Abstract
Colorectal cancer (CRC) is one of the most common types of malignancy and the third most commonly diagnosed form of cancer worldwide, ranking as the fourth leading cause of cancer-associated mortality. MicroRNA (miR)-576-5p has been reported to be highly expressed in patients with CRC; however, its biological role remains unclear. The present study aimed therefore to investigate the biological role and underlying mechanism of miR-576-5p in CRC cell line SW480. The viability of SW480 cells following transfection with miR-576-5p mimic or inhibitor was analyzed using MTT assay. Wound healing and Transwell assays were performed to determine the cell migratory and invasive abilities, respectively. A dual luciferase reporter assay was used to verify the predicted binding site between miR-576-5p and Wnt5a. Reverse transcription-quantitative PCR and western blotting were used to analyze the expression levels of miR-576-5p, E-cadherin, N-cadherin, vimentin, Snail1, Wnt5a, β-catenin, c-myc, cyclin D1 and p/t-c-Jun. Using bioinformatics analysis, high expression of miR-576-5p was found not only in tumor tissues, compared with the normal tissue, but also in CRC cells, compared with NCM460 cells. Furthermore, the inhibition of miR-576-5p expression significantly decreased the cell viability and the migratory and invasive abilities of SW480 cells, and suppressed the epithelial-to-mesenchymal transition (EMT). In addition, miR-576-5p could interact with Wnt5a and regulate the expression level of Wnt5a in order to influence the activity of Wnt/β-catenin signaling. The results from rescue experiments further demonstrated that the effect of miR-576-5p overexpression on cell metastasis and EMT was reversed by Wnt5a overexpression or treatment with XAV-939, which is an inhibitor of the Wnt/β-catenin signaling pathway. In conclusion, the findings from the present study suggested that inhibition of miR-576-5p may suppress SW480 cell metastasis and EMT by targeting Wnt5a and regulating the Wnt5a-mediated Wnt/β-catenin signaling pathway, providing a potential therapeutic target for the treatment of CRC.
Keywords: colorectal cancer, miR-576-5p, Wnt5a, epithelial-to-mesenchymal transition, Wnt/β-catenin signaling
Introduction
Colorectal cancer (CRC) is the third most diagnosed cancer worldwide and the fourth leading cause of cancer-related mortality, accounting for >1.4 million new cases and 700,000 deaths annually (1). Thanks to improved screening programs and standardized treatments, the mortality rate of CRC has declined in the past 30 years; however, the recurrence and metastasis still contribute to a poor response to CRC treatment, with only 50% of patients surviving 5 years (2–4). Better understanding of CRC pathogenesis is therefore urgently required in order to develop novel effective therapeutic strategies.
MicroRNAs (miRNAs/miRs) are a group of small, non-coding RNAs of 18–25 nucleotides in length, which can negatively regulate targeted genes at the post-transcriptional level by directly binding to their 3′-untranslated region (3′-UTR) (5). It has been reported that miRNAs are highly involved in various biological processes, including cell differentiation, apoptosis and metabolism (6). Previous studies demonstrated that aberrantly expressed miRNAs may be closely associated with various types of human cancer, in which miRNAs were reported to be involved in tumor initiation and development (7,8). For example, miR-324-3p is aberrantly high-expressed in gastric cancer, and overexpression of miR-324-3p promotes cell growth, migration and decreased apoptosis of gastric cancer (8); miR-125a is downregulated in cervical cancer, and serves an important role in its cell proliferation and progression (9). In addition, miRNAs have been reported to serve as effective biomarkers for the early diagnosis and prognosis of patients with cancer (10). A previous study demonstrated that miR-576-5p might be a potential tumor-promoting oncomiR in several types of human tumor (11). Kordass et al (11) reported that miR-576-5p enhances the invasion of melanoma cells in vitro. In addition, miR-576-5p was also found to be upregulated in esophageal cancer, where it was found to be involved in cancer cell migration and invasion, suggesting that it might serve as a predictor of cancer prognosis (12,13). However, the role of miR-576-5p in CRC remains unknown. In a previous study using a miRNA microassay from 50 patients with colon cancer and 44 healthy controls, the expression level of miR-576-5p in patients was significantly higher compared with controls (14). Furthermore, miR-576-5p expression levels are more highly expressed at TNM stage III/IV compared with TNM stage I/II, suggesting that miR-576-5p might be considered as an effective diagnostic marker for CRC (14). However, the exact role of miR-576-5p in CRC remains unclear and the biological functions of miR-576-5p are unknown. Further investigation is therefore required to determine whether miR-576-5p could regulate the biological functions of CRC cells, in addition to understanding the underlying mechanism.
The present study performed a series of in vitro experiments to determine the role and underlying mechanism of miR-576-5p in CRC and its effect on CRC development. The findings from this study may provide a novel therapeutic target and serve the development of novel strategy for the treatment of CRC.
Materials and methods
Data analyses and bioinformatics
miRNA-576-5p expression from 459 CRC samples and 8 normal samples (adjacent cancer tissues) were retrieved from the TCGA data portal (https://tcga-data.nci.nih.gov.).
Cell culture and transfection
The human normal colonic epithelial cell line NCM460 and the human CRC cell lines HCT116, SW620 and SW480, were obtained from The Cell Bank of Type Culture Collection of the Chinese Academy of Sciences. Cells were cultured in DMEM, supplemented with 10% FBS, and 1% penicillin-streptomycin (all from Gibco; Thermo Fisher Scientific, Inc.), and maintained in a humidified chamber with 5% CO2 at 37°C.
miR-576-5p mimic (miR-576-5p), miR-576-5p inhibitor (anti-miR-576-5p) and corresponding negative control (NC), as well as pcDNA3.1-Wnt5a (Wnt5a) and its negative control (vector), were synthesized by Shanghai GenePharma Co., Ltd. SW480 cells were seeded in 6-well plates at a density of 1×106 cells/well and cultured at 37°C for 24 h. SW480 cells were subsequently transfected with 100 nM miR-576-5p mimic, 100 nM miR-576-5p inhibitor, 100 nM negative control, as well as 10 nM pcDNA3.1-Wnt5a and its vector using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. After 48 h, the expression of miR-576-5p and Wnt5a could be observed, and the transfected cells were collected for subsequent experiments.
MTT assay
SW480 Cells were seeded into 96-well plates at a density of 1×103 cells/well and were cultured in a humidified incubator with 5% CO2 at 37°C for 48 h. Subsequently, 10 µl MTT solution (5 mg/ml; Beyotime Institute of Biotechnology) was added into each well and incubated for 4 h. Subsequently, 100 µl DMSO was then added to each well to dissolve the formazan crystals. The absorbance was measured at 490 nm using a microplate reader.
Wound healing assay
Cells were plated into 6-well plates in DMEM supplemented with 10% FBS. Once cells reached 85% confluence, a 20-µl pipette tip was used to make a wound in the cell monolayer. Cells were washed twice with PBS and incubated with complete medium without FBS. After 24 h, the wound healing process was monitored under a phase contrast microscope (magnification, ×100). The migratory distance was analyzed to quantify the cell migration ability.
Transwell assay
Transwell chambers (8-µm pore; Corning Inc.) precoated with 50 µl Matrigel (BD Biosciences) were placed into 24-well plates. SW480 cells (1×105) were plated into the upper chamber without serum, whereas complete medium supplemented with 10% FBS was placed into the lower chamber. Following incubation for 24 h, cells remaining in the upper chamber were removed carefully with a cotton swab. Cells that have invaded the lower chamber were stained with crystal violet and the invasive cells were counted under a light microscope (magnification, ×100).
Reverse transcription-quantitative (RT-q) PCR
Total RNA was extracted from cells using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's instructions. Total RNA was reverse transcribed into cDNA using the PrimeScript™ 1st Strand cDNA Synthesis kit (Takara Bio, Inc.). The expression levels were determined by qPCR using the 7500 Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The thermal cycling conditions were: Initial denaturation at 95°C for 10 min, followed by 32 cycles of denaturation at 95°C for 15 sec, annealing at 60°C for 30 sec and extension at 75°C for 40 sec. The primers were obtained from Sangon Biotech (Shanghai, China) and the primer sequences were: miR-576-5p, forward, 5′-TTGGGTCAAGAGTCAGAAGTTT-3′ and reverse, 5′-TGGCTTCTACTTGTCCTTTCC-3′; Wnt5a, forward, 5′-ATGCAGTACATTGGAGAAGGTG-3′, reverse, 5′-CGTCTCTCGGCTGCCTATTT-3′; U6, forward, 5′-AAAGCAAATCATCGGACGACC-3′, reverse, 5′-GTACAACACATTGTTTCCTCGGA-3′; GAPDH, forward, 5′-ATCATCCCTGCCTCTACTGG-3′; reverse, 5′-GTCAGGTCCACCACTGACAC-3′. Relative mRNA level was quantified using 2−ΔΔCq method (15). U6 and GAPDH were used as internal control.
Western blotting
Total protein was extracted from cells using RIPA lysis buffer (Cell Signaling Technology, Inc.). Total protein was measured using a bicinchoninic acid assay kit (Pierce; Thermo Fisher Scientific, Inc.). Equal amounts of proteins (30 µg/lane) were separated by 12% SDS-PAGE and subsequently transferred onto nitrocellulose membranes. Membranes were blocked with 5% nonfat dried milk at room temperature for 1 h and incubated with the primary antibodies against Wnt/β-catenin signaling-related proteins: Wnt5a (1:1,000; cat. no. ab179824), β-catenin (1:5,000; cat. no. ab32572), cyclin D1 (1:200; cat. no. ab16663), c-myc (1:1,000; cat. no. ab32072), p-c-Jun (1:1,000; cat. no. ab32385) and c-Jun (1:1,000; cat. no. ab40766); epithelial-to-mesenchymal transition (EMT)-related proteins: E-cadherin (1:1,000; cat. no. ab40772), vimentin (1:1,000; cat. no. ab92547), N-cadherin (1:5,000; cat. no. ab76011) and Snail (1:1,000; cat. no. ab216347); GAPDH (1:1,000; cat. no. ab8245 all from Abcam) at 4°C overnight. Membranes were then incubated with the corresponding horseradish peroxidase-conjugated anti-rabbit (1:2,000; cat. no. sc-2004) or anti-mouse IgG secondary antibodies (1:2,000; cat. no. sc-2005; both Santa Cruz Biotechnology, Inc.) at room temperature for 2 h. Bands were visualized using enhanced chemiluminescence system (Pierce; Thermo Fisher Scientific, Inc.) and analyzed using a LAS-4000 image document instrument (FUJIFILM Wako Pure Chemical Corporation). ImageJ software version 1.46 (National Institutes of Health) was used to analyze the gray value of protein bands and the relative expression levels were normalized to endogenous control GAPDH.
Dual-luciferase reporter assay
TargetScan (http://www.targetscan.org/) was used to predict the putative binding sites between miR-576-5p and Wnt5a, which was then verified by dual-luciferase reporter assay. Wild-type Wnt5a (Wnt5a-WT) and mutated Wnt5a (Wnt5a-MUT) were cloned into the pMIR-REPORT luciferase vectors (Ambion; Thermo Fisher Scientific, Inc.). After the SW480 cells reached a density of 80%, they were co-transfected with 100 ng Wnt5a-WT/Wnt5a-Mut and 150 nM miR-576-5p mimic/miR-NC using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. After transfection for 48 h, luciferase activity was analyzed using Dual-Luciferase Assay Kit (Promega Corporation). The relative luciferase activity was normalized to Renilla luciferase activity.
Statistical analysis
Statistical analysis was performed using SPSS 19.0 software (IBM Corp.) and data were presented as the means ± standard deviation. Statistical differences were determined using Student's t test for comparison between two groups and a one-way ANOVA followed by a Tukey's post hoc test for multiple comparisons. P<0.05 was considered to indicate a statistically significant difference.
Results
miR-576-5p expression level is upregulated in CRC tissues
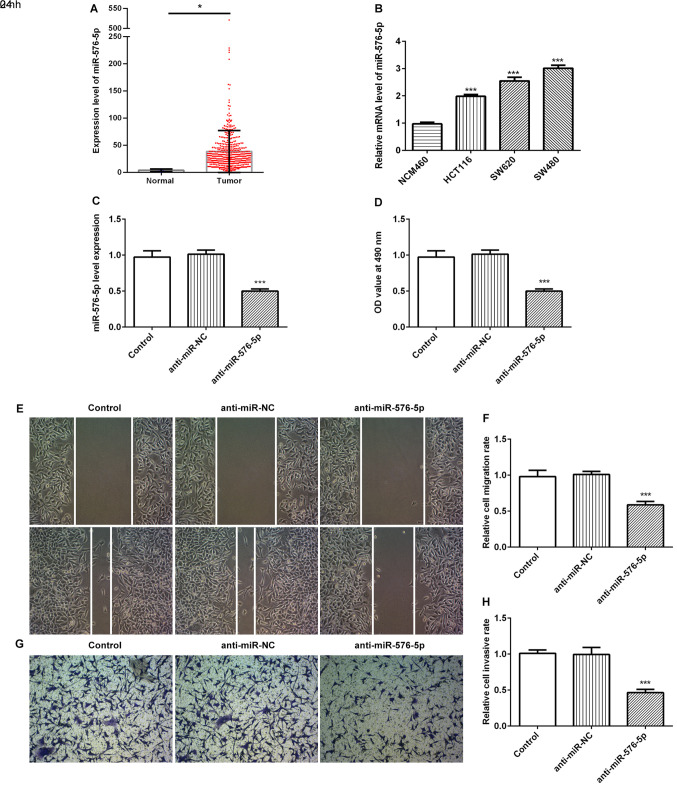
TCGA database was used to determine the expression level of miR-576-5p in clinical samples. As presented in Fig. 1A, the expression level of miR-576-5p in CRC tissues was significantly increased compared with that of normal tissues. Furthermore, miR-576-5p expression level was expressed at higher levels in CRC cell lines HCT116, SW620 and SW480 compared with the human normal colonic epithelial cell line NCM460 (Fig. 1B). Since SW480 cells expressed the highest level of miR-576-5p, this cell line was selected in subsequent experiments.
Figure 1.
miR-576-5p was upregulated in CRC and inhibition of miR-576-5p suppressed cell viability and migratory and invasive abilities. (A) Expression level of miR-576-5p from 459 CRC samples and 8 normal samples were retrieved from The Cancer Genome Atlas database. *P<0.05. (B) miR-576-5p level was detected in the CRC cell lines HCT116, SW620 and SW480, and the human normal colonic epithelial cell line NCM460 using RT-qPCR. ***P<0.001 vs. NCM460. (C) SW480 cells were transfected with miR-576-5p inhibitor and the level of miR-576-5p was detected by RT-qPCR. (D) Cell viability was determined by MTT assay. (E and F) Cell migratory ability was determined using wound healing assay (magnification, ×100). (G and H) Cell invasive ability was determined using Transwell assay (magnification, ×100). ***P<0.001 vs. anti-miR-NC. miR, microRNA; NC, negative control; CRC, colorectal cancer; RT-qPCR, reverse transcription quantitative PCR.
miR-576-5p inhibition inhibits cell viability and migratory and invasive abilities
To investigate the biological role of miR-576-5p in SW480 cells, miR-576-5p was inhibited (Fig. 1C). The cell viability was significantly decreased following inhibition of miR-576-5p (Fig. 1D). In addition, miR-576-5p inhibition significantly decreased the migratory and invasive abilities of SW480 cells in the wound healing and Transwell assays, respectively (Fig. 1E-H). miR-576-5p inhibition may therefore alter CRC cell biology.
Inhibition of miR-576-5p reduces EMT
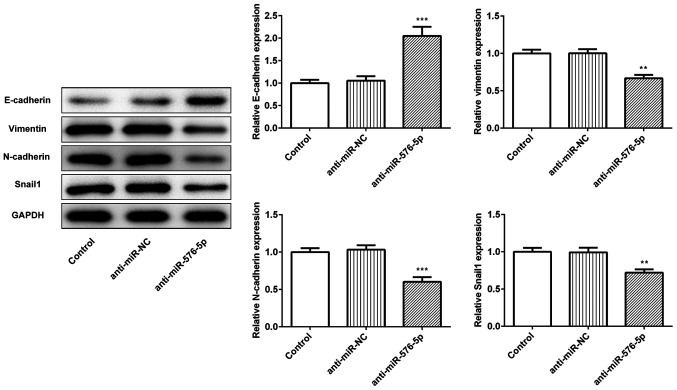
EMT serves a pivotal role in the development of metastatic tumors and it is responsible for most cancer-associated mortality cases, which are usually associated with cell invasion (16). In the present study, the protein expression of E-cadherin, vimentin, N-cadherin and Snail was assessed using western blotting to determine whether miR-576-5p could induce EMT in SW480 cells. The results demonstrated that the expression of the epithelial cell adhesion molecule E-cadherin was significantly upregulated following miR-576-5p was inhibited, whereas the expression of its transcriptional repressor Snail was downregulated (Fig. 2). Furthermore, upon inhibition of miR-576-5p, the expression of mesenchymal-associated proteins, including vimentin and N-cadherin, was significantly decreased, suggesting that miR-576-5p inhibition may successfully suppress EMT.
Figure 2.
Inhibition of miR-576-5p decreased epithelial-to-mesenchymal transition. Following miR-576-5p downregulation, the protein expression of E-cadherin, vimentin, N-cadherin and Snail were detected using western blotting. **P<0.01 and ***P<0.001 vs. anti-miR-NC. miR, microRNA; NC, negative control.
miR-576-5p targets Wnt5a and regulates the β-catenin signaling pathway
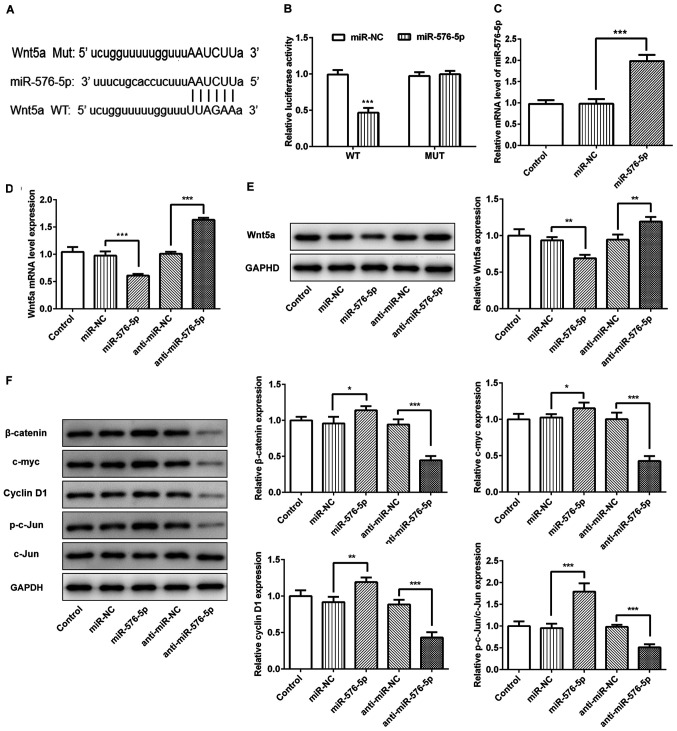
To further investigate the potential mechanism of miR-576-5p function in SW480 cells, TargetScan (http://www.targetscan.org/) was used to identify the putative genes which had a possible binding site on miR-576-5p. Wnt5a was predicted to have binding site on miR-576-5p (Fig. 3A). To verify the interaction between miR-576-5p and Wnt5a, 3′UTR reporter vectors (WT; Wnt5a 3′UTR) containing the predicted sequences were constructed. The dual luciferase reporter assay demonstrated that miR-576-5p mimic significantly decreased the relative Renilla luciferase activity, which was not the case when the predicted sites were mutated (MUT; Wnt5a 3′UTR; Fig. 3B). The mRNA level of miR-576-5p was significantly upregulated when cells were transfected with miR-576-5p mimic (Fig. 3C). The results from RT-qPCR and western blotting confirmed that the mRNA and protein expression levels of Wnt5a were regulated by miR-576-5p, as its expression levels were downregulated in the presence of the miR-576-5p mimic and upregulated following miR-576-5p inhibition (Fig. 3D and E). Wnt5a, one of the Wnt ligands, is known to act through the β-catenin-independent non-canonical signaling pathway (17). The present study investigated whether Wnt5a could activate or repress β-catenin signaling following the differential expression of miR-576-5p in cells. The results from western blotting demonstrated that miR-576-5p overexpression significantly increased the expression of β-catenin, c-Myc, cyclin D1 and phosphorylated (p)-c-Jun, whereas the inhibition of miR-576-5p had the opposite effect (Fig. 3F). Therefore, these results suggested that miR-576-5p may target Wnt5a and regulate the β-catenin signaling pathway.
Figure 3.
miR-576-5p targeted Wnt5a and regulated β-catenin signaling pathway. (A) Putative binding site of miR-576-5p and Wnt5a was predicted by TargetScan. (B) Dual-luciferase report assay was conducted to confirm the predicted binding site. (C) SW480 cells were transfected with miR-576-5p mimic and miR-576-5p level was determined using RT-qPCR. (D and E) miR-576-5p was upregulated or downregulated and mRNA level and protein expression of Wnt5a were measured using RTq-PCR and western blotting, respectively. (F) Protein expression of β-catenin, c-myc, cyclin D1, p-c-Jun and c-Jun were determined using western blotting. *P<0.05, **P<0.01 and ***P<0.001. miR, microRNA; NC, negative control; CRC, colorectal cancer; RT-qPCR, reverse transcription quantitative PCR; MUT, mutant; WT, wild-type; p, phosphorylated.
Wnt5a overexpression reverses the effects of miR-576-5p on SW480 cells
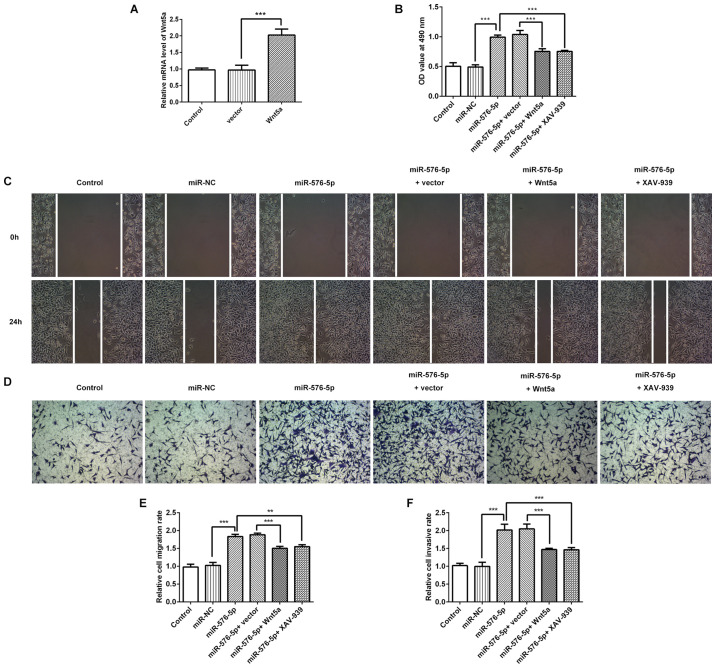
The contribution of Wnt5a and β-catenin signaling in miR-576-5p-mediated functions in SW480 cells was explored. pcDNA-Wnt5a was transfected into SW480 cells to overexpress Wnt5a (Fig. 4A) or cells were treated with XAV-939 to inhibit the activation of Wnt/β-catenin signaling. The cell viability and migratory and invasive abilities were subsequently evaluated. The results demonstrated that upregulated expression of miR-576-5p significantly improved SW480 cell viability, which was partially reversed by Wnt5a overexpression or treatment with XAV-939 (Fig. 4B). The results also showed that the effects of upregulated miR-576-5p on the cell migratory or invasive abilities were suppressed following Wnt5a overexpression or XAV-939 treatment (Fig. 4C-F). These findings suggested that miR-576-5p may exert its role on SW480 cell viability and migratory and invasive abilities by regulating Wnt5a and β-catenin signaling.
Figure 4.
Overexpression of Wnt5a reversed the effect of miR-576-5p on SW480 cells. SW480 cells were transfected with miR-576-5p mimic to overexpress miR-576-5p, with or without Wnt5a overexpression or treatment with XAV-939, the inhibitor of Wnt/β-catenin signaling. (A) After transfection with pcDNA3.1-Wnt5a, Wnt5a mRNA level was detected using reverse transcription quantitative PCR. (B) Cell viability was determined by MTT assay. (C and E) Cell migratory ability was determined using wound healing assay (magnification, ×100). (D and F) Cell invasive ability was determined using Transwell assay (magnification, ×100). **P<0.01 and ***P<0.001. miR, microRNA; NC, negative control.
miR-576-5p regulates EMT by targeting Wnt5a and activating Wnt/β-catenin signaling
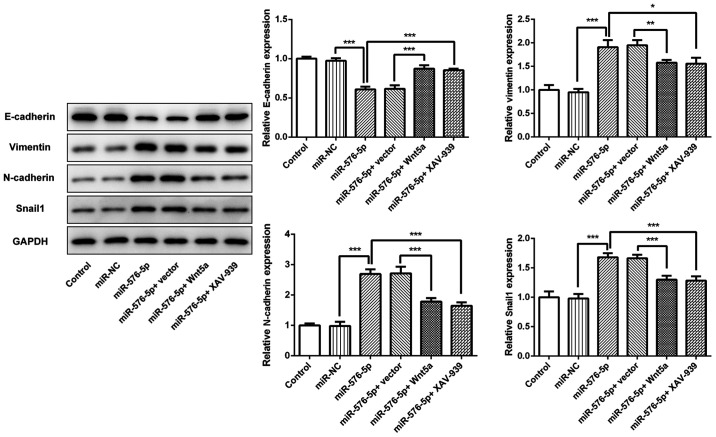
The effects of Wnt5a and XAV-939 on miR-576-5p-induced EMT were investigated. The results demonstrated that miR-576-5p mimic significantly decreased the expression of E-cadherin, and increased the expression of vimentin, N-cadherin and Snail1 (Fig. 5), suggesting that miR-576-5p may promote EMT in SW480 cells. miR-576-5p-mediated EMT could then be reversed by either Wnt5a overexpression or XAV-939 treatment, indicating that miR-576-5p mediated EMT in SW480 cells through Wnt5a and β-catenin signaling.
Figure 5.
miR-576-5p regulated EMT by targeting Wnt5a. SW480 cells were transfected with miR-576-5p mimic to overexpress miR-576-5p, with or without Wnt5a overexpression or XAV-939 treatment. Protein expression of E-cadherin, vimentin, N-cadherin and Snail in different groups were detected using western blotting. *P<0.05, **P<0.01 and ***P<0.001. miR, microRNA; NC, negative control.
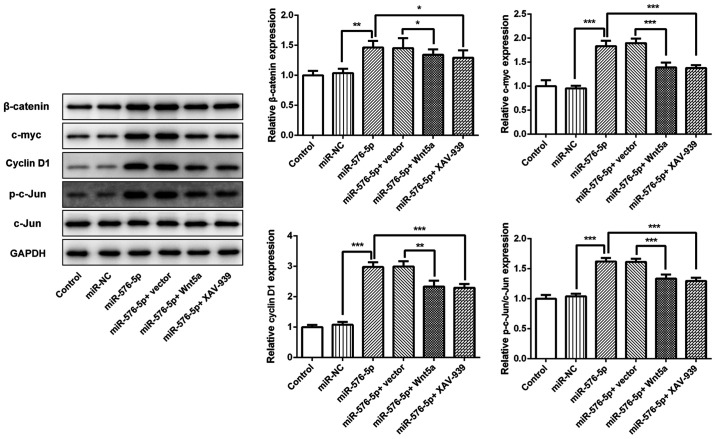
To confirm the association between miR-576-5p and Wnt5a, the effect of miR-576-5p mimic in the presence or absence of Wnt5a overexpression or XAV-939 treatment on Wnt/β-catenin signaling was evaluated. The results demonstrated that miR-576-5p significantly increased the expression of β-catenin, c-Myc, cyclin D1 and p-c-Jun (Fig. 6). These miR-576-5p-mediated changes were then reversed by either Wnt5a overexpression or XAV-939 treatment, indicating that miR-576-5p could mediate biological functions in SW480 cells via Wnt/β-catenin signaling.
Figure 6.
miR-576-5p activated Wnt/β-catenin signaling by targeting Wnt5a. SW480 cells were transfected with miR-576-5p mimic to overexpress miR-576-5p, with or without Wnt5a overexpression or XAV-939 treatment. Protein expression of β-catenin, c-myc, cyclin D1, p-c-Jun and c-Jun were determined using western blotting. *P<0.05, **P<0.01 and ***P<0.001. miR, microRNA; NC, negative control; p, phosphorylated.
Discussion
The aberrant expression or dysfunction of miRNAs has been reported to be closely associated with the progression of numerous types of human cancers, by regulating the molecular functions of cancer cells, including cell proliferation, metastatic ability and drug resistance (18,19). Increasing evidence has revealed that numerous miRNAs have a prominent role in CRC, serving as either diagnostic markers or therapeutic targets. For example, miR-92a can promote tumorigenesis of CRC and has become a useful biomarker for early detection of CRC in both serum and stool (20). Reduced miR-4319 is correlated with poor prognosis in CRC patients, and miR-4319 might become a therapeutic target for CRC treatment (21). However, the precise role of miR-576-5p in CRC remains unclear. The present study aimed therefore to understand the potential role of miR-576-5p in CRC. The results from the present study demonstrated that the expression level of miR-576-5p was upregulated in CRC cancer cells compared with normal colonic epithelial cells, which was consistent with a previous study (14). A miR-576-5p inhibitor was subsequently transfected into cancer cells to inhibit miR-576-5p expression and changes in SW480 cells were thus observed. The results demonstrated that miR-576-5p inhibition significantly decreased SW480 cell viability and migratory and invasive abilities. These results suggested that the inhibition of miR-576-5p may exert an anti-cancer effect by weakening the metastatic ability of CRC cells.
Distant metastases are major contributors to cancer-associated mortality, which often lead to therapeutic failure in patients (22). Cancer cell metastasis involves a series of reactions regulated by various factors, with EMT regarded as one of the most critical factors responsible for the infiltration and metastasis of ~90% of epithelial malignant tumors in humans (23). EMT consists in the differentiation of epithelial cells into mesenchymal cells, which is characterized by the loss of E-cadherin, a marker of epithelial characteristics, and the increase in N-cadherin, a marker of mesenchymal characteristics (24,25). In the present study, results from western blotting demonstrated that miR-576-5p inhibition significantly increased the expression of E-cadherin and decreased the expression of N-cadherin, vimentin and Snail1, suggesting that the inhibition of miR-576-5p may alleviate EMT in SW480 cells and effectively inhibit cancer cell metastasis, providing a potential therapeutic effect of miR-576-5p inhibition in CRC.
Subsequently, the present study investigated the potential mechanism responsible for the suppressive effect of miR-576-5p inhibition on cell viability, migratory and invasive abilities, and EMT. Wnt5a was identified as the direct target of miR-576-5p, which was regulated by miR-576-5p. Wnt5a, a member of the Wnt signaling protein family, was found to regulate various molecular functions through binding to different receptors on the cellular surface (26). Accumulating evidence has suggested that Wnt5a serves a crucial role in the pathogenesis of numerous types of human cancers, where Wnt5a exerts both oncogenic and tumor suppressive effects in various types of cancer (27). For instance, Wnt5a is highly expressed in patients with nasopharyngeal cancer, especially those with poor survival (28). Furthermore, Wnt5a expression level is positively correlated with the severity of melanoma as its expression increases following the progression of melanoma, suggesting that Wnt5a expression might be considered as a risk factor for the outcome of the diseases (29). However, Wnt5a was identified to serve as a tumor suppressor in hepatocellular carcinoma (HCC) and breast cancer. Several downstream signaling pathways involved in cancer cell metastasis were reported to be tightly controlled by Wnt5a in HCC and breast cancer (30–32). In CRC, Wnt5a is known to exert anti-cancer effects. For example, Li and Chen (33) reported that both mRNA and protein expression of Wnt5a was decreased in the highly metastatic human colon cancer cells, and Cheng et al (34) demonstrated that the expression level of Wnt5a was significantly downregulated in most patients with primary colon cancer. Restoring Wnt5a may therefore be considered as an innovative therapeutic target. In the present study, Wnt5a overexpression reversed miR-576-5p-induced EMT, cell viability and migratory and invasive abilities, suggesting that Wnt5a may be used to inhibit cell metastasis in SW480 cells.
Wnt5a, a member of the Wnt family of proteins, activates the canonical or non-canonical Wnt signaling pathway depending on the receptor context (35). As a key component of Wnt signaling, β-catenin serves as the marker of the pathway activation. In previous studies, Wnt5a was discovered to be an antagonist of the β-catenin-dependent Wnt signaling pathway (26,33). Furthermore, it was reported that Wnt/β-catenin signaling participates in the regulation of tumor signaling transduction and promotes the proliferation, differentiation and metastasis of tumor cells (36). In addition, Wnt/β-catenin signaling has been demonstrated to have a major impact on EMT during cancer progression. Upon the absence of Wnt, β-catenin and E-cadherin attach to the cell membrane (37,38). The loss of E-cadherin activates the Wnt pathway, which facilitates the translocation of β-catenin into the nucleus to initiate the transcription of various downstream target genes, including cyclin D1, c-Myc and c-Jun, which eventually promote the malignant progression of cells (37,38). Furthermore, c-myc is an important proto-oncogene associated with tumor occurrence and development. The cancer gene c-myc possesses the function of molecular switch in gene transcription and cell regulation, and is activated during the transition from colorectal adenoma to adenocarcinoma, leading to c-myc with abnormal activation, increased protein expression and promotion of cell proliferation (39). Activation of β-catenin is the key nuclear effector of Wnt pathway, and increased cytoplasmic and nuclear translocation of β-catenin promotes the combination between β-catenin and the T-cell factor (TCF)/lymphoid enhancer-binding factor (LEF) transcription factor family (40). c-myc is a well-established target gene of β-catenin/TCF transcription factor complex, and aberrant nuclear accumulation of β-catenin and constitutive upregulation of c-myc are believed to be the basis of colorectal tumorigenesis (41,42). In the present study, Wnt/β-catenin was activated following miR-576-5p overexpression. In addition, the results from the present study revealed that the upregulation of β-catenin, as well as of its downstream target genes c-Myc, cyclin D1 and c-Jun, may account for the suppression of Wnt5a by miR-576-5p, suggesting that miR-576-5p may activate Wnt/β-catenin signaling by silencing Wnt5a. To further validate the regulatory role of Wnt5a in CRC, Wnt5a was overexpressed in SW480 cells, and cells were treated with XAV-939, an inhibitor of the Wnt/β-catenin signaling pathway. The results demonstrated that both Wnt5a overexpression and XAV-939 treatment exhibited similar inhibitory effects on Wnt/β-catenin signaling. In addition, miR-576-5p exhibited promotional effects on cell viability, and migratory and invasive ability, in addition to EMT; however, the promotive effects were partly inhibited by overexpression of Wnt5a or XAV-939 treatment, suggesting that Wnt5a may serve as an antagonist of Wnt/β-catenin signaling pathway. Furthermore, the results suggested that miR-576-5p may affect cell metastasis by targeting Wnt5a and regulating Wnt5a-mediated Wnt/β-catenin signaling.
In conclusion, the present study revealed the biological role of miR-576-5p in CRC, and the inhibition of miR-576-5p was discovered to exert an anti-tumor effect by inhibiting cell viability, migration, invasion and EMT. In addition, miR-576-5p was demonstrated to directly target Wnt5a and regulate Wnt5a-mediated Wnt/β-catenin signaling. Furthermore, the effect of miR-576-5p in CRC cells may be reversed by Wnt5a overexpression or inhibition of the Wnt/β-catenin signaling using XAV-939. Taken together, these findings suggested that inhibition of miR-576-5p may suppress CRC cell viability, migration, invasion and EMT by targeting Wnt5a and regulating Wnt5a-mediated Wnt/β-catenin signaling, suggesting that miR-576-5p may be considered as a potential therapeutic target for CRC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the General Program of Zhejiang Natural Science Foundation (grant no. Y19H160049) and the Project of Medical and Health Science and Technology Plan in Zhejing Province (grant no. 2020KY460).
Availability of data and materials
All data generated or analyzed during the present study are included in this published article.
Authors' contributions
YZ and JL were responsible for conceiving and designing the study. JL, LL, JS and NZho participated in the literature collection and conducting the experiments. YF, NZha and QS analyzed the data and created the figures. JL and LL drafted the initial manuscript and YZ revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that there have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Lu CW, ZhouDDXieTHaoJ L, Pant OP, Lu CB, Liu XF. HOXAll antisense long noncoding RNA (HOXAll-AS): A promising lncRNA in human cancers. Cancer Med. 2018;7:3792–3799. doi: 10.1002/cam4.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McArdle CS, Hole DJ. Outcome following surgery for colorectal cancer: Analysis by hospital after adjustment for case-mix and deprivation. Br J Cancer. 2002;86:331–335. doi: 10.1038/sj.bjc.6600120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holm M, Saraswat M, Joenväärä S, Ristimäki A, Haglund C, Renkonen R. Colorectal cancer patients with different C-reactive protein levels and 5-year survival times can be differentiated with quantitative serum proteomics. PLoS One. 2018;13:e0195354. doi: 10.1371/journal.pone.0195354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 6.Tsuchiya S, Okuno Y, Tsujimoto G. MicroRNA: Biogenetic and functional mechanisms and involvements in cell differentiation and cancer. J Pharmacol Sci. 2006;101:267–270. doi: 10.1254/jphs.CPJ06013X. [DOI] [PubMed] [Google Scholar]
- 7.Bartels CL, Tsongalis GJ. MicroRNAs: Novel biomarkers for human cancer. Clin Chem. 2009;55:623–631. doi: 10.1373/clinchem.2008.112805. [DOI] [PubMed] [Google Scholar]
- 8.Sun GL, Li Z, Wang WZ, Chen Z, Zhang L, Li Q, Wei S, Li BW, Xu JH, Chen L, et al. miR-324-3p promotes gastric cancer development by activating Smad4-mediated Wnt/beta-catenin signaling pathway. J Gastroenterol. 2018;53:725–739. doi: 10.1007/s00535-017-1408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan Z, Cui H, Xu X, Lin Z, Zhang X, Kang L, Han B, Meng J, Yan Z, Yan X, Jiao S. MiR-125a suppresses tumor growth, invasion and metastasis in cervical cancer by targeting STAT3. Oncotarget. 2015;6:25266–25280. doi: 10.18632/oncotarget.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang C, Hu X, Alattar M, Zhao H. miRNA expression profiles associated with diagnosis and prognosis in lung cancer. Expert Rev Anticancer Ther. 2014;14:453–461. doi: 10.1586/14737140.2013.870037. [DOI] [PubMed] [Google Scholar]
- 11.Kordass T, Weber CEM, Eisel D, Pane AA, Osen W, Eichmüller SB. miR-193b and miR-30c-1* inhibit, whereas miR-576-5p enhances melanoma cell invasion in vitro. Oncotarget. 2018;9:32507–32522. doi: 10.18632/oncotarget.25986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ni XF, Zhao LH, Li G, Hou M, Su M, Zou CL, Deng X. MicroRNA-548-3p and MicroRNA-576-5p enhance the migration and invasion of esophageal squamous cell carcinoma cells via NRIP1 down-regulation. Neoplasma. 2018;65:881–887. doi: 10.4149/neo_2018_171206N803. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Chen J, Wang L, Chen L, Du Z, Zhu L, Cui M, Zhang M, Song L. Linc-PINT acted as a tumor suppressor by sponging miR-543 and miR-576-5p in esophageal cancer. J Cell Biochem. 2019;120:19345–19357. doi: 10.1002/jcb.28699. [DOI] [PubMed] [Google Scholar]
- 14.Wang YN, Chen ZH, Chen WC. Novel circulating microRNAs expression profile in colon cancer: A pilot study. Eur J Med Res. 2017;22:51. doi: 10.1186/s40001-017-0294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Ghahhari NM, Babashah S. Interplay between microRNAs and WNT/β-catenin signalling pathway regulates epithelial-mesenchymal transition in cancer. Eur J Cancer. 2015;51:1638–1649. doi: 10.1016/j.ejca.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 17.Kumawat K, Gosens R. WNT-5A: Signaling and functions in health and disease. Cell Mol Life Sci. 2016;73:567–587. doi: 10.1007/s00018-015-2076-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calin GA, Croce CM. MicroRNA-cancer connection: The beginning of a new tale. Cancer Res. 2006;66:7390–7394. doi: 10.1158/0008-5472.CAN-06-0800. [DOI] [PubMed] [Google Scholar]
- 19.Zhu W, Luo X, Fu H, Liu L, Sun P, Wang Z. MiR-3653 inhibits the metastasis and epithelial-mesenchymal transition of colon cancer by targeting Zeb2. Pathol Res Pract. 2019;215:152577. doi: 10.1016/j.prp.2019.152577. [DOI] [PubMed] [Google Scholar]
- 20.Chen E, Li Q, Wang H, Yang F, Min L, Yang J. MiR-92a promotes tumorigenesis of colorectal cancer, a transcriptomic and functional based study. Biomed Pharmacother. 2018;106:1370–1377. doi: 10.1016/j.biopha.2018.07.050. [DOI] [PubMed] [Google Scholar]
- 21.Huang L, Zhang Y, Li Z, Zhao X, Xi Z, Chen H, Shi H, Xin T, Shen R, Wang T. MiR-4319 suppresses colorectal cancer progression by targeting ABTB1. United European Gastroenterol J. 2019;7:517–528. doi: 10.1177/2050640619837440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodrigo JP, Martínez P, Allonca E, Alonso-Durán L, Suárez C, Astudillo A, García-Pedrero J. Immunohistochemical markers of distant metastasis in laryngeal and hypopharyngeal squamous cell carcinomas. Clin Exp Metastasis. 2014;31:317–325. doi: 10.1007/s10585-013-9630-5. [DOI] [PubMed] [Google Scholar]
- 23.Hugo H, Ackland ML, Blick T, Lawrence MG, Clements JA, Williams ED, Thompson EW. Epithelial-mesenchymal and mesenchymal-epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213:374–383. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 24.Li D, Tian B, Jin X. miR-630 inhibits epithelial-to-mesenchymal transition (EMT) by regulating the Wnt/β-catenin pathway in gastric cancer cells. Oncol Res. 2018;27:9–17. doi: 10.3727/096504018X15178732625479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montorsi L, Guizzetti F, Alecci C, Caporali A, Martello A, Atene CG, Parenti S, Pizzini S, Zanovello P, Bortoluzzi S, et al. Loss of ZFP36 expression in colorectal cancer correlates to wnt/β-catenin activity and enhances epithelial-to-mesenchymal transition through upregulation of ZEB1, SOX9 and MACC1. Oncotarget. 2016;7:59144–59157. doi: 10.18632/oncotarget.10828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kikuchi A, Yamamoto H, Sato A, Matsumoto S. Wnt5a: Its signalling, functions and implication in diseases. Acta Physiol (Oxf) 2012;204:17–33. doi: 10.1111/j.1748-1716.2011.02294.x. [DOI] [PubMed] [Google Scholar]
- 27.Asem MS, Buechler S, Wates RB, Miller DL, Stack MS. Wnt5a Signaling in Cancer. Cancers (Basel) 2016;8:79. doi: 10.3390/cancers8090079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin L, Yin YT, Zheng FJ, Peng LX, Yang CF, Bao YN, Liang YY, Li XJ, Xiang YQ, Sun R, et al. WNT5A promotes stemness characteristics in nasopharyngeal carcinoma cells leading to metastasis and tumorigenesis. Oncotarget. 2015;6:10239–10252. doi: 10.18632/oncotarget.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Da Forno PD, Pringle JH, Hutchinson P, Osborn J, Huang Q, Potter L, Hancox RA, Fletcher A, Saldanha GS. WNT5A expression increases during melanoma progression and correlates with outcome. Clin Cancer Res. 2008;14:5825–5832. doi: 10.1158/1078-0432.CCR-07-5104. [DOI] [PubMed] [Google Scholar]
- 30.Wang T, Liu X, Wang J. Up-regulation of Wnt5a inhibits proliferation and migration of hepatocellular carcinoma cells. J Cancer Res Ther. 2019;15:904–908. doi: 10.4103/jcrt.JCRT_886_18. [DOI] [PubMed] [Google Scholar]
- 31.Bi L, Liu X, Wang C, Cao Y, Mao R, Li P, Geng M. Wnt5a involved in regulation of the biological behavior of hepatocellular carcinoma. Int J Clin Exp Pathol. 2014;7:987–995. [PMC free article] [PubMed] [Google Scholar]
- 32.Prasad CP, Manchanda M, Mohapatra P, Andersson T. WNT5A as a therapeutic target in breast cancer. Cancer Metastasis Rev. 2018;37:767–778. doi: 10.1007/s10555-018-9760-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Q, Chen H. Silencing of Wnt5a during colon cancer metastasis involves histone modifications. Epigenetics. 2012;7:551–558. doi: 10.4161/epi.20050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng R, Sun B, Liu Z, Zhao X, Qi L, Li Y, Gu Q. Wnt5a suppresses colon cancer by inhibiting cell proliferation and epithelial-mesenchymal transition. J Cell Physiol. 2014;229:1908–1917. doi: 10.1002/jcp.24566. [DOI] [PubMed] [Google Scholar]
- 35.van Amerongen R, Mikels A, Nusse R. Alternative wnt signaling is initiated by distinct receptors. Sci Signal. 2008;1:re9. doi: 10.1126/scisignal.135re9. [DOI] [PubMed] [Google Scholar]
- 36.Howard S, Deroo T, Fujita Y, Itasaki N. A positive role of cadherin in Wnt/β-catenin signalling during epithelial-mesenchymal transition. PLoS One. 2011;6:e23899. doi: 10.1371/journal.pone.0023899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wijnhoven BP, Dinjens WN, Pignatelli M. E-cadherin-catenin cell-cell adhesion complex and human cancer. Br J Surg. 2000;87:992–1005. doi: 10.1046/j.1365-2168.2000.01513.x. [DOI] [PubMed] [Google Scholar]
- 38.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi L, Wu YX, Yu JH, Chen X, Luo XJ, Yin YR. Research of the relationship between β-catenin and c-myc-mediated Wnt pathway and laterally spreading tumors occurrence. Eur Rev Med Pharmacol Sci. 2017;21:252–257. [PubMed] [Google Scholar]
- 40.Valenta T, Hausmann G, Basler K. The many faces and functions of β-catenin. EMBO J. 2012;31:2714–2736. doi: 10.1038/emboj.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fang Y, Shen ZY, Zhan YZ, Feng XC, Chen KL, Li YS, Deng HJ, Pan SM, Wu DH, Ding Y. CD36 inhibits β-catenin/c-myc-mediated glycolysis through ubiquitination of GPC4 to repress colorectal tumorigenesis. Nat Commun. 2019;10:3981. doi: 10.1038/s41467-019-11662-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie C, Pan Y, Hao F, Gao Y, Liu Z, Zhang X, Xie L, Jiang G, Li Q, Wang E. C-Myc participates in β-catenin-mediated drug resistance in A549/DDP lung adenocarcinoma cells. APMIS. 2014;122:1251–1258. doi: 10.1111/apm.12296. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during the present study are included in this published article.