Abstract
Codonopsis pilosula is a kind of traditional Chinese medicine used to treat weak spleens, stomach problems, anemia, and fatigue. Polysaccharide is one of main components of Codonopsis pilosula. In this study, response surface methodology (RSM) was used to optimize the extraction parameters of Codonopsis pilosula polysaccharides (CPP) by fermentation. The exaction temperature (°C), yeast liquid volume (2 mg/ml, ml), and time (h) were employed effects. Results indicated that the best extraction conditions were the following: extraction temperature 24.75°C, yeast liquid volume 2.96 ml (5.92 mg), and a fermentation time of 21.03 hr. After purification with DE52 and Sephadex G‐100, the molecular structure was determined by ultraviolet–visible (UV) spectroscopy, Fourier transform infrared (FTIR) spectroscopy, and nuclear magnetic resonance (NMR) (1H and 13C). The monosaccharide composition of CPP1 was determined to be mannose (1.76%), glucose (97.38%), and arabinose (0.76%). CPP1 exhibited high antioxidant activities in scavenging ABTS radicals, ferreous ions, and superoxide ion radicals. Thus, CPP1 could be used as an antioxidant or functional food.
Keywords: antioxidation, codonopsis, polysaccharide, response surface method
Response surface methodology (RSM) was used to optimize the extraction parameters of Codonopsis pilosula polysaccharides (CPPs) by fermentation.

1. INTRODUCTION
Codonopsis pilosula (C. pilosula) is in the family Campanulaceae, its root is a commonly used Chinese medicine, and it is mainly planted in China, Japan, and Korea (Chen & Huang, 2018; Liu et al., 2018). As a traditional Chinese medicine, C. pilosula has been widely recorded in many ancient books. It has been mainly used for strengthening the spleen, moistening the lung, nourishing blood, engendering fluid, enhancing immune function, and modulating antitumor effect (Bai et al., 2018; Zou et al., 2014).
At present, the commonly used polysaccharide extraction methods include hot water extraction, acid–base extraction, and ultrasonic‐assisted extraction (Dou et al., 2019; Yilmaz & Sebnem, 2017). These methods have their advantages and disadvantages. We use yeast to grow and use the characteristics of oligosaccharides such as glucose and fructose. After fermenting C. pilosula, the yeast consumes monosaccharides and disaccharides in C.pilosula to improve the extraction efficiency and purity of polysaccharides and explore a new type of polysaccharide extraction method.
In this study, RSM was employed to estimate the influence of different extraction parameters (fermentation temperature, time, and yeast addition) on the yields of polysaccharides from C. pilosula. Column chromatography was used to purify water‐extracted polysaccharides and fermentation polysaccharides. Then, NMR, FTIR, high‐performance liquid chromatography (HPLC), and scanning electron microscopy (SEM) were employed to characterize the polysaccharide. SEM is an electron microscope developed after TEM. SEM is also one of the main instruments of microstructure analysis, which has been widely used in materials, metallurgy, minerals, biology, and other fields. However, SEM can only observe the micromorphology of the material surface and cannot obtain the information of the material interior. Furthermore, the antioxidant activities of polysaccharides were evaluated, including the metal iron ion scavenging ability, ABTS radical scavenging ability, and superoxide anion radical scavenging activity.
2. MATERIALS AND METHODS
2.1. Materials
C. pilosula was purchased from the local market in Hubei, China. The DEAE‐52 cellulose and Sephadex G‐100 were obtained from Solarbio (Beijing China). All chemicals were analytically pure grade.
2.2. Extraction of polysaccharide
C. pilosula was washed with distilled water and dried in the sun. Then, the dry C. pilosula was ground into powder. Then, to a 250‐ml shake flask, 1 g C. pilosula powder, 50 ml LB medium(peptone 10 g/L, yeast extract 5 g/L, NaCl 5 g/L, pH = 7), and 3 ml yeast sample solution (the beer active dry yeast from Angel Yeast Company and distilled water were mixed at the concentration of 2 mg/ml) were added. The sample was placed in the shaker (24.5°C, 200 rpm) for 21 hr. The fermentation broth was suction filtered to obtain an extract. The obtained extract was poured into a macroporous resin elution column for decolorization treatment, and using sevage solution (chloroform:n‐butanol, 4:1) protein was removed by separating with a separatory funnel. The supernatant was precipitated with fourfold volumes of 95% ethanol at 4°C for 16 hr. Precipitate was collected by centrifugation (2397 g, 10 min) and lyophilized to obtain crude polysaccharides, that is, CPP.
Measure 0, 0.2, 0.4, 0.6, 0.8, 1.0 ml of 0.1 g/L glucose standard solution (Accurately weigh 0.1 g glucose, add 100 ml distilled water to dissolve, and dilute to volume in a volumetric flask 100 ml) into the test tube, add distilled water to make the volume up to 1.0 ml in the volumetric flask, and then add 0.5 ml of 6% phenol (Accurately weigh 3 g of phenol, add 50 ml of distilled water to dissolve, and dilute to 10 ml in a 10 ml volumetric flask) solution And 2.5 ml concentrated sulfuric acid, heated in boiling water bath for 30 min, and finally measured the absorbance of the sample at 490 nm (Masuko et al., 2005). A glucose standard curve obtained with regression equation: y = 14.25x − 0.0599 (R 2 = .998), where y is absorbance at 490 nm and x is glucose concentration (mg/ml). The standard curve had a good linear relationship between 0.01–0.1 mg/ml.
Three single factors (fermentation temperature (°C), time (h), and yeast addition (ml)) were used for further extraction of CPP(Cai,et al., 2019). As shown in Table 1, on the basis results of single factor experiment, Box–Behnken design (BBD)‐RSM were used to investigate the yield of CPP (Table 1). An the polysaccharide yield of the experimental group based on the curve of glucose standard solution. The parameters of the model were estimated by the least square method through 17 measurement experiments, and then the model is established (Table 2). The absorbance at 490 nm of sugar by the phenol sulfuric acid method was used as the detection index. By applying multiple regression analysis to the experimental data, the response variable and the test variables were related by the second‐order polynomial equation. The obtained test data were used for multiple regression fitting and optimization of process parameters by using Design‐Expert 8.0 software. The best fermentation conditions were used for verification and detection, and compared with the ideal value of the software.
TABLE 1.
Levels and code of extraction variables used in Box–Behnken design
| Variable | Coded levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Exaction temperature (X1,°C) | 20 | 25 | 30 |
| Yeast liquid volume (X2, ml) | 2 | 3 | 4 |
| Exaction time (X3, h) | 16 | 20 | 24 |
TABLE 2.
Box–Behnken experimental design and the results for extraction yield of CPP
| Run | X1 | X2 | X3 | Absorbance | Yield (mg) |
|---|---|---|---|---|---|
| 1 | 30 | 3 | 24 | 0.541 | 16.561 |
| 2 | 25 | 4 | 24 | 0.533 | 16.286 |
| 3 | 20 | 2 | 20 | 0.534 | 16.320 |
| 4 | 25 | 3 | 20 | 0.565 | 17.387 |
| 5 | 20 | 3 | 24 | 0.551 | 16.905 |
| 6 | 25 | 2 | 24 | 0.535 | 16.355 |
| 7 | 20 | 4 | 20 | 0.520 | 15.838 |
| 8 | 25 | 2 | 16 | 0.527 | 16.079 |
| 9 | 25 | 3 | 20 | 0.569 | 17.525 |
| 10 | 25 | 3 | 20 | 0.560 | 17.215 |
| 11 | 25 | 3 | 20 | 0.563 | 17.319 |
| 12 | 30 | 2 | 20 | 0.522 | 15.907 |
| 13 | 30 | 4 | 20 | 0.527 | 16.079 |
| 14 | 25 | 4 | 16 | 0.522 | 15.907 |
| 15 | 20 | 3 | 16 | 0.534 | 16.320 |
| 16 | 30 | 3 | 16 | 0.544 | 16.664 |
| 17 | 25 | 3 | 20 | 0.561 | 17.250 |
2.3. Preparation of polysaccharides
CPPs (80 mg) were redissolved in ultrapure water (4 ml), and DEAE column (I.0 × 40 cm) was used for further classification. Gradient elution was carried out with 0, 0.05, 0.1, 0.2, 0.3, and 0.5 mol/L NaC1 solution at a flow rate of 1 ml/min, with 5 ml in each tube (Jin et al., 2016). The sugar of each tube was determined at 490 nm by the phenol sulfuric acid method. The major peak polysaccharide (CPP1) was concentrated and further purification through a column (1.0 × 40 cm) of Sephadex G‐100 at a flow rate of 0.5 ml/min. And then the purified polysaccharide fractions (CPP1) were analyzed, concentrated, and freeze‐dried.
2.4. UV spectroscopy and FTIR spectroscopy
The CPP1 sample solution was set to 0.1 g/ml, and full wavelength scanning was performed at 190–800 nm. Based on the spectrum, it was inferred whether the obtained polysaccharide component had free nucleic acids and proteins. CPP1 was mixed with spectrum grade potassium bromide powder and then milled into pellets for infrared spectrum determination. According to the previously reported method, FTIR spectra were recorded from 4,000 to 400 cm−1 by FTIR spectrometer (tensor 27, Bruker, Karlsruhe, Germany) (Jouraiphy et al., 2008).
2.5. Monosaccharide composition analysis
The monosaccharide composition of CPP1 was determined by PMP precolumn derivatization HPLC (Cai et al., 2018). The hydrolyzed CPP1(10 mg) or monosaccharide standard solution (50 μl) was mixed with 0.6 mol/L sodium hydroxide (50 μl), and then treated with a 0.5 mol/L methanolic solution of PMP (100 μl) at 70°C for 100 min. The product was neutralized with 100 μl hydrochloric acid (0.3 M), and distilled water was added to 1 ml. And then the reaction product was made up to extracted with 2 ml chloroform three times. Filtered the solution through 0.45 μM filter membrane and carried out monosaccharide composition analysis by HPLC.
2.6. SEM analysis
SEM (SU8010, Hitachi High‐Technologies Co., Tokyo, Japan) was employed to observe the molecular morphologies of CPP1. In brief, the CPP1 powder was placed on the sample stage and coated with gold powder, and the SEM images were observed under the high vacuum condition.
2.7. NMR analysis
The 1H and 13C NMR spectra of CPP1 were recorded using a Bruker AV‐400 NMR spectrometer (Bruker Instrumental Inc., Billerica, Massachusetts, USA) at 25°C, and the CPP1 was dissolved in dimethyl sulfoxide (DMSO).
2.8. Antioxidant activity analysis
The superoxide anion radical scavenging activity, ABTS radical scavenging activities, and ferrous iron ion scavenging ability of CPP1 were determined based on previously reported methods (Carter, 1971; Finkel et al., 2000; Peng et al., 2017; Re et al., 1999; Wang, Wang, et al., 2016; Wang, Ding, et al., 2016; Zhang et al., 2018; Zhu et al., 2018). 1, 2, 4, 8, and 10 mg/ml of CPP1 were dissolved in water.
2.8.1. Superoxide anion radical scavenging activity
7 mmol/L pyrogallol aqueous solution was prepared in advance. Take 0.3 ml of the prepared polysaccharide solution, add 0.9 ml of Tris‐HCl, 0.09 ml of pyrogallol, and 0.3 ml of 10 mol/L concentrated hydrochloric acid, respectively, and then react at room temperature for 20 min. Then, the absorbance A1 was determined at 420 nm. The absorbance of ultrapure water replacing polysaccharide solution is A0 and that of ultrapure water replacing chromogenic solution is A2.
2.8.2. ABTS radical scavenging activities
Accurately transfer 400 μl of the prepared polysaccharide solution into a 2 ml centrifuge tube, add 1,600 μl of diluted ABTS stock solution, react at room temperature for 10 min, detect its absorbance at 734 nm, and record it as A1. Accurately transfer 400 μl of each prepared polysaccharide solution, place 2 ml centrifuge tube, add 1,600 μl distilled water into each tube, react at room temperature for 10 min, and record the absorbance value as A2. Absorb 400 μl distilled water, add 1,600 μl ABTS stock solution, react at room temperature for 10 min, and record the absorbance value as A0.
2.8.3. Ferrous iron ion scavenging ability
Transfer 400 μl of the prepared polysaccharide solution, 80 μl phenanthroline test solution, 40 μl FeCl2 solution and 1.08 ml distilled water were added in order to react at a constant temperature for 2 hr. The absorbance was determined at 562 nm and recorded as A1. 400 μl of distilled water instead of the sample, the absorbance was determined at 562 nm and recorded as A0. Measure 400 μl polysaccharide solution, add 80 μl phenanthrazine test solution and 1.12 ml distilled water in order to react at constant temperature for 2 hr, and then determine the absorbance as A2.
The superoxide radical scavenging activity was calculated according to the following equation: Scavenging activity (%) = [1 − (A1 − A2)/A0] × 100%. The experiment was repeated three times with duplicate samples.
3. RESULTS AND DISCUSSION
3.1. Response surface analysis
Based on single factor investigation results, 17 runs of Box–Behnken test factors design and results of test factors are shown in Table 2. The results showed that the absorbance of CPP varied from 0.520 to 0.569, and the optimization conditions for extraction of CPP were as follows: extraction temperature of 24.75°C, yeast liquid volume of 2.96 ml (5.92 mg beer active dry yeast), and a fermentation time of 21.03 hr. The quadratic regression equation with the absorbance of CPP as the objective function was obtained:
The F‐test and P‐values were used to measure the significance of the coefficients of the model. As shown in Table 3, X1X2 and X1X3 were significant; X3 was highly significant. These data indicate that the model established by the experiment was feasible. The precision value of 19.927 indicates that the model can predict experimental results. An R 2 Adj value of .9703 indicates that the model can prove the prediction of 97.03% of the response value, and the determination coefficient R 2 of .9870 indicates that the model has a good degree of fit, and the absorbance value of the polysaccharide of C. pilosula can be analyzed and predicted. The R 2 Pred being equal to .9578 is not significantly different from the R 2 of .9870, indicating that there was no need to further optimize the response surface equation.
TABLE 3.
Analysis of variance of the experimental results of the BBD in extraction of CPP
| Variables | Sum of squares | df | Mean square | F‐value | p‐Value |
|---|---|---|---|---|---|
| Model | 0.004401932 | 9 | 0.000489104 | 59.08067581 | <.0001 |
| X1 | 3.125E‐06 | 1 | 3.125E‐06 | 0.377480587 | .5584 |
| X2 | 3.2E‐05 | 1 | 3.2E‐05 | 3.865401208 | .0900 |
| X3 | 0.000136125 | 1 | 0.000136125 | 16.44305436 | .0048 |
| X1X2 | 9.025E‐05 | 1 | 9.025E‐05 | 10.90163934 | .0131 |
| X1X3 | 0.0001 | 1 | 0.0001 | 12.07937877 | .0103 |
| X2X3 | 2.25E‐06 | 1 | 2.25E‐06 | 0.271786022 | .6182 |
| X1 2 | 0.000637011 | 1 | 0.000637011 | 76.94691431 | <.0001 |
| X2 2 | 0.002748642 | 1 | 0.002748642 | 332.0188911 | <.0001 |
| X3 2 | 0.000326063 | 1 | 0.000326063 | 39.38640389 | .0004 |
| Residual | 5.795E‐05 | 7 | 8.27857E‐06 | ||
| Lack of fit | 6.75E‐06 | 3 | 2.25E‐06 | 0.17578125 | .9076 |
| Pure error | 5.12E‐05 | 4 | 0.0000128 | ||
| R 2 = .9870 | R 2 Adj = .9703 | R 2 Pred = .9578 | CV = 0.53% | Adeq Precision = 19.927 | |
Design‐Expert (version 8.0) was used to create the relationship between the independent and dependent variables, and the 3D response surface and contour plots are shown in Figure 1. Figure 1A and a shown the response surface and contour map of X1 and X2 to the yield of CPP. The influence of each factor in the response surface is staggered, and the degree of its influence can be clearly reflected by the intensity of the contours in the contour map and the steep angle in the response surface. The denser contours indicate a steeper response surface and higher impact. The larger spacing between contours indicates a smaller impact. It can be seen in Figure 1 that the above three factors have an important impact on the results.
FIGURE 1.
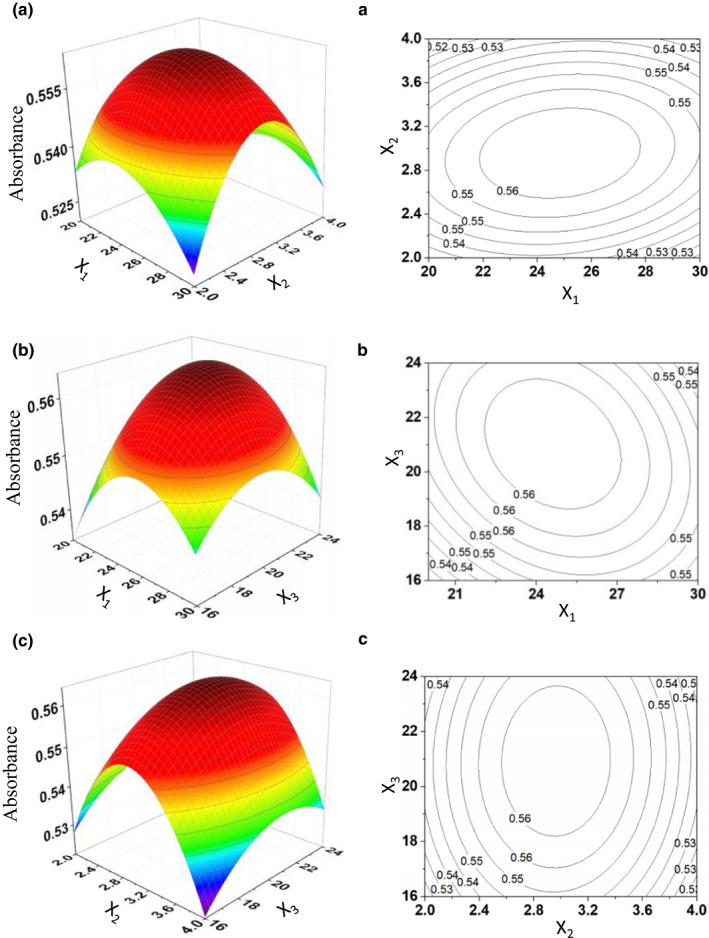
Response surface plots showing effects of variables on the extraction yield of CPP. A(a). The response surface of the effect of exaction temperature (X1, °C) and yeast liquid volume (X2, ml). B(b). The response surface of the effect of extraction temperature (X1, °C) and exaction time (X3, h). C(c). The response surface of the effect of the yeast liquid volume (X2, ml) and exaction time (X3, h)
In this experiment, the optimal extraction conditions of CPP were optimized by using RSM. At this time, the predicted absorbance value was 0.5642. The results of repeatability verification experiment were 0.552 (16.898 mg), 0.551(16.868 mg), 0.557(17.052), 0.550 (16.837), and 0.579(17.725). The average absorbance value of the experimental experiment was 0.5780, and the relative error was only 2.45%. Therefore, this model is reliable and can be used to optimize the extraction process of C. pilosula polysaccharides.
3.2. Separation and purification of polysaccharides
As shown in Figure 2a and b, crude CPPs showed three peaks after the purification of DE‐52, and the major peak was CPP1 (Figure 2a). Then, CPP1 was unimodal, symmetrical, and peaked in Sephadex G‐100 column chromatography (Figure 2b). Therefore, we obtain uniform and stable sample of CPP1.
FIGURE 2.
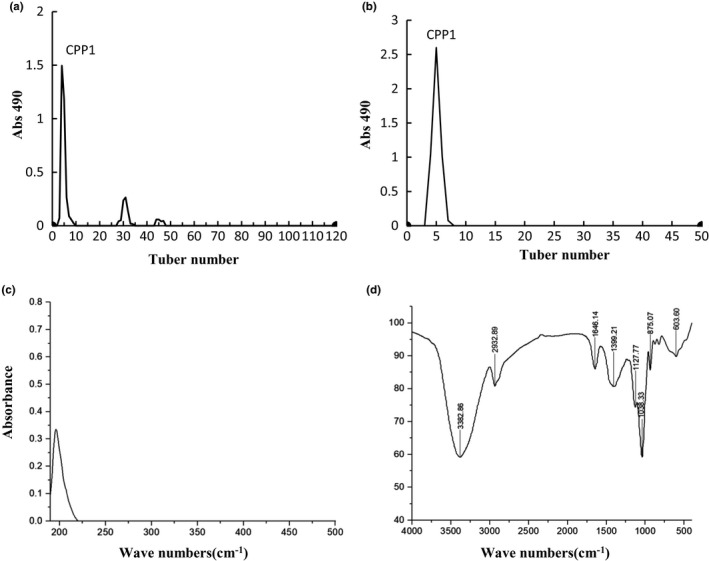
DEAE‐52 anion exchange chromatography (a) and Sephadex G‐100 size‐exclusion chromatography (b) of CPP; UV spectrum of CPP1 (c) and FTIR spectrum of CPP1 (d)
3.3. UV analysis and FTIR spectroscopy
As shown in Figure 2c, no absorption peak of CPP1 was detected at about 260 and 280 nm in the UV analysis in indicating that CPP1 was free of nucleic acids and protein. The FR‐IR spectrum of CPP1 is shown in Figure 2d (Jin et al., 2018). The absorption bands within the ranges 3,600–3,000, 3,000–2,800, 1,400–1,200, and 1,200–700 cm−1 were the characteristic absorption peaks of polysaccharides. The broad band at 3,382.86 cm−1 was the characteristic band of O–H tensile vibration, and the signal at 2,932.89 cm−1 was C–H tensile vibrations. The relatively strong absorption peak at 1,646.14 cm−1 indicated the existence of C–O bond, and the absorption peaks at 1,399.21 cm−1 was attributed to –CH2– shear vibration. The peak value in the range of 1,300–1,000 cm−1 was the characteristic of carbohydrates. In addition, the peak value of CPP1 at 875.07 cm−1 indicated that the type of sugar bond was β‐type glycosidic bonds. The results indicated that CPP‐1 possessed typical peaks of polysaccharide absorption.
3.4. NMR analysis
The structures of CPP1 was further analyzed by NMR. The 1H NMR spectra showed that the chemical shifts of CPP1 were mainly between 3.5 and 5.3 ppm (Zhu et al., 2011). As shown in Figure 3a, the signals in the range of 4.3–4.7 ppm in the 1H NMR spectrum indicated that CPP1 contained a β‐glycoside structure (Li et al., 2018). These results were consistent with the glycoside bond types of FTIR analysis. The weak peaks at 5.00–5.29 ppm were attributed to α‐Ara residues, and the peak at 4.90 ppm was attributed to α‐Glc residue (Li et al., 2017; Xu et al., 2016). In addition, signals between 3.62 and 3.35 ppm indicate the presence of methoxyl. As shown in the 13C NMR spectrum (Figure 3b), there were several isopropanol carbon signals in the range of 103.1 ppm, the signal near 19 ppm was expressed as methyl in acetyl, and the other non isopropanol carbons were appeared between 60.7 ppm to 80.9 ppm.
FIGURE 3.
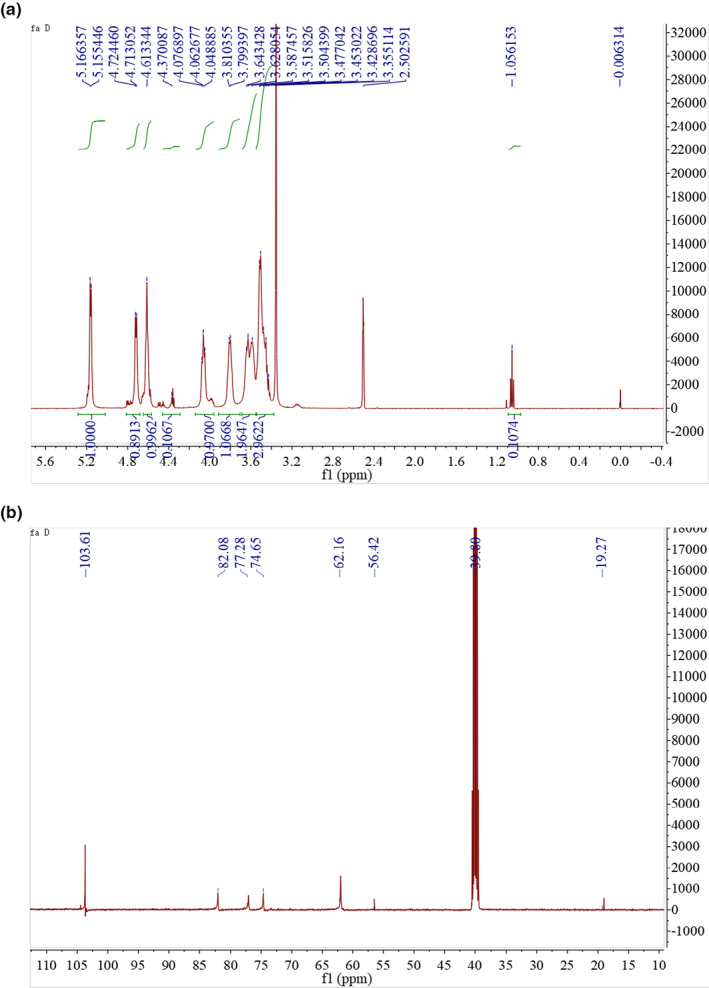
The 1H (A) and 13C spectra of CPP1 in DMSO at 25°C
3.5. Morphological properties
The SEM of CPP1 is shown in Figure 4. The polysaccharide has particle size and aggregates of irregular geometric shapes. The shapes and structures of polysaccharides may be influenced by product preparation or extraction and purification methods (Nep & Conway, 2010).
FIGURE 4.
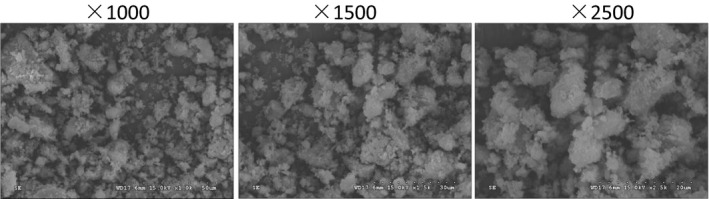
Scanning election microscope (SEM) photographs of CPP1
3.6. Monosaccharide composition analysis
Figure 5 shows the HPLC chromatograms of the release of PMP‐derived monosaccharides from CPP1 as well as those of eight standard monosaccharides. Based on the retention time of the derivatized monosaccharide standards, the monosaccharide composition of CPP1 was determined according to the retention time of the derived monosaccharide. CPP1 was mainly composed of mannose (Man), glucose (Glc), and arabinose (Ara), in the proportions of 1.76%, 97.38%, and 0.86%, respectively.
FIGURE 5.
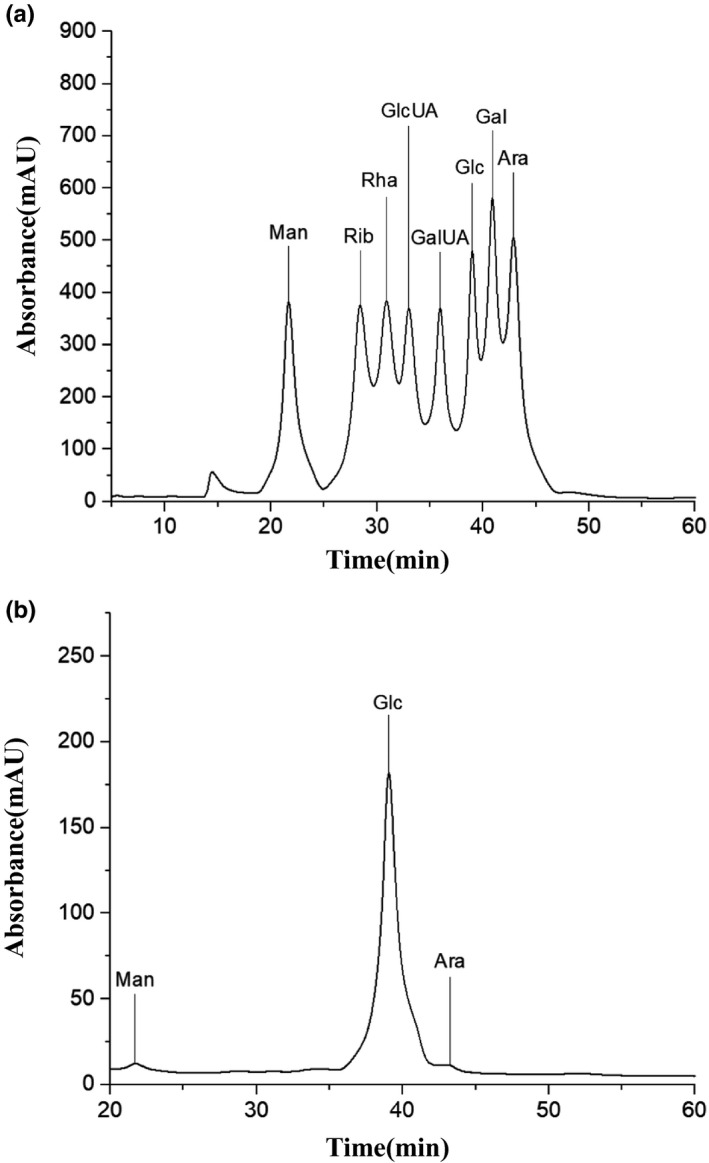
HPLC chromatograms of standard monosaccharides (a) and CPP1 hydrolysate (b)
3.7. In vitro antioxidant activities of CPP1
Polysaccharides are important natural product resources. Studies have shown that active polysaccharides have a variety of biological activities, including antioxidant, anti‐inflammatory, hypoglycemic, and hypolipidemic. Researchers are very concerned about the antioxidant capacity of active polysaccharides, especially in vitro antioxidant activity (Ahmad et al., 2020; Munir et al., 2020). The antioxidant mechanism can be summarized as follows: elimination of active free radicals, elimination of inactive oxidation factors, binding of metal ions, and so on. There are many factors affecting the antioxidant activity of polysaccharides, which may include the source, chemical structure, molecular weight, purity, and spatial conformation. The antioxidant test methods of polysaccharides in vitro can be summarized as DPPH radical scavenging ability, ABTS activity free radical scavenging ability, oxygen free radical scavenging ability, superoxide anion radical scavenging ability, and metal ion chelating ability (Yao et al., 2020).
Superoxide anion radicals are weak oxidants and therefore harmless to the body. However, excess superoxide radicals play an important role in the formation of secondary radicals such as hydrogen peroxide, hydroxyl radicals and singlet oxygen, which may lead to tissue damage. Superoxide radicals are the initial free radicals produced by the mitochondrial electron transport system. In addition, these free radicals can produce other strong free radicals, which may cause a variety of diseases (Vaz et al., 2011). Polysaccharides are a kind of highly effective free radical scavenger (Xiong et al., 2020). As shown in Figure 6a, CPP1 exhibited stronger superoxide anion radical scavenging activity. The maximum scavenging effect of CPP1 was 68.16 ± 0.23% at 10 mg/ml, which suggested that CPP1 was a good superoxide anion radical scavenger and protected the body from oxidative damage.
FIGURE 6.
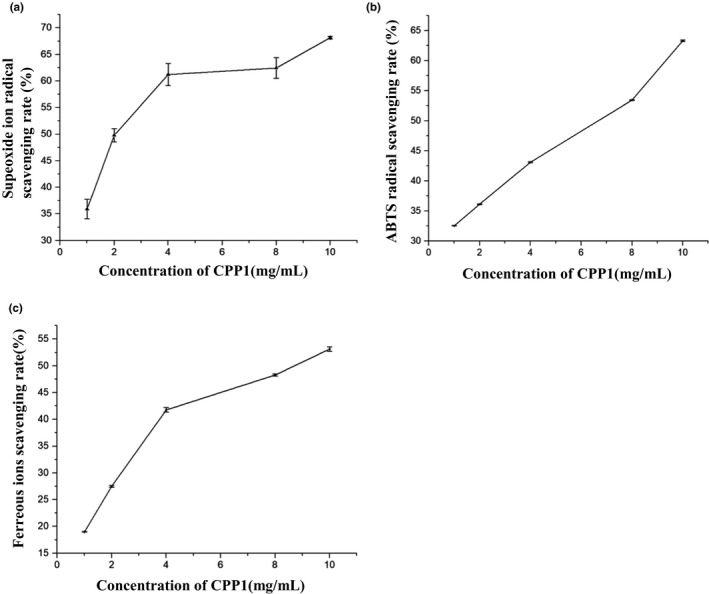
Antioxidant activity of CPP1. (a) Scavenging effects of CPP1 on superoxide ion radicals. Values are means ± SD (n = 3). (b) Scavenging effects of CPP1 on ABTS radicals. Values are means ± SD (n = 3). (c) Scavenging effects of CPP1 on metal ion radical scavenging rate. Value are means ± SD (n = 3)
ABTS assay is usually used to determine the total antioxidant capacity of individual compounds or complex mixtures of various plants (Katalinic et al., 2006). ABTS radical scavenging capability was one of the methods to investigate the antioxidant activity of polysaccharides (Patel et al., 2018). As shown in Figure 6b, the concentration‐dependent free radical scavenging activity of CPP1 was researched. At the concentration of 10 mg/ml, CPP1 had a scavenging effect of 63.32 ± 0.12% on ABTS.
Metal chelating activity is generally considered as an antioxidant mechanism because it reduces the concentration of catalytic transition metals in lipid peroxidation (Chen et al., 2012). Ferric zinc is a sensitive reagent that can be mixed with ferrous ions to form a colored species (iron (II)–ferric zinc complex). After antioxidants are introduced to the system, they compete with iron and zinc for ferrous ions, reducing the absorbance of the solution. The polysaccharide has some metal ion scavenging activity (Medlej et al., 2020). As shown in Figure 6c, the different concentrations of CPP1 (1, 2, 4, 6, 8, and 10 mg/ml) showed certain metal chelating ability (12.03%‐44%).
4. CONCLUSIONS
RSM method was used to optimize the process parameters of CPP extraction by fermentation. The optimum extraction conditions were obtained: fermentation temperature of 24.57°C, yeast addition of 5.92 mg, and fermentation time of 21.03 hr. RSM provided a valuable method for optimizing CPP extraction process. CPPs were purified by DE‐52, and CPP1 was characterized by UV, FTIR, HPLC, NMR, and SEM. The results showed that CPP1 was mainly composed of mannose, glucose, and arabinose, with the proportions of 1.76%, 97.38%, and 0.76%, respectively. The characteristic FTIR absorption peaks of polysaccharides were observed in the infrared spectra of CPP1. CPP1 showed higher free radical scavenging activity against superoxide anions, ABTS, and iron ions. The results showed that CPP1 could be used as a natural antioxidant.
5. ETHICAL GUIDELINES
Ethics approval was not required for this research.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Zhang XF and Yan YY designed the study. Yuan S, Xu CY, Xia J, and Feng YN collected data. All authors agreed the final version.
ACKNOWLEDGMENTS
This work was supported by Research start up project of Wuhan Polytechnic University (2017257).
Yuan S, Xu C‐Y, Xia J, Feng Y‐N, Zhang X‐F, Yan Y‐Y. Extraction of polysaccharides from Codonopsis pilosula by fermentation with response surface methodology. Food Sci Nutr. 2020;8:6660–6669. 10.1002/fsn3.1958
Contributor Information
Xi‐Feng Zhang, Email: zhangxf9465@163.com.
You‐Yu Yan, Email: yanyy75@163.com.
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- Ahmad, A. , Rehman, M. , Wali, A. , El‐Serehy, H. , Al‐Misned, F. , Maodaa, S. , Aljawdah, H. , Mir, T. , & Ahmad, P. (2020). Box‐Behnken response surface design of polysaccharide extraction from rhododendron arboreum and the evaluation of its antioxidant potential. Molecules, 25(17), 3835 10.3390/molecules25173835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, R. B. , Li, W. Y. , Li, Y. D. , Ma, M. , Wang, Y. P. , Zhang, J. , & Hu, F. D. (2018) .Cytotoxicity of two water‐soluble polysaccharides from Codonopsis Pilosula Nannf. Var. Modesta (Nannf.) L.T.Shen against human hepatocellular carcinoma HepG2 cells and its mechanism. International Journal of Biological Macromolecules, 120, 1544–1550. 10.1016/j.ijbiomac.2018.09.123 [DOI] [PubMed] [Google Scholar]
- Cai, L. L. , Chen, B. H. , Yi, F. L. , & Zou, S. S. (2019). Optimization of extraction of polysaccharide from dandelion root by response surface methodology: Structural characterization and antioxidant activity. International Journal of Biological Macromolecules, 140, 907–917. 10.1016/j.ijbiomac.2019.08.161 [DOI] [PubMed] [Google Scholar]
- Cai, L. , Zou, S. , Liang, D. , & Luan, L. (2018). Structural characterization, antioxidant and hepatoprotective activities of polysaccharides from Sophorae tonkinensis Radix. Carbohydrate Polymers, 184, 354–365. 10.1016/j.carbpol.2017.12.083 [DOI] [PubMed] [Google Scholar]
- Carter, P. (1971). Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (ferrozine). Analytical Biochemistry, 40(2), 450–458. 10.1016/0003-2697(71)90405-2 [DOI] [PubMed] [Google Scholar]
- Chen, H. , Ju, Y. , Li, J. , & Yu, M. (2012). Antioxidant activities of polysaccharides from Lentinus edodes and their significance for disease prevention. International Journal of Biological Macromolecules. 50, 214–218. 10.1016/j.ijbiomac.2011.10.027 [DOI] [PubMed] [Google Scholar]
- Chen, L. , & Huang, G. (2018). The antiviral activity of polysaccharides and their derivatives. International Journal of Biological Macromolecules, 115, 77–82. 10.1016/j.ijbiomac.2018.04.056 [DOI] [PubMed] [Google Scholar]
- Dou, Z. M. , Chen, C. , & Fu, X. (2019). The effect of ultrasound irradiation on the physicochemical properties and α‐glucosidase inhibitory effect of blackberry fruit polysaccharide. Food Hydrocolloids, 96, 568–576. 10.1016/j.foodhyd.2019.06.002 [DOI] [Google Scholar]
- Finkel, T. , Holbrook, N. J. ; Oxidants . (2000). Oxidative stress and the biology of ageing. Nature, 408, 239–247. [DOI] [PubMed] [Google Scholar]
- Jin, X. , Wang, Q. , Yang, X. , Guo, M. , Li, W. , Shi, J. , & Yu, J. (2018). Chemical characterisation and hypolipidaemic effects of two purified Pleurotus eryngii polysaccharides. International Journal of Food Science & Technology, 53(10), 2298–2307. 10.1111/ijfs.13821 [DOI] [Google Scholar]
- Jin, Y. X. , Hu, X. Y. , Zhang, Y. , & Liu, T. J. (2016). Studies on the purification of polysaccharides separated from Tremella fuciformis and their neuroprotective effect. Molecular Medicine Reports, 13(5), 3985–3992. 10.3892/mmr.2016.5026 [DOI] [PubMed] [Google Scholar]
- Jouraiphy, A. , Amir, S. , Winterton, P. , Gharous, M. E. , Revel, J. C. , & Hafidi, M. (2008). Structural study of the fulvic fraction during composting of activated sludge–plant matter: Elemental analysis, FTIR and 13C NMR. Bioresource Technology, 99(5), 1066–1072. 10.1016/j.biortech.2007.02.031 [DOI] [PubMed] [Google Scholar]
- Katalinic, V. , Milos, M. , Kulisic, T. , & Jukic, M. (2006). Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chemistry, 94, 550–557. 10.1016/j.foodchem.2004.12.004 [DOI] [Google Scholar]
- Li, B. , Zhang, N. , Wang, D. X. , Jiao, L. , Tan, Y. , Wang, J. , Li, H. , Wu, W. , & Jiang, D. C. (2018). Structural analysis and antioxidant activities of neutral polysaccharide isolated from Epimedium koreanum Nakai. Carbohydrate Polymers, 196, 246–253. 10.1016/j.carbpol.2018.05.037 [DOI] [PubMed] [Google Scholar]
- Li, N. , Liu, X. , He, X. , Wang, S. , Cao, S. , Xia, Z. , Xian, H. , Qin, L. , & Mao, W. (2017). Structure and anticoagulant property of a sulfated polysaccharide isolated from the green seaweed Monostroma angicava. Carbohydrate Polymers, 159, 195–206. 10.1016/j.carbpol.2016.12.013 [DOI] [PubMed] [Google Scholar]
- Liu, W. , Lv, X. , Huang, W. , Yao, W. , & Gao, X. (2018). Characterization and hypoglycemic effect of a neutral polysaccharide extracted from the residue of Codonopsis. Carbohydrate Polymers, 197, 215–226. 10.1016/j.carbpol.2018.05.067 [DOI] [PubMed] [Google Scholar]
- Masuko, T. , Minami, A. , Iwasaki, N. , Majima, T. , Nishimura, S. , & Lee, Y. C. (2005). Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Analytical Biochemistry, 339(1), 69–72. 10.1016/j.ab.2004.12.001 [DOI] [PubMed] [Google Scholar]
- Medlej, M. K. , Cherri, B. , Nasser, G. , Zaviska, F. , Hijazi, A. , Li, S. , & Pochat‐Bohatier, C. (2020). Optimization of polysaccharides extraction from a wild species of Ornithogalum combining ultrasound and maceration and their anti‐oxidant properties. International Journal of Biological Macromolecules, 161, 958–968. 10.1016/j.ijbiomac.2020.06.021 [DOI] [PubMed] [Google Scholar]
- Munir, N. , Mahmood, Z. , Yameen, M. , & Mustafa, G. (2020). Therapeutic response of epimedium gandiflorum's different doses to restore the antioxidant potential and reproductive hormones in male albino rats. Dose Response, 18(3), 1559325820959563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nep, E. I. , & Conway, B. R. (2010). Characterization of grewia gum, a potential pharmaceutical excipient. Journal of Excipients and Food Chemicals, 1, 30–40. [Google Scholar]
- Patel, M. K. , Tanna, B. , Mishra, A. , & Jha, B. (2018). Physicochemical characterization, antioxidant and anti‐proliferative activities of a polysaccharide extracted from psyllium (P. ovata) leaves. International Journal of Biological Macromolecules, 118, 976–987. 10.1016/j.ijbiomac.2018.06.139 [DOI] [PubMed] [Google Scholar]
- Peng, L. , Li, H. , & Meng, Y. (2017). Layer‐by‐layer structured polysaccharides‐based multilayerson cellulose acetate membrane: Towards better hemocompatibility, antibacterial and antioxidant activities. Applied Surface Science, 401, 25–39. 10.1016/j.apsusc.2016.12.235 [DOI] [Google Scholar]
- Re, R. N. , Pellegrini, A. , Proteggente, A. , Pannala, M. , Yang, C. , & Evans, R. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine. 26, 1231–1237. 10.1016/S0891-5849(98)00315-3 [DOI] [PubMed] [Google Scholar]
- Vaz, A. , Barros, L. , Martins, A. , Santos‐Buelga, C. , Vasconcelos, M. H. , & Ferreira, I. C. F. R. (2011). Chemical composition of wild edible mushrooms and antioxidant properties of their water soluble polysaccharidic and ethanolic fractions. Food Chemistry. 126, 610–616. 10.1016/j.foodchem.2010.11.063 [DOI] [Google Scholar]
- Wang, J. , Wang, C. , Li, W. , Pan, Y. , Yuan, G. , & Chen, H. (2016). Ball milling improves extractability and antioxidant properties of the active constituents of mushroom Inonotus obliquus powders. International Journal of Food Science Technology, 51(10), 2193–2200. [Google Scholar]
- Wang, L. , Ding, L. , Yu, Z. , Zhang, T. , Ma, S. , & Liu, J. (2016). Intracellular ROS scavenging and antioxidant enzyme regulating capacities of corn gluten meal‐derived antioxidant peptides in HepG2 cells. Food Research International, 90, 33–41. 10.1016/j.foodres.2016.10.023 [DOI] [PubMed] [Google Scholar]
- Xiong, C. , Li, P. , Luo, Q. , Yan, J. , Zhang, J. , Jin, X. , & Huang, W. (2020). Effect of γ‐irradiation on the structure and antioxidant activity of polysaccharide isolated from the fruiting bodies of Morchella sextelata. Bioscience Reports, 40(9), BSR20194522 https://pubmed.ncbi.nlm.nih.gov/32896857/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, L. , Zhang, Y. , & Wang, L. (2016). Structure characteristics of a water‐soluble polysaccharide purified from dragon fruit (Hylocereus undatus) pulp. Carbohydrate Polymers, 146, 224–230. 10.1016/j.carbpol.2016.03.060 [DOI] [PubMed] [Google Scholar]
- Yao, Y. L. , Shu, C. , Feng, G. , Wang, Q. , Yan, Y. Y. , Yi, Y. , Wang, H. X. , Zhang, X. F. , & Wang, L. M. (2020). Polysaccharides from Pyracantha fortuneana and its biological activity. International Journal of Biological Macromolecules, 150, 1162–1174. 10.1016/j.ijbiomac.2019.10.125 [DOI] [PubMed] [Google Scholar]
- Yilmaz, T. , & Sebnem, T. (2017). Modeling and Optimization of ultrasound assisted extraction parameters using response surface methodology for water soluble polysaccharide extraction from hazelnut skin. Journal of Food Processing and Preservation., 41, e12835 10.1111/jfpp.12835 [DOI] [Google Scholar]
- Zhang, L. , Hu, Y. , Duan, X. , Tang, T. , Shen, Y. , Hu, B. , Liu, A. , Chen, H. , Li, C. , & Liu, Y. (2018). Characterization and antioxidant activities of polysaccharides from thirteen boletus mushrooms. International Journal of Biological Macromolecules, 113, 1–7. 10.1016/j.ijbiomac.2018.02.084 [DOI] [PubMed] [Google Scholar]
- Zhu, B. W. , Li, D. M. , Zhou, D. Y. , Han, S. , Yang, J. F. , Li, T. , Ye, W. X. , & Greeley, G. H. (2011). Structural analysis and CCK‐releasing activity of a sulphated polysaccharide from abalone gonad (Haliotis Discus Hannai Ino) viscera. Food Chemistry, 125, 1273–1278. 10.1016/j.foodchem.2010.10.065 [DOI] [Google Scholar]
- Zhu, H. , Tian, L. , Zhang, L. , Bi, J. , Song, Q. , Yang, H. , & Qiao, J. (2018). Preparation, characterization and antioxidant activity of polysaccharide from spent Lentinus edodes substrate. International Journal of Biological Macromolecules, 112, 976–984. 10.1016/j.ijbiomac.2018.01.196 [DOI] [PubMed] [Google Scholar]
- Zou, Y.‐F. , Chen, X.‐F. , Malterud, K. E. , Rise, F. , Barsett, H. , Inngjerdingen, K. T. , Michaelsen, T. E. , & Paulsen, B. S. (2014). Structural features and complement fixing activity of polysaccharides from Codonopsis pilosula Nannf. Var. Modesta L.T.SHen roots. Carbohydrate Polymers, 113, 420–429. 10.1016/j.carbpol.2014.07.036 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are not shared.


