Abstract
Purpose
Nerve injury-induced pain is difficult to treat. In this study, we developed an alginate scaffold with human umbilical cord mesenchymal stem cell exosomes (EX-SC) to treat nerve injury-induced pain.
Materials and Methods
The scaffold was prepared and characterized for its physical traits and biocompatibility. In vitro studies of PC12 and HEK293 cells were used to evaluate the neuroprotective and neurotrophic effects of exosomes. Right L5/6 spinal nerve ligation (SNL) was performed in Sprague-Dawley rats to induce mechanical allodynia and thermal hyperalgesia, evaluated by von Frey hair and radiant heat tests. The EX-SC was wrapped around ligated L5/6 spinal nerves for treatment. Western blotting and immunofluorescence staining were used to evaluate neuron/glial activation, cytokines and neurotrophic factor of affected dorsal root ganglion (DRG).
Results
In cell culture assay, the exosomes induce neurite outgrowth of PC12 cells and protect PC12 and HEK293 cells against formaldehyde acid treatment. On post-ligation day 21, rats receiving EX-SC had significantly higher median (interquartile range) withdrawal threshold and latency [14.1 (13.7–15.5) g, 14.2 (13.7–15.3) s] than saline-SC-treated rats [2.1 (1.7–3.0) g, 2.0 (1.8–2.4) s, P=0.02 and 0.002]. The EX-SC also attenuated SNL-induced up-regulation of c-Fos, GFAP, Iba1, TNF-α and IL-1β, while enhancing the level of IL-10 and GDNF, in the ipsilateral L5/6 DRG. After implantation for 21 days, the EX-SC enhanced the expression of myelin basic protein and IL-10 in injured L5/6 axons.
Conclusion
We demonstrate the EX-SC possesses antinociceptive, anti-inflammation and pro-neurotrophic effects in the SNL pain model. It could be a promising therapeutic alternative for nerve injury-induced pain.
Keywords: exosome, nerve injury, neuropathic pain, scaffold, spinal nerve ligation, stem cell
Introduction
Peripheral nerve injury can be difficult to treat and usually leads to loss of nerve and end-organ functions. Peripheral nerve injury can also induce neuropathic pain, a debilitating neurological condition of high clinical relevance. Even though previous reports have proposed various types of bio-engineered nerve grafts to facilitate peripheral nerve regeneration,1 the overall clinical outcomes still remain suboptimal. Recently, stem cells have been used as seed cells in tissue-engineered nerve grafts because of their ability to differentiate into Schwann cells, secrete neurotrophic factors and assist in myelin formation.2 However, it has been reported that transplantation of olfactory mucosal stem cells into a spinal cord-injury patient formed a tumor at the injection side,3 suggesting potential risks of stem cell therapies.
Exosomes are cell membrane-enclosed 30–160 nm nanovesicles that are involved in intercellular communication.4 They contain cytokines and growth factors, signaling lipids, mRNAs, regulatory miRNA and lncRNA molecules, which can be transferred from producer cells to recipient cells, and can modify target cells and organ functions.5 Mesenchymal stem cells (MSCs)-derived exosomes have been found to promote comparable immune therapeutic activities as MSCs themselves, and they can influence tissue responses to injury, infection and disease.6 Extracellular vesicle-based therapeutics are currently being developed and clinically tested for the treatment of inflammatory diseases, autoimmune disorders and cancer.5
At present, microtechnology and nanotechnology provide three fundamentally different pathways for pursuing nerve injury repair: 1) as microstructure scaffolds to promote regeneration; 2) as direct repair by reconnecting axons; and 3) as biodegradable drug carriers for nerve repair. The natural biomaterial alginate isolated from the wall of brown seaweed can be made into a non-toxic/non-inflammatory, highly porous scaffold with relatively low cost. The bioresorbable alginate scaffold has been used for drug or cell delivery to provide spinal cord injury repair and axonal regeneration.7
Currently a combinatorial approach integrating biomaterial scaffolds and biomolecule delivery seems to be more promising for regeneration and functional recovery.7 In this study, we developed a sponge-like alginate scaffold that slowly releases human umbilical cord MSC (UCMSC) exosomes as a novel therapeutic approach for L5/6 spinal nerve ligation (SNL)-induced pain, a classical neuropathic pain model.8 The primary aim of this study is to examine the effects of this exosome-scaffold (EX-SC), wrapped around ligated nerves, on SNL-induced pain, neuron/glial activation and neuro-inflammation. The morphology, mechanical properties, biocompatibility, exosome carrier and release abilities of alginate scaffold were also examined.
Materials and Methods
This study adhered to the guidelines of International Association for the Study of Pain and was reported following the ARRIVE guidelines. The experiment was performed according to a protocol (MMH-A-S-107-14) approved by the Institutional Animal Care and Use Committee (Chairman: Prof. Chie-Pein Chen) on 16 March 2018 and a clinical trial (15MMHIS106) of Mackay Memorial Hospital, Taipei, Taiwan. The cell lines used in this study were HEK293 and PC12 cells (supplementary materials) and were purchased commercially from Bioresource Collection and Research Centre (BCRC, No. 60,019 and No. 60,048, Taiwan).
Isolation, Particle Size and Concentration Analysis of UCMSC Exosomes
The isolation of mesenchymal stem cell exosome is described in the supplementary materials. The particle size and concentration of UCMSC exosomes were analyzed by nanoparticle size and concentration analyzer (NanoSight NS300, Malvern Panalytical Ltd, Malvern, UK). Briefly, 300 μL dilution exosome sample (1:10,000 dilution) was loaded into NanoSight NS300 with a syringe pump and the sensitivity of the camera was set to auto 15. All data were collected in triplicate at 23°C.
Flow Cytometry of UCMSC Exosomes
The exosome detection processing was as previously reported.9 The 4 μm-diameter aldehyde/sulphate latex beads (Life Sciences, CA, USA) were incubated with purified anti-CD81 antibody (BD Biosciences, NJ, USA) for exosome binding. For allophycocyanin (APC) labeling, 50 μL anti-CD81 APC was added into each sample or matching isotype was added into control (BD Biosciences, NJ, USA). Samples were resuspended in 200 μL PBS buffer and analyzed by FACSCalibur system (BD Biosciences, NJ, USA). Ten thousand events were acquired using Cell Quest and the data were analyzed using FlowJo Software (Tree Star, Inc, Ashland, OR, USA).
Quantification of Proteins in UCMSC Exosomes
After two-step centrifuge isolation and purification, the UCMSC exosomes were in the top layer. The protein levels were analyzed with Milliplex Map human angiogenesis/growth factor magnetic bead panel 1 and Milliplex Map human cytokine/chemokine panel magnetic bead kits (Millipore, CA, USA) by Luminex Bio-Plex 200 system (Bio Rad, CA, USA). 25 µL of each exosome or standard sample was analyzed in the appropriate 96 wells, according to the manufacturer’s protocol. Data were analyzed using Bio-Plex ManagerTM (Bio Rad, CA, USA).
Animals
To be consistent with our previous intrathecal study9 and avoid the possible influence of the estrous cycle on nociceptive behavior,10 male Sprague-Dawley rats, weighing 200–250g on the day of surgery, were purchased from Bio LASCO Taiwan Co. They were housed as previously reported.11 Sample size calculations were used to estimate the smallest number of animals (n) needed to detect an arbitrarily chosen 60% increase in the mean of stabilized post-SNL behavioral data, when statistical significance was set at P<0.05 and power at 90%.
L5/6 Spinal Nerve Ligation and Implantation of Scaffold
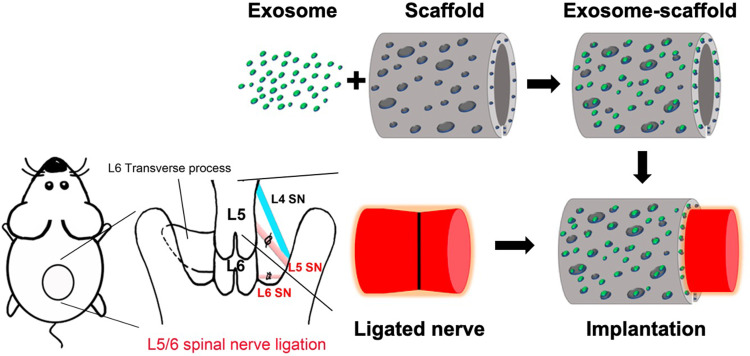
Right L5/6 SNL was executed as previously reported.8,9 Surgery was carried out under isoflurane anesthesia in 100% oxygen, induced at 5% and maintained at 2%. During the surgery, the percentage of isoflurane was increased if inadequate anesthesia, indicated by palpebral or pedal withdrawal response to a nociceptive stimulus, was observed. For the randomization, we used the List randomizer (random.org) to allocate the rats into five groups (Sham/saline, SNL/saline, SNL/exosome, SNL/exosome-gelfoam, SNL/exosome-scaffold) for scaffold implantation on day 3 after surgery or three groups (Sham/saline-scaffold, SNL/saline-scaffold, SNL/exosome-scaffold) for scaffold implantation on the day of surgery (n=6 in each group). In the sham group, the same wound exposure was performed without SNL. For scaffold implantation (illustrated in Figure 1), after making an incision on the scaffold, a 3 mm scaffold with saline or exosomes was wrapped around the L5 ligation site and a 1 mm scaffold around the L6 ligation site, with a total of 25 µL exosome solution (1 mg/mL) carried by scaffold. For the exosome-gelfoam group, 25 µL exosome solution (1 mg/mL) was dropped on gelfoam (4×4×3 mm). The exosome-gelfoam implantation followed the same processing as scaffold implantation. For the saline or exosome single injection group, 25 µL saline or exosome solution (1 mg/mL) was injected around L5/6 ligation sites.
Figure 1.
Schematic diagram illustrating the preparation and implantation of exosome-scaffold around ligated L5/6 spinal nerve (SN).
Von Frey Filament and Plantar Thermal Tests
To monitor the nociceptive behaviors, the following assessments were performed by an investigator blinded to the treatment groups once daily, between 9 a.m. and 3 p.m., before (day 0) and after surgery. The right hindpaw withdrawal threshold (WT) in response to normally innocuous mechanical stimuli was measured using von Frey filaments and “up-down” method as previously reported.9 The foot withdrawal latency (WL) to noxious heat stimuli was determined using the Analgesia Meter apparatus (IITC/Life Science Instruments, CA, USA) as previously reported.9 Four WLs collected at intervals of at least 5 min were averaged. Before both tests, rats were allowed at least 30 min acclimatization to the environment.
Western Blotting
After behavioral assessment, rats were culled with sodium pentobarbital (120 mg/kg, i.p.) on day 1 (for c-Fos, neuron activation marker),12 day 7 or 21 (for myelin basic protein, MBP) after surgery (n=3 in the indicated group). The right L5/6 spinal cord and dorsal root ganglion (DRG) sample procedures were performed, 40 μg total protein of each sample was analyzed and Western blot processing was as previously reported.11 The first antibodies used were: antiserum against c-Fos (1:1000, Cell Signaling, Danvers, USA), glial fibrillary acidic protein (GFAP, satellite glial cell marker, 1:10,000, Millipore, CA, USA), ionized calcium binding adaptor molecule 1 (Iba1, macrophage marker, 1:2000, Wako, Osaka, Japan), tumor necrosis factor alpha (TNF-α, 1:2000, R&D Systems, MN, USA), interleukin-1β (IL-1β, 1:10,000, Abcam, Cambridge, UK), IL-10 (1:1000, Cell Signaling, Boston, USA), glial cell line-derived neurotrophic factor (GDNF, 1:1000, R&D Systems, MN, USA), MBP (1:2000, GeneTex, Texas, USA) and actin (1:5000, Millipore, CA, USA). Images were captured and analyzed by cooled CCD system (LAS 4000, FujiFilm, Tokyo, Japan).
Immunofluorescence Staining
After behavioral assessment, rats were culled with sodium pentobarbital (120 mg/kg, i.p.) on day 3 (for exosome tracking), day 7 or 21 (for MBP) after surgery (n=3 in the indicated group). The ipsilateral L5 DRG and nerve sampling and immunostaining procedures followed those previously reported.9 To localize exosomes, exosomes were pre-labelled with Exo-green before scaffold absorption by kit (Exo-Glow labelling kit, SBI, CA, USA) and rats were culled on day 3 after surgery. All the slices were blocked with 2% goat serum, plus 0.3% Triton X-100 in 0.01 M PBS for 1 h at room temperature. Slices were then incubated with diluted primary antibodies against Iba1, GFAP, TNF-α, IL-1β, IL-10, GDNF or calcitonin gene related peptide (CGRP, 1:500, Peninsula Laboratories, CA, USA) overnight at 4°C. Next, the slices were incubated with appropriate secondary antibody (1:400, Alexa fluor 488, 594 or 647-conjugated donkey anti-goat, rabbit, mouse or guinea pig IgG, Jackson Immuno Research, West Grove, USA) for 1 h at room temperature. For isolectin-B4 (IB4) and DAPI (4ʹ,6-diamidino-2-phenylindole) labeling, procedures previously reported were followed.9 The specificity of the staining was tested by omission of primary antibodies or absorption with peptide antigens. Images were captured by confocal laser scanning microscope system (Leica TCS SP5, Germany) and processed with Adobe Photoshop 8.0 software (Adobe Systems, Mountain View, USA).
Statistical Analysis
Statistical comparisons were performed using GraphPad Prism 8 software. The behavioral data of each group (presented as median and IQR) and Western blot data were compared using Kruskal–Wallis test followed by Dunn’s multiple pairwise comparisons test. The others were expressed as Mean ± SD; n indicates the number of animals in each group. The data of cell culture were analyzed by one-way analysis of variance (ANOVA) with post hoc Tukey’s test. Student’s t-test was used when two treatment groups were compared. P<0.05 was considered statistically significant.
Results
Characterization and Neuroprotective Potential of UCMSC Exosome
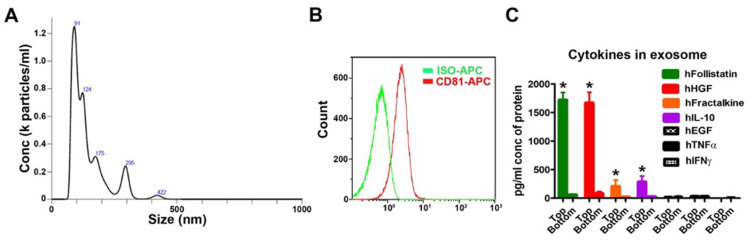
After two-step filters (0.22 μm and 100 kDa) isolation and purification, the particle size analysis of UCMSC exosomes showed the major sizes of vesicles was 91, 124 and 175 nm (Figure 2A), similar to the reported exosome sizes.4 In addition, the exosome membrane marker CD81 was detectable in exosome samples by flow cytometry (Figure 2B). All these data indicate the UCMSC exosomes were successfully isolated by the process. For quantification of proteins in the exosomes, some of the detected proteins have beneficial effects for the neuronal system, such as follistatin, hepatocyte growth factor (HGF), fractalkine/CX3CL1 and IL-10, while few epidermal growth factor (EGF), TNF-α and interferon gamma (IFN-γ) were detected in the exosome samples (Figure 2C). Next, we used formaldehyde acid (FA) treatment in cell culture assay to test the neuroprotective and anti-inflammatory abilities of UCMSC exosomes13 (supplementary Figure S1) and the characteristics of the alginate scaffold, including exosome adsorption/release, physical strength, biocompatibility and cytocompatibility, are provided in the supplementary data (Figures S2-S4).
Figure 2.
Characterization of human umbilical cord mesenchymal stem cell (UCMSC) exosomes. (A) Particle size analysis of UCMSC exosomes. (B) Exosome membrane marker CD81 were detectable in UCMSC exosome samples by flow cytometry. The X-axis is the fluorescence intensity. (C) The protein level of cytokines and growth factors in UCMSC exosomes. Human follistatin, HGF (hepatocyte growth factor), fractalkine and IL-10 (interleukin-10) were detectable in exosome samples by immunology multiplex assay. Top layer is exosome sample and bottom layer is flow-through part after centrifuge. Each column represents Mean ± SD, *P<0.05 compared with bottom layer (Student’s t-test, n=6 in each group).
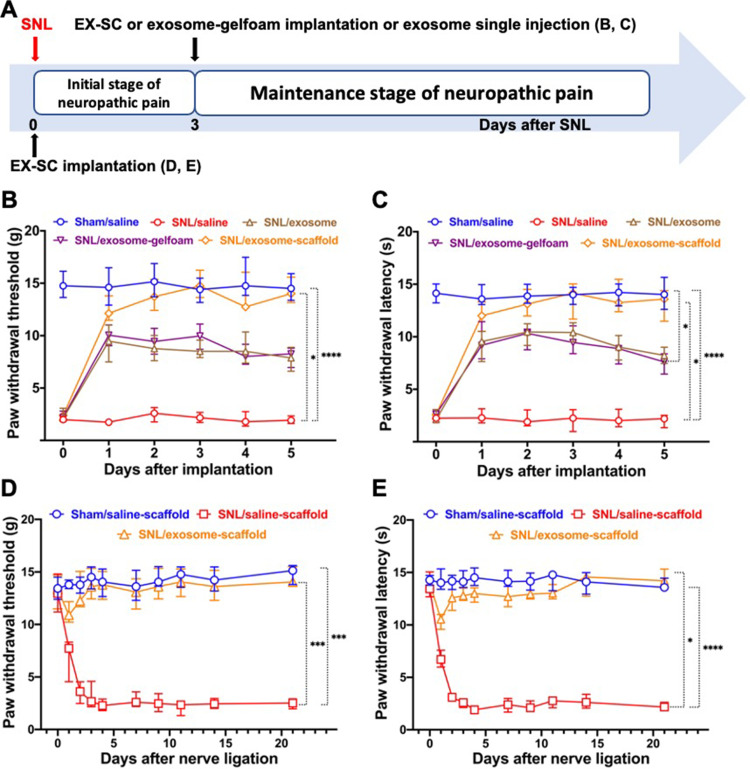
The Exosome-Scaffold Attenuates Nerve Ligation-Induced Pain
After SNL, we observed a decrease of threshold for tactile and thermal stimuli at the affected hindpaw. The threshold reached the minimum 3–4 days after SNL and maintained that level for weeks. The EX-SC implantation on day 3 after SNL (SNL/exosome-scaffold group) showed significant antinociceptive effects in SNL-induced mechanical allodynia and thermal hyperalgesia (P<0.05 vs SNL/saline group, Figure 3B and C Figure 3AandB). The antinociceptive effects of the SNL/exosome-scaffold group, but not the SNL/exosome-gelfoam group, were better than those of the SNL/exosome group (Figure 3B and C) (Figure 3AandB), suggesting the porous alginate scaffold might provide a protective environment for UCMSC exosomes in vivo.
Figure 3.
Implantation of stem cell exosome-scaffold (EX-SC) on ligated nerves attenuates L5/6 spinal nerve ligation (SNL)-induced pain. (A) The time course of surgery and implantation. (B and C) The analgesic effects of exosome-scaffold, exosome-gelfoam or exosome single injection on SNL-induced mechanical allodynia (B) and thermal hyperalgesia (C) with implantation on day 3 after surgery. (D and E) The preventive effects of EX-SC on SNL-induced mechanical allodynia (D) and thermal hyperalgesia (E) with implantation on the day of surgery. Data are presented as median and IQR (*P<0.05, ***P<0.001, ****P<0.0001 vs SNL/saline, Kruskal–Wallis test followed by Dunn’s multiple comparisons test, n=6 in each group).
Furthermore, it is shown that the EX-SC, implanted on the day of surgery (SNL/exosome-scaffold group), attenuated the development of SNL-induced mechanical allodynia and thermal hyperalgesia (Figure 3D and E)(Figure 3CandD). In the SNL/saline-scaffold group, both mechanical allodynia and thermal hyperalgesia developed on the first day and remained obvious on day 21 after SNL (Figure 3D and E) (Figure 3CandD). In contrast, the WTs and WLs of the SNL/exosome-scaffold group were significantly higher than those of the SNL/saline-scaffold group (P<0.05, Figure 3D and E Figure 3CandD). On postsurgery day 21, the median (IQR) WT and WL of the SNL/exosome-scaffold group were 14.1 (13.7–15.5) g and 14.2 (13.7–15.3) s, significantly higher than those of the SNL/saline-scaffold group [2.1 (1.7–3.0) g and 2.0 (1.8–2.4) s, P=0.02 and 0.002, Figure 3D and E Figure 3CandD]. Notably, there was no significant difference of WTs and WLs between the SNL/exosome-scaffold and Sham/saline-scaffold groups on postsurgery day 3 to 21 (Figure 3D and E Figure 3CandD).
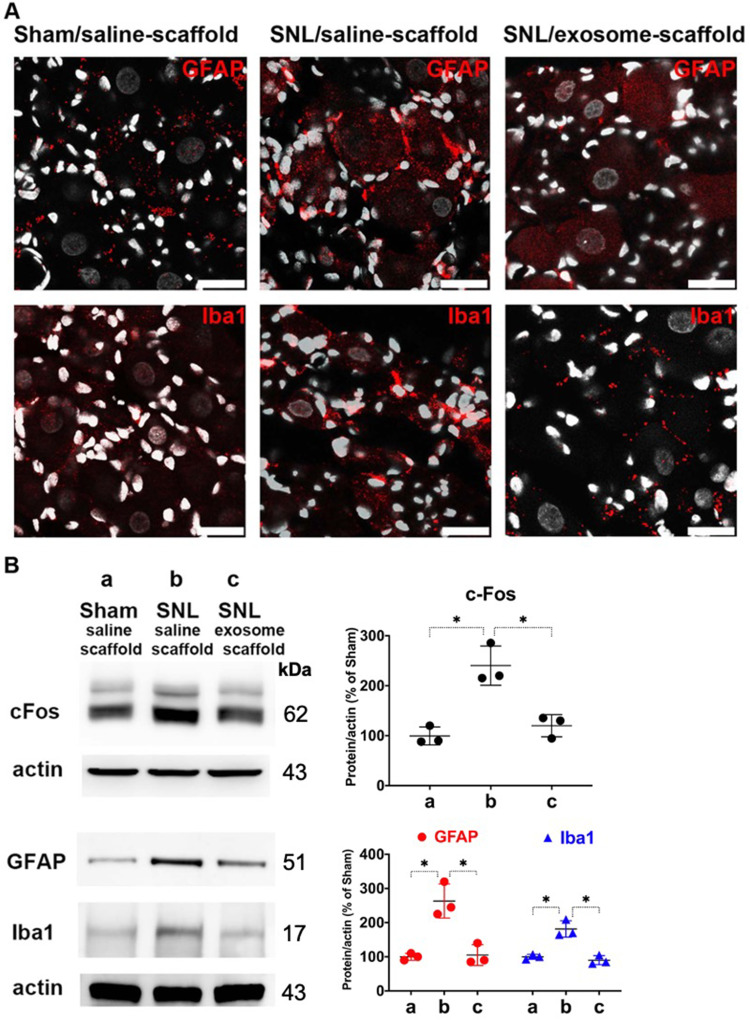
The Exosome-Scaffold Attenuates SNL-Induced Neuron and Glial Activation
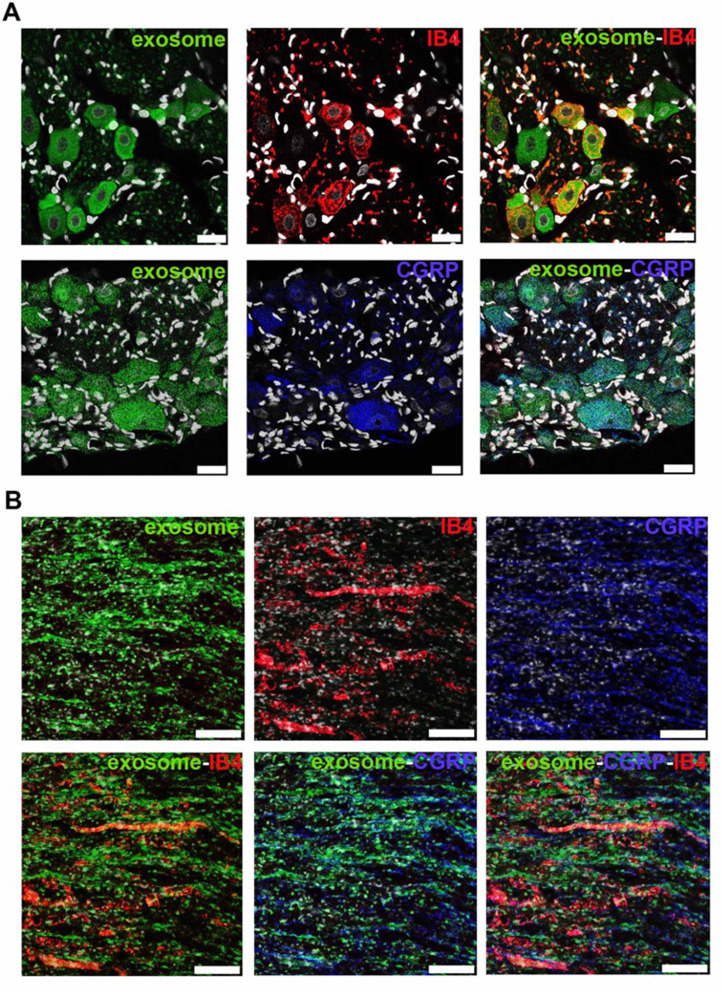
To investigate the localization of exosomes released from alginate scaffolds, we tracked the Exo-green-labelled exosomes in the ipsilateral L5 DRG and nerve of SNL/exosome-scaffold rats on day 3 after surgery. In the ipsilateral L5 DRG and nerve, some Exo-green-labelled exosomes were co-localized with IB4+ or CGRP+ nociceptors (Figure 4). Since the EX-SC showed antinociceptive effects (Figure 3) and the exosomes co-localized with nociceptors in SNL rats (Figure 4), we further examined if the EX-SC inhibits SNL-induced neuronal activation in the ipsilateral L5/6 DRGs. In Western blot assay, we observed that SNL-induced c-Fos over-expression in the ipsilateral L5/6 DRG on day 1 after surgery was significantly reversed in the EX-SC treatment group (P<0.05, Figure 5B).
Figure 4.
Human umbilical cord mesenchymal stem cell exosomes localize within nociceptors in ipsilateral L5 dorsal root ganglion and L5 axons on day 3 after L5/6 spinal nerve ligation and exosome-scaffold implantation. (A) Representative immunofluorescent image revealing Exo-green-labelled exosomes (green) localized within IB4+ (red) and CGRP+ (blue) nociceptors in ipsilateral L5 dorsal root ganglion. The white spots are DAPI nuclear staining. Scale bar: 25 μm. (B) Representative immunofluorescent image revealing Exo-green-labelled exosomes (green) localized within IB4+ (red) and CGRP+ (blue) nociceptors in injured L5 axons. Scale bar: 100 μm.
Figure 5.
Implantation of stem cell exosome-scaffold on ligated nerves attenuates L5/6 spinal nerve ligation (SNL)-induced neuron and glial activation in ipsilateral L5/6 dorsal root ganglion (DRG). (A) Representative immunofluorescent image showing SNL-induced GFAP (satellite glial cell marker) and Iba1 (macrophage marker) over-expression was attenuated by exosome-scaffold treatment on day 7 after surgery. The white spots are DAPI nuclear staining. Scale bar: 25 μm. (B) Left: representative Western blots of c-Fos (postsurgery day 1), GFAP and Iba1 (postsurgery day 7) of ipsilateral L5/6 DRG, actin used as internal control. Right: summarized graph depicting protein levels presented as raw data points and Mean ± SD, taking Sham/saline-scaffold group as 100% (*P<0.05, Kruskal–Wallis test followed by Dunn’s multiple pairwise comparisons test, n=3 in each group).
It is well known that nerve injury can activate glial cells in DRG. Figure 5A shows SNL-induced up-regulation of GFAP (satellite glial cell marker) and Iba1 (macrophage marker) in ipsilateral L5 DRG on postsurgery day 7 was attenuated by EX-SC treatment. Western blot data also revealed EX-SC treatment significantly reversed SNL-induced up-regulation of GFAP and Iba1 in the ipsilateral L5/6 DRGs (P<0.05, Figure 5B).
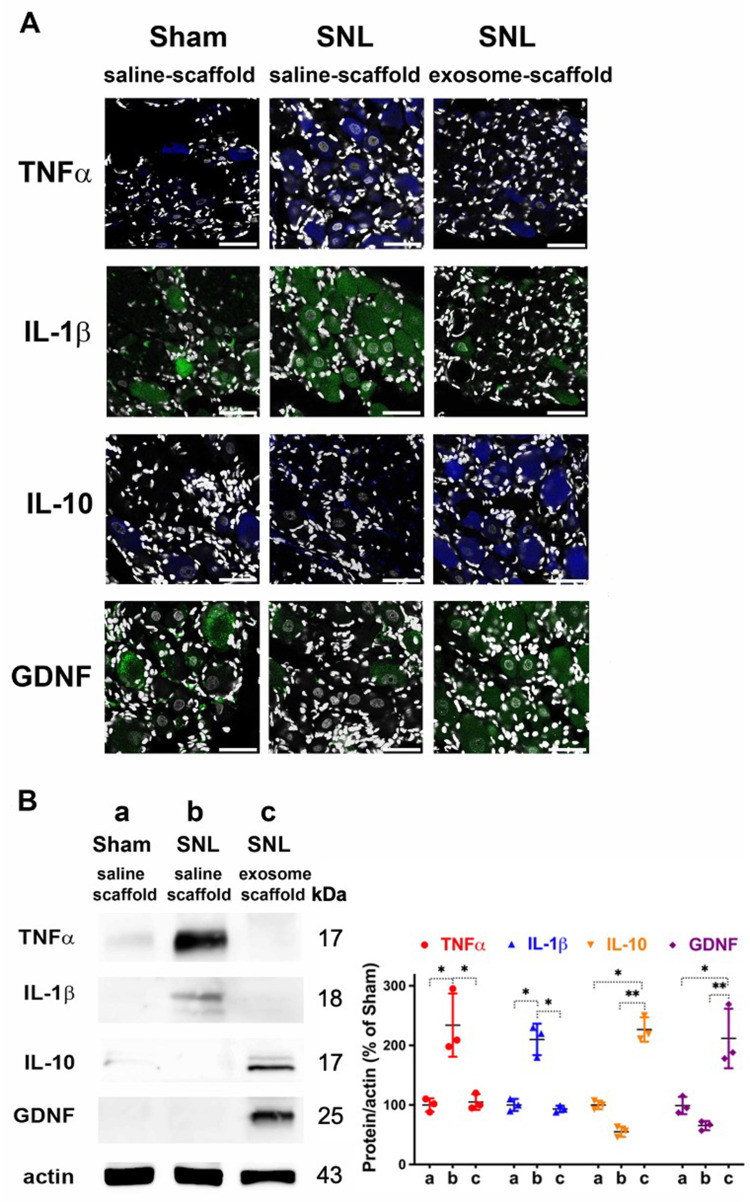
The Exosome-Scaffold Suppresses SNL-Induced Over-Expression of Inflammatory Cytokines but Enhances IL-10 and GDNF Expression
Our immunostaining data revealed SNL increased TNF-α and IL-1β in the ipsilateral L5 DRG, and EX-SC treatment attenuated this increase (Figure 6A). Western blot data further confirmed EX-SC significantly attenuated SNL-induced over-expression of TNF-α and IL-1β in the ipsilateral L5/6 DRGs on postsurgery day 7 (P<0.05, Figure 6B). In addition, we observed the EX-SC enhanced the expression of IL-10 and GDNF in the ipsilateral L5 DRG on postsurgery day 7 (Figure 6A). Western blot data also confirmed the EX-SC enhanced the expression of IL-10 and GDNF after SNL in the ipsilateral L5/6 DRGs (P<0.01, Figure 6B).
Figure 6.
Implantation of stem cell exosome-scaffold on ligated nerves suppresses L5/6 spinal nerve ligation (SNL)-induced inflammation on day 7 after surgery. (A) Representative immunofluorescent image showing the expression of TNF-α, IL-1β, IL-10 and GDNF in ipsilateral L5 DRG. The white spots are DAPI nuclear staining. Scale bar: 50 μm. (B) Left: representative Western blots of TNF-α, IL-1β, IL-10, GDNF and control protein actin in ipsilateral L5/6 DRG. Right: summarized graph depicting protein levels presented as raw data points and Mean ± SD, taking Sham/saline-scaffold group as 100% (*P<0.05, **P<0.01, Kruskal–Wallis test followed by Dunn’s multiple pairwise comparisons test, n=3 in each group).
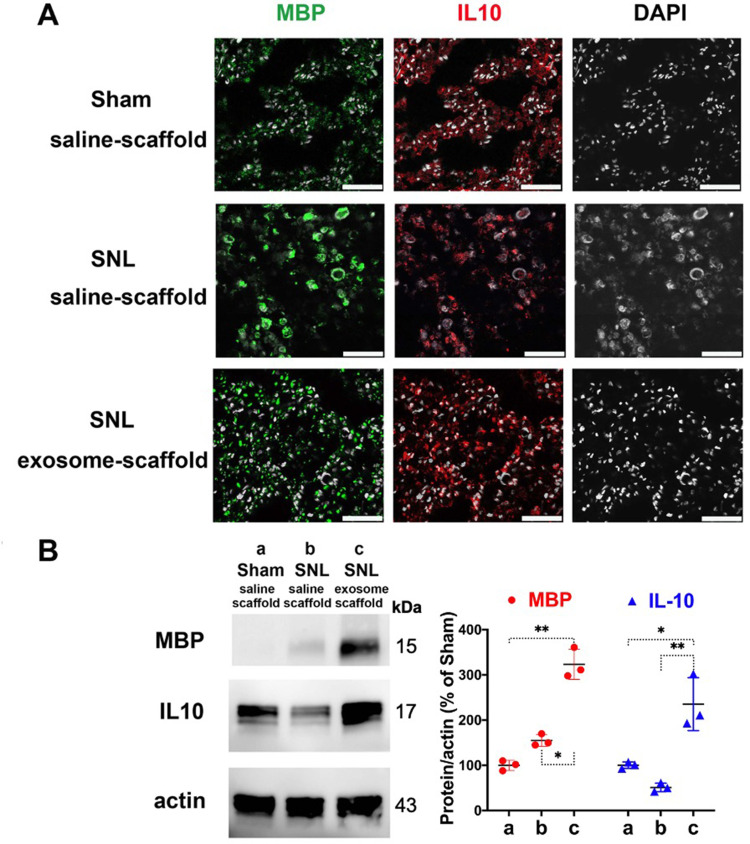
The Exosome-Scaffold Induces Myelination in Injured L5 Nerve
After implantation for 21 days, the EX-SC seemed to induce MBP expression in the injured L5 axons and the MBP distribution pattern is recovered, more like that in the Sham/saline-scaffold group (Figure 7A). Western blot also revealed the EX-SC significantly enhanced MBP expression on postsurgery day 21 (P<0.05, Figure 7B). The pro-regenerative immune mediator IL-10, which could support remyelination,14 was also significantly higher in the SNL/exosome-scaffold group than in the SNL/saline-scaffold group in the injured L5 axons on postsurgery day 21 (P<0.01, Figure 7B).
Figure 7.
Implantation of stem cell exosome-scaffold on ligated nerves enhances myelin basic protein (MBP) and interleukin-10 (IL-10) expression in injured nerves of L5/6 spinal nerve ligation (SNL) rats on day 21 after surgery. (A) Representative immunofluorescent image showing the expression of MBP, IL-10 and DAPI (white spots, nuclear staining) in ipsilateral L5 spinal nerve. Scale bar: 50 μm. (B) Left: representative Western blots of MBP, IL-10 and control protein actin in ipsilateral L5/6 spinal nerves. Right: summarized graph depicting protein levels presented as raw data points and Mean ± SD, taking Sham/saline-scaffold group as 100% (*P<0.05, **P<0.01, Kruskal–Wallis test followed by Dunn’s multiple pairwise comparisons test, n=3 in each group).
Discussion
The present study demonstrates the EX-SC successfully attenuated SNL-induced pain and enhanced axon regeneration in rat injured nerves. The sponge-like alginate scaffold showed excellent exosome adsorption and slow release ability. The immunostaining data revealed the exosomes located in the ipsilateral L5 DRG and axons, indicating they could be successfully released from scaffolds into injured nerves in vivo. The analgesic effects of EX-SC could be detectable as early as 24 h after implantation and remained obvious on post ligation day 21. After implantation for 21 days, the EX-SC enhanced MBP and IL-10 expression in the injured L5 axons. Herein, we demonstrate the EX-SC displays excellent antinociceptive and neuro-regenerative effects in SNL rats.
MSC exosome contains proteins and functional RNAs that exert neuroprotective and regenerative potential on damaged tissues.5 In this study, we isolated UCMSC exosomes using a size-based membrane filter technique. Compared with ultracentrifugation and exosome precipitation, sequential filtration allows the isolation of exosomes with high purity and functional integrity as a result of low manipulation forces.15 In our in vitro study, pre-treatment with exosome protects PC12 and HEK293 cells from FA-induced damage (Figure S1A), supporting neuro-protective and anti-inflammatory effects of UCMSC exosomes. In the UCMSC exosomes, we detected some cytokines and growth factors that have beneficial effects for the neuronal system, such as follistatin, HGF, fractalkine/CX3CL1 and IL-10 (Figure 2C). Follistatin is an endogenous activin-binding protein and offers considerable promise as a therapeutic in conditions as diverse as sepsis, liver fibrosis, acute lung injury, asthma, wound healing and ischemia-reperfusion injury.16 HGF is an anti-inflammatory cytokine and involved in remyelination.17 Fractalkine/CX3CL1 is involved in CX3CL1-CX3CR1 signaling, representing the most important communication channel between neurons and microglia.18 CX3CL1 has been reported to switch microglia from a detrimental phenotype to a neuroprotective one.19 We also identified 32 miRNA in the UCMSC exosomes (data not shown), 25 of them were predicted to be involved in pain regulation. Further transcriptome research of UCMSC exosomes are required to reveal detailed mechanisms.
In this report, the EX-SC exhibited excellent analgesic effects for SNL-induced pain. The analgesic effect could be detectable at 24 h after scaffold implantation in the neuropathic pain maintenance stage (Figure 3B and C Figure 3AandB) and initial stage (Figure 3D and E Figure 3CandD). On the second day after implantation, the EX-SC almost abolished SNL-induced nociceptive responses (Figure 3). It is also noted that the antinociceptive effects of EX-SC were better than those of exosome single injection (Figure 3B and C Figure 3AandB), indicating the scaffold can provide a suitable microenvironment to promote exosomes for pain treatment. Generally, exosomes can be quickly cleared from tissues with a duration of tracking of 1 min to 24 h in vivo.20 Moreover, the analgesic effects of EX-SC remained obvious on post ligation day 21 (Figure 3D and E Figure 3CandD), suggesting the EX-SC could induce long-lasting antinociceptive effects. These data support that the bioresorbable alginate scaffold is an excellent carrier for UCMSC exosomes and thus the EX-SC may be considered for future clinical trials, as a biodegradable conduit for peripheral nerve repair.21
In this study, the UCMSC exosomes were successfully released from alginate scaffold to nerve injury area. The Exo-green-labelled exosomes were detected in the ipsilateral L5 DRG and nerve and localized within IB4+ and CGRP+ nociceptors on post ligation day 3 (Figure 4). The EX-SC showed abilities to suppress SNL-induced c-Fos over-expression (Figure 5B), glial activation (Figure 5) and up-regulation of TNF-α and IL-1β (Figure 6). TNF-α is a potent pro-inflammatory cytokine and has been reported to activate the release of IL-1β, IL-6 and IL-8, leading to the development of inflammatory hyperalgesia.22 The EX-SC also enhanced GDNF expression in SNL rats (Figure 6), implicating its pro-neurotrophic ability. GDNF is a member of the neurotrophin family and promotes neuronal differentiation, survival and neurogenesis.23 All these data showed similar results as our previous study, in which we demonstrated antinociceptive effects of UCMSC exosomes via continuous intrathecal infusion for 7 days in SNL male rats.9 Nevertheless, according to the report of Mapplebeck et al.,24 future studies are needed to elucidate if there is sexual dimorphism for the effects of UCMSC exosomes. Herein, we provide another more clinically feasible and effective route/method of exosome application than intrathecal infusion.9 To our knowledge, this is the first study reporting the utilization of EX-SC in neuropathic pain.
Myelination of the nervous system is important for axonal function and structure stability.25 After nerve injury, remyelination involves reinvesting demyelinated axons with new myelin sheaths. Microglia/macrophages are major contributors to post-injury inflammation, but they may also promote neural repair when they are polarized towards an activated M2 state.26 Classically activated M1 macrophages/microglia are known to promote and exacerbate inflammation through their production of pro-inflammatory cytokines (i.e. IL-1β, TNF-α, IL-6, IL-12, IL-23 and IFN-γ), proteases and reactive oxygen species.27 In contrast, activated M2 macrophages/microglia are associated with phagocytosis of myelin debris and secretion of growth-promoting factors (i.e. IL-10 and insulin-like growth factor 1) that support axonal sprouting and remyelination.14,28
In this study, MBP and IL-10 were up-regulated in injured L5 axons after EX-SC treatment for 21 days (Figure 7), indicating the EX-SC could promote axon regeneration in injured nerves. MBP is a marker for myelin and localized in the myelin sheath surrounding myelinated axons. IL-10, an anti-inflammatory cytokine, is synthesized in microglia and astrocytes and can inhibit microglial secretion of pro-inflammatory cytokines such as TNF-α.29 Our cell culture data also revealed UCMSC exosomes could induce neurite outgrowth of PC12 cells (Figure S1C). In previous reports, MSCs implanted into injured neural tissue up-regulate M2 macrophages/microglia and improve neurological function recovery.30 Our data demonstrate the EX-SC possesses the potential to promote axon regeneration in injured nerves.
Conclusion
In conclusion, the locally applied EX-SC displays excellent antinociceptive effects for nerve injury-induced pain. It also inhibits SNL-induced neuro-inflammation and promotes expression of anti-inflammatory cytokine and neurotrophic factor. The combination of alginate scaffold and UCMSC exosomes could be a promising alternative for the treatment of neuropathic pain and nerve injury.
Acknowledgments
We thank Han-Yu Shen, Jia-Shun Hong and Zi-Hao Liu in Prof. Hsin-Yi Lin’s lab for scaffold preparation and characterization; Zhi-Xiang Li in Prof. Kuender D. Yang’s Lab for exosome preparation.
Presentation: Part of this work has been presented as poster at the annual meeting of Society for Neuroscience (Oct. 19-23, 2019), Chicago, USA.
Funding Statement
This work was supported by grants from Ministry of Science and Technology, Taiwan [MOST 105-2314-B-195-003-MY3 and 108-2314-B-195-006-MY3 (to J.K.C.); 106-2811-B-195-001 and 107-2811-B-195-500 (to S.J.S.); 108-2811-B-195-500 (to P.P.)]; Mackay Memorial Hospital, Taipei, Taiwan [MMH-E-108-15, MMH-E-109-15, MMH 10723, 10799, 107105, TT-10705, 10801 (to J.K.C.); MMH-E-107-05 (to K.D.Y.)]; and National Taipei University of Technology [NTUT-MMH Joint Research Program, NTUT-MMH-107-05, NTUT-MMH-108-01 and 108DMH0100032 (to H.Y.L.)].
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.Belanger K, Dinis TM, Taourirt S, Vidal G, Kaplan DL, Egles C. Recent strategies in tissue engineering for guided peripheral nerve regeneration. Macromol Biosci. 2016;16:472–481. doi: 10.1002/mabi.201500367 [DOI] [PubMed] [Google Scholar]
- 2.Ren Z, Wang Y, Peng J, Zhao Q, Lu S. Role of stem cells in the regeneration and repair of peripheral nerves. Rev Neurosci. 2012;23:135–143. doi: 10.1515/revneuro-2011-0069 [DOI] [PubMed] [Google Scholar]
- 3.Dlouhy BJ, Awe O, Rao RC, Kirby PA, Hitchon PW. Autograft-derived spinal cord mass following olfactory mucosal cell transplantation in a spinal cord injury patient: case report. J Neurosurg Spine. 2014;21:618–622. doi: 10.3171/2014.5.spine13992 [DOI] [PubMed] [Google Scholar]
- 4.Ludwig AK, Giebel B. Exosomes: small vesicles participating in intercellular communication. Int J Biochem Cell Biol. 2012;44:11–15. doi: 10.1016/j.biocel.2011.10.005 [DOI] [PubMed] [Google Scholar]
- 5.Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells. 2017;35:851–858. doi: 10.1002/stem.2575 [DOI] [PubMed] [Google Scholar]
- 6.Borger V, Bremer M, Ferrer-Tur R, et al. Mesenchymal stem/stromal cell-derived extracellular vesicles and their potential as novel immunomodulatory therapeutic agents. Int J Mol Sci. 2017;18:1450. doi: 10.3390/ijms18071450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiao G, Pan Y, Wang C, Li Z, Li Z, Guo R. A bridging SF/Alg composite scaffold loaded NGF for spinal cord injury repair. Mater Sci Eng C Mater Biol Appl. 2017;76:81–87. doi: 10.1016/j.msec.2017.02.102 [DOI] [PubMed] [Google Scholar]
- 8.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9 [DOI] [PubMed] [Google Scholar]
- 9.Shiue SJ, Rau RH, Shiue HS, et al. Mesenchymal stem cell exosomes as a cell-free therapy for nerve injury-induced pain in rats. Pain. 2019;160:210–223. doi: 10.1097/j.pain.0000000000001395 [DOI] [PubMed] [Google Scholar]
- 10.Chung JM, Kim HK, Chung K. Segmental spinal nerve ligation model of neuropathic pain. Methods Mol Med. 2004;99:35–45. doi: 10.1385/1-59259-770-X:035 [DOI] [PubMed] [Google Scholar]
- 11.Shiue SJ, Peng HY, Lin CR, Wang SW, Rau RH, Cheng JK. Continuous intrathecal infusion of cannabinoid receptor agonists attenuates nerve ligation-induced pain in rats. Reg Anesth Pain Med. 2017;42:499–506. doi: 10.1097/AAP.0000000000000601 [DOI] [PubMed] [Google Scholar]
- 12.Gao YJ, Ji RR. c-Fos and pERK, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? Open Pain J. 2009;2:11–17. doi: 10.2174/1876386300902010011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang XQ, Ren YK, Chen RQ, et al. Formaldehyde induces neurotoxicity to PC12 cells involving inhibition of paraoxonase-1 expression and activity. Clin Exp Pharmacol Physiol. 2011;38:208–214. doi: 10.1111/j.1440-1681.2011.05485.x [DOI] [PubMed] [Google Scholar]
- 14.Hu JG, Shi LL, Chen YJ, et al. Differential effects of myelin basic protein-activated Th1 and Th2 cells on the local immune microenvironment of injured spinal cord. Exp Neurol. 2016;277:190–201. doi: 10.1016/j.expneurol.2016.01.002 [DOI] [PubMed] [Google Scholar]
- 15.Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in exosome isolation techniques. Theranostics. 2017;7:789–804. doi: 10.7150/thno.18133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedger MP, de Kretser DM. The activins and their binding protein, follistatin-diagnostic and therapeutic targets in inflammatory disease and fibrosis. Cytokine Growth Factor Rev. 2013;24:285–295. doi: 10.1016/j.cytogfr.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 17.Moghadam S, Erfanmanesh M, Esmaeilzadeh A. Interleukin 35 and hepatocyte growth factor; as a novel combined immune gene therapy for multiple sclerosis disease. Med Hypotheses. 2017;109:102–105. doi: 10.1016/j.mehy.2017.09.017 [DOI] [PubMed] [Google Scholar]
- 18.Mecca C, Giambanco I, Donato R, Arcuri C. Microglia and aging: the role of the TREM2-DAP12 and CX3CL1-CX3CR1 axes. Int J Mol Sci. 2018;19:318. doi: 10.3390/ijms19010318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giunti D, Parodi B, Usai C, et al. Mesenchymal stem cells shape microglia effector functions through the release of CX3CL1. Stem Cells. 2012;30:2044–2053. doi: 10.1002/stem.1174 [DOI] [PubMed] [Google Scholar]
- 20.Gangadaran P, Hong CM, Ahn BC. Current perspectives on in vivo noninvasive tracking of extracellular vesicles with molecular imaging. Biomed Res Int. 2017;9158319. doi: 10.1155/2017/9158319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kusuhara H, Hirase Y, Isogai N, Sueyoshi Y. A clinical multi-center registry study on digital nerve repair using a biodegradable nerve conduit of PGA with external and internal collagen scaffolding. Microsurgery. 2019;39:395–399. doi: 10.1002/micr.30417 [DOI] [PubMed] [Google Scholar]
- 22.Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004;361:184–187. doi: 10.1016/j.neulet.2003.12.007 [DOI] [PubMed] [Google Scholar]
- 23.Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812 [DOI] [PubMed] [Google Scholar]
- 24.Mapplebeck JC, Beggs S, Salter MW. Sex differences in pain: A tale of two immune cells. Pain. 2016;157(Suppl 1):S2–6. doi: 10.1097/j.pain.0000000000000389 [DOI] [PubMed] [Google Scholar]
- 25.Nave KA, Trapp BD. Axon-glial signaling and the glial support of axon function. Annu Rev Neurosci. 2008;31:535–561. doi: 10.1146/annurev.neuro.30.051606.094309 [DOI] [PubMed] [Google Scholar]
- 26.Ransohoff RM. A polarizing question: do M1 and M2 microglia exist? Nat Neurosci. 2016;19:987–991. doi: 10.1038/nn.4338 [DOI] [PubMed] [Google Scholar]
- 27.Miron VE, Franklin RJ. Macrophages and CNS remyelination. J Neurochem. 2014;130:165–171. doi: 10.1111/jnc.12705 [DOI] [PubMed] [Google Scholar]
- 28.Gensel JC, Zhang B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 2015;1619:1–11. doi: 10.1016/j.brainres.2014.12.045 [DOI] [PubMed] [Google Scholar]
- 29.Sawada M, Suzumura A, Hosoya H, Marunouchi T, Nagatsu T. Interleukin-10 inhibits both production of cytokines and expression of cytokine receptors in microglia. J Neurochem. 1999;72:1466–1471. doi: 10.1046/j.1471-4159.1999.721466.x [DOI] [PubMed] [Google Scholar]
- 30.Xu C, Fu F, Li X, Zhang S. Mesenchymal stem cells maintain the microenvironment of central nervous system by regulating the polarization of macrophages/microglia after traumatic brain injury. Int J Neurosci. 2017;127:1124–1135. doi: 10.1080/00207454.2017.1325884 [DOI] [PubMed] [Google Scholar]