expression data were normalized using four reference/housekeeping genes (CsEF1, CsF-box, CsGAPDH, and CsSAND), as described above. The normalized transcript levels using the four reference genes were very similar to each other. Generally, the expression levels of most of the phytohormone biosynthetic genes increased after melatonin supplementation (Fig. 8), which supported our GC-MS findings.
Figure 8.
Relative expression of genes involved in the phytohormone biosynthesis pathways in Valencia sweet orange. Two-way HCA and its associated heat map diagrams of differential nonstandardized gene expression patterns of SAs (A), auxins (B), tJA (C), and ABA (D) after exogenous melatonin supplementation are shown. Rows represent the genes, whereas columns represent different treatments. Low expression levels are colored light blue and high expression levels are colored dark blue (see the scale at the right bottom corner of the heat maps). Treatments and genes in the HCA were organized using Ward’s minimum variance method (Ward, 1963). The full list of expressed genes, names, accession numbers, and primers is available in Supplemental Table S2.
Generally, all SAs biosynthetic genes were expressed at higher levels after melatonin supplementation, with a greater effect of the low melatonin concentrations (0.05 and 0.1 mm), which were clustered together (Euclidean distance was approximately 18; Fig. 8A). Chorismate synthase (CsCS) had the highest gene expression among all SAs biosynthetic genes (about 12.6-fold increase) when Valencia sweet orange trees were treated with 0.1 mm melatonin, followed by arogenate dehydratase/prephenate dehydratase1 (CsADT1) and tyrosine aminotransferase (CsTAT) at the same treatment (approximately 11.5-fold; Fig. 8A). Interestingly, CsCS catalyzes the last step in the shikimate pathway to produce chorismate, which is used for the biosynthesis of aromatic amino acids such as Phe (the precursor of SA) and Trp (the precursor of auxins and melatonin).
Likewise, the transcript levels of all auxin biosynthetic genes were induced after supplementation with 0.1 and 0.2 mm melatonin, which were clustered together (Euclidean distance was less than 2.5; Fig. 8B). The total HCA dendrogram among auxin biosynthetic genes showed that the 11 genes separated into three distinct clusters. Cluster I includes five genes that were highly expressed after melatonin supplementation, but less than 4-fold (Fig. 8B). Cluster II included three genes that were expressed up to 6-fold higher. Cluster III included another three genes that were expressed more than 6-fold higher (Fig. 8B). Interestingly, cluster III includes two Trp:pyruvate aminotransferase genes (CsTAA2 and CsTAA4) and the indole-3-pyruvate monooxygenase (CsYUC2). All of these genes are involved in the biosynthesis of IAA from Trp via indole-3-pyruvate as an intermediate compound. Furthermore, CsYUC2 had the highest transcript levels among all IAA biosynthetic genes (up to a 7.2-fold increase) when Valencia sweet orange trees were treated with any melatonin concentration except 1 mm.
All tJA biosynthesis genes were upregulated after melatonin supplementation with greater effect for the lowest concentration (0.05 mm melatonin), which clustered alone (Euclidean distance was more than 6; Fig. 8C). The total HCA dendrogram among the 10 tJA biosynthetic genes showed that nine genes separated into three distinct clusters, in addition to 3-ketoacyl-CoA thiolase (CsKAT), which clustered alone in the bottom of the HCA dendrogram. Cluster I included four genes, cluster II included three genes, and cluster III included acetate/butyrate-CoA ligase (CsAAE7) and enoyl-CoA hydratase2 (CsAIM2) only (Fig. 8C). Interestingly, these three genes (CsAAE7, CsAIM2, and CsKAT) catalyze the late steps in the JA biosynthesis pathway.
Furthermore, all ABA biosynthetic genes were highly expressed after melatonin supplementation, with greater effect for the low concentrations (0.05 and 0.1 mm melatonin), which clustered together (Euclidean distance was approximately 1.5; Fig. 8D). The six ABA biosynthetic genes separated into three distinct clusters. Cluster I included zeaxanthin epoxidase (CsZEP) and violaxanthin deepoxidase (CsVDE), cluster II included 9-cis-epoxycarotenoid dioxygenase (CsNCED) and abscisic aldehyde oxidase (CsAAO3), and cluster III included neoxanthin synthase (CsNSY) and short-chain alcohol dehydrogenase (or xanthoxin dehydrogenase [CsABA2]; Fig. 8D).
Melatonin Supplementation Diminished the Ca. L. asiaticus Population within the Infected Valencia Sweet Orange Leaves
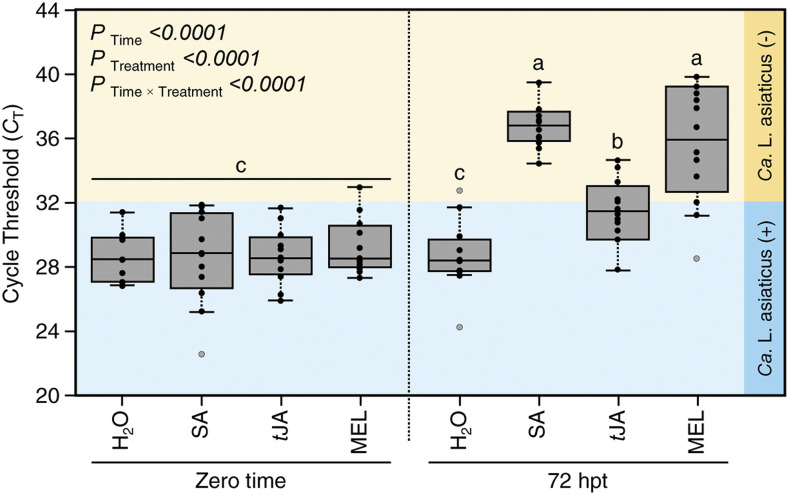
The effect of melatonin supplementation on the bacterial population of Ca. L. asiaticus within the detached leaves of Valencia sweet orange was investigated using quantitative PCR (qPCR) and expressed as cycle threshold (CT) values, which negatively reflect the bacterial population within the infected tissues. In agreement with our findings above, data presented in Figure 9B showed that the low melatonin concentrations (0.05 and 0.1 mm melatonin) significantly increased the CT values, particularly at 72 hpt, indicating a lower bacterial population of Ca. L. asiaticus. Furthermore, results from the second-degree polynomial regression analysis of the nonlinear relationship between exogenous melatonin concentrations and the CT values showed a positive and quadratic relationship (Fig. 9C). The relationship between exogenous melatonin rates and CT is described by the equation CT = −6.54x2 + 6.63x + 30.64 (R2 = 0.0442, R2adj = 0.0266, and P < 0.0001). Besides, the fit means model for each value showed that the CT value was higher when the detached leaves were treated with 0.1 mm melatonin (Fig. 9C), regardless of the sampling time.
Figure 9.
Effects of melatonin supplementation on the bacterial population of Ca. L. asiaticus in HLB-symptomatic detached leaves of Valencia sweet orange. A, Fully mature HLB-symptomatic detached leaves, with their complete petioles, were incubated in one of five concentrations of melatonin (0.05, 0.1, 0.2, 0.5, and 1 mm aqueous solution), in addition to the control (mock; Milli-Q water with 0 mm melatonin). A single leaf disc was collected from each leaf before the treatment (zero time) and at 24, 48, 72, and 96 hpt for Ca. L. asiaticus quantification. B, CT of reverse transcription quantitative PCR (RT-qPCR) for the detection of Ca. L. asiaticus in HLB-symptomatic detached leaves of Valencia sweet orange after treatment with different concentrations of melatonin. Data shown are means ± sd (n = 12). P < 0.05 indicates statistically significant differences among treatments, whereas P > 0.05 indicates no significant differences between them using Tukey’s HSD. C, Fit means model and quadratic polynomial regression analysis between exogenous melatonin concentrations and CT of RT-qPCR for the detection of Ca. L. asiaticus. Black dots represent the raw data (n = 6). The fit means line is presented as a solid line, while polynomial regression models are presented as dashed lines. The 95% confidence intervals for the estimated regression are gray shaded and edged by dotted lines. Regression equations, R2, R2adj, and P value based on the F test (P ≤ 0.05) were also obtained and are presented within the graph. D to I, Simple linear regression plots of hpt versus the CT of RT-qPCR for the detection of Ca. L. asiaticus in detached leaves after treatment with different concentrations of melatonin. White dots represent the raw data (n = 12). The fitted regression line is presented as a solid line, whereas the 95% confidence intervals for the estimated regression are gray shaded and edged by dotted lines. Regression equations, R2, R2adj, and P value based on the F test (P ≤ 0.05) were also obtained and are presented within the graphs.
Without melatonin supplementation, the SLR analysis showed a negative correlation between CT values and time posttreatment (Fig. 9D), which indicates higher bacterial titer. On the other hand, the linear regression of the CT values versus the time posttreatment showed a significant positive relationship between them after melatonin supplementation (Fig. 9, E–I). The lower melatonin concentrations (0.05 and 0.1 mm) showed a stronger positive correlation between CT values and the time posttreatment. For instance, the relationship between CT values and time posttreatment could be described by the equation CT = 0.075x + 29.17 (R2 = 0.4493, R2adj = 0.4399, and P < 0.0001) after the treatment with 0.05 mm melatonin (Fig. 9E), whereas it could be described by the equation CT = 0.087x + 29.6 (R2 = 0.5783, R2adj = 0.571, and P < 0.0001) after the treatment with 0.1 mm melatonin (Fig. 9F). However, the higher melatonin concentrations (0.2, 0.5, and 1 mm) showed a lower positive correlation between CT values and the time posttreatment (Fig. 9, G–I). In other words, there was a significant negative density-dependent relationship between the bacterial titer of Ca. L. asiaticus and melatonin content over time, which confirms the antibacterial role of melatonin against Ca. L. asiaticus within the infected tissues.
Endogenous Melatonin and SA Levels Are Higher in Ca. L. asiaticus-Tolerant Citrus Varieties
Our findings showed that the endogenous levels of melatonin and SA, but not tJA, were higher in Ca. L. asiaticus-tolerant varieties (Fig. 10). Briefly, the tolerant variety C. latipes had the highest melatonin content (941.56 ± 130.35 pg g−1 fresh weight), followed by Dancy tangerine, Mexican lime, and C. macrophylla (783.68 ± 100.52, 611.51 ± 29.91, and 393.93 ± 30.43 pg g−1 fresh weight, respectively; Fig. 10, A and B). On the other hand, the susceptible varieties, Valencia sweet orange and Duncan grapefruit (Citrus paradise), were lower in melatonin without significant differences between them (327.62 ± 23.19 and 254 ± 49.32 pg g−1 fresh weight, respectively; Fig. 10, A and B). Furthermore, the SLR showed that the endogenous melatonin content was positively correlated with the tolerance degree (Mel = 285.91x − 19.76; R2 = 0.8181, R2adj = 0.8127, and P < 0.0001; Fig. 10D).
Figure 10.
Endogenous contents of phytomelatonin, SA, and tJA detected in leaves of some citrus varieties with different degrees of tolerance to Ca. L. asiaticus using targeted GC-MS-SIM. A, D, and G, Representative chromatograms of melatonin (A), SA (D), and tJA (G) from different varieties. RT, Retention time. B, E, and H, Endogenous contents of melatonin (pg g−1 fresh weight; n = 6), SA (ng g−1 fresh weight; n = 6), and tJA (ng g−1 fresh weight; n = 6), respectively, from different varieties. Whiskers indicate the minimum and maximum values of the data, horizontal thick lines show the medians, black dots indicate the raw data, and boxes show the interquartile ranges (25th to 75th percentiles of the data). Different letters indicate statistically significant differences among varieties, while the same letter signifies no significant differences between them using Tukey’s HSD (P < 0.05). C, F, and I, Simple linear regression analysis between the HLB tolerance degree and endogenous content of melatonin (C), SA (F), and tJA (I). Dots represent the raw data. The fitted regression line is presented as a solid line. The 95% confidence intervals for the estimated regression are gray shaded and edged by dotted lines. Regression equations, R2, R2adj, and P value based on the F test (P ≤ 0.05) were also obtained and are presented within the graphs. FW, Fresh weight. Citrus varieties are as follows, from left to right: VS, Valencia sweet orange; DG, Duncan grapefruit (Citrus paradisi); CM, alemow (Citrus macrophylla); ML, Mexican lime (Citrus aurantifolia); CL, Khasi papeda (Citrus latipes); DT, Dancy tangerine (Citrus tangerina).
Likewise, SA was associated with citrus tolerance to Ca. L. asiaticus (Fig. 10, D and E). C. latipes had the highest SA content (1,453.51 ± 185.62 ng g−1 fresh weight) followed by Dancy tangerine, C. macrophylla, and Mexican lime (1,338.37 ± 131.29, 1,215.27 ± 176.51, and 1,116.28 ± 165.79 ng g−1 fresh weight, respectively), without significant differences between them (Fig. 10, D and E). On the other hand, the susceptible varieties, Duncan grapefruit and Valencia sweet orange, had the lowest SA content without significant differences between them (840.27 ± 59.59 and 774.65 ± 52.34 ng g−1 fresh weight, respectively; Fig. 10, D and E). Moreover, the SLR showed that the endogenous SA content was positively correlated with the tolerance degree (SA = 294.24x + 534.59; R2 = 0.7485, R2adj = 0.7411, and P < 0.0001; Fig. 10F).
On the other hand, no significant differences were observed in the endogenous content of tJA between all studied varieties (Fig. 10, G and H). Additionally, no significant correlation was observed between endogenous tJA content and tolerance levels (tJA = −6.76x + 272.82; R2 = 0.0179, R2adj = 0.0109, and P = 0.4363; Fig. 10C).
Melatonin and SA Supplementation Enhanced the Endogenous Content of Melatonin and SA of Ca. L. asiaticus Plants
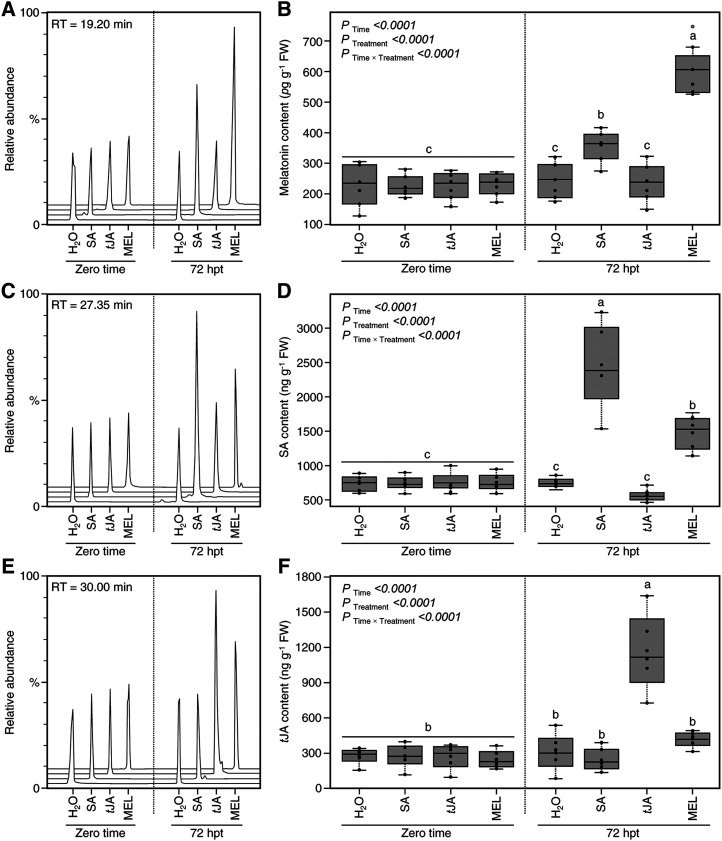
The impact of exogenous treatment with SA, tJA, and melatonin on their endogenous levels was investigated (Fig. 11). Generally, there were no significant differences between all treatments at the beginning of the experiment (zero time). However, at 72 hpt, the endogenous melatonin level was increased after the treatment with 0.1 mm melatonin (610.5 ± 79.87 pg g−1 fresh weight) or 0.25 mm SA (363.94 ± 47.79 pg g−1 fresh weight) but not tJA (238.98 ± 55.32 pg g−1 fresh weight), which was significantly similar to the control (246.45 ± 55.62 pg g−1 fresh weight; Fig. 11, A and B).
Figure 11.
Effects of exogenous application of melatonin, SA, and tJA on the endogenous contents of melatonin, SA, and tJA of Ca. L. asiaticus-infected Valencia sweet orange trees using targeted GC-MS-SIM. Citrus plants were treated with 200 mL of Milli-Q water (control), melatonin (0.1 mm), SA (0.1 mm), or tJA (0.1 mm) through foliar application. A, C, and E, Representative chromatograms of melatonin (A), SA (C), and tJA (E) at zero time and at 72 hpt. RT, Retention time. B, D, and F, Endogenous content of melatonin (pg g−1 fresh weight; n = 6), SA (ng g−1 fresh weight; n = 6), and tJA (ng g−1 fresh weight; n = 6), respectively, from Ca. L. asiaticus-infected Valencia sweet orange trees at zero time and at 72 hpt. Whiskers indicate the minimum and maximum values of the data, horizontal thick lines show the medians, black dots indicate the raw data, and boxes show the interquartile ranges (25th to 75th percentiles of the data). Different letters indicate statistically significant differences among varieties, while the same letter signifies no significant differences between them using Tukey’s HSD (P < 0.05). FW, Fresh weight.
Likewise, the endogenous levels of SA were elevated by 3.26-fold after treatment with 0.25 mm SA and about 2-fold after treatment with 0.1 mm melatonin. Interestingly, SA content slightly dropped after treatment with 0.1 mm tJA (564.02 ± 85.11 ng g−1 fresh weight); however, this reduction was not significant compared with the control (757.41 ± 62.32 ng g−1 fresh weight; Fig. 11, C and D). On the other hand, the exogenous application of tJA (0.1 mm) only induced the accumulation of endogenous tJA but not SA or melatonin (Fig. 11, E and F).
Melatonin and SA Supplementation Diminished the Ca. L. asiaticus Population within the Infected Citrus Plants
To confirm our findings of the antibacterial role of melatonin against Ca. L. asiaticus from the detached leaves experiment and to investigate if SA or tJA has a direct or indirect effect on the Ca. L. asiaticus infection, the effect of SA, tJA, and melatonin supplementation on the bacterial population of Ca. L. asiaticus within the infected Valencia sweet orange plants was investigated. In agreement with our findings above, data presented in Figure 12 showed that the exogenous application of melatonin (0.1 mm) or SA (0.25 mm) significantly increased the CT values at 72 hpt without significant differences between them, indicating a lower bacterial population of Ca. L. asiaticus within the infected plants.
Figure 12.
Exogenous application of melatonin, SA, and tJA on the bacterial population of Ca. L. asiaticus in HLB-infected Valencia sweet orange trees. CT of RT-qPCR for the detection of Ca. L. asiaticus in HLB-infected Valencia sweet orange trees after treatment with 200 mL of Milli-Q water (control), melatonin (0.1 mm), SA (0.1 mm), or tJA (0.1 mm) through foliar application is shown. Whiskers indicate the minimum and maximum values of the data, horizontal thick lines show the medians, black dots indicate the raw data, and boxes show the interquartile ranges (25th to 75th percentiles of the data). Different letters indicate statistically significant differences among varieties, while the same letter signifies no significant differences between them using Tukey’s HSD (P < 0.05).
DISCUSSION
One of our goals in this study was to develop a GC-MS-SIM-based method to determine the endogenous melatonin content in citrus leaf tissues after derivatization with MSTFA. This derivatization procedure produces a bis-TMS-melatonin derivative as a final product, with no indications of the presence of the mono-TMS-melatonin, and these results are in agreement with our previous study on D. citri (Nehela and Killiny, 2018) as well as the study of Nùñez-Vergara et al. (2001) on pharmaceutical tablets. Interestingly, the mass spectrum of the bis-TMS-melatonin derivative does not exist in the entries of the two mass spectral libraries employed in our lab: NIST 2011 (National Institute of Standards and Technology) and Wiley 9th edition (John Wiley and Sons). Although the citrus leaf extract is a complex matrix, our method was efficient in the simultaneous qualitative and quantitative determination of melatonin in Valencia sweet orange leaves. Melatonin was reported previously in citrus fruits (Johns et al., 2013) and juice (Fernández-Pachón et al., 2014); however, to the best of our knowledge, melatonin was never reported in Valencia sweet orange leaves previously. Herein, we detected melatonin from Valencia sweet orange leaves under different biotic stressors (Ca. L. asiaticus infection and D. citri infestation).
Our findings showed that Ca. L. asiaticus infection induced the accumulation of melatonin and upregulated the expression of its biosynthetic genes in the leaves of infected trees. These findings are in agreement with previous studies that showed that the endogenous melatonin level was elevated in Arabidopsis leaves upon infection with the virulent bacterial pathogen Pst-DC3000 (Shi et al., 2015) and the avirulent pathogen P. syringae pv tomato DC3000 expressing the elicitor avrRpt2 (Pst-avrRpt2; Lee et al., 2015; Lee and Back, 2016, 2017a). Likewise, the melatonin level was increased in watermelon (Citrullus lanatus) plants in response to the fungal powdery mildew pathogen Podosphaera xanthii (Mandal et al., 2018).
Exogenous melatonin application enhanced plant resistance against fungi and fungi-like organisms. For instance, exogenous melatonin treatment enhanced the resistance of apple trees to Marssonina apple blotch disease caused by D. mali (Yin et al., 2013), improved banana (Musa acuminata) responses to the Fusarium wilt disease caused by Fusarium oxysporum f. sp. cubense (Wei et al., 2017), and protected strawberry (Fragaria × ananassa) fruits against the postharvest decay caused by Botrytis cinerea and Rhizopus stolonifer (Aghdam and Fard, 2017). Furthermore, exogenous application of melatonin showed a significant reduction in hyphal growth and conidia development of the powdery mildew pathogen P. xanthii on watermelon, zucchini (Cucurbita pepo), and summer squash (Cucurbita pepo) leaves (Mandal et al., 2018). Likewise, melatonin significantly reduced the mycelial growth, sporulation intensity, and zoospore release of the soil-borne oomycete Phytophthora capsici in watermelon and other cucurbit plants (Mandal et al., 2018). Moreover, the addition of melatonin to the standard medium showed growth inhibition activity against several phytopathogenic fungi, including Alternaria spp., Botrytis spp., and Fusarium spp., and decreased the infection rate of Penicillium spp. on nonsterilized Lupinus albus seeds (Arnao and Hernández-Ruiz, 2015). In terms of fungi-like organisms, melatonin supplementation mitigated the negative effects of Phytophthora infestans, the causal agent of potato late blight (Zhang et al., 2017), and the soil-borne oomycete P. capsica, the causal agent of Phytophthora crown rot on watermelon and other cucurbits (Mandal et al., 2018). It has also been shown recently that melatonin supplementation might play a key role in the enhancement of host resistance against virus infection of numerous plants such as apple (Malus domestica; Chen et al., 2019a), rice (Oryza sativa; Lu et al., 2019), and tobacco (Nicotiana tabacum; Zhao et al., 2019). Taken together, these findings suggest that melatonin itself might be a defense molecule that can act as a biocide or as a biotic antistressor against phytopathogenic bacteria and fungi (Arnao and Hernández-Ruiz, 2015).
Our findings showed that melatonin has an antibacterial effect against Ca. L. asiaticus, since the low concentrations (between 0.05 and 0.1 mm) significantly reduced the bacterial titer within the HLB-symptomatic detached leaves of Valencia sweet orange. These findings are in agreement with our previous study, where we showed that exogenous melatonin reduced the Ca. L. asiaticus titer within the D. citri body (Nehela and Killiny, 2018). Although there is still debate concerning the antibacterial role of melatonin, previous in vitro studies in Animalia demonstrated that melatonin is effective against both gram-positive and gram-negative bacteria, such as Pseudomonas aeruginosa, Acinetobacter baumannii, and Staphylococcus aureus (Tekbas et al., 2008). In plants, previous studies showed that melatonin supplementation triggered the defense responses of Arabidopsis against the phytopathogenic gram-negative bacterium P. syringae pv tomato (Lee et al., 2014, 2015; Qian et al., 2015; Shi et al., 2015; Zhao et al., 2015; Lee and Back, 2016, 2017a) and cassava (Manihot esculenta) against Xanthomonas axonopodis pv manihotis (Wei et al., 2018a).
Furthermore, it has been shown recently that exogenous melatonin inhibited the growth of Xanthomonas oryzae pv oryzicola, the causal agent of rice bacterial leaf streak disease, in a dose-dependent manner (Chen et al., 2019b) and enhanced rice resistance against the bacterial pathogen (Xian et al., 2020). Melatonin application has been reported previously to inhibit the proliferation of several bacteria and fungi species (Atroshi et al., 1998; Oztürk et al., 2000; Wang et al., 2001; Tekbas et al., 2008). This inhibitory role might be due to the indole in its structure (Chen et al., 2019b). Previously, it was reported that indoles and their derivatives have an antibacterial effect (Raut et al., 2012; Yang et al., 2014; Chen et al., 2019b) through the inhibition of dimorphism (Raut et al., 2012) and biofilm formation (Raut et al., 2012; Yang et al., 2014) or the modulation of Ca2+ efflux (Yang et al., 2014). We suggest that the antibacterial role of melatonin against Ca. L. asiaticus might be because of its indole properties. However, more investigations are required to understand the molecular mechanisms behind the antibacterial role of melatonin against Ca. L. asiaticus, which is currently difficult due to the challenges associated with growing the bacterium in pure cultures.
Generally, melatonin is biosynthesized from the amino acid l-Trp via four enzymatic steps (Arnao and Hernández-Ruiz, 2006, 2018; Hardeland, 2008; Tan et al., 2014; Back et al., 2016; Nawaz et al., 2016; Dhole and Shelat, 2018; Lee and Back, 2019). In plants, phytomelatonin is synthesized in different subcellular locations, where l-Trp is first decarboxylated to form tryptamine by the cytosolic TDC (De Luca et al., 1989; Park et al., 2008; De Masi et al., 2017; Zhao et al., 2018; Facchiano et al., 2019). After Trp decarboxylation, tryptamine is hydroxylated by T5H (a cytochrome P450 monooxygenase), leading to the synthesis of serotonin, the key intermediate in melatonin synthesis (Kang et al., 2007; Fujiwara et al., 2010; Park et al., 2011, 2012). Subsequently, serotonin is converted into N-acetylserotonin via the activity of SNAT (Kang et al., 2013; Byeon et al., 2014; Lee et al., 2015; Back et al., 2016). The last step is to methylate N-acetylserotonin into phytomelatonin by either ASMT or COMT (Byeon et al., 2015; Back et al., 2016; Zheng et al., 2017; Yan et al., 2019). Interestingly, our findings showed that the transcript levels of two CsTDCs, CsT5H (or CsCYP71A1), two CsSNATs, seven CsASMTs, and three CsCOMTs were significantly increased after Ca. L. asiaticus infection more than for other treatments and after melatonin supplementation.
TDC was previously reported to be involved in plant defense responses against fungal diseases such as rice brown spot disease, caused by Bipolaris oryzae (Ishihara et al., 2008), and nematode diseases such as cereal cyst nematode, Heterodera avenae (Huang et al., 2018). Furthermore, the snat knockout mutant lines of Arabidopsis exhibited lower endogenous content of melatonin and SA and were more susceptible to the avirulent strains of Pst-avrRpt2 (Lee et al., 2015), whereas overexpression of OsSNAT1 or human SNAT elevated the endogenous levels of N-acetylserotonin and melatonin in transgenic rice plants and conferred resistance against abiotic stress and senescence (Kang et al., 2010; Lee and Back, 2017b). Likewise, the contribution of ASMT and COMT to host defense against abiotic stress has been reported previously (Park et al., 2013; Zuo et al., 2014; Byeon and Back, 2016; Xu et al., 2016; Zheng et al., 2017; Yan et al., 2019). However, the potential roles of melatonin biosynthetic genes in plant defense responses against phytopathogenic bacteria are poorly understood. Our findings showed that melatonin biosynthetic genes, particularly CsTDCs, CsSNATs, and CsASMTs, are involved in the citrus response to Ca. L. asiaticus through the induction of melatonin biosynthesis, which was positively correlated with SA content and its biosynthetic genes but not tJA. However, further studies are required to clarify the functional and regulatory roles of these genes in citrus in general and to better understand how they regulate SA-based defensive mechanisms.
Collectively, our findings showed that melatonin and its biosynthetic genes are involved in citrus response against the phytopathogenic bacterium Ca. L. asiaticus. Recently, the protective roles of melatonin against various phytopathogens have been reviewed (Moustafa-Farag et al., 2019). Although the antibacterial role of melatonin against several phytopathogenic bacteria has been reported previously (Lee et al., 2014, 2015; Qian et al., 2015; Shi et al., 2015; Zhao et al., 2015; Lee and Back, 2016, 2017a; Wei et al., 2016, 2018a), the molecular and biochemical mechanisms of melatonin-based defense are poorly characterized. Previous studies showed that melatonin defensive mechanisms involve the activation of the NO-dependent pathway (Shi et al., 2015; Lu et al., 2019), the modulation of carbohydrate, sugar, and glycerol metabolism (Qian et al., 2015; Zhao et al., 2015), the induction of hydrogen peroxide- and NO-mediated defense signaling (Lee and Back, 2017a), upstreaming transcription factors such as MAPK cascades and RAV transcription factors (Lee and Back, 2016; Wei et al., 2018a), the induction of pathogenesis- and defense-related genes, such as ENHANCED DISEASE SUSCEPTIBILITY1, PHYTOALEXIN DEFICIENT4, and PR1, PR2, and PR5 (Lee et al., 2014; Shi et al., 2015; Lee and Back, 2017a), and/or the modulation of stress-associated phytohormones, particularly SA, JA, and ET, as well as their biosynthetic genes (Lee et al., 2014, 2015; Lee and Back, 2017a).
Changes in host-derived phytohormones upon phytopathogen infection and/or insect infestation vary based on their feeding activity and trophic types (Glazebrook, 2005; Bari and Jones, 2009). For instance, the SA-mediated pathway is associated with defense response for biotrophic and hemibiotrophic phytopathogens, whereas the JA/ET-mediated pathway is associated with defense against necrotrophic phytopathogens and insect herbivory (Hatcher et al., 2004; Glazebrook, 2005; Bari and Jones, 2009). Our previous studies showed that Ca. L. asiaticus infection significantly induced the accumulation of SA, its precursor l-Phe, and its biosynthetic genes compared with D. citri-infested plants. On the other hand, the infestation with D. citri increased the level of tJA, its precursor linolenic acid, and its biosynthetic genes compared with Ca. L. asiaticus-infected plants (Nehela et al., 2018). The cross talk between these two pathways is possible and complicated. However, the interaction between SA and JA/ET is controversial. Some studies suggested that the SA- and JA/ET-mediated pathways are antagonistic (Bari and Jones, 2009), and the activation of one usually suppresses the other (Robert-Seilaniantz et al., 2007; Bari and Jones, 2009), while other studies have demonstrated that the SA-JA interaction is mutually synergistic (Kunkel and Brooks, 2002). Although several previous studies demonstrated the association between melatonin and SA-activated defense genes (Lee et al., 2014, 2015; Vielma et al., 2014; Qian et al., 2015), the cross talk between melatonin, SA, and/or JA is poorly studied. To the best of our knowledge, the interaction between these three compounds has not been reported previously.
In this study, we reported that phytomelatonin is involved in citrus response against HLB via modulation of stress-associated phytohormones and their biosynthetic genes. To summarize our findings, a hypothetical model for the cross talk between phytomelatonin and phytohormones in citrus was suggested and is presented in Figure 13. In this model, we propose that infection with the Ca. L. asiaticus bacterium and infestation with the D. citri vector, as well as exogenous melatonin supplementation, induced the biosynthesis of phytomelatonin and several phytohormone groups, including SAs, tJA, ABA, and auxins. Moreover, melatonin supplementation diminished the Ca. L. asiaticus population within the infected leaves and whole plants, which supports our hypothesis that melatonin might play an antibacterial role against Ca. L. asiaticus particularly and gram-negative bacteria in general.
Figure 13.
Hypothetical model of the cross talk between melatonin and different phytohormones of Valencia sweet orange leaves. In this model, we propose that infection with Ca. L. asiaticus bacterium and infestation with D. citri vector, as well as exogenous melatonin supplementation, might induce the biosynthesis of phytomelatonin and several phytohormone groups, including SAs, tJA, ABA, and auxins. Briefly, exogenous melatonin plays a dual role in the activation of both the SA-mediated pathway against pathogen infection and the JA-mediated pathway against herbivory, which are the most common defensive pathways in vascular plants. Besides these well-known pathways, our findings show that melatonin is involved in the activation of both ABA and auxin pathways. ABA and auxins play a key role in citrus defense response against HLB. The activation of four phytohormone pathways leads to complex cross talk among them, which interact synergistically or antagonistically together, and link back to the SA-mediated pathway and the JA-mediated pathway. Solid lines with arrows indicate well-established/confirmed pathways, dashed lines with whiskers signify negative reactions, and dotted lines represent hypothetical mechanisms or uncharacterized elements. DAHP, 3-Deoxy-D-arabino-heptulosonic acid 7-phosphate; TCA, tricarboxylic acid.
Furthermore, melatonin supplementation triggered the gene expression of all SAs biosynthetic genes, particularly CsCS. This enzyme catalyzes the last step in the shikimate pathway to produce chorismate, which is used for the biosynthesis of aromatic amino acids such as Phe (the precursor of SA) and Trp (the precursor of auxins and melatonin). Interestingly, our previous study showed that CsCS was highly upregulated after Ca. L. asiaticus infection (Nehela et al., 2018). On the other hand, even though the SLR modeling of endogenous melatonin and tJA content did not show a significant correlation between them, melatonin supplementation slightly induced the accumulation of tJA in Valencia sweet orange leaves and upregulated all tJA biosynthesis genes, particularly CsKAT. CsKAT is involved in the metabolism of fatty acids, which is part of JA biosynthesis. Interestingly, our previous study showed that CsKAT was highly upregulated only after D. citri infestation (Nehela et al., 2018). Taken together, these findings indicate that exogenous melatonin plays a key role in the activation of the SA-mediated pathway against pathogen infection, which is the most common defensive pathway in vascular plants (Glazebrook, 2005; Robert-Seilaniantz et al., 2007; Bari and Jones, 2009). Besides these well-known pathways, we suggest that melatonin might be involved in the activation of both ABA and auxin pathways. ABA and auxins play key roles in the citrus defense response against HLB (Nehela et al., 2018). The activation of four phytohormone pathways leads to complex cross talk among them, which interact synergistically or antagonistically and feeds back into the SA-mediated pathway and JA-mediated pathway. However, further research focusing on melatonin-ABA-auxin interactions and their roles in the activation of SA- and JA-mediated pathways is required for a better understanding of citrus phytohormonal response to HLB disease.
Our findings showed that the endogenous levels of melatonin and SA, but not tJA, were higher in Ca. L. asiaticus-tolerant varieties such as C. latipes and Dancy tangerine compared with susceptible varieties such as Valencia sweet orange and Duncan grapefruit. These findings suggest that both melatonin and SA biosynthesis pathways are more active in HLB-tolerant varieties. Our previous study showed that the phloem sap chemical composition of most of the tolerant cultivars such as C. latipes was higher in most of the metabolites, especially amino acids that are precursors for most of the secondary metabolites implicated in plant defense (Killiny and Hijaz, 2016). For instance, the phloem sap of the HLB-tolerant variety C. latipes had a higher l-Trp content compared with the moderately tolerant and susceptible varieties (Killiny and Hijaz, 2016). Interestingly, l-Trp is the precursor of auxins and melatonin (Arnao and Hernández-Ruiz, 2006, 2018; Hardeland, 2008; Tan et al., 2014; Back et al., 2016; Nawaz et al., 2016; Dhole and Shelat, 2018; Lee and Back, 2019), which supports the suggestion that the melatonin biosynthesis pathway is more active in HLB-tolerant varieties.
Furthermore, our findings showed that the endogenous levels of melatonin and SA were increased after treatment with melatonin or SA, but not tJA, which was significantly similar to the control. In other words, the exogenous application of melatonin increased the endogenous SA levels, and vice versa, the exogenous application of SA increased the endogenous melatonin levels, which indicates a strong correlation between them. Several previous studies demonstrated the role of melatonin in plant immunity against Pst-DC3000 in Arabidopsis and provided evidence that it requires SA as a key downstream component (Yin et al., 2013; Lee et al., 2014, 2015; Vielma et al., 2014; Qian et al., 2015; Shi et al., 2015, 2016; Zhao et al., 2015). Moreover, it has been reported that the melatonin-dependent defense response against Pst-DC3000 in Arabidopsis plants was associated with the SA-mediated defense signaling pathway (Lee et al., 2014, 2015; Vielma et al., 2014; Qian et al., 2015).
For instance, exogenous application of melatonin on the leaves of Arabidopsis and N. benthamiana induced various PR genes as well as a series of SA-activated defense genes compared with mock-treated plants (Lee et al., 2014). Likewise, melatonin activated numerous PR genes and other defense-related genes through the modulation of the levels of both SA and NO in infected plants (Yin et al., 2013; Shi et al., 2015, 2016; Zhao et al., 2015). Additionally, the SNAT (a key melatonin biosynthetic gene) knockout mutants not only exhibited decreased levels of melatonin but also had lower SA content along with a greater susceptibility to the bacterial pathogen (Lee et al., 2015). On the other hand, melatonin supplementation failed to induce defense-related genes in the nahG mutants of Arabidopsis but restored the induction of defense-related genes in the snat knockout mutants (Lee et al., 2015). Taken together, the evidence indicates the existence of cross talk between melatonin and other phytohormones and that the melatonin-elicited defense response that facilitates fine-tuning of resistance against phytopathogenic bacteria is SA dependent.
In conclusion, melatonin and SA biosynthesis pathways share a common precursor (chorismate; Fig. 13), which is generated from shikimic acid, and both play a relevant role in citrus physiology, particularly in aspects related to the response to biotic and abiotic stress. Our findings showed that both Ca. L. asiaticus infection and/or D. citri infestation significantly increased the endogenous melatonin and SA content and their biosynthetic genes of Valencia sweet orange leaves, with a greater effect of Ca. L. asiaticus alone. Moreover, Ca. L. asiaticus-tolerant and -moderately tolerant varieties had higher levels of both melatonin and SA but not tJA. All of these pieces of evidence support the potential role of melatonin in citrus response to different biotic stressors, including the phytopathogenic bacterium Ca. L. asiaticus and its insect vector, D. citri. Additionally, melatonin supplementation enhanced the endogenous content of stress-associated phytohormones, including SAs, auxins, tJA, and ABA and their biosynthetic genes. Likewise, the endogenous level of melatonin was increased after the treatment with SA but not tJA. These findings highlight the molecular and biochemical mechanisms of the melatonin-mediated defensive response and demonstrate its cross talk with other phytohormones. Furthermore, melatonin supplementation diminished the Ca. L. asiaticus population within the HLB-infected detached leaves and whole trees of Valencia sweet orange, which confirms the antibacterial role of melatonin against Ca. L. asiaticus within the infected tissues. To the best of our knowledge, the potential role of melatonin against citrus bacterial diseases in general, and HLB particularly, has not been reported previously. Melatonin is considered an eco-friendly molecule, which might be a promising alternative strategy to combat the citrus greening disease in particular and plant bacterial diseases in general. Finally, a thorough understanding of the melatonin-based defense mechanism in citrus might lead to a more comprehensive image of defense responses to HLB, critical for finding immediate and sustainable management strategies for HLB.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Valencia sweet orange (Citrus sinensis) was used as an experimental plant in this study. All trees were about 18 months old and 75 ± 10 cm tall at sampling time. All trees were grown in a U.S. Department of Agriculture-Animal and Plant Health Inspection Service (USDA-APHIS)-approved secured greenhouse (28°C ± 4°C, 60% ± 5% relative humidity, and 16/8-h light/dark photocycle) at the Citrus Research and Education Center, University of Florida (28°10′N, 81°71′E), in Lake Alfred. Weekly, trees were irrigated twice and fertilized once using 20-10-20 water-soluble fertilizer (Peter’s Florida Special).
Preparation of Candidatus Liberibacter asiaticus-Infected and/or Diaphorina citri-Infested Trees
To obtain the Ca. L. asiaticus-infected trees, healthy Valencia sweet orange trees (about 10 months old) were graft inoculated with budwood from a PCR-positive Ca. L. asiaticus-infected source and maintained in the same conditions described above. Eight months later, the infection was confirmed using PCR as described (Tatineni et al., 2008). For D. citri-infested and the double-attacked trees, 18-month-old healthy or Ca. L. asiaticus-infected trees exhibiting 15 cm of new flush were exposed to 100 healthy adult psyllids (PCR negative) per tree and caged individually using insect-rearing cages (60 × 60 × 90 cm; Bioquip) and maintained in a USDA-APHIS/Centers for Disease Control-approved secured growth room under the same conditions described above. Ca. L. asiaticus-free psyllids were obtained from our laboratory colony reared on Bergera koenegii plants, a nonhost for the pathogen. For all treatments, three leaves were collected from different positions on each tree. The collected leaves were chopped and mixed together and then kept at −80°C for further analysis.
Exogenous Application of Melatonin on Healthy Citrus Plants
Due to the lack of previous studies about melatonin application on citrus plants, our selection of tested concentrations for the exogenous application was based on the previous literature on other fruit trees such as apple (Malus domestica; Wang et al., 2012, 2013; Yin et al., 2013; Liang et al., 2018; Wei et al., 2018b; Zhang et al., 2019), pear (Pyrus communis; Liu et al., 2018, 2019; Zhai et al., 2018), wild jujube (Zizyphus acidojujuba; Ren et al., 2018), tamarillo (Cyphomandra betacea; Lin et al., 2018), and sweet cherry (Prunus avium; Tijero et al., 2019). Treatments with five concentrations of melatonin (powder, purity ≥ 98%; Sigma-Aldrich) consisted of 0.05, 0.1, 0.2, 0.5, and 1 mm, in addition to the control (Milli-Q water with 0 mm melatonin). Melatonin solutions were applied through root drench under low light intensity to avoid any degradation of melatonin under light conditions. Briefly, Valencia trees were irrigated with 150 mL of aqueous melatonin solution per tree. The treatments in excess of soil capacity were collected using plastic cups and added back to the soil the following day. All treatments (six biological replicates per each) were kept in the same growth room conditions as described previously. For leaf sampling, three leaves were collected from each tree from different positions before melatonin treatment (zero time) and at 12, 24, 48, 72, 96, and 120 hpt. The samples were stored at −80°C until analysis.
Melatonin Analysis Using GC-MS-SIM
Melatonin was analyzed using our reported method (Nehela and Killiny, 2018), developed from the published methods (Aboul-Enein et al., 1999; Hernández-Ruiz et al., 2004, 2005; Wójciak-kosior and Woźniak, 2008). Briefly, about 300 mg of leaf tissues was ground to a fine powder using liquid nitrogen. Melatonin was extracted with 1.5 mL of ethyl acetate containing 100 mg L−1 butylated hydroxytoluene (as an antioxidant) as described in our previous work (Nehela and Killiny, 2018). Subsequently, samples were derivatized with 50 μL of MSTFA (Thermo Fisher Scientific) containing 1% (v/v) chlorotrimethylsilane (98%; Acros Organics, Thermo Fisher Scientific) by incubating at 85°C for 45 min in a multiblock aluminum heater as described (Nùñez-Vergara et al., 2001). For the GC-MS analysis, 1 μL was injected into the GC-MS apparatus running in SIM mode using the same chromatographic conditions as described in our previous study (Nehela and Killiny, 2018). Peaks were identified by comparing their retention times, linear retention indices, and mass spectra with those of the authentic melatonin standard (powder, purity ≥98%; Sigma-Aldrich). In the SIM mode, seven characteristic ions were used for peak identification.
Phytohormone Analysis Using GC-MS-SIM
Citrus phytohormones were extracted using an extraction mixture of methanol:water:HCl (6 n; 80:19.9:0.1, v/v/v) as described (Nehela et al., 2016). After extraction, the supernatant was concentrated to 50 µL under a gentle nitrogen stream and derivatized using methyl chloroformate as described previously (Hijaz and Killiny, 2014; Nehela et al., 2016, 2018). After derivatization, the chloroform extract was concentrated to 20 µL under a nitrogen stream and a few milligrams of sodium sulfate (two to three crystals) was added to dry the organic phase. For GC-MS analysis, 1 µL was injected in SIM mode using the published thermal conditions, and three to five ions were used to identify each compound (Nehela et al., 2016). The abundances and ratios of the characteristic ions were similar to those of the authentic standards. TurboMass software version 6.1 (Perkin-Elmer) was used to analyze chromatograms. Identification of all phytohormones was performed by comparing their retention times, linear retention indices, and characteristic ions with those of authentic phytohormone standards.
Gene Expression Analysis Using RT-qPCR
Total RNA was extracted using Trizol reagent (Ambion, Life Technologies). Subsequently, we analyzed the expression of 15 genes involved in the melatonin biosynthetic pathway (Supplemental Table S1) and 38 genes involved in phytohormone biosynthetic pathways (Supplemental Table S2) in triplicate for each biological replicate for each treatment (n = 30), as described (Nehela et al., 2018; Nehela and Killiny, 2018). The relative expression of the consensus sequence among PCR products was done according to the 2−ΔΔCT method (Livak and Schmittgen, 2001). Four reference genes were used for normalization of gene expression: CsEF1, CsF-box, CsGAPC1, and CsSAND (Mafra et al., 2012; Wei et al., 2014a, 2014b).
Effect of Melatonin Supplementation on Ca. L. asiaticus Titer in Citrus Detached Leaves
The antibacterial activity of melatonin against Ca. L. asiaticus was examined using the detached leaves assay, which was used previously to study the effects of exogenous melatonin on abiotic and biotic stressors in several plant species, including Arabidopsis (Arabidopsis thaliana; Weeda et al., 2014), apple (Yin et al., 2013; Wei et al., 2018b), grape (Vitis vinifera; Shi et al., 2019), and perennial ryegrass (Lolium perenne; Zhang et al., 2016). Briefly, fully mature HLB-symptomatic leaves were detached with their complete petioles from 18-month-old trees of Ca. L. asiaticus-infected Valencia sweet orange. Collected leaves were covered immediately with wet absorbent gauze, placed on ice, and transferred to the laboratory. To investigate the effect of melatonin supplementation on Ca. L. asiaticus titer, the detached leaves were incubated separately in one of five concentrations of melatonin (0.05, 0.1, 0.2, 0.5, and 1 mm aqueous solution), in addition to the control (mock; Milli-Q water with 0 mm melatonin). All treatments (12 biological replicates per each) were kept in the same growth conditions as described above. For sampling, a single leaf disc (∼31 mm2) was collected from each leaf using a paper hole puncher before the treatment (zero time) and at 24, 48, 72, and 96 hpt (Fig. 9A). Leaf discs were placed individually into 2-mL screw-cap Lysing Matrix tubes (MP Biomedicals) and stored at −80°C until analysis.
Determination of Endogenous Levels of Melatonin, SA, and tJA in Some Citrus Varieties with Different Degrees of Tolerance to Ca. L. asiaticus
Seedlings from six Citrus species with different degrees of tolerance to Ca. L. asiaticus were used to investigate the endogenous levels of melatonin, SA, and tJA. These species included two susceptible varieties (Valencia sweet orange and Duncan grapefruit [Citrus paradisi]), two moderately tolerant varieties (alemow [Citrus macrophylla] and Mexican lime [Citrus aurantifolia]), and two tolerant varieties (Khasi papeda [Citrus latipes] and Dancy tangerine [Citrus tangerina]; Folimonova et al., 2009; Hijaz et al., 2020). Seedlings were grown from seeds as described in our previous study (Hijaz et al., 2020) and maintained under greenhouse conditions as described above. All seedlings were about 1 year old. Leaf samples from all varieties (six biological replicates per each) were collected from each tree from various positions as described above. The samples were stored at −80°C until analysis. Subsequently, the endogenous levels of melatonin were analyzed using our developed method (Nehela and Killiny, 2018), whereas SA, and tJA were extracted and analyzed as described by Nehela et al. (2016).
Exogenous Application of Melatonin, SA, and tJA on Ca. L. asiaticus-Infected Plants
Ca. L. asiaticus-infected Valencia sweet orange was used as an experimental plant in this study. Trees were ∼18 months old, 75 ± 10 cm tall, and maintained in a USDA-APHIS-approved secured greenhouse as described above. Aqueous solutions of 0.1 mm melatonin, 0.25 mm SA, or 0.1 mm tJA (Sigma-Aldrich) containing 0.1% (v/v) Tween 80 (Sigma-Aldrich) were applied, in addition to the control (Milli-Q water + 0.1% [v/v] Tween 80). Trees were treated using 200 mL of these aqueous solutions through foliar application to the point of runoff using a 2-gallon chemical sprayer (RYOBI One World Technologies). After the application, all treatments (six biological replicates per each) were kept under greenhouse conditions as described above. For leaf sampling, three leaves were collected from each tree from various positions before the treatment (zero time) and at 72 hpt. The samples were stored at −80°C until analysis. Subsequently, the endogenous levels of melatonin, SA, and tJA were analyzed as described previously (Nehela et al., 2016; Nehela and Killiny, 2018).
Ca. L. asiaticus Quantification Using qPCR
Ca. L. asiaticus quantification was carried out using the leaf disc assay as described recently (Etxeberria et al., 2019; Attaran et al., 2020). Briefly, individual leaf discs (12 biological replicates per treatment) were frozen by submerging in liquid nitrogen for 10 min, then homogenized twice with one metal ball using a TissueLyser II (Qiagen) without lysis buffer for 1 min of each run at 30 Hz. Subsequently, DNA was isolated from a single leaf disc using a manual extraction protocol developed in our laboratory. Briefly, 1,500 μL of the lysis buffer (0.1 mm NaCl, 10 mm Na-EDTA, and 50 mm Tris base, pH 9) along with dithiothreitol (0.16 g per 100 m) was added, and the tubes were inverted to mix. Samples were centrifuged at 2,500g for 1 min, then an aliquot of 1,300 μL of the supernatant was recovered into a new 2-mL Eppendorf tube. A total of 90 μL of SDS (10%, w/v) was added before incubation in a water bath at 65°C for 30 min. After incubation, 500 μL of 5 m potassium acetate (490.8 g of potassium acetate, 115 mL of glacial acetic acid, and Milli-Q water for a final volume of 1 L) was added, then samples were incubated on ice for 20 min. Samples were centrifuged at 15,000g for 10 min at 5°C, then an aliquot of 1,000 μL of the supernatant was recovered into a new 2-mL Eppendorf tube. The DNA was then precipitated by adding 500 μL of ice-cold isopropanol. Finally, DNA was collected by centrifugation at 16,000g for 10 min at 5°C, washed using 500 μL of ice-cold 70% (v/v) ethanol, dried, and resuspended in 20 μL of ultra-filtered DNase- and RNase-free water. The quantity and quality of extracted DNA were determined using a NanoDrop 2000 spectrophotometer (Thermo Scientific) and adjusted to 100 ng μL−1. DNA was used for RT-qPCR amplification using 16S rRNA primers HLBasf and HLBr, the probe HLBp, TaqMan PCR master mix, and SYBR green PCR master mix. The qPCR assay was performed on a 96-well plate using an ABI 7500 RT-qPCR system, and supplies were from Applied Biosystems as described (Killiny et al., 2017; Etxeberria et al., 2019; Attaran et al., 2020). The bacterial titer of Ca. L. asiaticus was expressed as CT values, which negatively reflect the bacterial population within the infected leaves.
Statistical Analysis
A completely randomized design was used as the experimental design throughout the study. In all experiments and based on the type of the experiment, six to 12 biological replicates and two technical replicates per treatment were analyzed. The technical replicates themselves were not used in the statistical analysis to avoid the possibility of pseudoreplication. All data were statistically analyzed according to the ANOVA technique, followed by posthoc pairwise comparisons between them using Tukey’s HSD (P ≤ 0.05). In addition, based on the assumptions of linearity, SLR analysis was performed to model the relationship between phytomelatonin content (as an independent variable) and the endogenous content of different phytohormones (as dependent variables) as well as to model the linear relationship between hpt (as an independent variable) and CT (as a dependent variable). The fitted regression line was expressed as a significant equation, as determined by the F test (P ≤ 0.05). Both the coefficient of determination (R2) and the adjusted coefficient of determination (R2adj) were also obtained. Furthermore, due to the observed nonlinear phenomena between exogenous melatonin concentrations (as an independent variable) and endogenous phytohormone content (as a dependent variable), and to understand the curvilinear relationship between them, data were fitted with a second-degree polynomial regression model (quadratic model). Likewise, the curvilinear relationship between exogenous melatonin concentrations (as an independent variable) and CT (as a dependent variable) was fitted using a second-degree polynomial regression model (quadratic model). For polynomial regression models, the 95% confidence intervals for the estimated regression, quadratic equation, R2, R2adj, and P value based on the F test (P ≤ 0.05) were obtained. The transcript levels of various genes involved in the biosynthesis of melatonin and different phytohormone groups are presented as heat maps combined with two-way HCA. HCA was performed using nonstandardized and standardized means of the matrices for all studied treatments. Distance and linkage were done using Ward’s minimum variance method (Ward, 1963).
Accession Numbers
Sequence data used for gene expression analysis from this article can be found in the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/gene/) data libraries under the following accession numbers: XM_006469752.3 (CsTDC1/CsAADC-like); XM_006479363.3 (CsTDC2); XM_015531938.2 (CsCYP71P1-like); XM_006481177.3 (CsSNAT-1); XM_006484231.3 (CsSNAT-2); XM_006467651.3 (CsASMT1-1, isoform 1); XM_006467653.3 (CsASMT1-2); XM_025094656.1 (CsASMT1-3); XM_006469074.3 (CsASMT2-1); XM_006469074.3 (CsASMT2-2); XM_006480390.3 (CsASMT2-3); XM_006486606.3 (CsASMT); XM_006478027.3 (CsCOMT-1); XM_006477837.2 (CsCOMT-like-1); XM_006477776.3 (CsCOMT-like-2); AY498567.1 (CsEF1); XM_006482390.1 (CsF-box); XM_006483974.2 (CsGAPC1); XM_006488024.2 (CsSAND); XM_006485798.2 (CsCS); XM_006483415.2 (CsCM2); XM_006482655.2 (CsCM3); XM_006467360.2 (CsADT1); XM_006469841.1 (CsTAT); XM_006476023.2 (CsAST-1); XM_006476024.2 (CsAST-2); XM_006481431.2 (CsPAL); NM_001288910.1 (CsAAT1); XM_006489736.1 (CsKAT); XM_006476586.2 (CsICS2); XM_015532734.1 (CsASA1); XM_006469235.2 (CsASA2); XM_006482255.1 (CsTS); XM_006470948.2 (CsTSA); XM_006493882.2 (CsTSB); XM_015529685.1 (CsTAA2); XM_006473060.2 (CsTAA4); XM_006479363.2 (CsTDC1); XM_006466708.2 (CsYUC2); XM_006480095.2 (CsYUC8); XM_006487697.2 (CsNIT4); XM_006480990.2 (CsFAD); XM_006483993.1 (CsLOX); NM_001288906.1 (CsAOS); NW_006260521.1 (CsAOC); XM_006488806.2 (CsAAE7); XM_006475468.2 (CsOPR3); XM_006477083.2 (CsACX1); XM_006488772.2 (CsAIM1); XM_006473681.2 (CsAIM2); XM_006480138.2 (CsKAT); XM_006466537.2 (CsZEP); NM_001288881.1 (CsVDE); NM_001288932.1 (CsNSY); NM_001288935.1 (CsNCED); NM_001288867.1 (CsABA2); and XM_006487736.2 (CsAAO3).
Supplemental Data
The following supplemental materials are available.
Supplemental Table S1. Primers used for gene expression analysis of melatonin biosynthetic genes by RT-qPCR.
Supplemental Table S2. Primers used for gene expression analysis of genes involved in the biosynthesis of four major groups of phytohormones in Valencia sweet orange by RT-qPCR.
Acknowledgments
We thank the members of our laboratory for helpful discussions and comments.
Footnotes
This work was supported by the USDA National Institute of Food and Agriculture (grant no. 2016–70016–24844 to N.K.).
References
- Aboul-Enein HY, Doneanu C, Covaci A(1999) Capillary GC-MS determination of melatonin in several pharmaceutical tablet formulations. Biomed Chromatogr 13: 24–26 [DOI] [PubMed] [Google Scholar]
- Aghdam MS, Fard JR(2017) Melatonin treatment attenuates postharvest decay and maintains nutritional quality of strawberry fruits (Fragaria×anannasa cv. Selva) by enhancing GABA shunt activity. Food Chem 221: 1650–1657 [DOI] [PubMed] [Google Scholar]
- Arnao MB, Hernández-Ruiz J(2006) The physiological function of melatonin in plants. Plant Signal Behav 1: 89–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnao MB, Hernández-Ruiz J(2007) Melatonin promotes adventitious- and lateral root regeneration in etiolated hypocotyls of Lupinus albus L. J Pineal Res 42: 147–152 [DOI] [PubMed] [Google Scholar]
- Arnao MB, Hernández-Ruiz J(2015) Functions of melatonin in plants: A review. J Pineal Res 59: 133–150 [DOI] [PubMed] [Google Scholar]
- Arnao MB, Hernández-Ruiz J(2018) Melatonin and its relationship to plant hormones. Ann Bot 121: 195–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atroshi F, Rizzo A, Westermarck T, Ali-vehmas T(1998) Effects of tamoxifen, melatonin, coenzyme Q10, and L-carnitine supplementation on bacterial growth in the presence of mycotoxins. Pharmacol Res 38: 289–295 [DOI] [PubMed] [Google Scholar]
- Attaran E, Berim A, Killiny N, Beyenal H, Gang DR, Omsland A(2020) Controlled replication of ‘Candidatus Liberibacter asiaticus’ DNA in citrus leaf discs. Microb Biotechnol 13: 747–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back K, Tan DX, Reiter RJ(2016) Melatonin biosynthesis in plants: Multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J Pineal Res 61: 426–437 [DOI] [PubMed] [Google Scholar]
- Bari R, Jones JDG(2009) Role of plant hormones in plant defence responses. Plant Mol Biol 69: 473–488 [DOI] [PubMed] [Google Scholar]
- Bisson MMA, Bleckmann A, Allekotte S, Groth G(2009) EIN2, the central regulator of ethylene signalling, is localized at the ER membrane where it interacts with the ethylene receptor ETR1. Biochem J 424: 1–6 [DOI] [PubMed] [Google Scholar]
- Bové JM.(2006) Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. J Plant Pathol 88: 7–37 [Google Scholar]
- Bové JM, Ayres AJ(2007) Etiology of three recent diseases of citrus in São Paulo State: Sudden death, variegated chlorosis and huanglongbing. IUBMB Life 59: 346–354 [DOI] [PubMed] [Google Scholar]
- Byeon Y, Back K(2014) An increase in melatonin in transgenic rice causes pleiotropic phenotypes, including enhanced seedling growth, delayed flowering, and low grain yield. J Pineal Res 56: 408–414 [DOI] [PubMed] [Google Scholar]
- Byeon Y, Back K(2016) Low melatonin production by suppression of either serotonin N-acetyltransferase or N-acetylserotonin methyltransferase in rice causes seedling growth retardation with yield penalty, abiotic stress susceptibility, and enhanced coleoptile growth under anoxic conditions. J Pineal Res 60: 348–359 [DOI] [PubMed] [Google Scholar]
- Byeon Y, Choi GH, Lee HY, Back K(2015) Melatonin biosynthesis requires N-acetylserotonin methyltransferase activity of caffeic acid O-methyltransferase in rice. J Exp Bot 66: 6917–6925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byeon Y, Lee HY, Lee K, Park S, Back K(2014) Cellular localization and kinetics of the rice melatonin biosynthetic enzymes SNAT and ASMT. J Pineal Res 56: 107–114 [DOI] [PubMed] [Google Scholar]
- Carrillo-Vico A, Lardone PJ, Alvarez-Sánchez N, Rodríguez-Rodríguez A, Guerrero JM(2013) Melatonin: Buffering the immune system. Int J Mol Sci 14: 8638–8683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Wang MR, Li JW, Feng CH, Cui ZH, Zhao L, Wang QC(2019a) Exogenous application of melatonin improves eradication of apple stem grooving virus from the infected in vitro shoots by shoot tip culture. Plant Pathol 68: 997–1006 [Google Scholar]
- Chen X, Sun C, Laborda P, He Y, Zhao Y, Li C, Liu F(2019b) Melatonin treatments reduce the pathogenicity and inhibit the growth of Xanthomonas oryzae pv. oryzicola. Plant Pathol 68: 288–296 [Google Scholar]
- Cipolla-Neto J, Amaral FG, Afeche SC, Tan DX, Reiter RJ(2014) Melatonin, energy metabolism, and obesity: A review. J Pineal Res 56: 371–381 [DOI] [PubMed] [Google Scholar]
- De Luca V, Marineau C, Brisson N(1989) Molecular cloning and analysis of cDNA encoding a plant tryptophan decarboxylase: Comparison with animal dopa decarboxylases. Proc Natl Acad Sci USA 86: 2582–2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Masi L, Castaldo D, Pignone D, Servillo L, Facchiano A(2017) Experimental evidence and in silico identification of tryptophan decarboxylase in Citrus genus. Molecules 22: 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhole AM, Shelat HN(2018) Phytomelatonin: A plant hormone for management of stress. J Anal Pharm Res 7: 188–190 [Google Scholar]
- Dubbels R, Reiter RJ, Klenke E, Goebel A, Schnakenberg E, Ehlers C, Schiwara HW, Schloot W(1995) Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J Pineal Res 18: 28–31 [DOI] [PubMed] [Google Scholar]
- Etxeberria E, Gonzalez P, Singerman A, Ebert T(2019) An improved method to track changes of Candidatus Liberibacter asiaticus titer in HLB-affected citrus trees. HortScience 54: 1357–1360 [Google Scholar]
- Facchiano A, Pignone D, Servillo L, Castaldo D, De Masi L(2019) Structure and ligands interactions of citrus tryptophan decarboxylase by molecular modeling and docking simulations. Biomolecules 9: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Pachón MS, Medina S, Herrero-Martín G, Cerrillo I, Berná G, Escudero-López B, Ferreres F, Martín F, García-Parrilla MC, Gil-Izquierdo A(2014) Alcoholic fermentation induces melatonin synthesis in orange juice. J Pineal Res 56: 31–38 [DOI] [PubMed] [Google Scholar]
- Folimonova SY, Robertson CJ, Garnsey SM, Gowda S, Dawson WO(2009) Examination of the responses of different genotypes of citrus to huanglongbing (citrus greening) under different conditions. Phytopathology 99: 1346–1354 [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Maisonneuve S, Isshiki M, Mizutani M, Chen L, Wong HL, Kawasaki T, Shimamoto K(2010) Sekiguchi lesion gene encodes a cytochrome P450 monooxygenase that catalyzes conversion of tryptamine to serotonin in rice. J Biol Chem 285: 11308–11313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galano A, Tan DX, Reiter RJ(2011) Melatonin as a natural ally against oxidative stress: A physicochemical examination. J Pineal Res 51: 1–16 [DOI] [PubMed] [Google Scholar]
- Glazebrook J.(2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43: 205–227 [DOI] [PubMed] [Google Scholar]
- Gottwald TR.(2010) Current epidemiological understanding of citrus huanglongbing. Annu Rev Phytopathol 48: 119–139 [DOI] [PubMed] [Google Scholar]
- Hardeland R.(2008) Melatonin, hormone of darkness and more: Occurrence, control mechanisms, actions and bioactive metabolites. Cell Mol Life Sci 65: 2001–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, Pandi-Perumal SR(2011) Melatonin: A pleiotropic, orchestrating regulator molecule. Prog Neurobiol 93: 350–384 [DOI] [PubMed] [Google Scholar]
- Hatcher PE, Moore J, Taylor JE, Tinney GW, Paul ND(2004) Phytohormones and plant-herbivore-pathogen interactions: Integrating the molecular with the ecological. Ecology 85: 59–69 [Google Scholar]
- Hattori A, Migitaka H, Iigo M, Itoh M, Yamamoto K, Ohtani-Kaneko R, Hara M, Suzuki T, Reiter RJ(1995) Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem Mol Biol Int 35: 627–634 [PubMed] [Google Scholar]
- Hernández-Ruiz J, Cano A, Arnao MB(2004) Melatonin: A growth-stimulating compound present in lupin tissues. Planta 220: 140–144 [DOI] [PubMed] [Google Scholar]
- Hernández-Ruiz J, Cano A, Arnao MB(2005) Melatonin acts as a growth-stimulating compound in some monocot species. J Pineal Res 39: 137–142 [DOI] [PubMed] [Google Scholar]
- Hijaz F, Al-Rimawi F, Manthey JA, Killiny N(2020) Phenolics, flavonoids and antioxidant capacities in Citrus species with different degree of tolerance to huanglongbing. Plant Signal Behav 15: 1752447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijaz F, El-Shesheny I, Killiny N(2013) Herbivory by the insect Diaphorina citri induces greater change in citrus plant volatile profile than does infection by the bacterium, Candidatus Liberibacter asiaticus. Plant Signal Behav 8: 25677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijaz F, Killiny N(2014) Collection and chemical composition of phloem sap from Citrus sinensis L. Osbeck (sweet orange). PLoS ONE 9: e101830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Li L, Zheng M, Chen F, Long H, Deng G, Pan Z, Liang J, Li Q, Yu M, et al. (2018) The tryptophan decarboxylase 1 gene from Aegilops variabilis No.1 regulate the resistance against cereal cyst nematode by altering the downstream secondary metabolite contents rather than auxin synthesis. Front Plant Sci 9: 1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara A, Hashimoto Y, Tanaka C, Dubouzet JG, Nakao T, Matsuda F, Nishioka T, Miyagawa H, Wakasa K(2008) The tryptophan pathway is involved in the defense responses of rice against pathogenic infection via serotonin production. Plant J 54: 481–495 [DOI] [PubMed] [Google Scholar]
- Jan JE, Reiter RJ, Wasdell MB, Bax M(2009) The role of the thalamus in sleep, pineal melatonin production, and circadian rhythm sleep disorders. J Pineal Res 46: 1–7 [DOI] [PubMed] [Google Scholar]
- Johns NP, Johns J, Porasuphatana S, Plaimee P, Sae-Teaw M(2013) Dietary intake of melatonin from tropical fruit altered urinary excretion of 6-sulfatoxymelatonin in healthy volunteers. J Agric Food Chem 61: 913–919 [DOI] [PubMed] [Google Scholar]
- Kang K, Lee K, Park S, Byeon Y, Back K(2013) Molecular cloning of rice serotonin N-acetyltransferase, the penultimate gene in plant melatonin biosynthesis. J Pineal Res 55: 7–13 [DOI] [PubMed] [Google Scholar]
- Kang K, Lee K, Park S, Kim YS, Back K(2010) Enhanced production of melatonin by ectopic overexpression of human serotonin N-acetyltransferase plays a role in cold resistance in transgenic rice seedlings. J Pineal Res 49: 176–182 [DOI] [PubMed] [Google Scholar]
- Kang S, Kang K, Lee K, Back K(2007) Characterization of rice tryptophan decarboxylases and their direct involvement in serotonin biosynthesis in transgenic rice. Planta 227: 263–272 [DOI] [PubMed] [Google Scholar]
- Killiny N, Hijaz F(2016) Amino acids implicated in plant defense are higher in Candidatus Liberibacter asiaticus-tolerant citrus varieties. Plant Signal Behav 11: e1171449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killiny N, Hijaz F, Ebert TA, Rogers ME(2017) A plant bacterial pathogen manipulates its insect vector’s energy metabolism. Appl Environ Microbiol 83: e03005-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killiny N, Nehela Y(2017a) Metabolomic response to huanglongbing: Role of carboxylic compounds in Citrus sinensis response to ‘Candidatus Liberibacter asiaticus’ and its vector, Diaphorina citri. Mol Plant Microbe Interact 30: 666–678 [DOI] [PubMed] [Google Scholar]
- Killiny N, Nehela Y(2017b) One target, two mechanisms: The impact of “Candidatus Liberibacter asiaticus” and its vector, Diaphorina citri, on citrus leaf pigments. Mol Plant Microbe Interact 30: 543–556 [DOI] [PubMed] [Google Scholar]
- Kolar J, Johnson CH, Machackova I(2003) Exogenously applied melatonin (N-acetyl-5-methoxytryptamine) affects flowering of the short-day plant Chenopodium rubrum. Physiol Plant 118: 605–612 [Google Scholar]
- Kunkel BN, Brooks DM(2002) Cross talk between signaling pathways in pathogen defense. Curr Opin Plant Biol 5: 325–331 [DOI] [PubMed] [Google Scholar]
- Lee HY, Back K(2016) Mitogen-activated protein kinase pathways are required for melatonin-mediated defense responses in plants. J Pineal Res 60: 327–335 [DOI] [PubMed] [Google Scholar]
- Lee HY, Back K(2017a) Melatonin is required for H2O2- and NO-mediated defense signaling through MAPKKK3 and OXI1 in Arabidopsis thaliana. J Pineal Res 62: e12379. [DOI] [PubMed] [Google Scholar]
- Lee HY, Byeon Y, Back K(2014) Melatonin as a signal molecule triggering defense responses against pathogen attack in Arabidopsis and tobacco. J Pineal Res 57: 262–268 [DOI] [PubMed] [Google Scholar]
- Lee HY, Byeon Y, Tan DX, Reiter RJ, Back K(2015) Arabidopsis serotonin N-acetyltransferase knockout mutant plants exhibit decreased melatonin and salicylic acid levels resulting in susceptibility to an avirulent pathogen. J Pineal Res 58: 291–299 [DOI] [PubMed] [Google Scholar]
- Lee K, Back K(2017b) Overexpression of rice serotonin N-acetyltransferase 1 in transgenic rice plants confers resistance to cadmium and senescence and increases grain yield. J Pineal Res 62: e12392. [DOI] [PubMed] [Google Scholar]
- Lee K, Back K(2019) Melatonin-deficient rice plants show a common semidwarf phenotype either dependent or independent of brassinosteroid biosynthesis. J Pineal Res 66: e12537. [DOI] [PubMed] [Google Scholar]
- Lerner AB, Case JD, Takahashi Y, Lee TH, Mori W(1958) Isolation of melatonin, the pineal gland factor that lightens melanocytes. J Am Chem Soc 80: 2587 [Google Scholar]
- Liang B, Ma C, Zhang Z, Wei Z, Gao T, Zhao Q, Ma F, Li C(2018) Long-term exogenous application of melatonin improves nutrient uptake fluxes in apple plants under moderate drought stress. Environ Exp Bot 155: 650–661 [Google Scholar]
- Lin L, Li J, Chen F, Liao M, Tang Y, Liang D, Xia H, Lai Y, Wang X, Chen C, et al. (2018) Effects of melatonin on the growth and cadmium characteristics of Cyphomandra betacea seedlings. Environ Monit Assess 190: 119. [DOI] [PubMed] [Google Scholar]
- Liu J, Yue R, Si M, Wu M, Cong L, Zhai R, Yang C, Wang Z, Ma F, Xu L(2019) Effects of exogenous application of melatonin on quality and sugar metabolism in ‘Zaosu’ pear fruit. J Plant Growth Regul 38: 1161–1169 [Google Scholar]
- Liu J, Zhai R, Liu F, Zhao Y, Wang H, Liu L, Yang C, Wang Z, Ma F, Xu L(2018) Melatonin induces parthenocarpy by regulating genes in gibberellin pathways of ‘Starkrimson’ pear (Pyrus communis L.). Front Plant Sci 9: 946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD(2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ ΔC(T)) method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Lu R, Liu Z, Shao Y, Sun F, Zhang Y, Cui J, Zhou Y, Shen W, Zhou T(2019) Melatonin is responsible for rice resistance to rice stripe virus infection through a nitric oxide-dependent pathway. Virol J 16: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mafra V, Kubo KS, Alves-Ferreira M, Ribeiro-Alves M, Stuart RM, Boava LP, Rodrigues CM, Machado MA(2012) Reference genes for accurate transcript normalization in citrus genotypes under different experimental conditions. PLoS ONE 7: e31263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal MK, Suren H, Ward B, Boroujerdi A, Kousik C(2018) Differential roles of melatonin in plant-host resistance and pathogen suppression in cucurbits. J Pineal Res 65: e12505. [DOI] [PubMed] [Google Scholar]
- Meldau S, Ullman-Zeunert L, Govind G, Bartram S, Baldwin IT(2012) MAPK-dependent JA and SA signalling in Nicotiana attenuata affects plant growth and fitness during competition with conspecifics. BMC Plant Biol 12: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustafa-Farag M, Almoneafy A, Mahmoud A, Elkelish A, Arnao MB, Li L, Ai S(2019) Melatonin and its protective role against biotic stress impacts on plants. Biomolecules 10: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mur LAJ, Kenton P, Atzorn R, Miersch O, Wasternack C(2006) The outcomes of concentration-specific interactions between salicylate and jasmonate signaling include synergy, antagonism, and oxidative stress leading to cell death. Plant Physiol 140: 249–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz MA, Huang Y, Bie Z, Ahmed W, Reiter RJ, Niu M, Hameed S(2016) Melatonin: Current status and future perspectives in plant science. Front Plant Sci 6: 1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehela Y, Hijaz F, Elzaawely AA, El-Zahaby HM, Killiny N(2016) Phytohormone profiling of the sweet orange (Citrus sinensis (L.) Osbeck) leaves and roots using GC-MS-based method. J Plant Physiol 199: 12–17 [DOI] [PubMed] [Google Scholar]
- Nehela Y, Hijaz F, Elzaawely AA, El-Zahaby HM, Killiny N(2018) Citrus phytohormonal response to Candidatus Liberibacter asiaticus and its vector Diaphorina citri. Physiol Mol Plant Pathol 102: 24–35 [Google Scholar]
- Nehela Y, Killiny N(2018) Infection with phytopathogenic bacterium inhibits melatonin biosynthesis, decreases longevity of its vector, and suppresses the free radical-defense. J Pineal Res 65: e12511. [DOI] [PubMed] [Google Scholar]
- Nehela Y, Killiny N(2019) ‘Candidatus Liberibacter asiaticus’ and its vector, Diaphorina citri, augment the tricarboxylic acid cycle of their host via the γ-aminobutyric acid shunt and polyamines pathway. Mol Plant Microbe Interact 32: 413–427 [DOI] [PubMed] [Google Scholar]
- Nùñez-Vergara LJ, Squella JA, Sturm JC, Baez H, Camargo C(2001) Simultaneous determination of melatonin and pyridoxine in tablets by gas chromatography-mass spectrometry. J Pharm Biomed Anal 26: 929–938 [DOI] [PubMed] [Google Scholar]
- Oztürk AI, Yilmaz O, Kirbağ S, Arslan M(2000) Antimicrobial and biological effects of ipemphos and amphos on bacterial and yeast strains. Cell Biochem Funct 18: 117–126 [DOI] [PubMed] [Google Scholar]
- Park M, Kang K, Park S, Back K(2008) Conversion of 5-hydroxytryptophan into serotonin by tryptophan decarboxylase in plants, Escherichia coli, and yeast. Biosci Biotechnol Biochem 72: 2456–2458 [DOI] [PubMed] [Google Scholar]
- Park S, Byeon Y, Back K(2013) Functional analyses of three ASMT gene family members in rice plants. J Pineal Res 55: 409–415 [DOI] [PubMed] [Google Scholar]
- Park S, Kang K, Lee SW, Ahn MJ, Bae JM, Back K(2011) Production of serotonin by dual expression of tryptophan decarboxylase and tryptamine 5-hydroxylase in Escherichia coli. Appl Microbiol Biotechnol 89: 1387–1394 [DOI] [PubMed] [Google Scholar]
- Park S, Lee K, Kim YS, Back K(2012) Tryptamine 5-hydroxylase-deficient Sekiguchi rice induces synthesis of 5-hydroxytryptophan and N-acetyltryptamine but decreases melatonin biosynthesis during senescence process of detached leaves. J Pineal Res 52: 211–216 [DOI] [PubMed] [Google Scholar]
- Qian Y, Tan DX, Reiter RJ, Shi H(2015) Comparative metabolomic analysis highlights the involvement of sugars and glycerol in melatonin-mediated innate immunity against bacterial pathogen in Arabidopsis. Sci Rep 5: 15815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raut JS, Shinde RB, Karuppayil MS(2012) Indole, a bacterial signaling molecule, exhibits inhibitory activity against growth, dimorphism and biofilm formation in Candida albicans. Afr J Microbiol Res 6: 6005–6012 [Google Scholar]
- Ren S, Deng Q, Peng J, Lin L, Zhang H(2018) Effects of exogenous melatonin on growth and cadmium content of Zizyphus acidojujuba seedlings. IOP Conf Ser Earth Environ Sci 199: 042006 [Google Scholar]
- Robert-Seilaniantz A, Navarro L, Bari R, Jones JD(2007) Pathological hormone imbalances. Curr Opin Plant Biol 10: 372–379 [DOI] [PubMed] [Google Scholar]
- Sarropoulou VN, Therios IN, Dimassi-Theriou KN(2012) Melatonin promotes adventitious root regeneration in in vitro shoot tip explants of the commercial sweet cherry rootstocks CAB-6P (Prunus cerasus L.), Gisela 6 (P. cerasus × P. canescens), and MxM 60 (P. avium × P. mahaleb). J Pineal Res 52: 38–46 [DOI] [PubMed] [Google Scholar]
- Shi H, Chen Y, Tan DX, Reiter RJ, Chan Z, He C(2015) Melatonin induces nitric oxide and the potential mechanisms relate to innate immunity against bacterial pathogen infection in Arabidopsis. J Pineal Res 59: 102–108 [DOI] [PubMed] [Google Scholar]
- Shi H, Wei Y, He C(2016) Melatonin-induced CBF/DREB1s are essential for diurnal change of disease resistance and CCA1 expression in Arabidopsis. Plant Physiol Biochem 100: 150–155 [DOI] [PubMed] [Google Scholar]
- Shi X, Xu S, Mu D, Sadeghnezhad E, Li Q, Ma Z, Zhao L, Zhang Q, Wang L(2019) Exogenous melatonin delays dark-induced grape leaf senescence by regulation of antioxidant system and senescence associated genes (SAGs). Plants (Basel) 8: 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Zhang N, Wang J, Zhang H, Li D, Shi J, Li R, Weeda S, Zhao B, Ren S, et al. (2015) Melatonin promotes ripening and improves quality of tomato fruit during postharvest life. J Exp Bot 66: 657–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan DX, Zheng X, Kong J, Manchester LC, Hardeland R, Kim SJ, Xu X, Reiter RJ(2014) Fundamental issues related to the origin of melatonin and melatonin isomers during evolution: Relation to their biological functions. Int J Mol Sci 15: 15858–15890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatineni S, Sagaram US, Gowda S, Robertson CJ, Dawson WO, Iwanami T, Wang N(2008) In planta distribution of ‘Candidatus Liberibacter asiaticus’ as revealed by polymerase chain reaction (PCR) and real-time PCR. Phytopathology 98: 592–599 [DOI] [PubMed] [Google Scholar]
- Tekbas OF, Ogur R, Korkmaz A, Kilic A, Reiter RJ(2008) Melatonin as an antibiotic: New insights into the actions of this ubiquitous molecule. J Pineal Res 44: 222–226 [DOI] [PubMed] [Google Scholar]
- Tijero V, Muñoz P, Munné-Bosch S(2019) Melatonin as an inhibitor of sweet cherries ripening in orchard trees. Plant Physiol Biochem 140: 88–95 [DOI] [PubMed] [Google Scholar]
- Tiryaki I, Keles H(2012) Reversal of the inhibitory effect of light and high temperature on germination of Phacelia tanacetifolia seeds by melatonin. J Pineal Res 52: 332–339 [DOI] [PubMed] [Google Scholar]
- Turner JG, Ellis C, Devoto A(2002) The jasmonate signal pathway. Plant Cell 14(Suppl): S153–S164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchendu EE, Shukla MR, Reed BM, Saxena PK(2013) Melatonin enhances the recovery of cryopreserved shoot tips of American elm (Ulmus americana L.). J Pineal Res 55: 435–442 [DOI] [PubMed] [Google Scholar]
- Vielma JR, Bonilla E, Chacín-Bonilla L, Mora M, Medina-Leendertz S, Bravo Y(2014) Effects of melatonin on oxidative stress, and resistance to bacterial, parasitic, and viral infections: A review. Acta Trop 137: 31–38 [DOI] [PubMed] [Google Scholar]
- Wang HX, Liu F, Ng TB(2001) Examination of pineal indoles and 6-methoxy-2-benzoxazolinone for antioxidant and antimicrobial effects. Comp Biochem Physiol C Toxicol Pharmacol 130: 379–388 [DOI] [PubMed] [Google Scholar]
- Wang N, Trivedi P(2013) Citrus huanglongbing: A newly relevant disease presents unprecedented challenges. Phytopathology 103: 652–665 [DOI] [PubMed] [Google Scholar]
- Wang P, Sun X, Li C, Wei Z, Liang D, Ma F(2013) Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J Pineal Res 54: 292–302 [DOI] [PubMed] [Google Scholar]
- Wang P, Yin L, Liang D, Li C, Ma F, Yue Z(2012) Delayed senescence of apple leaves by exogenous melatonin treatment: Toward regulating the ascorbate-glutathione cycle. J Pineal Res 53: 11–20 [DOI] [PubMed] [Google Scholar]
- Ward JH.(1963) Hierarchical grouping to optimize an objective function. J Am Stat Assoc 58: 236–244 [Google Scholar]
- Weeda S, Zhang N, Zhao X, Ndip G, Guo Y, Buck GA, Fu C, Ren S(2014) Arabidopsis transcriptome analysis reveals key roles of melatonin in plant defense systems. PLoS ONE 9: e93462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Li QT, Chu YN, Reiter RJ, Yu XM, Zhu DH, Zhang WK, Ma B, Lin Q, Zhang JS, et al. (2015) Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J Exp Bot 66: 695–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Chen C, Yu Q, Gady A, Yu Y, Liang G, Gmitter FG(2014a) Novel expression patterns of carotenoid pathway-related genes in citrus leaves and maturing fruits. Tree Genet Genomes 10: 439–448 [Google Scholar]
- Wei X, Chen C, Yu Q, Gady A, Yu Y, Liang G, Gmitter FG Jr.(2014b) Comparison of carotenoid accumulation and biosynthetic gene expression between Valencia and Rohde Red Valencia sweet oranges. Plant Sci 227: 28–36 [DOI] [PubMed] [Google Scholar]
- Wei Y, Chang Y, Zeng H, Liu G, He C, Shi H(2018a) RAV transcription factors are essential for disease resistance against cassava bacterial blight via activation of melatonin biosynthesis genes. J Pineal Res 64: e12454. [DOI] [PubMed] [Google Scholar]
- Wei Y, Hu W, Wang Q, Zeng H, Li X, Yan Y, Reiter RJ, He C, Shi H(2017) Identification, transcriptional and functional analysis of heat-shock protein 90s in banana (Musa acuminata L.) highlight their novel role in melatonin-mediated plant response to Fusarium wilt. J Pineal Res 62: e12367. [DOI] [PubMed] [Google Scholar]
- Wei Y, Zeng H, Hu W, Chen L, He C, Shi H(2016) Comparative transcriptional profiling of melatonin synthesis and catabolic genes indicates the possible role of melatonin in developmental and stress responses in rice. Front Plant Sci 7: 676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z, Gao T, Liang B, Zhao Q, Ma F, Li C(2018b) Effects of exogenous melatonin on methyl viologen-mediated oxidative stress in apple leaf. Int J Mol Sci 19: 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wójciak-kosior M, Woźniak A(2008) Quantitative determination of melatonin in Lamium album flos. Herba Pol 54: 13–18 [Google Scholar]
- Wu Y, Zhang D, Chu JY, Boyle P, Wang Y, Brindle ID, De Luca V, Després C(2012) The Arabidopsis NPR1 protein is a receptor for the plant defense hormone salicylic acid. Cell Rep 1: 639–647 [DOI] [PubMed] [Google Scholar]
- Xian C, Laborda P, Liu F(2020) Exogenous melatonin enhances rice plant resistance against Xanthomonas oryzae pv. oryzae. Plant Dis 104: 1701–1708 [DOI] [PubMed] [Google Scholar]
- Xu W, Cai SY, Zhang Y, Wang Y, Ahammed GJ, Xia XJ, Shi K, Zhou YH, Yu JQ, Reiter RJ, et al. (2016) Melatonin enhances thermotolerance by promoting cellular protein protection in tomato plants. J Pineal Res 61: 457–469 [DOI] [PubMed] [Google Scholar]
- Yan Y, Sun S, Zhao N, Yang W, Shi Q, Gong B(2019) COMT1 overexpression resulting in increased melatonin biosynthesis contributes to the alleviation of carbendazim phytotoxicity and residues in tomato plants. Environ Pollut 252: 51–61 [DOI] [PubMed] [Google Scholar]
- Yang C, Yu Y, Sun W, Xia C(2014) Indole derivatives inhibited the formation of bacterial biofilm and modulated Ca2+ efflux in diatom. Mar Pollut Bull 88: 62–69 [DOI] [PubMed] [Google Scholar]
- Yin L, Wang P, Li M, Ke X, Li C, Liang D, Wu S, Ma X, Li C, Zou Y, et al. (2013) Exogenous melatonin improves Malus resistance to Marssonina apple blotch. J Pineal Res 54: 426–434 [DOI] [PubMed] [Google Scholar]
- Zhai R, Liu J, Liu F, Zhao Y, Liu L, Fang C, Wang H, Li X, Wang Z, Ma F, et al. (2018) Melatonin limited ethylene production, softening and reduced physiology disorder in pear (Pyrus communis L.) fruit during senescence. Postharvest Biol Technol 139: 38–46 [Google Scholar]
- Zhang H, Wang L, Shi K, Shan D, Zhu Y, Wang C, Bai Y, Yan T, Zheng X, Kong J(2019) Apple tree flowering is mediated by low level of melatonin under the regulation of seasonal light signal. J Pineal Res 66: e12551. [DOI] [PubMed] [Google Scholar]
- Zhang HJ, Zhang N, Yang RC, Wang L, Sun QQ, Li DB, Cao YY, Weeda S, Zhao B, Ren S, et al. (2014) Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in cucumber (Cucumis sativus L.). J Pineal Res 57: 269–279 [DOI] [PubMed] [Google Scholar]
- Zhang J, Li H, Xu B, Li J, Huang B(2016) Exogenous melatonin suppresses dark-induced leaf senescence by activating the superoxide dismutase-catalase antioxidant pathway and down-regulating chlorophyll degradation in excised leaves of perennial ryegrass (Lolium perenne L.). Front Plant Sci 7: 1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Zhao B, Zhang HJ, Weeda S, Yang C, Yang ZC, Ren S, Guo YD(2013) Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J Pineal Res 54: 15–23 [DOI] [PubMed] [Google Scholar]
- Zhang S, Zheng X, Reiter RJ, Feng S, Wang Y, Liu S, Jin L, Li Z, Datla R, Ren M(2017) Melatonin attenuates potato late blight by disrupting cell growth, stress tolerance, fungicide susceptibility and homeostasis of gene expression in Phytophthora infestans. Front Plant Sci 8: 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Wang R, Liu D, Wu Y, Sun J, Tao J(2018) Melatonin and expression of tryptophan decarboxylase gene (TDC) in herbaceous peony (Paeonia lactiflora Pall.) flowers. Molecules 23: 1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Xu L, Su T, Jiang Y, Hu L, Ma F(2015) Melatonin regulates carbohydrate metabolism and defenses against Pseudomonas syringae pv. tomato DC3000 infection in Arabidopsis thaliana. J Pineal Res 59: 109–119 [DOI] [PubMed] [Google Scholar]
- Zhao L, Chen L, Gu P, Zhan X, Zhang Y, Hou C, Wu Z, Wu YF, Wang QC(2019) Exogenous application of melatonin improves plant resistance to virus infection. Plant Pathol 68: 1287–1295 [Google Scholar]
- Zheng X, Tan DX, Allan AC, Zuo B, Zhao Y, Reiter RJ, Wang L, Wang Z, Guo Y, Zhou J, et al. (2017) Chloroplastic biosynthesis of melatonin and its involvement in protection of plants from salt stress. Sci Rep 7: 41236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo B, Zheng X, He P, Wang L, Lei Q, Feng C, Zhou J, Li Q, Han Z, Kong J(2014) Overexpression of MzASMT improves melatonin production and enhances drought tolerance in transgenic Arabidopsis thaliana plants. J Pineal Res 57: 408–417 [DOI] [PubMed] [Google Scholar]