Abstract
Background:
Ovarian cancer patients enrolled in phase I trials are typically platinum resistant, heavily pretreated, with a poor prognosis. We assessed prognostic factors and survival in women with recurrent ovarian cancer treated in phase I clinical trials.
Methods:
We performed a retrospective analysis of patients treated from 2008 through 2018 at the University of Colorado Cancer Center. Patient characteristics, treatment and toxicity related survival data were assessed. Descriptive statistics and Cox proportional hazards models were utilized to identify risk factors associated with survival time.
Results:
132 patients were treated on phase I clinical trials. Patients had a median age of 59 years (range 33–88) with a median of 5.5 (range 1–13) previous chemotherapy lines. 53/132 (40%) of patients were treated on multiple phase I trials with a median of 1 (range 0–5). Overall response rate was 14.7%. Median overall survival was 11.3 months (95% CI: 9.1–13.4). Two patients died on trial due to progression of disease while no patients died due to treatment-related toxicity. Independent risk factors predicting shorter survival were elevated CA-125 (HR 2.8; 95% CI: 1.6–5.2) and albumin <3.5 g/dL (HR 2.5; 95% CI: 1.65–3.79). BMI >25 predicted longer survival (HR 0.65; 95% CI: 0.44–0.96).
Conclusions:
In this single institution series, patients with heavily pretreated ovarian cancer treated in Phase I clinical trials experienced a median overall survival of 11.3 months. Phase I clinical trials represent a reasonable treatment option for heavily pretreated ovarian cancer patients with a preserved performance status when available.
Keywords: Ovarian Cancer, Clinical Trial, Phase I, Phase 1, Recurrent Ovarian Cancer
Precis:
Phase I clinical trials are a safe and effective therapy option for women with recurrent platinum resistant ovarian cancer. Phase I trial enrollment should be considered, when available, for women with this disease.
Background:
Ovarian cancer incidence and mortality rates have been declining over the past decade according to SEER data analysis. However, five-year survival for advanced stage disease remains less than 30% (1). Over 80% of women diagnosed with ovarian cancer will recur, with an eventual progression to platinum-resistant disease. Cytotoxic chemotherapy has been the mainstay of treatment for recurrent disease with incorporation of targeted therapies such as VEGF and PARP inhibitors. The majority of ovarian cancer patients will receive multiple lines of systemic therapy through their disease course. When available, clinical trials are often incorporated into patient management. NCCN guidelines recommend that a clinical trial is the best management for patients with cancer and encourage enrollment when available (2).
Later phase clinical trials are always preferred by both providers and patients due to a perceived higher likelihood of clinical benefit with more predictable toxicity and phase I clinical trials are often only considered for patients who have exhausted other clinical options. Phase I trials are designed to evaluate toxicity profiles and determine a recommended phase II dose for future studies. An historical viewpoint of phase I trials has questioned the ethics of enrolling patients on to trials designed for safety rather than efficacy (3, 4). However, data from phase I trials over the past decade have demonstrated not only favorable safety profiles, but also more therapeutic benefit than in the past (5, 6). In a heavily pretreated ovarian cancer patient population, therapeutic options are often only expected to demonstrate efficacy on the order of months as described the AURELIA trial (7). There is a paucity of literature evaluating clinical outcomes for ovarian cancer patients treated in phase I clinical trials. We evaluated both the safety and clinical outcomes of patients enrolled in phase I clinical trials at the University of Colorado Cancer Center. Our aim was to further characterize this population with a focused analysis of their survival.
Methods:
A retrospective analysis of all ovarian cancer patients enrolled in phase I clinical trial at the University of Colorado Cancer Center from January 2008 through December 2018 was performed. Patents were identified through an IRB-approved phase I registry and electronic medical records were reviewed. Data was collected regarding patient characteristics, treatment, and clinical outcomes. Only patients who were enrolled and received treatment in a phase I trial were included in the analysis. By nature of clinical trial enrollment, all patients were age ≥18 and were enrolled on an IRB-approved phase I clinical trial at our institution. Initial tumor pathology and surgical outcomes were recorded along with laboratory and clinical assessment at time of trial enrollment. Clinical response was reported as the best response defined by RECIST imaging assessment.
Statistical Analysis:
Statistical analysis was performed using IBM SPSS statistical software. Chi-square and Cox regression multivariate analysis were utilized to evaluate associated factors with survival time. Kaplan- Meier estimations were utilized to report survival outcomes. Progression-free survival (PFS) and overall survival (OS) were defined as time from trial enrollment until documented disease progression, death, or lost to follow-up.
Results:
Patient characteristics
One hundred thirty-two patients with ovarian cancer were identified. A total of 222 data points were collected as multiple patients enrolled on more than one phase I clinical trial. Median age was 59 years (range 33–88) and all patients had an ECOG performance status of 0 or 1. Patient characteristics are demonstrated in Table 1. Laboratory analysis at time of trial enrollment are reported as greater than or less than institutional laboratory normal values (Table 2). Multivariate analysis demonstrates that CA-125 >35 U/mL (HR 2.8; 95%CI: 1.6–5.2) and albumin <3.5 g/dL (HR 2.5; 95% CI 1.65–3.79) predict shorter survival while BMI >25 kg/m2 (HR 0.65; 95%CI 0.44–0.96) predicts longer survival. Median BMI was 24.2 kg/m2 (range 15.9–55.3). Only 5 patients had a BMI <18 kg/m2.
Table 1:
Patient characteristics of all patients with ovarian cancer enrolled in phase I clinical trial over a 10 year time period.
| Variable | N (132) | % |
|---|---|---|
| Age | ||
| Median: 59 | Range: 33–88 | |
| BMI | ||
| Median: 25.7 | Range: 11.6–55.3 | |
| Race | ||
| White | 117 | 88.6 |
| Asian | 1 | 0.7 |
| Black | 6 | 4.5 |
| Other | 6 | 4.5 |
| Unknown | 2 | 1.5 |
| Histology | ||
| Serous | 96 | 72.7 |
| Endometrioid | 5 | 3.8 |
| Clear Cell | 3 | 2.3 |
| Mucinous | 3 | 2.3 |
| Small Cell | 1 | 0.8 |
| Neuroendocrine | 1 | 0.8 |
| Squamous | 1 | 0.8 |
| Carcinosarcoma | 4 | 3.0 |
| Unrecorded | 18 | 13.6 |
| Initial Stage | ||
| I | 6 | 4.5 |
| II | 5 | 3.8 |
| III | 78 | 59.1 |
| IV | 35 | 26.5 |
| Unrecorded | 8 | 6.1 |
| Number of Prior Phase I Trials | ||
| 0 | 78 | 59.1 |
| 1 | 43 | 32.6 |
| 2 | 8 | 6.1 |
| 3 | 2 | 1.5 |
| 4 | 1 | 0.8 |
| Number of prior therapy regimens | ||
| 1–2 | 13 | 9.8 |
| 3–4 | 33 | 25.0 |
| 5–7 | 65 | 49.2 |
| 8–10 | 14 | 10.6 |
| >10 | 4 | 3.0 |
| Unrecorded | 3 | 2.3 |
Table 2:
Laboratory assessment at the time of trial enrollment for 222 individual patient enrollments. Variable frequencies are reports as at, above or below respective normal values per institutional standards.
| Variable | Percent (%) |
|---|---|
| CA-125 | |
| <35 | 13.5 |
| ≥35 | 82.9 |
| ECOG Performance Status | |
| 0 | 25.7 |
| 1 | 74.3 |
| BMI | |
| <25 | 45.9 |
| ≥25 | 53.2 |
| WBC (10*9/L) | |
| <11 | 94.6 |
| ≥11 | 5.4 |
| Hemoglobin (g/dL) | |
| <12 | 40.1 |
| ≥12 | 59.9 |
| ANC (10*9/L) | |
| <1.5 | 2.7 |
| 1.5–8 | 92.3 |
| >8 | 5 |
| Platelet Count (10*9/L) | |
| <150 | 7 |
| 151–400 | 84 |
| >400 | 9 |
| Creatinine (mg/dL) | |
| <1.2 | 92.3 |
| ≥1.2 | 7.7 |
| Albumin (g/dL) | |
| <3.5 | 71.6 |
| ≥3.5 | 28.4 |
| LDH (U/L) | |
| <190 | 38.7 |
| ≥190 | 37.8 |
| Not recorded | 23.4 |
| ALT (U/L) | |
| <52 | 92.3 |
| ≥52 | 7.7 |
| AST (U/L) | |
| <39 | 89.2 |
| ≥39 | 10.8 |
| Total Bilirubin (mg/dL) | |
| <1.3 | 99.5 |
| ≥1.3 | 0.5 |
Previous treatment
At the time of trial enrollment, patients had a median of 5.5 (range 1–13) prior lines of therapy. As described above, many patients also enrolled on more than one phase I clinical trial at our institution. The median number of prior phase I trials was 1 with a range of 0–4. 41% of patients enrolled on more than one phase I trial. There were 74 unique clinical trials that patients were enrolled onto. All trials were inclusive of multiple tumor types or “all comers” trials. No trial was gynecologic or ovarian cancer specific. Trial therapies were highly variant with cytotoxic, targeted, and combination regimens. 24/222 (10.8%) of patients received cytotoxic therapy alone, 165/222 (74.3%) received a targeted therapy, and 33/222 (14.9%) received a combination of targeted and cytotoxic therapy. No treatment modality demonstrated obvious superiority.
Toxicity
No patient died due to treatment-related toxicity related to an investigational agent. Two patients died while on trial related to progression of disease. Only 19/222 (9%) patients came off study due to treatment related toxicities. 75% (166/222) of patients came off of trial due to progression of disease.
Clinical outcomes
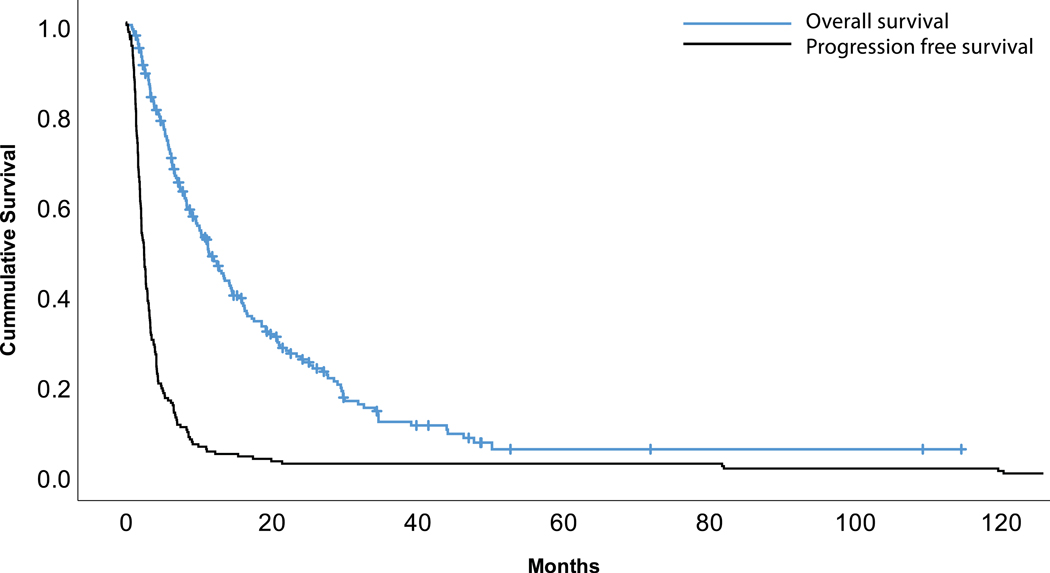
Of 222 patient enrollments, 204 were evaluable for our efficacy assessment based on a recorded best response by RECIST criteria (Table 3). Two patients (1%) obtained a complete response (CR), 28 (13.7%) had a partial response (PR), 68 (33.3%) had stable disease (SD), and 106 (52%) had progressive disease (PD). Overall response rate (ORR) as defined as patients with CR + PR was 14.7%. Survival outcomes demonstrated a median PFS of 2.5 months (95%CI: 2.1–2.9) and a median OS of 11.3 months (95%CI: 9.1–13.4). Kaplan-Meier curves illustrate these survival rates (Figure 1).
Table 3:
Response for 204 evaluable patients as reported by best response on clinical trial by RECIST criteria.
| N | % | |
|---|---|---|
| Complete Response (CR) | 2 | 1 |
| Partial Response (PR) | 28 | 13.7 |
| Stable Disease (SD) | 68 | 33.3 |
| Progressive Disease (PD) | 106 | 52 |
| Overall Response Rate (ORR) | 14.7 |
Figure 1:
Kaplan-Meier curves for progression free survival (PFS) and overall survival (OS).
Discussion:
Clinical trial design has significantly evolved over the past several decades, specifically with an aim to bring modern therapeutic options to patients in a timely manner. The backbone of trial design has been to sequence from phase I-III with an ultimate goal of improved patient outcomes, a favorable safety profile and FDA approval. Establishing the safety of new drugs or new combinations of drugs is imperative prior to exposing large number of patients to a potentially beneficial or non-beneficial therapeutic option.
In the late 1990’s and early 2000’s, concern regarding the perceived toxicity and lack of potential for therapeutic benefit of phase I trials spawned multiple publications evaluating safety and ultimately citing a consistent toxicity-related death rate of 0.5% (8–10). This rate has remained relatively stable over the past several decades within the limited published literature (11, 12). In this single institution study, the rate of treatment discontinuation due to treatment-related toxicities was low at 9% and we did not observe any treatment-related deaths. The rate of treatment discontinuation compares favorably to previous reports citing 12% trial withdrawal due to toxicity (11) and rates of serious toxicity ranging from 10–14% (5, 10). While not distinctly different, this is consistent with trends of phase I clinical trials having fewer overall severe toxicities. Roberts et al demonstrated that over a 10 year period evaluating phase I clinical trials, the death rate due to toxicity and severe toxicity rate both decreased (10). They attributed potential causality due to several indications including an evolution from cytotoxic therapy trials to targeted therapeutic trials, as well an increase in attention to safety in clinical trial design. Our data are unique in confirming a favorable toxicity profile for phase I agents generally in a patient population homogeneous in the sense of having a single primary malignancy (ovarian cancer) previously treated with multiple lines of systemic therapy. We recognize that our patient population has the potential for self-selection towards a healthier patient cohort due to their eligibility for clinical trial enrollment. All patients in our cohort had a performance status of 0–1 and selection bias is likely present given poor historic outcomes for heavily pretreated ovarian cancer patients.
In addition to an acceptable predicted level of toxicity, the potential for clinical benefit of treatment with an investigational drug on a phase I trial is an important consideration. Our patient population was heavily pretreated, yet still demonstrated promising clinical outcomes. We observed an ORR of 14.7% and median OS of 11.3 months. By nature of the disease, all recurrent ovarian cancer patients will become platinum-resistant with a subsequent median OS of approximately 12 months (14). Other single tumor type phase I analyses, as well as large evaluations in survival of phase I patients, have evaluated responses and survival data. MD Anderson individually analyzed their clinical outcomes of patients with breast and colorectal cancers who were treated on phase I trials and reported an OS of 6.7 and 6.4 months respectively (15, 16). Evaluations of survival in phase I patient cohorts of all tumor types demonstrate OS of 8–10 months (12, 17, 18). It is important to recognize that phase I trials are not designed for efficacy outcomes analysis and there is great heterogeneity in regards to eligibility criteria and patient populations on a study to study level. Despite all of these limitations in our single institution study, response rates and overall survival for ovarian cancer patients treated in phase I studies does support this as a reasonable option for heavily pretreated patients.
Unique to our study, BMI >25 demonstrated a survival benefit for overweight women. This is contrary to a wealth of literature citing obesity as an increased risk of mortality in ovarian cancer (19–23). This was not a primary outcome for our analyses and was found on multivariate analyses evaluating for the patient characteristics associated with outcome. One area of discrepancy is that BMI for this study was recorded at the time of initiation of trial while other studies have evaluated pre-diagnosis BMI. As expected, and consistent with existing literature, nutritional status as recorded by albumin levels in our study demonstrate that lower albumin levels are associated with increased mortality. It is not clear why an elevated BMI demonstrates survival benefit in our cohort. We hypothesize that obese patients towards end of life have a higher nutritional reserve than those without, which may contribute to longer survival but acknowledge that further evaluation of this phenomenon is warranted.
In conclusion, this study is the first to evaluate the ovarian cancer patient population from a perspective of those treated on phase I clinical trials. We demonstrate that at our institution patients treated in a phase I clinical trial have acceptable outcomes for this difficult patient population. The NCCN guidelines advocate for clinical trial enrollment in this patient population and we concordantly agree that phase I trials should be highly considered for all recurrent ovarian cancer patients when available to them.
Acknowledgements:
We would like to acknowledge the University of Colorado Women’s Cancer Developmental Therapeutics Program for institutional support of this research proposal.
No funding was directly associated with this manuscript. This work was supported by the National Institutes of Health (NIH) and the National Cancer Institute (NCI) through 5P30CA046934–25 (University of Colorado Cancer Center Support Grant) and 1K23CA172691–01A1 (J.R. Diamond).
There are no relative potential conflicts of interests for any of the authors in relation to this manuscript. Dr. Bradley Corr discloses that he sits on advisory boards for Genentech, Novocure, Merck & GSK/Tesaro. He also has research funding supplied by Clovis.
Footnotes
Dr. Eckhardt was associated with the University of Colorado during patient collection period but is currently associated with Dell Medical School, The University of Texas at Austin.
References:
- 1.Howlader N NA, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER Cancer Statistics Review, 1975–2016, National Cancer Institute; Available from: https://seer.cancer.gov/csr/1975_2016/. [Google Scholar]
- 2.Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Barroilhet L, Behbakht K, Berchuck A, Berek JS, Chen LM, Cristea M, DeRosa M, ElNaggar AC, Gershenson DM, Gray HJ, Hakam A, Jain A, Johnston C, Leath CA III, Liu J, Mahdi H, Matei D, McHale M, McLean K, O’Malley DM, Penson RT, Percac-Lima S, Ratner E, Remmenga SW, Sabbatini P, Werner TL, Zsiros E, Burns JL, Engh AM. NCCN Guidelines Insights: Ovarian Cancer, Version 1.2019. Journal of the National Comprehensive Cancer Network : JNCCN. 2019;17(8):896–909. Epub 2019/08/08. doi: 10.6004/jnccn.2019.0039. [DOI] [PubMed] [Google Scholar]
- 3.Agrawal M, Emanuel EJ. Ethics of phase 1 oncology studies: reexamining the arguments and data. JAMA : the journal of the American Medical Association. 2003;290(8):1075–82. Epub 2003/08/28. doi: 10.1001/jama.290.8.1075. [DOI] [PubMed] [Google Scholar]
- 4.Lipsett MB. On the nature and ethics of phase I clinical trials of cancer chemotherapies. JAMA : the journal of the American Medical Association. 1982;248(8):941–2. Epub 1982/08/27. [PubMed] [Google Scholar]
- 5.Horstmann E, McCabe MS, Grochow L, Yamamoto S, Rubinstein L, Budd T, Shoemaker D, Emanuel EJ, Grady C. Risks and benefits of phase 1 oncology trials, 1991 through 2002. N Engl J Med. 2005;352(9):895–904. Epub 2005/03/05. doi: 10.1056/NEJMsa042220. [DOI] [PubMed] [Google Scholar]
- 6.Kurzrock R, Benjamin RS. Risks and benefits of phase 1 oncology trials, revisited. N Engl J Med. 2005;352(9):930–2. Epub 2005/03/05. doi: 10.1056/NEJMe058007. [DOI] [PubMed] [Google Scholar]
- 7.Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, Sorio R, Vergote I, Witteveen P, Bamias A, Pereira D, Wimberger P, Oaknin A, Mirza MR, Follana P, Bollag D, Ray-Coquard I. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32(13):1302–8. Epub 2014/03/19. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- 8.Bachelot T, Ray-Coquard I, Catimel G, Ardiet C, Guastalla JP, Dumortier A, Chauvin F, Droz JP, Philip T, Clavel M. Multivariable analysis of prognostic factors for toxicity and survival for patients enrolled in phase I clinical trials. Ann Oncol. 2000;11(2):151–6. Epub 2000/04/13. doi: 10.1023/a:1008368319526. [DOI] [PubMed] [Google Scholar]
- 9.Decoster G, Stein G, Holdener EE. Responses and toxic deaths in phase I clinical trials. Ann Oncol. 1990;1(3):175–81. Epub 1990/01/01. doi: 10.1093/oxfordjournals.annonc.a057716. [DOI] [PubMed] [Google Scholar]
- 10.Roberts TG Jr., Goulart BH, Squitieri L, Stallings SC, Halpern EF, Chabner BA, Gazelle GS, Finkelstein SN, Clark JW. Trends in the risks and benefits to patients with cancer participating in phase 1 clinical trials. JAMA : the journal of the American Medical Association. 2004;292(17):2130–40. Epub 2004/11/04. doi: 10.1001/jama.292.17.2130. [DOI] [PubMed] [Google Scholar]
- 11.Arkenau HT, Olmos D, Ang JE, de Bono J, Judson I, Kaye S. Clinical outcome and prognostic factors for patients treated within the context of a phase I study: the Royal Marsden Hospital experience. Br J Cancer. 2008;98(6):1029–33. Epub 2008/03/20. doi: 10.1038/sj.bjc.6604218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Italiano A, Massard C, Bahleda R, Vataire AL, Deutsch E, Magne N, Pignon JP, Vassal G, Armand JP, Soria JC. Treatment outcome and survival in participants of phase I oncology trials carried out from 2003 to 2006 at Institut Gustave Roussy. Ann Oncol. 2008;19(4):787–92. Epub 2007/11/29. doi: 10.1093/annonc/mdm548. [DOI] [PubMed] [Google Scholar]
- 13.Moore KN, Secord AA, Geller MA, Miller DS, Cloven N, Fleming GF, Wahner Hendrickson AE, Azodi M, DiSilvestro P, Oza AM, Cristea M, Berek JS, Chan JK, Rimel BJ, Matei DE, Li Y, Sun K, Luptakova K, Matulonis UA, Monk BJ. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019;20(5):636–48. Epub 2019/04/06. doi: 10.1016/S1470-2045(19)30029-4. [DOI] [PubMed] [Google Scholar]
- 14.Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384(9951):1376–88. Epub 2014/04/29. doi: 10.1016/S0140-6736(13)62146-7. [DOI] [PubMed] [Google Scholar]
- 15.Hong DS, Patel JC, Wheler J, Naing A, Garrido-Laguna I, Falchook G, Fu S, Tsimberidou AM, Kopetz S, Win S, Kurzrock R. Outcomes in 144 patients with colorectal cancer treated in a phase I clinic: the MD Anderson Cancer Center experience. Clin Colorectal Cancer. 2012;11(4):297–303. Epub 2012/04/28. doi: 10.1016/j.clcc.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Wheler J, Tsimberidou AM, Moulder S, Cristofanilli M, Hong D, Naing A, Pathak R, Liu S, Feng L, Kurzrock R. Clinical outcomes of patients with breast cancer in a phase I clinic: the M. D. Anderson cancer center experience. Clin Breast Cancer. 2010;10(1):46–51. Epub 2010/02/06. doi: 10.3816/CBC.2010.n.006. [DOI] [PubMed] [Google Scholar]
- 17.Wheler J, Tsimberidou AM, Hong D, Naing A, Falchook G, Piha-Paul S, Fu S, Moulder S, Stephen B, Wen S, Kurzrock R. Survival of 1,181 patients in a phase I clinic: the MD Anderson Clinical Center for targeted therapy experience. Clin Cancer Res. 2012;18(10):2922–9. Epub 2012/03/29. doi: 10.1158/1078-0432.CCR-11-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wheler J, Tsimberidou AM, Hong D, Naing A, Jackson T, Liu S, Feng L, Kurzrock R. Survival of patients in a Phase 1 Clinic: the M. D. Anderson Cancer Center experience. Cancer. 2009;115(5):1091–9. Epub 2009/01/24. doi: 10.1002/cncr.24018. [DOI] [PubMed] [Google Scholar]
- 19.Kim SJ, Rosen B, Fan I, Ivanova A, McLaughlin JR, Risch H, Narod SA, Kotsopoulos J. Epidemiologic factors that predict long-term survival following a diagnosis of epithelial ovarian cancer. Br J Cancer. 2017;116(7):964–71. Epub 2017/02/17. doi: 10.1038/bjc.2017.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagle CM, Dixon SC, Jensen A, Kjaer SK, Modugno F, deFazio A, Fereday S, Hung J, Johnatty SE, Australian Ovarian Cancer Study G Fasching PA, Beckmann MW, Lambrechts D, Vergote I, Van Nieuwenhuysen E, Lambrechts S, Risch HA, Rossing MA, Doherty JA, Wicklund KG, Chang-Claude J, Goodman MT, Ness RB, Moysich K, Heitz F, du Bois A, Harter P, Schwaab I, Matsuo K, Hosono S, Goode EL, Vierkant RA, Larson MC, Fridley BL, Hogdall C, Schildkraut JM, Weber RP, Cramer DW, Terry KL, Bandera EV, Paddock L, Rodriguez-Rodriguez L, Wentzensen N, Yang HP, Brinton LA, Lissowska J, Hogdall E, Lundvall L, Whittemore A, McGuire V, Sieh W, Rothstein J, Sutphen R, Anton-Culver H, Ziogas A, Pearce CL, Wu AH, Webb PM, Ovarian Cancer Association C. Obesity and survival among women with ovarian cancer: results from the Ovarian Cancer Association Consortium. Br J Cancer. 2015;113(5):817–26. Epub 2015/07/08. doi: 10.1038/bjc.2015.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Protani MM, Nagle CM, Webb PM. Obesity and ovarian cancer survival: a systematic review and meta-analysis. Cancer Prev Res (Phila). 2012;5(7):901–10. Epub 2012/05/23. doi: 10.1158/1940-6207.CAPR-12-0048. [DOI] [PubMed] [Google Scholar]
- 22.Zamorano AS, Hagemann AR, Morrison L, Lee JA, Liao LM, Brinton LA, Park Y, Toriola AT. Pre-diagnosis body mass index, physical activity and ovarian cancer mortality. Gynecol Oncol. 2019;155(1):105–11. Epub 2019/08/07. doi: 10.1016/j.ygyno.2019.07.025. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Y, Chlebowski R, LaMonte MJ, Bea JW, Qi L, Wallace R, Lavasani S, Walsh BW, Anderson G, Vitolins M, Sarto G, Irwin ML. Body mass index, physical activity, and mortality in women diagnosed with ovarian cancer: results from the Women’s Health Initiative. Gynecol Oncol. 2014;133(1):4–10. Epub 2014/04/01. doi: 10.1016/j.ygyno.2014.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]