Abstract
Acute ischemic stroke is one of the leading causes of death and long-term disability in the United States. Intravenous thrombolysis with recombinant tissue-type plasminogen activator (rt-PA) has been the mainstay of acute therapy. However, multiple randomized clinical trials have been published that have shown higher rates of recanalization and improved functional outcomes with endovascular therapy compared with intravenous rt-PA in patients with ischemic stroke from large vessel occlusion. This article provides an update and discusses the role of endovascular therapy in management of acute ischemic stroke.
Introduction
Stroke is the leading cause of serious long-term disability and the fifth most common cause of death in the U.S. Ischemic stroke accounts for approximately 87% of all strokes in the U.S.1 For almost two decades, prompt administration of intravenous fibrinolytic treatment with recombinant tissue plasminogen activator (rt-PA), within 4.5 hours of the onset of symptoms in eligible patients, has been the standard of care in management of acute ischemic stroke.2 Despite improvement in functional outcomes at three to six months in patients who receive rt-PA, 3–5 the efficacy of IV rt-PA in achieving recanalization continues to be limited (Table 1). Limitations of rt-PA include - a narrow therapeutic time window (within 3 – 4.5 hours), resistance of an old or large thrombus to fibrinolysis, risks of systemic and cerebral hemorrhage and lower rates of recanalization in patients with proximal vessel occlusion. 4,7,12,13 This led to evolution of endovascular approaches devised to reduce clot burden, improve vessel recanalization, and hence improve functional outcomes. Table 2 describes the most commonly used terms in endovascular management of acute ischemic stroke.
Table 1.
Results of Treatment with IV rt-PA in Acute Ischemic Stroke
| Rate of recanalization | 20 – 46%6 |
| Rate of death or disability | 47 – 61%4,7–10 |
| Percentage of eligible patients who fail to receive rt-PA | 18–25%11 |
rt-PA – Recombinant tissue plasminogen activator
Table 2.
Description of Selective Stroke Terminology
| Time Last Known well | The time prior to hospital arrival at which the patient was last known to be without the signs and symptoms of the current stroke or at his or her baseline state of health. |
| Endovascular Treatment | Catheter based approach that may include administration of intra-arterial thrombolytic, and/or mechanical clot retrieval (thrombectomy). |
| The National Institute of Health Stroke Scale (NIHSS) | A 42-point score that objectively quantifies severity of neurological impairment from acute stroke. |
| Recanalization | Complete or partial restoration of cerebral blood flow as determined on pre and post cerebral angiography. |
| Alberta Stroke Program Early CT Score (ASPECTS) | A 10-point quantitative topographic CT scan score to determine early ischemic changes (<3 hours from symptoms onset) in anterior circulation stroke. |
| Modified Rankin Score (mRS) | A 6-point disability score most widely used in stroke clinical trials to document outcome measure. |
| Functional Independence | Defined by a mRS score of 0 – 2 |
| Symptomatic Intracerebral Hemorrhage (sICH) | Radiographic appearance of hemorrhage associated with neurological deterioration post thrombolytic/endovascular therapy. |
Clinical Evidence
In 2013 three randomized controlled trials of endovascular treatment of acute ischemic stroke with primarily intra-arterial (IA) fibrinolysis and/or first generation-mechanical embolectomy devices were published: The Interventional Management of Stroke III (IMS III), 13 Magnetic Resonance and Recanalization of Stroke Clots Using Embolectomy (MR RESCUE)14 and Local versus Systemic Thrombolysis for Acute Ischemic Stroke (SYNTHESIS EXPANSION).15 The results showed that endovascular therapy was non superior to the standard treatment with intravenous rt-PA alone with similar safety outcomes and no significant differences in functional independence. On further analysis it was observed that patient selection criteria used in the these trials (included patients with minor ischemic stroke with NIHSS < 6), lack of arterial occlusion confirmation on imaging (CT or MR angiography), delayed time of endovascular intervention, mechanical thrombectomy as a sole endovascular treatment option and use of old generation thrombectomy devices/stent retrievers; may have contributed towards a lack of clinical benefit of endovascular therapy as compared to medical management.16
Learning from the shortcomings of these trials, in 2015 five landmark randomized controlled trials demonstrated improved clinical outcomes and rates of recanalization of occluded artery with use of new generation mechanical thrombectomy devices (stent retrievers) in acute ischemic stroke with large vessel occlusion in the anterior circulation (Table 3). This led to a paradigm shift in the management of acute ischemic stroke and formed the basis for updating the American Heart Association (AHA)/American Stroke Association guidelines in 2015, establishing the use of endovascular therapy in patients with acute ischemic stroke with a large vessel occlusion.2 Later a meta-analysis, comprising data from 1,287 patients was performed by the Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials (HERMES) collaborators.22 The results showed that the proportion of patients achieving functional independence at 90 days (defined as modified Rankin Score of 0–2) was 46.0% in the intervention group (mechanical thrombectomy) compared with 26.5% in the control group (odds ratio: 2.35; 95% confidence interval [CI]: 1.85 to 2.98; p <0.0001). These findings further emphasized the benefit of endovascular treatment for management of acute ischemic stroke in anterior circulation large vessel occlusion over medical management and helped paved the way for establishing endovascular therapy as a standard of care for management of acute ischemic stroke in select patient population.
Table 3.
Summary of five major Randomized Controlled Trials of Endovascular Therapy in Acute Ischemic Stroke
| MR CLEAN17 | ESCAPE18 | SWIFT PRIME19 | EXTEND-IA20 | REVASCAT21 | |
|---|---|---|---|---|---|
| No of patients | 500 | 316 | 196 | 70 | 206 |
| Age (years) | ≥18 | ≥18 | 18–80 | ≥18 | 18–85 |
| NIHSS inclusion criteria | ≥2 | >5 | 8–29 | None | ≥6 |
| Treatment Arm | IV rt-PA + IA UK/rt-PA/device | Stent retriever ± IV rt-PA | Stent retriever ± IV rt-PA | Stent retriever ± IV rt-PA | Stent retriever ± IV rt-PA |
| Control Arm (Standard care) | ± IV rt-PA | ± IV rt-PA | ± IV rt-PA | ± IV rt-PA | ± IV rt-PA |
| IV rt-PA use | 87% | 72.7% | 100% | 100% | 68% |
| Median time from stroke onset to groin puncture (min) | 260 | 200 | 224 | 210 | 269 |
| Use of Stent retriever | 81.5% | 86.1% | 89% | 77% | 95% |
| Rate of recanalization [TICI 2b/3a] | 59% | 72.4% | 88% | 86% | 66% |
| Functional Independence (mRS 0–2) | 32.6% vs. 19.1% | 53.0% vs. 29.3% | 60.0% vs. 35% | 71% vs. 40% | 43.7% vs. 28.2% |
| Mortality/sICH | No significant difference in death or sICH | Mortality at 90 days: 10.4% (treatment arm) vs. 19% (control arm). No significant difference in sICH | No significant difference in death or sICH | No significant difference in death or sICH | No significant difference in death or sICH |
MR CLEAN - Multicenter Randomized Control Trial of Endovascular treatment for Acute Ischemic Stroke; ESCAPE - Endovascular Treatment for Small Core and Anterior Circulation Proximal Occlusion With Emphasis on Minimizing CT to Recanalization Times; SWIFT PRIME- Solitaire FR With the Intention for Thrombectomy as Primary Endovascular Treatment of Acute Ischemic Stroke; EXTEND-IA - Extending the Time for Thrombolysis in Emergency Neurological Deficits–Intra-Arterial; REVASCAT - Randomized Trial of Revascularization With Solitaire FR Device Versus Best Medical Therapy in the Treatment of Acute Stroke Due to Anterior Circulation Large Vessel Occlusion Presenting Within 8 Hours of Symptom Onset; NIHSS - National Institutes of Health Stroke Scale; IV rt-PA - intravenous tissue plasminogen activator; IA - intra-arterial; UK – urokinase; TICI - Thrombolysis in Cerebral Infarction scale; mRS - modified Rankin scale; sICH – Spontaneous intracerebral hemorrhage.
Neuroimaging
As a rule, all patients suspected of having acute ischemic stroke should obtain neuroimaging on first arrival to a hospital before initiating any specific therapy. As per the 2019 AHA/ASA guidelines, both non-contrast CT (NCCT) and magnetic resonance (MR) imaging (MRI) is effective to exclude underlying hemorrhage and determine whether the patients with acute ischemic stroke are candidates for thrombolytic or endovascular therapy.23 However, NCCT head is preferred as the initial imaging test due to low cost, wide spread availability and faster speed of acquisition of images. The Alberta Stroke Program Early CT Score (ASPECTS)- a 10-point quantitative, topographic CT score is a simple and reliable method of assessing the extent of ischemic changes on NCCT which aids in rapid identification of patients who would benefit from endovascular therapy.24 Furthermore, for patients who meet criteria for endovascular therapy, a non-invasive intracranial vascular study – CT angiogram (CTA) or MR angiogram (MRA) of the head and neck; is strongly recommended during the initial imaging evaluation of acute stroke patients, without delaying administration of intravenous rt-PA (Figure 1).23 Obtaining CTA or MRA of head and neck allows rapid identification of large vessel occlusion, presence of collaterals, clinically significant vascular disease (e.g., atherosclerotic stenosis) as well as the aortic arch and great vessel anatomy. When evaluating patients with acute ischemic stroke within 6 hours of last known normal with large vessel occlusion and an Alberta Stroke Program Early Computed Tomography Score (ASPECTS) of ≥6, selection for mechanical thrombectomy based on CT and CTA or MRI and MRA is recommended in preference to obtaining additional imaging such as perfusion studies. However, in selected patients with AIS within 6 to 24 hours of last known normal who have large vessel occlusion (LVO) in the anterior circulation; obtaining CT perfusion (CTP), Diffusion Restriction Imaging (DWI-MRI), or MRI perfusion (MR-perfusion) is recommended to aid in patient selection for mechanical thrombectomy, but only when patients meet eligibility criteria from one of the trials (DAWN or DEFUSE)25,26 [Table 3] that showed benefit from mechanical thrombectomy in this extended time window [Class of recommendation I, Level of Evidence A].23 With recent advances in the management of acute ischemic stroke, multi-modal CT protocols including CT, CTA, and CTP are being made available increasingly in many centers which can help in quick decision making before attempting endovascular therapy.
Figure 1.
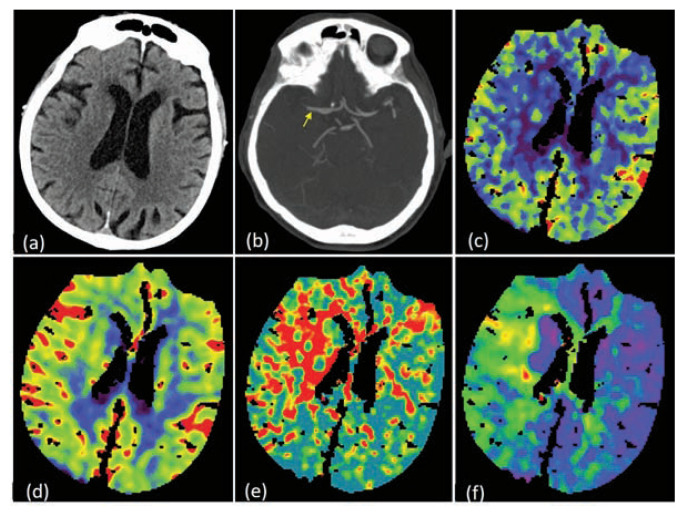
75-year old female presented with sudden onset left sided weakness with left facial droop. Time of onset of symptoms was 3.5 hours prior to coming to the hospital. Patient did not receive IV rt-PA as she was on Xarelto due to underlying atrial fibrillation. NIHSS score on admission was 7. NCCT head was negative for any intracranial bleed or early signs of ischemia (a). CTA head and neck showed occlusion (yellow arrow) of the right proximal M1 segment of MCA (b). CT perfusion study showed a large penumbra [reduced regional cerebral blood flow (c) with preserved regional cerebral blood volume (d), elevated mean transmit time (MTT) (e) and elevated Tmax (f)].
Patient Selection Criteria
As per the most recent American Heart Association (AHA)/American Stroke Association guidelines published in 2019,23 selected patients with acute ischemic stroke with onset of symptoms between 0–6 hours, should undergo mechanical thrombectomy (with a stent retriever) if they meet all of the following criteria [Class of recommendation I, Level of Evidence A]:
Pre-stroke modified Rankin Score (mRS) of 0 to 1 (functional independence)
Patients ≥ 18 years
Stroke severity on NIHSS ≥ 6
Computed tomography (CT) brain without evidence of large infarct suggested by Alberta Stroke Program Early CT Score (ASPECTS) of ≥ 6
Imaging proven causative occlusion of the Internal carotid artery (ICA) or proximal segment (M1) of middle cerebral artery (MCA).
Treatment can be initiated (groin puncture) within 6 hours of symptom onset.
In 2018 two major randomized controlled trials were published: DAWN (DWI or CTP Assessment with Clinical Mismatch in the Triage of Wake-Up and Late Presenting Strokes Undergoing Neurointervention with Trevo), 25 and DEFUSE 3 (The Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke); 26 which showed encouraging findings resulting in extending the time window for endovascular therapy in acute ischemic stroke within 6–24 hours from symptom onset. The DAWN trial used clinical-core mismatch, which is a mismatch between the severity of clinical deficit (NIHSS) and infarct volume on diffusion-weighted magnetic resonance imaging (MRI) or perfusion CT imaging (CTP), as an eligibility criteria to select patients with large anterior circulation vessel occlusion for treatment with mechanical thrombectomy between 6 and 24 hours from last known normal. The study demonstrated a benefit in functional outcome at 90 days in the treatment group (mechanical thrombectomy plus standard care) as compared to standard care alone (mRS score 0–2, 49% vs 13%; adjusted difference, 33% [95% CI, 21–44]. Whereas, the DEFUSE-3 trial used perfusion-core mismatch and maximum core size as imaging criteria to select patients with large anterior circulation occlusion 6 to 16 hours from last known normal for mechanical thrombectomy. The results showed a benefit in functional outcome at 90 days in the endovascular-therapy group as compared to the medical-therapy group alone (mRS score 0–2, 44.6% versus 16.7%; RR, 2.67 [95% CI, 1.60–4.48]; P<0.0001). Current guidelines recommend mechanical thrombectomy (preferably with a stent retriever) in selected patients with acute ischemic stroke within 6 to 16 hours of last known normal who have large vessel occlusion in the anterior circulation and meet other DAWN or DEFUSE 3 eligibility criteria (Table 4) [Class of recommendation I, level of evidence A].
Table 4.
Summary of DAWN and DEFUSE -3 Eligibility Criteria
| DAWN Criteria (6 to 24 hours)25 | DEFUSE-3 (6 to 16 hours)26 |
|---|---|
|
|
DAWN - DWI or CTP Assessment with Clinical Mismatch in the Triage of Wake-Up and Late Presenting Strokes Undergoing Neurointervention with Trevo; DEFUSE 3 - The Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke; NIHSS - National Institute of Health Stroke Scale; mRS - Modified Rankin Scale; MCA - Middle Cerebral Artery; ICA -Internal Carotid Artery; MRA - Magnetic Resonance Angiography; CTA - Computed Tomography Angiography; MR-DWI - Magnetic Resonance-Diffusion Weighted Imaging; CTP - Computed Tomography Perfusion
Other Indications for Endovascular Treatment
Vertebrobasilar Circulation Stroke
Approximately 20% of all ischemic strokes occur in the vertebrobasilar circulation, which includes acute basilar artery occlusion (BAO), and upto 80–90% of patients with acute basilar artery occlusion have a fatal and poor outcome.27 Unlike anterior circulation ischemic stroke, data on use of endovascular therapy and its efficacy in achieving successful revascularization in large vessel occlusion within vertebrobasilar circulation is sparse. However, small single-centre studies have shown good functional outcomes following basilar thrombectomy ranging from 30% to 48% and lower mortality (approximately 30 percent) than expected when compared with outcomes among patients who did not receive endovascular therapy.28–30 Due to lack of randomized controlled trials on endovascular treatment in patients with basilar artery occlusion, management of such patients should be guided by the severity of the symptoms, time of onset of symptoms, age, clinical status of the patient and presence of collaterals after careful evaluation of non-invasive vessel imaging.
Tandem Occlusions
Tandem occlusions, defined as simultaneous occurrence of an intracranial large vessel occlusion and a high-grade stenosis or occlusion of the ipsilateral proximal internal carotid artery (at the level of the cervical internal carotid artery), accounts for 10–20% of large vessel strokes.31,32 Previous studies have shown that patients with tandem occlusion (cervical ICA-MCA) have a lower likelihood of recanalization and poor outcomes as compared to isolated MCA occlusion after treatment with intravenous thrombolysis.32,33 Consequently, endovascular treatment has evolved as a more effective treatment option with or without prior intravenous rt-PA. However, there is uncertainty regarding the best approach (anterograde vs. retrograde) for treating tandem occlusions in acute ischemic stroke due to lack of randomized multicenter trials evaluating the different approaches and its effect on clinical outcomes. In a systematic review by Mbabuike et al.34 patients with acute ischemic stroke with tandem occlusions, a distal to proximal revascularization approach appeared to be more practical and effective with an advantage of decreased time to reperfusion and hence better functional outcome. The optimum approach for management of tandem occlusions and choice of therapy for management of proximal occlusion should be ascertained on an individual case to case basis after careful evaluation of the clinical severity, age, presence or absence of collaterals and bleeding risks.
Cerebral Angiography
Endovascular access is obtained through the common femoral artery in most cases. However, in patients with severe ilio-femoral arterial occlusions, unfavorable aortic arch anatomy, extremely tortuous vessels; alternate routes like trans-radial or trans-brachial can be used. After achieving access, a standard diagnostic catheter can then be used to engage the carotid arteries at which time digital subtraction angiography (DSA) is performed to visualize the cerebral vessels focusing on the specific artery of interest, its distal branches, site of occlusion and presence or absence of collaterals (Figure 2 and Figure 3). Other supplementary catheters like micro-catheter to pass the site of occlusion and large bore catheters for aspiration can then be utilized depending on the vessel caliber and site of occlusion.
Figure 2.
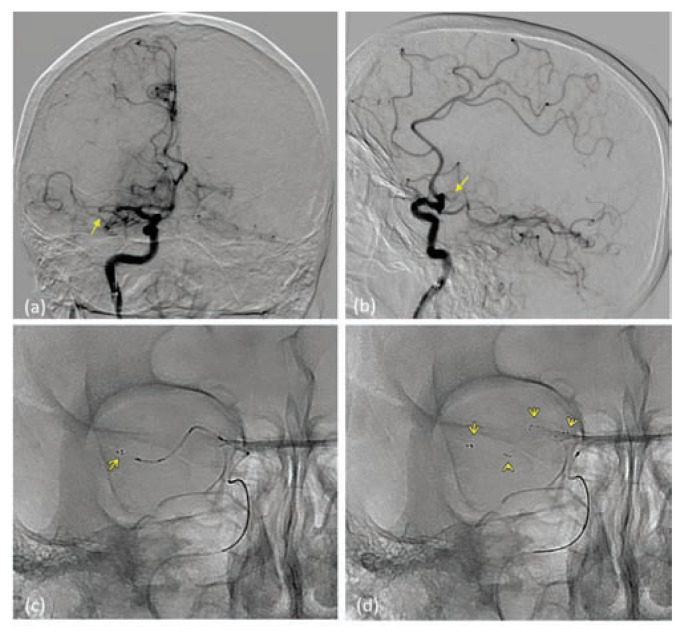
(a) and (b) - Selective right internal carotid injection showing occlusion (yellow arrow) in the proximal M1 segment of right MCA on axial (a) and lateral (b) projections. (c) and (d) - The solitaire revascularization device is seen deployed (yellow arrows highlighting the radiopaque markers) through the micro-catheter and across the occluding thrombus.
Figure 3.
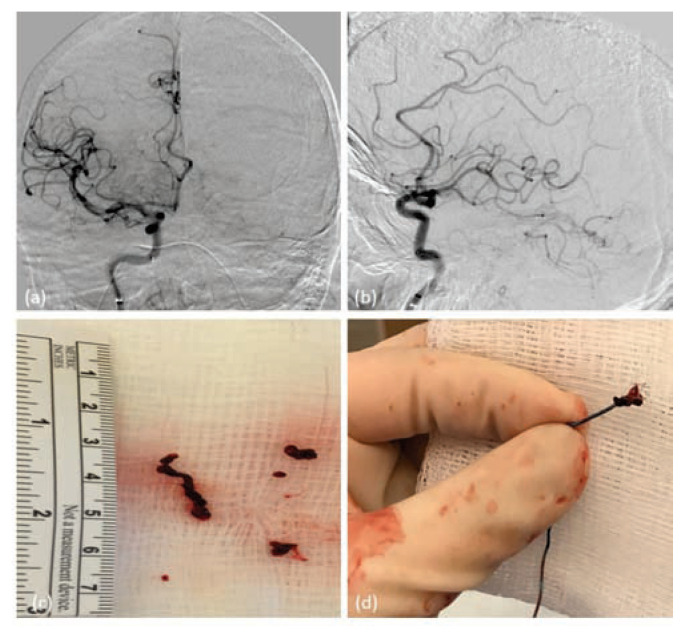
(a) and (b) – Selective Right Internal carotid injection showing complete recanalization of the previously occluded right middle cerebral artery M1 segment [TICI 3] with flow visualized in both superior and inferior divisions of middle cerebral artery. (c) and (d) – Shows the clot fragment retrieved after a combination of manual aspiration and removal of stent retriever.
Endovascular Recanalization Techniques
Endovascular therapy for management of acute ischemic stroke from a large vessel occlusion, has evolved rapidly over the years due to availability of newer generation thrombectomy devices and advancements in the catheter technology. Historically, intra-arterial fibrinolytic therapy with use of recombinant pro-urokinase in the vessel of interest, to achieve clot lysis, was used to achieve recanalization.35,36
Neurointerventionalists than began attempting mechanical thrombus disruption by repeated probing of the thrombus by a micro guidewire, microcatheter or snares. This when combined with use of intra-arterial fibrinolytic therapy leads to an increase in the surface area on which fibrinolytic agents can act, thus increasing efficacy of thrombolytic therapy [Figure 4 (a) and (b)]. 37–39 However, there is a risk of clot fragmentation and distal embolization with this approach leading to suboptimal rates of reperfusion. Intracranial stenting within the occluded segment using the Enterprise vascular reconstructive device (Codman, Raynham, Massachusetts, USA) was also tested as a potential tool to achieve mechanical clot disruption and partial restoration of blood flow in acute ischemic stroke.40,41 However, its use has been limited due to concomitant use of dual antiplatelets during the procedure and risks of bleeding.
Figure 4.
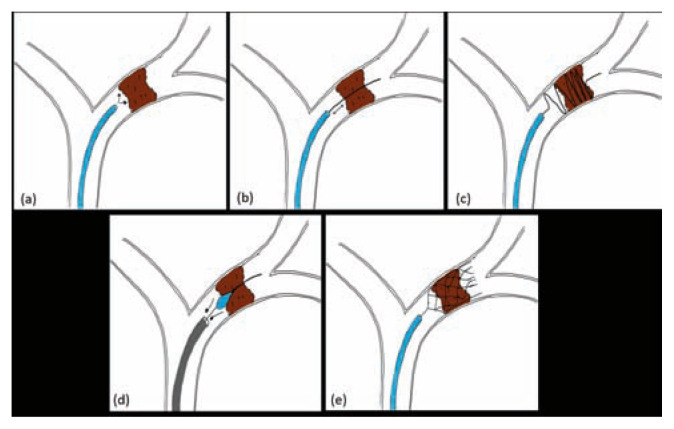
Illustration of different endovascular approaches used for management of acute ischemic stroke. (a) intra-arterial rt-PA, (b) Microwire manipulation, (c) Merci retriever, (d) Penumbra Aspiration, (e) Solitaire stent retriever.
Mechanical Thrombectomy Devices
The Merci retriever (Concentric Medical, Mountain View, California, USA), was the first stroke device to be approved by the FDA for mechanical thrombectomy in acute ischemic stroke patients. The Merci device (Mechanical Embolus Removal in Cerebral Ischemia) works primarily by advancing through the occlusion/thrombus using a micro-catheter and deploying distal to the thrombus [Figure 4 (c)]. This is followed by withdrawal of the Merci retriever with the thrombus captured by its helical loops, alongside the micro-catheter, using manual aspiration with a large syringe to reverse the flow and further aspirate any clot debris. However, thrombectomy with Merci device was associated with lower rates of recanalization ranging between 46%–55%.42,43
The next generation of mechanical thrombectomy devices, stent retriever, brought a paradigm shift in the endovascular management of acute ischemic stroke. Solitaire (ev3 Endovascular, Plymouth, Minnesota, USA) and Trevo Pro (Stryker Neurovascular, Kalamazoo Michigan, USA) are the most common stent retrievers used in the recent thrombectomy trials that showed a higher recanalization rates and a shorter time to treatment in ischemic stroke from large vessel occlusions.17–21,25,26 Stent retrievers are re-sheathable, re-constrainable, self-expanding stents mounted on a wire and are deployed within the thrombus through a microcatheter. The radial force of the stent compresses the thrombus against the vessel wall allowing restoration of the blood flow immediately. Leaving the device in place, for up to 5 mins, promotes engagement of the device with thrombus. The micro-catheter and stent are then withdrawn gently under continuous aspiration using a large syringe through the guiding-catheter to prevent the development of an embolism [Figure 4 (e)]. The use of stent retrievers is indicated in preference to the coil retrievers such as Merci device for mechanical thrombectomy 23
More recently, large caliber aspiration catheters have been made available [ACE 68, 5MAX, 5MAX ACE (Penumbra Inc., Alameda, CA, USA), AXS Catalyst 6 (Stryker), Sofia 6F (MicroVention, Aliso Viejo, CA, USA)] that can be advanced easily in the cerebral vasculature and can be placed at the level of thrombus over a microcatheter and is used to aspirate the thrombus directly using a syringe or Penumbra aspiration pump that is part of the Penumbra thrombectomy/aspiration system [Figure 4 (d)].44
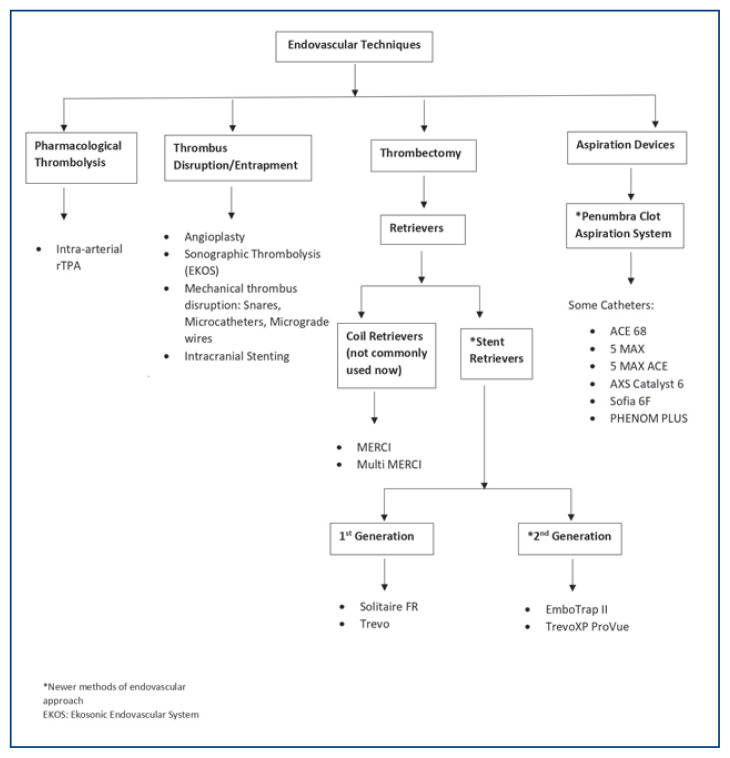
Figure 5 summaries the different endovascular revascularization strategies available currently. In general, the technical goal of the thrombectomy procedure should be reperfusion to a modified Thrombolysis in Cerebral Infarction (mTICI) grade 2b/3 angiographic result [2b= antegrade reperfusion of more than half of the previously occluded target artery ischemic territory, 3= complete antegrade reperfusion of the previously occluded target artery ischemic territory with absence of visualized occlusion in all distal branches],45 to maximize the probability of a good functional clinical outcome [Figure 3 (a) and (b)]. The use of adjunctive salvage techniques, including IA fibrinolysis, may be reasonable to achieve mTICI 2b/3 angiographic results if completed within 6h of symptom onset.23
Figure 5.
Summary of the different endovascular treatment options available currently. rTPA – Recombinant tissue plasminogen activator; EKOS – Ekosonic endovascular system; * - Newer generation thrombectomy devices.
Choice of Anesthesia
Both general anesthesia and conscious sedation can be used during endovascular therapy in acute ischemic stroke patients. However, the choice between general anesthesia and conscious sedation should be individualized based on clinical characteristics, tolerance of procedure and patient risk factors. A recent meta-analysis showed that use of general anesthesia may be associated with poorer outcomes as compared to conscious sedation in the setting of endovascular therapy.46 However, general anesthesia may be preferable in patients with depressed level of consciousness, respiratory compromise, and uncooperative or agitated patients for airway protection.
Post Procedure Care
All patients who undergo endovascular therapy for acute ischemic stroke should be admitted to neurological critical care units for closer monitoring. Low threshold should be kept for obtaining a non-contrast CT head to detect hemorrhagic transformation, particularly in patients who develop neurological decline post procedure. There is no consensus regarding the appropriate timing of obtaining follow up CT head and can range from immediately post procedure to 24 hours.
There is very limited data available to guide BP management during and after the procedure in patients who undergo mechanical thrombectomy. The ESCAPE protocol states that SBP ≥150 mm Hg is probably useful in promoting and keeping collateral flow adequate while the artery remains occluded.18 The DAWN protocol recommends maintaining SBP <140 mm Hg in the first 24 hours in subjects who are reperfused after mechanical thrombectomy.27 According to current AHA guidelines, it might be reasonable to maintain BP at a level < 180/105 mm Hg in patients who undergo mechanical thrombectomy with successful reperfusion.23
Vascular groin complications can arise from use of multiple or large bore catheters and should be monitored regularly. A standardized protocol should be followed diligently by the nursing staff and physicians for assessment of vascular complications and maintenance of hemostasis at the puncture site either with use of manual compression or vascular closure devices. Other post-procedural complications can include cardiac arrhythmias, temperature dysregulation, respiratory failure, hemorrhagic transformation, malignant cerebral edema, stroke evolution, and vessel re-thrombosis.
Conclusion
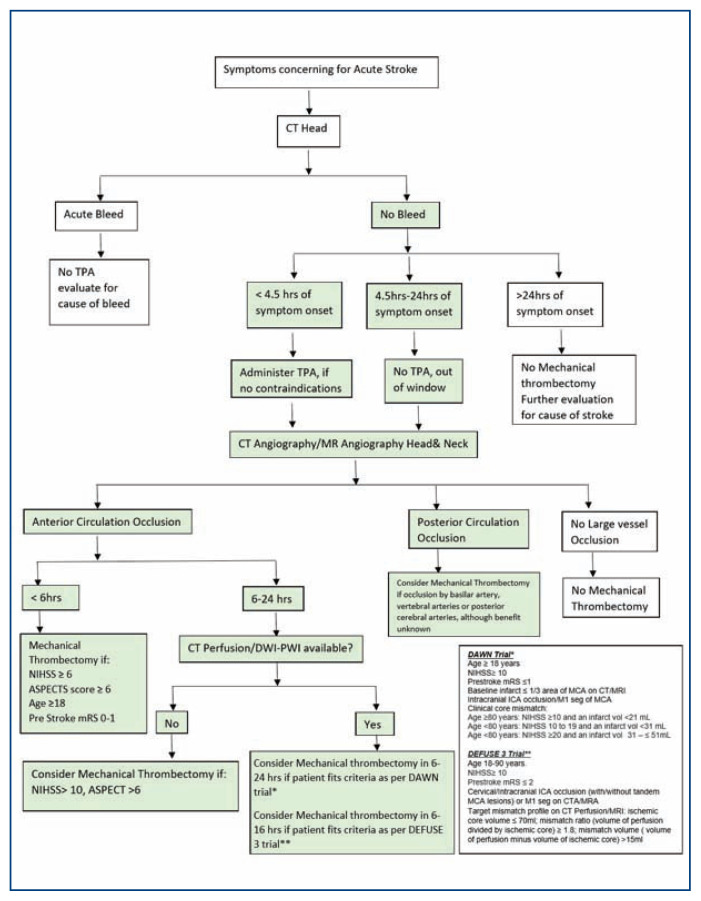
Endovascular therapy for management of acute ischemic stroke has evolved tremendously over the recent years and is currently considered standard of care in selected patients with large vessel occlusion within the anterior circulation. Figure 6 depicts a flowchart summarizing the imaging and patient selection criteria for endovascular therapy in patients with acute ischemic stroke. Diagnostic advancements in imaging with availability of multi model imaging protocols including CT perfusion, MR-DWI and MR-perfusion has made it possible to extend the time window for endovascular therapy up to 24 hours in select patient population, leading to improved functional outcomes. Recognition of large vessel related stroke and timely transfer of patients to specialized stroke centers with endovascular intervention capabilities, can help improve patient outcomes and improve stroke related morbidity.
Figure 6.
Flowchart summarizing the imaging and patient selection criteria for endovascular therapy in patients with acute ischemic stroke.
NIHSS - National Institute of Health Stroke Scale; mRS - Modified Rankin Scale; ASPECTS -Alberta Stroke Program Early Computed Tomography Score; MCA- Middle Cerebral artery; ICA – Internal carotid artery; DWI/PWI – Diffusion weighted perfusion imaging.
Footnotes
Kunal Bhatia, MD, (above), MSMA member since 2020, Sachin Bhagavan, MD, Navpreet Bains, DO, Brandi French, MD, Farhan Siddiq, MD, Camilo R. Gomez, MD, and Adnan I. Qureshi, MD are all with University of Missouri - Columbia School of Medicine/MU Health Care in Columbia, Missouri.
Disclosure
None reported.
References
- 1.Benjamin EJ, Muntner P, Alonso A, et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139(10):e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Powers WJ, Derdeyn CP, Biller J, et al. American Heart Association/American Stroke Association Focused Update of the 2013 Guidelines for the Early Management of Patients With Acute Ischemic Stroke Regarding Endovascular Treatment: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2015;46(10):3020–35. doi: 10.1161/STR.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 3.Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384(9958):1929–35. doi: 10.1016/S0140-6736(14)60584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375(9727):1695–703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 5.Wardlaw JM, Murray V, Berge E, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet. 2012;379(9834):2364–72. doi: 10.1016/S0140-6736(12)60738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38(3):967–73. doi: 10.1161/01.STR.0000258112.14918.24. [DOI] [PubMed] [Google Scholar]
- 7.The National Institute of Neurological Disorders and Stroke Rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 8.Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS) JAMA. 1995;274(13):1017–25. [PubMed] [Google Scholar]
- 9.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352(9136):1245–51. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 10.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–29. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 11.Messé SR, Khatri P, Reeves MJ, et al. Why are acute ischemic stroke patients not receiving IV tPA? Results from a national registry. Neurology. 2016;87(15):1565–1574. doi: 10.1212/WNL.0000000000003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhatia R, Hill MD, Shobha N, et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke. 2010;41(10):2254–8. doi: 10.1161/STROKEAHA.110.592535. [DOI] [PubMed] [Google Scholar]
- 13.Broderick JP, Palesch YY, Demchuk AM, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368(10):893–903. doi: 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kidwell CS, Jahan R, Gornbein J, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013;368(10):914–923. doi: 10.1056/NEJMoa1212793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciccone A, Valvassori L, Nichelatti M, et al. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013;368(10):904–913. doi: 10.1056/NEJMoa1213701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qureshi AI, Abd-Allah F, Aleu A, et al. Endovascular treatment for acute ischemic stroke patients: implications and interpretation of IMS III, MR RESCUE, and SYNTHESIS EXPANSION trials: A report from the Working Group of International Congress of Interventional Neurology. J Vasc Interv Neurol. 2014;7(1):56–75. [PMC free article] [PubMed] [Google Scholar]
- 17.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 18.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019–30. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 19.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285–95. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 20.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009–18. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 21.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296–306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 22.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723–31. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]
- 23.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–e418. doi: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 24.Pexman JHW, Barber PA, Hill MD, et al. Use of the Alberta stroke program early CT score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR Am J Neuroradiol. 2001;22:1534–42. [PMC free article] [PubMed] [Google Scholar]
- 25.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 26.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708–718. doi: 10.1056/NEJMoa1713973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung S, Mono ML, Fischer U, et al. Three-month and long-term outcomes and their predictors in acute basilar artery occlusion treated with intra-arterial thrombolysis. Stroke. 2011;42(7):1946–1951. doi: 10.1161/STROKEAHA.110.606038. [DOI] [PubMed] [Google Scholar]
- 28.Nagel S, Kellert L, Mohlenbruch M, Bosel J, Rohde S, Ringleb P. Improved clinical outcome after acute basilar artery occlusion since the introduction of endovascular thrombectomy devices. Cerebrovasc Dis. 2013;36(5–6):394–400. doi: 10.1159/000356185. [DOI] [PubMed] [Google Scholar]
- 29.Mohlenbruch M, Stampfl S, Behrens L, et al. Mechanical thrombectomy with stent retrievers in acute basilar artery occlusion. AJNR Am J Neuroradiol. 2014;35(5):959–64. doi: 10.3174/ajnr.A3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baek JM, Yoon W, Kim SK, et al. Acute basilar artery occlusion: outcome of mechanical thrombectomy with Solitaire stent within 8 hours of stroke onset. AJNR Am J Neuroradiol. 2014;35(5):989–93. doi: 10.3174/ajnr.A3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubiera M, Ribo M, Delgado-Mederos R, et al. Tandem internal carotid artery/middle cerebral artery occlusion: an independent predictor of poor outcome after systemic thrombolysis. Stroke. 2006;37(9):2301–5. doi: 10.1161/01.STR.0000237070.80133.1d. [DOI] [PubMed] [Google Scholar]
- 32.Malik AM, Vora NA, Lin R, et al. Endovascular treatment of tandem extracranial/intracranial anterior circulation occlusions: preliminary single-center experience. Stroke. 2011;42(6):1653–7. doi: 10.1161/STROKEAHA.110.595520. [DOI] [PubMed] [Google Scholar]
- 33.Kim YS, Garami Z, Mikulik R, Molina CA, Alexandrov AV CLOTBUST Collaborators. Early recanalization rates and clinical outcomes in patients with tandem internal carotid artery/middle cerebral artery occlusion and isolated middle cerebral artery occlusion. Stroke. 2005;36(4):869–71. doi: 10.1161/01.STR.0000160007.57787.4c. [DOI] [PubMed] [Google Scholar]
- 34.Mbabuike N, Gassie K, Brown B, Miller DA, Tawk RG. Revascularization of tandem occlusions in acute ischemic stroke: review of the literature and illustrative case. Neurosurg Focus. 2017;42(4):E15. doi: 10.3171/2017.1.FOCUS16521. [DOI] [PubMed] [Google Scholar]
- 35.del Zoppo GJ, Higashida RT, Furlan AJ, et al. PROACT: a phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke. PROACT Investigators. Prolyse in Acute Cerebral Thromboembolism. Stroke. 1998 Jan;29(1):4–11. doi: 10.1161/01.str.29.1.4. [DOI] [PubMed] [Google Scholar]
- 36.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke. JAMA. 1999;282(21):2003–11. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- 37.Barnwell SL, Clark WM, Nguyen TT, O’Neill OR, Wynn ML, Coull BM. Safety and efficacy of delayed intraarterial urokinase therapy with mechanical clot disruption for thromboembolic stroke. AJNR Am J Neuroradiol. 1994;15(10):1817–22. [PMC free article] [PubMed] [Google Scholar]
- 38.Qureshi AI, Siddiqui AM, Suri MF, et al. Aggressive mechanical clot disruption and low-dose intra-arterial third-generation thrombolytic agent for ischemic stroke: a prospective study. Neurosurgery. 2002;51(5):1319–27. doi: 10.1097/00006123-200211000-00040. [DOI] [PubMed] [Google Scholar]
- 39.Sorimachi T, Fujii Y, Tsuchiya N, et al. Recanalization by mechanical embolus disruption during intra-arterial thrombolysis in the carotid territory. AJNR Am J Neuroradiol. 2004;25(80):1391–402. [PMC free article] [PubMed] [Google Scholar]
- 40.Levy EI, Siddiqui AH, Crumlish A, et al. First Food and Drug Administration-approved prospective trial of primary intracranial stenting for acute stroke SARIS (stent-assisted recanalization in acute ischemic stroke) Stroke. 2009;40(11):3552–6. doi: 10.1161/STROKEAHA.109.561274. [DOI] [PubMed] [Google Scholar]
- 41.Kelly ME, Furlan AJ, Fiorella D. Recanalization of an acute middle cerebral artery occlusion using a self-expanding, reconstrainable, intracranial microstent as a temporary endovascular bypass. Stroke. 2008;39(6):1770–3. doi: 10.1161/STROKEAHA.107.506212. [DOI] [PubMed] [Google Scholar]
- 42.Flint AC, Duckwiler GR, Budzik RF, et al. Mechanical thrombectomy of intracranial internal carotid occlusion: pooled results of the MERCI and Multi MERCI Part I trials. Stroke. 2007;38(4):1274–80. doi: 10.1161/01.STR.0000260187.33864.a7. [DOI] [PubMed] [Google Scholar]
- 43.Smith WS. Safety of mechanical thrombectomy and intravenous tissue plasminogen activator in acute ischemic stroke. Results of the multi Mechanical Embolus Removal in Cerebral Ischemia (MERCI) trial, part I. AJNR Am J Neuroradiol. 2006;27(6):1177–82. [PMC free article] [PubMed] [Google Scholar]
- 44.Yoo AJ, Frei D, Tateshima S, et al. The Penumbra Stroke System: a technical review. J Neurointerv Surg. 2012;4(3):199–205. doi: 10.1136/neurintsurg-2011-010080. [DOI] [PubMed] [Google Scholar]
- 45.Zaidat OO, Yoo AJ, Khatri P, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44(9):2650–63. doi: 10.1161/STROKEAHA.113.001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campbell BCV, van Zwam WH, Goyal M, et al. Effect of general anaesthesia on functional outcome in patients with anterior circulation ischaemic stroke having endovascular thrombectomy versus standard care: a meta-analysis of individual patient data. Lancet Neurol. 2018;17(1):47–53. doi: 10.1016/S1474-4422(17)30407-6. [DOI] [PubMed] [Google Scholar]