Abstract
Pneumonia accounts for ~1.3 million mortalities in children per year worldwide. MicroRNAs are implicated in several diseases, including cancer and pneumonia; however, the role of let7f-5p in pneumonia is not completely understood. In the present study, lipopolysaccharide (LPS) was used to establish an in vitro pneumonia model in A549 and WI-38 cells. The reverse transcription-quantitative PCR (RT-qPCR) and western blotting results demonstrated that let7f-5p expression levels were significantly decreased, whereas MAPK6 expression levels were significantly increased in the peripheral venous blood of patients with pneumonia and in LPS-induced A549 and WI-38 cells compared with healthy volunteers and control cells, respectively. Furthermore, the dual-luciferase reporter assay demonstrated that let7f-5p targeted the 3′-untranslated region of MAPK6. The ELISA and RT-qPCR results demonstrated that let7f-5p mimic ameliorated LPS-induced inflammatory injury in A549 and WI-38 cells, as demonstrated by decreased expression levels of proinflammatory cytokines, including TNF-α and IL-6. In addition, the Cell Counting Kit-8 assay results indicated that let7f-5p mimic ameliorated LPS-induced reductions in cell viability, and the western blotting results demonstrated that let7f-5p mimic reversed LPS-induced activation of the STAT3 signaling pathway. Notably, the aforementioned let7f-5p-mediated effects were reversed by MAPK6 overexpression. Collectively, the results of the present study suggested that let7f-5p inhibited inflammation by targeting MAPK6 in the in vitro pneumonia model, thus let7f-5p may serve as a potential novel therapeutic target for pneumonia.
Keywords: pneumonia, let7f-5p, MAPK6, STAT3 signaling pathway
Introduction
Pneumonia is a lower respiratory illness, which is characterized by the following symptoms: Cough, fever, chest pain and in severe cases, heart failure (1). Pneumonia is a common infectious disease, with high morbidity rates (~0.02%) worldwide, particularly in children (2,3). Inflammation is a key defense mechanism to injury that prevents the entry of hazardous substances into the body; however, inflammation also displays the potential to cause injury (4). The respiratory tract is easily infected on account of poor immune function in pediatric cases (5). Inflammation from endotoxins is a leading cause of pneumonia (6). As a potent endotoxin for inflammation, lipopolysaccharide (LPS) is the primary bioactive component of the cell wall of gram-negative bacterium (7). Therefore, developing novel therapeutic strategies to inhibit the progression of pneumonia is important.
MicroRNAs (miRNAs/miRs) are a subgroup of non-coding RNAs ≤200 nucleotides in length, which control gene expression at the transcriptional and post-transcriptional levels, thus affecting cellular processes (8). miRNAs, such as miR-3941, have been reported to regulate inflammatory responses and diseases (9). In addition, certain miRNAs have been identified in the pathogenesis of pneumonia. For example, Abd-El-Fattah et al (10) demonstrated that miR-155, miR-21 and miR-197 are upregulated in patients with pneumonia, but their targets and respective functions have not been identified. In 2017, Huang et al (11) identified miRNA biomarkers for pneumonia via RNA-sequencing and bioinformatics analysis, which demonstrated that let7f is downregulated in the peripheral blood of patients with severe pneumonia. Therefore, the present study aimed to investigate the role of let7f in pneumonia.
Materials and methods
Serum samples
As pneumonia is also frequent in children (2), the present study aimed to identify a potential therapeutic target for pneumonia in children. Peripheral venous blood (3 ml) was collected from 29 healthy children (22 male patients and 7 female patients; age range, 1–8 years; mean age, 4.7 years) and 29 patients with pneumonia (22 male patients and 7 female patients; age range, 1–7 years; mean age, 3.8 years) at Guizhou Provincial People's Hospital (Guiyang, China) between March 2016 and February 2018. The exclusion criteria were as follows: i) Patients with immunodeficiency, tuberculosis infection or asthma; and ii) patients with respiratory tract infection or inflammatory disease. Patients aged 1–8 years who were diagnosed with pneumonia were included in the present study. Blood samples were centrifuged at 1,000 × g for 10 min at 4°C and the supernatant was collected for subsequent experimentation. The present study was approved by the Ethical Committee of Guizhou Provincial People's Hospital (approval no. 2016011605). The parents or legal guardians of all participants provided written informed consent.
Cell culture
The human lung adenocarcinoma A549 cell line and normal human fibroblast WI-38 cell line were purchased from American Type Culture Collection. Cells were maintained in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin (all purchased from Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C with 5% CO2.
Cell transfection
The full length of MAPK6 was reconstructed into a pcDNA3.1 empty vector (Invitrogen; Thermo Fisher Scientific, Inc.). let7f-5p mimic (5′-UUGAUAUGUUAGAUGAUGGAGU-3′) and negative control (NC) mimic (5′-UCACAACCUCCUAGAAAGAGUAGA-3′) were synthesized by Shanghai GenePharma Co., Ltd. A549 and WI-38 cells (1×104 cells/well) were transfected with 2 µg pcDNA3.1, 2 µg pcDNA3.1-MAPK6, 100 nM NC mimic or 100 nM let7f-5p mimic using Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. After transfection for 48 h at 37°C, cells were used for subsequent experiments.
LPS treatment
Briefly, cells were seeded (2×105 cells/well) into 6-well plates and cultured at 37°C for 24 h. To establish the in vitro pneumonia model, WI-38 cells were incubated with 10 µg/ml LPS (Beijing Solarbio Science & Technology Co., Ltd.) for 12 h at 37°C as previously described (12,13), whereas A549 cells were incubated with 10 µg/ml LPS for 12 h at 37°C as previously described (14).
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from peripheral venous blood, and A549 and WI-38 cells using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA was reverse transcribed into cDNA using the PrimeScript™ RT reagent kit (cat. no. RR047A; Takara Biotechnology Co., Ltd.), according to the manufacturer's protocol. Subsequently, qPCR was performed using the SYBR Premix Ex Taq (cat. no. RR003A; Takara Biotechnology Co., Ltd.). The following thermocycling conditions were used for qPCR: Initial denaturation at 94°C for 5 min; followed by 40 cycles of degeneration at 94°C for 30 sec, annealing at 60°C for 30 sec and extension at 72°C for 1 min. The following primers were used for qPCR: let7f-5p forward, 5′-TTGATATGTTAGATGATGGAGT-3′ and reverse, 5′-ACTCCATCATCTAACATATCAA-3′; IL-6 forward, 5′-AGCCACTCACCTCTTCAGAACGAA-3′ and reverse, 5′-TACTCATCTGCACAGCTCTGGCTT-3′; TNF-α forward, 5′-GCCAATGGCATGGATCTCAAAG-3′ and reverse, 5′-CAGAGCAATGACTCCAAAGT-3′; U6 forward, 5′-GTGCTCGCTTCGGCAGCACAT-3′ and reverse, 5′-AATATGGAACGCTTCACGAAT-3′; MAPK6 forward, 5′-GTACACATGTGTTATCTACCTCA-3 and reverse, 5′-TACAATAAACGCTGGCTAA-3′; and GAPDH forward, 5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse, 5′-TGGTGAAGACGCCAGTGGA-3′. let7f-5p and IL-6/TNF-α/MAPK6 mRNA expression levels were quantified using the 2−ΔΔCq method (15) and normalized to the internal reference genes U6 and GAPDH, respectively.
Western blotting
Total protein was extracted from peripheral venous blood, and A549 and WI-38 cells using RIPA lysis buffer (Sigma-Aldrich; Merck KGaA) supplemented with protease inhibitors. Total protein was quantified using the BCA method (Beyotime Institute of Biotechnology). Equal amounts of protein (15 µg/lane) were separated via 8% SDS-PAGE and transferred onto PVDF membranes. Following blocking with 5% non-fat milk at room temperature for 2 h, the membranes were incubated overnight at 4°C with primary antibodies (all purchased from Cell Signaling Technology, Inc.) targeted against: MAPK6 (cat. no. 4067; 1:1,000), STAT3 (cat. no. 12640; 1:1,000), phosphorylated-STAT3 (cat. no. 9145; 1:1,000) and GAPDH (cat. no. 5174; 1:1,000). Following washing three times with TBST, the membranes were incubated with HRP-conjugated goat anti-rabbit secondary antibodies (cat. no. 7074; 1:3,000; Cell Signaling Technology, Inc.) at room temperature for 1 h. Protein bands were visualized using an ECL system (Beyotime Institute of Biotechnology). Protein expression was semi-quantified using ImageJ software (version 1.50; National Institutes of Health).
Cell viability assay
A549 and WI-38 cell viability were assessed using the Cell Counting Kit-8 (CCK-8) detection kit (cat. no. CSP04; Dojindo Molecular Technologies, Inc.) according to the manufacturer's protocol. Briefly, cells were seeded (5×103 cells/well) into 96-well plates and cultured for 24 h at 37°C. Subsequently, 20 µl CCK-8 solution was added to each well for 1 h at 37°C. The absorbance was measured at a wavelength of 450 nm using a microplate reader.
ELISA
A549 and WI-39 cell culture media were collected. The levels of inflammatory cytokines, including IL-6 (cat. no. D6050) and TNF-α (cat. no. MTA00B), were detected using ELISA kits (R&D Systems, Inc.) according to the manufacturer's protocol.
Dual-luciferase reporter assay
TargetScan software (version 7.1; www.targetscan.org/vert_71) was used to predict the complementary binding sites between let7f-5p and the 3′-untranslated region (UTR) of MAPK6. To validate the interaction between let7f-5p and MAPK6, A549 and WI-38 cells were seeded (1×104 cells/well) into 24-well plates and co-transfected with wild-type (WT) MAPK6 (0.2 mg) or mutant MAPK6 (0.2 mg) pmiRGLO dual-luciferase vectors (Promega Corporation) and let7f-5p mimic (20 nM) or NC mimic (20 nM) using Lipofectamine 2000. Following incubation for 24 h at 37°C, luciferase activities were detected using a Dual-Luciferase Reporter assay system (Promega Corporation) according to the manufacturer's protocol. Firefly luciferase activity was normalized to Renilla luciferase activity.
Statistical analysis
Statistical analyses were performed using GraphPad software (version 6.0; GraphPad Software, Inc.). All experiments were performed in triplicate. Data are presented as the mean ± SD. The unpaired Student's t-test was performed to compare the difference of let7f-5p and MAPK6 between healthy volunteers and patients with pneumonia. The unpaired Student's t-test was performed to compare differences between two groups for in vitro experiments. One-way ANOVA followed by Tukey's post hoc test was used to compare differences among multiple groups. Pearson's correlation analysis was performed to determine the correlation between let7f-5p and MAPK6 in the peripheral venous blood of patients with pneumonia. P<0.05 was considered to indicate a statically significant difference.
Results
let7f-5p expression is negatively correlated with MAPK6 expression in patients with pneumonia
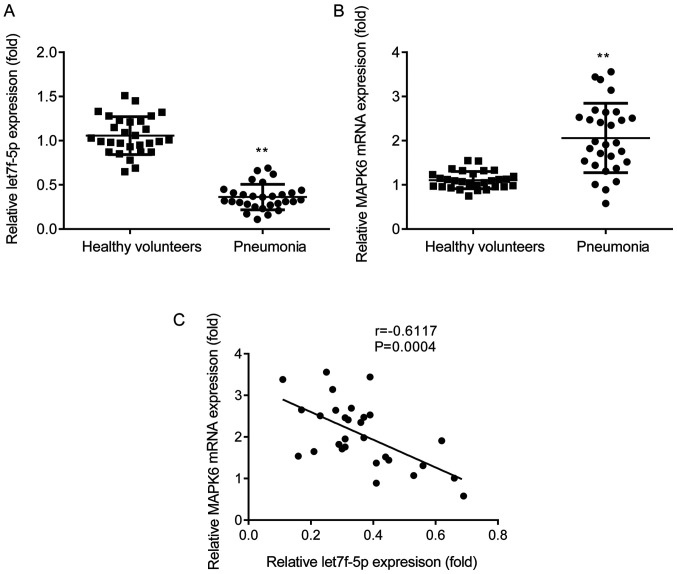
The expression profiles of let7f-5p and MAPK6 in patients with pneumonia and healthy volunteers were assessed via RT-qPCR. let7f-5p expression was significantly decreased (Fig. 1A) and MAPK6 expression was significantly increased (Fig. 1B) in the peripheral venous blood of patients with pneumonia compared with healthy volunteers. Pearson's correlation analysis demonstrated that let7f-5p expression was negatively correlated with MAPK6 expression in patients with pneumonia (Fig. 1C).
Figure 1.
let7f-5p expression is negatively correlated with MAPK6 expression in patients with pneumonia. Reverse transcription-quantitative PCR analysis was performed to detect the expression levels of (A) let7f-5p and (B) MAPK6 in the peripheral venous blood of patients with pneumonia and healthy volunteers. (C) Pearson's correlation analysis was performed to determine the correlation between let7f-5p and MAPK6. **P<0.01 vs. healthy volunteers.
LPS-induced inflammatory injury in A549 and WI-38 cells
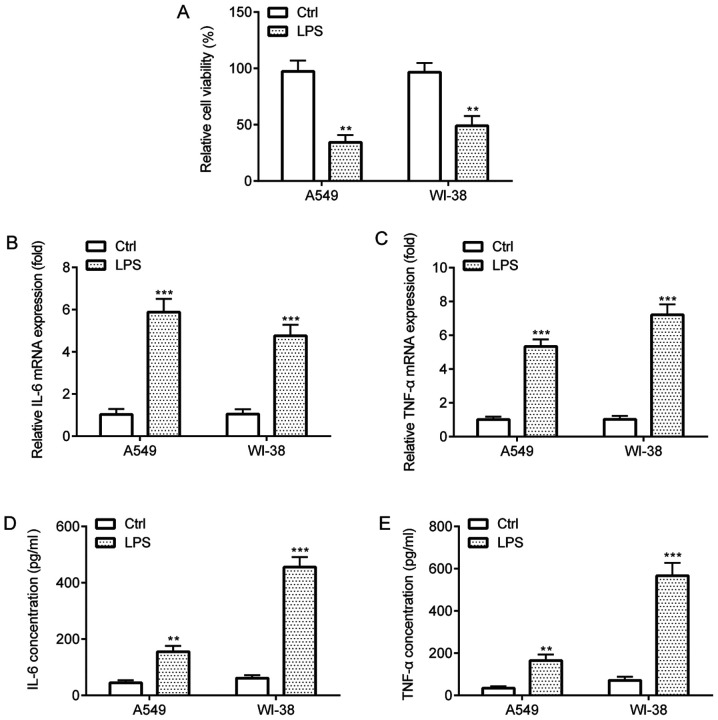
The effects of LPS on A549 and WI-38 cells were assessed by performing CCK-8 and ELISA assays. The CCK-8 assay results indicated that LPS treatment significantly decreased cell viability compared with the control group (Fig. 2A). In addition, the RT-qPCR and ELISA results indicated that LPS treatment significantly increased the expression and release of proinflammatory cytokines TNF-α and IL-6 in A549 and WI-38 cells compared with the control group (Fig. 2B-E). Collectively, the results suggested that LPS induced inflammatory injury in A549 and WI-38 cells.
Figure 2.
LPS-induced inflammatory injury in A549 and WI-38 cells. (A) Cell Counting Kit-8 assay was performed to assess cell viability in A549 and WI-38 cells. Reverse transcription-quantitative PCR was performed to measure (B) IL-6 and (C) TNF-α mRNA expression levels in A549 and WI-38 cells. ELISAs were performed to assess the concentrations of (D) IL-6 and (E) TNF-α in the cell culture medium of A549 and WI-38 cells. **P<0.01 and ***P<0.001 vs. Ctrl. LPS, lipopolysaccharide; Ctrl, control.
let7f-5p and MAPK6 expression in LPS-induced inflammatory injury
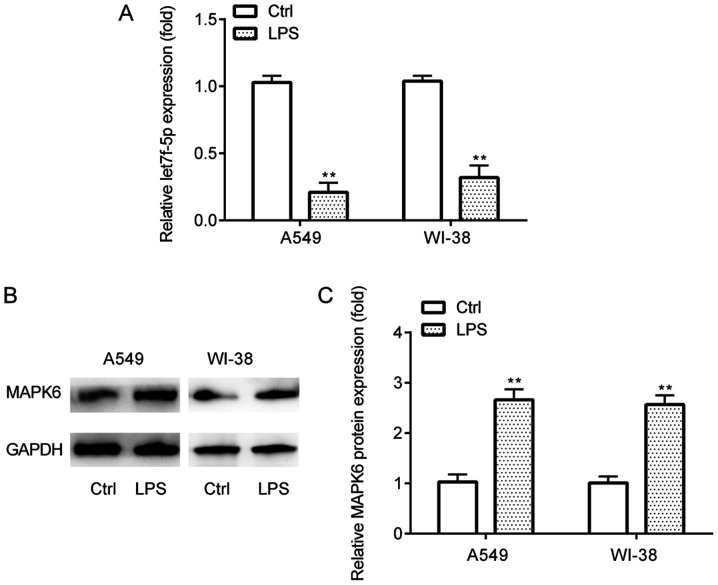
The expression profiles of let7f-5p and MAPK6 in the in vitro pneumonia model in A549 and WI-38 cells were assessed via RT-qPCR and western blotting. The results demonstrated that let7f-5p expression levels were significantly lower (Fig. 3A), whereas MAPK6 protein expression levels were significantly higher (Fig. 3B and C) in LPS-treated A549 and WI-38 cells compared with the control group.
Figure 3.
let7f-5p and MAPK6 expression in LPS-induced inflammatory injury. (A) Reverse transcription-quantitative PCR was performed to detect let7f-5p expression levels in A549 and WI-38 cells. MAPK6 protein expression levels were (B) determined via western blotting and (C) semi-quantified in A549 and WI-38 cells. **P<0.01 vs. Ctrl. LPS, lipopolysaccharide; Ctrl, control.
let7f-5p targets MAPK6
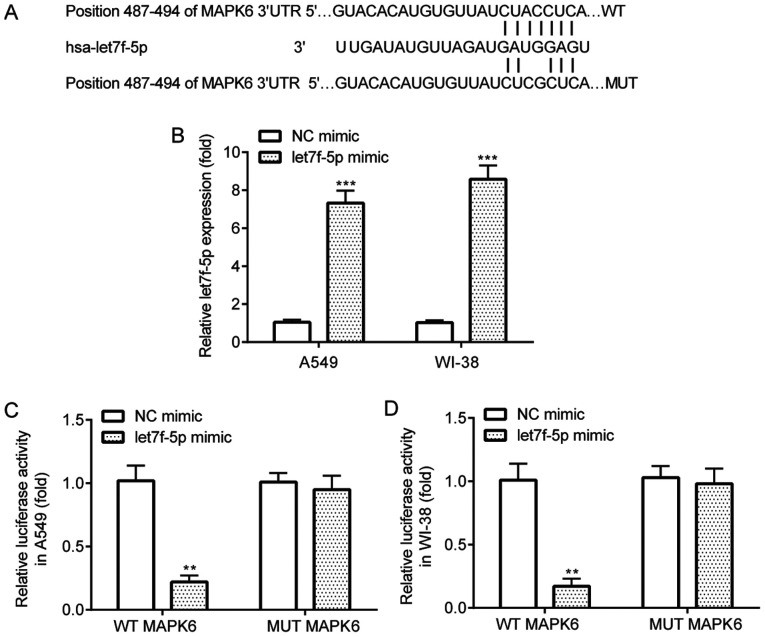
The interaction between let7f-5p and MAPK6 was verified by performing the dual-luciferase reporter assay. TargetScan software identified complementary binding sites between let7f-5p and the 3′UTR of MAPK6 (Fig. 4A). The results demonstrated that let7f-5p mimic significantly increased let7f-5p expression in A549 and WI-38 cells compared with NC mimic (Fig. 4B). The dual-luciferase reporter assay results indicated that compared with NC mimic, let7f-5p mimic significantly decreased the luciferase activity of WT MAPK6 in A549 and WI-38 cells, but did not significantly alter the luciferase activity of MUT MAPK6 in A549 or WI-38 cells (Fig. 4C and D).
Figure 4.
let7f-5p targets MAPK6. (A) TargetScan software was used to predict the complementary binding sites between let7f-5p and MAPK6. (B) Reverse transcription-quantitative PCR was performed to assess the transfection efficiency of let7f-5p mimic in A549 and WI-38 cells. Dual-luciferase reporter assays were performed to validate the interaction between let7f-5p and MAPK6 in (C) A549 and (D) WI-38 cells. **P<0.01 and ***P<0.001 vs. NC mimic. NC, negative control; UTR, untranslated region; WT, wild-type; MUT, mutant.
let7f-5p attenuates LPS-induced inflammatory injury by targeting MAPK6
The effects of let7f-5p and MAPK6 in the LPS-induced in vitro pneumonia model were investigated in A549 and WI-38 cells. The RT-qPCR and western blotting results demonstrated that pcDNA3.1-MAPK6 significantly increased MAPK6 mRNA and protein expression levels (Fig. 5A-C) compared with pcDNA3.1 in A549 and WI-38 cells. The CCK-8 assay results suggested that let7f-5p mimic significantly ameliorated LPS-induced reductions in cell viability in A549 and WI-38 cells, but let7f-5p mimic-mediated effects were significantly reversed by MAPK6 overexpression (Fig. 6A). The RT-qPCR and ELISA results demonstrated that let7f-5p mimic significantly ameliorated LPS-induced TNF-α and IL-6 expression and release in A549 and WI-38 cells, which was also significantly reversed by transfection with pcDNA3.1-MAPK6 (Fig. 6B-E).
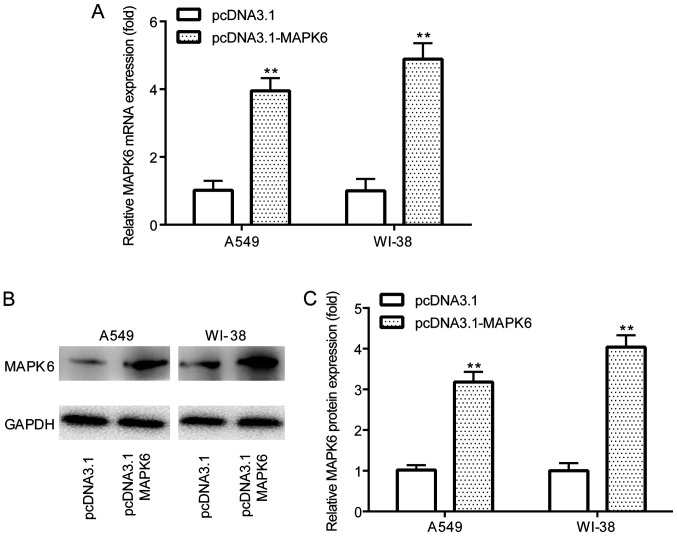
Figure 5.
pcDNA3.1-MAPK6 increases MAPK6 expression. (A) Reverse transcription-quantitative PCR was performed to assess the transfection efficiency of pcDNA3.1-MAPK6 in A549 and WI-38 cells. pcDNA3.1-MAPK6 transfection efficiency in A549 and WI-38 cells was also (B) determined via western blotting and (C) semi-quantified. **P<0.01 vs. pcDNA3.1.
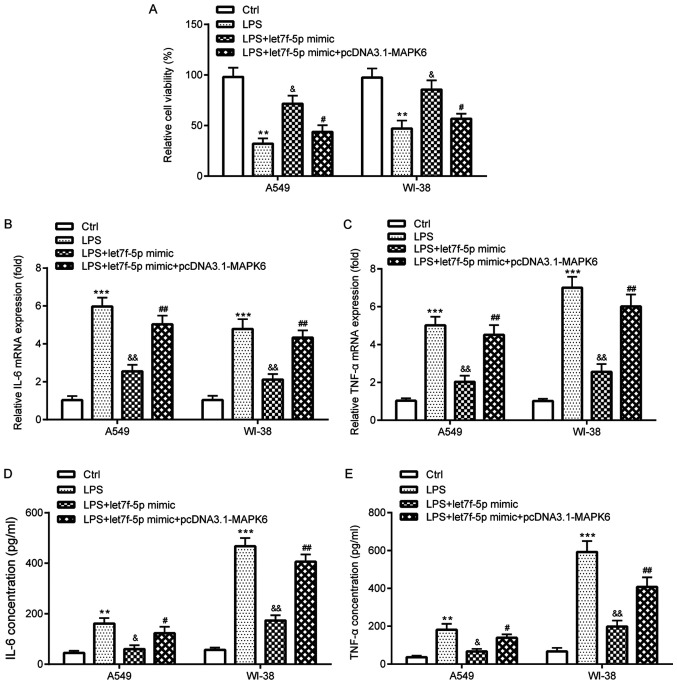
Figure 6.
let7f-5p attenuates LPS-induced inflammatory injury by targeting MAPK6. (A) Cell Counting Kit-8 assay was performed to assess cell viability in A549 and WI-38 cells. Reverse transcription-quantitative PCR was performed to determine (B) IL-6 and (C) TNF-α mRNA expression levels in A549 and WI-38 cells. ELISAs were performed to assess the concentrations of (D) IL-6 and (E) TNF-α in the cell culture medium of A549 and WI-38 cells. **P<0.01 and ***P<0.001 vs. Ctrl; &P<0.05 and &&P<0.01 vs. LPS; #P<0.05 and ##P<0.01 vs. LPS + let7f-5p mimic. LPS, lipopolysaccharide; Ctrl, control.
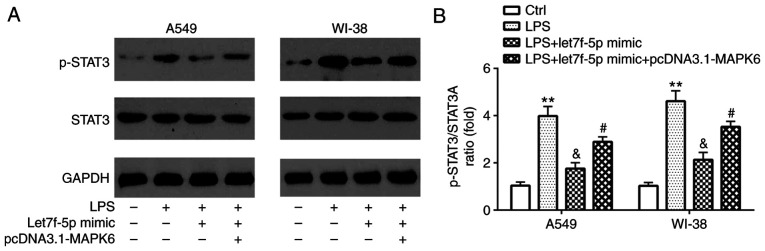
STAT3 is involved in let7f-5p/MAPK6-mediated regulation of LPS-induced inflammatory injury
Compared with the control group, LPS significantly increased STAT3 activation in A549 and WI-38 cells, which was reversed by transfection with let7f-5p mimic (Fig. 7A and B). However, let7f-5p mimic-mediated effects on STAT3 activation were significantly reversed by transfection with pcDNA3.1-MAPK6.
Figure 7.
STAT3 is involved in let7f-5p/MAPK6-mediated regulation of LPS-induced inflammatory injury. STAT3 protein expression levels were (A) determined by western blotting and (B) semi-quantified in A549 and WI-38 cells. **P<0.01 vs. Ctrl; &P<0.05 vs. LPS; #P<0.05 vs. LPS + let7f-5p mimic. LPS, lipopolysaccharide; Ctrl, control; p, phosphorylated.
Discussion
Pneumonia is a common infectious disease, with high mortality and morbidity rates worldwide (2,3); therefore, developing novel therapeutic strategies for the management of pneumonia is important. Targeted therapy is extensively applied in the treatment of several diseases (16). For example, the let7 family participates in multiple carcinogenic signaling pathways (17). let-7 serves as a tumor suppressor, whereby let-7 downregulation promotes lung cancer cell proliferation (18), whereas let-7b and let-7c are crucial for lung restoration in influenza pneumonia model mice (18). let7f inhibition facilitates neuroprotection in ischemic stroke (19), and let7f expression is elevated following ischemia-reperfusion (20), but decreased in patients with papillary thyroid cancer (21). By performing RNA-sequencing and bioinformatics analysis, a previous study demonstrated that let7f expression is decreased in the peripheral blood of patients with severe pneumonia compared with healthy volunteers (11). More recently, it has been reported that let7f expression is decreased in the blood of extracellular vesicles of alcohol-drinkers without liver injury compared with non-drinkers (22). To the best of our knowledge, the present study was the first to investigate the role of let7f-5p in pneumonia.
MAPK6 serves as a promoter of several diseases: Small nucleolar RNA host gene 6 facilitates breast cancer cell proliferation and metastasis by regulating the miR-26a-5p/MAPK6 axis (23); miR-144-3p inhibits the progression of cervical cancer by targeting MAPK6 (24); nuclear paraspeckle assembly transcript 1 enhances myocardial ischemia-reperfusion injury via the miR-495-3p/MAPK6 axis (25); and miR-26a-5p negatively regulates neuropathic pain by targeting MAPK6 (26). As for the function of MAPK6 in inflammation, hierarchical clustering in tongue tissue with hyperplasia suggests that cytokine-mediated inflammation may be associated with MAPK6 (27). However, the exact role of MAPK6 in pneumonia is not completely understood.
The present study aimed to investigate the role of let7f-5p and MAPK6 by recruiting healthy volunteers and patients with pneumonia, and using in vitro pneumonia model. The results demonstrated that let7f-5p expression was significantly decreased, whereas MAPK6 expression was significantly increased in the peripheral venous blood of patients with pneumonia and in LPS-induced WI-38 and A549 cells compared with healthy volunteers and control cells, respectively. The results also demonstrated that let7f-5p targeted MAPK6 in WI-38 and A549 cells, and let7f-5p expression was negatively correlated with MAPK6 expression in the peripheral venous blood of patients with pneumonia.
Pneumonia is associated with inflammation (6). TNF-α, a predominant cytokine that is produced by monocytes and macrophages, is an important inflammatory mediator (28,29). TNF-α initiates the inflammatory response by inducing local infiltration, neutrophil chemotaxis, phagocytosis and killing of pathogens (30). IL-6, an important cytokine that is produced by monocytes, macrophages and lymphocytes, is a pivotal mediator during the acute phase of inflammatory response (28,30). The results of the present study demonstrated that, compared with the control group, LPS treatment significantly increased the expression levels of TNF-α and IL-6 in A549 and WI-38 cells, which were reversed by transfection with let7f-5p mimic. However, let7f-5p mimic-mediated effects were reversed by MAPK6 overexpression.
The STAT3 signaling pathway is pivotal for the inflammatory response (31). During acute inflammatory injury, the STAT3 signaling pathway is implicated in lung injury (32) and LPS-induced inflammatory injury, as well as in the circular RNA (circ)_0038467/miR-338-3p axis (33). The results of the present study demonstrated that LPS significantly increased STAT3 activation compared with the control group, which was reversed by transfection with let7f-5p mimic. Moreover, let7f-5p mimic-mediated effects on STAT3 activation were reversed by MAPK6 overexpression.
As for the association between the let7f-5p/MAPK6/STAT3 axis and inflammation, several reports in other inflammation-related diseases have been published. In 2019, Tan et al (34) reported that let7f-5p attenuated inflammation in systemic lupus erythematosus by targeting NLR family pyrin domain containing 3. In 2020, Yao et al (35) demonstrated that MAPK6 was involved in circ_0000285-induced inflammation in diabetic nephropathy. The STAT3 signaling pathway is pivotal for the inflammatory response (31). In 2019, Li et al (36) reported that let7f-5p reduced Th17 differentiation in multiple sclerosis by targeting STAT3. In 2018, Kim et al (37) demonstrated that orientin repressed breast cancer cell invasion via the MAPK6/STAT3 signaling pathway (37). However, the relationship between let7f-5p and MAPK6, as well as the interactions between let7f-5p and MAPK6, let7f-5p and STAT3 or MAPK6 and STAT3 in pneumonia have not been previously reported. Therefore, to the best of our knowledge, the present study indicated for the first time that the let7f-5p/MAPK6/STAT3 axis may serve an inhibitory role in inflammation in pneumonia.
The present study had two key limitations. The results of the present study demonstrated that let7f-5p expression was significantly decreased in patients with pneumonia and in LPS-induced A549 and WI-38 cells compared with healthy volunteers and control cells, respectively. Therefore, the present study aimed to investigate the effect of let7f-5p overexpression on pneumonia and to assess whether MAPK6 overexpression could rescue the effects of let7f-5p overexpression on pneumonia. The results indicated that let7f-5p inhibited pneumonia-associated inflammation in vitro by targeting MAPK6. However, the effects of MAPK6 knockdown or knockout on inflammation could be similar to the effects mediated by let7f-5p overexpression, but this was not investigated in the present study, thus further investigation is required. Secondly, although the WI-38 cell line is widely used for the study of pneumonia in vitro (13,38,39), a future study using a normal non-cancerous human lung cell line should be performed to verify the results of the present study.
Collectively, the results of the present study suggested that let7f-5p inhibited pneumonia-associated inflammation in vitro by targeting MAPK6 and inactivating the STAT3 signaling pathway. Therefore, let7f-5p may serve as a potential target for anti-inflammatory therapeutic strategies for pneumonia.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Respiratory Disease Clinical Research Center of Guizhou Province (grant no. 2016-2907) and the Science and Technology Project of Guizhou Province (grant nos. 2017-1100 and 2019-1195).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
LX, QS and ZO performed the experiments and analyzed the data. XZ and CZ designed the present study and supervised the experiments. CZ drafted the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethical Committee of Guizhou Provincial People's Hospital (approval no. 2016011605). The parents or legal guardians of all participants provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Cillóniz C, Torres A, Niederman M, van der Eerden M, Chalmers J, Welte T, Blasi F. Community-acquired pneumonia related to intracellular pathogens. Intensive Care Med. 2016;42:1374–1386. doi: 10.1007/s00134-016-4394-4. [DOI] [PubMed] [Google Scholar]
- 2.Korppi M. Diagnosis and treatment of community-acquired pneumonia in children. Acta Paediatr. 2012;101:702–704. doi: 10.1111/j.1651-2227.2012.02648.x. [DOI] [PubMed] [Google Scholar]
- 3.Simonetti AF, Viasus D, Garcia-Vidal C, Carratalà J. Management of community-acquired pneumonia in older adults. Ther Adv Infect Dis. 2014;2:3–16. doi: 10.1177/2049936113518041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim GD. Myricetin inhibits angiogenesis by inducing apoptosis and suppressing PI3K/Akt/mTOR signaling in endothelial cells. J Cancer Prev. 2017;22:219–227. doi: 10.15430/JCP.2017.22.4.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu B, Shen Y, Qiao J, Cui Q. Geniposide attenuates Staphylococcus aureus-induced pneumonia in mice by inhibiting NF-κB activation. Microb Pathog. 2017;112:117–121. doi: 10.1016/j.micpath.2017.09.050. [DOI] [PubMed] [Google Scholar]
- 6.Rojas M, Woods CR, Mora AL, Xu J, Brigham KL. Endotoxin-induced lung injury in mice: Structural, functional, and biochemical responses. Am J Physiol Lung Cell Mol Physiol. 2005;288:L333–L341. doi: 10.1152/ajplung.00334.2004. [DOI] [PubMed] [Google Scholar]
- 7.Didier D, Jean-Damien R. Acute lung injury and bacterial infection. Clin Chest Med. 2005;26:105–112. doi: 10.1016/j.ccm.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 8.Wong KY, Huang X, Chim CS. DNA methylation of microRNA genes in multiple myeloma. Carcinogenesis. 2012;33:1629–1638. doi: 10.1093/carcin/bgs212. [DOI] [PubMed] [Google Scholar]
- 9.Fei S, Cao L, Pan L. microRNA3941 targets IGF2 to control LPS-induced acute pneumonia in A549 cells. Mol Med Rep. 2018;17:4019–4026. doi: 10.3892/mmr.2017.8369. [DOI] [PubMed] [Google Scholar]
- 10.Abd-El-Fattah AA, Sadik NA, Shaker OG, Aboulftouh ML. Differential microRNAs expression in serum of patients with lung cancer, pulmonary tuberculosis, and pneumonia. Cell Biochem Biophys. 2013;67:875–884. doi: 10.1007/s12013-013-9575-y. [DOI] [PubMed] [Google Scholar]
- 11.Huang S, Feng C, Zhai YZ, Zhou X, Li B, Wang LL, Chen W, Lv FQ, Li TS. Identification of miRNA biomarkers of pneumonia using RNA-sequencing and bioinformatics analysis. Exp Ther Med. 2017;13:1235–1244. doi: 10.3892/etm.2017.4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai D, Han A, Cong S. The effect of down-regulation of CCL5 on lipopolysaccharide-induced WI-38 fibroblast injury: A potential role for infantile pneumonia. Iran J Basic Med Sci. 2018;21:449–454. doi: 10.22038/IJBMS.2018.27165.6640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Z, Zhua Y, Gao G, Zhang Y. Long noncoding RNA SNHG16 targets miR-146a-5p/CCL5 to regulate LPS induced WI-38 cell apoptosis and inflammation in acute pneumonia. Life Sci. 2019;228:189–197. doi: 10.1016/j.lfs.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Wang Q, Li D, Han Y, Ding X, Xu T, Tang B. MicroRNA-146 protects A549 and H1975 cells from LPS-induced apoptosis and inflammation injury. J Biosci. 2017;42:637–645. doi: 10.1007/s12038-017-9715-4. [DOI] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Pérez-Herrero E, Fernández-Medarde A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharm. 2015;93:52–79. doi: 10.1016/j.ejpb.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Cao L, Wang Y, Wang X, Liu N, You Y. Regulation of let-7 and its target oncogenes (Review) Oncol Lett. 2012;3:955–960. doi: 10.3892/ol.2012.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan KS, Choi H, Jiang X, Yin L, Seet JE, Patzel V, Engelward BP, Chow VT. Micro-RNAs in regenerating lungs: An integrative systems biology analysis of murine influenza pneumonia. BMC Genomics. 2014;15:587. doi: 10.1186/1471-2164-15-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selvamani A, Sathyan P, Miranda RC, Sohrabji F. An antagomir to microRNA Let7f promotes neuroprotection in an ischemic stroke model. PLoS One. 2012;7:e32662. doi: 10.1371/journal.pone.0032662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Niu X, Hu J, Xing H, Sun M, Wang J, Jian Q, Yang H. After myocardial ischemia-reperfusion, miR-29a, and Let7 could affect apoptosis through regulating IGF-1. Biomed Res Int. 2015;2015:245412. doi: 10.1155/2015/245412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Damanakis AI, Eckhardt S, Wunderlich A, Roth S, Wissniowski TT, Bartsch DK, Di Fazio P. MicroRNAs let7 expression in thyroid cancer: Correlation with their deputed targets HMGA2 and SLC5A5. J Cancer Res Clin Oncol. 2016;142:1213–1220. doi: 10.1007/s00432-016-2138-z. [DOI] [PubMed] [Google Scholar]
- 22.Eguchi A, Franz N, Kobayashi Y, Iwasa M, Wagner N, Hildebrand F, Takei Y, Marzi I, Relja B. Circulating extracellular vesicles and their miR ‘Barcode’ differentiate alcohol drinkers with liver injury and those without liver injury in severe trauma patients. Front Med (Lausanne) 2019;6:30. doi: 10.3389/fmed.2019.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lv P, Qiu X, Gu Y, Yang X, Xu X, Yang Y. Long non-coding RNA SNHG6 enhances cell proliferation, migration and invasion by regulating miR-26a-5p/MAPK6 in breast cancer. Biomed Pharmacother. 2019;110:294–301. doi: 10.1016/j.biopha.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 24.Wu J, Zhao Y, Li F, Qiao B. miR-144-3p: A novel tumor suppressor targeting MAPK6 in cervical cancer. J Physiol Biochem. 2019;75:143–152. doi: 10.1007/s13105-019-00681-9. [DOI] [PubMed] [Google Scholar]
- 25.Luo M, Sun Q, Zhao H, Tao J, Yan D. Long noncoding RNA NEAT1 sponges miR-495-3p to enhance myocardial ischemia-reperfusion injury via MAPK6 activation. J Cell Physiol. 2020;235:105–113. doi: 10.1002/jcp.28791. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Su Z, Liu HL, Li L, Wei M, Ge DJ, Zhang ZJ. Effects of miR-26a-5p on neuropathic pain development by targeting MAPK6 in in CCI rat models. Biomed Pharmacother. 2018;107:644–649. doi: 10.1016/j.biopha.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Liu YC, Ho HC, Lee MR, Lai KC, Yeh CM, Lin YM, Ho TY, Hsiang CY, Chung JG. Early induction of cytokines/cytokine receptors and Cox2, and activation of NF-κB in 4-nitroquinoline 1-oxide-induced murine oral cancer model. Toxicol Appl Pharmacol. 2012;262:107–116. doi: 10.1016/j.taap.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 28.Khan J, Noboru N, Young A, Thomas D. Pro and antiinflammatory cytokine levels (TNF-α, IL-1β, IL-6 and IL-10) in rat model of neuroma. Pathophysiology. 2017;24:155–159. doi: 10.1016/j.pathophys.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Berg AS, Inchley CS, Fjaerli HO, Leegaard TM, Lindbaek M, Nakstad B. Clinical features and inflammatory markers in pediatric pneumonia: A prospective study. Eur J Pediatr. 2017;176:629–638. doi: 10.1007/s00431-017-2887-y. [DOI] [PubMed] [Google Scholar]
- 30.Dai JP, Wang QW, Su Y, Gu LM, Zhao Y, chen XX, Chen C, Li WZ, Wang GF, Li KS. Emodin inhibition of influenza A virus replication and influenza viral pneumonia via the Nrf2, TLR4, p38/JNK and NF-kappaB pathways. Molecules. 2017;22:1754. doi: 10.3390/molecules22101754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu Q, Zeng K, Ma X, Song F, Jiang Y, Tu P, Wang X. Resokaempferol-mediated anti-inflammatory effects on activated macrophages via the inhibition of JAK2/STAT3, NF-κB and JNK/p38 MAPK signaling pathways. Int Immunopharmacol. 2016;38:104–114. doi: 10.1016/j.intimp.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Gao H, Ward PA. STAT3 and suppressor of cytokine signaling 3: Potential targets in lung inflammatory responses. Expert Opin Ther Targets. 2007;11:869–880. doi: 10.1517/14728222.11.7.869. [DOI] [PubMed] [Google Scholar]
- 33.Liu G, Wan Q, Li J, Hu X, Gu X, Xu S. Circ_0038467 regulates lipopolysaccharide-induced inflammatory injury in human bronchial epithelial cells through sponging miR-338-3p. Thorac Cancer. 2020;11:1297–1308. doi: 10.1111/1759-7714.13397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan W, Gu Z, Leng J, Zou X, Chen H, Min F, Zhou W, Zhang L, Li G. Let-7f-5p ameliorates inflammation by targeting NLRP3 in bone marrow-derived mesenchymal stem cells in patients with systemic lupus erythematosus. Biomed Pharmacother. 2019;118:109313. doi: 10.1016/j.biopha.2019.109313. [DOI] [PubMed] [Google Scholar]
- 35.Yao T, Zha D, Hu C, Wu X. Circ_0000285 promotes podocyte injury through sponging miR-654-3p and activating MAPK6 in diabetic nephropathy. Gene. 2020;747:144661. doi: 10.1016/j.gene.2020.144661. [DOI] [PubMed] [Google Scholar]
- 36.Li ZH, Wang YF, He DD, Zhang XM, Zhou YL, Yue H, Huang S, Fu Z, Zhang LY, Mao ZQ, et al. Let-7f-5p suppresses Th17 differentiation via targeting STAT3 in multiple sclerosis. Aging (Albany NY) 2019;11:4463–4477. doi: 10.18632/aging.102093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim SJ, Pham TH, Bak Y, Ryu HW, Oh SR, Yoon DY. Orientin inhibits invasion by suppressing MMP-9 and IL-8 expression via the PKCα/ERK/AP-1/STAT3-mediated signaling pathways in TPA-treated MCF-7 breast cancer cells. Phytomedicine. 2018;50:35–42. doi: 10.1016/j.phymed.2018.09.172. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Zhu Y, Gao G, Zhou Z. Knockdown XIST alleviates LPS-induced WI-38 cell apoptosis and inflammation injury via targeting miR-370-3p/TLR4 in acute pneumonia. Cell Biochem Funct. 2019;37:348–358. doi: 10.1002/cbf.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang L, Dong L, Tang Y, Li M, Zhang M. miR-146b protects against the inflammation injury in pediatric pneumonia through MyD88/NF-κB signaling pathway. Infect Dis (Lond) 2020;52:23–32. doi: 10.1080/23744235.2019.1671987. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.